Abstract
Purpose:
CT screening can reduce death from lung cancer. We sought to improve the diagnostic accuracy of lung cancer screening using ultrasensitive methods and a lung cancer specific gene panel to detect DNA methylation in sputum and Plasma.
Experimental Design:
This is a case-control study of subjects with suspicious nodules on CT imaging. Plasma and sputum were obtained pre-operatively. Cases (n=150) had pathological confirmation of node negative (stage I and IIA) non-small cell lung cancer. Controls (n=60) had non-cancer diagnoses. We detected promoter methylation using quantitative methylation-specific real-time PCR and Methylation on Beads for cancer-specific genes (SOX17, TAC1, HOXA7, CDO1, HOXA9, and ZFP42).
Results:
DNA methylation was detected in plasma and sputum more frequently in people with cancer compared to controls (p<0.001) for 5 of 6 genes. The sensitivity and specificity for lung cancer diagnosis using the best individual genes was 63–86% and 75–92% in sputum respectively and 65–76% and 74–84% in plasma. A three-gene combination of the best individual genes has sensitivity and specificity of 98% and 71% using sputum and 93% and 62% using plasma. Area under the Receiver Operating Curve for this panel was 0.89 95% CI (0.80–0.98) in sputum and 0.77 95% CI (0.68–0.86) in plasma. Independent blinded random forest prediction models combining gene methylation with clinical information correctly predicted lung cancer in 91% of subjects using sputum detection and 85% of subjects using plasma detection.
Conclusions:
High diagnostic accuracy for early stage lung cancer can be obtained using methylated promoter detection in sputum or plasma.
Keywords: Lung cancer screening, epigenetics, gene promoter hypermethylation, early detection, molecular biomarkers
Introduction
The National Lung Screening Trial (NLST) demonstrated a 20% reduction in lung cancer mortality using low-dose computed tomography (CT) screening.(1) This survival benefit comes at the price of detecting many indeterminate pulmonary nodules with an overall false positive rate of 96.4%. (1, 2) The likelihood that a nodule is malignant increases with size,(3) with a challenge in management for the indeterminate nodules from 7–29mm, with a risk of malignancy between 1.7% and 22%.(3) This has led to a cautious adoption of CT screening, because complications, and even deaths, result from further diagnostic procedures.(4) One approach to improve the specificity of CT screening involves the use of cancer specific biomarkers from sputum and plasma. Previous studies have examined DNA methylation as a biomarker for cancer risk, but limited sensitivity and/or specificity were insufficient for lung cancer screening.(5–16)
Reduced sensitivity of methylation detection may occur from technical limitations. Extraction methods for DNA have been inefficient for small amounts of DNA,(17, 18) a particular problem for bodily fluids. We recently developed Methylation-on-Beads (MOB), which reduces sample loss thereby potentially increasing sensitivity.(19, 20) Another issue for detection is the use of loci with low frequencies of altered DNA methylation, leading to an inability to detect changes in biofluids. We recently identified six genes (SOX17, TAC1, HOXA7, CDO1, HOXA9, ZFP42) using the Cancer Genome Atlas (TCGA) (21) with highly prevalent DNA methylation in lung squamous and adenocarcinoma, but not in normal lung tissue (22, 23) one of which (CDO1) has been described elsewhere.(22, 23) These were chosen solely based upon high frequency methylation cancer specific methylation and developed into assays using MOB and real-time Methylation-Specific PCR (qMSP) to determine the diagnostic accuracy for lung cancer detection in sputum and plasma.
Material and Methods
Study Population
The study population consists of a prospective, observational cohort of 651 participants, initiated in 2007 within the Johns Hopkins Lung Cancer Specialized Program of Research Excellence (SPORE). From this cohort, 210 study patients had early stage node negative tumors (T1-T2N0) and samples adequate for analysis. Institutional review board approval was obtained prior to study initiation (NA_00005998), and all patients signed informed consent. Surgical resection with curative intent and pathological analyses of suspected lung cancer lesions were completed in all patients, and staged according to revised TNM guidelines classification criteria.(24) Cases had pathologically confirmed lung cancer. Controls were defined as patients histologically confirmed not to have cancer. Pack-years of cigarette smoking was defined as the average number of packs smoked per day times the number of years smoked. Nodule size was obtained from the pathological report, and nodule volumewas calculated using the ellipsoid volume formula (Volume = 4/3 × π × radius A × radius B × radius C).
Plasma and Sputum Collection
Prior to surgery, 20 ml of plasma was collected in tubes containing sodium heparin (Bectin Dickinson, Franklin Lakes) and then stored at −80°C. For sputum collection, two cups containing Saccomanno’s fixative solution were used for each patient as previously described.(8, 11, 25) Subjects were asked to provide an early morning spontaneous sputum at home in two cups for 3 consecutive days within 1 week prior to pulmonary resection.(11, 26) Five milliliters of sputum was collected, washed with Saccomanos’ solution, vortexed, centrifuged and stored at −80°C.(8)
DNA Isolation and Bisulfite Conversion
DNA extraction from tumor, plasma and sputum was performed using MOB, a process that allows DNA extraction and bisulfite conversion in a single tube via the use of silica super magnetic beads.(20) This approach yields a 1.5 to 5-fold improvement in extraction efficiency compared to traditional conventional techniques.(27) We optimized the protocol previously described for plasma, (27) using 1.5 ml of plasma and 375 μl (800units/ml, NEBL p8107s) of proteinase K. For DNA extraction from sputum, we modified the protocol used for plasma by adding 200 μl of sample to 300 μl of Buffer AL and 40 μl of Proteinase K and by incubating them together at the same temperature (50 °C for 2 hours). After digestion, 300 μl of IPA and 150 μl of beads were added. The lysate was incubated and rotated for 10 minutes before adding 5 μl of carrier RNA, and incubating for an additional 5 minutes.(27)
DNA Methylation Analysis
The genomic sequence for the genes and 1000 bases upstream was obtained from the UCSC genomic browser website.(28) The primers and hybridization probes for methylation analysis were designed based on this sequence by using Primer3 (v.0.4.0).(29, 30) All primer and probe sequences are listed in supplementary Table S1. The analysis was performed using quantitative real-time Methylation Specific PCR, and normalized to a control x-Actin assay.(18) Each reaction was performed in a 25 μl PCR mixture consisting of 2 μl of bisulfite converted DNA, 300 nM R-sense primer, 300 nM F-anti-sense primer, 100nM probe, 100 nM of fluorescein reference dye (Life Technologies), 1.67mM dNTPs (VWRQuotation), and 1 μl of Platinum Taq® DNA Polymerase (invitrogen). Master mix contained 16.6mM (NH4)2SO4, 67mM Tris pH 8.8, 6.7 mM MgCl2 and 10mM β-mercaptoethanol in a nuclease-free DI water solution. Amplification reactions were performed using 96 well-plates (MicroAmp®) in triplicate. Thermo cycling conditions were: 95°C for 5 min, 50 cycles at 95°C for 15 seconds, and 65°C for 1min and 72°C for 1 min. An ABI StepOnePlus Real-Time PCR system was used (Applied Bio Systems, examples shown in Supplemental Figure S1).
With the extremely low levels of DNA methylation in plasma and sputum, replicates for some samples produced no detectable methylation as expected. To incorporate this information into the final quantification of methylation, we calculated the 2−ΔCT for each methylation detection replicate comparing it to the mean Ct for β-Actin (ACTB). For replicates which were not detected (ND), a CT of 100 was used, creating a near zero value for 2−ΔCT. The mean 2−ΔCT value was calculated with the formula:
Statistical Analysis
Quantitative data are expressed as median (interquartile range) for continuous, non-parametric variables and frequency (percentage) for categorical variables. For inter-group comparison, the Wilcoxon rank sum test was used for continuous data and the Fisher´s exact test for categorical data.
Data was analyzed using two approaches. The first approach is the ROC analysis using the 2−ΔCT values for individual genes to determine the performance of each individual marker (R statistic software, version 3.0.2, Vienna, Austria).(31) The area under the curve was reported with 95% CIs. The three best performing genes were selected for diagnostic accuracy for lung cancer detection, based on receiver operator classification (ROC) curves and were used for combined detection. Sensitivity and specificity values were obtained from the presence or absence of detectable methylation as a cutoff.
The second approach utilized a non-parametric machine learning method, random forest, to estimate the prediction accuracy in an independent validation dataset by combining the methylation data and clinical risk factors: nodule size, age, pack-year, COPD status and FVC values. Subjects were randomly selected as a training set (67%) and a test set (33%). A statistician (PH), blinded to the diagnoses of the test set, used the training set to build three random forest prediction models: (1) used sputum, clinical, and demographic variables, (2) used plasma and clinical variables, (3) used only clinical and demographic variables. The random forest model consisted of 5000 trees, each using a random sample of the training data. The remaining training data was used for internal cross-validation. Each random forest model provides two predictions: the cancer status (a binary prediction) and the probability of cancer (a continuous prediction). The three random forest models were then applied to the test set data. Prediction accuracy was reported as the proportion of test set subjects correctly predicted by the random forest classification models, allowing calculation of sensitivity, specificity, and ROC analysis.
Results
Characteristics of the Patients
Two hundred and ten patients fulfilled inclusion criteria, with 150 node negative early stage lung cancer subjects and 60 controls with non-cancerous lung lesions (Table 1). Clinical and demographic variables were similar in cases and controls with the exception of age, number of pack-year and nodule size (cm) as well as volume (cm3). Subjects with lung cancer were older than controls (67 vs. 73 years, p=0.007), smoked more (30 vs. 19.5 pack years, p=0.01), and had larger nodules (2.0 vs. 1.5 cm, p=0.01). The proportion of smokers, former smokers and never smokers was not different between cases and controls.
Table 1.
Baseline Characteristics of the 210 Subjects.
| Patient Characteristics | Cancer (N=150) |
Control (N=60) |
p Value | ||
|---|---|---|---|---|---|
| Age at surgery (years) (IQR) | 68 (62–75) | 63 (55–73) | 0.007 | ||
| Gender | |||||
| Male (%) | 63 (42%) | 33 (55%) | 0.094 | ||
| Female (%) | 87 (58%) | 27 (45%) | |||
| Race | |||||
| White (%) | 120 (80%) | 51 (85%) | 0.087 | ||
| Black (%) | 19 (13%) | 3 (5%) | |||
| Other (%) | 11 (7%) | 6 (10%) | |||
| Stage | |||||
| IA-IB (%) | 136 (91%) | NA | NA | ||
| IIA (%) | 14 (9%) | NA | |||
| Histology | |||||
| Adenocarcinoma (%) | 121 (81%) | NA | NA | ||
| Squamous-cell (%) | 26 (17%) | NA | |||
| Adenosquamous (%) | 3 (2%) | NA | |||
| Smoking status | |||||
| Current (%) | 27 (18%) | 7 (12%) | 0.176 | ||
| Former (%) | 87 (58%) | 34 (57%) | |||
| Never (%) | 31 (21%) | 19 (32%) | |||
| Pack-year (IQR) | 30 (10–50) | 20 (0–35) | 0.010 | ||
| COPD (%) | 41 (27%) | 12 (20%) | 0.370 | ||
| FEV1 % Predicted (IQR) | 84 (70–99) | 85 (70–100) | 0.861 | ||
| FVC % Predicted (IQR) | 92 (80–103) | 87 (80–110) | 0.682 | ||
| FEV1/FVC % Ratio (IQR) | 73 (68–78) | 77 (70–79) | 0.080 | ||
| Nodule size (cm) | 2 (1.5–3) | 1.5 (1.1–3) | 0.01 | ||
| < 1cm | 6 (4%) | 13 (22%) | 0.001 | ||
| 1–2 cm | 52 (35%) | 19 (32%) | |||
| > 2 cm | 92 (61%) | 28 (47%) | |||
| Nodule volume (cm3) | 4.19 (1.77–14-14) | 1.6 (0.52–18.12) | 0.001 | ||
Abbreviations: Chronic obstructive pulmonary disease: COPD, Forced Expiratory Volume in one second: FEV1, Forced vital capacity: FVC, Interquartile range: IQR.
Nodule size % <1cm, 1–2, >2cm
Detection of DNA Methylation
We first measured DNA methylation for these genes in tumor tissue, confirming our previous study suggesting these genes were methylated in the majority of lung tumors (Figure 1). Methylation in sputum was detected more frequently in all 6 genes in cancer patients compared to controls (Figure 1), which for some patients was quantitatively similar to lung tumor tissues, but in some cases was at levels previously below conventional methods of detection. For 5 of the 6 genes, (SOX17, TAC1, HOXA7, CDO1, and ZFP42) this was statistically significant (p < 0.001), while HOXA9 showed a lack of specificity. Methylation of all 6 genes was detected more frequently in plasma in cases compared to controls (p < 0.001). The worst performing gene was HOXA9 in plasma, which showed a lack of specificity as was seen in the sputum. We determined the sensitivity and specificity in this cohort using the presence or absence of detectable methylation as a cutoff, without considering the quantitation of methylation. This resulted in good sensitivity and specificities (Table 2), showing that the sensitivity and specificity for lung cancer diagnosis using individual genes from sputum ranged from 63–93% and 42–92% respectively and from plasma from 33–91% and 52–94%.
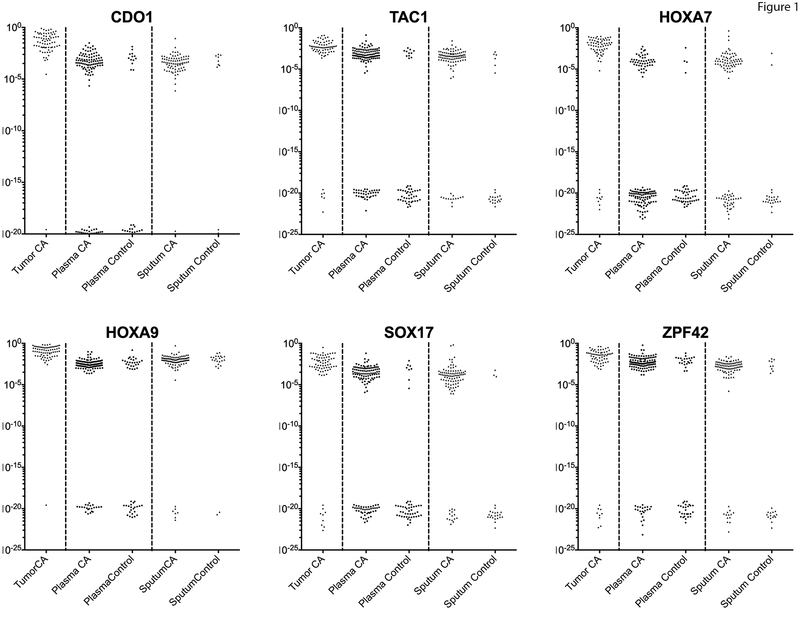
Figure 1. Methylation detection values of the studied genes.
This scatter plot shows the converted ΔCT methylation values in a logarithmic scale. These values show a bimodal distribution with the lower group the values corresponding to those samples with no detectable amplification (ND). The majority of lung tumor samples have high levels of methylation, as expected from the previous study. Plasma and sputum samples from cancer patients have detectable methylation which varies from levels nearing that of tumor samples to those at the limits of detection (10−5-10−6), while some patients are undetectable. The majority of controls have undetectable methylation at these loci, although some patients do have detectable methylation that is quantitatively similar to cancer patients. HOXA9 methylation is detectable in most control patients, especially in the sputum, suggesting this change is present in the lung epithelium and not as specific for the detection of cancer.
Table 2.
Gene Methylation Detection in Sputum and Plasma
| Cancer (n=90) | Control (n=24) | |||||
| Sputum | n | Sensitivity | n | Specificity | PPV | NPV |
| SOX17 | 76 | 84% | 3 | 88% | 96% | 60% |
| TAC1 | 77 | 86% | 6 | 75% | 93% | 58% |
| HOXA7 | 57 | 63% | 2 | 92% | 97% | 40% |
| CDO1 | 70 | 78% | 8 | 67% | 90% | 44% |
| HOXA9 | 84 | 93% | 22 | 8% | 79% | 25% |
| ZFP42 | 78 | 87% | 9 | 63% | 90% | 56% |
| TAC1, HOXA7, SOX17 | 88 | 98% | 7 | 71% | 93% | 89% |
| Cancer (n=125) | Control (n=50) | |||||
| Plasma | n | Sensitivity | n | Specificity | PPV | NPV |
| SOX17 | 91 | 73% | 8 | 84% | 92% | 55% |
| TAC1 | 95 | 76% | 11 | 78% | 90% | 57% |
| HOXA7 | 42 | 34% | 4 | 92% | 91% | 36% |
| CDO1 | 81 | 65% | 13 | 74% | 86% | 46% |
| HOXA9 | 108 | 86% | 27 | 46% | 80% | 58% |
| ZFP42 | 105 | 84% | 23 | 54% | 82% | 57% |
| CDO1, TAC1, SOX17 | 116 | 93% | 19 | 62% | 86% | 78% |
Abbreviations: positive predictive value: PPV; negative predictive value: NPV.
Gene Methylation and Lung Cancer Diagnostic Accuracy
ROC curves for lung cancer detection were obtained for each single gene; using the normalized methylation ΔCt values calculated as described in methods (Supplemental Table S2, ROC curves in Supplemental figure S2 & S3). By determining the best quantitative cutoff, the sensitivity and specificity for lung cancer diagnosis from single methylated genes in sputum ranged 63–93% and 42–92% respectively and in plasma from 33–91% and 52–94% and was very similar to that obtained reported in table 2, with the exception of HOXA9 where quantitative cutoffs improved performance. The AUC values were 0.56–0.89 in sputum samples and 0.60–0.78 in plasma samples.
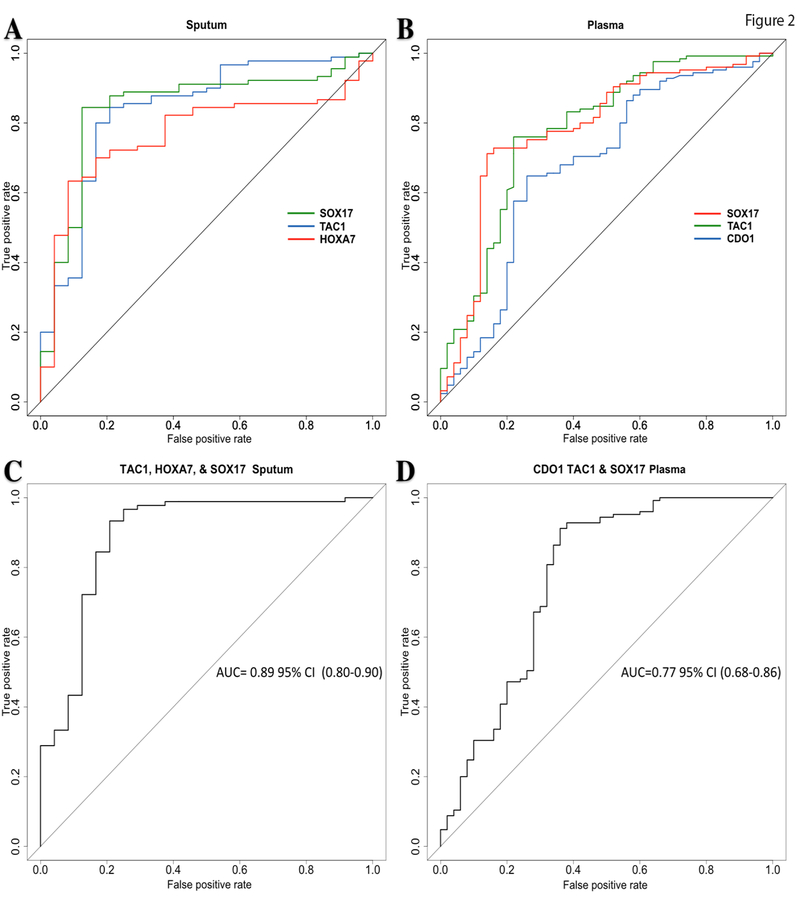
The genes with the largest AUC in sputum were: TAC1 AUC: 0.84 95% CI (0.74–0.94), HOXA7 AUC: 0.77 95% CI (0.67–0.86) and SOX17 AUC: 0.84 95% CI (0.75–0.94) (Figure 2A), with sensitivities and specificities for TAC1 86% and 75%; HOXA7 63% and 92%; and SOX17 84% and 88 respectively. The positive and negative predictive values for these three genes were: TAC1 93% and 58%; HOXA7 97% and 40%; SOX17 96% and 60% respectively.
Figure 2. Receiver operator classification curves for lung cancer detection.
A. ROC curves comparing the 3 genes with the largest areas under the curve for sputum. B. ROC curves comparing the 3 genes with the largest areas under the curve for Plasma. C. ROC of the combined methylation status of the genes from sputum with the largest area under the curve. D. ROC of the combined methylation status of the genes from Plasma with the largest area under the curve.
Abbreviations: area under the curve: AUC, 95 % confidence interval: 95% CI.
In plasma, the genes with the largest areas under the curve (AUC) were: CDO1 AUC: 0.68 95% CI (0.58–0.77), TAC1 AUC: 0.78 (0.70–0.86) and SOX17 AUC: 0.78 95% CI (0.70–0.86) (Figure 2B), with corresponding sensitivities and specificities of: CDO1 65% and 74%; TAC1 76% and 78%; SOX17 73% and 84% respectively. The positive and negative predictive values for these genes were: CDO1 86% and 46%; TAC1 90% and 57%; SOX17 92% and 55% respectively.
The sensitivity and specificity obtained from the combination of the three best performing markers (TAC1, HOXA17 and SOX17) in sputum was 93% and 89%, respectively with a corresponding ROC AUC of 0.89 95% CI (0.80–0.98) (Figure 2C). In plasma, the combination of CDO1, TAC1 and SOX17 showed a sensitivity, specificity and AUC of 86%, 78% and 0.77, 95% CI (0.68–0.86) respectively (Figure 2D).
Smokers subset analysis
Since CT screening for lung cancer is currently recommended for current and ex-smokers, we explored the diagnostic accuracy when only smokers were considered (n=155; 114 with cancer and 41 without cancer) (Supplemental Table S4). The results in only smokers were similar to the entire study population for the prevalence of methylated patients, sensitivity, specificity and AUC (Supplemental Table S5). AUC in smokers only was 0.89 95% CI (0.79–0.99) for the combination of the methylation status of the best three genes from sputum and AUC 0.85 95% CI (0.76–0.94) from the best three genes from plasma (Supplemental Table S5).
Independent Prediction Accuracy Performance
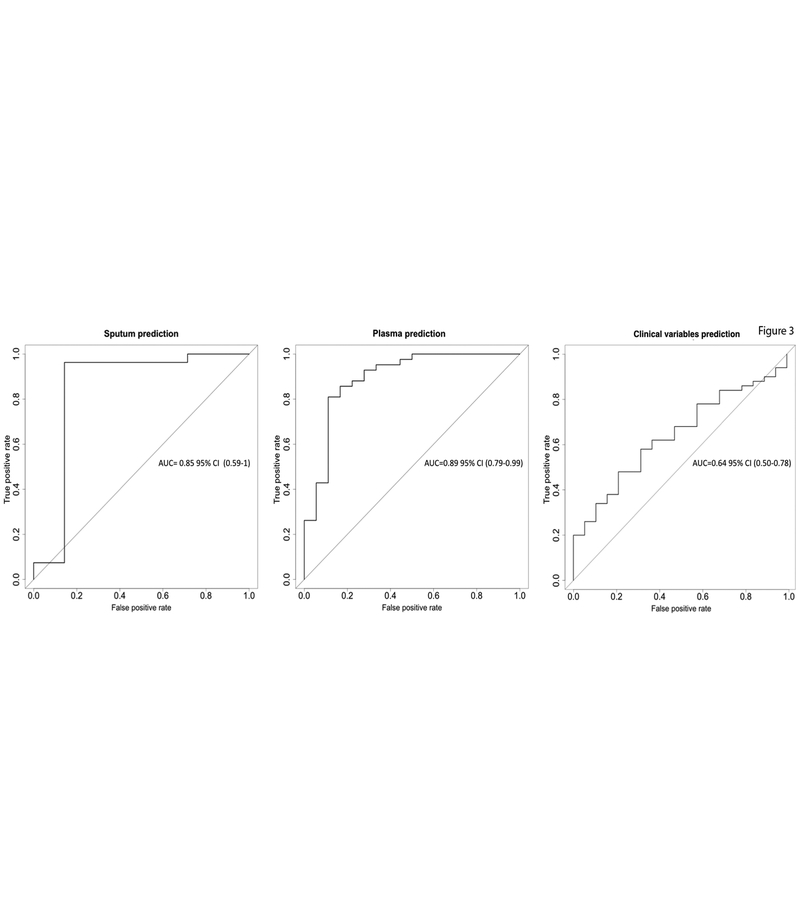
While the above analysis looked at individual gene methylation in cases and controls to detect cancer, independent blinded random forest prediction models was used to consider all DNA methylation biomarkers in combination with clinical risk factors. Risk factors included in the first two random forest prediction models were methylation Ct values from all six genes, age, pack-year, COPD status and FVC values. The methylation Ct values were not included in the last prediction model. The randomly selected training dataset has 140 subjects with 99 (70.7%) cancers and 41 (29.3%) controls. The independent test set has 70 subjects with 51 (72.9%) cancers and 19 (27.1%) controls. In the variable of importance output of the first two random forest prediction models, methylation Ct values were ranked as more important variables than demographic and clinical variables (Supplemental Figure S4). Supplemental Table S3 summarizes the prediction accuracies of these three models when they were applied to the independent test set patients. With sputum samples, the random forest model correctly predicted lung cancer in 91% of subjects in the test subset. The corresponding AUC was 0.85 95% CI (0.59–1.0) (Figure 3). The sensitivity and specificity of the prediction in the testing subset from the ROC curve were 0.93 and 0.86, respectively. Using plasma samples, the random forest model correctly predicted lung cancer in 85% of subjects in the testing subset. The corresponding AUC was 0.89 95% CI (0.79–0.99) (Figure 3). The sensitivity and specificity of the prediction in the testing subset from the ROC curve were 0.93 and 0.67, respectively. Using clinical and demographic risk factors alone, the accuracies were lower than the first two models with a diagnostic accuracy of 68%, AUC of 0.64, PPV of 75% and a NPV of 38% (Figure 3 and Supplemental Table S3).
Figure 3. Receiver operator classification curves for cancer predictions.
ROC curves assessing the accuracy of the predictions for lung cancer performed on the testing subset by using as predictors the ΔCt values for all six genes, age, pack-year, COPD status and FVC values. The left plot is obtained using sputum samples, the middle one, using plasma samples and the right one, the ROC curve for the clinical predictors alone.
Discussion
High diagnostic accuracy for early stage lung cancer can be obtained using a panel of methylated promoter genes and an ultrasensitive detection strategy based on MOB in sputum or plasma., This assay has several characteristics which make it clinically useful (i) the sensitivity and specificity in sputum and plasma exceeds the diagnostic accuracy required by most clinical standards(32, 33) (ii) it can be performed with minute quantities of DNA from sputum or plasma (iii) it can help distinguish malignant versus benign nodules, addressing the current problem of high false positive scans in lung cancer screening. This discrimination is independent of age, pack-year and even nodule size, which allows the detection of early stage lung cancer in smokers. Finally, as a PCR-based assay, it is simple and relatively inexpensive.
Previous studies have sought to improve lung cancer risk assessment by the use of molecular biomarkers obtained from plasma and sputum.(8, 10, 11, 25, 26, 34, 35) However none of these tests have been used clinically because their achieved sensitivities and specificities were usually not high enough for clinical decision-making. (8, 10, 11, 25, 26, 34–38) With improvements in DNA extraction methods and processing for methylation detection, along with the use of highly prevalent cancer specific methylation targets, we have overcome these limitations. Direct comparisons between serum and plasma for detection of DNA methylation have not been conducted in this study, but the use of plasma may reduce the amount of lymphocyte DNA present for analysis.
Despite the improved sensitivity of this approach, there are some patients with undetectable DNA methylation in either blood or sputum. In examining these non-detectable patients, this is not related to clinical characteristics, including smoking status (see similar detection in only smokers,(Supplemental data) . This does not appear to be related to PCR failure or assay efficiency, which was assessed for each assay using appropriate controls (Methods). We also examined whether tumor size and therefore tumor burden affected our ability to detect DNA methylation in the plasma or sputum. There was no statistical difference in tumor size between cancer patients with or without detectable DNA methylation. (Supplemental Table S6), and notably nodules less than 2 cm were readily detected.
In this study, detection of methylation in sputum samples was slightly better than the detection of these same genes in plasma. The access of early cancers to the airways may be one explanation for this difference. Indeed, changes in the airways form the basis for the AEGIS Study, which reported an improved diagnostic yield of bronchoscopy using gene-expression classifiers from epithelial cells collected during bronchoscopy.(38) The AUC, sensitivities and specificities reported in the AEGIS Study were lower than we report here. In our model where methylation markers from plasma were considered simultaneously with age and number of pack-years, we observed a predictive accuracy close to that of sputum. This suggests that plasma could substitute for sputum in lung cancer detection in those cases where sputum cannot be obtained.
According to the NLST, the chances of having lung cancer with a positive CT screening are less than 5%.(1, 2) This is because lung cancer with CT screening in the NLST study yielded a 71% sensitivity but a 63% specificity with a 96.4% false positive rate.(1, 2) Our current findings suggest that methylation detection with a few genes from either plasma and sputum could potentially guide management of positive CT screening results. Although our study included some patients who would not meet current lung cancer screening guidelines (non-smokers), we observed similar detection rates when only smokers were analyzed. Replication and external validation of our findings in a large, prospective, multicenter case control trial are essential before this approach can be adopted.
Conclusion
This study shows that high sensitivity and specificity detection of early stage NSCLC can be obtained using a panel of methylated promoter genes in plasma and sputum, and that the methylation level of these genes is associated with a high lung cancer risk independent of age, pack-year and nodule size. If confirmed in a validation study, this panel could be used as an adjunct to CT screening, identifying patients at high risk for lung cancer, reducing false positive results, unnecessary tests, and improving the diagnosis of lung cancer at an earlier stage.
Supplementary Material
Translational relevance.
The National Lung Screening Trial demonstrated a 20% reduction in lung cancer mortality using low-dose computed tomography screening. Diagnostic accuracy of screening could be improved using cancer specific biomarkers from sputum and plasma. We developed Methylation-on-Beads (MOB), reducing sample loss with potentially increased sensitivity. We used MOB and real-time quantitative Methylation-Specific PCR (qMSP) to detect the promoter methylation using genes frequently methylated in SOX17, TAC1, HOXA7, CDO1, HOXA9, and ZFP42. This study demonstrates that high diagnostic accuracy of early stage NSCLC can be obtained using a panel of methylated promoter genes in plasma and sputum, and that the methylation level of these genes is associated with a high lung cancer risk independent of age, pack-year and nodule size. This panel could be used to identify patients at high risk for lung cancer, reducing false positive results, unnecessary tests, and improving the diagnosis of lung cancer at an earlier stage.
Acknowledgments:
Funding: DOD W81WH-12-1-0323 (to JGH and MVB) and NCI P50CA058184 (to JGH, MVB, and SBB)
Footnotes
Conflict of Interest Statement: The authors of this manuscript report no relationship to disclose. The data in this manuscript were in part presented in the abstract/poster form at the 2015 Meeting of the American Association for Cancer Research (AACR) in Philadelphia, Pennsylvania 2015 Meeting of the International Association of Lung Cancer (IASLC) in Denver, Colorado
References
- 1.National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tammemagi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gierada DS, Pinsky P, Nath H, Chiles C, Duan F, Aberle DR. Projected outcomes using different nodule sizes to define a positive CT lung cancer screening examination. Journal of the National Cancer Institute. 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esteller M, Sanchez-Cespedes M, Rosell R, Sidransky D, Baylin SB, Herman JG. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Cancer Res. 1999;59:67–70. [PubMed] [Google Scholar]
- 6.Wong IH, Lo YM, Zhang J, Liew CT, Ng MH, Wong N, et al. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res. 1999;59:71–3. [PubMed] [Google Scholar]
- 7.Palmisano WA, Divine KK, Saccomanno G, Gilliland FD, Baylin SB, Herman JG, et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000;60:5954–8. [PubMed] [Google Scholar]
- 8.Belinsky SA, Liechty KC, Gentry FD, Wolf HJ, Rogers J, Vu K, et al. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006;66:3338–44. [DOI] [PubMed] [Google Scholar]
- 9.Brock MV, Hooker CM, Ota-Machida E, Han Y, Guo M, Ames S, et al. DNA methylation markers and early recurrence in stage I lung cancer. New England Journal of Medicine. 2008;358:1118–28. [DOI] [PubMed] [Google Scholar]
- 10.Ostrow KL, Hoque MO, Loyo M, Brait M, Greenberg A, Siegfried JM, et al. Molecular analysis of plasma DNA for the early detection of lung cancer by quantitative methylation-specific PCR. Clin Cancer Res. 2010;16:3463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leng S, Do K, Yingling CM, Picchi MA, Wolf HJ, Kennedy TC, et al. Defining a gene promoter methylation signature in sputum for lung cancer risk assessment. Clin Cancer Res. 2012;18:3387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Shen Y, Wang M, Tang D, Luo Y, Jiao W, et al. Identification of the methylation of p14ARF promoter as a novel non-invasive biomarker for early detection of lung cancer. Clin Transl Oncol. 2013;16:581–9. [DOI] [PubMed] [Google Scholar]
- 13.Sandoval J, Mendez-Gonzalez J, Nadal E, Chen G, Carmona FJ, Sayols S, et al. A prognostic DNA methylation signature for stage I non-small-cell lung cancer. J Clin Oncol. 2013;31:4140–7. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y, Kim D-H. CpG Island Hypermethylation as a Biomarker for the Early Detection of Lung Cancer. (null). New York, NY: Springer New York; 2014. p. 141–71. [DOI] [PubMed] [Google Scholar]
- 15.Nawaz I, Qiu X, Wu H, Li Y, Fan Y, Hu L-F, et al. Development of a multiplex methylation specific PCR suitable for (early) detection of non-small cell lung cancer. Epigenetics. 2014;9:1138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Dai W, Kwong DL-w, Szeto CYY, Wong EH-w, Ng WT, et al. Epigenetic markers for noninvasive early detection of nasopharyngeal carcinoma by methylation-sensitive high resolution melting. Int J Cancer. 2014;136:E127–E35. [DOI] [PubMed] [Google Scholar]
- 17.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eads CA, Danenberg KD, Kawakami K, Saltz LB, Blake C, Shibata D, et al. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey VJ, Keeley BP, Razavi CR, Griffiths E, Carraway HE, Wang TH. DNA methylation detection using MS-qFRET, a quantum dot-based nanoassay. Methods. 2010;52:237–41. [DOI] [PubMed] [Google Scholar]
- 20.Bailey VJ, Zhang Y, Keeley BP, Yin C, Pelosky KL, Brock M, et al. Single-tube analysis of DNA methylation with silica superparamagnetic beads. Clin Chem. 2010;56:1022–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wrangle J, Machida EO, Danilova L, Hulbert A, Franco N, Zhang W, et al. Functional identification of cancer-specific methylation of CDO1, HOXA9, and TAC1 for the diagnosis of lung cancer. Clin Cancer Res. 2014;20:1856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz-Lagares A, Mendez-Gonzalez J, Hervas D, Saigi M, Pajares MJ, Garcia D, et al. A Novel Epigenetic Signature for Early Diagnosis in Lung Cancer. Clin Cancer Res. 2016;22:3361–71. [DOI] [PubMed] [Google Scholar]
- 24.Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-small cell lung cancer, version 1.2015. J Natl Compr Canc Netw. 2014;12:1738–61. [DOI] [PubMed] [Google Scholar]
- 25.Belinsky SA, Klinge DM, Dekker JD, Smith MW, Bocklage TJ, Gilliland FD, et al. Gene promoter methylation in plasma and sputum increases with lung cancer risk. Clin Cancer Res. 2005;11:6505–11. [DOI] [PubMed] [Google Scholar]
- 26.Prindiville SA, Byers T, Hirsch FR, Franklin WA, Miller YE, Vu KO, et al. Sputum cytological atypia as a predictor of incident lung cancer in a cohort of heavy smokers with airflow obstruction. Cancer Epidemiol Biomarkers Prev. 2003;12:987–93. [PubMed] [Google Scholar]
- 27.Keeley B, Stark A, Pisanic TR 2nd, Kwak R, Zhang Y, Wrangle J, et al. Extraction and processing of circulating DNA from large sample volumes using methylation on beads for the detection of rare epigenetic events. Clin Chim Acta. 2013;425:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genome Bioinformatics Group of UC Santa Cruz. UCSC Genome Bioinformatics. 2015. [cited; Available from:http://genome.uscs.edu
- 29.Brandes JC, Carraway H, Herman JG. Optimal primer design using the novel primer design program: MSPprimer provides accurate methylation analysis of the ATM promoter. Oncogene. 2007;26:6229–37. [DOI] [PubMed] [Google Scholar]
- 30.Untergrasser ACI, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG Primer3web. 2012. [cited; Available from: http://primer3.ut.ee/ [DOI] [PMC free article] [PubMed]
- 31.Team RC. R: A language and environment for statistical computing R-Project Org, Version 3.0.2 ed. Vienna Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 32.Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, et al. The case for early detection. Nat Rev Cancer. 2003;3:243–52. [DOI] [PubMed] [Google Scholar]
- 33.Belinsky SA. Gene-promoter hypermethylation as a biomarker in lung cancer. Nat Rev Cancer. 2004;4:707–17. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy TC, Proudfoot SP, Franklin WA, Merrick TA, Saccomanno G, Corkill ME, et al. Cytopathological analysis of sputum in patients with airflow obstruction and significant smoking histories. Cancer Res. 1996;56:4673–8. [PubMed] [Google Scholar]
- 35.Kennedy TC, Proudfoot SP, Piantadosi S, Wu L, Saccomanno G, Petty TL, et al. Efficacy of two sputum collection techniques in patients with air flow obstruction. Acta Cytol. 1999;43:630–6. [DOI] [PubMed] [Google Scholar]
- 36.Ahrendt SA, Chow JT, Xu LH, Yang SC, Eisenberger CF, Esteller M, et al. Molecular detection of tumor cells in bronchoalveolar lavage fluid from patients with early stage lung cancer. Journal of the National Cancer Institute. 1999;91:332–9. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez-Cespedes M, Esteller M, Wu L, Nawroz-Danish H, Yoo GH, Koch WM, et al. Gene promoter hypermethylation in tumors and serum of head and neck cancer patients. Cancer Res. 2000;60:892–5. [PubMed] [Google Scholar]
- 38.Silvestri GA, Vachani A, Whitney D, Elashoff M, Porta Smith K, Ferguson JS, et al. A Bronchial Genomic Classifier for the Diagnostic Evaluation of Lung Cancer. N Engl J Med. 2015;373:243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.