Abstract
Objectives:
Leptin is a hormone produced by adipose tissue that promotes satiety, and some evidence suggests that greater early life leptin exposure prevents excessive adiposity gain in later life. However, few studies have analyzed dynamic changes in leptin throughout childhood in relation to later cardio-metabolic health. Our study aims to identify distinct leptin trajectories in childhood, and to examine their associations with cardio-metabolic outcomes in adolescence.
Methods:
Among children in the Project Viva cohort born 1999–2002 in Massachusetts, we used latent class growth models to identify leptin trajectories independent of maternal BMI, child sex, race/ethnicity, size at birth and current age and size among 1360 children with leptin measured at least once at birth, early childhood (mean 3.3±SD 0.3 years), or mid-childhood (7.9±0.8 years). At research visits in early adolescence (13.2±0.9 years), we assessed cardio-metabolic outcomes including adiposity measures, fasting biomarkers, and blood pressure among 855 children. We then applied multiple regression models to examine associations of the leptin trajectories with these cardio-metabolic outcomes in early adolescence, adjusting for child age at outcome, maternal age, education, prenatal smoking and glucose,, total gestational weight gain and paternal BMI.
Results:
The latent class growth model identified 3 distinct leptin trajectories: “low stable” (n=1031, 75.8%), “high-decreasing” (n=219, 16.1%) and “intermediate-increasing” (n=110, 8.1%). In adjusted models, the intermediate-increasing leptin trajectory was associated with higher early adolescence adiposity measures (e.g. BMI z-score: 0.62 units; 95% confidence interval: 0.28, 0.96 and odds of obesity: 2.84: 1.17, 6.94), but lower systolic blood pressure (−0.46 z-score units; −0.74, −0.18), compared to the low-stable group.
Conclusions:
Our findings on leptin trajectories in childhood suggest important differences and associations with later metabolic outcomes.
Keywords: Leptin trajectories, childhood, early adolescence, cardio-metabolic risks, childhood obesity
INTRODUCTION
Leptin, a hormone synthesized primarily in adipose tissue, acts on the hypothalamus to regulate satiety and long-term energy balance.1, 2 Plasma leptin levels correlate positively with adiposity in newborns, children and adults.3–5 Higher plasma leptin is associated with obesity-related inflammation biomarkers not only in adipose tissue but also in the circulatory system.6–8 However, few studies have examined the extent to which early life leptin levels predict later cardio-metabolic risk markers including adiposity, blood pressure and biomarkers of insulin resistance.
We have previously reported that higher maternal and cord blood leptin were associated with lower offspring adiposity from early childhood to early adolescence.3, 5 However, higher plasma leptin in early childhood was associated with greater adiposity in later childhood in our cohort as well as in others.3, 9–11 These studies however, only analyzed the associations of leptin measured at a certain (static) time point with either current or subsequent adiposity. Since leptin reflects real-time adiposity accumulation and is associated with subsequent adiposity, the dynamic changes in leptin levels throughout early life (from birth to mid-childhood) independent of BMI, may predict subsequent cardio-metabolic outcomes in adolescence.
Individual children may exhibit distinct leptin trajectories, which may differentially influence the development of adverse cardio-metabolic outcomes later in life. Two cohorts—the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) (n=80) and the Europe Childhood Obesity Project (CHOP) (n=459)—have described longitudinal changes of leptin in children.10, 12 These studies both measured leptin since birth or infancy and followed the children until mid-childhood (8–9 years). Both studies reported three leptin trajectories based on unadjusted leptin values, and found adiposity measures across early and mid-childhood (e.g. higher BMI and waist circumference) distinguished different courses of leptin trajectories.10 However, these two studies used different trajectory plotting methods and did not account for maternal and offspring major determinants in their leptin trajectories, which makes the distinctive patterns inconsistent with each other.
Recent progress in statistical techniques such as latent class growth modeling (LCGM) make it possible to study the potential heterogeneity in longitudinal changes of leptin in childhood.13 LCGM is a semi-parametric statistical technique that is used to analyze longitudinal data based on structural equation modelling,14 and it assumes that individuals in the sample need not come from a single underlying population, but rather from multiple, latent (unobserved) subgroups. Therefore, in our Project Viva cohort, we aimed to: 1) develop our leptin trajectories using the LCGM method, after standardization for major maternal and fetal determinants; 2) investigate the predictive values for comprehensive cardio-metabolic outcomes at early adolescence with different courses of leptin trajectories. We hypothesized that distinct leptin trajectories from birth to mid-childhood would be predictive of early adolescent adiposity, blood pressure, and biomarkers of cardio-metabolic risk.
MATERIALS AND METHODS
Study population
Project Viva is an observational, prospective birth cohort that recruited pregnant women at in-person visits during the first trimester of pregnancy from 8 obstetric offices of Atrius Harvard Vanguard Medical Associations, a multi-site group practice in Eastern Massachusetts since 1999.15 The study methodology has previously been published.15 We conducted follow-up in-person research visits at late 2nd trimester (26–28 weeks gestation), delivery, early childhood (mean 3.3±standard deviation 0.3 years), mid-childhood (7.9±0.8 years), and early adolescence (13.2±0.9 years).
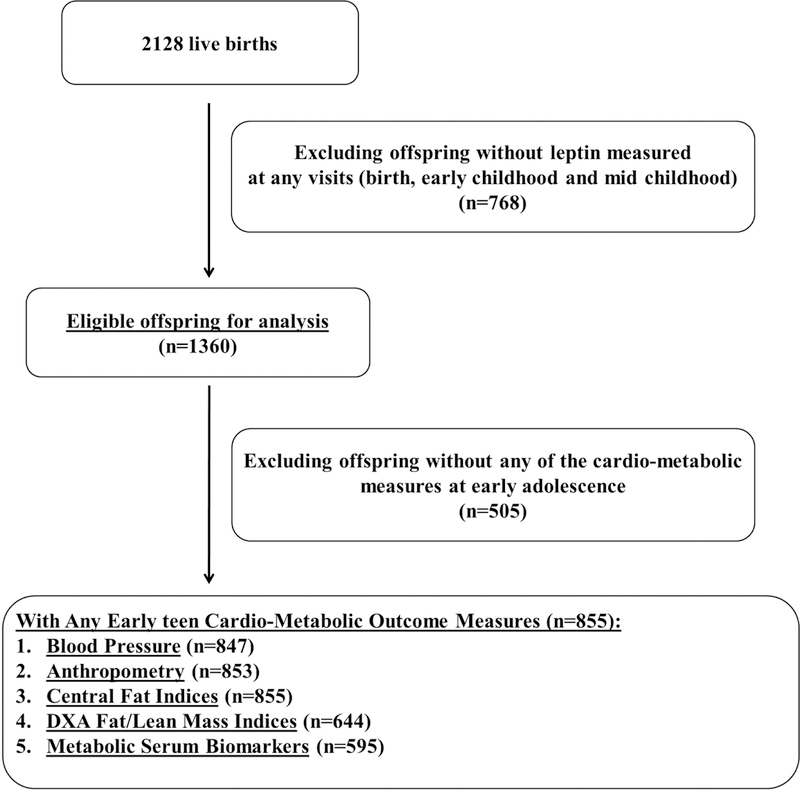
Among 2128 live births, we excluded 768 without leptin measured at birth, early childhood or mid-childhood. Thus, 1360 eligible offspring were included for leptin trajectory analysis. For each subsequent follow-up visit, outcomes varied based on different sample sizes for adiposity (n=644–855), blood pressure (n=847), and plasma biomarker (n=595) outcomes, as shown in Figure 1.
Figure 1.
Study recruitment and follow-up flow from birth to early adolescent in Project Viva.
Mothers provided written informed consent at enrollment and each postnatal follow-up visit, and children proved verbal assent at the early adolescent visit. The institutional review board of Harvard Pilgrim Health Care approved the project, which we conducted in line with ethical standards established by Declaration of Helsinki.
Exposure - Offspring Leptin Measures
We collected cord blood at birth and offspring blood at early childhood and mid-childhood via venipuncture, and immediately refrigerated whole blood samples and spun them within 24 hours according to a standard protocol.3, 5 Plasma leptin was measured using an immunoassay (Linco Research Inc, St Charles, MO) as described elsewhere.3, 5
Outcomes - Cardio-Metabolic Measures at Early Adolescence
Adiposity Measures
Trained research assistants measured standing height and weight according to standardized protocols.3, 5, 15, 16 We calculated BMI as weight divided by height squared (kg/m2). We further calculated age- and sex-specific height and BMI z-scores using Centers for Disease Control and Prevention growth charts.17 We defined children as being obese if their BMI was above the 95th percentile for their age and sex.18 Waist and hip circumferences were measured to the nearest 0.1 mm using a Hoechstmass measuring tape (Hoechstmass Balzer GmbH, Sulzbach, Germany). Subscapular (SS) and triceps (TR) skinfolds (mm) were measured to the nearest 0.2 mm with Holtain Tanner Skinfold Calipers (Holtain Limited, London, UK).3 We calculated waist-to-hip ratio, and sum (SS+TR) and ratio (SS/TR) of the two skinfolds. We performed whole body DXA scans with a Hologic Model Discovery A fan-beam scanner (Hologic, Bedford, Massachusetts). A single trained investigator assessed fat mass index, lean mass index and trunk fat index according to a standardized protocol.3, 15
Blood Pressure—
Trained research assistants measured blood pressure (BP) using biannually calibrated automated oscillometric monitors (The Omron HEM-907XL, Illinois, US) and appropriate cuff sizes on the child’s upper arm at 1-minute intervals 5 times.19 We included the mean of the 5 measurements for both systolic (SBP) and diastolic blood pressure (DBP) to improve precision when quantifying individual BP variability.19 We further calculated age-, sex- and height specific SBP and DBP percentiles and z-scores according to the NHANES 2004 BP reference for children and adolescents.20 We defined children as having pre-hypertension (HTN)/HTN if SBP or DBP was ≧90th percentile according to the same reference.20
Plasma Biomarkers and Metabolic Score—
Trained technicians collected fasting blood specimens. We centrifuged all samples within 24 hours and stored plasma aliquots at −80°C. Fasting glucose, insulin, c-peptide, high-density lipoprotein (HDL), total cholesterol, triglycerides, alanine aminotransferase (ALT) and C-reactive protein (CRP) were all measured according to standard protocols.21 We calculated insulin resistance using the homeostasis model assessment (HOMA-IR),22 and metabolic risk score using z scores internally standardized for age and sex for waist circumference, SBP, triglycerides, HDL and HOMA-IR, as reported elsewhere.23
Covariates
Antenatal Maternal Characteristics—
At recruitment, mothers reported their age, race/ethnicity, pre-pregnancy weight, height, smoking history (smoking during pregnancy, past smoker and never smoker), personal history of chronic systemic disease including diabetes and HTN, highest education (which we categorized as lower than college degree and college degree and above), exercise during pregnancy, and father’s weight and height via questionnaires and interviews. We used measured weights recorded in the outpatient medical records and self-reported pre-pregnancy weight to calculate total gestational weight gain (GWG). Clinicians assessed maternal non-fasting blood sample for oral glucose challenge test (GCT), in which venous blood was sampled 1 hour after a 50 gram oral glucose load.15 At the same time point, we collected additional blood specimens, which we assayed for maternal leptin and adiponectin as described above.
Offspring Characteristics from Birth to Mid-Childhood—
We extracted birth weight and delivery date from hospital medical records and calculated birth weight for gestational age z-score using national reference data from the United States Natality database.18 Mothers reported their child’s race/ethnicity, which we categorized as white, black, Hispanic, Asian or others. Trained research assistants measured height and at early and mid-childhood and we calculated BMI and BMI z-score at each time point.
Statistical Analysis
We first plotted child leptin trajectories in two steps.
Step 1: Calculation of leptin residuals—
We estimated variation in leptin that was attributable to major determinants (e.g. maternal pre-pregnancy BMI, birth weight, gestational age, offspring sex, offspring BMI) of offspring leptin based on previous publications.3, 5, 12, 18, 24–26 We also considered, but did not include, gestational weight gain, maternal smoking during pregnancy, and route of delivery, because these characteristics were not related to leptin across childhood. In separate models, we regressed child leptin measured at each time point on maternal pre-pregnancy BMI, and child sex, race/ethnicity and birth weight for gestational age z-score for all visits (at birth, early and mid-childhood), and additionally child age and BMI at early and mid-childhood visits, based on associations of these factors with leptin levels in our dataset (Table S1). We used the residuals from these models to derive the leptin trajectories, based on the example of nutritional epidemiology.36 We describe the characteristics of recruited and non-recruited subjects of data completion, according to leptin trajectories and cardio-metabolic outcomes at early adolescence, in Table S2.
We also generated crude leptin trajectories, i.e. without adjusting for maternal characteristics or child BMI (Figure S1). However, since BMI is a strong driver of both leptin and our subsequent outcomes, and we were primarily interested in effects of leptin levels independent of BMI, we were concerned that these trajectories were substantially confounded by BMI. Therefore we used the residual approach as descried above in our subsequent analyses.
Step 2: latent class growth model—
We applied a latent class growth model (LCGM)13 to the leptin residuals to identify leptin trajectories that followed a similar pattern of change over time from birth to mid-childhood. LCGM is a semi-parametric statistical technique that is used to analyze longitudinal data based on structural equation modelling,14 and it assumes that individuals in the sample need not come from a single underlying population, but rather from multiple, latent (unobserved) subgroups. The aim of the LCGM is to identify distinct trajectory patterns with the appropriate polynomial order that represents the heterogeneity in trajectories. The number of groups and orders of the polynomials were determined by the Bayesian Information Criterion (BIC). A further criterion is that each trajectory should include at least 5% of all participants, so that it would be large enough to be clinically important.13, 27 Quadratic-shaped trajectories were fitted to allow for curved developmental patterns. We searched for the optimal number of groups using a forward classifying approach, which starts with a one-class solution and then adds further classes until both criterions were met. We coded distinct trajectories as a categorical variable (with k number of categories) and named them based on their visual appearance.
Finally, we performed multiple regression analyses to study the association of leptin trajectories from birth to mid-childhood and cardio-metabolic outcomes at early adolescence. We adjusted models as follows, based on prior publications: Model 1—adjusted for child age at the early adolescent visit; Model 2—Model 1 and additionally adjusted for maternal age at enrollment, education, smoking, glucose levels at 2nd trimester GCT, and total GWG as well as paternal BMI. Correlations among these covariates were generally low and so we did not have concern for collinearity. Because we had already adjusted for maternal pre-pregnancy BMI, child sex, child race/ethnicity, birth weight for gestational age z-score and subsequent child age and size in determining leptin trajectories, we did not also include these variables in our final models. We analyzed adiposity, blood pressure, and biomarker outcomes as continuous measures using linear regression. We used logistic regression for dichotomous outcomes, and thus calculated odds ratios (OR) for childhood pre-HTN/HTN (vs. normal BP) and childhood obesity (BMI >95th vs. <=95th percentile) for each distinct leptin trajectory. Although we performed separate models for each of our outcomes, we did not explicitly account for multiple testing as the correlation between leptin and cardio-metabolic risks have been well established32–35 and there are high correlations among our early adolescence cardio-metabolic outcomes. Rather, we have focused on patterns of associations consistent within each other outcomes and were conservative about isolated significant findings.
We also performed sensitivity analyses by including additional covariates such as maternal pre-pregnancy diabetes, pre-pregnancy hypertension, gestational hypertensive disorders, parity, 2nd trimester leptin, 2nd trimester total energy intake and adiponectin, household income, child pre-term status as well as changes in BMI between different time points (birth, early- and mid-childhood) in the final adjusted model. We further tested maternal gestational diabetes as a potential effect modifier in our final model, as previous studies have shown that gestational diabetes links with both cord leptin level28, 29 and infant and childhood adiposity.30, 31
To illustrate the robustness of the trajectory patterns, we repeated the analyses and conducted kappa agreement tests by using: 1. Children without missing leptin values at any of the visits (n=182) or missing only one leptin value (n=641); 2. Only adjusted for same set of variables at each timepoint (i.e. maternal pre-pregnancy BMI, child sex, child race/ethnicity and birth weight for gestational age z score, but not current child age or BMI at leptin measurement). We performed statistical analyses using SAS (version 9.4, SAS Institute, Cary NC, US) and STATA (version 11.1, STATA Corp, Texas, US).
RESULTS
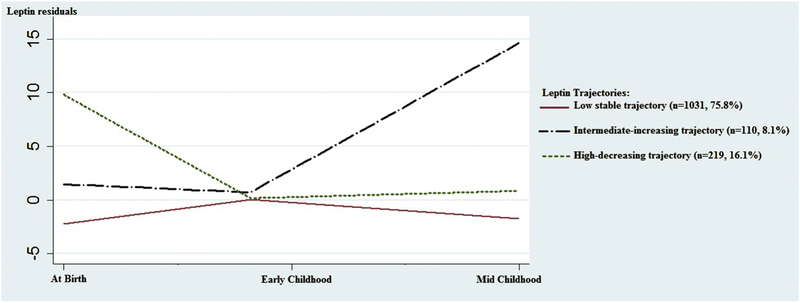
Among the 1360 offspring with at least one leptin measured at birth, early childhood and mid-childhood visits, we identified three distinct leptin trajectories using the LCGM approach, which we named a “low-stable” group (n=1031, 75.8%), a “high-decreasing” group (n=219, 16.1%) and an “intermediate-increasing” group (n=110, 8.1%) (Figure 2). Descriptive data of age and leptin residuals, and comparisons among residual leptin values at each time point are shown in Table S3. In sensitivity tests, leptin trajectories did not substantively change when we limited our sample size to offspring with leptin measured at all three visits (n=182) or at any two of the three visits (n=641) or calculate leptin residuals using the same set of variables at birth, early childhood and mid-childhood visits (Figures S2-4; Tables S4–6).
Figure 2. Leptin trajectories (n=1360).
We identified three distinct leptin trajectories using the LCGM approach: a “low-stable” group (n=1031, 75.8%) in red solid line, a “high-decreasing” group (n=219, 16.1%) in black dotted line and an “intermediate-increasing” group (n=110, 8.1%) in a green dotted line, among 1360 offspring with at least one leptin measured at birth, early childhood and mid-childhood visits.
Table 1 shows the distributions of maternal and offspring characteristics, and offspring cardio-metabolic measures at early adolescence for each leptin trajectory. Since maternal pre-pregnancy BMI and birth weight for gestational age z-score were included during the generation of residuals, these variables did not differ across the three leptin trajectories. Maternal GCT result and total GWG showed similar means and standard deviation (SD) in each leptin trajectory. Offspring SBP z-score was similar between the low-stable and high-decreasing group, and lowest in the intermediate-increasing group. Childhood pre-HTN/HTN was less prevalent in intermediate-increasing group, while childhood obesity was more prevalent in the same group. Among the three leptin trajectories, most of the adiposity measures and biomarkers seemed to have the greatest values in intermediate-increasing group, while HDL-cholesterol was lowest in this group.
Table 1.
Maternal and Offspring Characteristics and Cardio-Metabolic Measures in Early Adolescence in Project Viva (n=855)
| Leptin trajectories |
||||
|---|---|---|---|---|
| Characteristics | Total (n=855) | Low–stable (n=732) |
High–decreasing (n=87) |
Intermediate–increasing (n=36) |
| mean (SD) or n (%) |
mean (SD) or n (%) |
mean (SD) or n (%) |
mean (SD) or n (%) |
|
| Perinatal characteristics | ||||
| Maternal | ||||
| Age at enrollment, years | 32.4 (5.1) | 32.4 (5.1) | 32.7 (4.6) | 30.9 (6.1) |
| Pre–pregnancy BMI, kg/m2 | 24.8 (5.2) | 24.8 (5.1) | 25.1 (5.6) | 25.6 (5.4) |
| Smoking status, % | ||||
| . Never | 600 (70.3) | 511 (70.0) | 63 (72.4) | 26 (72.2) |
| . Former | 178 (20.9) | 154 (21.1) | 17 (19.5) | 7 (19.4) |
| . During pregnancy | 75 (8.8) | 65 (8.9) | 7 (8.0) | 3 (8.3) |
| > College Degree, % | ||||
| . No | 255 (29.9) | 216 (29.6) | 24 (27.6) | 15 (41.7) |
| . Yes | 598 (70.1) | 514 (70.4) | 63 (72.4) | 21 (58.3) |
| 2nd tri–glucose, mg/dl | 113.2 (27.5) | 113.0 (27.7) | 116.1 (26.3) | 110.8 (27.1) |
| Total GWG, kg | 15.4 (5.3) | 15.4 (5.2) | 14.8 (5.5) | 16.3 (6.2) |
| Maternal race/ethnicity, % | ||||
| . Black | 130 (15.2) | 108 (14.8) | 14 (16.1) | 8 (22.2) |
| . Hispanic | 53 (6.2) | 47 (6.4) | 3 (3.4) | 3 (8.3) |
| . Asian | 39 (4.6) | 36 (4.9) | 1 (1.1) | 2 (5.6) |
| . White | 590 (69.2) | 505 (69.2) | 66 (75.9) | 19 (52.8) |
| . Other | 41 (4.8) | 34 (4.7) | 3 (3.4) | 4 (11.1) |
| Paternal BMI, kg/m2 | 26.5 (4.0) | 26.4 (3.9) | 26.8 (5.0) | 26.8 (3.7) |
| Offspring Characteristics | ||||
| At birth | ||||
| BW/GA z–score | 0.23 (0.96) | 0.20 (0.94) | 0.54 (1.00) | 0.14 (1.04) |
| Gestational age, weeks | 39.6 (1.7) | 39.5 (1.7) | 39.9 (1.4) | 40.0 (1.1) |
| Female, % | ||||
| . No | 432 (50.5) | 381 (52.0) | 38 (43.7) | 13 (36.1) |
| . Yes | 423 (49.5) | 351 (48.0) | 49 (56.3) | 23 (63.9) |
| Child race/ethnicity, % | ||||
| . Black | 135 (15.8) | 113 (15.5) | 13 (14.9) | 9 (25.0) |
| . Hispanic | 39 (4.6) | 33 (4.5) | 3 (3.4) | 3 (8.3) |
| . Asian | 24 (2.8) | 21 (2.9) | 1 (1.1) | 2 (5.6) |
| . White | 556 (65.1) | 473 (64.7) | 64 (73.6) | 19 (52.8) |
| . Other | 100 (11.7) | 91 (12.4) | 6 (6.9) | 3 (8.3) |
| Leptina, ng/dl | 7.4 | 6.0 | 19.1 | 7.7 |
| (3.8–12.3) | (3.3, 9.1) | (16.3–22.1) | (4.6–11.4) | |
| At early childhood | ||||
| Age, years | 3.3 (0.3) | 3.2 (0.3) | 3.2 (0.3) | 3.3 (0.6) |
| Waist circumference, cm | 51.3 (3.6) | 51.2 (3.5) | 50.9 (3.3) | 52.4 (6.1) |
| BMI z–score | 0.45 (0.99) | 0.46 (0.98) | 0.26 (1.01) | 0.62 (0.95) |
| Leptina, ng/dl | 1.4 | 1.4 | 1.4 | 2.2 |
| (1.0–2.3) | (0.9–2.2) | (1.1–2.1) | (1.3, 3.0) | |
| At mid–childhood | ||||
| Age, years | 7.9 (0.8) | 7.9 (0.8) | 8.0 (0.9) | 8.1 (1.0) |
| Waist circumference, cm | 59.9 (8.5) | 59.4 (8.0) | 60.5 (9.5) | 67.7 (11.3) |
| BMI z–score | 0.40 (1.01) | 0.37 (0.98) | 0.31 (1.02) | 1.12 (1.12) |
| Leptina, ng/dl | 3.2 | 2.9 | 5.1 | 23.9 |
| (2.2–5.9) | (2.1–4.7) | (2.8–12.1) | (20.0–30.7) | |
| At early–adolescence | ||||
| Growth and Adiposity measures | ||||
| Height z–score | 0.37 (1.02) | 0.38 (1.04) | 0.24 (0.89) | 0.62 (0.86) |
| BMI z–score | 0.38 (1.08) | 0.35 (1.07) | 0.33 (1.13) | 1.09 (0.96) |
| Obesity (>95th %tile), % | ||||
| . No | 740 (86.8) | 642 (87.8) | 73 (84.9) | 25 (69.4) |
| . Yes | 113 (13.2) | 89 (12.2) | 13 (15.1) | 11 (30.6) |
| Waist circumference, cm | 73.1 (12.0) | 72.7 (11.6) | 73.0 (13.2) | 81.2 (14.6) |
| Hip circumference, cm | 88.8 (10.8) | 88.5 (10.3) | 87.9 (13.0) | 97.2 (11.4) |
| SS+TR skinfolds, mm | 28.3 (13.9) | 27.6 (13.1) | 29.9 (16.1) | 38.8 (19.0) |
| DXA_total fat index, kg/m2 | 6.3 (3.1) | 6.2 (2.9) | 6.8 (3.5) | 9.2 (4.7) |
| DXA_total lean index, kg/m2 | 14.9 (2.1) | 14.9 (2.1) | 14.7 (2.0) | 16.2 (2.3) |
| DXA_trunk fat mass, kg/m2 | 2.4 (1.5) | 2.3 (1.4) | 2.6 (1.6) | 3.8 (2.3) |
| Blood pressure measures | ||||
| SBP z–score | −0.17 (0.81) | −0.15 (0.81) | −0.15 (0.82) | −0.56 (0.69) |
| DBP z–score | −0.17 (0.64) | −0.18 (0.64) | −0.12 (0.65) | −0.24 (0.58) |
| HTN or pre–HTN, % | ||||
| Yes | 83 (9.8) | 70 (9.6) | 11 (12.8) | 2 (5.6) |
| Plasma biomarkers | ||||
| Fasting glucose, mg/dl | 93.0 (24.0) | 93.0 (25.5) | 91.5 (13.0) | 95.1 (10.8) |
| Insulin, uU/mL | 16.3 (17.9) | 15.7 (18.4) | 17.4 (11.6) | 23.3 (17.9) |
| HOMA–IR | 3.4 (3.9) | 3.3 (4.0) | 3.7 (2.5) | 5.4 (4.9) |
| C–peptide, ng/ml | 2.2 (1.1) | 2.1 (1.1) | 2.3 (0.9) | 2.8 (1.4) |
| HDL, mg/dl | 55.5 (13.5) | 56.0 (13.5) | 54.7 (13.0) | 48.9 (12.8) |
| Total cholesterol, mg/dl | 156.2 (28.3) | 156.4 (28.1) | 158.1 (30.6) | 150.4 (27.2) |
| Triglyceride, mg/dl | 70.4 (31.5) | 69.7 (31.1) | 75.0 (34.4) | 73.2 (32.7) |
| ALT, u/l | 18.8 (9.2) | 18.7 (7.6) | 18.1 (6.3) | 21.4 (26.2) |
| hsCRP, mg/L | 0.9 (1.9) | 0.9 (1.9) | 0.7 (0.9) | 1.8 (3.1) |
| Leptina, ng/dl | 6.7 (3.1–16.2) |
6.0 (2.9–14.8) |
9.4 (3.7–20.6) |
16.5 (9.0–50.2) |
| Metabolic risk scoreb, units | −0.00 (0.63) | −0.03 (0.62) | 0.08 (0.58) | 0.34 (0.70) |
Abbreviation: SBP, systolic blood pressure; DBP, diastolic blood pressure, HTN, hypertension.
BMI, body mass index; SS+TR, sum of subscapular and triceps skinfold thicknesses; HDL,high-density lipoprotein; ALT, alanine aminotransferase; hsCRP, high sensitivity C-reactive protein.
Median [IQR] are shown for leptin value measured at early adolescence.
Metabolic risk score using z scores internally standardized for age and sex for waist circumference, SBP, triglycerides, HDL and HOMA-IR.
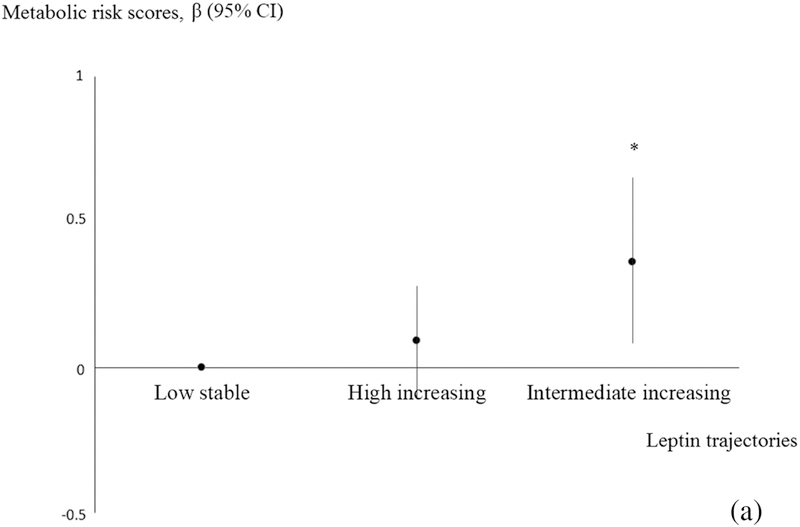
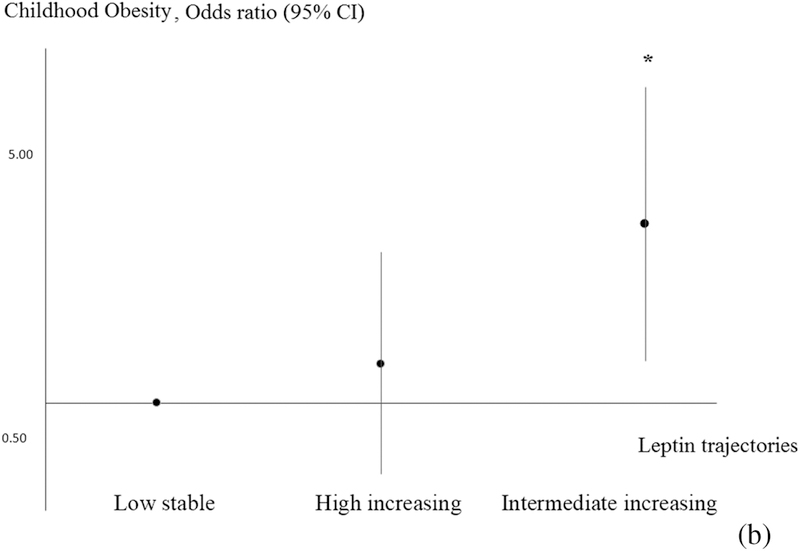
Associations of offspring leptin trajectories with cardio-metabolic outcomes in early adolescence are summarized in Table 2. In analyses adjusted for age at outcome, leptin trajectories predicted many cardio-metabolic outcomes in early adolescence. After adjusting for potential confounders, associations were either modestly attenuated or did not appreciably change. The intermediate-increasing leptin trajectory was associated with lower SBP z-score (−0.46 units; 95% CI: −0.74, −0.18), but otherwise higher BMI z-score (0.62 units; 0.28, 0.96), higher waist circumference (7.28 cm; 3.47, 11.09), higher SS+TR (9.37 mm, 4.92, 13.82), higher total fat index (2.71 kg/m2; 1.62, 3.80), higher insulin level (2.23 IU; 1.26 3.20), higher CRP (0.94 mg/L; 0.17, 1.71), higher ALT (3.68 u/L; 0.07, 7.29) and greater global metabolic risk scores (0.33 units; 0.08, 0.57) (Figure 3) as well as lower HDL (−6.25 mg/dl; −11.57, −0.99), compared with the low-stable trajectory. Offspring in the intermediate-increasing trajectory also had higher odds (OR 2.84; 95% CI: 1.17, 6.94) of obesity at early adolescence compared with offspring in the low stable trajectory (Figure 4). Interestingly, both the intermediate-increasing (16.37 ng/ml, 95% CI: 11.26, 21.49) and high-decreasing (6.10, 95% CI: 2.35, 9.85) trajectories were associated with higher leptin level at early adolescence, although the association was stronger for the intermediate-increasing group.
Table 2.
Multivariable associations of early life leptin trajectories with cardio-metabolic outcomes in early adolescence (n=855)
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Early adolescent cardio–metabolic measures | Low–stable | High–decreasing | Intermediate–increasing | Low–stable | High–decreasing | Intermediate–increasing |
| (n=732) | (n=87) | (n=36) | (n=732) | (n=87) | (n=36) | |
| β (95% CI) | β (95% CI) | |||||
| Growth and adiposity measures | ||||||
| Height z–score | 0.0 (ref) | −0.13 (−0.36, 0.10) | 0.24 (−0.10, 0.58) | 0.0 (ref) | −0.15 (−0.38, 0.09) | 0.14 (−0.21, 0.49) |
| BMI z–score | 0.0 (ref) | −0.02 (−0.26, 0.22) | 0.75 (0.39, 1.10) | 0.0 (ref) | −0.01 (−0.24, 0.22) | 0.62 (0.28, 0.96) |
| Waist circumference, cm | 0.0 (ref) | −0.15 (−2.78, 2.48) | 8.37 (4.42,12.32) | 0.0 (ref) | 0.06 (−2.46, 2.58) | 7.28 (3.47,11.08) |
| Hip circumference, cm | 0.0 (ref) | −1.26 (−3.54, 1.03) | 8.61 (5.18,12.04) | 0.0 (ref) | −1.13 (−3.32, 1.07) | 7.49 (4.18,10.80) |
| SS+TR skinfolds, mm | 0.0 (ref) | 2.18 (−0.90, 5.25) | 11.21 (6.61,15.81) | 0.0 (ref) | 2.53 (−0.42, 5.47) | 9.36 (4.91,13.81) |
| SS:TR ratio | 0.0 (ref) | 0.02 (−0.03, 0.08) | 0.15 (0.07, 0.23) | 0.0 (ref) | 0.03 (−0.02, 0.08) | 0.14 (0.06, 0.21) |
| DXA total fat, % | 0.0 (ref) | 1.91 (−0.09, 3.91) | 6.08 (3.30, 8.86) | 0.0 (ref) | 2.16 (0.20, 4.12) | 5.52 (2.81, 8.24) |
| DXA total fat index, kg/m2 | 0.0 (ref) | 0.64 (−0.19, 1.46) | 3.04 (1.90, 4.19) | 0.0 (ref) | 0.70 (−0.08, 1.49) | 2.71 (1.62, 3.80) |
| DXA total lean index, kg/m2 | 0.0 (ref) | −0.15 (−0.69, 0.39) | 1.34 (0.59, 2.09) | 0.0 (ref) | −0.16 (−0.68, 0.37) | 1.03 (0.30, 1.76) |
| DXA_trunk fat mass, kg/m2 | 0.0 (ref) | 0.27 (−0.12, 0.66) | 1.44 (0.89, 1.98) | 0.0 (ref) | 0.30 (−0.07, 0.67) | 1.26 (0.74, 1.78) |
| Blood pressure measures | ||||||
| SBP z–score | 0.0 (ref) | 0.00 (−0.18, 0.18) | −0.41 (−0.68,−0.14) | 0.0 (ref) | 0.01 (−0.18, 0.20) | −0.46 (−0.74,−0.18) |
| DBP z–score | 0.0 (ref) | 0.05 (−0.09, 0.20) | −0.07 (−0.28, 0.15) | 0.0 (ref) | 0.04 (−0.11, 0.19) | −0.09 (−0.31, 0.14) |
| Serum biomarkers | ||||||
| Fasting glucose, mg/dl | 0.0 (ref) | −2.68 (−9.64, 4.27) | 2.36 (−6.86,11.58) | 0.0 (ref) | −1.83 (−6.59, 2.92) | 3.34 (−3.10, 9.77) |
| Insulin, uU/mL | 0.0 (ref) | 1.13 (−3.79, 6.05) | 7.51 (0.75,14.27) | 0.0 (ref) | 1.93 (−2.17, 6.02) | 7.11 (1.48,12.75) |
| HOMA–IR | 0.0 (ref) | 0.33 (−0.83, 1.49) | 2.17 (0.59, 3.75) | 0.0 (ref) | 0.61 (−0.09, 1.30) | 2.23 (1.26, 3.20) |
| C−peptide, ng/ml | 0.0 (ref) | 0.12 (−0.18, 0.42) | 0.72 (0.31, 1.13) | 0.0 (ref) | 0.17 (−0.12, 0.46) | 0.68 (0.27, 1.08) |
| HDL, mg/dl | 0.0 (ref) | −0.80 (−4.49, 2.89) | −7.00 (−12.1, −1.93) | 0.0 (ref) | −0.25 (−4.08, 3.58) | −6.22 (−11.5, −0.96) |
| Total cholesterol, mg/dl | 0.0 (ref) | 2.68 (−5.12,10.48) | −5.84 (−16.6, 4.88) | 0.0 (ref) | 5.25 (−2.84,13.34) | −4.17 (−15.3, 6.96) |
| Triglyceride, mg/dl | 0.0 (ref) | 5.18 (−3.57,13.93) | 3.44 (−8.58,15.46) | 0.0 (ref) | 6.76 (−2.21,15.73) | 5.54 (−6.80,17.88) |
| ALT, u/l | 0.0 (ref) | −0.37 (−2.91, 2.17) | 2.75 (−0.74, 6.24) | 0.0 (ref) | −0.11 (−2.74, 2.51) | 3.68 (0.07, 7.28) |
| hsCRP, mg/L | 0.0 (ref) | −0.19 (−0.73, 0.35) | 0.91 (0.17, 1.65) | 0.0 (ref) | −0.14 (−0.69, 0.41) | 0.94 (0.18, 1.71) |
| Leptinǂ, ng/dl | 0.0 (ref) | 5.04 (1.31, 8.76) | 16.66 (11.54,21.78) | 0.0 (ref) | 5.99 (2.28, 9.70) | 16.37 (11.27,21.47) |
| Global metabolic risk | ||||||
| Metabolic risk scorea, units | 0.0 (ref) | 0.09 (−0.09, 0.27) | 0.37 (0.12, 0.62) | 0.0 (ref) | 0.12 (−0.06, 0.29) | 0.33 (0.08, 0.57) |
| OR (95% CI) | OR (95% CI) | |||||
| Obesity | 1.0 (ref) | 1.28 (0.68, 2.40) | 3.17 (1.51, 6.67) | 1.0 (ref) | 1.30 (0.63, 2.68) | 2.84 (1.17, 6.94) |
| Pre–HTN/HTN | 1.0 (ref) | 1.27 (0.64, 2.53) | 0.55 (0.13, 2.35) | 1.0 (ref) | 1.34 (0.66, 2.71) | 0.31 (0.04, 2.31) |
Abbreviation: OR, odds ratio; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; Circum-, circumference; HDL, high-density lipoprotein; ALT, alanine aminotransferase; hsCRP, C-reactive protein.
Model 1, adjusted for child age at early adolescent visit.
Model 2, Model 1 + maternal age at enrollment, college degree, smoking during pregnancy, 2nd trimester glucose, and total gestational weight gain and paternal BMI.
Metabolic risk score using z scores internally standardized for age and sex for waist circumference, SBP, triglycerides, HDL and HOMA-IR.
Figure 3.
Estimates of associations between distinctive leptin trajectories and metabolic risk scores.
Figure 4.
Estimates of associations between distinctive leptin trajectories and odds of obesity.
Additional adjustment for maternal pre-gestational chronic hypertension, type 1 or type 2 diabetes mellitus, gestational hypertensive disorders, maternal total energy intake, total maternal physical activity during pregnancy, parity, pre-term birth or changes in BMI between different time points did not appreciably change the observed associations. We did not find a significant interaction between leptin trajectories and maternal gestational diabetes.
DISCUSSION
Our study identified three patterns of longitudinal change in offspring leptin from birth to mid-childhood. Compared with a low-stable group, those in the intermediate-increasing group had higher levels of several cardio-metabolic risk markers at early adolescence, including greater central and overall adiposity, lower HDL cholesterol, higher inflammation, higher liver function tests, and higher global metabolic risk scores. The odds of obesity in early adolescence was almost threefold higher in this group. However, somewhat paradoxically, this group did have lower blood pressure z scores, which are standardized by height.
We identified two cohorts— CHAMACOS (n=80, age=6 months) and CHOP (n=459, age=8–9 years)—that have previously described longitudinal changes of leptin in children using unadjusted leptin values.10, 12 Even though the researchers found a series of perinatal characteristics such as pre-pregnancy overweight, maternal smoking and formula feeding distinguished different courses of leptin trajectories,10, 12 such leptin trajectories were generated based on a relatively small sample size across a relatively wide age range. Without controlling for age, sex and race/ethnicity, leptin trajectories reported in these two studies were not in agreement with each other. In addition, leptin is highly driven by maternal pre-pregnancy BMI, birth weight and offspring BMI, and such factors are major predictors for offspring metabolic outcomes. If these characteristics are not accounted for, the associations are likely driven by these factors other than offspring leptin itself. Therefore, in this study, we modified the LCGM model by using leptin residuals, instead of leptin crude data, for plotting the leptin trajectories.
With leptin trajectories derived from these residual models, we detected evidence for “leptin rebound” in the intermediate-increasing trajectory, and this trajectory pattern predicted poorer cardio-metabolic outcomes in adolescence, including obesity. There is substantial evidence showing that metabolic programming may begin prenatally, yet continues to be modified during early infancy and childhood.37–40 Leptin plays a critical role in energy homeostasis,41, 42 and has been proposed as a potential programming modulator of childhood obesity.37, 38, 40 Depending on available energy resources, leptin acts on the hypothalamus to promote adaptive reactions that shape the pattern of early growth after delivery.43 After adjusting for offspring BMI at early and mid-childhood, leptin trajectories reflected more on the adaptive reactions on leptin sensitivity or resistance in vivo. Since the leptin trajectory with this rebound pattern was highly related to childhood obesity, a possible degree of leptin resistance or decreased leptin sensitivity may have developed even though this group did not experience high leptin concentrations at birth.3 It is not clear what predicted this “leptin rebound” shape, in particular why it was not associated with high leptin in the umbilical cord blood. Future studies are warranted to look at longer follow-up on the dynamic leptin changes in offspring, and to study whether such a pattern implies an opportunity for obesity intervention during early childhood.
Interestingly, our study also suggested an unexpected inverse relationship between children with intermediate-increase leptin trajectory and blood pressure, which does not support the assumption that higher leptin level leads to elevated blood pressure as described in other studies.44, 45 A wide array of blood pressure regulation pathways might underlie such intriguing findings in our cohort. A recent study showed that higher leptin level was associated with lower risk of stroke in the top waist-hip ratio quartile group.46 The authors suggested that hyperleptinemia generated in the course of diet induced obesity may protect non-adipose tissues from lipotoxicity and oxidative damage.47 Furthermore, a recent study reported that leptin did not induce elevated blood pressure in diet-induced obese mice, possibly via leptin related vascular counter-regulatory pathways against HTN,48 or even leptin resistance in obese individuals as mentioned above.3 In such circumstances, it might explain why the “intermediate-increasing” leptin trajectory was associated with childhood obesity yet with lower SBP.
Our study has many strengths including its relatively large sample size, prospective study design, long follow-up from birth to early adolescence, ability to analyse dynamic patterns of leptin independent of predictive factors such as maternal leptin, child birth weight, gestational age, sex, race/ethnicity, and age and BMI at each leptin measurement time point. We assessed a wide range of cardio-metabolic outcome measures at early adolescence using standardized protocols and highly trained staff. We also collected information on a large number of other covariates including maternal socio-demographics, diet and other behaviours. However, there are also some limitations. First, there are missing data in leptin values at each time point. In the LCGM approach, missing data are assumed to be missing at random, and are estimated by full information maximum likelihood using all available observations. In fact, trajectories derived from the complete-case analysis showed substantial agreement with the trajectories derived from the entire sample, suggesting the robustness of the trajectories. Second, a considerable number of offspring were excluded due to the lack of outcome observations at early adolescence. Selection bias cannot be excluded. Third, we were unable to account for other potential confounders during early life, such as child’s diet or physical activity Lastly, leptin has a permissive role in puberty initiation, which may alter some of the metabolic observations among early adolescent subjects, who may have had more advanced pubertal stage than others. However, as would puberty lie in the causal pathway between leptin trajectory patterns and subsequent metabolic outcomes, we chose not to adjust for it in our analysis. Further studies are warranted to look into the potential mediating role of puberty.
Conclusion
Our analysis of this prospective cohort demonstrated three different trajectories in offspring leptin from birth to mid-childhood. The intermediate-increasing leptin trajectory was associated with increased levels of several cardio-metabolic risk markers, yet with lower systolic blood pressure at early adolescence. The cause of “leptin rebound” in early childhood remains unknown, but may imply an opportunity for obesity intervention during early childhood. Future studies warrant ongoing follow-up of outcomes related to dynamic leptin changes in early life.
Supplementary Material
Highlights.
We identified three distinct leptin trajectories from birth to mid-childhood.
Such trajectories are low-stable, high-decreasing and intermediate-increasing.
The intermediate-increasing trajectory was associated with early teen cardio-metabolic risk.
Distinctive leptin trajectories may lead to different cardio-metabolic outcomes in early teen
ACKNOWLEDGEMENTS
The authors thank A/Prof. Jessica Young and Dr. Andres Cardenas (Harvard Pilgrim Health Care Institute, Boston, US) for their advice on some of the statistical analyses. The authors also thank Prof. Wong Tien Yin (Professor, Singapore Eye Research Institute, Singapore National Eye Centre) for their great support and mentorship in this study.
Support: Project Viva is supported by the National Institutes of Health [R01 HD034568 and UG3 OD023286]. Dr. Oken is additionally supported by National Institutes of Health [K24 HD069408 and P30 DK092924]. Dr. Mantzoros is supported by National Institute of Health [K24 DK081913]. Dr. Li is funded by Singapore National Medical Research Council Transition Award [NMRC TA/0027/2014], Singapore National Medical Research Council Research Training Fellowship [NMRC/Fellowship/0029/2016] and Singapore National Medical Research Council Centre Grant [NMRC/CG/C008A/2017_KKH].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors declared no conflict of interest.
REFERENCES
- [1].Harris RB. Direct and indirect effects of leptin on adipocyte metabolism. Biochim Biophys Acta 2014;1842:414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Miehle K, Stepan H, Fasshauer M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin Endocrinol (Oxf) 2012;76:2–11. [DOI] [PubMed] [Google Scholar]
- [3].Boeke CE, Mantzoros CS, Hughes MD, S LR-S, Villamor E, Zera CA, et al. Differential associations of leptin with adiposity across early childhood. Obesity (Silver Spring) 2013;21:1430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kettaneh A, Heude B, Romon M, Oppert JM, Borys JM, Balkau B, et al. High plasma leptin predicts an increase in subcutaneous adiposity in children and adults. Eur J Clin Nutr 2007;61:719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics 2009;123:682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sabin MA, Holly JM, Shield JP, Turner SJ, Grohmann MJ, Stewart CE, et al. Mature subcutaneous and visceral adipocyte concentrations of adiponectin are highly correlated in prepubertal children and inversely related to body mass index standard deviation score. J Clin Endocrinol Metab 2006;91:332–5. [DOI] [PubMed] [Google Scholar]
- [7].Sbarbati A, Osculati F, Silvagni D, Benati D, Galie M, Camoglio FS, et al. Obesity and inflammation: evidence for an elementary lesion. Pediatrics 2006;117:220–3. [DOI] [PubMed] [Google Scholar]
- [8].Winer JC, Zern TL, Taksali SE, Dziura J, Cali AM, Wollschlager M, et al. Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J Clin Endocrinol Metab 2006;91:4415–23. [DOI] [PubMed] [Google Scholar]
- [9].Jois A, Navarro P, Ortega-Senovilla H, Gavela-Perez T, Soriano-Guillen L, Garces C. Relationship of high leptin levels with an adverse lipid and insulin profile in 6–8 year-old children in Spain. Nutr Metab Cardiovasc Dis 2015;25:1111–6. [DOI] [PubMed] [Google Scholar]
- [10].Volberg V, Heggeseth B, Harley K, Huen K, Yousefi P, Dave V, et al. Adiponectin and leptin trajectories in Mexican-American children from birth to 9 years of age. PLoS One 2013;8:e77964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mantovani RM, Rocha NP, Magalhaes DM, Barbosa IG, Teixeira AL, Simoes ESAC. Early changes in adipokines from overweight to obesity in children and adolescents. J Pediatr (Rio J) 2016;92:624–30. [DOI] [PubMed] [Google Scholar]
- [12].Gruszfeld D, Kulaga Z, Wierzbicka A, Rzehak P, Grote V, Martin F, et al. Leptin and Adiponectin Serum Levels from Infancy to School Age: Factors Influencing Tracking. Child Obes 2016;12:179–87. [DOI] [PubMed] [Google Scholar]
- [13].Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010;6:109–38. [DOI] [PubMed] [Google Scholar]
- [14].Schwandt A, Hermann JM, Rosenbauer J, Boettcher C, Dunstheimer D, Grulich-Henn J, et al. Longitudinal Trajectories of Metabolic Control From Childhood to Young Adulthood in Type 1 Diabetes From a Large German/Austrian Registry: A Group-Based Modeling Approach. Diabetes Care 2016. [DOI] [PubMed]
- [15].Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. Cohort profile: project viva. Int J Epidemiol 2015;44:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Louer AL, Simon DN, Switkowski KM, Rifas-Shiman SL, Gillman MW, Oken E. Assessment of Child Anthropometry in a Large Epidemiologic Study. J Vis Exp 2017. [DOI] [PMC free article] [PubMed]
- [17].Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002:1–190. [PubMed] [Google Scholar]
- [18].Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Perng W, Rifas-Shiman SL, Kramer MS, Haugaard LK, Oken E, Gillman MW, et al. Early Weight Gain, Linear Growth, and Mid-Childhood Blood Pressure: A Prospective Study in Project Viva. Hypertension 2016;67:301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555–76. [PubMed] [Google Scholar]
- [21].Perng W, Gillman MW, Mantzoros CS, Oken E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol 2014;24:793–800 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Antuna-Puente B, Disse E, Rabasa-Lhoret R, Laville M, Capeau J, Bastard JP. How can we measure insulin sensitivity/resistance? Diabetes & metabolism 2011;37:179–88. [DOI] [PubMed] [Google Scholar]
- [23].Haugaard LK, Baker JL, Perng W, Belfort MB, Rifas-Shiman SL, Switkowski K, et al. Growth in Total Height and Its Components and Cardiometabolic Health in Childhood. PLoS One 2016;11:e0163564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fonseca VM, Sichieri R, Moreira ME, Moura AS. Early postnatal growth in preterm infants and cord blood leptin. J Perinatol 2004;24:751–6. [DOI] [PubMed] [Google Scholar]
- [25].Ong KK, Ahmed ML, Sherriff A, Woods KA, Watts A, Golding J, et al. Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab 1999;84:1145–8. [DOI] [PubMed] [Google Scholar]
- [26].Simpson J, Smith AD, Fraser A, Sattar N, Lindsay RS, Ring SM, et al. Programming of adiposity in childhood and adolescence: associations with birth weight and cord blood adipokines. J Clin Endocrinol Metab 2016:jc20162342. [DOI] [PMC free article] [PubMed]
- [27].Twisk J, Hoekstra T. Classifying developmental trajectories over time should be done with great caution: a comparison between methods. J Clin Epidemiol 2012;65:1078–87. [DOI] [PubMed] [Google Scholar]
- [28].Fatima SS, Alam F, Chaudhry B, Khan TA. Elevated levels of chemerin, leptin, and interleukin-18 in gestational diabetes mellitus. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 2017;30:1023–8. [DOI] [PubMed] [Google Scholar]
- [29].Silva NY, Tennekoon KH, Senanayake L, Karunanayake EH. Cord blood leptin levels in normal pregnancies, pregnancy induced hypertension and gestational diabetes mellitus. The Ceylon medical journal 2008;53:79–82. [DOI] [PubMed] [Google Scholar]
- [30].Logan KM, Gale C, Hyde MJ, Santhakumaran S, Modi N. Diabetes in pregnancy and infant adiposity: systematic review and meta-analysis. Archives of disease in childhood Fetal and neonatal edition 2017;102:F65–F72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhao P, Liu E, Qiao Y, Katzmarzyk PT, Chaput JP, Fogelholm M, et al. Maternal gestational diabetes and childhood obesity at age 9–11: results of a multinational study. Diabetologia 2016;59:2339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ebenibo S, Edeoga C, Owei I, Dagogo-Jack S. Basal and Dynamic Leptin Secretion: Association with Cardiometabolic Risk and Body Weight Trajectories in African-Americans and European-Americans. Frontiers in endocrinology 2018;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gonzaga NC, Medeiros CC, de Carvalho DF, Alves JG. Leptin and cardiometabolic risk factors in obese children and adolescents. Journal of paediatrics and child health 2014;50:707–12. [DOI] [PubMed] [Google Scholar]
- [34].Li WC, Hsiao KY, Chen IC, Chang YC, Wang SH, Wu KH. Serum leptin is associated with cardiometabolic risk and predicts metabolic syndrome in Taiwanese adults. Cardiovascular diabetology 2011;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Palhares HM, da Silva AP, Resende DC, Pereira GA, Rodrigues VJ, Borges MF. Evaluation of clinical and laboratory markers of cardiometabolic risk in overweight and obese children and adolescents. Clinics (Sao Paulo, Brazil) 2017;72:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brown CC, Kipnis V, Freedman LS, Hartman AM, Schatzkin A, Wacholder S. Energy adjustment methods for nutritional epidemiology: the effect of categorization. Am J Epidemiol 1994;139:323–38. [DOI] [PubMed] [Google Scholar]
- [37].Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004;304:108–10. [DOI] [PubMed] [Google Scholar]
- [38].McMillen IC, Muhlhausler BS, Duffield JA, Yuen BS. Prenatal programming of postnatal obesity: fetal nutrition and the regulation of leptin synthesis and secretion before birth. Proc Nutr Soc 2004;63:405–12. [DOI] [PubMed] [Google Scholar]
- [39].Socha P, Hellmuth C, Gruszfeld D, Demmelmair H, Rzehak P, Grote V, et al. Endocrine and Metabolic Biomarkers Predicting Early Childhood Obesity Risk. Nestle Nutr Inst Workshop Ser 2016;85:81–8. [DOI] [PubMed] [Google Scholar]
- [40].Stocker CJ, Cawthorne MA. The influence of leptin on early life programming of obesity. Trends Biotechnol 2008;26:545–51. [DOI] [PubMed] [Google Scholar]
- [41].Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 1998;395:763–70. [DOI] [PubMed] [Google Scholar]
- [42].Harris RB. Leptin--much more than a satiety signal. Annu Rev Nutr 2000;20:45–75. [DOI] [PubMed] [Google Scholar]
- [43].Karakosta P, Roumeliotaki T, Chalkiadaki G, Sarri K, Vassilaki M, Venihaki M, et al. Cord blood leptin levels in relation to child growth trajectories. Metabolism 2016;65:874–82. [DOI] [PubMed] [Google Scholar]
- [44].Ma D, Feitosa MF, Wilk JB, Laramie JM, Yu K, Leiendecker-Foster C, et al. Leptin is associated with blood pressure and hypertension in women from the National Heart, Lung, and Blood Institute Family Heart Study. Hypertension 2009;53:473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, et al. Leptin mediates the increase in blood pressure associated with obesity. Cell 2014;159:1404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Saber H, Himali JJ, Shoamanesh A, Beiser A, Pikula A, Harris TB, et al. Serum Leptin Levels and the Risk of Stroke: The Framingham Study. Stroke 2015;46:2881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Unger RH. Hyperleptinemia: protecting the heart from lipid overload. Hypertension 2005;45:1031–4. [DOI] [PubMed] [Google Scholar]
- [48].Belin de Chantemele EJ, Mintz JD, Rainey WE, Stepp DW. Impact of leptin-mediated sympathoactivation on cardiovascular function in obese mice. Hypertension 2011;58:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.