Abstract
Purpose
Survival after metastatic cancer has improved at the cost of increased presentation with metastatic spinal disease. For patients with pathologic spinal fractures and/or spinal cord compression, surgical intervention may relieve pain and improve quality of life. Surgery is generally considered to be inappropriate if anticipated survival is < 3 months. The aim of this international multicenter study was to analyze data from patients who died within 3 months or 2 years after surgery, to identify preoperative factors associated with poor or good survival, and to avoid inappropriate selection of patients for surgery in the future.
Patients and Methods
A total of 1,266 patients underwent surgery for impending pathologic fractures and/or neurologic deficits and were prospectively observed. Data collected included tumor characteristics, preoperative fitness (American Society of Anesthesiologists advisory [ASA]), neurologic status (Frankel scale), performance (Karnofsky performance score [KPS]), and quality of life (EuroQol five-dimensions questionnaire [EQ-5D]). Outcomes were survival at 3 months and 2 years postsurgery. Univariable and multivariable logistic regression analyses were used to find preoperative factors associated with short-term and long-term survival.
Results
In univariable analysis, age, emergency surgery, KPS, EQ-5D, ASA, Frankel, and Tokuhashi/Tomita scores were significantly associated with short survival. In multivariable analysis, KPS and age were significantly associated with short survival (odds ratio [OR], 1.36; 95% CI, 1.15 to 1.62; and OR, 1.14; 95% CI, 1.02 to 1.27, respectively). Associated with longer survival in univariable analysis were age, number of levels included in surgery, KPS, EQ-5D, Frankel, and Tokuhashi/Tomita scores. In multivariable analysis, the number of levels included in surgery (OR, 1.21; 95% CI, 1.06 to 1.38) and primary tumor type were significantly associated with longer survival.
Conclusion
Poor performance status at presentation is the strongest indicator of poor short-term survival, whereas low disease load and favorable tumor histology are associated with longer-term survival.
INTRODUCTION
Because of improvements in systemic therapy, survival of patients with metastasized cancer has improved substantially in the past decade.1 As a result, the number of patients with symptomatic spinal metastases is increasing, which necessitates the development of new strategies to improve the quality of the remaining lifespan for individual patients.2 Currently, painful spinal metastases without signs of gross mechanical instability or spinal cord compression can usually be treated successfully with external beam irradiation.3 With increasing mechanical instability or in the presence of pathologic fractures and/or symptomatic spinal cord compression, surgical intervention may be warranted to provide mechanical stability and/or to decompress neural structures.4
In general, spine surgeons select less-demanding interventions for patients with limited life expectancy and save larger interventions for patients with relatively good prognosis.5,6 After the introduction of less-invasive techniques, minimum life expectancy required for patients to benefit from surgery has decreased as time to recovery and surgical demand is reduced.7 Now, spine surgeons may perform palliative surgery with the goal of increasing quality of life, provided the patient has a reasonable chance to survive for at least 3 months.8 Patients with life expectancies < 3 months are generally regarded as unsuitable for surgery as the risks and drawbacks, including pre- and postoperative complications, managing postoperative pain, and time required for recovery, are considered to outweigh the benefits of intervention, and patients are subsequently referred for radiotherapy or palliative medical care.5,8,9 Similarly, patients who undergo highly demanding surgery need a sufficiently large life expectancy to recover and to benefit from these larger procedures. Because of a lack of solid selection criteria, surgeons currently rely mainly on subjective clinical and radiologic assessments to determine the extent of surgery deemed appropriate. The number of patients who do not receive optimal surgical care as a result of under- or overtreatment is not known; therefore, it would be valuable to identify preoperative factors associated with exceedingly short survival to learn which patients may not benefit from surgery and to also identify factors associated with prolonged survival to help select patients who will potentially benefit from larger interventions. The aim of this study was to review those patients who underwent spinal surgery for symptomatic spinal metastases, but who died within 3 months of surgery, and contrast this with long-term survivors (> 2 years). Patients who died within 3 months of surgery might be considered as having received surgery inappropriately and, therefore, we set out to identify preoperative factors which might indicate that anticipated survival does not justify the risk of surgery.
PATIENTS AND METHODS
For this prospective, observational, longitudinal cohort study, patients with surgically treated symptomatic spinal metastases were consecutively included in 23 internationally recognized spine centers. Indications for surgery were back pain caused by mechanical instability and/or neurologic symptoms as assessed by the treating spine surgeon and according to institutional practices and guidelines.10 All principal investigators adhered to the principle that to perform any type of spine surgery, life expectancy for individual patients should be no less than 3 months as established by the treating oncologist. Participating centers were located in Belgium, Canada, People’s Republic of China, Denmark, France, Germany, Japan, the Netherlands, Spain, South Korea, the United Kingdom, and the United States. All centers collected predefined parameters prospectively and entered anonymized data via a secure internet database.10,11 Institutional review board approval was obtained for all centers following local regulations. Data collection started in March 2001 and continued until October 2014 when the database was closed for data analysis to answer our specific research questions. Anonymous case records were kept for individual patients and included detailed information on primary tumor histology, oncologic treatment received previously, preoperative characteristics, and surgical, discharge, and follow-up data. Although patients with multiple myeloma can be regarded as a distinct category, they were included because of similarity in management. Patients were observed from enrollment up to 2 years after surgery or death. Table 1 gives a detailed listing of collected parameters. Primary outcome was survival at 3 months postsurgery and secondary outcome was survival at 2 years postsurgery. To ensure reliability of primary and secondary outcomes, participating investigators reviewed all their case records after closure of the database with special emphasis on vital statistics—being dead or alive—for each patient.
Table 1.
Parameters Scored and Their Definitions
| Parameter | Definition |
|---|---|
| Age at surgery | Years |
| Gender | Male and female |
| Surgical priority | Emergency (< 1 day), urgent (1-3 days), scheduled (> 3 days) |
| Spinal levels affected | No. of levels |
| Karnofsky performance score | Scale of 0-100 |
| EQ-5D index | EuroQol five-dimensions questionnaire |
| Frankel category | Frankel grading system (A/B/C/D/E) |
| Tokuhashi score | 0-15 points |
| Tomita score | 2-10 points |
| Paralysis = yes | Wheelchair bound or confined to bed |
| ASA score | American Society for Anesthesiologists score (1-4) |
| Histologic tumor diagnosis | Breast, colorectal, renal, lung, prostate, myeloma, gastric, liver, bladder, lymphoma, melanoma, sarcoma, thyroid, miscellaneous |
| No. of extraspinal metastasis locations | 0-4 |
| Preoperative pain level | Numeric rating scale (0-10) |
| Intraoperative complications | Vascular; neurologic; visceral |
| Postoperative complications, including directly related to surgical intervention | Hematoma, infection, implant failure, CSF leakage, wound breakdown, neurologic deterioration, other |
| Cause of death | Related to disease, unrelated to disease, related to spine treatment, related to spine surgery, other/not known |
Statistical Analysis
Data distributions were reviewed before analysis of results and calculations of statistical parameters. Univariable and multivariable logistic regression analyses of cases without missing data were performed to assess factors associated with dying within 3 months after surgery and factors associated with survival after surgery for > 2 years. Pertinent variables—considered directly relevant to the outcome but not obviously interrelated or similar to other variables—were included in multivariable analysis if observed at least 400 times. Sensitivity analyses were performed to test the influence of missing values on results. P values < .05 were considered significant. All statistical tests were performed with Stata 13.1 software (STATA, College Station, TX; Computing Resource Center, Santa Monica, CA).
RESULTS
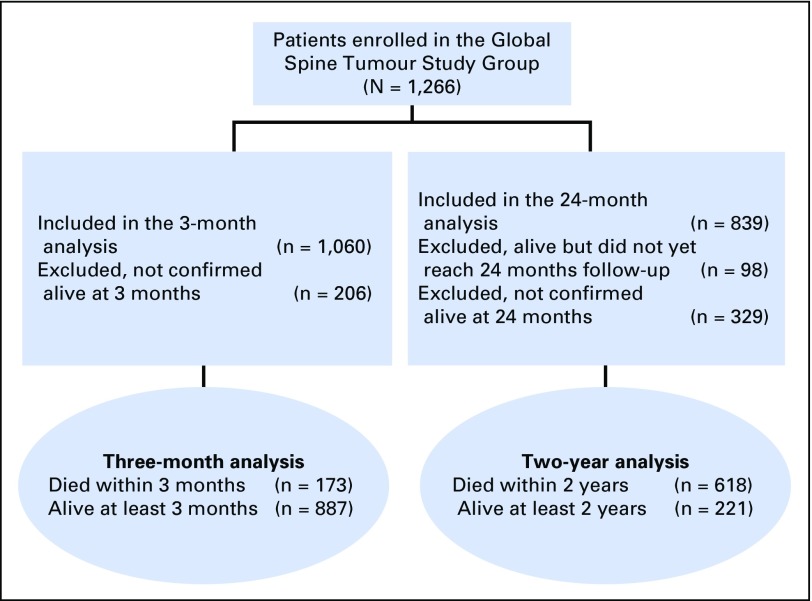
A total of 1,266 patients were included in the database. The cohort used in the analysis is under ongoing follow-up. Of 1,266 patients, 1,060 patients have been observed past 3 months or have died within this period; 839 have been observed past 2 years or have died in this period (Fig 1). Univariable and multivariable regression analyses are exclusively run on these two, smaller groups. Average age of the patients at time of surgery was 60.7 ± 12.3 years and 56.5% of the population was male. Surgical interventions were performed on a scheduled basis in 51.4% and urgent or emergency basis in 48.6% of patients. The five most frequent histologic tumor types were (in order): cancer of the breast, unknown primary, prostate, kidney, and lung. Mean preoperative Karnofsky performance score (KPS) was 60.2 ± 20.5. Neurologic status was normal (Frankel E) in 39.8%, mildly impaired (Frankel D) in 34.9%, and grossly impaired (Frankel C/B/A) in 25.3% of patients. Median number of spinal levels that warranted surgical intervention was one. The majority of surgical procedures (84.4%) were performed intralesionally, which reflected the predominantly palliative character of the intervention. Table 2 gives a detailed listing of baseline characteristics of all patients.
Fig 1.
CONSORT diagram.
Table 2.
Baseline Characteristics for All Patients
| Characteristic | Observed No. | Overall (N = 1,266) | Alive (n = 648) | Died (n = 618) |
|---|---|---|---|---|
| Age at surgery, mean (SD), years | 1,266 | 60.7 (12.3) | 59.6 (12.5) | 61.8 (11.9) |
| Male, No. (%) | 1,261 | 715 (56.5) | 349 (53.9) | 366 (59.2) |
| First surgery type, No. (%) | 941 | |||
| Emergency | 163 (17.3) | 76 (14.2) | 87 (21.4) | |
| Scheduled | 484 (51.4) | 297 (55.6) | 187 (46.0) | |
| Urgent | 294 (31.2) | 161 (30.2) | 133 (32.7) | |
| Spinal levels affected, median (IQR) | 1,266 | 1 (1-3) | 1 (1-3) | 2 (1-3) |
| Preoperative KPS score, mean (SD) | 1,000 | 60.2 (20.5) | 65.0 (20.5) | 55.6 (19.4) |
| Preoperative EQ-5D, median (IQR) | 902 | 0.38 (0.16-0.69) | 0.44 (0.20-0.71) | 0.31 (0.12-0.60) |
| Preoperative Frankel category, No. (%) | 1,239 | |||
| A/B/C | 314 (25.3) | 128 (20.0) | 186 (31.0) | |
| D | 432 (34.9) | 217 (33.9) | 215 (35.8) | |
| E | 494 (39.8) | 295 (46.1) | 199 (33.2) | |
| Tokuhashi score, No. (%) | 877 | |||
| 0-8 | 380 (43.0) | 166 (33.2) | 214 (55.9) | |
| 9-11 | 351 (39.8) | 219 (43.8) | 132 (34.5) | |
| 12-15 | 152 (17.3) | 115 (23.0) | 37 (9.7) | |
| Tomita score, No. (%) | 1,110 | |||
| ≤ 3 | 373 (33.6) | 260 (44.1) | 113 (21.7) | |
| 4/5 | 249 (22.4) | 144 (24.4) | 105 (20.1) | |
| 6/7 | 252 (22.7) | 119 (20.2) | 133 (25.5) | |
| ≥ 8 | 238 (21.4) | 67 (11.4) | 171 (32.8) | |
| Paralysis, yes, No. (%) | 1,266 | 352 (27.8) | 143 (22.1) | 209 (33.8) |
| ASA, No. (%) | 1,031 | |||
| 1 | 123 (11.9) | 91 (15.9) | 32 (7.0) | |
| 2 | 457 (44.3) | 275 (48.0) | 182 (39.7) | |
| 3 | 403 (39.1) | 195 (34.0) | 208 (45.4) | |
| 4 | 48 (4.7) | 12 (2.1) | 36 (7.9) | |
| Metastatic tumor diagnosis, No. (%) | ||||
| Breast | 234 (18.5) | 148 (22.8) | 86 (13.9) | |
| Colorectal | 54 (4.3) | 15 (2.3) | 39 (6.3) | |
| Renal | 163 (12.9) | 88 (13.6) | 75 (12.1) | |
| Lung (any) | 163 (12.9) | 67 (10.3) | 96 (15.5) | |
| Prostate | 168 (13.3) | 67 (10.3) | 101 (16.3) | |
| Myeloma | 85 (6.7) | 66 (10.2) | 19 (3.1) | |
| Gastric | 18 (1.4) | 3 (0.5) | 15 (2.4) | |
| Liver | 21 (1.7) | 9 (1.4) | 12 (1.9) | |
| Bladder | 15 (1.2) | 3 (0.5) | 12 (1.9) | |
| Lymphoma | 21 (1.7) | 17 (2.6) | 4 (0.7) | |
| Melanoma | 27 (2.1) | 15 (2.3) | 12 (1.9) | |
| Sarcoma | 18 (1.4) | 7 (1.1) | 11 (1.8) | |
| Thyroid | 37 (2.9) | 24 (3.7) | 13 (2.1) | |
| Other specified | 59 (4.7) | 25 (3.9) | 34 (5.5) | |
| Other unknown | 183 (14.5) | 94 (14.5) | 89 (14.4) | |
| Metastasis locations, No. (%) | 1,266 | |||
| 0 | 891 (70.4) | 495 (76.4) | 396 (64.1) | |
| 1 | 228 (18.0) | 109 (16.8) | 119 (19.3) | |
| 2 | 102 (8.1) | 31 (4.8) | 71 (11.5) | |
| 3 | 39 (3.1) | 12 (1.9) | 27 (4.4) | |
| 4 | 6 (0.5) | 1 (0.2) | 5 (0.8) |
Abbreviations: ASA, American Society of Anesthesiologists; EQ-5D, EuroQol five-dimensions questionnaire; IQR, interquartile range; KPS, Karnofsky performance score; SD, standard deviation.
Patients Who Survived < 3 Months
Descriptive analysis of the group of survivors at 3 months versus those who had died at that point showed that patients who had died within 3 months were in a significantly worse condition preoperatively with more emergency cases, lower preoperative KPS, lower preoperative EQ-5D, more severe neurologic deficits, worse Tokuhashi and Tomita categorical scores, worse ASA scores, and more often presented with unfavorable tumor histology and extraspinal and visceral metastases. Table 3 displays detailed descriptive analysis of the two groups of patients at 3 months postsurgery.
Table 3.
Descriptive Analysis of Survivors Versus Nonsurvivors at 3 Months
| Variable | Alive (n = 887) | Died (n = 173) | P |
|---|---|---|---|
| Age at surgery, mean (SD), years | 60.0 (12.3) | 63.3 (11.3) | .001 |
| Male, No. (%) | 491 (55.4) | 116 (67.1) | .004 |
| First surgery type, No. (%) | < .001 | ||
| Emergency | 109 (17.2) | 38 (29.9) | |
| Scheduled | 349 (55.0) | 47 (37.0) | |
| Urgent | 177 (27.9) | 42 (33.1) | |
| Preoperative KPS, mean (SD) | 62.3 (20.4) | 47.6 (16.9) | <.001 |
| Preoperative EQ-5D, median (IQR) | 0.44 (0.20-0.70) | 0.18 (0.06-0.44) | .001 |
| Preoperative Frankel category, No. (%) | < .001 | ||
| A/B/C | 188 (21.6) | 72 (42.9) | |
| D | 301 (34.6) | 60 (35.7) | |
| E | 381 (43.8) | 36 (21.4) | |
| Tokuhashi score, No. (%) | < .001 | ||
| 0-8 | 232 (39.3) | 82 (66.1) | |
| 9-11 | 241 (40.8) | 38 (30.7) | |
| 12-15 | 118 (20.0) | 4 (3.2) | |
| Tomita score, No. (%) | < .001 | ||
| ≤ 3 | 291 (36.6) | 21 (15.0) | |
| 4/5 | 183 (23.0) | 19 (13.6) | |
| 6/7 | 169 (21.3) | 41 (29.3) | |
| ≥ 8 | 152 (19.1) | 59 (42.1) | |
| Paralysis, yes, No. (%) | 217 (24.5) | 85 (49.1) | < .001 |
| ASA, No. (%) | .007 | ||
| 1 | 91 (12.6) | 7 (5.4) | |
| 2 | 305 (42.3) | 48 (37.2) | |
| 3 | 293 (40.6) | 62 (48.1) | |
| 4 | 32 (4.4) | 12 (9.3) | |
| Intraoperative complications, yes, No. (%) | 63 (7.1) | 11 (6.4) | .73 |
| Surgical complications, yes, No. (%) | 149 (19.2) | 49 (30.6) | .001 |
| Metastatic tumor diagnosis, No. (%) | < .001 | ||
| Breast | 183 (20.6) | 14 (8.1) | |
| Colorectal | 37 (4.2) | 11 (6.4) | |
| Renal | 115 (13.0) | 18 (10.4) | |
| Lung (any) | 108 (12.2) | 31 (17.9) | |
| Prostate | 114 (12.9) | 35 (20.2) | |
| Myeloma | 59 (6.7) | 7 (4.1) | |
| Gastric | 14 (1.6) | 4 (2.3) | |
| Liver | 14 (1.6) | 3 (1.7) | |
| Bladder | 6 (0.7) | 7 (4.1) | |
| Lymphoma | 13 (1.5) | 1 (0.6) | |
| Melanoma | 19 (2.1) | 2 (1.2) | |
| Sarcoma | 15 (1.7) | 2 (1.2) | |
| Thyroid | 27 (3.0) | 3 (1.7) | |
| Other specified | 40 (4.5) | 6 (3.5) | |
| Other unknown | 123 (13.9) | 29 (16.8) | |
| Metastasis locations, No. (%) | < .001 | ||
| 0 | 641 (72.3) | 92 (53.2) | |
| 1 | 157 (17.7) | 38 (22.0) | |
| 2 | 64 (7.2) | 28 (16.2) | |
| 3 | 23 (2.6) | 12 (6.9) | |
| 4 | 2 (0.2) | 3 (1.7) | |
| Complications, yes, No. (%) | 254 (28.6) | 67 (38.7) | < .001 |
Abbreviations: ASA, American Society of Anesthesiologists; EQ-5D, EuroQol five-dimensions questionnaire; IQR, interquartile range; KPS, Karnofsky performance score; SD, standard deviation.
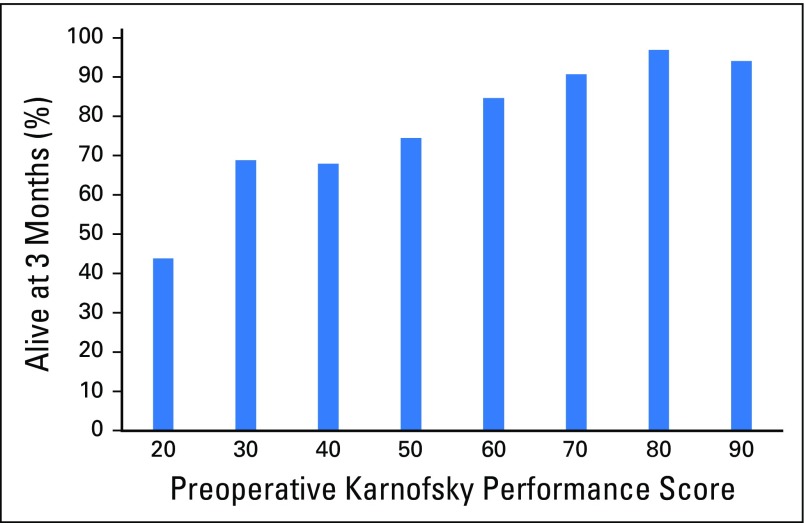
Comparison of characteristics from patients who had a follow-up visit at, at least, 3 months or were discharged at this point (n = 887) with those who had died by 3 months (n = 173) demonstrated the following factors associated with dying within 3 months in univariable analysis: age per 5-year increase (odds ratio [OR], 1.12; 95% CI, 1.04 to 1.20; P = .001), emergency surgery (OR, 2.06; 95% CI, 1.34 to 3.17; P = .002), preoperative KPS per 10-unit decrease (OR, 1.51; 95% CI, 1.36 to 1.67; P < .001), preoperative EQ-5D per unit decrease (OR, 7.47; 95% CI, 3.77 to 14.79; P < .001), and preoperative ASA, Frankel, and Tokuhashi and Tomita categorical scores. Table 4 gives a detailed overview of these factors. In multivariable analysis, however, preoperative KPS per 10-unit decrease and age were the only significant factors associated with dying within 3 months (OR, 1.36; 95% CI, 1.15 to 1.62; P < .001; and OR, 1.14; 95% CI, 1.02 to 1.27; P = .02, respectively). Appendix Figure A1 (online only) presents a graphical representation of 3-month survival stratified by preoperative KPS. Causes of death for the 173 patients who died within 3 months were systemic progression of cancer (n = 146; 84.4%), a result of complications of spine surgery within 30 days of surgery (n = 7; 4.0%), and not known or not specified (n = 20; 11.6%).
Table 4.
Logistic Regression Analysis Exploring Factors Associated With Dying Within 3 Months After Surgery
| Variable | No. | Unadjusted Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | ||
| Age at surgery (per 5-year increase) | 1,060 | 1.12 (1.04 to 1.20) | .001 | 1.14 (1.02 to 1.27) | .02 |
| First surgery type (odds for emergency) | 762 | 2.06 (1.34 to 3.17) | .002 | 1.20 (0.67 to 2.15) | .54 |
| Spinal levels affected (per additional level) | 1,060 | 1.05 (0.99 to 1.11) | .11 | 1.04 (0.97 to 1.13) | .27 |
| Preoperative KPS (per 10-unit decrease) | 843 | 1.51 (1.36 to 1.67) | < .001 | 1.36 (1.15 to 1.62) | < .001 |
| Preoperative EQ-5D (per unit decrease) | 730 | 7.47 (3.77 to 14.79) | < .001 | — | — |
| Preoperative Frankel category | 1,038 | < .001 | .41 | ||
| E | Ref | Ref | |||
| D | 2.11 (1.36 to 3.28) | 1.53 (0.78 to 3.00) | |||
| A/B/C | 4.05 (2.62 to 6.27) | 1.55 (0.73 to 3.27) | |||
| Tokuhashi category (surgery) | 715 | < .001 | — | — | |
| 12-15 | Ref | ||||
| 9-11 | 4.65 (1.62 to 13.34) | ||||
| 0-8 | 10.43 (3.73 to 29.14) | ||||
| Tomita category (surgery) | 935 | < .001 | — | — | |
| ≤ 3 | Ref | ||||
| 4/5 | 1.44 (0.75 to 2.75) | ||||
| 6/7 | 3.36 (1.92 to 5.88) | ||||
| ≥ 8 | 5.38 (3.15 to 9.19) | ||||
| Paralysis (odds for paralysis) | 1,060 | 2.98 (2.13 to 4.17) | < .001 | — | — |
| ASA | 850 | .007 | — | .77 | |
| 1 | Ref | Ref | |||
| 2 | 2.05 (0.89 to 4.68) | 1.06 (0.42 to 2.68) | |||
| 3 | 2.75 (1.22 to 6.22) | 1.10 (0.43 to 2.81) | |||
| 4 | 4.88 (1.77 to 13.46) | 1.93 (0.48 to 7.82) | |||
| Complications (odds for complications) | 1,060 | 1.58 (1.12 to 2.21) | .009 | 1.33 (0.79 to 2.23) | .28 |
Abbreviations: ASA, American Society of Anesthesiologists; EQ-5D, EuroQol five-dimensions questionnaire; KPS, Karnofsky performance score; Ref, reference.
Patients Who Survived > 2 Years
Descriptive analysis for patients who survived > 2 years postsurgery compared with those who had died by 2 years after surgery showed survivors to have a favorable preoperative condition with lower age, better preoperative KPS and EQ-5D scores, less (and less severe) neurologic deficits, better ASA, Frankel, and Tokuhashi and Tomita categorical scores, more favorable tumor histology, and less extraspinal and visceral metastases (Table 5).
Table 5.
Descriptive Analysis of Survivors Versus Nonsurvivors at 2 Years
| Variable | Alive (n = 221) | Died (n = 618) | P |
|---|---|---|---|
| Age at surgery, years, mean (SD) | 57.6 (12.9) | 61.8 (11.9) | < .001 |
| Male, No. (%) | 119 (53.9) | 366 (59.2) | .17 |
| First surgery type, No. (%) | .001 | ||
| Emergency | 27 (14.4) | 87 (21.4) | |
| Scheduled | 118 (62.8) | 187 (46.0) | |
| Urgent | 43 (22.9) | 133 (32.7) | |
| Spinal levels affected, median (IQR) | 1 (1-3) | 2 (1-3) | .14 |
| Preoperative KPS, mean (SD) | 64.9 (21.1) | 55.6 (19.4) | < .001 |
| Preoperative EQ-5D, median (IQR) | 0.46 (0.26-0.77) | 0.31 (0.12-0.60) | .001 |
| Preoperative Frankel category, No. (%) | < .001 | ||
| A/B/C | 36 (16.4) | 186 (31.0) | |
| D | 72 (32.7) | 215 (35.8) | |
| E | 112 (50.9) | 199 (33.2) | |
| Tokuhashi score, No. (%) | < .001 | ||
| 0-8 | 50 (27.6) | 214 (55.9) | |
| 9-11 | 86 (47.5) | 132 (34.5) | |
| 12-15 | 45 (24.9) | 37 (9.7) | |
| Tomita score, No. (%) | < .001 | ||
| ≤ 3 | 99 (50.0) | 113 (21.7) | |
| 4/5 | 44 (22.2) | 105 (20.1) | |
| 6/7 | 36 (18.2) | 133 (25.5) | |
| ≥ 8 | 19 (9.6) | 171 (32.8) | |
| Paralysis, yes, No. (%) | 52 (23.5) | 209 (33.8) | .005 |
| ASA, No. (%) | < .001 | ||
| 1 | 32 (16.5) | 32 (7.0) | |
| 2 | 92 (47.4) | 182 (39.7) | |
| 3 | 68 (35.1) | 208 (45.4) | |
| 4 | 2 (1.0) | 36 (7.9) | |
| Intraoperative complications, yes, No. (%) | 12 (5.4) | 37 (6.0) | .76 |
| Surgical complications, yes, No. (%) | 49 (22.2) | 131 (21.2) | .76 |
| Metastatic tumor diagnosis, No. (%) | < .001 | ||
| Breast | 56 (25.3) | 86 (13.9) | |
| Colorectal | 2 (0.9) | 39 (6.3) | |
| Renal | 28 (12.7) | 75 (12.1) | |
| Lung (any) | 19 (8.6) | 96 (15.5) | |
| Prostate | 27 (12.2) | 101 (16.3) | |
| Myeloma | 29 (13.1) | 19 (3.1) | |
| Gastric | 1 (0.5) | 15 (2.4) | |
| Liver | 2 (0.9) | 12 (1.9) | |
| Bladder | 0 (0.0) | 12 (1.9) | |
| Lymphoma | 5 (2.3) | 4 (0.6) | |
| Melanoma | 6 (2.7) | 12 (1.9) | |
| Sarcoma | 4 (1.8) | 11 (1.8) | |
| Thyroid | 4 (1.8) | 13 (2.1) | |
| Other specified | 1 (0.5) | 34 (5.5) | |
| Other unknown | 37 (16.7) | 89 (14.4) | |
| Metastasis locations, No. (%) | .006 | ||
| 0 | 164 (72.2) | 396 (64.1) | |
| 1 | 42 (19.0) | 119 (19.3) | |
| 2 | 10 (4.5) | 71 (11.5) | |
| 3 | 5 (2.3) | 27 (4.4) | |
| 4 | 0 | 5 (0.8) | |
| Complications, yes, No. (%) | 55 (24.9) | 202 (32.7) | .031 |
Abbreviations: ASA, American Society of Anesthesiologists; EQ-5D, EuroQol five-dimensions questionnaire; IQR, interquartile range; KPS, Karnofsky performance score; SD, standard deviation.
Comparison of patients who survived for at least 2 years (n = 221) with patients who died within 2 years after surgery (n = 618) demonstrated in univariable analysis the following factors associated with dying within 2 years after surgery: age per 5-year increase (OR, 1.14; 95% CI, 1.07 to 1.22; P < .001), number of affected spinal levels included in surgery per additional level (OR, 1.09; 95% CI, 1.02 to 1.17; P = .007), preoperative KPS per 10-unit decrease (OR, 1.26; 95% CI, 1.15 to 1.37; P = .04), preoperative EQ-5D per unit decrease (OR, 3.74; 95% CI, 2.05 to 6.80; P < .001), and preoperative Frankel and Tokuhashi and Tomita categorical scores. Table 6 gives a detailed overview of these factors. In multivariable analysis, the number of affected spinal levels included in surgery (per additional level: OR, 1.21; 95% CI, 1.06 to 1.38; P < .005) and primary tumor type were significant factors associated with dying in 2 years. Of all patients with one affected spinal level included in surgery, 34.9% were alive at 2 years after surgery, whereas of all patients with two or more affected spinal levels included in surgery, 22.7% were alive at that point. Sensitivity analyses performed for both short- and long-term survival cohorts did not show any change in the significance of the results (Appendix Tables A1 and A2, online only).
Table 6.
Logistic Regression Analysis Exploring Factors Associated With Dying in 2 Years After Surgery
| Variable | No. | Unadjusted Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | ||
| Age at surgery (per 5-year increase) | 839 | 1.14 (1.07 to 1.22) | < .001 | 1.01 (0.99 to 1.23) | .07 |
| First surgery type (odds for emergency) | 595 | 1.62 (1.01 to 2.60) | .04 | 0.66 (0.35 to 1.27) | .22 |
| Spinal levels affected (per additional level) | 839 | 1.09 (1.02 to 1.17) | .007 | 1.21 (1.06 to 1.38) | .005 |
| Preoperative KPS (per 10-unit decrease) | 676 | 1.26 (1.15 to 1.37) | .04 | 1.13 (0.97 to 1.31) | .12 |
| Preoperative EQ-5D (per unit decrease) | 576 | 3.74 (2.05 to 6.80) | < .001 | — | — |
| Preoperative Frankel category | 820 | < .001 | .27 | ||
| E | Ref | Ref | |||
| D | 1.68 (1.18 to 2.40) | 1.38 (0.78 to 2.47) | |||
| A/B/C | 2.91 (1.90 to 4.45) | 1.93 (0.86 to 4.30) | |||
| Tokuhashi category (surgery) | 564 | < .001 | — | — | |
| 12-15 | Ref | ||||
| 9-11 | 1.87 (1.12 to 3.12) | ||||
| 0-8 | 5.21 (3.06 to 8.87) | ||||
| Tomita category (surgery) | 720 | < .001 | — | — | |
| ≤ 3 | Ref | ||||
| 4/5 | 2.09 (1.34 to 3.26) | ||||
| 6/7 | 3.24 (2.05 to 5.11) | ||||
| ≥ 8 | 7.88 (4.57 to 13.6) | ||||
| Paralysis (odds for paralysis) | 839 | 1.66 (1.17 to 2.36) | .004 | — | — |
| ASA | 652 | < .001 | — | .76 | |
| 1 | Ref | Ref | |||
| 2 | 1.98 (1.14 to 3.43) | 1.22 (0.58 to 2.57) | |||
| 3 | 3.06 (1.74 to 5.36) | 1.03 (0.46 to 2.28) | |||
| 4 | 18 (3.99 to 81.14) | 1 (—) | |||
| Complications (odds for complications) | 839 | 1.47 (1.03 to 2.08) | .03 | 1.22 (0.70 to 2.13) | .48 |
Abbreviations: ASA, American Society of Anesthesiologists; EQ-5D, EuroQol five-dimensions questionnaire; KPS, Karnofsky performance score; Ref, reference.
DISCUSSION
In this study, preoperative factors associated with short- and long-term survival were investigated in a prospectively observed cohort of 1,266 patients who underwent surgery for treatment of symptomatic spinal metastases. Our results suggest that patients dying within 3 months after surgery were in a significantly worse physical condition preoperatively than were those who survived beyond the first 3 months, independent of unfavorable primary tumors or visceral metastases. Second, longer-term survivors were in a relatively good condition preoperatively and required surgery for only a limited number of spinal metastases, and their survival was associated with more favorable primary tumor types. Experienced clinicians may have an intuitive feel for when a patient is not a suitable candidate for surgery. Our results suggest that this intuition has a scientific basis, as the main predictor of short-term survival is functional status. KPS has previously also been shown to be a predictor of quality of life for patients who were surgically treated for spinal metastases and may therefore become a predominant prognostic variable for decision making.6
Spinal metastases are common (≥ 70%) in patients with end-stage cancer.12 Approximately 5% to 10% of patients with spinal metastases require surgical intervention as a result of mechanically unstable painful lesions and/or impending neurologic deficits.13 Clinical outcome of patients who require surgical treatment of spinal metastases depends predominantly on appropriate patient selection. This process is complex and involves the synthesis of several factors, many of which are uncertain, unreliable, or unreproducible, to determine the risk:benefit ratio of surgery. Calculation of the risk:benefit ratio is dependent upon the accuracy with which the factors involved can be determined. Unfortunately, many preoperative factors that are thought to be relevant for clinical outcome are not only difficult to quantify but also often interdependent (performance, for example, depends on the presence of neurologic deficits and/or pain) or are not yet known. As a result, some patients may not receive operative treatment, although they could have benefitted from it, whereas others undergo surgery only to succumb quickly because of rapid progression of disease. To help predict survival and guide treatment in patients with metastatic spinal disease, various scoring systems have been developed.14-18 Some of these systems were designed for specific histologic tumor types, whereas others rely on general biologic tumor behavior or extent of metastatic spread to predict outcome. These scoring systems, however, do not provide information for two key questions that arise frequently in clinical practice and are strongly related with short- and long-term survival. First, will a specific patient benefit from surgical intervention or will surgery lead to accelerated systemic progression of the malignant disease and subsequent death within 3 months? Second, should the surgical procedure for this specific patient be performed with longer-term survival in mind? For example, does the patient need to achieve bony fusion of the instrumented spine segments?
With regard to deciding whether to operate for spinal metastases, several studies have shown that physicians are not consistent when given the task of estimating the life expectancy of a patient and often overestimate survival of patients with cancer.19 This overestimation may lead to the decision to perform surgery on patients who will not live long enough to benefit from the procedure. More so, the surgical procedure itself may negatively influence survival in patients with cancer to the extent that the expected remaining lifespan that was assumed preoperatively is shortened. Evidence is emerging that mechanisms of perioperative immunosuppression as a result of factors that include, but are not limited to, surgical stress and injury, anesthetic agents, and blood transfusions may impair immunosurveillance and lead to inflammatory reactions that might promote metastatic growth.20 Results of our study show that the majority of patients who died within 3 months (146 of 173; 84.4%) succumbed because of rapid progression of the malignant process, rather than surgical complications. This suggests that survival may have been influenced, in part, by systemic effects of the surgical procedure and the balance between the malignant process and tumor control by immunosurveillance.20 This balance may be more delicate and easily tipped in patients with poor preoperative physical condition, as our results clearly show worse survival for patients with unfavorable preoperative parameters, including KPS, ASA, and neurologic status. In addition, it is possible that patients who are referred by oncologists for palliative spinal surgery and are estimated to have a remaining life span of approximately ≤ 6 months will not have many chemotherapeutic options remaining to counter accelerated systemic progression of disease.
As patients undergo surgery for spinal metastases are treated in a palliative setting to improve quality of life, minimizing surgical insult is imperative. The extent and execution of the index surgery, however, should be sufficient to function as intended for the remaining life of the patient, thus avoiding any revision or repeat surgeries. For patients with longer life expectancy, performing more demanding index surgery may be preferable over less demanding, lower risk operations that may not be long-lasting and carry a higher risk of repeat surgery. The promising results of immunotherapy for tumors that were previously considered to be therapy resistant may lead to a change from tumor histology to biologic tumor behavior after treatment as the important prognostic factor for long-term survival after surgical treatment of spinal metastases.21
The current study shows better survival for patients who undergo surgery when still in reasonably good physical shape. Because most surgical procedures for patients with spinal metastases need to be performed in specialized centers, appropriate and timely referral is of paramount importance for good clinical outcome.22 As a general rule, patients with a malignancy in the medical history who develop back and neck pain and/or neurologic deficits have symptomatic spinal metastases until proven otherwise and require immediate work-up, including appropriate imaging of the entire spinal column. The recently developed Spinal Instability Neoplastic Score is a useful instrument to help to determine which patients should then be referred to a spine surgery service.23,24
Although our current study showed consistency over multiple preoperative domains of improved survival for fitter patients, several biases may be present. First, although each participating center provided up-to-date information on vital statistics, inaccuracies pertaining to the actual status of the patient—being dead or alive—cannot be ruled out as many patients were referred back to their own referring hospital or home physicians. As the chance of the patient being registered as alive in the database but being dead in reality is much higher than the opposite; this potential inaccuracy could have led to an overestimation of effect of the end points. Second, as baseline characteristics show, the cohort of patients was heterogeneous, with a wide range in age, tumor histology, and neurologic status at admission. The common factor for all patients was the need for surgical treatment of symptomatic spinal metastases. For each patient, indication to proceed with surgery was based on the need to decompress neural structures and/or fixate unstable spinal segments, together with sufficient fitness to endure the procedure (as assessed preoperatively by the anesthesiologist and surgeon), and a presumed life expectancy of > 3 months (as assessed by the referring oncologist).8 All these factors can be biased by the experience and preference of the anesthesiologist, surgeon, and oncologist, institutional preference, and patient wishes. Finally, during multivariable analysis, only two factors, KPS and age, were shown to be significantly and independently associated with short-term survival, and two factors, number of affected spinal segments involved in the surgical procedure and primary tumor type, were predominant for longer-term survival. Because the results from the univariable analysis yielded many highly significant factors, the low number of significant factors in the multivariable analysis suggests a high level of interdependence of preoperative variables. For example, factors such as Frankel score and paralysis would overlap. We suggest that the plethora of significant factors in the univariable analysis, which all point in the same direction, indicate that fairly strong conclusions can be drawn from our work for the factors associated with survival in both the short term and long term.
In conclusion, results from this large prospective cohort study strongly suggest that survival depends on general preoperative fitness for palliative surgery of symptomatic spinal metastases. In particular, a low preoperative KPS is significantly and independently associated with poor short-term survival (< 3 months), and a limited number of spinal metastases involved in the surgical procedure and favorable primary tumor type are significantly and independently associated with longer-term survival.
Appendix
Fig A1.
Relation between preoperative Karnofsky performance score and poor survival.
Table A1.
Sensitivity Analysis of Multivariable Logistic Regression Model of Table 4
| Variable | Missing Patients Coded as Having Died | Missing Patients Coded as Being Alive | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| Age at surgery (per 5-year increase) | 1.13 (1.04 to 1.22) | .002 | 1.11 (1.00 to 1.23) | .05 |
| First surgery type (odds for emergency) | 0.84 (0.53 to 1.32) | .44 | 1.30 (0.74 to 2.30) | .36 |
| Spinal levels affected (per additional level) | 1.01 (0.94 to 1.08) | .89 | 1.05 (0.97 to 1.14) | .19 |
| Preoperative KPS (per 10-unit decrease) | 1.13 (1.01 to 1.26) | .04 | 1.37 (1.16 to 1.61) | < .001 |
| Preoperative Frankel category | .04 | .65 | ||
| E | Ref | Ref | ||
| D | 1.56 (0.99 to 2.56) | 1.35 (0.70 to 2.62) | ||
| A/B/C | 1.96 (1.14 to 3.37) | 1.33 (0.64 to 2.76) | ||
| ASA | .51 | .65 | ||
| 1 | Ref | Ref | ||
| 2 | 1.13 (0.63 to 2.03) | 1.09 (0.44 to 2.75) | ||
| 3 | 0.85 (0.46 to 1.59) | 1.21 (0.48 to 3.07) | ||
| 4 | 1.27 (0.43 to 3.72) | 2.17 (0.57 to 8.29) | ||
| Complications (odds for complications) | 1.00 (0.68 to 1.45) | .98 | 1.41 (0.85 to 2.33) | .18 |
Abbreviations: ASA, American Society of Anesthesiologists; KPS, Karnofsky performance score; Ref, reference.
Table A2.
Sensitivity Analysis of Multivariable Logistic Regression Model of Table 6
| Variable | Missing Patients Coded as Having Died | Missing Patients Coded as Being Alive | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| Age at surgery (per 5-year increase) | 1.08 (0.99 to 1.18) | .08 | 1.04 (0.96 to 1.12) | .32 |
| First surgery type (odds for emergency) | 0.84 (0.49 to 1.46) | .54 | 0.70 (0.45 to 1.11) | .13 |
| Spinal levels affected (per additional level) | 1.13 (1.01 to 1.27) | .03 | 1.18 (1.08 to 1.30) | < .001 |
| Preoperative KPS (per 10-unit decrease) | 1.07 (1.01 to 1.83) | .05 | 1.12 (1.00 to 1.25) | .06 |
| Preoperative Frankel category | .35 | .84 | ||
| E | Ref | Ref | ||
| D | 1.22 (0.73 to 2.03) | 1.06 (0.68 to 1.65) | ||
| A/B/C | 1.68 (0.83 to 3.37) | 1.18 (0.68 to 2.04) | ||
| ASA | .71 | .46 | ||
| 1 | Ref | Ref | ||
| 2 | 0.85 (0.46 to 1.58) | 1.25 (0.71 to 2.19) | ||
| 3 | 0.75 (0.38 to 1.49) | 1.37 (0.75 to 2.48) | ||
| 4 | 1 (—) | 2.61 (0.76 to 8.94) | ||
| Complications (odds for complications) | 1.12 (0.70 to 1.80) | .64 | 1.29 (0.88 to 1.89) | .19 |
Abbreviations: ASA, American Society of Anesthesiologists; KPS, Karnofsky performance score; Ref, reference.
Footnotes
Supported by the Global Spine Tumour Study Group, a registered charity of England and Wales, Charity Commission number 1134934, and DePuy Synthes. This study was performed in part at University College London Biomedical Research Centre, which receives funding from the National Institute for Health Research.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Jorrit-Jan Verlaan, David Choi, Katsuro Tomita
Collection and assembly of data: David Choi, Todd Albert, Mark Arts, Laurent Balabaud, Cody Bunger, Jacob Maciej Buchowski, Chung Kee Chung, Maarten Hubert Coppes, Hugh Alan Crockard, Bart Depreitere, Michael George Fehlings, James Harrop, Norio Kawahara, Eun Sang Kim, Chong-Suh Lee, Yee Leung, Zhongjun Liu, Antonio Martin-Benlloch, Eric Maurice Massicotte, Christian Mazel, Bernhard Meyer, Wilco Peul, Nasir A. Quraishi, Yasuaki Tokuhashi, Christian Ulbricht, Michael Wang, F. Cumhur Oner
Data analysis and interpretation: Jorrit-Jan Verlaan, David Choi, Anne Versteeg, Bernhard Meyer
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Characteristics of Patients Who Survived < 3 Months or > 2 Years After Surgery for Spinal Metastases: Can We Avoid Inappropriate Patient Selection?
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Jorrit-Jan Verlaan
No relationship to disclose
David Choi
Research Funding: DePuy Synthes (Inst)
Anne Versteeg
No relationship to disclose
Todd Albert
Employment: DePuy Synthes
Stock or Other Ownership: Paradigm Spine
Consulting or Advisory Role: DePuy Synthes
Patents, Royalties, Other Intellectual Property: Royalties from DePuy Synthes, royalties from Biomet Spine
Mark Arts
Consulting or Advisory Role: InSpine, Biomet, Silony, Amedica, EIT, QMediq
Research Funding: Zimmer Biomet (Inst), EIT (Inst), Intrinsics (Inst)
Patents, Royalties, Other Intellectual Property: EIT, Silony
Travel, Accommodations, Expenses: Amedica
Laurent Balabaud
No relationship to disclose
Cody Bunger
No relationship to disclose
Jacob Maciej Buchowski
Consulting or Advisory Role: Advance Medical, Corelink, DePuy Synthes, Gerson Lehrman Group, Globus Medical, K2M, Medtronic, Stryker
Speakers' Bureau: Broadwater, Vertical Health, DePuy Synthes, Globus Medical, Orthofix, Stryker
Patents, Royalties, Other Intellectual Property: Wolters Kluwer, Globus Medical
Expert Testimony: Various entities
Other Relationship: AO Foundation (parent organization to AO Spine)
Chung Kee Chung
No relationship to disclose
Maarten Hubert Coppes
No relationship to disclose
Hugh Alan Crockard
No relationship to disclose
Bart Depreitere
Research Funding: Johnson & Johnson
Michael George Fehlings
No relationship to disclose
James Harrop
Consulting or Advisory Role: DePuy Synthes, Bioventus
Norio Kawahara
No relationship to disclose
Eun Sang Kim
Honoraria: Takeda Pharmaceuticals, Hanmi Medicare
Research Funding: Yuhan Corporation (Inst)
Travel, Accommodations, Expenses: B. Braun Medical
Chong-Suh Lee
No relationship to disclose
Yee Leung
No relationship to disclose
Zhongjun Liu
No relationship to disclose
Antonio Martin-Benlloch
No relationship to disclose
Eric Maurice Massicotte
Honoraria: AO Spine
Christian Mazel
Stock or Other Ownership: Amplitude
Honoraria: Medtronic, AO Spine, Zimmer Spine, DePuy Synthes
Consulting or Advisory Role: Clariance, AO Spine
Travel, Accommodations, Expenses: DePuy Synthes, Medtronic, Clariance, Zimmer Spine
Bernhard Meyer
Honoraria: Medtronic, DePuy Synthes, BrainLAB, Ulrich Medical
Consulting or Advisory Role: Ulrich Medical, Spineart
Research Funding: Ulrich Medical (Inst), Medtronic (Inst), Relivant (Inst)
Travel, Accommodations, Expenses: Medtronic, DePuy Synthes
Wilco Peul
Research Funding: Medtronic (Inst), Paradigm (Inst), Aesculap (Inst)
Nasir A. Quraishi
Honoraria: AO Spine, Medtronic, DePuy Synthes
Research Funding: Medtronic (Inst), Depuy Synthes (Inst)
Travel, Accommodations, Expenses: AO Spine, Medtronic
Yasuaki Tokuhashi
No relationship to disclose
Katsuro Tomita
No relationship to disclose
Christian Ulbricht
No relationship to disclose
Michael Wang
No relationship to disclose
F. Cumhur Oner
Research Funding: DePuy Synthes (Inst)
Travel, Accommodations, Expenses: AO Spine
REFERENCES
- 1.Rosen LS Gordon D Tchekmedyian NS, etal: Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: A randomized, phase III, double-blind, placebo-controlled trial Cancer 100:2613–2621,2004 [DOI] [PubMed] [Google Scholar]
- 2.Jensen AO Jacobsen JB Nørgaard M, etal: Incidence of bone metastases and skeletal-related events in breast cancer patients: A population-based cohort study in Denmark BMC Cancer 11:29,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Linden YM Lok JJ Steenland E, etal: Single fraction radiotherapy is efficacious: A further analysis of the Dutch Bone Metastasis Study controlling for the influence of retreatment Int J Radiat Oncol Biol Phys 59:528–537,2004 [DOI] [PubMed] [Google Scholar]
- 4.Patchell RA Tibbs PA Regine WF, etal: Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial Lancet 366:643–648,2005 [DOI] [PubMed] [Google Scholar]
- 5.Tomita K Kawahara N Kobayashi T, etal: Surgical strategy for spinal metastases Spine 26:298–306,2001 [DOI] [PubMed] [Google Scholar]
- 6.Choi D Fox Z Albert T, etal: Prediction of quality of life and survival after surgery for symptomatic spinal metastases: A multicenter cohort study to determine suitability for surgical treatment Neurosurgery 77:698–708, discussion 708,2015 [DOI] [PubMed] [Google Scholar]
- 7.Park HY Lee SH Park SJ, etal: Minimally invasive option using percutaneous pedicle screw for instability of metastasis involving thoracolumbar and lumbar spine: A case series in a single center J Korean Neurosurg Soc 57:100–107,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klimo P, Jr, Schmidt MH: Surgical management of spinal metastases Oncologist 9:188–196,2004 [DOI] [PubMed] [Google Scholar]
- 9.Eap C Tardieux E Goasgen O, etal: Tokuhashi score and other prognostic factors in 260 patients with surgery for vertebral metastases Orthop Traumatol Surg Res 101:483–488,2015 [DOI] [PubMed] [Google Scholar]
- 10.Choi D Crockard A Bunger C, etal: Review of metastatic spine tumour classification and indications for surgery: The consensus statement of the Global Spine Tumour Study Group Eur Spine J 19:215–222,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Global Spine Tumour Study Group : www.gstsg.org
- 12.Coleman RE: Clinical features of metastatic bone disease and risk of skeletal morbidity Clin Cancer Res 12:6243s–6249s,2006 [DOI] [PubMed] [Google Scholar]
- 13.Coleman RE: Skeletal complications of malignancy Cancer 80:1588–1594,1997. suppl 8 [DOI] [PubMed] [Google Scholar]
- 14.Westhoff PG de Graeff A Monninkhof EM, etal: An easy tool to predict survival in patients receiving radiation therapy for painful bone metastases Int J Radiat Oncol Biol Phys 90:739–747,2014 [DOI] [PubMed] [Google Scholar]
- 15.van der Linden YM Dijkstra SP Vonk EJ, etal: Prediction of survival in patients with metastases in the spinal column: Results based on a randomized trial of radiotherapy Cancer 103:320–328,2005 [DOI] [PubMed] [Google Scholar]
- 16.Lee CH Chung CK Jahng TA, etal: Which one is a valuable surrogate for predicting survival between Tomita and Tokuhashi scores in patients with spinal metastases? A meta-analysis for diagnostic test accuracy and individual participant data analysis J Neurooncol 123:267–275,2015 [DOI] [PubMed] [Google Scholar]
- 17.Wibmer C Leithner A Hofmann G, etal: Survival analysis of 254 patients after manifestation of spinal metastases: Evaluation of seven preoperative scoring systems Spine 36:1977–1986,2011 [DOI] [PubMed] [Google Scholar]
- 18.Bollen L Wibmer C Wang M, etal: Molecular phenotype is associated with survival in breast cancer patients with spinal bone metastases Clin Exp Metastasis 32:1–5,2015 [DOI] [PubMed] [Google Scholar]
- 19.Glare P Virik K Jones M, etal: A systematic review of physicians’ survival predictions in terminally ill cancer patients BMJ 327:195–198,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horowitz M Neeman E Sharon E, etal: Exploiting the critical perioperative period to improve long-term cancer outcomes Nat Rev Clin Oncol 12:213–226,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forde PM, Kelly RJ, Brahmer JR: New strategies in lung cancer: Translating immunotherapy into clinical practice Clin Cancer Res 20:1067–1073,2014 [DOI] [PubMed] [Google Scholar]
- 22.Kim CH Chung CK Jahng TA, etal: Resumption of ambulatory status after surgery for nonambulatory patients with epidural spinal metastasis Spine J 11:1015–1023,2011 [DOI] [PubMed] [Google Scholar]
- 23.Fisher CG DiPaola CP Ryken TC, etal: A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group Spine 35:E1221–E1229,2010 [DOI] [PubMed] [Google Scholar]
- 24.Fourney DR Frangou EM Ryken TC, etal: Spinal instability neoplastic score: An analysis of reliability and validity from the Spine Oncology Study Group J Clin Oncol 29:3072–3077,2011 [DOI] [PubMed] [Google Scholar]