Abstract
Transient nociceptive stimuli elicit robust phase-locked local field potentials (LFPs) in the human insula. However, these responses are not preferential for nociception, as they are also elicited by transient non-nociceptive vibrotactile, auditory, and visual stimuli. Here, we investigated whether another feature of insular activity, namely gamma-band oscillations (GBOs), is preferentially observed in response to nociceptive stimuli. Although nociception-evoked GBOs have never been explored in the insula, previous scalp electroencephalography and magnetoencephalography studies suggest that nociceptive stimuli elicit GBOs in other areas such as the primary somatosensory and prefrontal cortices, and that this activity could be closely related to pain perception. Furthermore, tracing studies showed that the insula is a primary target of spinothalamic input. Using depth electrodes implanted in nine patients investigated for epilepsy, we acquired insular responses to brief thermonociceptive stimuli and similarly arousing non-nociceptive vibrotactile, auditory, and visual stimuli (59 insular sites). As compared to non-nociceptive stimuli, nociceptive stimuli elicited a markedly stronger enhancement of GBOs (150-300 ms post-stimulus) at all insular sites, suggesting that this feature of insular activity is preferential for thermonociception. Although this activity was also present in temporal and frontal regions, its magnitude was significantly greater in the insula as compared to these other regions.
Keywords: Pain, Nociception, Insula, Gamma-band oscillations, Local field potentials
Tracing studies have shown that the insula is a primary target for inputs ascending through the spinothalamic tract (Vogt et al. 1979; Willis 1985; Apkarian and Shi 1994; Craig 1996; Treede et al. 1999; Brooks and Tracey 2005), and several studies have suggested or assumed that the insula could play a key role in the ability to experience pain (Ostrowsky et al. 2002; Frot and Mauguière 2003; Isnard et al. 2004, 2011; Brooks and Tracey 2007; Mazzola et al. 2009, 2012; Garcia-Larrea et al. 2010; Garcia-Larrea 2012; Frot et al. 2014; Moayedi 2014; Segerdahl et al. 2015a). Supporting a role of the insula in pain perception is the observation, in a small subset of patients, that insular seizures and direct electrical stimulation of the insula can elicit pain-related sensations (Isnard et al. 2004, 2011; Mazzola et al. 2012). There are also case reports describing lesions of the insula leading to a selective impairment of pain perception (Garcia-Larrea et al. 2010). However, these reports have been recently questioned by studies finding no relationship between insular damage and the ability to perceive pain (Baier et al. 2014; Feinstein et al. 2016).
There is thus, at present, no consensus on the role of the insula in pain perception (Davis et al. 2015; Segerdahl et al. 2015b). Considering its heterogeneous cytoarchitecture and widespread anatomical connections (Augustine 1996; Cauda et al. 2011; Cerliani et al. 2012; Chang et al. 2013; Morel et al. 2013), it is widely acknowledged that the function of the insula is not limited to pain and nociception. Instead, the insula appears to be involved in the processing of a range of non-nociceptive sensory inputs, as well as in a number of cognitive, affective, interoceptive, and homeostatic functions (Davis et al. 1998; Craig et al. 2000; Wicker et al. 2003; Brass and Haggard 2010; Lamm and Singer 2010; Sterzer and Kleinschmidt 2010; Craig 2011; Furl and Averbeck 2011; Heydrich and Blanke 2013). Baliki et al. (Baliki et al. 2009) proposed that sensory inputs integrate in the insula to generate a magnitude estimation signal, providing a unifying explanation for the role of the insula in the numerous tasks in which it has been implicated. Consistently, the insula was also shown to be activated in contexts requiring to compare the intensities or sizes of different stimuli such as numbers, letters, and visual stimuli (Fulbright et al. 2003; Göbel et al. 2004). The insula has also been related to the detection of salience, and was suggested to constitute a hub connecting sensory areas to other networks involved in the processing and integration of external and internal information (Yantis 2008; Menon and Uddin 2010).
There is also evidence that distinct sub-regions of the insula respond differently to nociceptive and non-nociceptive stimuli, leading some authors to propose that specific subregions of the insula might be selectively involved in pain perception (Davis et al. 1998; Craig 2002, 2003b; Garcia-Larrea 2012; Segerdahl et al. 2015a). However, there is no agreement on which subregions that would be. Davis et al. (Davis et al. 1998) suggested that the anterior insula is more strongly involved in the perception of pain, whereas the posterior insula would be more related to the perception of innocuous tactile and thermal stimuli. Craig (Craig 2002, 2003a, 2005) suggested that the posterior insula comprises an interoceptive system giving rise to distinct feelings that originate from inside the body, such as pain, itch, temperature, muscular and visceral sensations, and vasomotor activity. Garcia-Larrea et al. (Garcia-Larrea 2012) and Segerdahl et al. (Segerdahl et al. 2015a) have argued for a specific role in pain of the dorsal posterior insula and neighboring parietal operculum. Other aspects of functional specialization in the insula have been reported. The right anterior insula has been associated with sympathetic responses such as those triggered by noxious stimuli, arousal, and the experience of basic emotions that are accompanied by vocalization or intense bodily action (Phillips et al. 1997; Damasio et al. 2000; Craig 2005; Wattendorf et al. 2016). In contrast, the left anterior insula was suggested to be predominantly associated with parasympathetic responses (Craig 2005).
Using intracerebral electroencephalographic (EEG) recordings performed in epileptic patients, several studies have shown that brief nociceptive stimuli perceived as painful elicit robust phase-locked local field potentials (LFPs) in the human insula. Because the magnitude of these LFPs correlated with the intensity of pain (Frot et al. 2007), it was suggested that these responses reflect processes that are specific for pain (Frot et al. 2007, 2014; Garcia-Larrea 2012). However, finding that a given brain response is always observed following the presentation of a painful stimulus does not justify the conclusion that this brain response is specific for pain. Demonstrating specificity for pain requires to also show that this response is not elicited by stimuli that are not painful, but matched in terms of other characteristics, such as their salience, valence, or behavioral relevance (Iannetti and Mouraux 2010; Legrain et al. 2011). In fact, also using intracerebral recordings with depth electrodes implanted in the human insula, we recently showed that salient non-nociceptive and non-painful vibrotactile, auditory, and visual stimuli can elicit robust phase-locked LFPs in the anterior and posterior insula (Liberati et al. 2016) appearing as large biphasic waves, with an insular topographical distribution largely identical to the one of LFPs elicited by nociceptive stimuli. Furthermore, using a blind source separation procedure, we showed that nociceptive phase-locked LFPs could be largely explained by multimodal neural activity also contributing to non-nociceptive LFPs, suggesting that these responses relate to a function of the insula that is largely unspecific for pain.
Here, our aim was to explore whether other features of insular activity sampled using intracerebral EEG, namely gamma-band oscillations (GBOs, >40 Hz), might reflect insular processes that are selective for thermonociception. GBOs have been consistently observed in a wide array of brain regions, and in response to a variety of stimuli and experimental paradigms (Womelsdorf et al. 2006; Fries 2009; Karns and Knight 2009; Crone et al. 2011; Herrmann and Kaiser 2011; Buzsáki and Wang 2012; Buzsáki and Schomburg 2015). Whether nociceptive stimuli elicit GBOs in the human insula is currently unknown, but other studies using scalp EEG or magnetoencephalography (MEG) have suggested that painful nociceptive stimuli elicit GBOs in several other brain regions, such as the primary somatosensory cortex (S1) and prefrontal areas (Gross et al. 2007; Hauck et al. 2007; Schulz et al. 2012; Zhang et al. 2012). Furthermore, these studies suggested that the magnitude of nociception-evoked GBOs can correlate with pain perception (Gross et al. 2007; Hauck et al. 2007; Schulz et al. 2012; Zhang et al. 2012), and can be dissociated from the magnitude of phase-locked nociception-evoked brain potentials (Zhang et al. 2012).
More generally, low-frequency activity sampled using intracerebral EEG has been shown to predominantly reflect synaptic activity, whereas high-frequency activities such as GBOs are thought to predominantly reflect spiking activity from neuronal aggregates (Friedman-Hill et al. 2000; Frien et al. 2000; Brosch et al. 2002; Miller et al. 2012; Nourski et al. 2015; Nozaradan et al. 2016). Further supporting the view that low- and high-frequency activities reflect functionally-distinct processes is the finding that they can be modulated differentially (e.g. phase-locked low-frequency LFPs and non-phase-locked high-frequency GBOs elicited by acoustic stimulation in the primary and secondary auditory cortices) (Nourski et al. 2015; Nozaradan et al. 2016). GBOs have been related to numerous cognitive functions including perceptual binding (Gray et al. 1989), selective attention (Fries et al. 2001), and working memory (Pesaran et al. 2002). Furthermore, the synchronization of gamma frequencies has been hypothesized to form a temporal code that dynamically “binds” spatially segregated neurons into assemblies representing higher-order stimulus properties (Malsburg 1995; Engel and Singer 2001; Crone et al. 2011).
Using time-frequency analysis of the signals sampled from 59 intracerebral contacts located in the insula of nine patients undergoing invasive EEG recordings for the diagnostic workup of partial epilepsy, we found that nociceptive thermal stimuli elicit an early-latency enhancement of GBOs (40-90 Hz) in the insula, in the time interval between 150 and 300 ms post-stimulus. This increase was markedly greater than the magnitude of post-stimulus GBOs following non-nociceptive vibrotactile, auditory, and visual stimulation. In contrast, stimulus-evoked phase-locked LFPs elicited by nociceptive stimuli were not of greater magnitude than the phase-locked LFPs elicited by non-nociceptive stimuli (see also Liberati et al. 2016). Taken together, our results suggest that nociception-evoked GBOs in the human insula reflect cortical activity that is preferentially involved in nociception and/or the processing of spinothalamic input.
Materials and Methods
Participants
Nine patients (six females, mean age: 30, range: 19–43 years) suffering from intractable focal epilepsy were recruited at the Department of Neurology of the Saint Luc University Hospital (Brussels, Belgium). Electrophysiological data from six of these patients was recently used to show that low-frequency stimulus-evoked phase-locked LFPs recorded from the human insula are not specific for nociception (Liberati et al. 2016). None of the patients had a history of psychiatric illness or cognitive dysfunction, and all patients had a normal neurological examination with no sensory deficit, with the exception of one patient with documented hyperacusis (Patient 7). All patients were investigated using depth electrodes implanted for the recording of brain activity in various regions suspected to be the origin of the seizures, including wide portions of the anterior and posterior insula (Ad-Tech depth electrodes with an “MRI-friendly” titanium body). The intracerebral EEG was recorded from ten insulae (eight left, two right; one of the patients had a bilateral insular implantation), with a total of 59 electrode contacts located in the insula (Table 1). In addition, all patients had at least one additional electrode implanted in a region outside the insula, either in the temporal lobe (with a total of 126 contacts) or in the frontal lobe (with a total of 106 contacts) (Table 2). None of the participants presented ictal discharge onset in the insula during the recordings, and low voltage fast activity was never present in this area during spontaneous seizures. All participants gave written informed consent. All experimental procedures were approved by the local Research Ethics Committee (B403201316436) and performed in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Table 1. Localization of insular electrode contacts.
| Patient | Hemisphere | Contact | Description | MNI coordinates |
|---|---|---|---|---|
| 1 | Right | 1 | Anterior insular cortex, first short gyrus | 39, 17, -17 |
| 2 | Anterior insular cortex, first short gyrus | 38, 17, -12 | ||
| 3 | Anterior insular cortex, subcortical topography of the first short gyrus | 38, 17, -5 | ||
| 4 | Anterior insular cortex / subcortical pars triangularis of the frontal gyrus | 38, 18, 1 | ||
| 5 | Anterior insular cortex / subcortical pars triangularis of the frontal gyrus | 38, 18, 5 | ||
| 6 | Anterior insular cortex / subcortical pars triangularis of the frontal gyrus | 38, 18, 11 | ||
| 2 | Left | 1 | Anterior insular cortex, lateral fissure between the orbital portion of the inferior frontal gyrus and the first short gyrus | -35, 18, -10 |
| 2 | Anterior insular cortex, first short gyrus | -35, 15, -10 | ||
| 3 | Posterior insular cortex, subcortical topography of the first long gyrus | -36, 9, -10 | ||
| 4 | Posterior insular cortex, subcortical topography of the first long gyrus | -36, 3, -9 | ||
| 5 | Posterior insular cortex, subcortical topography of the second long gyrus | -36, -2, -9 | ||
| 6 | Posterior insular cortex, subcortical topography of the second long gyrus | -37, -7, -7 | ||
| 7 | Posterior insular cortex, subcortical topography of the second long gyrus | -37, -12, -7 | ||
| 8 | Posterior insular cortex, intersection between the insula and the root of the Heschl gyrus (superior temporal gyrus) | -38, -17, -6 | ||
| 3 | Left | 1 | Anterior insular cortex, second short gyrus | -32, 8, -10 |
| 2 | Anterior insular cortex, second short gyrus | -32, 8, -6 | ||
| 3 | Anterior insular cortex, second short gyrus | -31, 8, 0 | ||
| 4 | Anterior insular cortex, superior portion of the second short gyrus | -31, 8, 5 | ||
| 5 | Anterior insular cortex, superior portion of the circular fold | -30, 8, 11 | ||
| 6 | Anterior insular cortex, superior portion of the circular fold | -30, 8, 17 | ||
| 4a | Left | 1 | Anterior insular cortex, subcortical topography between the first short gyrus and the second short gyrus | -38, 9, -4 |
| 2 | Anterior insular cortex, subcortical topography between the second short gyrus and the third short gyrus | -37, 4, -3 | ||
| 3 | Anterior insular cortex, subcortical topography of the third short gyrus | -37, 0, -3 | ||
| 4 | Posterior insular cortex, subcortical topography of the first long gyrus | -36, -5, -3 | ||
| 5 | Posterior insular cortex, subcortical topography of the first long gyrus | -35, -13, -3 | ||
| 6 | Posterior insular cortex, subcortical topography of the first long gyrus | -35, -18, -2 | ||
| 7 | Posterior insular cortex, subcortical topography of the second long gyrus | -34, -22, -1 | ||
| 4b | Right | 1 | Anterior insular cortex, base of the second short gyrus | 30, 7, -8 |
| 2 | Anterior insular cortex, base of the second short gyrus | 30, 1, -7 | ||
| 3 | Posterior insular cortex, inferior portion of the first long gyrus | 30, -4, -6 | ||
| 4 | Posterior insular cortex, inferior portion of the first long gyrus | 30, -9, -5 | ||
| 5 | Posterior insular cortex, inferior portion of the second long gyrus | 30, -15, -5 | ||
| 6 | Posterior insular cortex, adjacent to the posterior portion of the lentiform nucleus | 30, -20, -4 | ||
| 5 | Left | 1 | Anterior insular cortex, adjacent to the opercular portion of the inferior frontal gyrus | -34, 22, -2 |
| 2 | Anterior insular cortex, first short gyrus | -35, 16, -2 | ||
| 3 | Anterior insular cortex, transition between the first and second short gyri | -35, 11, -2 | ||
| 4 | Anterior insular cortex, second short gyrus | -35, 5, -2 | ||
| 5 | Posterior insular cortex, subcortical portion of the first long gyrus | -36, -1, -3 | ||
| 6 | Posterior insular cortex, subcortical portion of the first long gyrus | -36, -6, -3 | ||
| 7 | Posterior insular cortex, subcortical portion of the second long gyrus | -37, -12, -3 | ||
| 8 | Posterior insular cortex, subcortical portion of the second long gyrus | -37, -17, -3 | ||
| 9 | Posterior insular cortex, subcortical portion of the circular fold, adjacent to the superior temporal gyrus | -37, -23, -3 | ||
| 6 | Left | 1 | Anterior insular cortex, first short gyrus, posterior to the circular fold | -30, 25, -1 |
| 2 | Anterior insular cortex, subcortical portion of the second short gyrus | -30, 19, -1 | ||
| 3 | Anterior insular cortex, third short gyrus | -30, 17, -1 | ||
| 4 | Posterior insular cortex, first long gyrus | -31, 13, -2 | ||
| 5 | Posterior insular cortex, second long gyrus | -32, 9, -2 | ||
| 7 | Left | 1 | Posterior insular cortex, dorsal portion of the long gyri / operculum | -36, -16, 15 |
| 8 | Left | 1 | Anterior insular cortex, adjacent to the anterior portion of the circular sulcus | -35, 10, -9 |
| 2 | Anterior insular cortex, first short gyrus | -36, -1, -6 | ||
| 3 | Anterior insular cortex, second short gyrus | -37, -11, -3 | ||
| 4 | Posterior insular cortex, first long gyrus | -37, -21, 0 | ||
| 5 | Posterior insular cortex, adjacent to the posterior portion of the circular sulcus | -37, -31, 3 | ||
| 9a | Left | 1 | Posterior insular cortex, external capsule | -33, -12, 13 |
| 2 | Posterior insular cortex, transition between the long gyrus of the insula and the cortex from the parietal operculum, at the level of the circular gyrus | -37, -12, 15 | ||
| 3 | Posterior insular cortex, transition between the long gyrus of the insula and the cortex from the parietal operculum, at the level of the circular gyrus | -41, -12, 16 | ||
| 4 | Posterior insular cortex / parietal operculum | -49, -12, 16 | ||
| 9b | Left | 1 | Posterior insular cortex, most posterior long gyrus | -38, -9, -5 |
| 2 | Posterior insular cortex, most posterior long gyrus | -42, -9, -5 |
Table 2. Contacts located in the temporal and frontal regions.
| Subject | Hemisphere | Lobe | Location | Number of contacts |
|---|---|---|---|---|
| 1 | Right | Frontal | Lateral superior frontal gyrus | 16 |
| Lateral medial frontal gyrus | 16 | |||
| Orbitofrontal cortex | 8 | |||
| 2 | Left | Temporal | Mesiotemporal cortex | 8 |
| 3 | Left | Temporal | Basal temporal cortex | 8 |
| Mesiotemporal cortex | 8 | |||
| Frontal | Lateral frontal cortex | 32 | ||
| 4 | Left | Temporal | Mesiotemporal cortex | 21 |
| Right | Temporal | Mesiotemporal cortex | 17 | |
| 5 | Left | Temporal | Mesiotemporal cortex | 8 |
| Temporopolar cortex | 8 | |||
| Frontal | Lateral frontal cortex | 16 | ||
| 6 | Left | Temporal | Mesiotemporal cortex | 4 |
| Lateral temporal cortex | 8 | |||
| Temporopolar cortex | 8 | |||
| Basal temporal cortex | 12 | |||
| 7 | Left | Frontal | Superior frontal cortex | 8 |
| Dorsolateral frontal cortex | 10 | |||
| 8 | Left | Temporal | Temporopolar cortex | 4 |
| Mesiotemporal cortex | 4 | |||
| 9 | Left | Temporal | Temporopolar cortex | 4 |
| Mesiotemporal cortex | 4 |
Electrode implantation and anatomical electrode contact localization
For each patient, a tailored implantation strategy was planned according to the regions considered most likely to be ictal onset sites or propagation sites. Target areas, including the insular cortex, were reached using commercially available bipolar depth electrodes (AdTech, Racine, WI, U.S.A.; contact length: 2.4 mm; contact spacing: 5 mm) implanted using a frameless stereotactic technique through burr holes. The placement was guided by a neuronavigation system based on 3D T1-weighted (3D-T1W) magnetic resonance imaging (MRI) sequence performed in a 1.5 T scanner (Gradient Echo; flip angle: 15°; TR: 7.5 s; TE minimum full; 3.1 -13 ms; slice thickness: 1 mm; FOV: 24 cm; matrix: 224x224; number of slices: 162). To accurately identify the locations of each electrode, a post-implantation 3D-T1W MRI sequence was performed either immediately after surgery or on the following day. This MRI scan, which was conducted for clinical diagnostic purposes, is considered safe, as the implanted electrodes are made of an “MRI friendly” titanium body. Individual contact locations were identified with the help of multiplanar reformations. The anterior insula (29 contacts) was identified as the region encompassing the short insular gyri (anterior, middle, and posterior), the pole of the insula, and the transverse insular gyrus. The posterior insula (30 contacts) was identified as the region composed of the anterior and posterior long insular gyri (Naidich et al. 2004). To obtain the MNI coordinates of each insular electrode contact, individual MRI scans were normalized to a standard T1 template in MNI space using BrainVoyager 20.2 / QX 3.2 (Brain Innovation, Maastricht, The Netherlands). The DICOM images of each patient are available at the OSF online repository at the address https://osf.io/nzeea/.
Procedure
The study was conducted at the patient bedside. Before the beginning of the experiment, the procedure was explained to the patient, who was exposed to a small number of test stimuli for familiarization. The experiment consisted of two sessions of four blocks each, one session per side of stimulation (contralateral and ipsilateral to the implanted insular electrode). In each block, the patient received stimuli belonging to one of four sensory modalities: nociceptive, vibrotactile, auditory, and visual. Each block comprised 40 stimuli. The order of the blocks was randomized across participants. To reduce expectation of the stimuli, the inter-stimulus interval (ISI) was large, variable, and self-paced by the experimenter (5-10 s). Participants were instructed to fixate a black cross (3 x 3 cm) placed in front of them, at a distance of ~ 2 m, 30° below eye level, for the whole duration of each block. To ensure that each stimulus was perceived, and to maintain vigilance across time, participants were asked to press a button immediately after perceiving the stimulation. To have a measure of the perceived magnitude of the stimuli, subjects provided a verbal rating of the intensity of each stimulus using a numerical scale ranging from 0 (defined as “no sensation at all”) to 10 (defined as “the highest intensity I can imagine”). Ratings from one participant were lost due to a computer failure. At the end of each block, participants were asked to report whether they had perceived the stimuli as painful.
Sensory stimuli
Nociceptive somatosensory stimuli consisted in 50-ms pulses of radiant heat generated by a CO2 laser (wavelength: 10.6 μm). The stimuli were applied on the hand dorsum. The laser beam was transmitted via an optic fiber. Focusing lenses were used to set the beam diameter at target site to 6 mm. The laser stimulator was equipped with a radiometer providing a continuous measure of the target skin temperature, used in a feedback loop to regulate laser power output, which was adjusted to raise the target skin temperature to 62.5°C in 10 ms, and to maintain this temperature for 40 ms. To prevent nociceptor fatigue or sensitization, the laser beam was manually displaced after each stimulus (Schlereth et al. 2001). All participants reported that the laser stimuli elicited a clear painful pinprick sensation. This target temperature was chosen following a series of pilot tests performed on 10 healthy subjects, with the aim of finding an intensity at which the stimuli are always perceived as painful and clearly pricking, and detected with reaction times compatible with the conduction velocity of Aδ fiber nociceptors (Bromm and Treede 1984).
Non-nociceptive somatosensory stimuli consisted in 50-ms vibrations at 250 Hz, delivered via a recoil-type vibrotactile transducer driven by a standard audio amplifier (Haptuator, Tactile Labs Inc., Canada) and positioned on the palmar side of the index fingertip. Auditory stimuli were loud, lateralized sounds (0.5 left/right amplitude ratio) delivered through an earphone. The sounds consisted in 50-ms tones at 800 Hz and ~105 dB SPL. Visual stimuli were 50-ms punctuate flashes of light delivered by means of a light-emitting diode (LED) with a 12 lm luminous flux, a 5.10 cd luminous intensity, and a 120° visual angle (GM5BW97333A, Sharp Corporation, Japan), placed on the hand dorsum. The intensities of the non-nociceptive vibrotactile, auditory and visual stimuli were chosen after a series of pilot tests performed on healthy subjects in which intensities were adjusted such that none of the non-nociceptive stimuli would be systematically perceived as more or less intense than the others, or more or less intense than the nociceptive stimulus. The chosen parameters of stimulation were also guided by the results of previous studies using the same types of stimuli (Mouraux and Iannetti 2009; Mouraux et al. 2011; Liberati et al. 2016).
Intracerebral recordings
The intracerebral EEG recordings were performed using a DeltaMed Natus (Paris, France) acquisition system (AC coupling), using a reference electrode located between Cz and Pz. Additional bipolar channels were used to record electromyographic activity (EMG: two electrodes measuring respectively bicipital and tricipital contraction of the patient's arm) and electrocardiographic activity (EKG: two channels, utilizing two electrodes respectively located on the right and left side of the sternum, one electrode located centrally under the sternum, and one electrode on the right lateral side of the chest). EMG and EKG are normally measured to help the interpretation of the EEG signals and assess the behavioral manifestations of seizures. All signals were acquired at a 512 Hz sampling rate, and analyzed offline using Letswave 6 (https://www.letswave.org/) (Mouraux and Iannetti 2008).
Analysis of intracerebral insular recordings
The continuous recordings obtained from each insular contact were band-pass filtered (0.3 – 40 Hz) for analysis in the time domain and high-passed filtered (>20 Hz) for analysis in the time-frequency domain, and then segmented into 1.5-s epochs (-0.5 to 1.0 s relative to stimulus onset). In the time domain, the recordings were baseline corrected using a -0.5 to 0 s reference interval relative to stimulus onset.
Trials contaminated by artefacts were corrected using an independent component analysis (ICA) algorithm (Makeig et al. 1997) or removed after visual inspection. 1.7 ± 1.5 epochs (mean ± standard deviation) were rejected in the nociceptive modality; 3.3 ± 2.7 epochs were rejected in the vibrotactile modality; 2.1 ± 1.9 epochs were rejected in the auditory modality; and 2.7 ± 2.0 epochs were rejected in the visual modality. Separate average waveforms were then computed for each insular contact and stimulus type (nociceptive, vibrotactile, auditory, and visual). Within these average waveforms, the peak-to-peak magnitude of the large biphasic wave elicited by each type of stimulus was used as a measure of the magnitude of the low-frequency stimulus-evoked phase-locked LFP.
For the analysis in the time-frequency domain, a time-frequency representation of each high-pass filtered intracerebral EEG epoch was obtained using a short-term Fourier transform (STFT) with a fixed 200-ms width Hanning window, chosen to achieve a good tradeoff between time resolution and frequency resolution in the range of gamma-band frequencies (Gross et al. 2007; Schulz et al. 2011; Zhang et al. 2012). The STFT yielded, for each trial, a complex time-frequency spectral estimate F(t, f) at each point (t, f) of the time-frequency plane extending from -0.5 to 1.0 s in the time domain, and from 20 to 150 Hz (in steps of 1 Hz) in the frequency domain. After averaging the single-trial time-frequency maps, the average magnitude of the stimulus-induced changes in oscillation amplitude was estimated as follows (Pfurtscheller and Lopes da Silva 1999; Zhang et al. 2012; Hu et al. 2014):
where P(t, f) = |F(t, f)|2 is an estimate of signal amplitude at each time-frequency point (t, f) and R(f) is the average amplitude of the signal enclosed within the pre-stimulus reference interval (-0.4 to -0.1 s before the onset of the stimulus), for each estimated frequency f. This yielded, for each insular electrode contact and modality of stimulation, a time-frequency representation of the average stimulus-induced changes of intracerebral EEG signal (event-related percentage of change in signal amplitude, ER%)(Pfurtscheller and Lopes da Silva 1999).
The magnitudes of stimulus-evoked low-frequency phase-locked LFPs recorded from the insular contacts contralateral to the location of the sensory stimulus were compared using a linear mixed models (LMM) analysis as implemented in IBM SPSS Statistics 22 (Armonk, NY: IBM Corp) with the fixed factors ‘modality’ (four levels: nociceptive, vibrotactile, auditory, and visual) and ‘contact location’ (two levels: anterior and posterior insula). The magnitudes of stimulus-evoked GBOs were also compared using a LMM analysis with the fixed factors ‘modality’ (four levels: nociceptive, vibrotactile, auditory, and visual), ‘contact location’ (two levels: anterior and posterior insula), ‘frequency range’ (two levels: 40-90 Hz and 90-140 Hz), and ‘latency’ (4 levels: 150-300 ms, 300-450 ms, 450-600 ms, 600-750 ms). To assess whether differences in pre-stimulus GBO amplitude across modalities could have yielded differences in the post-stimulus ER% values computed across modalities, we also compared the average baseline amplitude (from -0.4 to -0.1 ms before stimulation onset) using a LMM analysis with the factors ‘modality’ (four levels: nociceptive, vibrotactile, auditory, and visual), ‘contact location’ (two levels: anterior and posterior insula); and ‘frequency range’ (two levels: 40-90 Hz and 90-140 Hz).
Analysis of intracerebral recordings outside the insula
One patient (Patient 3) had, in addition to a depth electrode in the left insula, an electrode grid over the left sensorimotor cortex, with 6 contacts above the hand motor area. Because patients were instructed to press a button as quickly as possible after perceiving each stimulus, and because part of the observed GBO activities recorded from the insula could have been related to movement preparation and/or execution, the time-frequency distribution of stimulus-evoked GBOs recorded from the insula of that patient was compared to the time-frequency distribution of stimulus-evoked GBOs recorded from the hand motor area, i.e. an area strongly involved in both movement preparation and execution (Carrillo-de-la-Peña et al. 2008).
Finally, to assess whether stimulus-evoked GBOs recorded from the insula could be distinguished from stimulus-evoked GBOs recorded from other brain regions, the magnitude of insular GBOs was compared with the magnitude of GBOs recorded at contacts located in the temporal and frontal lobes (126 and 106 contacts respectively, after excluding contacts located in the temporal and frontal operculum as these might have sampled insular activity), contralateral to stimulation, using a LMM with the fixed factors ‘modality’ (four levels: nociceptive, vibrotactile, auditory, and visual), ‘region’ (three levels: insula, temporal, frontal), ‘frequency range’ (two levels: 40-90 Hz and 90-140 Hz), and ‘latency’ (4 levels: 150-300 ms, 300-450 ms, 450-600 ms, 600-750 ms).
Analysis of electrocardiographic signals
The EKG was used to derive heart rate variability as a measure of stimulus-evoked arousal (Malmstrom et al. 1965; Taylor and Epstein 1967; Epstein 1971). The EKG activity was analyzed offline using Letswave 6. The continuous EKG recordings were filtered using a 0.67-40 Hz band-pass filter. The 0.67 Hz lower-frequency cutoff corresponds to a minimum heart rate of 40 beats per minute (bpm) and the 40 Hz high-frequency cutoff allows eliminating muscle noise (Luo and Johnston 2010; Sansone et al. 2010; Shing-Hong 2010). The Pan Tompkins algorithm was used to recognize the QRS complexes in the EKG signal based on slope, amplitude and width (Pan and Tompkins 1985). The time intervals between two QRS complexes were then used to generate a continuous waveform expressing the instantaneous heart rate as a function of time. This waveform was then segmented into 15 s epochs (-5 to 10 s relative to stimulus onset), and averaged according to stimulus type (nociceptive, vibrotactile, auditory, and visual). Finally, within these average waveforms, the difference between the maximum and minimum heart rate value during the 5 seconds that followed the onset of each stimulus was computed as an indicator of stimulus-triggered heart rate variability. The difference between the maximum and minimum heart rate value during the 5 seconds preceding the onset of each stimulus was computed as a baseline.
A LMM analysis was then performed to evaluate the effect of the fixed factors ‘modality’ (four levels: nociceptive, vibrotactile, auditory, and visual) and ‘time interval’ (two levels: 5 seconds before the stimulus, 5 seconds after the stimulus) on the magnitude of heart rate variability.
In all analyses, the contextual variable ‘subject’ was added to the LMM models, to account for the variation of the regression model intercept across participants. Parameters were estimated using restricted maximum likelihood (REML) (Twisk 2005). Main effects were compared using the Bonferroni confidence interval adjustment.
Results
Intensity of perception
On average, the ratings of intensity of the stimuli (nociceptive: 5.7 ± 1.8; vibrotactile: 4.2 ± 1.6; auditory: 5.5 ± 3.2; visual: 5.1 ± 1.5; mean ± standard deviation) were not significantly different across modalities (F=1.3, p=.312). However, for all subjects, nociceptive stimuli were systematically described as eliciting a clear burning/pricking sensation, and systematically qualified as painful. Conversely, all subjects described the auditory, vibrotactile, and visual stimuli as intense but not painful, with the exception of one subject with documented hyperacusis (with one single electrode contact in the dorsal posterior insula, see Table 1, Subject 7), who described the auditory stimuli as painful and annoying.
Heart rate variability
Given the relatively short time interval between two successive stimuli, it is likely that stimulus-induced changes in heart rate did not have enough time to completely return to baseline. Nevertheless, previous reports have shown that heart rate variability can be measured wit ISIs as short as 5 seconds (Graham and Clifton 1966), and the LMM analysis showed a significant effect of ‘time interval’ (F=7.77, p=.007) on the heart rate variability, which was greater during the 5 seconds that followed the stimulus (nociceptive: 5.5 ± 2.2 bpm; vibrotactile: 5.5 ± 2.9 bpm; auditory: 5.7 ± 2.5 bpm; visual: 5.5 ± 2.6 bpm) compared to the 5 seconds preceding the stimulus (nociceptive: 4.6 ± 1.8 bpm; vibrotactile: 4.2 ± 2.5 bpm; auditory: 5.0 ± 2.9 bpm; visual: 3.6 ± 1.6 bpm). The effect of ‘modality’ (F=1.0, p=.379) and the interaction between ‘modality’ and ‘time interval’ (F=0.1, p=.945) were not significant. This indicates that all stimuli elicited a significant change in heart rate variability, and that this change in heart rate variability was of the same order of magnitude across the different modalities.
Intracerebral responses recorded from the insula
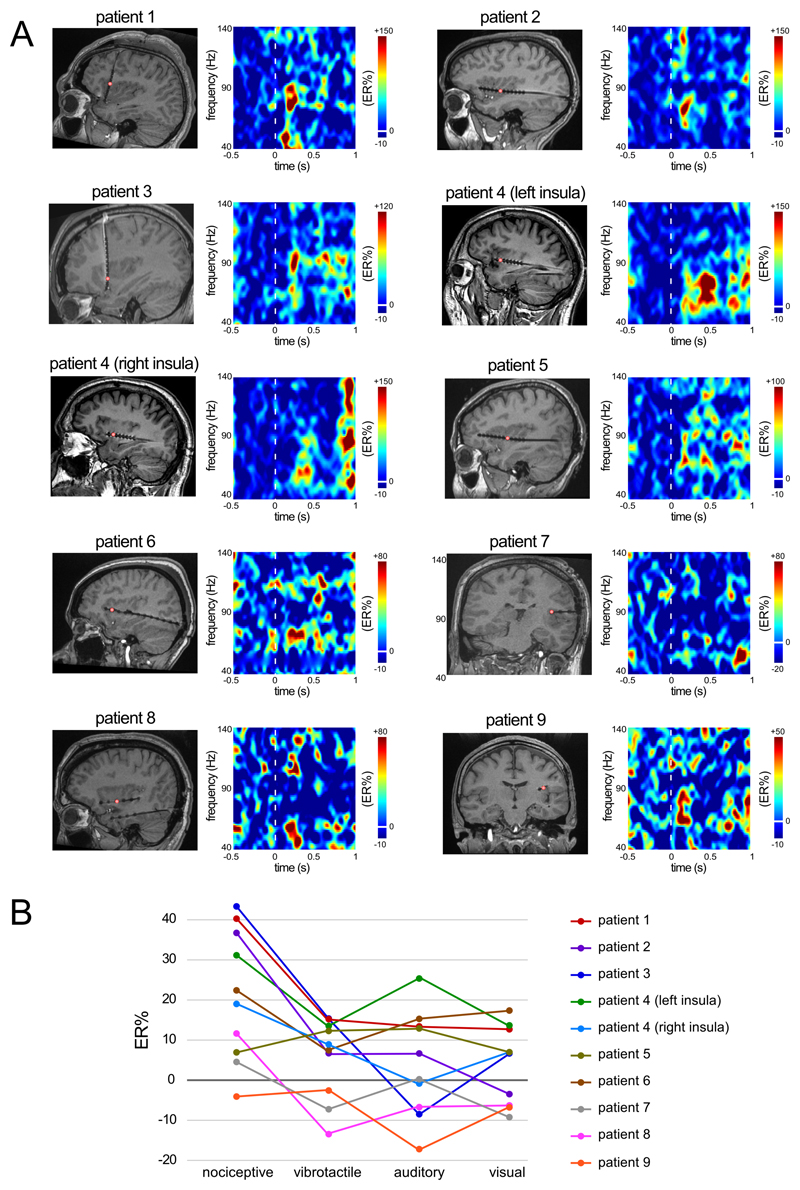
Time-frequency analysis of the intracerebral EEG recordings obtained at insular contacts showed that nociceptive stimuli elicited a strong enhancement of GBOs in the insula, which was not observed in response to non-nociceptive vibrotactile, auditory, and visual stimuli (Figure 1). The LMM analysis of the magnitudes of insular GBOs showed a main effect of ‘modality’ (F=71.36, p<.001), a main effect of ‘frequency’ (F=57.79, p<.001), and a main effect of ‘latency’ (F=5.70, p=.001). The LMM also showed a significant interaction between ‘modality’ and ‘latency’ (F=5.75, p<.001), and a significant interaction between ‘modality’ and ‘location’ (F=6.75, p<.001). Post hoc comparisons showed that, regardless of sensory modality, GBOs were significantly greater in magnitude in the 40-90 Hz frequency range compared to the 90-140 Hz frequency range. Moreover, nociceptive GBOs were significantly greater in the 150-300 ms time interval compared to the 300-450 ms (p=.005), 450-600 ms (p=.001), and 600-750 ms (p<.001) time intervals. Most importantly, in both the anterior and posterior insula, the magnitude of nociceptive GBOs was significantly greater than the magnitude of vibrotactile, auditory, and visual GBOs (all p<.001; see Figure 2). Whereas vibrotactile, auditory, and visual GBOs were greater in magnitude in the posterior insula compared to the anterior insula (p=.028, p=.009, and p=.027), nociceptive GBOs were greater in magnitude in the anterior insula compared to the posterior insula (p=.013). Figure 1B shows the magnitude of the post-stimulus change in GBO amplitude averaged at all contacts of each individual insula, for each modality of stimulation, considering the 40-90 Hz frequency range and the 150-300 ms post-stimulus interval.
Figure 1. Enhancement of gamma-band oscillations (GBOs) in response to nociceptive stimuli.
A. Time-frequency representations of the change in GBOs (40-140 Hz, expressed as percentage of change relative to baseline, ER%) elicited by nociceptive stimulation in the insula of 9 patients (10 insulae: one patient underwent a bilateral insular implantation), at the electrode contacts at which the increase in GBOs elicited by nociceptive stimuli was most pronounced. B. ER% values averaged at all contacts of each individual insula, for each modality of stimulation, considering the 40-90 Hz frequency range and the 150-300 ms post-stimulus interval.
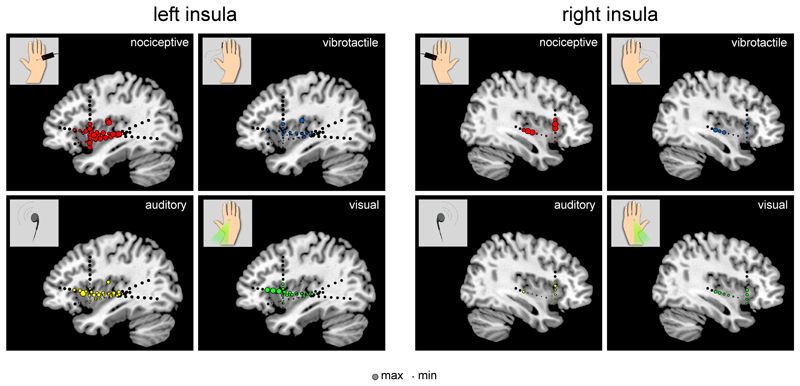
Figure 2. Magnitude of GBOs elicited by nociceptive, vibrotactile, auditory, and visual stimuli in the contralateral left or right insula.
The sizes of the circles represent the magnitudes of the post-stimulus change in GBO magnitude (40-90 Hz, 150-300 ms). Max and Min correspond to the maximum and minimum ER% values observed across modalities and electrode contacts, separately for each insula. At the majority of insular locations, and both in the left and in the right insula, the post-stimulus GBO enhancement was much more pronounced after nociceptive stimulation, as compared to tactile, auditory and visual stimuli. This preferential enhancement following nociceptive stimulation was not restricted to a specific subregion of the insula and, instead, was observed in both the anterior and posterior portions of the insula.
The LMM analysis of the magnitude of pre-stimulus GBOs showed no effect of ‘location’ (F=1.52, p=.218), but a main effect of ‘modality’ (F=3.12, p=.026), a main effect of ‘frequency’ (F=142.86, p<.001), and a significant interaction between ‘frequency’ and ‘modality’ (F=2.87, p=.036). Post-hoc comparisons showed that in the 40-90 Hz frequency range, the magnitude of pre-stimulus GBOs in the visual condition was significantly smaller than the magnitude of pre-stimulus GBOs in the vibrotactile (p=.023) and auditory (p=.001) conditions. The magnitude of pre-stimulus GBOs in the nociceptive condition was not significantly different from the magnitudes of pre-stimulus GBOs in the vibrotactile, auditory, and visual conditions, indicating that the preferential enhancement of post-stimulus GBOs following nociceptive stimulation was not due to a difference in pre-stimulus GBO magnitude.
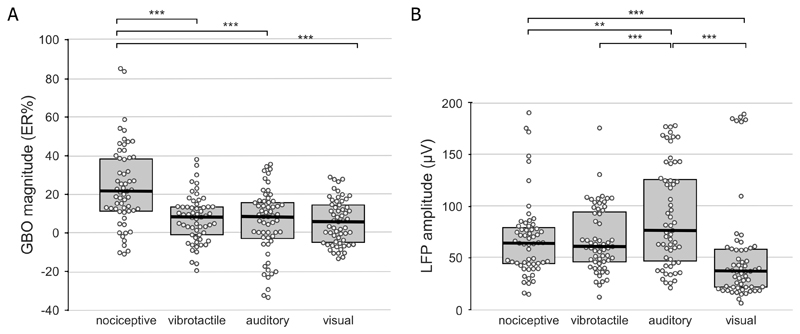
As reported in Liberati et al. (Liberati et al. 2016), all four types of stimuli elicited a large biphasic phase-locked LFP, having a same topographical distribution across insular contacts (average peak-to-peak amplitudes: nociceptive: 68 ±37 μV; vibrotactile: 67 ±31 μV; auditory: 90 ±49 μV; visual: 52 ±49 μV). The LMM analysis showed a main effect of ‘modality’ (F=13.66, p<.001) with no effect of ‘contact location’ (F=1.35, p=.246) on the amplitude of the phase-locked LFPs. Post-hoc comparisons showed that the amplitude of the auditory phase-locked LFP was significantly larger than the amplitudes of the nociceptive (p=.002), vibrotactile (p=.001), and visual (p<.001) phase-locked LFPs, and that the amplitude of the nociceptive phase-locked LFP was significantly larger than the amplitude of the visual phase-locked LFP (p=.043).
Figure 3A shows the average post-stimulus change in GBO amplitude recorded at each insular contact across modalities (ER% values; 40-90 Hz and 150-300 ms post-stimulus). Figure 3B shows the average amplitude of phase-locked LFPs recorded across modalities at the same insular contacts. The clear dissociation between GBOs (which were preferentially enhanced following nociceptive stimulation) and phase-locked LFPs (which were similarly elicited by all types of stimuli) can also be observed in Figure 4.
Figure 3. Magnitude of insular responses to nociceptive, vibrotactile, auditory, and visual stimuli.
A. Swarm plots showing the post-stimulus change in GBO amplitude elicited by the different types of stimuli, and expressed as percentage of change relative to baseline (ER%), recorded across subjects from each electrode contact located in the insula, considering the 40-90 Hz frequency range and the 150-300 ms post-stimulus interval. B. Swarm plots showing the amplitude of phase-locked LFPs recorded across subjects from each insular contact. In both panels, the boxplots display the first quartile, the median, and the third quartile of the distribution of response magnitude. Note that the magnitude of GBOs elicited by nociceptive stimuli in the insula is significantly greater than the amplitude of GBOs elicited by non-nociceptive vibrotactile, auditory, and visual stimuli. In contrast, the magnitude of the phase-locked LFPs elicited at the same contacts were not greater in response to nociceptive stimuli. *** p ≤.001; ** p=.002 (paired sample t-tests).
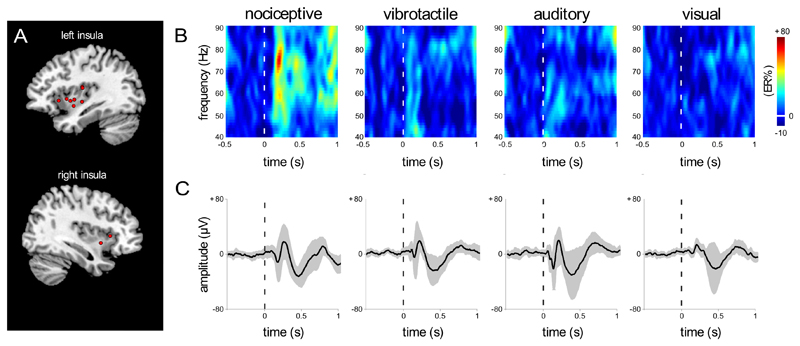
Figure 4. Dissociation between gamma band oscillations (GBOs) and low-frequency phase-locked local field potentials (LFPs) recorded in the human insula in response to nociceptive, tactile, auditory, and visual stimulation.
A. Selected contacts at which, for each explored insula (on both the left and the right hemisphere), GBOs elicited by nociceptive stimuli were more pronounced (same contacts as displayed in Figure 1). B. Time-frequency representation of the changes in oscillatory power (40-90 Hz) elicited by nociceptive, tactile, auditory, and visual stimuli at the insular locations shown in A (group level average percentage change in amplitude; ER%). As compared to non-nociceptive stimuli, brief nociceptive stimuli elicit a greater post-stimulus increase in GBO power. Single-subject GBO responses elicited by nociceptive stimuli at the indicated insular contacts are shown in Figure 1. C. Phase-locked LFPs recorded at the same insular locations shown in A, in response to the four kinds of stimuli (group level average; confidence intervals stated at the 95% confidence level are shown in gray). The dissociation between GBOs (exhibiting a strong increase only following nociceptive stimulation) and phase-locked LFPs (presenting similar magnitudes across modalities) indicates that GBOs and phase-locked LFPs reflect different neural processes.
Intracerebral responses recorded outside the insula
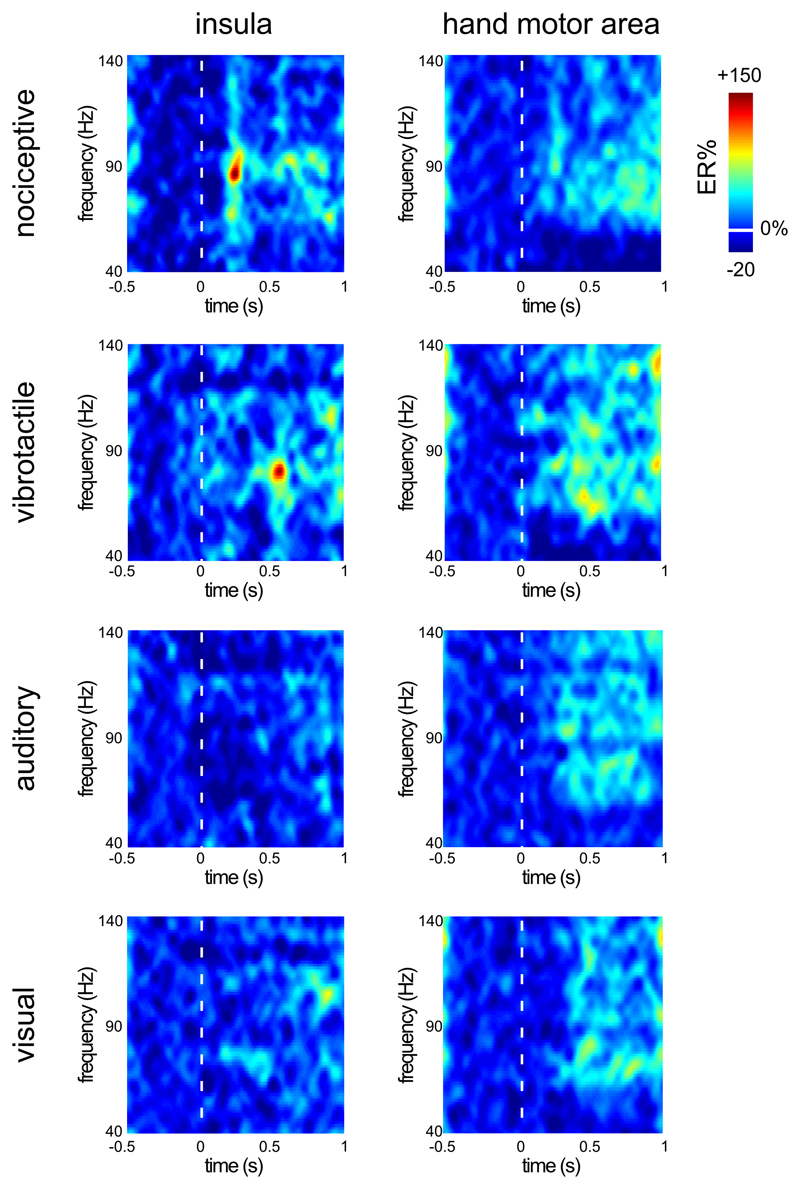
In Patient 3, the comparison of the post-stimulus GBOs recorded from the insula to the post-stimulus GBOs recorded over the hand motor area showed that, as for the other patients, nociceptive stimuli elicited an early latency enhancement of GBOs. The magnitude of post-stimulus GBOs (latency: 150-300 ms) recorded at insular contacts was markedly greater following nociceptive stimulation (43 ±12 ER%) as compared to non-nociceptive vibrotactile (16 ±14 ER%), auditory (-8 ±11 ER%), and visual (7 ±10 ER%) stimulation (Figure 6). In contrast, at contacts located in the hand motor area, an increase of GBO amplitude was observed for all modalities (nociceptive: 19 ±15 ER%; vibrotactile: 37 ±21 ER%; auditory: 28 ±17 ER%; visual: 25 ±18 ER%), having a later latency (300-750 ms) and a broader frequency range (40-140 Hz) than the GBO response recorded from the insula.
Figure 6. Comparison between GBOs recorded from the insula and GBOs recorded from the hand motor area of one patient.
The time-frequency maps show the post-stimulus change in GBO amplitude (40-140 Hz) in the insula and in the hand motor area (averaged across contacts) of Patient 3. Such as for the other patients, the magnitude of the stimulus-evoked enhancement of GBOs recorded at insular contacts (latency: 150-300 ms) was markedly greater following nociceptive stimulation compared to non-nociceptive vibrotactile, auditory, and visual stimulation. In contrast, in the hand motor area, a late latency increase of GBO power was observed for all modalities, at a later latency (300-750 ms), and within a broader frequency range (40-140 Hz) than the GBO response recorded from the insula.
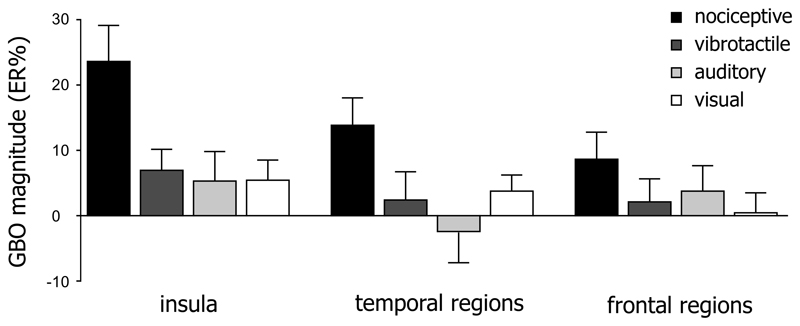
The results of the LMM analysis performed to compare the magnitude of GBOs recorded in the insula with the magnitude of GBOs recorded in temporal and frontal regions (excluding the temporal and frontal operculum) showed significant interactions between ‘region’ and ‘modality’ (F=24.13, p<.001) and between ‘region’ and ‘frequency’ (F=24.35, p<.001). Post-hoc comparisons showed that, in the insula, but also in temporal regions and in frontal regions, nociceptive GBOs were greater in magnitude than vibrotactile, auditory, and visual GBOs (see Table 3); that nociceptive GBOs recorded from the insula were significantly greater than nociceptive GBOs recorded from the frontal and temporal regions (both p<.001); that the magnitude of GBOs was greater in the 40-90 Hz frequency range compared to the 90-140 Hz frequency range in the insula and in temporal regions (both p<.001), but not in frontal regions (p=.175). Figure 5 shows the difference between nociceptive, vibrotactile, auditory, and visual GBOs recorded from the insula, in temporal regions, and in frontal regions, considering the 150-300 ms time interval and the 40-90 Hz frequency range.
Table 3. Pairwise comparisons of the magnitude of gamma-band oscillations elicited by nociceptive stimuli and the magnitude of gamma-band oscillations elicited by non-nociceptive vibrotactile, auditory, and visual stimuli, respectively in the insula, in temporal regions, and in frontal regions.
| insula | temporal regions | frontal regions | ||||
|---|---|---|---|---|---|---|
| mean difference (ER%) |
p-value | mean difference (ER%) |
p-value | mean difference (ER%) |
p-value | |
| nociceptive vs. vibrotactile | 8.6 | p<.001 | 5.4 | p<.001 | 3.6 | p=.001 |
| nociceptive vs. auditory | 11.8 | p<.001 | 12.7 | p<.001 | 0.2 | p=1 |
| nociceptive vs. visual | 9.4 | p<.001 | 3.9 | p<.001 | 4.3 | p<.001 |
Figure 5. Comparison between GBOs recorded from the insula and GBOs recorded from frontal and temporal regions (150-300 ms post-stimulus; 40-90 Hz frequency range).
The magnitude of GBOs recorded from the insula was significantly greater than the magnitude of GBOs recorded from temporal and frontal regions. In all brain regions, the magnitude of GBOs elicited by nociceptive stimuli was significantly greater than the magnitude of GBOs elicited by vibrotactile, auditory, and visual stimuli. Error bars represent confidence intervals stated at the 95% confidence level.
Discussion
The present study shows that in the posterior and anterior human insula, brief nociceptive stimuli perceived as painful do not only elicit a low-frequency LFP phase-locked to the stimulus onset, but also elicit an early-latency (150-300 ms) enhancement of GBOs maximal in the 40-90 Hz frequency range. Remarkably, there was an evident dissociation between the low-frequency phase-locked LFPs and GBOs: unlike the phase-locked LFPs, insular GBOs appeared to reflect activity that is preferential for nociception.
As in our previous study (Liberati et al. 2016), non-nociceptive tactile, auditory, and visual stimuli elicited phase-locked LFPs, appearing as large biphasic waves with a spatial distribution across insular contacts indistinguishable from the spatial distribution of the LFPs elicited by nociceptive stimuli. Because the insula is thought to contribute to a large number of cognitive, affective, interoceptive, and homeostatic functions (Davis et al. 1998; Craig et al. 2000; Wicker et al. 2003; Brass and Haggard 2010; Lamm and Singer 2010; Sterzer and Kleinschmidt 2010; Craig 2011; Furl and Averbeck 2011; Heydrich and Blanke 2013), we hypothesized that phase-locked insular LFPs elicited by painful nociceptive stimuli reflect processes that are unspecific for pain, such as salience-related processes involved in arousal or bottom-up attention (Liberati et al. 2016).
Contrasting with the phase-locked LFPs, nociceptive stimuli elicited a clear early-latency enhancement of GBOs at insular contacts, and a similar response was not observed following non-nociceptive vibrotactile, auditory, and visual stimulation. This indicates that nociception-evoked GBOs in the insula reflect activity that is preferentially involved in the processing of spinothalamic input, nociception, and/or the perception of pain. Importantly, the magnitude of GBOs preceding the presentation of nociceptive stimuli did not differ significantly from the magnitude of GBOs preceding vibrotactile, auditory and visual stimuli, indicating that the preferential increase of GBO magnitude after nociceptive stimulation was not due to differences in baseline GBO activity.
The latency and frequency range of nociception-evoked GBOs recorded in the insula are similar to the latency and frequency range of nociception-evoked GBOs identified in other brain regions using scalp EEG and MEG (Gross et al. 2007; Hauck et al. 2007; Schulz et al. 2012, 2015; Zhang et al. 2012; Ploner et al. 2016; Fardo et al. 2017). Their early latency (150-300 ms) indicates that these activities are triggered by input conveyed by thinly-myelinated Aδ fibers (Gross et al. 2007). Indeed, when stimulating the hand dorsum, input conveyed by C fibers would be expected to elicit responses having a much greater latency (Opsommer et al. 1999). On average, nociception-evoked GBOs recorded from the anterior insula were greater in magnitude than nociception-evoked GBOs recorded from the posterior insula. This is consistent with previous studies suggesting that the anterior insula is more strongly involved in the perception of pain (Davis et al. 1998), but also at odds with studies suggesting a stronger involvement of the dorsal posterior insula in nociception (Segerdahl et al. 2015a). Given the sparse spatial sampling of intracerebral EEG, this anterior-posterior difference should be interpreted with great caution. Notwithstanding, nociception-evoked GBOs were widespread in both the anterior and the posterior insula, suggesting that they did not originate from a restricted “pain-specific” subregion of the insula.
Another important point to consider is whether the preferential enhancement of GBOs following laser stimulation was truly due to the nociceptive nature of the laser stimulus (or the painful quality of the elicited sensation), or whether it could have resulted from another feature distinguishing the laser stimulus from the other stimuli, such as a difference in salience or intensity. On average, patients provided similar ratings of intensity for the different stimuli, indicating that none of the four types of stimuli was systematically perceived as more intense than the others – a circumstance that could have constituted a bias in the interpretation of our findings. However, intensity ratings are entirely subjective measures, which can vary largely across participants and, possibly, within the same participant. Furthermore, because stimuli that are painful are also inherently salient, painfulness and salience cannot be completely dissociated. The strongest evidence that the preferential enhancement of GBOs following laser stimulation compared to non-nociceptive vibrotactile, auditory, and visual stimulation was not due to a gross difference in the intensity or salience of the different stimuli is that all four types of stimuli elicited consistent and robust low-frequency phase-locked LFPs, of the same order of magnitude. The fact that nociceptive and non-nociceptive stimuli elicited comparable changes in heart rate variability further suggests that the nociceptive stimuli were not systematically more salient or arousing than the other stimuli. Indeed, stimulus-evoked changes in heart rate variability is another response to the stimulus which can be expected to relate to stimulus arousal and valence (Malmstrom et al. 1965; Taylor and Epstein 1967; Epstein 1971). Nevertheless, further studies should explore the potential relationship between stimulus-evoked responses from the insula and different measures of arousal such as changes in skin conductance and pupillometry.
One question that cannot be addressed with the present results is whether the nociception-evoked GBOs we observed were related to the nociceptive nature of the stimuli, to the painful quality of the elicited sensation, or to the fact that the stimuli conveyed thermal information. This latter hypothesis is consistent with a recent scalp EEG and MEG study that identified cortical generators of cold-evoked GBOs within a distributed network of sources including operculo-insular regions, parietal regions, and frontal regions (Fardo et al. 2017), as well as with the proposition that the dorsal middle/posterior insula is preferentially involved in thermoception (Craig et al. 2000; Hua et al. 2005). In the present study, however, nociception-evoked GBOs were not limited to opercular or dorsal portions of the insula, but were widespread throughout the insula. Further studies could explore, using depth electrodes implanted in the insula, whether innocuous cold (i.e. non-painful thermal stimuli conveyed by the spinothalamic system) or mechanical pinprick stimuli (i.e. a non-thermal but nevertheless painful stimuli also conveyed by the spinothalamic system) elicit a similar enhancement of GBOs.
Participants were asked to press a button as soon as they perceived the stimuli. This raises the possibility that post-stimulus GBOs could reflect, at least partly, activity related to movement preparation and/or execution. However, GBOs related to the preparation or execution of a motor response could not explain the greater magnitude of nociceptive GBOs compared to non-nociceptive vibrotactile, auditory, and visual GBOs, as movement-related activity would be expected to affect the responses to all stimuli equally. Moreover, the comparison of post-stimulus GBOs recorded from insular contacts and post-stimulus GBOs recorded from the hand motor area of one patient showed that, in the insula, nociceptive stimuli elicited an early-latency enhancement of GBOs which was not present after non-nociceptive vibrotactile, auditory, and visual stimulation, whereas in the hand motor area, all stimuli elicited a distinct later-latency and longer-lasting enhancement of GBOs, possibly related to movement preparation or execution.
Studies have suggested that blood-oxygen-level dependent (BOLD) signals measured using MRI often correlate with the magnitude of GBOs (Logothetis et al. 2001; Logothetis 2002; Foucher et al. 2003). Therefore, if nociceptive stimuli elicit a selective enhancement of GBOs in the insula, one may wonder why previous fMRI studies found that brief nociceptive and non-nociceptive tactile, auditory, and visual stimuli similar to those used in the present study elicit insular BOLD responses that are spatially overlapping and largely indistinguishable (Mouraux et al. 2011). However, the relationship between GBO activity and BOLD signal appears to be nonlinear, and changes in GBO activity can occur with no (or very subtle) changes in the BOLD signal (Muthukumaraswamy and Singh 2008). Moreover, there are discrepancies in the literature relative to the range of oscillation frequencies that are most closely linked to the BOLD signal (Pan et al. 2013), which include low gamma (<60 Hz) (Goense and Logothetis 2008; Hutchison et al. 2015), mid-gamma (60-120 Hz) (Conner et al. 2011), and high gamma (up to 250 Hz) (Murayama et al. 2010). Several experiments performed in humans have shown that GBOs and BOLD signals can be functionally decoupled, and that an increase in the magnitude of GBO responses is not always sufficient to drive a subsequent BOLD response (Singh et al. 2000; Adjamian et al. 2004; Muthukumaraswamy and Singh 2008, 2009). A likely explanation could be that, as compared to the baseline GBO activity, the stimulus-induced enhancement of GBOs is relatively small and, most importantly, very short-lasting.
The magnitude of nociception-evoked GBOs recorded in the insula was significantly greater than the magnitude of nociception-evoked GBOs recorded in temporal and frontal regions. Nevertheless, post-stimulus GBOs preferential for nociceptive stimuli as compared to non-nociceptive tactile, auditory and visual stimuli were also observed in these regions. Because temporal and frontal electrode contacts were located in structurally and functionally diverse areas, it is difficult to draw conclusions on the functional significance of these responses. What can be said is that the stronger magnitude of nociception-evoked GBOs recorded in the insula is compatible with a strong involvement of that brain structure in nociception (Ostrowsky et al. 2002; Frot and Mauguière 2003; Isnard et al. 2004, 2011; Brooks and Tracey 2007; Mazzola et al. 2009, 2012; Garcia-Larrea et al. 2010; Garcia-Larrea 2012; Frot et al. 2014; Moayedi 2014; Segerdahl et al. 2015a), and that finding nociception-evoked GBOs outside the insula is also supported by previous scalp EEG and MEG studies, showing that nociceptive stimuli can elicit GBOs originating from S1 and prefrontal regions (Gross et al. 2007; Hauck et al. 2007; Schulz et al. 2012; Zhang et al. 2012). The observation that nociceptive stimuli can elicit GBOs both inside and outside the insula is also compatible with the recent proposal that pain is an intrinsically dynamic process that emerges from synchronized activity within a network of neurons or brain areas, i.e., the so-called “pain connectome”, which would integrate all cognitive, affective, and sensorimotor aspects of pain, thereby shaping cognition and behavior (Kucyi and Davis 2015, 2016). Indeed, one of the hypothesized functions of GBOs is the selective and flexible coupling of neighboring or distant cortical regions – a mechanism referred to as communication through coherence (Engel et al. 2001; Varela et al. 2001; Fries 2005; Buzsáki 2010; Buzsáki and Schomburg 2015). In this framework, the enhancement of GBO activity following nociceptive stimulation could constitute the mean through which this network synchronizes its activity across brain regions, therefore serving as a distinctive feature of insular activity preferential for nociception and/or the processing of spinothalamic input in humans.
Funding
GL, MA, and AM received support from a European Research Council (ERC) Starting Grant (PROBING-PAIN 336130). GL also received support from the Fonds Spécial de Recherche (FSR) of the Belgian Walloon Region, from the Fonds de Recherche Clinique (Université catholique de Louvain), and from the Fonds National de la Recherche Scientifique (FNRS, Belgium). AK received support from the Belgian Walloon Region (CWALity program – Neurosense project). DM received support from the FNRS (Belgium). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author contributions
GL and AM developed the concept, designed the experiment, and performed the data analyses. GL, AK, and MA acquired the psychophysical and electrophysiological data. DM contributed to the data analysis. MMS provided the localization of all contact electrodes required for data analysis, and contributed to the creation of the figures. SFS contributed to the conceptualization of the study, writing of the paper, and recruitment of patients. JGRV and CR planned and performed the surgical implantation of the electrodes. GL and AM wrote the paper. All authors discussed and revised the manuscript.
Competing financial interests
The authors declare that no competing interests exist.
References
- Adjamian P, Holliday IE, Barnes GR, Hillebrand A, Hadjipapas A, Singh KD. Induced visual illusions and gamma oscillations in human primary visual cortex. Eur J Neurosci. 2004;20:587–592. doi: 10.1111/j.1460-9568.2004.03495.x. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Shi T. Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J Neurosci. 1994;14:6779–6795. doi: 10.1523/JNEUROSCI.14-11-06779.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Baier B, Eulenburg P, Geber C, Rohde F, Rolke R, Maihöfner C, Birklein F, Dieterich M. Insula and sensory insular cortex and somatosensory control in patients with insular stroke. Eur J Pain. 2014;18:1385–1393. doi: 10.1002/j.1532-2149.2014.501.x. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol. 2009;101:875–887. doi: 10.1152/jn.91100.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Haggard P. The hidden side of intentional action: the role of the anterior insular cortex. Brain Struct Funct. 2010;214:603–610. doi: 10.1007/s00429-010-0269-6. [DOI] [PubMed] [Google Scholar]
- Bromm B, Treede RD. Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum Neurobiol. 1984;3:33–40. [PubMed] [Google Scholar]
- Brooks JCW, Tracey I. The insula: a multidimensional integration site for pain. Pain. 2007;128:1–2. doi: 10.1016/j.pain.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Brooks J, Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathways. J Anat. 2005;207:19–33. doi: 10.1111/j.1469-7580.2005.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch M, Budinger E, Scheich H. Stimulus-related gamma oscillations in primate auditory cortex. J Neurophysiol. 2002;87:2715–2725. doi: 10.1152/jn.2002.87.6.2715. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Schomburg EW. What does gamma coherence tell us about inter-regional neural communication? Nat Neurosci. 2015;18:484–489. doi: 10.1038/nn.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Wang X-J. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-de-la-Peña MT, Galdo-Alvarez S, Lastra-Barreira C. Equivalent is not equal: primary motor cortex (MI) activation during motor imagery and execution of sequential movements. Brain Res. 2008;1226:134–143. doi: 10.1016/j.brainres.2008.05.089. [DOI] [PubMed] [Google Scholar]
- Cauda F, Agata FD’, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Cerliani L, Thomas RM, Jbabdi S, Siero JCW, Nanetti L, Crippa A, Gazzola V, Arceuil HD’, Keysers C. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Hum Brain Mapp. 2012;33:2005–2034. doi: 10.1002/hbm.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner CR, Ellmore TM, Pieters TA, DiSano MA, Tandon N. Variability of the relationship between electrophysiology and BOLD-fMRI across cortical regions in humans. J Neurosci. 2011;31:12855–12865. doi: 10.1523/JNEUROSCI.1457-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. An ascending general homeostatic afferent pathway originating in lamina I. Prog Brain Res. 1996;107:225–242. doi: 10.1016/s0079-6123(08)61867-1. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003a;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig ADB. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003b;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- Craig ADB. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci (Regul Ed) 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Craig ADB. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Crone NE, Korzeniewska A, Franaszczuk PJ. Cortical γ responses: searching high and low. Int J Psychophysiol. 2011;79:9–15. doi: 10.1016/j.ijpsycho.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Davis KD, Bushnell MC, Iannetti GD, Lawrence K, St, Coghill R. Evidence against pain specificity in the dorsal posterior insula. F1000Res. 2015;4:362. doi: 10.12688/f1000research.6833.1. [version 1; referees: 3 approved] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol. 1998;80:1533–1546. doi: 10.1152/jn.1998.80.3.1533. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci (Regul Ed) 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Epstein S. Heart rate, skin conductance, and intensity ratings during experimentally induced anxiety: habituation within and among days. Psychophysiology. 1971;8:319–331. doi: 10.1111/j.1469-8986.1971.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Fardo F, Vinding MC, Allen M, Jensen TS, Finnerup NB. Delta and gamma oscillations in operculo-insular cortex underlie innocuous cold thermosensation. J Neurophysiol. 2017 doi: 10.1152/jn.00843.2016. jn.00843.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Khalsa SS, Salomons TV, Prkachin KM, Frey-Law LA, Lee JE, Tranel D, Rudrauf D. Preserved emotional awareness of pain in a patient with extensive bilateral damage to the insula, anterior cingulate, and amygdala. Brain Struct Funct. 2016;221:1499–1511. doi: 10.1007/s00429-014-0986-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher JR, Otzenberger H, Gounot D. The BOLD response and the gamma oscillations respond differently than evoked potentials: an interleaved EEG-fMRI study. BMC Neurosci. 2003;4:22. doi: 10.1186/1471-2202-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman-Hill S, Maldonado PE, Gray CM. Dynamics of striate cortical activity in the alert macaque: I. Incidence and stimulus-dependence of gamma-band neuronal oscillations. Cereb Cortex. 2000;10:1105–1116. doi: 10.1093/cercor/10.11.1105. [DOI] [PubMed] [Google Scholar]
- Frien A, Eckhorn R, Bauer R, Woelbern T, Gabriel A. Fast oscillations display sharper orientation tuning than slower components of the same recordings in striate cortex of the awake monkey. European Journal of Neuroscience. 2000;12:1453–1465. doi: 10.1046/j.1460-9568.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci (Regul Ed) 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Frot M, Faillenot I, Mauguière F. Processing of nociceptive input from posterior to anterior insula in humans. Hum Brain Mapp. 2014;35:5486–5499. doi: 10.1002/hbm.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frot M, Magnin M, Mauguière F, Garcia-Larrea L. Human SII and posterior insula differently encode thermal laser stimuli. Cereb Cortex. 2007;17:610–620. doi: 10.1093/cercor/bhk007. [DOI] [PubMed] [Google Scholar]
- Frot M, Mauguière F. Dual representation of pain in the operculo-insular cortex in humans. Brain. 2003;126:438–450. doi: 10.1093/brain/awg032. [DOI] [PubMed] [Google Scholar]
- Fulbright RK, Manson SC, Skudlarski P, Lacadie CM, Gore JC. Quantity determination and the distance effect with letters, numbers, and shapes: a functional MR imaging study of number processing. AJNR Am J Neuroradiol. 2003;24:193–200. [PMC free article] [PubMed] [Google Scholar]
- Furl N, Averbeck BB. Parietal cortex and insula relate to evidence seeking relevant to reward-related decisions. J Neurosci. 2011;31:17572–17582. doi: 10.1523/JNEUROSCI.4236-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Larrea L. The posterior insular-opercular region and the search of a primary cortex for pain. Neurophysiol Clin. 2012;42:299–313. doi: 10.1016/j.neucli.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Perchet C, Creac’h C, Convers P, Peyron R, Laurent B, Mauguière F, Magnin M. Operculo-insular pain (parasylvian pain): a distinct central pain syndrome. Brain. 2010;133:2528–2539. doi: 10.1093/brain/awq220. [DOI] [PubMed] [Google Scholar]
- Göbel SM, Johansen-Berg H, Behrens T, Rushworth MFS. Response-selection-related parietal activation during number comparison. J Cogn Neurosci. 2004;16:1536–1551. doi: 10.1162/0898929042568442. [DOI] [PubMed] [Google Scholar]
- Goense JBM, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol. 2008;18:631–640. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- Graham FK, Clifton RK. Heart-rate change as a component of the orienting response. Psychol Bull. 1966;65:305–320. doi: 10.1037/h0023258. [DOI] [PubMed] [Google Scholar]
- Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Gross J, Schnitzler A, Timmermann L, Ploner M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol. 2007;5:e133. doi: 10.1371/journal.pbio.0050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck M, Lorenz J, Engel AK. Attention to painful stimulation enhances gamma-band activity and synchronization in human sensorimotor cortex. J Neurosci. 2007;27:9270–9277. doi: 10.1523/JNEUROSCI.2283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Kaiser J. EEG γ-band responses reflect human behavior: an overview. Int J Psychophysiol. 2011;79:1–2. doi: 10.1016/j.ijpsycho.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Heydrich L, Blanke O. Distinct illusory own-body perceptions caused by damage to posterior insula and extrastriate cortex. Brain. 2013;136:790–803. doi: 10.1093/brain/aws364. [DOI] [PubMed] [Google Scholar]
- Hua LH, Strigo IA, Baxter LC, Johnson SC, Craig ADB. Anteroposterior somatotopy of innocuous cooling activation focus in human dorsal posterior insular cortex. Am J Physiol Regul Integr Comp Physiol. 2005;289:R319–R325. doi: 10.1152/ajpregu.00123.2005. [DOI] [PubMed] [Google Scholar]
- Hu L, Xiao P, Zhang ZG, Mouraux A, Iannetti GD. Single-trial time-frequency analysis of electrocortical signals: baseline correction and beyond. Neuroimage. 2014;84:876–887. doi: 10.1016/j.neuroimage.2013.09.055. [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Hashemi N, Gati JS, Menon RS, Everling S. Electrophysiological signatures of spontaneous BOLD fluctuations in macaque prefrontal cortex. Neuroimage. 2015;113:257–267. doi: 10.1016/j.neuroimage.2015.03.062. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back) Exp Brain Res. 2010;205:1–12. doi: 10.1007/s00221-010-2340-1. [DOI] [PubMed] [Google Scholar]
- Isnard J, Guénot M, Sindou M, Mauguière F. Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study. Epilepsia. 2004;45:1079–1090. doi: 10.1111/j.0013-9580.2004.68903.x. [DOI] [PubMed] [Google Scholar]
- Isnard J, Magnin M, Jung J, Mauguière F, Garcia-Larrea L. Does the insula tell our brain that we are in pain? Pain. 2011;152:946–951. doi: 10.1016/j.pain.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Karns CM, Knight RT. Intermodal auditory, visual, and tactile attention modulates early stages of neural processing. J Cogn Neurosci. 2009;21:669–683. doi: 10.1162/jocn.2009.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci. 2015;38:86–95. doi: 10.1016/j.tins.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. The Neural Code for Pain: From Single-Cell Electrophysiology to the Dynamic Pain Connectome. Neuroscientist. 2016 doi: 10.1177/1073858416667716. [DOI] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Struct Funct. 2010;214:579–591. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Liberati G, Klöcker A, Safronova MM, Ferrão Santos S, Ribeiro Vaz J-G, Raftopoulos C, Mouraux A. Nociceptive Local Field Potentials Recorded from the Human Insula Are Not Specific for Nociception. PLoS Biol. 2016;14:e1002345. doi: 10.1371/journal.pbio.1002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond, B, Biol Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Luo S, Johnston P. A review of electrocardiogram filtering. J Electrocardiol. 2010;43:486–496. doi: 10.1016/j.jelectrocard.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Makeig S, Jung TP, Bell AJ, Ghahremani D, Sejnowski TJ. Blind separation of auditory event-related brain responses into independent components. Proc Natl Acad Sci U S A. 1997;94:10979–10984. doi: 10.1073/pnas.94.20.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmstrom EJ, Opton E, Lazarus RS. Heart rate measurement and the correlation of indices of arousal. Psychosom Med. 1965;27:546–556. doi: 10.1097/00006842-196511000-00005. [DOI] [PubMed] [Google Scholar]
- von der Malsburg C. Binding in models of perception and brain function. Curr Opin Neurobiol. 1995;5:520–526. doi: 10.1016/0959-4388(95)80014-x. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Isnard J, Peyron R, Guénot M, Mauguière F. Somatotopic organization of pain responses to direct electrical stimulation of the human insular cortex. Pain. 2009;146:99–104. doi: 10.1016/j.pain.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Isnard J, Peyron R, Mauguière F. Stimulation of the human cortex and the experience of pain: Wilder Penfield’s observations revisited. Brain. 2012;135:631–640. doi: 10.1093/brain/awr265. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Foster BL, Honey CJ. Does rhythmic entrainment represent a generalized mechanism for organizing computation in the brain? Front Comput Neurosci. 2012;6:85. doi: 10.3389/fncom.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayedi M. All roads lead to the insula. Pain. 2014;155:1920–1921. doi: 10.1016/j.pain.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Morel A, Gallay MN, Baechler A, Wyss M, Gallay DS. The human insula: Architectonic organization and postmortem MRI registration. Neuroscience. 2013;236:117–135. doi: 10.1016/j.neuroscience.2012.12.076. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix”. Neuroimage. 2011;54:2237–2249. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD. Across-trial averaging of event-related EEG responses and beyond. Magn Reson Imaging. 2008;26:1041–1054. doi: 10.1016/j.mri.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Murayama Y, Biessmann F, Meinecke FC, Müller K-R, Augath M, Oeltermann A, Logothetis NK. Relationship between neural and hemodynamic signals during spontaneous activity studied with temporal kernel CCA. Magn Reson Imaging. 2010;28:1095–1103. doi: 10.1016/j.mri.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Singh KD. Spatiotemporal frequency tuning of BOLD and gamma band MEG responses compared in primary visual cortex. Neuroimage. 2008;40:1552–1560. doi: 10.1016/j.neuroimage.2008.01.052. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Singh KD. Functional decoupling of BOLD and gamma-band amplitudes in human primary visual cortex. Hum Brain Mapp. 2009;30:2000–2007. doi: 10.1002/hbm.20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidich TP, Kang E, Fatterpekar GM, Delman BN, Gultekin SH, Wolfe D, Ortiz O, Yousry I, Weismann M, Yousry TA. The insula: anatomic study and MR imaging display at 1.5 T. AJNR Am J Neuroradiol. 2004;25:222–232. [PMC free article] [PubMed] [Google Scholar]
- Nourski KV, Steinschneider M, Rhone AE, Oya H, Kawasaki H, Howard MA, McMurray B. Sound identification in human auditory cortex: Differential contribution of local field potentials and high gamma power as revealed by direct intracranial recordings. Brain Lang. 2015;148:37–50. doi: 10.1016/j.bandl.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaradan S, Mouraux A, Jonas J, Colnat-Coulbois S, Rossion B, Maillard L. Intracerebral evidence of rhythm transform in the human auditory cortex. Brain Struct Funct. 2016 doi: 10.1007/s00429-016-1348-0. [DOI] [PubMed] [Google Scholar]
- Opsommer E, Masquelier E, Plaghki L. Determination of nerve conduction velocity of C-fibres in humans from thermal thresholds to contact heat (thermode) and from evoked brain potentials to radiant heat (CO2 laser) Neurophysiologie Clinique/Clinical Neurophysiology. 1999;29:411–422. doi: 10.1016/S0987-7053(00)87265-2. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguière F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb Cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE Trans Biomed Eng. 1985;32:230–236. doi: 10.1109/TBME.1985.325532. [DOI] [PubMed] [Google Scholar]
- Pan W-J, Thompson GJ, Magnuson ME, Jaeger D, Keilholz S. Infraslow LFP correlates to resting-state fMRI BOLD signals. Neuroimage. 2013;74:288–297. doi: 10.1016/j.neuroimage.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat Neurosci. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Ploner M, Sorg C, Gross J. Brain Rhythms of Pain. Trends Cogn Sci (Regul Ed) 2016 doi: 10.1016/j.tics.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone M, Mirarchi L, Bracale M. Adaptive removal of gradients-induced artefacts on ECG in MRI: a performance analysis of RLS filtering. Med Biol Eng Comput. 2010;48:475–482. doi: 10.1007/s11517-010-0596-z. [DOI] [PubMed] [Google Scholar]
- Schlereth T, Magerl W, Treede R. Spatial discrimination thresholds for pain and touch in human hairy skin. Pain. 2001;92:187–194. doi: 10.1016/s0304-3959(00)00484-x. [DOI] [PubMed] [Google Scholar]
- Schulz E, May ES, Postorino M, Tiemann L, Nickel MM, Witkovsky V, Schmidt P, Gross J, Ploner M. Prefrontal Gamma Oscillations Encode Tonic Pain in Humans. Cereb Cortex. 2015;25:4407–4414. doi: 10.1093/cercor/bhv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz E, Tiemann L, Schuster T, Gross J, Ploner M. Neurophysiological coding of traits and states in the perception of pain. Cereb Cortex. 2011;21:2408–2414. doi: 10.1093/cercor/bhr027. [DOI] [PubMed] [Google Scholar]
- Schulz E, Tiemann L, Witkovsky V, Schmidt P, Ploner M. γ Oscillations are involved in the sensorimotor transformation of pain. J Neurophysiol. 2012;108:1025–1031. doi: 10.1152/jn.00186.2012. [DOI] [PubMed] [Google Scholar]
- Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci. 2015a;18:499–500. doi: 10.1038/nn.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula is not an island in pain but subserves a fundamental role - Response to: “Evidence against pain specificity in the dorsal posterior insula” by Davis et al. F1000Res. 2015b;4:1207. doi: 10.12688/f1000research.7287.1. [version 1; referees: 2 approved]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing-Hong L. Motion Artifact Reduction in Electrocardiogram Using Adaptive Filter. Journal of medical and biological engineering. 2010;31:67–72. [Google Scholar]
- Singh KD, Smith AT, Greenlee MW. Spatiotemporal frequency and direction sensitivities of human visual areas measured using fMRI. Neuroimage. 2000;12:550–564. doi: 10.1006/nimg.2000.0642. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Kleinschmidt A. Anterior insula activations in perceptual paradigms: often observed but barely understood. Brain Struct Funct. 2010;214:611–622. doi: 10.1007/s00429-010-0252-2. [DOI] [PubMed] [Google Scholar]
- Taylor SP, Epstein S. The measurement of autonomic arousal. Some basic issues illustrated by the covariation of heart rate and skin conductance. Psychosom Med. 1967;29:514–525. doi: 10.1097/00006842-196709000-00010. [DOI] [PubMed] [Google Scholar]
- Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Twisk JWR. A practical guide. Cambridge University Press; Cambridge, UK: 2005. Applied multilevel analysis. [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979;204:205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- Wattendorf E, Westermann B, Lotze M, Fiedler K, Celio MR. Insular cortex activity and the evocation of laughter. J Comp Neurol. 2016;524:1608–1615. doi: 10.1002/cne.23884. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Willis WD. The pain system. The neural basis of nociceptive transmission in the mammalian nervous system. Pain Headache. 1985;8:1–346. [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- Yantis S. The Neural Basis of Selective Attention: Cortical Sources and Targets of Attentional Modulation. Curr Dir Psychol Sci. 2008;17:86–90. doi: 10.1111/j.1467-8721.2008.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Hu L, Hung YS, Mouraux A, Iannetti GD. Gamma-band oscillations in the primary somatosensory cortex--a direct and obligatory correlate of subjective pain intensity. J Neurosci. 2012;32:7429–7438. doi: 10.1523/JNEUROSCI.5877-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]