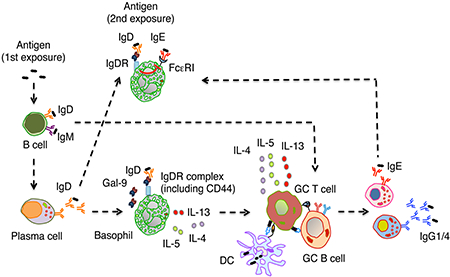
Summary
B cells thwart antigenic aggressions by releasing immunoglobulin M (IgM), IgG, IgA and IgE, which deploy well-understood effector functions. In contrast, the role of secreted IgD remains mysterious. We found that some B cells generated IgD-secreting plasma cells following early exposure to external soluble antigens such as food proteins. Secreted IgD targeted basophils by interacting with the CD44-binding protein galectin-9. When engaged by antigen, basophil-bound IgD incresed basophil secretion of interleukin-4 (IL-4), IL-5 and IL-13, which facilitated the generation of T follicular helper type-2 cells expressing IL-4. These germinal center T cells enhanced IgG1 and IgE but not IgG2a and IgG2b responses to the antigen initially recognized by basophil-bound IgD. In addition, IgD ligation by antigen attenuated allergic basophil degranulation induced by IgE co-ligation. Thus, IgD may link B cells with basophils to optimize humoral T helper type-2-mediated immunity against common environmental soluble antigens.
eTOC Blurb
The function of IgD has been mysterious. Shan et al. find that IgD recognized food antigens and targeted basophils through galectin-9. IgD ligation by antigen induced basophil secretion of IL-4, IL-5 and IL-13, which amplified Th2 cell-mediated IgG1 and IgE production by B cells. IgD also constrained IgE-mediated basophil degranulation.
Graphical abstract
Introduction
B cells of the adaptive immune system generate humoral protection by releasing immunoglobulin M (IgM), IgG, IgA and IgE. Each of these antibodies consists of variable (V) heavy (H) and light (L) chain regions, which recognize antigen, and an H chain constant (C) region, which mediates specific biological functions (Lu et al., 2018). Besides co-opting soluble innate effector proteins such as complement, collectins, pentraxins and ficolins, the C region engages Fc receptors (FcRs) from various innate effector cells to maximize antigen clearance (Chorny et al., 2016; Holmskov et al., 2003).
Humoral immunity also involves IgD, an antibody with enigmatic effector function (Chen et al., 2009; Choi et al., 2017; Rouaud et al., 2014). IgD emerged with IgM at the time of the inception of the adaptive immune system, when the lack of FcRs may have forced antibodies to develop FcR-independent defensive strategies (Flajnik, 2018). Unlike IgM but similar to the mucosal antibody IgA, IgD does not recruit pro-inflammatory complement proteins (Gutzeit et al., 2018; Lu et al., 2018), raising the possibility that IgD may have diverged from IgM to implement non-inflammatory protection at mucosal sites of antigen entry, including gills in fishes and nasopharyngeal cavities in mammals (Gutzeit et al., 2018). This response could involve IgD recruitment of lectins (Holmskov et al., 2003).
IgD is best known for its function as a B cell antigen receptor (BCR) (Gutzeit et al., 2018). Mature B cells emerging from the bone marrow acquire IgD receptors of the same specificity as IgM receptors through alternative splicing of a long precursor mRNA (Gutzeit et al., 2018). IgD and IgM receptor engagement by antigen initiates cognate B cell interactions with T follicular helper (Tfh) cells at the T-B follicular border (Victora and Nussenzweig, 2012). B cells emerging from these interactions downregulate surface IgD and either differentiate into short-lived extrafollicular IgM-secreting plasma cells or enter a germinal center (GC) program fostering somatic hypermutation (SHM) and class switch DNA recombination (CSR), which are dependent on the DNA-editing enzyme activation-induced cytidine deaminase (AID) (Victora and Nussenzweig, 2012). While SHM introduces point mutations within V region-encoding V(D)J genes to promote the selection of high-affinity BCRs, CSR replaces Cμ with Cγ, Cα or Cε genes encoding the CH region of IgM, IgG, IgA or IgE to diversify the antibody effector functions. Besides inducing memory B cells, the GC generates long-lived IgG-secreting plasma cells that home to the bone marrow (Victora and Nussenzweig, 2012).
Additional plasma cells secrete IgD in nasopharyngeal lymphoid tissues after undergoing CSR from Cμ to Cδ (Chen et al., 2009; Choi et al., 2017; Rouaud et al., 2014). In humans, secreted IgD binds to basophils and, when cross-linked in vitro, activates basophil release of interleukin-4 (IL-4) through a yet unknown IgD receptor (IgDR) (Chen et al., 2009; Gutzeit et al., 2018). Together with IL-5 and IL-13, the T helper type-2 (Th2) cytokine IL-4 promotes dendritic cell-orchestrated B cell responses involving IgG1 (in mice) or IgG4 (in humans) and IgE production (Wynn, 2015). Of note, IgE protects against toxins and helminths and does so in cooperation with basophils and mast cells (Karasuyama et al., 2009; Palm et al., 2013). Although early studies suggest that secreted IgD also plays an immune-amplifying role (Gutzeit et al., 2018; Xue et al., 1984), the specific contribution of IgD to immunity remains elusive.
In this report we have shown that B cells mounted early IgD responses upon exposure to small soluble antigens such as food proteins. Secreted IgD bound to basophils and mast cells after interacting with galectin-9, a galactose-binding lectin that linked IgD with the immunomodulating proteoglycan CD44 on basophils. Ligation of basophil-bound IgD by antigen triggered a basophil-regulated Th2 cell-mediated protective program that enhanced B cell production of antigen-specific IgG1 and IgE while decreasing IgE-induced basophil degranulation. Thus, secreted IgD may link B cells with basophils to maximize Th2 cell-mediated immune surveillance against common soluble antigens.
Results
IgD Enhances Basophil Expression of IL-4
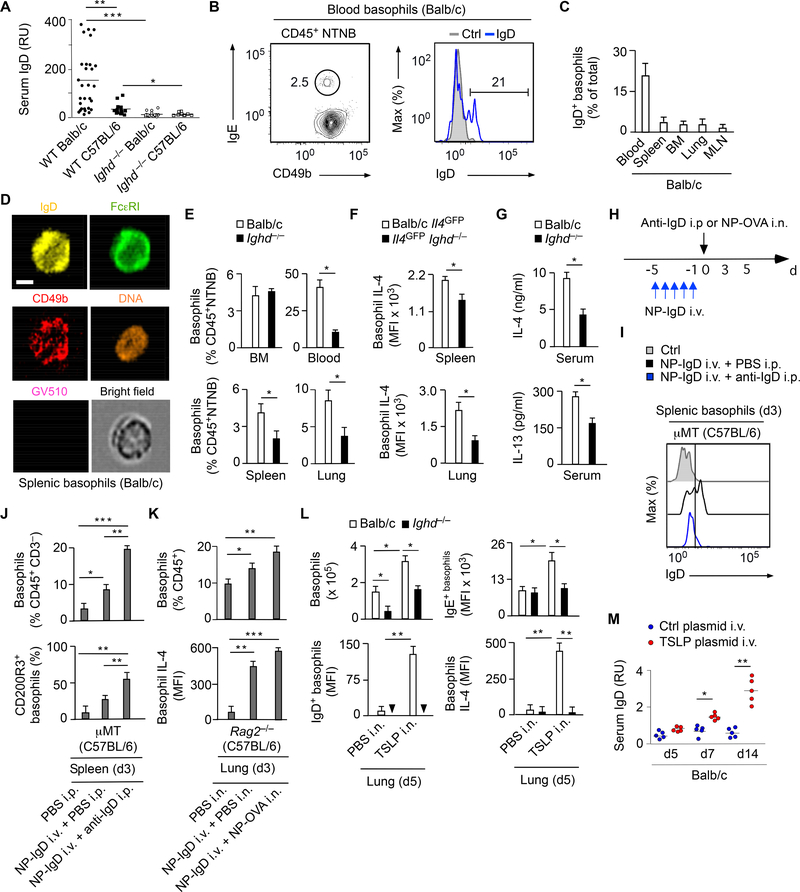
We first verified whether IgD elicited basophil release of IL-4 in vivo. As shown by ELISA, serum IgD was detected in wild type (WT) but not control IgD-deficient (Ighd−/−) mice and its concentration was higher in Th2 cell-biased Balb/c than TH1 cell-biased C57BL/6 strains (Figure 1A). Standard and imaging flow cytometry (FCM) assays detected both surface and intracellular IgD in a subset of basophils from various tissues of WT Balb/c mice (Figure 1B and 1D and Figure S1A). Next, we analyzed basophils in IgD-deficient Ighd−/− mice, which have a largely normal immune system (Roes and Rajewsky, 1993). These mice were crossed with Il4GFP reporter mice, which express green-fluorescent protein (GFP) in response to Il4 gene activation (Mohrs et al., 2001). FCM showed that Ighd−/− mice had fewer peripheral but not bone marrow basophils and that peripheral basophils from Ighd−/−Il4GFP mice expressed less IL-4 compared to controls (Figure 1E and 1F). Consistent with the involvement of basophils in Th2 cell-mediated immunity (Karasuyama et al., 2009), Ighd−/− mice also had less serum IL-4 and IL-13 compared to controls (Figure 1G). Thus, IgD binds to basophils and increases basophil expression of IL-4.
Figure 1. IgD Ligation by Antigen Elicits Basophil Activation.
(A) ELISA of serum IgD from WT Balb/c (n=29), WT C57BL/6 (n=15), Ighd−/− Balb/c (n=20) or Ighd−/− C57BL/6 (n=10) mice.
(B) Left: FCM of CD49b and IgE on basophils (black gate) from circulating CD45+NTNB cells of a WT Balb/c mouse. NTNB, non-T non-B. Right: FCM of IgD on circulating basophils (blue open profile) from a WT Balb/c mouse. Ctrl, isotype-matched control (gray solid profile).
(C) FCM of IgD+ basophils from peripheral blood, spleen, bone marrow (BM), lung or mesenteric lymph nodes (MLNs) of WT Balb/c mice (n=5).
(D) Imaging FCM of IgD, FcεRI and CD49b from a representative viable Ghost Dye Violet 510 (GV510)− splenic basophil of a WT Balb/c mouse. Scale bar, 5 μm.
(E) FCM quantification of FcεRI+CD49b+ basophils from BM, blood, spleen or lung CD45+NTNB cells of WT Balb/c (n=10) or Ighd−/− (n=10) mice.
(F) FCM of Il4-driven GFP expression in splenic or lung FcεRI+CD49b+ basophils from WT Balb/c Il4GFP (n=5) or Il4GFPIghd−/− (n=5) mice. MFI, mean fluorescence intensity.
(G) ELISA of IL-4 and IL-13 from serum of WT Balb/c or Ighd−/− mice (n=10).
(H) Schematics of i.v. reconstitution μMT or Rag2−/− mice with NP-reactive IgD (NP-IgD) followed by i.p. injection of anti-IgD or i.n. inoculation of NP-OVA.
(I) FCM of IgD on splenic FcεRI+CD49b+ basophils from a μMT mouse before (ctrl) or after reconstitution as in (H). PBS, control phosphate buffer solution (PBS).
(J, K) FCM quantification of total, CD200R3+ or IL-4+ basophils from the spleen of μMT C57BL/6 mice (n=10) (J) or the lungs of IgD-deficient Rag2−/− (n=10) C57BL/6 mice (K) treated as in (H).
(L) FCM quantification of total, IgD+, IgE+ or IL-4+ basophils from the lungs of WT Balb/c (n=5) or Ighd−/− (n=5) mice 5 d following i.n. exposure to PBS or recombinant TSLP.
(M) ELISA of serum IgD from Balb/c mice following i.v. injection of control empty or TSLP-encoding plasmids (n=10).
Data summarize two-three experiments with 5–10 mice per each experimental group (A, C, E-G, J-M) or show one experiment of at least three with similar results (B, D, I). Results are presented as mean ± SEM; *p < 0.05, **p < 0.01 (two-tailed unpaired Student’s t test). See also Figure S1.
IgD Ligation by Antigen Activates Basophils
Next, we determined whether basophil expression of IL-4 increases upon IgD ligation by antigen. IgDdeficient μMT or Rag2−/− mice, which lack B cells or both B and T cells, respectively, were reconstituted with IgD secreted by B1-δ8 cells (Figure S1B-E), a class-switched hybridoma specific for the hapten 4hydroxy-3-nitrophenylacetyl (NP) (Neuberger and Rajewsky, 1981). As shown by FCM, NP-specific IgD (NP-IgD) bound splenic basophils following intravenous (i.v.) administration into μMT mice (Figure 1H and 1I). Nonspecific ligation of basophil-bound NP-IgD following intraperitoneal (i.p.) injection of a goat polyclonal antibody to IgD (anti-IgD) induced down-regulation of basophil-bound NP-IgD as well as expansion and activation of splenic basophils from IgD-reconstituted μMT mice, including increased basophil expression of CD200R3 (Figure 1I and 1J and Figure S1F). Similarly, specific ligation of basophil-bound NP-IgD by NP-haptenated ovalbumin (NP-OVA) made available by intranasal (i.n.) inoculation stimulated an increase of both the frequency and IL-4 expression of lung basophils from IgDreconstituted Rag2−/− mice (Figure 1K and Figure S1F).
Having shown that IgD ligation by antigen enhanced basophil expression of IL-4 and knowing that some oral antigens initiate IL-3-independent Th2 cell-mediated responses through the epithelial cytokine thymic stromal lymphopoietin (TSLP) (Siracusa et al., 2011), we verified whether IgD modulated IL-4 expression by lung basophils exposed to exogenous TSLP. Compared to controls, Ighd−/− mice inoculated with i.n. TSLP showed fewer lung basophils, which bound less IgE and expressed less IL-4 (Figure 1L). The functional relationship between IgD and TSLP was consistent with the detection of TSLP in human tonsillar districts inhabited by IgD+IgM− plasma cells (Figure S1G). These data suggest that TSLP stimulates B cell release of IgD in the context of an IgE-inducing humoral Th2 cell-mediated response involving IL-4 expression by basophils. This conclusion was further strengthened by the observation that i.v. inoculation of a TSLP-encoding plasmid strongly induced serum IgD (Figure 1M). Thus, ligation of basophil-bound IgD by antigen may enhance humoral Th2 cell immunity through a mucosal TSLPregulated mechanism involving increased basophil expression of IL-4.
IgD Enhances Some But Not all Humoral Th2 Cell Responses
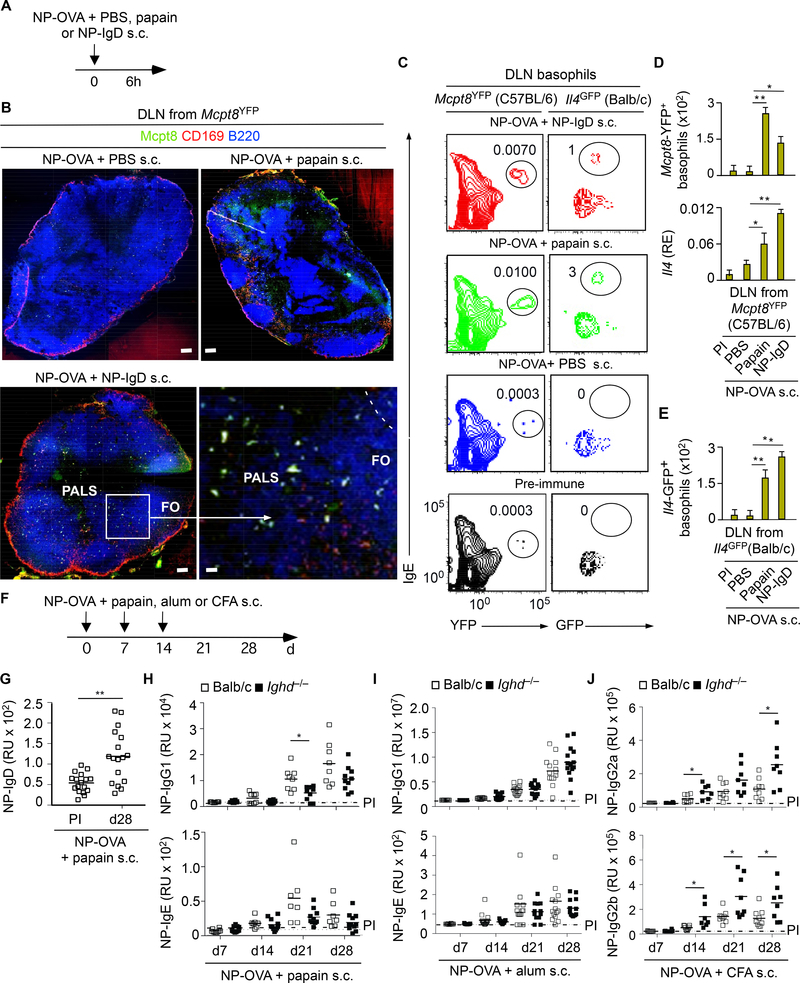
The Th2 cell-amplifying function of IgD was further evaluated in a subcutaneous (s.c.) immunization model involving co-inoculation of NP-OVA with exogenous NP-IgD or papain (Figure 2A), a Th2 cellinducing protease that activates basophils (Karasuyama et al., 2009). This experiment was initially performed in Mcpt8YFP reporter mice expressing yellow fluorescent protein (YFP) under the control of the basophil-specific mast cell protease 8 (Mcpt8) promoter. As shown by tissue immunofluorescence analysis (IFA), FCM and quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR), Mcpt8YFP mice exhibited comparable basophil recruitment as well as basophil expression of IL-4 in the homolateral draining lymph node upon s.c. inoculation of NP-OVA with either papain or exogenous NPIgD (Figure 2B-D). In an identical immunization setting, NP-IgD or papain comparably induced IL-4 in basophils from the draining lymph node of reporter Il4GFP mice (Figures 2E). Thus, IgD deploys basophil-activating adjuvant-like Th2 cell signals similar to those induced by papain.
Figure 2. IgD Ligation by Antigen Induces Basophil Expression of IL-4.
(A) Schematics of s.c. immunization with NP-OVA combined with control PBS, papain or NP-IgD.
(B) Confocal imaging of CD169 (subcapsular sinus macrophage molecule, red), B220 (B cell molecule, blue) and Mcpt8 (YFP, yellow) from draining lymph node (DLN) of a C57BL/6 Mcpt8YFP mouse immunized for 6 h as in (A). FO, follicle; dashed line, follicular border. Original magnification, ×5; rightbottom panel, ×40. Scale bars, 50 μm (top and bottom-left panels) or 5 μm (bottom-right panel).
(C-E) FCM quantification of total YFP+ or GFP+ basophils and qRT-PCR quantification of Il4 transcripts encoding IL-4 from the DLN of C57BL/6 Mcpt8YFP (n=5) mice (C, D) or Balb/c Il4GFP (n=10) mice (E) 6 h following s.c. immunization as in (A). qRT-PCR results (D, bottom graph) are presented as relative expression (RE) compared to mRNA for glyceraldeheyde phosphate dehydrogenase (GAPDH).
(F) Schematics of s.c. immunization with NP-OVA combined with papain, alum or CFA.
(G) ELISA of serum NP-specific IgD from WT Balb/c mice (n=17) following s.c. immunization with NP-OVA and papain. PI, pre-immune (day −1 relatively to the onset of immunization (day 0).
(H) ELISA of serum NP-specific IgG1 and IgE from WT Balb/c (n=12) or Ighd−/− (n=16) mice following s.c. immunization with NP-OVA and papain.
(I) ELISA of serum NP-specific IgG1 and IgE from WT Balb/c (n=15) or Ighd−/− (n=15) mice following s.c. immunization with NP-OVA and alum.
(J) ELISA of serum NP-specific IgG2a and IgG2b from Balb/c (n=10) or Ighd−/− (n=10) mice following s.c. immunization with NP-OVA and CFA.
Data show one experiment of three with similar results (B, C) or summarize results from two experiments with 5–10 (D, E) or 10–17 (G-J) mice per experimental group. Results are presented as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001 (two-tailed unpaired Student’s t test).
Given that s.c. papain activates basophils similarly to IgD ligation by antigen, we next verified whether papain could elicit a specific secretory IgD response. Indeed, s.c. immunization of WT Balb/c mice with NP-OVA and papain induced NP-specific serum IgD (Figure 2F and 2G). Next, knowing that IgD-stimulated basophils express more IL-4, we assessed whether IgD could enhance papain-adjuvanted humoral Th2 cell responses. Compared to WT controls, Ighd−/− mice showed less NP-specific serum IgG1 but not IgE upon s.c. immunization with NP-OVA and papain (Figure 2H). In contrast, WT and Ighd−/− mice mounted comparable NP-specific IgG1 and IgE responses upon s.c. immunization with NP-OVA and alum (Figure 2I), a Th2 cell-polarizing adjuvant distinct from papain (Wynn, 2015). Finally, NPspecific IgG2a and IgG2b were increased in Ighd−/− mice s.c. immunized with NP-OVA and the TH1 cellpolarizing complete Freund adjuvant (CFA) (Figure 2J). Thus, IgD enhances some but not all humoral Th2 cell-mediated responses through a mechanism involving basophils.
IgD Increases Th2 Cell Polarization in Germinal Centers
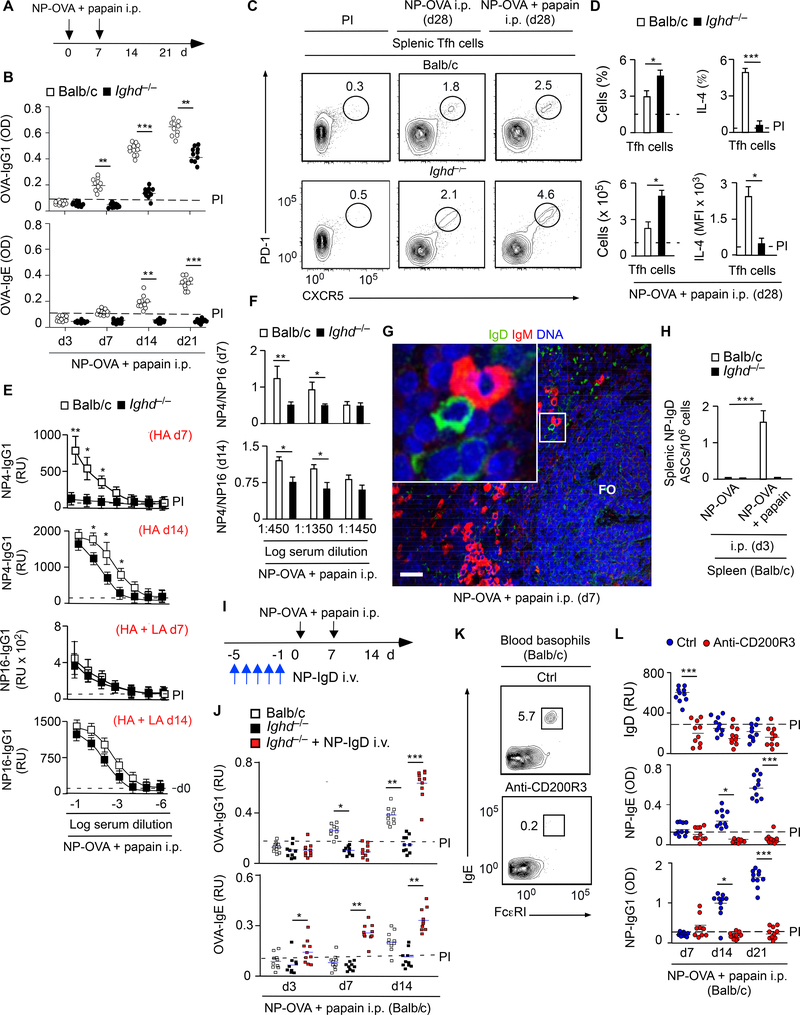
The humoral Th2 cell-inducing function of IgD was further explored in an intraperitoneal (i.p.) immunization model. ELISAs showed that, compared to WT controls, Ighd−/− mice induced less IgG1 and IgE specific to OVA upon i.p. immunization with NP-OVA and papain (Figure 3A and 3B). This effect did not stem from depletion of Tfh cells or T follicular regulatory (TFR) cells (Victora and Nussenzweig, 2012). Indeed, FCM showed that these GC-residing T cells were increased in the spleen from immunized Ighd−/− mice, but expressed less IL-4 compared to controls (Figure 3C and 3D and Figure S2A-C). This perturbation was coupled with reduced GC production of high-affinity IgG1 to the NP hapten (Figure 3E and 3F).
Figure 3. Ligation of Basophil-Bound IgD by Antigen Enhances IgG1 and IgE Responses.
(A) Schematics of i.p. immunization with NP-OVA and papain.
(B) ELISA of serum OVA-specific IgG1 and IgE from WT Balb/c (n=10) or Ighd−/− (n=10) mice immunized as in (A). Dashed line, maximum antibody concentration in pre-immune (PI) mice. PI, day −1 relatively to the onset of immunization (day 0).
(C-D) FCM quantification of total and IL-4-expressing splenic PD-1highCXCR5high Tfh cells from WT Balb/c (n=5) or Ighd−/− (n=5) mice immunized as in (A).
(E, F) ELISA of serum NP-specific IgG1 from WT Balb/c (n=10) or Ighd−/− (n=10) mice immunized as in (A). BSA haptenated with 4 or 16 NPs was used to measure high-affinity (HA) and both HA and lowaffinity (LA) IgG1, respectively.
(G) IFA of splenic tissue from immunized Balb/c mice stained for IgD (green), IgM (red) and nuclei (blue) following i.p. immunization as in (B). Inset: IgD+IgM− plasmablast next to IgD−IgM+ plasmablast. FO, follicle. Original magnification, ×10 with ×2 enlargement. Scale bar, 50 μm.
(H) ELISPOT of spleen ASCs expressing NP-specific IgD from WT Balb/c (n=5) or Ighd−/− (n=5) mice 3 d following i.p. immunization with PBS or NP-OVA and papain.
(I) Schematics of i.v. reconstitution with NP-reactive IgD (NP-IgD) followed by i.p. immunization with NP-OVA and papain.
(J) ELISA of serum OVA-specific IgG1 and IgE from WT Balb/c controls (n=10), Ighd−/− mice (n=10) or NP-IgD-reconstituted Ighd−/− mice (n=10) following i.p. immunization with NP-OVA and papain as in (I).
(K) FCM of circulating FcεRI+IgE+ basophils from WT Balb/c mice after i.v. injection of a control (ctrl) IgG2b antibody or a basophil-depleting anti-CD200R3 antibody.
(L) ELISA of serum total IgD as well as serum NP-specific IgG1 and IgE in control (n=10) or basophil-depleted (n=10) WT Balb/c mice after i.p. immunization with NP-OVA and papain.
Data summarize results from two experiments with 5–10 mice per experimental group (B-F, H, J, L) or show one experiment of three-five with similar results (C, G, K). Results are presented as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001 (two-tailed unpaired Student’s t test). See also Figure S2.
We then verified whether i.p. immunization with NP-OVA and papain could induce antigen-specific IgD. Tissue IFA and immunospot demonstrated early but transient extrafollicular induction of plasma cells containing intracellular IgD but not IgM specific to OVA-associated NP in immunized WT control but not Ighd−/− mice (Figure 3G and 3H and Figure S2D). Thus, similar to s.c. immunization, i.p. immunization induced antigen-specific IgD in the context of a papain-adjuvanted humoral Th2 cell response. Next, we ascertained whether reconstitution of Ighd−/− mice with secreted NP-IgD was sufficient to rescue humoral Th2 cell responses to i.p. NP-OVA combined with papain (Figure 3I). We found that secreted NP-IgD completely restored OVA or NP-specific serum IgG1 and IgE responses in immunized Ighd−/− mice (Figure 3J and Figure S2E). This rescue effect was specific, because NP-IgD did not increase serum IgG1 and IgE in non-immunized WT or Ighd−/− mice (Figure S2F). Thus, IgD amplifies IgG1 and IgE responses to certain soluble antigens by enhancing Th2 cell polarization in GCs.
IgD Augments Humoral Th2 Cell-Mediated Responses through Basophils
We then ascertained whether IgD needed basophils to amplify humoral Th2 cell-mediated responses. As shown by qRT-PCR, splenic basophils from Ighd−/− mice i.p. immunized with NP-OVA and papain had less transcripts for IL-4 and IL-13 compared to controls (Figure S2G). Next, we determined whether basophil-depleted mice phenocopied Ighd−/− mice following i.p. immunization with NP-OVA and papain. Compared to controls, WT mice i.v. injected with a basophil-depleting anti-CD200R3 antibody showed less total IgD and less IgG1 and IgE responses to NP-associated OVA (Figure 3K and 3L and Figure S2H). Thus, mouse IgD amplifies papain-induced humoral Th2 cell-mediated responses through a mechanism involving basophils.
Secreted IgD Suffices to Enhance Humoral Th2 Cell Responses
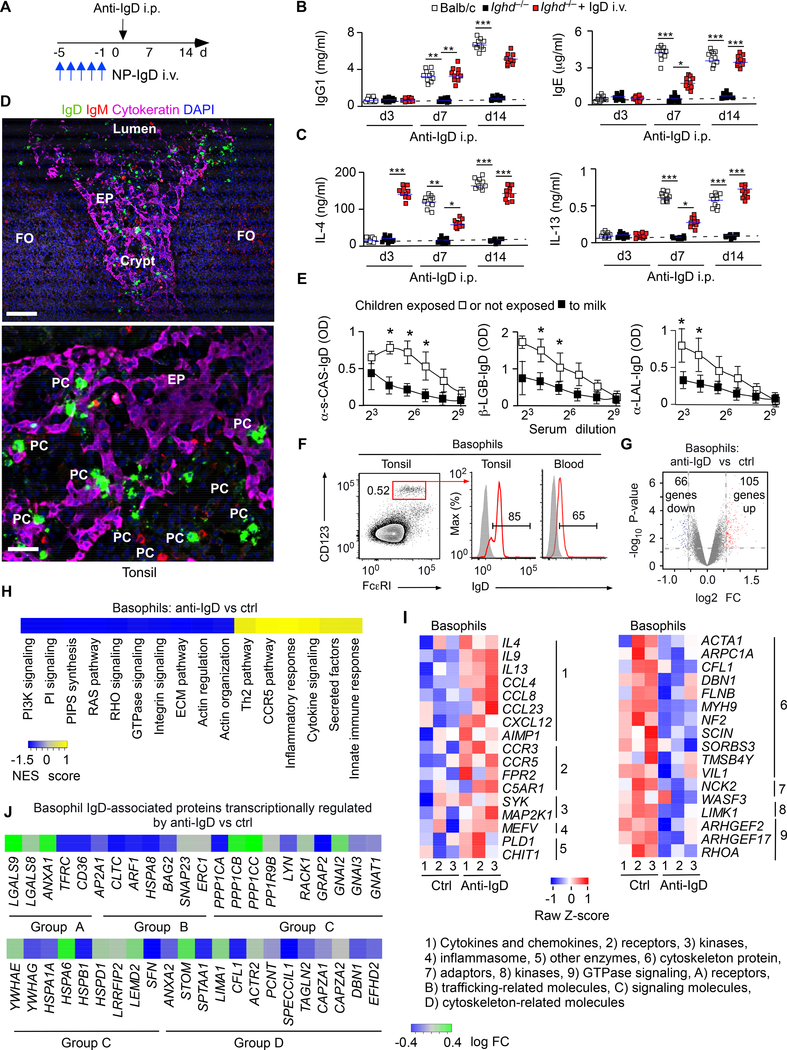
Having shown that specific ligation of secreted IgD by antigen augments humoral Th2 cell responses in Ighd−/− mice, we asked whether non-specific ligation of secreted IgD by anti-IgD could exert a comparable effect. Unlike WT Balb/c mice i.p. treated with a control antibody (Figure S3A and B), WT Balb/c mice i.p. inoculated with anti-IgD induced serum IgG1, IgE and IgD as well as serum IL-4, IL-13 and IL-21 (Figure 4A-C and Figure S3C and S3D), a B cell-activating cytokine from Tfh cells (Victora and Nussenzweig, 2012). This polyclonal humoral Th2 cell-mediated response required IgD ligation, because anti-IgD did not increase serum IgG1 and IgE in Ighd−/− mice (Figure 4B and 4C). In addition, secreted IgD was sufficient to promote humoral Th2 cell-mediated immunity, because reconstitution of Ighd−/− mice with soluble IgD rescued serum IgG1 and IgE as well as IL-4, IL-13 and IL-21 induction by anti-IgD (Figure 4B and 4C and Figure S3C and S3D). Moreover, this IgD-induced response was Th2 cell-specific, because anti-IgD did not augment humoral Th1 cell-mediated responses, including serum IgG2a and IgG2b (Figure S3E). Furthermore, the IgD-induced humoral Th2 cell-mediated response required T cell-dependent induction of the CSR-inducing enzyme AID by GC B cells, because anti-IgD did not stimulate serum IgG1 and IgE in T cell-deficient Tcrb−/− or AID-deficient Aicda−/− mice (Figure S3F and S3G). Finally, the induction of serum IL-4 by anti-IgD decreased upon diphtheria toxin (DT)mediated depletion of basophils in Mcpt8DTR mice (Figure S3H and S3I), which express the DT receptor (DTR) under the control of the basophil-specific Mcpt8 promoter. Thus, ligation of basophil-bound IgD activates a GC pathway that enhances humoral Th2 cell-mediated immunity.
Figure 4. Basophil-Bound IgD Interacts with Galectin-9 and Its Ligation Induces Th2 Cell-Associated Cytokine Expression But Inhibits Cytoskeleton Remodeling.
(A) Schematics of i.p. anti-IgD treated mice i.v. reconstituted or not with secreted IgD.
(B-C) ELISA of serum IgG1, IgE (B), IL-4 and IL-13 (C) from WT Balb/c (n=10) or Ighd−/− (n=10) mice in the presence or absence of i.v. reconstitution with secreted IgD (NP-IgD), followed by i.p. injection of anti-IgD. Dashed line, maximum antibody concentration in pre-immune (PI) mice. PI, day −1 relatively to onset of anti-IgD treatment (day 0).
(D) IFA of human tonsillar tissue stained for IgD (green), IgM (red), cytokeratin (purple) and nuclei (blue). EP, epithelium; FO, follicle; PC, plasma cell. Original magnification, ×20 (top) or ×63 (bottom). Scale bars, 50 μm (top) and 5 μm (bottom).
(E) ELISA of IgD specific to α-s-casein, β-lactoglobulin (β-LGB) or α-lactalbumin (α-LAL) from plasma of FPIES children with (n=5) or without (n=5) dietary milk restrictions.
(F) FCM of IgD (red open profile) bound to human tonsillar or circulating CD123+FcεRI+ basophils (red gate). Gray open profiles, control isotype-matched antibody with irrelevant binding activity.
(G) Microarray analysis of genes expressed by human basophils upon IgD cross-linking. The Volcano plot represents genes differentially expressed by basophils treated with anti-IgD or a F(ab’)2 control antibody (ctrl). Red and blue dots, up-regulated and down-regulated genes, respectively; FC, fold change. (H) Heat map of coordinated gene sets identified by gene set enrichment analysis in human basophils treated as in (G). NES (normalized enrichment score) indicates correlation between individual gene sets. Positive correlation, NES > 0 (yellow gradient); negative correlation, NES < 0 (blue gradient).
(I) Heat maps depicting changes in FC expression of coordinated “Basophil-Th2 pathway” (left) or “Actin filament-based processes” (right) gene sets from human basophils treated as in (G). The corresponding gene set enrichment analysis is further detailed in Fig. S4D and S4E. Bottom numbers, biological replicates. Right numbers, functions. Red, high expression; blue, low expression.
(J) Heat map of mRNAs for IgD-bound proteins identified by mass spectrometry after immunoprecipitation of human basophil lysates with ctrl or anti-IgD. Letters indicate functional groups.
Data summarize results from two-three experiments with 10 mice per experimental group (B, C), show one experiment of at least three with similar results (D, F), or depict results from one experiment with at least three biological replicates (G, H, I, J). Results are presented as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001 (two-tailed unpaired Student’s t test). See also Figures S3 and S4.
IgD Targets Common Environmental Antigens
Given that IgD amplifies basophil-regulated humoral Th2 cell-mediated responses, we determined the reactivity of IgD. FCM and IFA detected IgD class-switched IgD+IgM− B cells and IgD+IgM− plasma cells accumulating intracellular IgD in human tonsillar crypts (Figure 4D and Figure S4A and S4B), a mucosal site exposed to both airborne and oral antigens. Accordingly, ELISA showed that serum IgD antibodies recognized milk-derived α-s-casein, β-lactoglobulin and α-lactalbumin, and indeed were more abundant in children with food protein-induced enterocolitis syndrome (FPIES) exposed to milk compared to FPIES children under dietary milk restriction (Figure 4E). Finally, FCM detected IgD on both tonsillar and circulating basophils (Figure 4F). Thus, IgD may arm basophils with an adaptive immune recognition system for common small soluble antigens, including food proteins.
IgD Induces Basophil Th2 Cell-Like Responses But Inhibits Cytoskeleton Remodeling
Given that anti-IgD up-regulates IL-4 expression by IgD-coated mouse basophils, we analyzed the gene expression profile of IgD-coated human basophils exposed to anti-IgD by gene microarray. Anti-IgD down- and up-regulated 66 and 106 basophil transcripts, respectively (Figure 4G and Figure S4C and Table S1). Gene set enrichment analysis followed by gene ontology analysis found that anti-IgD induced coordinated gene sets implicated in Th2 cell-mediated immunity, chemotaxis, inflammation and signaling, including genes encoding IL-4 and IL-13 (Figure 4H and 4I and Table S1). In addition, antiIgD inhibited gene sets implicated in cytoskeleton remodeling via phosphoinositide 3-kinase (PI3-K), RAS and small GTPases (Figure 4H and 4I and Table S1). Thus, IgD ligation stimulates transcriptional programs that mediate basophil induction of Th2 cell-associated cytokines as well as basophil inhibition of cytoskeleton remodeling, which might have a negative influence on granule mobilization.
IgD Binds to Basophils Via Galectin-9 and CD44
Next, we explored how IgD binds to human basophils by studying IgD-associated proteins obtained by immunoprecipitation (IP). These proteins were identified by mass spectrometry and thereafter correlated with changes of their corresponding transcript upon basophil stimulation with anti-IgD (Figure 4J). Group A proteins mediated receptor stabilization, cytokine induction, inhibition of degranulation (galectin-9), autophagy (galectin-8), suppression of inflammation (annexin-1), iron metabolism, antibody binding, cell activation (transferrin receptor C or CD71), and endocytosis (CD36). Group B proteins mediated clathrin-mediated receptor endocytosis and intracellular trafficking, whereas group C proteins encompassed both positive and negative regulators of receptor-mediated signaling (e.g., LYN). Finally, group D proteins mediated receptor anchoring to the cytoskeleton and cytoskeleton remodeling.
Of note, IgD ligation by anti-IgD transcriptionally up-regulated degranulation inhibitors such as galectin-9 (Niki et al., 2009), but down-regulated degranulation inducers, such as LYN (Gould and Sutton, 2008) (Figure 4J). Given its ability to bind IgE (Niki et al., 2009), galectin-9 was further explored in relationship to its capacity to bind IgD. IP followed by immunoblotting (IB) showed that human IgD interacted with galectin-9 through a mechanism that was blocked by galactose and lactose but not glucose (Figure 5A). In addition, FCM demonstrated that the human IgD-galectin-9 complex bound to human basophil-like KU812 cells more effectively than IgD alone did and showed that this binding decreased upon incubation of KU812 cells with lactose but not glucose (Figure 5B and 5C).
Figure 5. IgD Binds to Basophils through Galectin-9 and CD44.
(A) IB of galectin-9 following IP of human IgD and galectin-9 protein mix with control (ctrl) or anti-IgD antibodies. Prior to IP, the protein mix was supplemented with control PBS, glucose or lactose.
(B) FCM of human IgD on human KU812 cells cultured for 30 min with control medium alone (ctrl), IgD or an IgD-galectin-9 complex formed by pre-incubating IgD with galectin-9 for 10 min.
(C) FCM of human IgD or galectin-9 on KU812 cells cultured with IgD-galectin-9 in the presence of medium alone (ctrl), glucose or lactose for 30 min.
(D) FCM of human IgD or CD44 on KU812 cells treated with scrambled (ctrl) or CD44-targeting small interfering RNA (siRNA) and later incubated with or without (ctrl) IgD-galectin-9. MFI, mean fluorescence intensity.
(E) Confocal imaging of human basophils stained for IgD (blue), galectin-9 (green) and CD44 (red). Scale bar, 0.5 μm.
(F) FCM of IgD on human basophils incubated with or without (ctrl) IgD-galectin-9 for 30 min.
(G) ELISA of IL-4 from human basophils incubated with medium alone (ctrl) and with or without the IgD-galectin-9 complex in the presence or absence of IgD cross-linking by anti-IgD for 18 h.
(H, I) FCM of IgD+ basophils from the spleen or lung of WT C57BL/6, Lgals9−/− (H) or Cd44−/− (I) mice.
(J, K) ELISA of serum IgG1 and IgE to NP from WT Balb/c (n=10), Lgals9−/− (n=8) (J) or Cd44−/− (n=10)
(K) mice following s.c. immunization with NP-OVA and papain. Dashed line, maximum antibody concentration in pre-immune (PI) mice. PI, day −1 relatively to the onset of anti-IgD treatment (day 0).
(L) ELISA of serum total IgG1 and IgE from WT C57BL/6 controls (n=10) and Lgals9−/− mice (n=8) following i.p. injection of anti-IgD.
Data show one experiment of at least three with similar results (A, B, E, F) or summarize three experiments (C, D), one experiment with three biological replicates per experimental group (G) or twothree experiments with 8–10 mice per experimental group (H-L). Results are presented as mean ± SEM; *p < 0.05, **p < 0.01, ***p < 0.001 (two-tailed unpaired Student’s t test). See also Figures S5.
As shown by recently published studies (Panda et al., 2018; Wu et al., 2014), additional experiments indicated that galectin-9 linked IgD to basophils via CD44. Indeed, the pre-formed IgD-galectin-9 complex bound less to KU812 cells after knocking down CD44 expression through RNA interference (Figure 5D). Accordingly, imaging FCM and confocal microscopy showed co-localization of IgD with galectin-9 and CD44 in human basophils (Figure 5E and Figure S4D and S4E). Finally, FCM and ELISA showed that, compared to untreated controls, human basophils stripped of pre-bound endogenous IgD and later incubated with a pre-formed exogenous IgD-galectin-9 complex displayed more surface IgD and secreted more IL-4 upon IgD ligation (Figure 5F and 5G). In the absence of IgD ligation, IgDgalectin-9 induced little or no IL-4 (Figure 5G).
Consistent with these data, IgD-coated basophils were reduced in the spleen and/or lung of galectin-9deficient Lgals9−/− or CD44-deficient Cd44−/− mice (Figure 5H and 5I). Similar to Ighd−/− or basophildepleted mice, Cd44−/− mice showed weaker NP-specific IgE and IgG1 responses following s.c. immunization with NP-OVA and papain, whereas in Lgals9−/− mice such responses decreased in a nonsignificant manner (Figure 5J and 5K). This marginal decrease could indicate that IgD binds to different tandem-repeat galectins depending on the immunization route (Rabinovich and Toscano, 2009). In agreement with this possibility, ELISAs showed that mouse NP-IgD bound to tandem-repeat galectin-4 in addition to galectin-9 but neither to prototypic galectin-1 and galectin-7 nor chimeric galectin-3 nor sialic acid-binding immunoglobulin-type lectin-1 (SIGLEC-1), SIGLEC-2, SIGLEC-E or SIGLEC-F (Figure S5A).
Given our earlier data showing that mouse basophils up-regulated galectin-9 and that anti-IgD elicited basophil-regulated humoral Th2 cell-mediated responses, we measured IgG1 and IgE induction by antiIgD in Lgals9−/− mice. These mice showed no induction of serum IgG1 and IgE following i.p. injection of anti-IgD (Figure 5L). In contrast, IgG1 and IgE were comparable in anti-IgD-treated WT DBA controls and Fcer1a−/− mice (Figure S5B), which lack the IgE-binding and IL-4-inducing FcεRI receptor (Gould and Sutton, 2008). Notwithstanding the existence of evidence indicating IgE interaction with galectin-9 and FcεRI modulation by CD44 (Kim et al., 2008; Niki et al., 2009), these findings make unlikely an involvement of FcεRI in IgG1 and IgE induction by anti-IgD. In agreement with earlier findings obtained with human basophils, FCM and ELISA showed that mouse basophils bound NP-IgD-galectin-9 but not NP-IgD and that NP-IgD-galectin-9 ligation by NP-OVA enhanced IL-3-induced basophil release of IL-4, IL-5 and IL-13 (Figure S5C and S5D). Thus, IgD binds galectin-9, which could interact with glycans from IgD and CD44 through two distinct carbohydrate-recognition domains (CRDs). In this way, galectin-9 may bridge IgD to a CD44-stabilized, clathrin-coated and cytoskeleton-linked IgD receptor (IgDR) complex that may include CD71 (Figure S5E), a signal-transducing receptor (Chen et al., 2015). Besides being endocytosed into antigen-degrading compartments, this IgDR complex may induce IL-4, IL-5 and IL-13 via multiple signal transducers (Figure S5E), including LYN (Gould and Sutton, 2008).
IgD Inhibits IgE-Mediated Basophil Degranulation
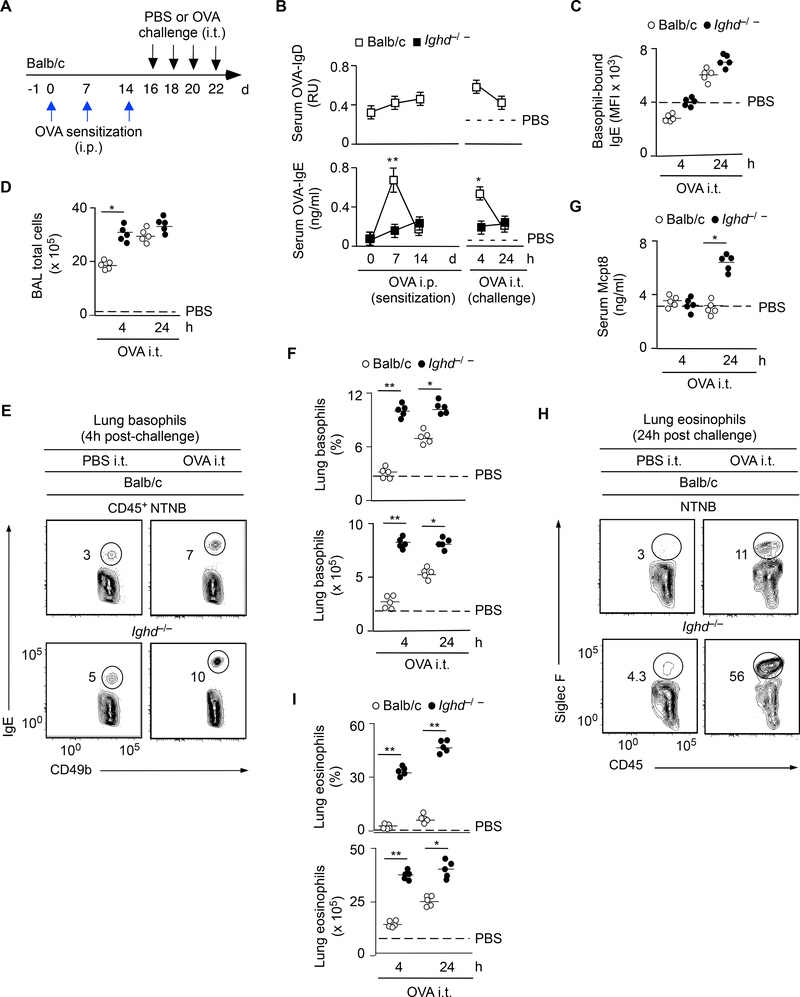
Besides enhancing humoral Th2 cell-mediated responses, cross-linking of human IgD inhibited basophil gene programs linked to IgE-induced degranulation, which occurs upon secondary exposure to IgEtargeted antigens (Gould and Sutton, 2008). The relationship of IgD with IgE was further explored in a mouse model of acute lung inflammation (Figure 6A). Compared to WT controls, Ighd−/− mice induced less OVA-specific serum IgE seven days following OVA i.p. sensitization and four hours following four intratracheal (i.t.) challenges with OVA (Figure 6B). Despite showing normal IgE-coated lung basophils (Figures 6C), challenged Ighd−/− mice had more lung inflammation, including more total cells in bronchoalveolar lavage fluids, more lung basophils, more lung eosinophils and more serum Mcpt8 (Figure 6D-I), a product of basophil degranulation.
Figure 6. IgD Attenuates Acute Lung Inflammation Following Secondary Antigen Exposure.
(A) Schematics of i.p. OVA sensitization followed by a challenge consisting of four consecutive i.t. inoculations of OVA or control PBS.
(B) ELISA of OVA-specific IgD and IgE from serum of WT Balb/c (n=10) or Ighd−/− (n=10) mice treated as in (A). Dashed line, maximum antibody concentration 4 hs after the last i.t. inoculation of PBS. (C) FCM quantitation of IgE on l basophils from lungs of WT Balb/c (n=5) or Ighd−/− (n=5) mice treated as in (A).
(D) Microscopic quantitation of total cells from the bronchoalveolar lavage (BAL) of WT Balb/c (n=5) or Ighd−/− (n=5) mice treated as in (A).
(E, F) FCM quantitation of CD49b+IgE+ basophils from lungs of WT Balb/c (n=10) or Ighd−/− (n=10) mice treated as in (A). NTNB, non-T non-B.
(G) ELISA of Mcpt8 from serum of WT Balb/c (n=5) or Ighd−/− (n=5) mice treated as in (A).
(H, I) FCM quantitation of CD45+Siglec-F+ eosinophils from lungs of WT Balb/c (n=5) or Ighd−/− (n=5) mice treated as in (A).
Data summarize two experiments with at least five mice per experimental group. Results are presented as mean ± SEM; *p < 0.05, **p < 0.01 (two-tailed unpaired Student’s t test).
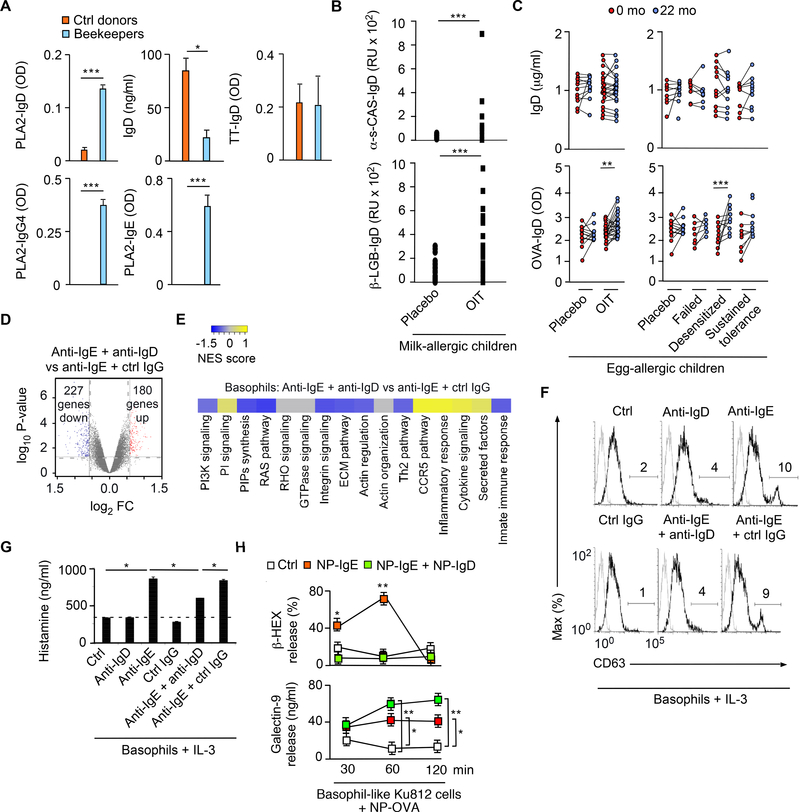
The inhibition of IgE-induced basophil degranulation by IgD was further explored in humans. Compared to healthy individuals, beekeepers tolerant to the bee venom antigen phospholipase A2 (PLA2) showed increased serum IgD to PLA2, but conserved IgD to tetanus toxin and reduced total IgD (Figure 7A). Protective PLA2-specific responses further included IgE and IgG4 (Figure 7A), which is equivalent to mouse IgG1 (Gould and Sutton, 2008). Similarly, milk-allergic children treated for 12 months with oral immunotherapy (OIT) mounted increased IgD responses to α-s-casein and β-lactoglobulin compared to placebo-treated controls (Figure 7B). Moreover, egg-allergic children treated for 22 months with OIT mounted increased IgD responses to OVA compared to placebo controls (Figure 7C). In this OIT group, OVA-reactive IgD was increased in desensitized children but not in children unresponsive to OIT or with sustained tolerance to an OVA challenge (Figure 7C).
Figure 7. IgD Ligation by Antigen Attenuates IgE-induced Basophil Degranulation.
(A) ELISA of IgD, IgG4 and IgE to PLA2 or tetanus toxin (TT) from plasma of healthy non-allergic controls (n=4) or beekeepers (n=4).
(B) ELISA of IgD to α-casein or β-lactoglobulin from plasma of allergic children at baseline (0 months) and following 22 months of treatment with placebo (n=5) or OIT (n=5).
(C) ELISA of total IgD or IgD to OVA from plasma of allergic children at baseline (0 months) and following 22 months of treatment with placebo (n=15) or OIT (n=40). Left and right panels: total (top) or OVA-specific (bottom) IgD concentrations by treatment group and treatment outcome, respectively.
(D) Microarray analysis of genes from human basophils exposed for 3 h to anti-IgE and control (ctrl) IgG or anti-IgD. Red and blue dots in volcano plot show up-regulated and down-regulated genes, respectively; FC, fold change.
(E) Heat map showing enhancement or depletion of coordinated gene sets identified by gene set enrichment analysis of human basophils treated as in (D). NES (normalized enrichment score) > 0 (yellow gradient) or < 0 (blue gradient) indicates positive or negative correlation between individual gene sets. (F, G) FCM of surface CD63 (F) and ELISA of histamine (G) from IL-3-stimulated human basophils incubated with control (ctrl) medium alone, ctrl irrelevant IgG, anti-IgD and/or anti-IgE for 6 h.
(H) ELISA of β-hexosaminidase (β-HEX) and galectin-9 from human KU812 cells exposed to NP-OVA following incubation with irrelevant IgG (ctrl) or NP-IgE combined or not with NP-IgD complexed to galectin-9.
Data show one experiment with 4–15 donors per treatment group (A-C), depict one experiment with three biological replicates per condition (D-G), or summarize two experiments with three biological replicates per experimental group (H). Results are presented as mean ± SEM; *p < 0.05, **p < 0.01, *** p < 0.001 (Wilcoxon matched pairs signed-rank test or two-tailed Student’s t test). See also Figures S6 and S7.
Next, we stimulated human basophils with F(ab’)2 anti-IgE with or without F(ab’)2 anti-IgD for two hours to assess the role of IgD in IgE-induced degranulation. IgD ligation down-regulated 227 and upregulated 180 transcripts in IgE-co-stimulated basophils, including mRNA encoding the signaling inhibitor dual specificity protein phosphatase 2 (Figure 7D and Figure S6A). Gene set enrichment analysis followed by gene ontology analysis further determined that IgD ligation inhibited IgE-induced coordinated gene sets implicated in cytoskeleton remodeling, including mRNAs related to phospoinoside3 kinase (PI3K), RAS and RHO signaling (Figure 7E). Accordingly, IgD ligation impaired IgE-induced surface relocation of the granule-associated protein CD63 as well as extracellular release of the granuleassociated pro-inflammatory mediator histamine in IL-3-co-stimulated human basophils (Figure 7F-G).
Similar results were obtained in human basophil-like KU812 cells pre-coated with NP-reactive IgE and IgD. Co-ligation of IgD by NP-OVA reduced IgE-induced basophil release of the granule-associated enzyme β-hexosaminidase, but increased basophil release of galectin-9 (Figure 7H). Like basophils (Chen et al., 2009), human mast cells were proximal to tonsillar IgD-secreting plasma cells, exhibited surface IgD and galectin-9, and attenuated anti-IgE-induced degranulation upon exposure to anti-IgD (Figure S6B-E). Thus, basophil-bound IgD not only enhances Th2 cell-mediated IgG and IgE responses, but also mitigates IgE-induced basophil and mast cell degranulation. Besides competing with IgE for antigen, basophil-bound IgD may inhibit IgE-mediated cytoskeleton remodeling and degranulation by inducing FcεRI signaling inhibitors (Figure S6F and Figure S7).
Discussion
We have shown here that certain soluble antigens induced plasma cells secreting IgD. This antibody bound to basophils through the CD44-interacting protein galectin-9. Ligation of secreted IgD by antigen increased basophil release of IL-4, IL-5 and IL-13, which promoted Tfh cell-dependent B cell production of IgE as well as IgG1 with high affinity for the antigen initially targeted by IgD. Besides amplifying humoral Th2 cell-mediated responses, basophil-bound IgD attenuated IgE-induced basophil degranulation. In this way, IgD may dampen the immunogenicity of innocuous environmental antigens.
Antibodies regulate immunity in addition to neutralizing and clearing antigen. Indeed, mouse studies have shown that IgM and IgG3 increase antibody responses through complement receptors, whereas IgG1, IgG2a, IgG2b, IgA and IgE enhance or suppress antibody responses via FcRs (Lu et al., 2018). The role of IgD remains unknown, but pioneering works suggest that IgD amplifies humoral immunity through glycans from the Cδ1 and Cδ3 domains (Amin et al., 1993; Gutzeit et al., 2018; Xue et al., 1984). Accordingly, we found that IgD augmented IgG1 and IgE responses through a mechanism involving IgD binding to basophils via galectin-9, a lectin encompassing two distinct CRDs (Rabinovich and Toscano, 2009). This protein bridged IgD with CD44, an immunomodulatory receptor expressed by activated immune cells, including basophils (Kim et al., 2008; Panda et al., 2018; Wu et al., 2014).
Besides binding to galectin-9, IgD interacted with galectin-4 and possibly galectin-8, which are tandem repeat galectin subfamily members similar to galectin-9 (Rabinovich and Toscano, 2009). However, IgD did not interact with galectin-1, galectin-3 and galectin-7, which belong to the prototypic or chimeric galectin subfamilies (Rabinovich and Toscano, 2009). This binding pattern may confer specific tissue or cell tropism to IgD while preserving its functional flexibility. Consistently, the number of lung but not spleen IgD-coated basophils decreased in galectin-9-deficient mice. In contrast, both spleen and lung IgDcoated basophils were undetectable in CD44-deficient mice, suggesting that CD44 represents a common acceptor molecule for multiple tandem repeat galectins. As suggested by published studies (Kim et al., 2008; Niki et al., 2009; Wu et al., 2014), these lectins may link IgD to CD44 to stabilize a basophil IgDR complex that regulates cytokine secretion and degranulation.
Although broadly expressed by activated immune cells, CD44 may generate context-dependent signals by clustering with specific receptors, such as FcεRI from basophils and mast cells (Kim et al., 2008; Niki et al., 2009). However, IgD did not require FcεRI to enhance humoral Th2 cell-mediated responses, which indeed were conserved in anti-IgD-treated Fcer1a−/− mice. Yet, IgD might leverage the CD44-binding properties of galectin-9 to modulate other FcεRI-induced functions, including degranulation (Kim et al., 2008; Niki et al., 2009). In addition to transmitting autonomous immunomodulating signals (Panda et al., 2018), CD44 interacts with the immunoregulatory TGF-β receptor to stabilize its functional output (Wu et al., 2014). This implies that IgD may engage signal-transducing proteins additional to CD44.
In agreement with this possibility, IgD co-immunoprecipitated with the CD71 transferrin receptor, which delivers activating signals in addition to regulating iron metabolism (Chen et al., 2015). Besides transferrin, CD71 binds IgA and does so through glycans similar to those associated with IgD (Swenson et al., 1998). Of note, CD71 undergoes ligation-induced endocytosis via clathrin-coated vesicles (Kaksonen and Roux, 2018). Accordingly, IgD also associated with clathrin, the clathrin adaptor protein AP-2, and the ADP-rybosylation factor ARF1, a regulator of clathrin-mediated endosomal trafficking (Kaksonen and Roux, 2018). Thus, besides delivering basophil-regulating signals via CD44 and possibly CD71, the IgDR complex may internalize IgD-bound antigens by undergoing clathrin-mediated endocytosis. This scenario is supported by our findings showing IgD co-localization with galectin-9 and CD44 within basophil membrane, sub-membrane as well as intracellular compartments and could account for the rapid degradation of IgD-bound antigens within endosomes from basophils as well as mast cells.
In mice, basophil release of IL-4, IL-5 and IL-13 followed engagement of IgD by antigen. In humans, proximal signaling elements of this pathway may include LYN, which mediates IL-4-inducing signals from the high-affinity IgER, commonly known as FcεRI (Gould and Sutton, 2008). In addition to IL-4, cross-linking of human basophil-bound IgD up-regulated Th2 cell response-related genes such as IL-9 and IL-13. This finding correlated with the ability of mouse basophils to enhance Th2 cell-type IgG1 and IgE responses upon IgD ligation by antigen. It also echoed early studies showing that IgD has immuneamplifying properties that encompass antibody induction (Xue et al., 1984).
In mice, secreted IgD was sufficient to enhance some but not all IgG1 and IgE responses through an AID-mediated GC pathway involving IL-4-expressing Tfh cells. Indeed, IgG1 production decreased in Ighd−/− mice immunized with the basophil-activating adjuvant papain, but not with the dendritic cellactivating adjuvant alum (Karasuyama et al., 2009; Wynn, 2015). The phenotype of Ighd−/− mice correlated with decreased basophil expression of IL-4, impaired IgG1 and IgE class switching, and reduced IgG1 affinity maturation. However, Ighd−/− mice restored this antigen-specific response as well as that non-specifically induced by anti-IgD following reconstitution with secreted IgD. Thus, IgD can deploy basophil-regulated antibody-enhancing signals independently of its BCR function.
Secreted IgD mimicked the Th2 cell-inducing properties of papain by inducing basophil recruitment and up-regulation of IL-4, IL-5 and IL-13 upon engagement by antigen and this response correlated with the early emergence of extrafollicular IgD-secreting plasma cells. Unlike their murine counterparts (Rouaud et al., 2014), human IgD+IgM− plasma cells are highly mutated (Gutzeit et al., 2018), which could reflect chronic exposure of mucosal IgD-inductive sites to food antigens (Chen et al., 2009; Choi et al., 2017). In this regard, we found that children assuming cow’s milk with their diet had more serum IgD to milk proteins compared to children restricting milk from their diet. Thus, food antigens may constitute a major target of humoral IgD responses in humans. The Th2 cell-inducing nature of mouse IgD responses to mucosal antigens was suggested by the homeostatically elevated serum concentration of IgD in Th2 cell-biased Balb/c mice compared to Th1 cell-biased C57BL/6 mice and by the induction of IgD in mice inoculated with exogenous TSLP, a Th2 cell-inducing mucosal cytokine that triggers basophil activation in an IL-3-independent manner (Siracusa et al., 2011).
Besides eliciting basophil release of Th2 cell-associated cytokines, IgD constrained IgE-mediated basophil and mast cell degranulation. Accordingly, IgD deficiency enhanced OVA-induced acute lung inflammation as well as basophil degranulation in OVA-sensitized Ighd−/− mice. Conversely, OVAspecific IgD increased in allergic children desensitized to OVA by OIT. Similarly, beekeepers tolerant to the bee venom toxin PLA2 mounted enhanced PLA2-specific IgD responses. As also shown by others (Palm et al., 2013), beekeepers additionally produced PLA2-specific IgE and IgG4, which is the human equivalent of mouse IgG1 and a hallmark of protection against allergy (Gould and Sutton, 2008). Thus, IgD may co-emerge with IgE from a protective Th2 cell pathway targeting some soluble antigens.
How IgD mitigates degranulation remains unclear, but IgD ligation down-regulated the expression of gene products involved in IgE-mediated cytoskeleton remodeling and granule exocytosis (Gould and Sutton, 2008), including the signal transducers phosphoinositide-3 kinase, RAS and RHO. Concomitantly, IgD up-regulated the expression of gene products encoding signal inhibitors such as DUSP2. Finally, the association of IgD with the synaptosome-associated protein of 23 kDa SNAP23 suggests that IgD could also perturb the docking of granules to the plasma membrane.
In summary, our data show that some common soluble antigens, such as certain food proteins, induce B cell release of IgD, which binds to basophils through the CD44-interacting protein galectin-9. By eliciting basophil release of IL-4, IL-5 and IL-13, IgD promotes protective humoral Th2 cell-mediated responses while constraining IgE-mediated basophil degranulation. Thus, IgD-harnessing strategies, including administration of galectin-9 (de Kivit et al., 2012), could attenuate acute IgE-dependent allergic reactions to food antigens and might represent a revolutionizing approach to allergy prophylaxis.
STAR Methods
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Andrea Cerutti (acerutti@imim.es).
Experimental Model and Subject Details
Human Blood and Tissue Specimens
Plasma samples were collected from children (5–11 years of age) with FPIES undergoing a supervised food challenge. In this cohort, 5 children were restricting milk from their diet due to history of milkrelated FPIES but passed the food challenge, whereas 5 children were not due to milk-unrelated FPIES. Additional plasma samples were collected from 55 children (5–11 years of age) with egg allergy enrolled in a double-blind placebo-controlled OIT trial. In this trial, 40 children received daily OIT with egg white powder, whereas 15 children received placebo control. In the OIT group, a dose escalation phase was followed by a maintenance phase of 2 g egg white powder daily. All participants underwent an oral food challenge at 10 months with a 5 g cumulative dose of egg white powder. Children in the OIT group continued treatment and underwent a second oral food challenge at 22 months with a cumulative dose of 10 g egg white powder. Those who passed the 22 month challenge without symptoms discontinued OIT for 4–6 weeks and underwent a third oral food challenge at 24 months to determine if desensitization was sustained. Subjects were categorized as having failed treatment if they were unable to tolerate OIT or if they reacted during the challenge at 22 months (n = 10). Subjects passing the challenge at 22 months but reacting to the challenge at 24 months were categorized as desensitized (n = 19). Subjects passing the challenge at both time-points were categorized as having sustained unresponsiveness (n = 11). More plasma samples were collected from 4 beekeepers and 4 age-matched healthy donors. Finally, archived tonsillar tissue sections and fresh tonsillar mononuclear cells were obtained from 5 non-allergic patients who underwent tonsillectomy due to reactive hypertrophy. The Institutional Review Board of Mount Sinai School of Medicine, Swiss Institute for Allergy and Asthma Research and Hospital del Mar approved the use of plasma and tissue samples and patients provided informed consent.
Mice
12–18 weeks old animals matched for age, gender and genetic background were used according to protocols approved by The Animal Care and Use Committee of Mount Sinai School of Medicine. Ighd−/−, Il4GFP (Jackson Laboratories) and Aicda−/− (Jayanta Chaudhuri, Memorial Sloan Kettering Cancer Center) mice were on Balb/c background, whereas Lgals9−/− (Michael Croft, La Jolla Institute for Allergy and Immunology), Cd44−/− (Jackson Laboratories), Mcpt8DTR (Stephen J. Galli, Stanford University), Mcpt8YFP, and Tcrb−/− mice were on C57BL/6 background. Ighd−/− × Il4GFP (4get) mice were generated by backcrossing Ighd−/− mice onto 4get genetic background for > 10 generations. Fcer1a−/− mice (Jackson Laboratories) were on a DBA background. WT BALB/c, C57BL/6 and DBA mice (Jackson Laboratories) were used as controls. Mice were maintained under SPF conditions in a barrier facility at Mount Sinai School of Medicine.
Method Details
Cells
Human basophils were purified from circulating or tonsillar mononuclear cells by Ficoll density centrifugation followed by negative selection using EasySep Human Basophil Enrichment kit (Stem Cell Technologies). Basophil purity was >98% and viability ranged from 90% to 95%. Human B cells were analyzed as in published studies (Chen et al., 2009). Mouse mononuclear cells were isolated from spleen, lung, lymph nodes and bone marrow as reported previously (Shan et al., 2013). CD45+CD49b+IgE+(or FcεRI+)CD117− basophils were FACSorted from non-T-non-B (NTNB) CD3−CD4−CD8α−CD19−NK1.1−TER119− cells.
Standard Flow Cytometry
Cells were stained with various combinations of monoclonal antibodies (mAbs) or polyclonal antibodies (pAbs) (Tables S2 and S3). Cells were incubated at 4°C with Fc-blocking reagent (Miltenyi Biotec) before the addition of the appropriate ‘cocktails’ of fluorochrome-labeled mAbs or pAbs. A BD Cytofix/Cytoperm Kit was used to perform intracellular stainings (BD Biosciences). 4’−6-diamindine-2’-phenylindole (DAPI; Boehringer Mannheim), 7-aminoactinomycin D (7-AAD; BD Pharmingen), or LiveDead Kit (Invitrogen) were used to exclude dead cells and all gates were drawn to give ≤ 1% positive cells in the sample stained with control antibodies. Cells were acquired with a BD LSR Fortessa Cell Analyzer (BD Biosciences) and data were analyzed with FlowJo V10 software (TreeStar).
Imaging Flow Cytometry
Untouched human basophils were stripped of pre-bound endogenous IgD as described in published studies (Chen et al., 2009) and incubated in PBS with or without 50 μg/ml monoclonal IgD from human myeloma (Athens Research and Technology), 2.5 μg/ml galectin-9 (R&D Systems), or 50 μg/ml IgD premixed with 2.5 μg/ml galectin-9 on ice for 15 minutes. Following washing, basophils were sequentially stained with F(ab)2 to IgD biotin (Southern Biotech) and streptavidin (Life Technologies) along with antiFcɛRI FITC (clone: AER-37; eBioscience) and anti-CD44 PE (clone: IM7; Biolegend). Following additional washing, stained cells were fixed with 1 % paraformaldehyde, stained with 100 ng/ml DAPI (Sigma-Aldrich), and imaged. A similar strategy was used to image basophils included in splenocytes from Balb/c mice. These cells were incubated with an Fc-blocking 2.4G2 mAb (Tonbo Bioscience) and subsequently stained with anti-FcɛRI FITC (clone: MAR-1), anti-IgD PE (clone: 11–26c.2a) (BD Biosciences), anti-CD49b APC (clone: DX5) (Biolegend), and Ghost Violet 510 viability dye (Tonbo Bioscience). Following washing, stained cells were fixed with 1 % paraformaldehyde and stained with 5 μg/ml 7-AAD (Tonbo Bioscience).
FACSorting
For human basophil sorting, cell suspensions were incubated at 4ºC with Fc-blocking reagent (Miltenyi Biotec) or blocking 2.4G2 mAb to FcγRIII/II (BD PharMingen) and then stained for 30 min with the following antibody combination: anti-CD45 AF700 (clone: HI30), anti-FcεRI FITC (clone: CRA1), antiCD123 PE (clone: 6H6), anti-HLA-DR PerCpCy5.5 (clone: L243), anti-CD19 eFluor450 (clone: HIB19), anti-IgD PE-Cy7 (clone: IA6–2). Basophils were sorted with a FACSAria II (BD Biosciences) after exclusion of dead cells through DAPI staining. The purity of cells sorted this way was consistently >95%.
Cell and Tissue Imaging
Human and mouse cells were imaged using an Amnis ImageStream X Mark II imaging flow cytometer (EMD Millipore) and data were analyzed using IDEAS 6.1 (EMD Millipore) after excluding non-single cells and cells out of focus. Human and mouse tissue sections were processed as reported previously (Shan et al., 2013). Briefly, formalin-fixed paraffin-embedded (FFPE) human or mouse tissue sections were treated with xylene, a decreasing alcohol gradient, and distilled water to rehydrate the tissue. Heatinduced epitope retrieval was performed for 15 min. in citrate buffer (pH 6) or Tris-EDTA (pH 9). Antigen-retrieved FFPE or OCT-embedded tissue sections were processed as previously reported (Shan et al., 2013) and stained with various combinations of mAbs and/or pAbs (Tables S2 and S3). Biotinylated antibodies were detected with streptavidin-Alexa Fluor conjugates. Nuclear DNA was visualized with DAPI and coverslips were applied with FluorSave reagent (Merck Millipore) or ProLong Gold Antifade reagent (Invitrogen). Fluorescent images were acquired with a Leica TCS SP5 Upright laser scanning confocal microscope (Leica Microsystems) and further analyzed with ImageJ software. Alternatively, images were acquired with an epi-fluorescence Eclipse Ni-U microscope (Nikon Instruments) with NIS Elements BR software (Nikon Instruments).
Mass Spectrometry
To identify the IgD receptor, buffy coats from five healthy donors provided by the New York Blood Bank were pooled and processed with a Basophil Isolation Kit (Miltenyi Biotech) to MACSort 20 × 106 circulating basophils (purity > 95%). Cells were divided into two 10 × 106 fractions. In the first fraction, basophils were incubated with a biotinylated goat 2032–08 F(ab’)2 pAb to IgD (Southern Biotech) to label pre-bound IgD. In the second fraction, basophils were treated with biotinylated 0110–08 goat F(ab’)2 control IgG (Southern Biotech). These fractions were lysed in a buffer containing 1% NP-40 and supernatants immunoprecipitated with streptavidin-conjugated sepharose beads (GE Healthcare). Immunoprecipitated proteins were resolved by one-dimensional SDS-PAGE and stained by SYPRO Ruby. Differentially immunoprecipitated protein bands were identified using a 4000 Q Trap (SciEx) mass spectrometer.
Binding Assays
Primary circulating human basophils were first stripped of pre-bound endogenous IgD as previously described (Chen et al., 2009) and then incubated for 15 min with 50 μg/ml monoclonal IgD (Athens Research and Technology) pre-mixed or not with 10 μg/ml galectin-9 (R&D Systems) for 15 min. Binding of IgD was detected by staining cells with FITC-conjugated goat 2032–02 F(ab’)2 pAb to IgD (Southern Biotech) or control FITC-conjugated goat F(ab’)2 IgG. A similar method was followed to measure binding of exogenous IgD or IgD-galectin-9 to human basophil-like KU812 cells, except that no stripping of endogenous pre-bound IgD was necessary.
Cultures and Reagents
Primary human or mouse basophils and human basophil-like KU812 cells were cultured in complete RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Life Technologies). Human basophils were stimulated with 30 ng/ml IL-3 (Prepotech). Endogenous IgD and IgE pre-bound to human basophils were cross-linked with 25 μg/ml anti-IgD (clone: IA6–2; BD Biosciences) and 2 μg/ml anti-IgE (clone: G7–18, BD Biosciences). As a control, basophils or KU812 cells were exposed to 25 μg/ml IgG2a pAb with irrelevant binding specificity (Santa Cruz Biotechnology). KU812 cells were also incubated with 1 μg/ml NP-OVA (Biosearch Technologies) before or after pre-incubation with 50 μg/ml control human IgE with irrelevant specificity (AbD Serotec), 50 μg/ml chimeric human NP-reactive IgE (AbD Serotec), and/or 50 μg/ml mouse NP-reactive IgD from B1-δ8 hybridoma pre-complexed with 25 μg/ml human galectin-9 (R&D Systems). This binding strategy obviated the lack of human NP-reactive IgD and was based on preliminary data showing conserved binding of human or mouse galectin-9 to mouse or human IgD. Mouse basophils were stimulated with 10 ng/ml IL-3 (Prepotech), 1 μg/ml NP-conjugated OVA (Biosearch Technologies), 50 μg/ml mouse NP-reactive IgD pre-incubated or not with 25 μg/ml mouse galectin-9 (R&D Systems).
Cytometric Bead Arrays and ELISAs for Cytokine Measurements
A BD Cytometric Bead Array Th1Th2Th17 Cytokine Kit as well as Human IL-4IL-13 Quantikine ELISA (R&D Systems) kits were used to measure human IL-4. A Human Galectin-9 Quantikine ELISA (R&D Systems) was used to measure human galectin-9. A Histamine (HIS) ELISA kit and an Hexosaminidase A ELISA Kit (HEXA) (antibodies-online.com) were used to measure human histamine and αhexosaminidase, respectively. Mouse Th1Th2Th17Th22 13 PLEX Kit (eBioscience) as well as Mouse IL4IL-13IL-25IL-33 DuoSet ELISA (R&D Systems) kits were used to measure IL-4, IL-5, IL-13 and IL-21 from the serum of WT or IgD mice immunized with NP-OVA and papain or treated with anti-IgD before or after reconstitution with exogenous NP-IgD. A Mast Cell Protease 8 (MCPT8) ELISA Kit (antibodies-online.com) was used to measure mouse serum Mcpt8.
Global Transcriptome Analysis
Total cellular RNA was isolated with the RNeasy Micro kit (QIAGEN) from sorted basophils by following the manufacturer’s protocol. RNA integrity was assessed using Agilent 2100 Bioanalyzer (Agilent). Amplification, labeling and hybridizations were performed according to the protocol GeneChip WT Pico Reagent Kit (P/N 703262, Rev.1 2015) and then hybridized to GeneChip Human Gene 2.0 ST Array (Thermo Fisher) in a GeneChip Hybridization Oven 640. Washing and scanning were performed using the Expression Wash, Stain and Scan User Manual (P/N 702731 Rev. 3) in the Affymetrix GeneChip System including GeneChip Fluidics Station 450 and GeneChip Scanner 3000 7G (Affymetrix). After quality control, raw data were background corrected, quantile-normalized and summarized to a gene-level using the robust multi-chip average (RMA) system. For the detection of differentially expressed genes, a linear model was fitted to the data and empirical Bayes moderated statistics were calculated using the limma package from Bioconductor Genes (Wettenhall et al., 2006) with a p value less than 0.05 and with an absolute fold change value above 1.5 were selected as significant. Analyses were performed with R (v.3.2.3) and standard packages. Functional analyses were performed using the Comparison Upstream Regulator Analysis tool in the Ingenuity Pathway Analysis package (Ingenuity Systems, www.ingenuity.com). Gene Set Enrichment Analysis was performed along with the Molecular Signature Database (MSigDB) (Subramanian et al., 2005) and used to identify similarities and differences among samples.
Immunoprecipitation and Immunoblotting
To determine IgD interaction with galectin-9 in a cell-free protein-protein assay, 2 μg/ml human monoclonal IgD from multiple myeloma (Athens Research and Technology) was mixed with 2 μg/ml human recombinant galectin-9 (R&D Systems) in the presence or absence of control PBS, glucose or lactose (Sigma) for 30 min. Then, this protein mix was first incubated with biotin-conjugated goat pAbs with either an irrelevant specificity or a specific reactivity against human IgD and later exposed to SAcoated sepharose beads (Table S3). Denatured proteins were fractionated by SDS-PAGE, transferred onto PVDF membranes, and immunoblotted with a mAb to galectin-9 (Table S3). To analyze human IgD interaction with galectin-9 through CD44, 5 × 106 basophil-like KU812 cells were incubated with or without a pre-formed IgD-galectin-9 complex prepared as described earlier and then homogenized in lysis buffer with a Dounce homogeneizer. Next, equal amounts of protein lysates were sequentially incubated with goat biotin-conjugated pAb to human IgD and SA-coated beads. Denatured proteins were fractionated through SDS-PAGE, transferred onto PVDF membranes (BioRad) and immunoblotted with mAbs to human IgD, galectin-9 or CD44 (Table S3) and appropriate secondary antibodies as reported previously (Shan et al., 2013). Proteins were detected with an enhanced chemiluminescence detection system (Amersham).
ELISAs
Total human IgD was measured with the Human IgD ELISA Quantitation Set (Bethyl Laboratories). To measure total human IgG4 and IgE, 96-well plates were first coated with 1 μg/ml mouse RJ4 or TN142 mAbs, respectively, and later sequentially incubated with plasma and 0.4 μg/ml horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG pAb (Pierce). To measure total mouse IgD, plates were first coated with goat 21–1281 pAb to mouse IgD (American Research Products) and later sequentially incubated with serum and HRP-conjugated goat 21–1284 pAb to mouse IgD (American Research Products). To measure human or mouse antigen-specific IgG, IgE or IgD, plates (Immulon 4HBX, Thermo Scientific) coated with 5 μg/ml NP (4)-BSA, NP (7)-BSA, NP (16)-BSA, NP (20)-BSA, (Bioresearch Technologies) (Table S4) were incubated overnight at 4°C. OVA-specific IgE was measured through a LEGEND MAX Mouse OVA Specific IgE ELISA Kit (BioLegend). Diluted serum samples were added overnight at 4°. After washing the plates, horseradish peroxidase (HRP)-conjugated goat IgG pAbs to mouse IgG1, IgE, IgG2a, IgG2b (Southern Biotech) or IgD (American Research Product) were incubated for 2 h at room temperature. Plates were developed with TMB Microwell Peroxidase Substrate System (KPL) and read at 405 nm (Biotek Epoch) using Gen5 Microplate reader software 2.01. An appropriate control serum or IgD purified from he supernatants of the B1-δ8 hybridoma were used as standards to quantify the concentration of total antibodies, whereas a hyper-immune serum was used as reference to calculate the concentration of antigen-specific antibodies. To detect the binding of mouse IgD to galectins or Siglecs, plates were first coated with 5 μg/ml mouse galectin-1, galectin-3, galectin-4, galectin-7, galectin 9, Siglec-1, Siglec-2, Siglec-E, or Siglec-F (R&D Systems). Next, coated plates were washed and incubated with serial 2-fold dilutions of IgD purified from the supernatants of the mouse IgD class-switched B1-δ8 cells. Finally, plates were washed and incubated with HPR-conjugated goat anti-IgD (American Research Products). Wells coated with mouse IgD but no galectins or Siglecs were used as positive controls. Reactions were developed with the HRP substrate TMB (Life Technologies) and stopped with 2N H2SO4 to measure optical density (OD) at 450 nm using a Berthold Mithras LB940 spectrophotometer (Berthold Technologies).
ELISPOT
MultiScreen HTS IP 0.45 μm ELISPOT plates (Millipore) were coated with 1 μg/ml NP-BSA or IgD in PBS. The next day, plates were washed twice with RPMI 1640 and blocked with 10% FCS in RPMI 1640 at 37°C for 2 h. Splenocytes were added to the wells and incubated overnight at 37°C in a 5% CO2 incubator. The following day, cells were discarded and plates washed twice with deionized H2O and three times with PBS containing 0.1% Tween. Spots corresponding to ASCs were detected by adding an HRPconjugated goat anti-mouse antibody and AEC Substrate Set (Beckton Dickinson). Color development was stopped with water and spots were counted using C.T.L. Immunospot (Cellular Technology Limited).
Isolation of Secreted IgD
Mouse NP-reactive IgD class-switched B1-δ8 hybridoma cells (from Michael Neuberger, Cambridge University) were stained with FITC-conjugated 11–26c.2a mAb to IgD (BD Pharmingen) and analyzed for IgD expression by FCM. Gated IgDhi cells were seeded at 1 cell/well into 96-well plates by FACSorting and cultured with RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) to obtain IgDhi subclones. More than 200 subclones were screened by ELISA and 4H11 was selected for elevated IgD but no IgM secretion and incubated into a CELLine CL1000 bioreactor to obtain large amounts of NPspecific IgD. To affinity purify IgD, concentrated supernatant was clarified by centrifugation at 3000 × g for 60 minutes at 4°C, followed by filtration through a 0.22 μm Millex-GP filter (Merck Millipore). NP (Biosearch Technologies) was coupled to EAH Sepharose 4B (GE Healthcare) with 1-ethyl-3-(3dimethylaminopropyl) carbodiimide hydrochloride (Pierce). 0.5M of NaCl was added to the clarified supernatant that was incubated with NP-EAH Sepharose 4B overnight at 4ºC with end over end rotation. Unbound material was washed using DPBS 0.5M NaCl and IgD was eluted with DPBS 4M MgCl2. Finally, IgD was concentrated using an Amicon Ultra-4 Centrifugal Filter Unit with Ultracel 50K membrane (Merck Millipore) and quantified by measuring absorbance at 280 nm. 1 μg/mL corresponded to an OD of 1.5. Concentrated IgD preparations did not contain endotoxin, which was measured using a QCL-1000 Limulus Amebocyte Lysate kit from Lonza (Catalogue Number 50–647U).
qRT-PCRs
qRT-PCRs were performed as previously described (Shan et al., 2013) using appropriate primer pairs (Table S5).
Immunizations
Mice were immunized by s.c. injection of 50 μg NP-OVAL (Bioresearch Technologies) and 50 μg papain from Carica papaya (Sigma) on day 0, 7 and 14; by s.c. injection of 50 μg NP-OVAL and Imject alum (Thermo Scientific) on day 0, 7 and 14; by s.c. injection of 50 μg NP-OVAL and 50 μg Imject CFA (Thermo Scientific) on day 0 followed by 50 μg NP-OVAL on day 7 and 14; by i.p. injection with 50 μg grade-VI NP-OVA (Sigma) and 50 μg papain (Sigma) on day 0 and 7; or by i.p. injection of 1 mg goat anti-mouse IgD or 1 mg control goat IgG.
IgD Reconstitution
IgD-deficient Ighd−/− or Ighd−/−Il4GFP mice and B cell-deficient μMT or Rag2 mice were i.v. injected for 3–5 consecutive days before immunization (from day −5 to day −1) with 100 μg NP-IgD purified from NPreactive IgD class-switched B1-δ8 cells.
Basophil Depletion
Basophils from Mcpt8DTR mice were depleted by i.v. injection of 2.5 μg DT (Sigma). Basophils from WT Balb/c mice were depleted by i.v. injection of 30 μg Ba103 mAb to CD200R3 (Hycult Biotech) for 5 consecutive days before immunization (from day −5 to day −1) and for 5 alternate days (from day 1 to day 9) after immunization. A rat IgG2b RTK4530 mAb (Hycult Biotech) was used as control.
Acute Lung Inflammation
Mice were sensitized through i.p. injection of 20 μg grade-V OVA (Sigma) mixed with 2 μg aluminium hydroxide gel (Invitrogen) on day 0, 7 and 14 and challenged through i.t. inoculation of 0.1% OVA or control sterile PBS on day 16, 18, 20 and 22. Bronchoalveolar (BAL) fluid, blood and lung cells were collected for analysis 4 and 24 h following the last i.t. challenge.
Statistical Analysis
Statistical significance was assessed with a two-tailed unpaired Student’s t-test. Mann-Whitney U-test and Wilcoxon matched-pairs signed-rank test were used for analysis of non-parametric data. Results were analyzed with Prism software (Graph Pad) and p values of less than 0.05 were considered significant.
Data and Software Availability
The Gene Expression Omnibus (GEO) accession number for the global gene transcriptional analysis reported in this paper is GSE115296.
Supplementary Material
Highlights.
IgD binds to basophils through the CD44-binding protein galectin-9
IgD ligation by antigen elicits basophil release of Th2 cell-associated cytokines
IgD-activated basophils enhance B cell production of IgG1 and IgE
IgD interferes with IgE-mediated basophil degranulation
Acknowledgments
For access to CoFAR3 specimens, we thank the Consortium of Food Allergy Research Investigators (Wesley Burks, Donald Leung, Stacie Jones, Scott Sicherer, Robert Wood), the EMMES statistical coordinating center (Robert Lindblad), NIAID (Marshall Plaut, Wendy Davidson), and the Mucosal Immunology Studies Team at NAID (Annette L. Rothermel). For provision of other specimens, we thank Anna Nowak-Wegrzyn. Supported by US National Institutes of Health grants U01 AI95613, R01 AI57653 and P01 AI61093 to A.C.; by European Advanced Grant ERC-2011-ADG-20110310 and Ministerio de Ciencia e Innovación grant SAF2011-25241 to A.C.; and by the Swiss National Foundation grant 320030_159870 and European 7th frame work project MeDALL: Mechanisms of the Development of Allergy (grant 261357) to M.A.
Footnotes
Competing Interests Statement
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin AR, Tamma SM, Swenson CD, Kieda CC, Oppenheim JD, Finkelman FD, and Coico RF (1993). The immunoaugmenting properties of murine IgD reside in its C delta 1 and C delta 3 regions: potential role for IgD-associated glycans. Int Immunol 5, 607–614. [DOI] [PubMed] [Google Scholar]
- Chen AC, Donovan A, Ned-Sykes R, and Andrews NC (2015). Noncanonical role of transferrin receptor 1 is essential for intestinal homeostasis. Proc Natl Acad Sci U S A 112, 11714–11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, Edholm ES, Santini PA, Rath P, Chiu A, et al. (2009). Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol 10, 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Wang KW, Zhang D, Zhan X, Wang T, Bu CH, Behrendt CL, Zeng M, Wang Y, Misawa T, et al. (2017). IgD class switching is initiated by microbiota and limited to mucosa-associated lymphoid tissue in mice. Proc Natl Acad Sci U S A 114, E1196–E1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorny A, Casas-Recasens S, Sintes J, Shan M, Polentarutti N, Garcia-Escudero R, Walland AC, Yeiser JR, Cassis L, Carrillo J, et al. (2016). The soluble pattern recognition receptor PTX3 links humoral innate and adaptive immune responses by helping marginal zone B cells. J Exp Med 213, 2167–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kivit S, Saeland E, Kraneveld AD, van de Kant HJ, Schouten B, van Esch BC, Knol J, Sprikkelman AB, van der Aa LB, Knippels LM, et al. (2012). Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy 67, 343–352. [DOI] [PubMed] [Google Scholar]
- Flajnik MF (2018). A cold-blooded view of adaptive immunity. Nat Rev Immunol 18, 438–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, and Sutton BJ (2008). IgE in allergy and asthma today. Nat Rev Immunol 8, 205–217. [DOI] [PubMed] [Google Scholar]
- Gutzeit C, Chen K, and Cerutti A (2018). The enigmatic function of IgD: some answers at last. Eur J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmskov U, Thiel S, and Jensenius JC (2003). Collectins and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol 21, 547–578. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, and Roux A (2018). Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 19, 313–326. [DOI] [PubMed] [Google Scholar]
- Karasuyama H, Mukai K, Tsujimura Y, and Obata K (2009). Newly discovered roles for basophils: a neglected minority gains new respect. Nat Rev Immunol 9, 9–13. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lee YS, Hahn JH, Choe J, Kwon HJ, Ro JY, and Jeoung D (2008). Hyaluronic acid targets CD44 and inhibits FcepsilonRI signaling involving PKCdelta, Rac1, ROS, and MAPK to exert anti-allergic effect. Mol Immunol 45, 2537–2547. [DOI] [PubMed] [Google Scholar]
- Lu LL, Suscovich TJ, Fortune SM, and Alter G (2018). Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol 18, 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, and Locksley RM (2001). Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15, 303–311. [DOI] [PubMed] [Google Scholar]
- Neuberger MS, and Rajewsky K (1981). Switch from hapten-specific immunoglobulin M to immunoglobulin D secretion in a hybrid mouse cell line. Proc Natl Acad Sci USA 78, 1138–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki T, Tsutsui S, Hirose S, Aradono S, Sugimoto Y, Takeshita K, Nishi N, and Hirashima M (2009). Galectin-9 is a high affinity IgE-binding lectin with anti-allergic effect by blocking IgE-antigen complex formation. J Biol Chem 284, 32344–32352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, and Medzhitov R (2013). Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity 39, 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda SK, Facchinetti V, Voynova E, Hanabuchi S, Karnell JL, Hanna RN, Kolbeck R, Sanjuan MA, Ettinger R, and Liu YJ (2018). Galectin-9 inhibits TLR7-mediated autoimmunity in murine lupus models. J Clin Invest 128, 1873–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich GA, and Toscano MA (2009). Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol 9, 338–352. [DOI] [PubMed] [Google Scholar]
- Roes J, and Rajewsky K (1993). Immunoglobulin D (IgD)-deficient mice reveal an auxiliary receptor function for IgD in antigen-mediated recruitment of B cells. J Exp Med 177, 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaud P, Saintamand A, Saad F, Carrion C, Lecardeur S, Cogne M, and Denizot Y (2014). Elucidation of the enigmatic IgD class-switch recombination via germline deletion of the IgH 3’ regulatory region. J Exp Med 211, 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, He B, Cassis L, Bigas A, Cols M, et al. (2013). Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, et al. (2011). TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 477, 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson CD, Patel T, Parekh RB, Tamma SM, Coico RF, Thorbecke GJ, and Amin AR (1998). Human T cell IgD receptors react with O-glycans on both human IgD and IgA1. Eur J Immunol 28, 2366–2372. [DOI] [PubMed] [Google Scholar]
- Victora GD, and Nussenzweig MC (2012). Germinal centers. Annu Rev Immunol 30, 429–457. [DOI] [PubMed] [Google Scholar]
- Wettenhall JM, Simpson KM, Satterley K, and Smyth GK (2006). affylmGUI: a graphical user interface for linear modeling of single channel microarray data. Bioinformatics 22, 897–899. [DOI] [PubMed] [Google Scholar]
- Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, Zhu C, Hirashima M, Anderson AC, and Kuchroo VK (2014). Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 41, 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA (2015). Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol 15, 271–282. [DOI] [PubMed] [Google Scholar]
- Xue B, Coico R, Wallace D, Siskind GW, Pernis B, and Thorbecke GJ (1984). Physiology of IgD. IV. Enhancement of antibody production in mice bearing IgD-secreting plasmacytomas. J Exp Med 159, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Gene Expression Omnibus (GEO) accession number for the global gene transcriptional analysis reported in this paper is GSE115296.