Abstract
Purpose of review:
Obesity represents the primary challenge to improving cardiovascular health, and suppression of resting metabolic rate (RMR) is implicated in the maintenance of obesity. Increasing evidence supports a major role for the renin-angiotensin system (RAS) within the brain in the control of RMR.
Recent findings:
The angiotensin II (ANG) Agtr1a receptor co-localizes with the leptin receptor (Lepr) primarily within cells of the arcuate nucleus (ARC) of the hypothalamus that also express Agouti-related peptide (Agrp). This sub-population of Agtr1a receptors is required for stimulation of thermogenic sympathetic nervous activity and RMR, but not the suppression of food intake or increasing blood pressure, in response to various stimuli including high fat diet, deoxycorticosterone acetate + salt, and leptin. Agtr1a is localized to a specific subset (SST3) of Agrp neurons within the ARC.
Summary:
The RAS within the ARC is implicated specifically in RMR control, primarily through Agtr1a localized to the SST3 subset of Agrp neurons. Ongoing research is focused on understanding the unique anatomical projections, neurotransmitter utilization, and signal transduction pathways of Agtr1a within this subset of neurons. Understanding these projections and molecular mechanisms may identify therapeutic targets for RMR and thus obesity, independent of blood pressure and appetite.
Keywords: Resting metabolism, Energy expenditure, Agtr1a, Angiotensin
Introduction
Obesity remains a major health concern and economic burden for the global populace. Despite decades of efforts to halt disease progression, the World Health Organization has published that approximately 39% of the world’s population is either overweight (body mass index (BMI) 25 to 30) or obese (BMI >30), translating into a global health burden of >$2 trillion dollars USD (1, 2). A recent study examining the association between BMI and mortality in the United States concluded that the increase in BMI between 1998 to 2011 has reduced the life expectancy at age 40 by 0.9 year in 2011, and warns that continued increases in BMI will jeopardize future gains in life expectancy (3). Obesity is a major risk factor for type II diabetes mellitus as well as cardiovascular diseases, including hypertension (4). The link between obesity and hypertension was first noted in a prospective data analysis from the Framingham Heart Study in the late 1960s (5). Alarmingly, subsequent studies have reported that while ~34% of humans with normal BMI exhibit hypertension, approximately 78% of humans with obesity are diagnosed with hypertension (6). Thus, improvements in cardiovascular outcomes are expected, secondary to the identification of actionable therapeutic target(s) central to the development and maintenance of obesity. This review therefore aims to outline the rationale for pursuing the study of resting metabolic rate (RMR), and the current understanding of the role of the brain renin-angiotensin system in RMR control.
Suppression of resting metabolic rate in the pathogenesis and maintenance of obesity
The obesity epidemic is attributable to the complex interplay among social factors, genotypes and phenotypes, which ultimately lead to an imbalance between energy intake and energy expenditure. Beyond diet and exercise, many anti-obesity interventions (including bariatric surgery and pharmacological agents to suppress caloric intake) exist, yet there are currently no safe and effective drugs available that stimulate resting energy expenditure.
Resting metabolic rate (RMR), the rate of energy expenditure at rest, accounts for approximately 70% of all energy expended by healthy humans. Long-term follow-up studies examining energy balance of obese adult humans who have undergone intensive behaviorally-mediated weight loss identify the adaptive suppression of RMR as the single dominant factor in weight maintenance & regain (7). Given this evidence, it is unsurprising that most anti-obesity therapeutics currently approved by the FDA (eg - orlistat, lorcaserin, liraglutide, etc.), which act to reduce food intake or fat absorption, fail to elicit a sustained meaningful reduction (<6%) in body weight (8). In contrast, numerous studies have touted the efficacy of 2,4-dinitrophenol (2,4-DNP), a potent RMR stimulator, as proof-of-concept evidence for RMR stimulation as a robust approach to weight loss despite the dangerous pharmacokinetics of this particular compound that preclude its use clinically (9, 10). While evidence implicates RMR suppression in the pathogenesis and maintenance of obesity and the utility of stimulating RMR as a powerful anti-obesity therapeutic approach, the underlying cellular and molecular processes controlling RMR remain unclear.
Research over the past two decades examining the genetic basis of obesity has identified loss-of-function mutations in several key components of the leptin/melanocortin pathway in patients with hyperphagia and severe early-onset obesity, including the adipose-derived hormone leptin, the leptin receptor (LEPR), pro-opiomelanocortin (POMC), and the melanocortin 4 receptor (MC4R). The most commonly mutated gene implicated in monogenetic obesity is MC4R, encoding the melanocortin 4 receptor, with a prevalence of ~6% in severely obese patients (11). Central MC4R activation by α-melanocyte stimulating hormone induces powerful satiety signals to inhibit feeding behavior, as well as increase sympathetic output to stimulate energy expenditure (12). Critically, however, sympathetic activation by MC4R agonists such as LY2112688 also increases blood pressure (BP) and heart rate, thereby limiting the therapeutic use and continued development of first-generation MC4R agonists for obesity treatment (13, 14).
Recent studies evaluating the efficacy and safety profile of the second-generation MC4R agonist, Setmelanotide, in obese patients with POMC, LEPR, or MC4R deficiency demonstrate that it suppresses hyperphagia and body weight without adverse cardiovascular effects (15–17). It has been suggested that the MC4R-mediated modulation of food intake and body mass are dependent on Gαq signaling, while cardiovascular responses appear to require Gαs signaling (15–23). The finding that differential second messenger signaling pathways are activated by MC4R to elicit metabolic versus cardiovascular effects illustrates a critically important concept: Biased activation of the melanocortin system, to selectively engage specific second-messenger signaling cascades in MC4R-expressing cells, and/or activate only specific subsets of MC4R neurons, may hold great therapeutic potential – but greater understanding of the relevant signaling network(s) is required.
The Renin-Angiotensin System
The renin-angiotensin system (RAS) exists as a circulating hormone system as well as a local paracrine signaling system within various tissues including brain and adipose. Angiotensin II (ANG) activates at least two G-protein Coupled Receptors (GPCRs), the ANG type 1 (AGTR1) and type 2 (AGTR2) receptors. In contrast to humans, there two isoforms of Agtr1 within rodents –Agtr1a and Agtr1b (24–26). It has been established that Agtr1a is essential for the BP response effects of the brain RAS, whereas Agtr1b receptors are critical for the dipsogenic effects of central ANG action within rodents (27). While the role of the RAS in cardiovascular control has been well defined, growing evidence demonstrates a multimodal role for the RAS in energy homeostasis, and more specifically, RMR control (28). Activation of Agtr1a in the brain stimulates energy expenditure through increasing RMR, but activation of Agtr2 in adipocytes suppresses RMR (29, 30).
The sympathetic nervous system orchestrates a complex homeostatic control of white adipose tissue (WAT) and brown adipose tissue (BAT) in response to caloric availability by modulating the sympathetic outflow to these fat depots. In response to thermal (cold) challenge, BAT dissipates chemical energy as heat, thus serving as a critical site for heat production. The thermogenic capacity of BAT corresponds to the presence of uncoupling proteins encoded by the UCP1 gene. By uncoupling respiration from ATP production, uncoupling proteins dissipate the energy of substrate oxidation as heat (reviewed in (31)). As noted above, Agtr2 activation in adipocytes suppresses RMR, which is mediated in part by abrogating norepinephrine-induced UCP1 production (30).
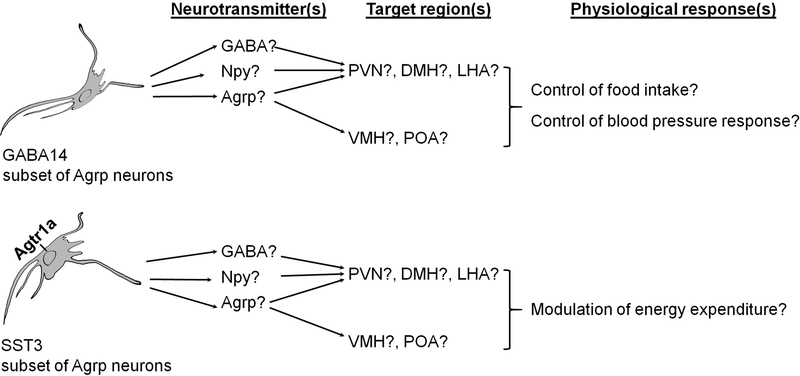
The brain RAS is required for RMR control by various stimuli. Inhibition of angiotensin converting enzyme with captopril or blockade of Agtr1a with losartan within the brain, or whole-body genetic knockout of Agtr1a all result in loss of thermogenic sympathetic nerve activity (SNA) responses to acute injections of leptin (32). Agtr1a is expressed by neurons within the arcuate nucleus (ARC) which also express Lepr and Agrp. Genetic disruption of Agtr1a in Lepr- or Agrp-expressing cells abolishes thermogenic SNA and RMR responses to leptin, high-fat diet (HFD), and deoxycorticosterone acetate (DOCA)-salt stimuli (33). Because pharmacological inhibition of the brain RAS attenuates BP responses to these types of stimuli (34–36), it was surprising to discover that BP responses to DOCA-salt remained intact in mice lacking Agtr1a in Lepr-expressing cells. This establishes a specific role for the subpopulation of Agrp neurons, and Agtr1a expressed on this subset of neurons, in RMR control, and implicates other populations of Agtr1a-expressing neurons in BP control. Although Agrp neurons are known to play in the control of feeding behavior, we consistently fail to detect any alterations in food intake behaviors in mice lacking Agtr1a within Lepr- or Agrp-expressing cells. Collectively, these observations identify divergent mechanisms by which ANG in the brain contributes to the control of RMR versus BP, and identifies the brain RAS as a major integrator of RMR control through its actions on leptin-sensitive ARC Agrp neurons. Further, the determination that Agtr1a on Agrp neurons contributes to RMR control without effect upon food intake or BP led us to hypothesize the existence of distinct ANG-sensitive ARC Agrp neuronal subtype(s) essential for RMR control but decoupled from food intake behaviors and BP control. In support of this hypothesis, in silico reanalysis of a publicly-available single-cell RNA-sequencing dataset describing the transcriptomes of cells within the mouse hypothalamus, identifies at least two distinct populations of Agrp neurons: the “GABA14” and “SST3” subtypes (37, 38). Intriguingly, only the SST3 subtype of Agrp neurons expresses Agtr1a, and this cell type exhibits one of the highest levels of expression of Agtr1a among all cell types within the mouse hypothalamus. The presence of neurons coexpressing Agrp and Sst has also been reported by Campbell and colleagues (39). Notably, deletion of Crhr1 from all AgRP neurons resulted in alterations in thermogenesis without effects on food intake and body weight, and Crhr1 is highly expressed within Agrp+Sst+ neurons, underscoring the potential for this subtype of Agrp neurons in metabolic control (39, 40). The existence of the “GABA14” and “SST3” Agrp neuronal subpopulations has prompted our team to propose a series of questions to further understand the specific contributions of these cells to energy homeostasis:
Do GABA14 and SST3 subtypes of Agrp neurons project to different brain nuclei to control specific physiological effects?
Agrp neurons are critical controllers of appetite, and respond to circulating satiety and hunger signals such as leptin and ghrelin. In addition to expressing Agrp, these neurons also express neuropeptide Y (Npy) as well as the genes to produce and package γ-aminobutyric acid (GABA). In response to leptin, Agrp neuronal activity is decreased, and Agrp and Npy production are suppressed. This results in the disinhibition of Mc4r-expressing second-order neurons, and the suppression of feeding (41). Further, a role for Agrp neurons in energy expenditure control has been suggested with the observation that mice with specific deletion of the vesicular GABA transporter in Agrp neurons are resistant to HFD-induced weight gain, due to increased oxygen consumption (42).
While the presynaptic inputs to Agrp neurons of the ARC have been identified, the targets of efferent projections from these neurons involved in RMR control remain relatively unresolved (43). As we have recently reviewed, Agrp neurons send projections to numerous nuclei implicated in energy balance control, including the PVN, the dorsomedial nucleus of hypothalamus (DMH), the lateral hypothalamic area (LHA), ventromedial hypothalamus (VHL), the preoptic area (POA), the locus coeruleus (LC) as well as the periaqueductal gray (PAG) (44). The PVN is implicated in the control of feeding and BP (45, 46). Thus, it is plausible that the GABA14 subset of Agrp neurons projects to second order neurons of the PVN critical for feeding control and/or BP control, while the ANG-sensitive SST3 subset of Agrp neurons projects to a unique set of second order neurons specifically involved in RMR modulation. To the best of our knowledge, however, there have been no studies examining differential efferent projections from subpopulations of Agrp neurons which differentiate RMR, BP, and food intake. Future studies designed to specifically elucidate the distinguishing molecular features, projections, and relative utilization of neurotransmitters between GABA14 and SST3 Agrp neurons are needed (Figure 1).
Figure 1. Localization of Agtr1a and hypothesized role(s) for distinct subtypes of Agrp neurons within the arcuate nucleus.
Agtr1a is expressed in the SST3, but not GABA14, subset of Agrp neurons. Clarification of the neurotransmitter(s) and efferent projections utilized by the GABA14 and SST3 subsets of AgRP neurons will provide critical insight into the integrative control of energy expenditure, versus blood pressure and food intake. Agouti-related peptide (Agrp), dorsomedial hypothalamus (DMH), γ-aminobutyric acid (GABA), lateral hypothalamic area (LHA), neuropeptide Y (Npy), paraventricular nucleus of hypothalamus (PVN), preoptic area (POA), ventromedial hypothalamus (VMH).
What is the second-messenger signaling cascade activated by Agtr1a within the RMR-modulating “SST3” subtype of Agrp neurons?
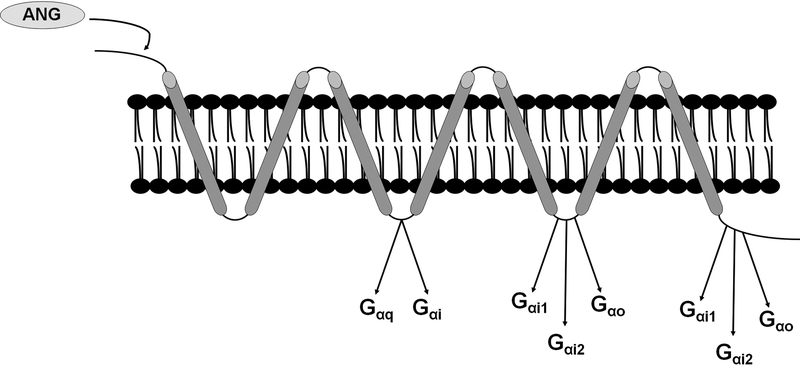
As noted above, our recent publications implicate the brain RAS in RMR control through actions at the Agtr1a specifically expressed on ARC Agrp neurons. The “SST3” subtype of Agrp neurons but not the GABA14 subtype of Agrp neurons expresses Agtr1a. This receptor is a prototypical GPCR that can couple to Gαq, Gαi1, Gαi2 and Gα12/13 pathways (Figure 2). Using site-directed mutagenesis, Shibata and colleagues identified a conserved sequence within second intracellular loop of the human AGTR1 critical for association with both Gαq and Gαi, whereas the last 50 amino acids of the cytoplasmic tail coupling to Gαi but not Gαq (47). The observation that Agtr1a couples to both Gαq and Gαi is consistent with earlier mapping studies of G protein coupling sites in the rat Agtr1a by Shirai et al (48). The SST3 cell expresses genes encoding Gαi (Gnai1, Gnai2) and Gαq (Gnaq) (37), and thus it is possible that Agtr1a may couple to either (or both) of these pathways to control RMR.
Figure 2. Structure of the AGTR1 with documented sites of interaction with Gα subunits highlighted.
Structural mapping studies performed by Shibata et al (47) and Shirai et al (48) identified critical regions within the human AGTR1 and rodent Agtr1a essential for association and activation of Gαq, Gαi and Gαo.
Typically, activation of Gαq results in increased intracellular calcium ([Ca2+]i), whereas Gα12/13 activates Rho/Rho-kinase signaling cascades, and Gαi decreases intracellular cyclic-adenosine monophosphate (cAMP) levels. Thus, the molecular, cellular and physiological output of Agtr1a activation critically depends upon which G-protein it couples. This coupling interaction is often both tissue- and cell-type dependent. In vascular smooth muscle cells (VSMC), Agtr1a can couple to Gαq and Gα12/13, as its activation results in [Ca2+]i-mediated activation of myosin light chain kinase as well as Rho/Rho-kinase-dependent myosin light chain phosphatase inhibition, respectively (49, 50). More recently, using bioluminescence resonance energy transfer biosensors, Saulière et al. further confirmed the ability of ANG, as well as the biased agonist [1Sar4Ile8Ile]-angiotensin II (SII), to facilitate the coupling of Agtr1a to both Gαq and Gαi, and induce the G-protein independent recruitment of β-arrestin, in cells with exogenous as well as endogenous Agtr1a expression (51). Agtr1a can also transactivate receptor tyrosine kinases, such as the epidermal growth factor receptor to facilitate VSMC hypertrophy and migration (52, 53). The complex intracellular signaling cascades afforded by Agtr1a activation also include serine/threonine and tyrosine kinases, including ERK, PI3K, JAK and c-SRC. As it is beyond the scope of this review, we wish to direct interested readers to a recent review highlighting the novel Agtr1a signaling partners by Forrester and colleagues (54).
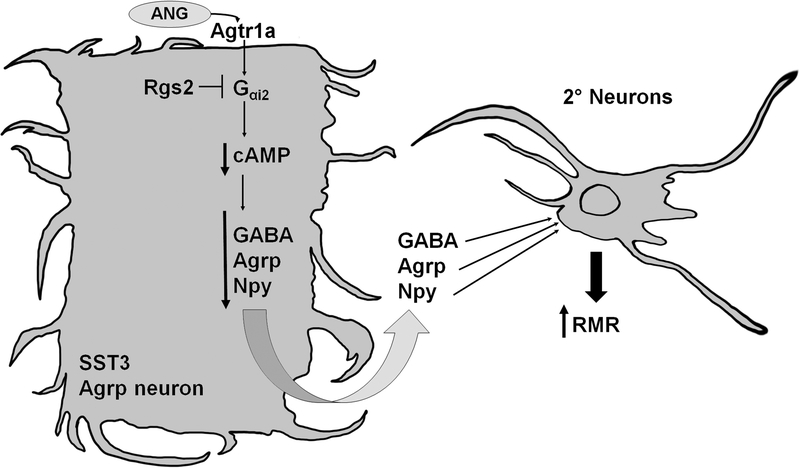
Termination of G-protein signaling can be accelerated by Regulators of G-protein Signaling (RGS), which are GTPase activating proteins that function as endogenous terminators of Gαi and Gαq second-messenger signaling by increasing the intrinsic GTPase activity of Gα subunits to facilitate the GTP-to-GDP exchange (55–57). Rgs2 is a potent suppressor of Agtr1a signaling in various cell types, and it is the dominant isoform expressed within Agrp neurons (37, 58). Roles for Rgs2 and Gαi2 in the modulation of RMR have been suggested from studies demonstrating that mice expressing an RGS-insensitive Gαi2 mutant allele exhibit increased energy expenditure, whereas Rgs2-deficient mice are resistant to weight gain (59, 60). We therefore hypothesize specific roles for Gαi2 and Rgs2 within Agrp neurons to mediate and modulate Agtr1a signaling, and studies are ongoing (Figure 3).
Figure 3. Hypothesized second-messenger signaling cascade of Agtr1a within the SST3 subtype of Agrp neurons.
Agtr1a within SST3 Agrp neurons may couple to a Gαi2 cascade that is sensitive to inhibition by the regulator of G protein signaling-2 (Rgs2), resulting in decreased intracellular cyclic adenosine monophosphate (cAMP) concentrations and repression of genes encoding the neurotransmitters Agouti-related peptide (Agrp), γ-aminobutyric acid (GABA), neuropeptide Y (Npy). This would disinhibit second-order neurons in the paraventricular nucleus, dorsomedial hypothalamus, lateral hypothalamic area, ventromedial hypothalamus and/or preoptic area, ultimately leading to the stimulation of resting metabolic rate (RMR).
Is there a role for dysregulated Agtr1a signaling in the pathogenesis of obesity and selective leptin resistance?
A phenomenon, termed “selective leptin resistance (SLR),” is observed in experimental animal models fed a HFD and in morbidly obese humans without concurrent deficiency in leptin (reviewed in (61)). Further, higher circulating leptin levels are documented in patients who have regained lost weight following an energy-restriction-based intervention as compared to patients who have been successful in maintaining weight loss (62). With SLR following 10 weeks of HFD feeding, mice are desensitized to the metabolic actions (ie – thermogenic adipose SNA) of leptin, while the cardiovascular responses (eg – renal SNA) are preserved (63). While molecular mechanism(s) underlying SLR remained undefined, several possibilities have been hypothesized. First, the inability for leptin to cross the blood brain barrier (BBB) limits its access to specific portions of the central nervous system and neuronal targets. Thus, site-specific alterations in BBB permeability/transport of leptin may contribute to SLR. Second, dysregulation of leptin and/or its multiple independent second-messenger signaling pathways within specific neurons may also contribute to the development of SLR. Our recent work underscores a pivotal role for the Agtr1a in ARC Agrp neurons in RMR stimulation in response to various stimuli, including HFD and leptin (33). Given the critical role of RMR suppression in the maintenance of obesity (7), and the specific role of Agtr1a in this neuronal population in RMR but not BP control (33), we hypothesize a role for dysregulated Agtr1a signaling (possibly including dysregulated Gαi2 and Rgs2) in SST3 neurons in the pathogenesis of obesity and SLR, and therefore ultimately obesity-associated cardiovascular disease.
Conclusions
The brain RAS is critically involved in energy homeostasis, in addition to its well-documented roles in cardiovascular control. The Agtr1a receptor within a subset of ARC Agrp neurons is critical for RMR stimulation in response to many stimuli, but is not required for cardiovascular control by the brain RAS. Understanding the molecular and neuroanatomical characteristics of the Agtr1a-expressing subset of Agrp neurons, and the unique signal transduction pathway(s) for Agtr1a in these specific cells, will provide essential insights into the fundamental control of RMR necessary for the development of safe and efficacious new anti-obesity therapeutics.
Key Points.
Suppression of resting metabolic rate (RMR) is implicated in the maintenance of obesity, yet no safe and effective anti-obesity therapeutics are currently available that stimulate RMR
Increasing evidence demonstrates a role for the renin-angiotensin (RAS) within the arcuate nucleus of the hypothalamus (ARC) in the control of RMR
The angiotensin Agtr1a receptor is expressed in one (“SST3”) subset of neurons within the ARC that express the leptin receptor (LepR) and Agouti-related peptide (Agrp), but Agtr1a is absent from the other subset of Lepr- / Agrp-expressing cells of the ARC (“GABA14” subset)
The activation of Agtr1a in the SST3 subset of Agrp neurons is absolutely required for thermogenic autonomic and RMR responses to an array of stimuli including leptin, high fat diet, and deoxycorticosterone (DOCA)-salt, but dispensable for appetite and blood pressure responses
Because of opposing actions of the brain RAS (stimulates RMR) and circulating/adipose RAS (suppresses RMR), therapeutically targeting Agtr1a signaling in SST3 neurons to stimulate RMR will require increased understanding of the unique second-messenger pathways activated by Agtr1a within, and the anatomical projections and neurotransmitter utilization of, the SST3 cell
Acknowledgments
The authors acknowledge ongoing related collaborations and the intellectual contributions of Drs. Curt D. Sigmund, Kamal Rahmouni, Julien A. Sebag, Huxing Cui, Anne E. Kwitek, and Allyn L. Mark of the University of Iowa.
This work was supported by grants from the National Institutes of Health / National Heart, Lung & Blood Institute (HL134850, HL084207), the American Heart Association (15SFRN23730000, 18EIA33890055, 19POST34380239), and the Roy J. Carver Trust (JLG).
Footnotes
The authors declare no relevant conflicts of interest.
References
- 1.Dobbs RSC, Thompson F, Manyika J, Woetzel J, Child P, McKenna S, Spatharou A Overcoming obesity: An initial economic analysis. McKinsey Global Institute; 2014. November 2014. [Google Scholar]
- 2.W.H.O. Obesity and Overweight: Key Facts World Health Organization: World Health Organization; 2018. [Available from: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
- 3.Preston SH, Vierboom YC, Stokes A. The role of obesity in exceptionally slow US mortality improvement. Proc Natl Acad Sci U S A. 2018;115(5):957–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. Jama. 2010;303(3):235–41. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Brand N, Skinner JJ Jr., Dawber TR, McNamara PM. The relation of adiposity to blood pressure and development of hypertension. The Framingham study. Ann Intern Med. 1967;67(1):48–59. [DOI] [PubMed] [Google Scholar]
- 6.Bramlage P, Pittrow D, Wittchen HU, Kirch W, Boehler S, Lehnert H, et al. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. American journal of hypertension. 2004;17(10):904–10. [DOI] [PubMed] [Google Scholar]
- 7.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring, Md). 2016;24(8):1612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, Loos RJF, et al. Obesity. Nat Rev Dis Primers. 2017;3:17034. [DOI] [PubMed] [Google Scholar]
- 9.Colman E. Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regulatory toxicology and pharmacology : RTP. 2007;48(2):115–7. [DOI] [PubMed] [Google Scholar]
- 10.Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM. 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 2011;7(3):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–95. [DOI] [PubMed] [Google Scholar]
- 12.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. [DOI] [PubMed] [Google Scholar]
- 13.do Carmo JM, da Silva AA, Wang Z, Fang T, Aberdein N, Perez de Lara CE, et al. Role of the brain melanocortins in blood pressure regulation. Biochim Biophys Acta. 2017;1863(10 Pt A):2508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360(1):44–52. [DOI] [PubMed] [Google Scholar]
- 15.Kuhnen P, Clement K, Wiegand S, Blankenstein O, Gottesdiener K, Martini LL, et al. Proopiomelanocortin Deficiency Treated with a Melanocortin-4 Receptor Agonist. The New England journal of medicine. 2016;375(3):240–6. [DOI] [PubMed] [Google Scholar]
- 16.Clement K, Biebermann H, Farooqi IS, Van der Ploeg L, Wolters B, Poitou C, et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nature medicine. 2018;24(5):551–5. [DOI] [PubMed] [Google Scholar]
- 17.Collet TH, Dubern B, Mokrosinski J, Connors H, Keogh JM, Mendes de Oliveira E, et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Molecular metabolism. 2017;6(10):1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar KG, Sutton GM, Dong JZ, Roubert P, Plas P, Halem HA, et al. Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R- and MC4R-deficient C57BL/6J mice. Peptides. 2009;30(10):1892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62(2):490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemmensen C, Finan B, Fischer K, Tom RZ, Legutko B, Sehrer L, et al. Dual melanocortin-4 receptor and GLP-1 receptor agonism amplifies metabolic benefits in diet-induced obese mice. EMBO molecular medicine. 2015;7(3):288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen KY, Muniyappa R, Abel BS, Mullins KP, Staker P, Brychta RJ, et al. RM-493, a melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals. The Journal of clinical endocrinology and metabolism. 2015;100(4):1639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayers KL, Glicksberg BS, Garfield AS, Longerich S, White JA, Yang P, et al. Melanocortin 4 Receptor Pathway Dysfunction in Obesity: Patient Stratification Aimed at MC4R Agonist Treatment. The Journal of clinical endocrinology and metabolism. 2018;103(7):2601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YQ, Shrestha Y, Pandey M, Chen M, Kablan A, Gavrilova O, et al. G(q/11)alpha and G(s)alpha mediate distinct physiological responses to central melanocortins. The Journal of clinical investigation. 2016;126(1):40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandberg K, Ji H, Clark AJ, Shapira H, Catt KJ. Cloning and expression of a novel angiotensin II receptor subtype. J Biol Chem. 1992;267(14):9455–8. [PubMed] [Google Scholar]
- 25.Iwai N, Inagami T. Identification of two subtypes in the rat type I angiotensin II receptor. FEBS Lett. 1992;298(2–3):257–60. [DOI] [PubMed] [Google Scholar]
- 26.Elton TS, Stephan CC, Taylor GR, Kimball MG, Martin MM, Durand JN, et al. Isolation of two distinct type I angiotensin II receptor genes. Biochem Biophys Res Commun. 1992;184(2):1067–73. [DOI] [PubMed] [Google Scholar]
- 27.Davisson RL, Oliverio MI, Coffman TM, Sigmund CD. Divergent functions of angiotensin II receptor isoforms in the brain. The Journal of clinical investigation. 2000;106(1):103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Littlejohn NK, Grobe JL. Opposing tissue-specific roles of angiotensin in the pathogenesis of obesity, and implications for obesity-related hypertension. American journal of physiology Regulatory, integrative and comparative physiology. 2015;309(12):R1463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, et al. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension. 2011;57(3):600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Littlejohn NK, Keen HL, Weidemann BJ, Claflin KE, Tobin KV, Markan KR, et al. Suppression of Resting Metabolism by the Angiotensin AT2 Receptor. Cell reports. 2016;16(6):1548–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Echtay KS, Bienengraeber M, Mayinger P, Heimpel S, Winkler E, Druhmann D, et al. Uncoupling proteins: Martin Klingenberg’s contributions for 40years. Arch Biochem Biophys. 2018;657:41–55. [DOI] [PubMed] [Google Scholar]
- 32.Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, et al. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. American journal of physiology Heart and circulatory physiology. 2012;303(2):H197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claflin KE, Sandgren JA, Lambertz AM, Weidemann BJ, Littlejohn NK, Burnett CM, et al. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. The Journal of clinical investigation. 2017;127(4):1414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubo T, Yamaguchi H, Tsujimura M, Hagiwara Y, Fukumori R. Blockade of angiotensin receptors in the anterior hypothalamic preoptic area lowers blood pressure in DOCA-salt hypertensive rats. Hypertension research : official journal of the Japanese Society of Hypertension. 2000;23(2):109–18. [DOI] [PubMed] [Google Scholar]
- 35.Itaya Y, Suzuki H, Matsukawa S, Kondo K, Saruta T. Central renin-angiotensin system and the pathogenesis of DOCA-salt hypertension in rats. The American journal of physiology. 1986;251(2 Pt 2):H261–8. [DOI] [PubMed] [Google Scholar]
- 36.Park CG, Leenen FH. Effects of centrally administered losartan on deoxycorticosterone-salt hypertension rats. Journal of Korean medical science. 2001;16(5):553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sapouckey SA, Deng G, Sigmund CD, Grobe JL. Potential mechanisms of hypothalamic renin-angiotensin system activation by leptin and DOCA-salt for the control of resting metabolism. Physiological genomics. 2017;49(12):722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romanov RA, Zeisel A, Bakker J, Girach F, Hellysaz A, Tomer R, et al. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nature neuroscience. 2017;20(2):176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20(3):484–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuperman Y, Weiss M, Dine J, Staikin K, Golani O, Ramot A, et al. CRFR1 in AgRP Neurons Modulates Sympathetic Nervous System Activity to Adapt to Cold Stress and Fasting. Cell Metab. 2016;23(6):1185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature neuroscience. 2008;11(9):998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507(7491):238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morselli LL, Claflin KE, Cui H, Grobe JL. Control of Energy Expenditure by AgRP Neurons of the Arcuate Nucleus: Neurocircuitry, Signaling Pathways, and Angiotensin. Current hypertension reports. 2018;20(3):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin C, Li J, Tang K. The Paraventricular Nucleus of the Hypothalamus: Development, Function, and Human Diseases. Endocrinology. 2018;159(9):3458–72. [DOI] [PubMed] [Google Scholar]
- 46.Zhang B, Nakata M, Lu M, Nakae J, Okada T, Ogawa W, et al. Protective role of AgRP neuron’s PDK1 against salt-induced hypertension. Biochem Biophys Res Commun. 2018;500(4):910–6. [DOI] [PubMed] [Google Scholar]
- 47.Shibata T, Suzuki C, Ohnishi J, Murakami K, Miyazaki H. Identification of regions in the human angiotensin II receptor type 1 responsible for Gi and Gq coupling by mutagenesis study. Biochemical and biophysical research communications. 1996;218(1):383–9. [DOI] [PubMed] [Google Scholar]
- 48.Shirai H, Takahashi K, Katada T, Inagami T. Mapping of G protein coupling sites of the angiotensin II type 1 receptor. Hypertension. 1995;25(4 Pt 2):726–30. [DOI] [PubMed] [Google Scholar]
- 49.Harris DM, Cohn HI, Pesant S, Zhou RH, Eckhart AD. Vascular smooth muscle G(q) signaling is involved in high blood pressure in both induced renal and genetic vascular smooth muscle-derived models of hypertension. Am J Physiol Heart Circ Physiol. 2007;293(5):H3072–9. [DOI] [PubMed] [Google Scholar]
- 50.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14(1):64–8. [DOI] [PubMed] [Google Scholar]
- 51.Sauliere A, Bellot M, Paris H, Denis C, Finana F, Hansen JT, et al. Deciphering biased-agonism complexity reveals a new active AT1 receptor entity. Nat Chem Biol. 2012;8(7):622–30. [DOI] [PubMed] [Google Scholar]
- 52.Eguchi S, Dempsey PJ, Frank GD, Motley ED, Inagami T. Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J Biol Chem. 2001;276(11):7957–62. [DOI] [PubMed] [Google Scholar]
- 53.Ohtsu H, Dempsey PJ, Frank GD, Brailoiu E, Higuchi S, Suzuki H, et al. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26(9):e133–7. [DOI] [PubMed] [Google Scholar]
- 54.Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, et al. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol Rev. 2018;98(3):1627–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR. RGS2/G0S8 is a selective inhibitor of Gqalpha function. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osei-Owusu P, Sabharwal R, Kaltenbronn KM, Rhee MH, Chapleau MW, Dietrich HH, et al. Regulator of G protein signaling 2 deficiency causes endothelial dysfunction and impaired endothelium-derived hyperpolarizing factor-mediated relaxation by dysregulating Gi/o signaling. The Journal of biological chemistry. 2012;287(15):12541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perschbacher KJ, Deng G, Fisher RA, Gibson-Corley KN, Santillan MK, Grobe JL. Regulators of G protein signaling in cardiovascular function during pregnancy. Physiol Genomics. 2018;50(8):590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsuzaki N, Nishiyama M, Song D, Moroi K, Kimura S. Potent and selective inhibition of angiotensin AT1 receptor signaling by RGS2: roles of its N-terminal domain. Cellular signalling. 2011;23(6):1041–9. [DOI] [PubMed] [Google Scholar]
- 59.Huang X, Charbeneau RA, Fu Y, Kaur K, Gerin I, MacDougald OA, et al. Resistance to diet-induced obesity and improved insulin sensitivity in mice with a regulator of G protein signaling-insensitive G184S Gnai2 allele. Diabetes. 2008;57(1):77–85. [DOI] [PubMed] [Google Scholar]
- 60.Nunn C, Zhao P, Zou MX, Summers K, Guglielmo CG, Chidiac P. Resistance to age-related, normal body weight gain in RGS2 deficient mice. Cellular signalling. 2011;23(8):1375–86. [DOI] [PubMed] [Google Scholar]
- 61.Mark AL. Selective leptin resistance revisited. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305(6):R566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crujeiras AB, Goyenechea E, Abete I, Lage M, Carreira MC, Martinez JA, et al. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab. 2010;95(11):5037–44. [DOI] [PubMed] [Google Scholar]
- 63.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54(7):2012–8. [DOI] [PubMed] [Google Scholar]