Abstract
Background:
Pregnancy and childbirth are associated with lumbopelvic pain and instability. Fatigability of the lumbopelvic stabilizing muscles after childbirth is unknown, and no clinical tests exist to assess this important metric of muscle function.
Objectives:
To compare fatigability of the lumbopelvic stabilizing muscles in postpartum and nulligravid (control) women using the Active Straight Leg Raise (ASLR) Fatigue Task, and to determine if fatigability is associated with inter-recti distance (IRD), physical function, and pain/disability.
Study design:
Longitudinal case-control study
Methods:
Twenty-nine nulligravid (25.4 ± 9.1 years) and 31 postpartum women (31.4 ± 5.2 years; vaginal delivery n=18) were tested at two time points, 16 weeks apart (postpartum women tested at 8–10 and 24–26 weeks postpartum). Muscular function was assessed with manual muscle testing (MMT), the ASLR Test, and a new ASLR Fatigue Task. Other measures included IRD, rectus abdominis thickness, physical activity, and six-minute walk distance.
Results:
Postpartum women were 23% more fatigable (p=0.028) and were weaker (MMT) (p<0.001) than controls up to 26 weeks postpartum. The ASLR fatigue task (time-to-failure) was associated with smaller IRD, greater rectus abdominis thickness, higher physical activity levels, greater MMT strength, and further distance walked in six minutes (p<0.05).
Conclusion:
Postpartum women (up to 6 months) had greater fatigability of the lumbopelvic stabilizing muscles and lower physical function than nulligravid women, suggesting core muscle function and fatigability should be assessed after pregnancy and childbirth. The ASLR Fatigue Task could be a clinically useful tool to determine fatigability of the lumbopelvic stabilizing muscles in women postpartum.
Keywords: Abdominal muscles, pregnancy, women’s health
Introduction
Pregnancy and childbirth can have a detrimental impact on the musculoskeletal system of the mother, and have been associated with several physical impairments which are commonly treated by physical therapists, such as low back pain, pelvic girdle pain, and incontinence.1–5 Hormonal changes during pregnancy lead to joint laxity, particularly in the pelvic joints.6 Pregnant women frequently experience an increase in inter-recti distance (IRD; increased separation of the rectus abdominis muscles) 7–9 that potentially disrupts force transmission of the abdominal muscles10 and persists up to one year postpartum.9 Regardless of delivery type, further damage to pelvic or abdominal structures occurs during child birth. With vaginal delivery, disruption of the pelvic joints and/or injury to the pelvic floor muscles can occur, especially when forceps or vacuum interventions are needed.11 Cesarean delivery causes further trauma to the fascia and musculature of the anterior abdominal wall.12 An additional factor that may impact neuromuscular function and recovery is physical activity: approximately 60% of pregnant and 80% of postpartum women report decreased physical activity levels compared to pre-pregnancy.13 Despite these musculoskeletal and lifestyle changes, the impact of pregnancy and method of delivery on the function of the abdominal muscles in the postpartum period is not well quantified.
The abdominal muscles and pelvic floor muscles contribute to stability of the spine and pelvis,14,15 and dysfunction of these muscle groups has been linked with pain syndromes.16–20 The fascial integrity of the abdominal wall has also been shown to be critical to the transfer of abdominal muscle force,10 and this integrity can be assessed by measuring IRD. Currently, the Active Straight Leg Raise (ASLR) test—which involves lifting and lowering a fully-extended leg in supine while maintaining a neutral lumbar spine— is used as a clinical measure of lumbopelvic stability, the ability to activate the abdominal muscles, and posterior pelvic pain severity in both pregnant and postpartum populations.21–24 However, standard postpartum care in the United States does not include these tests or other assessments of musculoskeletal function.25
Clinical measures of abdominal muscle function, such as manual muscle testing (MMT), are often subjective and insensitive, and frequently limited to assessing strength alone. Sustained and intermittent abdominal muscle contractions, however, are often required during daily functional tasks and ultimately result in muscle fatigue. Muscle fatigue, also known as fatigability, is typically quantified by two methods: 1) the reduction in maximal force or power of a muscle in response to muscle contraction,26 and 2) the duration a motor task can be successfully maintained, i.e. time-to-task failure.26,27 Fatigability is often overlooked in clinical assessment. We previously reported that the trunk flexor muscles of postpartum women are 71% and 52% more fatigable at 8 and 26 weeks postpartum, respectively, than women whom had never been pregnant, during an intermittent isometric contraction task performed at 50% of maximal strength .28 This fatigue task of the trunk flexors was performed in a torque dynamometer (Biodex Systems) in upright sitting that targets the rectus abdominis and the oblique muscles; however, many clinicians have limited or no access to this equipment, and time constraints make this test less clinically appealing. Thus, we created a clinically accessible tool to assess fatigability of the lumbopelvic stabilizing muscles, which are functionally important for lifting/carrying tasks and stabilization of the trunk during limb movements. To accomplish this, the ASLR test was modified into a fatigue task to assess fatigability of the lumbopelvic stabilizing muscles. The standard ASLR test involves a five-second hold of the leg (heel 20 cm from the ground with knee fully-extended) while supine and, thus, does not involve a sustained contraction of the lumbopelvic musculature, which is often required in daily tasks.
The purposes of this study were: (1) to compare fatigability of the lumbopelvic stabilizing muscles during a sustained contraction with the new ASLR Fatigue Task in nulligravid women to women who were up to 26 weeks postpartum; (2) to determine the impact of mode of delivery on fatigability (assessed with the ASLR Fatigue Task); (3) to determine if fatigability changes over time as postpartum women recover from childbirth and return to pre-pregnancy activities; and (4) to establish test-retest reliability of the newly developed ASLR Fatigue Task in nulligravid women. We hypothesized that postpartum women would be more fatigable (shorter time-to-task failure) than nulligravid women for the newly developed ASLR Fatigue Task at both time points, and the postpartum women who delivered via Cesarean section would have greater fatigability than the women who had a vaginal delivery. We also hypothesized that the postpartum women (both delivery types) would demonstrate improved fatigability (increase in time-to-task failure) by the 24–26 week testing point, due to natural recovery after childbirth. Given the role for the lumbopelvic stabilizing muscles during walking, we hypothesized that the ASLR Fatigue Task (time-to-task failure) would be associated with performance on the six-minute walk test, physical activity levels and IRD. Due to previous studies showing decreased physical activity levels during and after pregnancy, we also compared fatigability of a subgroup of postpartum women and control women who were matched for physical activity levels. Given the role of the abdominal muscles in pelvic floor function, low back pain and pelvic girdle pain, we used questionnaires to quantify pelvic floor function, pain, and low back pain-related disability.
Methods
Participants.
Twenty-nine women who had never been pregnant (nulligravid; 25.4 ± 9.1 years) and 31 postpartum women (31.4 ± 5.2 years; vaginal delivery n=18) participated in the study. All participants were free from neurological disorders, cardiovascular disease, chronic pain diagnoses, or other significant medical or orthopedic conditions (such as exercise induced asthma, fractures, severe scoliosis) which may have impacted study outcomes. Participants did not use medications known to influence neuromuscular excitability, including anti-depressants. Use of pain medication, anti-inflammatory medications, and alcohol was restricted for 12 hours and caffeine for 2 hours prior to testing. All participants provided written informed consent. Participants completed two experimental sessions, separated by one to seven days. Postpartum women completed testing between 8–10 weeks after delivery and returned between 24–26 weeks postpartum. Nulligravid women completed their initial testing sessions and returned 16–18 weeks later to repeat the testing sessions. Study approval was obtained by the Institutional Review Boards at Marquette University, the Medical College of Wisconsin, and the Office of Clinical Research and Innovative Care Compliance at Froedtert Hospital.
An overview of the study design is summarized in Figure 1. Session one included a dual x-ray absorptiometry (DXA) scan to estimate body composition, MMT, ultrasound measurement of IRD, the ASLR test, and the ASLR Fatigue Task. Session two involved performance of the six-minute walk test and completion of multiple questionnaires. Participants also wore a triaxial accelerometer for four days to quantify physical activity levels.
FIGURE 1.
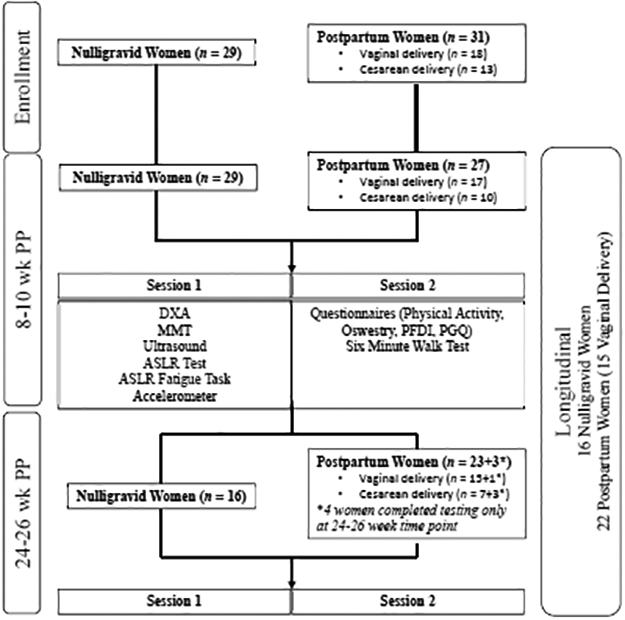
Overview of study design and subject participation. DXA= dual x-ray absorptiometry; MMT= Manual Muscle Testing; ASLR= Active Straight Leg Raise; PFDI= Pelvic Floor Distress Inventory; PGQ= Pelvic Girdle Questionnaire
Standard Clinical Measurements of Abdominal Muscle Function
Manual Muscle Testing (MMT).
Participants were positioned on a plinth in supine with legs extended, without a pillow underneath their head. Participants were instructed to lift their head and shoulders up off the table as far as possible without moving their legs. Strength was graded on a 1–5 scale29, as described by Hislop.
Active Straight Leg Raise Test.
While lying supine on a plinth, participants were asked to raise one leg (with knee extended) 20 cm (heel to plinth), hold for 5 seconds, and slowly lower the leg back to the plinth. Stability of the spine and pelvis were assessed with an inflatable air bladder under the lumbopelvic region, inflated to 40 mmHg. Participants were asked to rate difficulty of raising the leg on a scale of 0–5 (0=not difficult at all, 5=unable to lift leg). For ratings above zero, the test was repeated with the examiner providing manual compression to the pelvis. If the difficulty rating decreased with manual compression, the test was considered positive for instability of the pelvis.21,22,24,30,31
Novel Clinical Measurement of Fatigability of the Lumbopelvic Stabilizers
Active Straight Leg Raise Fatigue Task.
Participants performed the ASLR test prior to performing the ASLR Fatigue Task. If difficulty of raising the leg was equal bilaterally, the dominant leg (self-reported) was used for the fatigue task. If difficulty ratings were different between legs, or if the participant had a positive test and/or a painful side, the leg that was less difficult (or not painful) was used. Participants lifted the heel of the test leg to 20 cm for as long as possible. Participants were required to maintain pressure in the cuff beneath their back as close to 40 mmHg as possible. Visual feedback of cuff pressure was provided throughout the task, but no instruction was given on how to affect cuff pressure. Task failure was defined as an inability to maintain heel height ≥10 cm off the plinth and/or a change in cuff pressure ≥20 mmHg. One attempt was performed.
Functional Test: Six-Minute Walk
To assess muscular and cardiovascular endurance during functional mobility, the six-minute walk test was performed.32 Participants walked as quickly as possible, without running, for six minutes on an indoor walking track. Standard instructions and encouragement were provided for each participant. The distance walked was measured.
Physical Activity
Self-reported Physical Activity.
Participants self-reported physical activity from the previous 12 months using the Physical Activity Questionnaire33. The questionnaire evaluates both recreational and occupational physical activity in metabolic equivalents per hour per week (MET·hour·week−1).
Accelerometry.
Physical activity around the time of testing was quantified using triaxial accelerometers (Actigraph, Pensacola, FL) worn around the waist for four days. Average minutes per day of moderate intensity exercise was determined (ActiLife software, Actigraph) in accordance with American College of Sports Medicine physical activity recommendations.34
Pain and Disability Questionnaires
Pain was assessed with the McGill Short Form Pain Questionnaire35 prior to performance of the six-minute walk test. The impact of low back and pelvic girdle pain on daily function was assessed with the Oswestry Low Back Disability Questionnaire36,37 and the Pelvic Girdle Questionnaire.38 Pelvic floor symptoms were assessed with the Pelvic Floor Distress Inventory41
Body Composition
A GE Lunar iDXA (GE Healthcare, Little Chalfont, United Kingdom) was used to estimate body composition. Participants were scanned in supine with hips and forearms in neutral position.
Inter-Recti Distance and Muscle thickness
IRD and muscle thickness were assessed while the participant was supine on a plinth44 with a GE vivid e ultrasound machine (8 LRS linear probe; GE Healthcare, Little Chalfont, United Kingdom). Thickness of the right rectus abdominis muscle belly was measured at 2.5 cm above and below the umbilicus. IRD was measured at 4 cm above, 2.5 cm above, 2.5 cm below, and 4 cm below the umbilicus. Images were recorded at end expiration with recommended transducer orientation.45 For IRD, three images were obtained at each site and averaged. For muscle thickness, the largest measurement was used. Images were excluded from analysis if the muscular borders were not clearly defined. For women whose IRD exceeded the viewing range of the transducer, the maximal width of the transducer viewing rage (3.85 cm) was recorded.
Statistical Analysis
Data within the text and tables are presented as means ± standard deviation (SD) and in figures as means ± standard error of the mean (SEM). Independent samples t-tests were used to compare subject characteristics (age, height, weight, body composition), ASLR time-to-task failure, six-minute walk test performance, physical activity (self-reported and accelerometer), IRD, and rectus abdominis thickness between: 1) nulligravid vs. postpartum women, and 2) vaginal vs. Cesarean section delivery, separately at each time point (8–10 vs. 24–26 weeks postpartum). Nonparametric tests were used to compare MMT strength grades (Mann-Whitney U), questionnaire results (Mann-Whitney U) and ASLR test outcome (Chi-square) between groups. Spearman’s Rho nonparametric correlation was used to test associations between variables that were not ordinal level data (such as MMT) or did not demonstrate a linear relationship (such as IRD). Spearman correlation was also used to assess the association between fatigability and age. Pearson correlation was used to determine test-retest reliability of the newly developed ASLR Fatigue Task in nulligravid women. Pearson Correlation was used for the remaining correlation analyses.
A subgroup of 9 controls and 7 postpartum women (vaginal delivery, n=5) were matched for physical activity over the previous 12 months using the physical activity questionnaire. Activity matching was only possible at the initial timepoint (8–10 weeks postpartum), due to low numbers of matched individuals at the follow up. A similar statistical approach, as listed above, was used to compare data.
In a subgroup of participants (16 control women, 22 postpartum women [15 vaginal delivery]), the effect of time on fatigability was assessed by comparing time-to-task failure between the 8 and 26 weeks postpartum time points with repeated measures analysis of variance (ANOVA) with group (control, vaginal delivery, Cesarean delivery) as between-subjects factor.
Results
Participants.
Twenty-nine control women and 27 postpartum women (vaginal delivery n=17) completed testing at the initial (8–10 weeks postpartum) time point. Sixteen control women and 26 postpartum women (vaginal delivery n=16) completed testing at the follow up time point (24–26 weeks postpartum). Seven control women and 5 postpartum women (vaginal delivery n=2, Cesarean delivery n=3) were lost to follow up due to schedule conflicts. Six control women completed only the six-minute walk test, questionnaires, ASLR test, and ASLR Fatigue Task as part of a separate study and did not return for follow up. Three women from the Cesarean delivery group and one woman from the vaginal delivery group completed testing only at the 26-week time point. A flow diagram of subject participation is presented in Figure 1.
Descriptive data.
Subject characteristics, including height, weight, and ultrasound data (IRD and rectus abdominis thickness), are presented for nulligravid vs postpartum women in Table 1 and for vaginal delivery vs Cesarean delivery in Table 2. Nulligravid women were younger, weighed less, had lower BMI and less body fat, had thicker rectus abdominis muscle above the umbilicus, demonstrated smaller IRD, and were more physically active than postpartum women (p<0.05). Women in the vaginal delivery group were similar in age, number of pregnancies, weight, BMI, body fat percentage, abdominal muscle thickness, IRD, and physical activity (p>0.05) to the women in the Cesarean delivery group. Clinical assessments, including ASLR test and questionnaires, are presented for postpartum vs control in Table 3 and for vaginal delivery vs Cesarean delivery in Table 4. Nulligravid women had higher MMT grades, lower Oswestry scores, less pelvic floor dysfunction, less pelvic girdle pain, and walked a further distance than postpartum women (p<0.05). At 8–10 weeks postpartum, women who delivered via Cesarean section had lower Oswestry scores, less pelvic floor dysfunction, and less pelvic girdle pain than women who delivered vaginally (p<0.05). However, by 26 weeks postpartum, there was no difference between the Cesarean and vaginal delivery groups (p>0.05).
TABLE 1.
Nulligravid vs Postpartum Participant Characteristics.
| INITIAL (8–10 wks postpartum) | FOLLOW UP (24–26 wks postpartum) | |||
|---|---|---|---|---|
| Nulligravid (n=29) | Postpartum (n=27) | Nulligravid (n=16) | Postpartum (n=26) | |
| Age (yrs) | 25.4 ± 9.1 | 31.3 ± 5.4* | 25.4 ± 5.8 | 32.1 ± 5.3* |
| Weight (kg) | 64.4 ± 11.7 | 75.1 ± 13.2* | 63.4 ± 7.5 | 71.5 ± 14.6* |
| Height (cm) | 165.9 ± 8.1 | 164.3 ± 4.8 | 166.4 ± 8.1 | 164.1 ± 4.8 |
| BMI (kg/m2) | 23.1 ± 3.4 (n=23) | 27.9 ± 4.9* | 22.8 ± 2.1 | 26.9 ± 5.3* |
| Body Fat % | 32.2 ± 5.4 (n=23) | 38.9 ± 6.5* | 32.7 ± 5.1 | 36.8 ± 8.6 |
| RA muscle thickness (2.5 cm above umbilicus) (cm) | 1.0 ± 0.2 (n=23) | 0.8 ± 0.2* | 1.0 ± 0.1 | 0.8 ± 0.1* |
| RA muscle thickness (2.5 cm below umbilicus) (cm) | 1.0 ± 0.2 (n=23) | 0.8 ± 0.2* | 0.9 ± 0.1 | 0.8 ± 0.2 |
| IRD 4 cm above umbilicus (cm) | 1.1 ± 0.4 (n=23) | 2.3 ± 1.1 (n=22)* | 1.1 ± 0.6 | 2.3 ± 1.0 (n=25)* |
| IRD 2.5 cm above umbilicus (cm) | 1.1 ± 0.4 (n=23) | 2.5 ± 1.1 (n=21)* | 1.0 ± 0.5 | 2.4 ± 1.0 (n=24)* |
| IRD 2.5 cm below umbilicus (cm) | 0.5 ± 0.2 (n=23) | 1.8 ± 0.9 (n=19)* | 0.5 ± 0.2 (n=15) | 2.0 ± 1.0 (n=21)* |
| IRD 4 cm below umbilicus (cm) | 0.4 ± 0.2 (n=23) | 1.5 ± 1.1 (n=22)* | 0.3 ± 0.2 | 1.4 ± 1.1 (n=23)* |
| Average moderate intensity physical activity (min·day−1) | 37.9 ± 23.2 (n=18) | 16.2 ± 15.5 (n=19)* | 29.3 ± 14.1 (n=8) | 16.7 ± 10.5 (n=13)* |
| Self-reported physical activity over the previous 12 months (MET·hours·week−1) | 41.2 ± 28.4 (n=27) | 20.7 ± 18.9 (n=25)* | 33.6 ± 24.3 (n=15) | 12.0 ± 9.8 (n=23)* |
Wks=weeks; yrs=years; kg=kilogram; cm=centimeter; m=meter; RA=rectus abdominis; IRD=inter-recti distance; MET=metabolic equivalents; min=minutes.
indicates p<0.05 (between-groups).
TABLE 2.
Cesarean vs Vaginal Delivery Participant Characteristics.
| 8–10 wks postpartum | 24–26 wks postpartum | |||
|---|---|---|---|---|
| Vaginal Delivery (n=17) | Cesarean Delivery (n=10) | Vaginal Delivery (n=16) | Cesarean Delivery (n=10) | |
| Age (yrs) | 30.6 ± 6.2 | 32.5 ± 3.6 | 31.3 ± 6.0 | 33.3 ± 4.0 |
| Weeks Postpartum (Session 1) | 8.6 ± 1.0 | 8.6 ± 0.4 | 25.3 ± 1.6 | 25.1 ± 0.7 |
| Weeks Postpartum (Session 2) | 9.4 ± 1.2 | 9.4 ± 0.5 | 26.1 ± 1.7 | 26.2 ± 0.6 |
| Duration of pregnancy (weeks) | 39.3 ± 1.0 (n=13) | 38.0 ± 1.2* (n=9) | 39.3 ± 1.1 (n=12) | 38.3 ± 1.4 |
| Fundal height prior to delivery (cm) | 38.7 ± 1.4 (n=6) | 37.0 ± 1.2 (n=5) | 38.7 ± 1.3 (n=7) | 37.0 ± 1.2* |
| Total Number of pregnancies | 2.2 ± 1.2 (n=16) | 3.2 ± 1.9 (n=9) | 2.1 ± 1.1 | 3.0 ± 2.1 |
| Weight (kg) | 74.5 ± 14.8 | 76.2 ± 10.7 | 70.8 ± 15.8 | 72.7 ± 13.0 |
| Height (cm) | 164.8 ± 4.3 | 163.3 ± 5.6 | 165.4 ± 4.6 | 162.1 ± 4.8 |
| BMI (kg/m2) | 27.5 ± 5.6 | 28.6 ± 3.7 | 26.3 ± 5.8 | 27.7 ± 4.5 |
| Body Fat % | 38.8 ± 7.2 | 39.1 ± 5.5 | 36.5 ± 8.9 | 37.5 ± 8.6 |
| RA muscle thickness (2.5 cm above umbilicus) (cm) | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.1 |
| RA muscle thickness (2.5 cm below umbilicus) (cm) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 |
| IRD 4 cm above umbilicus (cm) | 2.5 ± 1.2 (n=16) | 1.9 ± 0.7 (n=6) | 2.3 ± 1.1 | 2.3 ± 0.9 |
| IRD 2.5 cm above umbilicus (cm) | 2.7 ± 1.1 (n=16) | 2.0 ± 1.0 (n=5) | 2.4 ± 1.1 (n=15) | 2.4 ± 0.7 (n=9) |
| IRD 2.5 cm below umbilicus (cm) | 1.8 ± 0.9 (n=15) | 1.6 ± 1.2 (n=4) | 1.9 ± 0.9 (n=16) | 2.1 ± 1.3 (n=5) |
| IRD 4 cm below umbilicus (cm) | 1.3 ± 1.1 (n=16) | 2.0 ± 1.2 (n=6) | 1.2 ± 1.1 | 1.7 ± 1.3 |
| Average minutes/day of moderate intensity physical activity | 17.1 ± 19.1 (n=10) | 15.3 ± 11.2 (n=9) | 17.0 ± 11.9 | 16.0 ± 7.8 |
| Self-reported physical activity over the previous 12 months (MET·hours·week−1) | 21.4 ± 16.6 (n=16) | 19.4 ± 23.4 (n=9) | 10.4 ± 8.1 | 14.4 ± 12.0 |
Wks=weeks; yrs=years; kg=kilogram; cm=centimeter; m=meter; RA=rectus abdominis; IRD=inter-recti distance; MET=metabolic equivalents.
indicates p<0.05 (between-groups).
TABLE 3.
Postpartum vs Nulligravid Clinical Assessments.
| INITIAL (8–10 wks postpartum) | FOLLOW UP (24–26 wks postpartum) | |||
|---|---|---|---|---|
| Nulligravid (n=29) | Postpartum (n=27) | Nulligravid (n=16) | Postpartum (n=26) | |
| MMT (AU) | 4.3 ± 1.0 | 2.7 ± 1.2* | 4.5 ± 0.7 | 2.9 ± 1.1* |
| (+) ASLR test | 23% | 37% | 12.5% | 44%* |
| UL (+) | 18.20% | 22% | 12.50% | 20% |
| B (+) | 4.50% | 15% | 0.00% | 24% |
| (−) ASLR test | 77% | 63% | 87.5% | 56% |
| Oswestry (%) | 1.7 ± 3.2 | 4.6 ± 5.4* | 0.8 ± 1.8 | 5.0 ± 7.2* |
| PFDI (AU) | 8.2 ± 17.6 (n=10) | 38.6 ± 42.6* (n=24) | 6.1 ± 12.2 (n=4) | 28.7 ± 24.7* (n=26) |
| PGQ (AU) | 0.4 ± 1.3 (n=10) | 4.4 ± 6.9* (n=23) | 0 (n=4) | 3.3 ± 6.2 (n=26) |
| 6MWT (m) | 689 ± 57 | 640 ± 65* | 692.5 ± 56.1 | 648.7 ± 61.4* |
MMT=Manual Muscle Testing; AU=Arbitrary Units; (+) ASLR test=positive Active Straight Leg Raise test (lifting leg was easier with external pelvic compression); (−) ASLR test = negative Active Straight Leg Raise test (no change in difficulty of lifting leg with external pelvic compression); PFDI=Pelvic Floor Distress Inventory; PGQ=Pelvic Girdle Questionnaire; 6MWT=Six Minute Walk Test; m=meters; wks= weeks; PP = postpartum.
indicates p<0.05 (between-groups). Within-group changes for the Oswestry, PFDI, and PGQ did not meet MCD/MCID for either group (nulligravid or postpartum).
TABLE 4.
Cesarean vs Vaginal Delivery Clinical Assessments.
| 8–10 wks postpartum | 24–26 wks postpartum | |||
|---|---|---|---|---|
| Vaginal Delivery (n=17) | Cesarean Delivery (n=10) | Vaginal Delivery (n=16) | Cesarean Delivery (n=10) | |
| MMT (AU) | 2.9 ± 1.1 | 2.4 ± 1.4 | 3.0 ± 1.1 | 2.7 ± 1.2 |
| (+) ASLR test | 41% | 30% | 44% | 44% |
| UL (+) | 23.50% | 20% | 19% | 22% |
| B (+) | 17.60% | 10% | 25% | 22% |
| (−) ASLR test | 59% | 70% | 56% | 56% |
| Oswestry (%) | 6.4 ± 6.0 | 1.6 ± 2.5* | 5.8 ± 8.0 | 3.9 ± 6.1 |
| PFDI (AU) | 43.8 ± 34.0 | 30.9 ± 49.8* | 30.7 ± 27.5 | 25.4 ± 20.3 |
| PGQ (AU) | 7.3 ± 8.6 | 1.4 ± 2.8* | 2.2 ± 4.9 | 5.1 ± 7.8 |
| 6MWT (m) | 645 ± 62.0 | 631.8 ± 71.3 | 655.6 ± 53.2 | 637.6 ± 74.5 |
MMT=Manual Muscle Testing; Wks=weeks; AU=Arbitrary Units; (+) ASLR test=positive Active Straight Leg Raise test (lifting leg was easier with external pelvic compression); (−) ASLR test = negative Active Straight Leg Raise test (no change in difficulty of lifting leg with external pelvic compression); PFDI=Pelvic Floor Distress Inventory; PGQ=Pelvic Girdle Questionnaire; 6MWT=Six Minute Walk Test.
indicates p<0.05 (between-groups). Within-group changes for the Oswestry, PFDI, and PGQ did not meet MCD/MCID for either group (vaginal delivery or Cesarean delivery).
Clinical Measures of Abdominal Muscle Function
Manual Muscle Testing.
Postpartum women demonstrated lower MMT strength grades than control women at 8 and 26 weeks postpartum (p<0.001, Table 3). Even when matched for physical activity, postpartum women had lower MMT strength grades than controls (p=0.001). There was no difference in MMT strength grades between the vaginal delivery and Cesarean delivery groups at 8 weeks or 26 weeks postpartum (p=0.115 and p=0.397, respectively, Table 4).
Active Straight Leg Raise Test.
At the initial time point, 23% of control women and 37% of postpartum women had a positive ASLR test (p=0.280, Table 3). Of the women who had positive ASLR tests, 40% of the postpartum women and 20% of the control women had bilateral positive ASLR tests. At follow up, 12.5% of the control women and 44% of the postpartum women tested positive for impaired lumbopelvic stability (p=0.035, Table 3). At the follow up time point, 55% in the postpartum group of the positive ASLR tests were bilateral positives, while no control women were bilateral positive.
At 8 weeks postpartum, 41% of women who had a vaginal delivery and 30% of women who had a Cesarean delivery had positive ASLR tests (p=0.692, Table 4). Forty-three percent of the women who delivered vaginally had bilateral positive ASLR tests, and 33% of the women in the Cesarean group had bilateral instability. At 26 weeks postpartum, 44% of women in both the vaginal delivery and the Cesarean delivery groups had positive ASLR tests (p=1.00, Table 4). Of the women in the vaginal delivery group with a positive ASLR test, 57% had a positive test bilaterally. Fifty percent of the women in the Cesarean group with a positive ASLR test were positive bilaterally.
Active Straight Leg Raise Fatigue Task.
Postpartum women at 8 weeks had a shorter time-to-task failure (more fatigable) than control women (109 ± 50 s vs 161 ± 63 s, p=0.001) and this persisted at 26 weeks postpartum (125 ± 45 s vs 163 ± 63 s, p=0.028; Figure 2A). There was no difference in time-to-task failure between vaginal and Cesarean delivery at 8 weeks (114 ± 52 s vs 101 ± 49 s, respectively, p=0.512) or 26-weeks postpartum (113 ± 38 s vs 144 ± 50 s, respectively, p=0.086; Figure 2B).
FIGURE 2.

Fatigability of the lumbopelvic stabilizing muscles at both time points (8–10 weeks [Initial] and 24–26 weeks [Follow Up] postpartum) using the ASLR fatigue task. Postpartum women had shorter time-to-task failure than control women at both time points (A), with no difference between a vaginal and Cesarean delivery (B). Women with a positive ASLR test (reduced difficulty of lifting lower extremity when external compression provided to pelvis), did not differ in time-to-task failure compared with women with a negative ASLR test (no change in difficulty or lifting lower extremity with or without external compression to pelvis) (C).
At 8-weeks postpartum, the 7 postpartum women who were matched for physical activity with the 9 controls had greater fatigability compared to controls (113 ± 22 s vs 164 ± 29 s, respectively, p=0.002).
Subjects were also grouped by ASLR test status (positive vs negative test). There was no difference in time-to-task failure between women with a positive and negative ASLR test at the 8-week time point (126 ± 47 s vs 142 ± 69 s, respectively, p=0.431) or 26-week time points (123 ± 52 s vs 147 ± 56 s, respectively, p=0.179; Figure 2C). Furthermore, no group demonstrated a change in ASLR time-to-task failure from the 8 to 26-week time point (time effect p=0.603; time × group effect p=0.567).
Test-retest reliability, assessed in the nulligravid group with Pearson correlation, had an r value of 0.810 (p<0.001).
Associations
Performance on the ASLR Fatigue Task was associated, at both 8 and 26-week time points, with body fat, IRD, rectus abdominis thickness, self-reported physical activity over the previous year, and MMT. A shorter time-to-task failure was associated with higher body fat percentage (8 weeks: r=−0.601, p<0.001, Figure 3A; 26 weeks: r=−0.468, p=0.002, Figure 3B). Women with a greater IRD 2.5 cm below the umbilicus had a shorter time-to-task failure (8 weeks: rs=−0.443, p=0.003, Figure 3C; 26 weeks: rs=−0.508, p=0.002; Figure 3D). Lower self-reported physical activity over the previous year was associated with increased fatigability (8 weeks: r=0.345, p=0.017, Figure 3E; 26 weeks: r=0.376, p=0.020, Figure 3F), as were lower MMT strength grades (8 weeks: rs=0.532, p<0.001; 26 weeks: rs=0.360, p=0.026). Thinner rectus abdominis muscle belly at 2.5 cm above the umbilicus was also associated with greater fatigability (8 weeks: r=0.332, p=0.018, Figure 3G; 26 weeks: r=0.404, p=0.010; Figure 3H). Women who walked a shorter distance in six minutes were also more fatigable (shorter time-to-task failure) at 8 weeks postpartum (8 weeks: r=0.451, p=0.001), although this did not reach significance at 26 weeks (r=0.307, p=0.051).
FIGURE 3.

Longer time-to-task failure on the ASLR fatigue task was associated at both time points with lower body fat (A & B), smaller inter-recti distance (C & D), greater self-reported physical activity over the previous year (E & F), and thicker rectus abdominis muscle (G & H).
Because there was a statistically significant difference in age between nulligravid and postpartum women, the potential association between fatigability and age in this young cohort of women was assessed with Spearman correlation. Age was not associated with fatigability (time-to-task-failure) at the initial (8–10 weeks postpartum) or follow up time point (24–26 weeks postpartum) for all participants (rs= −0.118, p=0.482 and rs= −0.034, p=0.840, respectively), only nulligravid women (rs=0.266, p=0.319 and rs=0.094, p=0.729, respectively), and only postpartum women (rs=−0.025, p=0.913 and rs=0.072, p=0.751, respectively).
Discussion
The novel finding of this study was that postpartum women demonstrated greater fatigability of the lumbopelvic stabilizing muscles than nulligravid controls, up to 26 weeks after childbirth, as assessed by the newly-developed ASLR Fatigue Task. The greater fatigability of the postpartum women was independent of lumbopelvic stability, as there was no difference in time-to-task failure between women who had a positive and a negative ASLR test. There was no difference in fatigability of the lumbopelvic stabilizing muscles between delivery types. Postpartum women who were matched for physical activity levels with the control women exhibited greater fatigability, suggesting that decreased physical activity is not the sole mechanism responsible for the large fatigability of the lumbopelvic stabilizing muscles of postpartum women. Women who were less fatigable with the ASLR Fatigue Task, however, reported greater physical activity over the preceding year, walked a longer distance in six minutes, had lower body fat, a smaller IRD, a thicker rectus abdominis muscle, and a higher MMT strength grade.
This is the first study to establish the ASLR Fatigue Task as a potential clinical measure of fatigability of the lumbopelvic stabilizing muscles. This test showed that postpartum women are more fatigable than nulligravid women, and that this increased fatigability was still present ~6 months after childbirth. The finding of increased fatigability in postpartum women is consistent with our previous findings of greater fatigability of trunk flexor muscles for an intermittent, isometric trunk flexion fatiguing task in the Biodex dynamometer.28 The short time-to-task failure of the ASLR Fatigue Task, along with the fact that it requires minimal additional equipment (pressure biofeedback unit, stopwatch, ruler, and plinth), and its good test-retest reliability, make it a practical clinical test.
Performance on the ASLR Fatigue Task was positively associated the MMT strength grade. Postpartum women were weaker than control women when assessed with MMT at 8 weeks and 26 weeks postpartum, which is consistent with a previous study from our group that demonstrated lower isometric trunk flexion strength in postpartum women using the Biodex dynamometer.28
Fatigability of the lumbopelvic stabilizing muscles was also inversely associated with IRD at both 8 weeks and 26 weeks postpartum such that women with a smaller IRD were less fatigable. The association between fatigability and IRD may be partially explained by an impaired ability of the anterior abdominal wall fascia to transfer abdominal muscle force.10 The thinning of the connective tissue caused by pregnancy hormones and substantial stretch of the abdominal wall may compromise the integrity of the fascia, and some of the force generated by the abdominal muscles may be reduced due to ineffective connective tissue.
Postpartum women reported increased pain and disability as well as impaired muscle function on questionnaires compared to nulligravid women. While postpartum women in this study demonstrated Oswestry scores that were statistically higher than control women, these results were not clinically significant (<6% disability; ≤20% is considered mild disability). Further investigation of the fatigability of the lumbopelvic stabilizing muscles in women (postpartum and nulligravid) with clinically significant Oswestry scores would be beneficial to the understanding of the role of fatigability of this muscle group with low back pain and disability. Postpartum women also demonstrated impaired pelvic floor function and increased pelvic girdle symptoms compared with controls. While women in the vaginal delivery group had higher Oswestry, PFDI, and PGQ scores than women in the Cesarean delivery group at 8 weeks postpartum, no difference in these questionnaires was observed between delivery types at 26 weeks postpartum. This lack of difference at 26 weeks was driven by improvement in the vaginal delivery group, while scores for the Cesarean delivery group did not change across time. These findings suggest that vaginal birth is a risk factor for low back pain, pelvic floor dysfunction, and pelvic girdle pain only in the immediate postpartum period, which is in contrast to the popular opinion that Cesarean delivery provides long-term protection against these impairments.50 However, this study only tested women up to 6 months postpartum. Future research should assess the impact of mode of delivery on pain, disability, and pelvic floor dysfunction after the postpartum year.
Women who were more fatigable during the ASLR Fatigue Task also walked a shorter distance in six minutes, which was significant at the 8-week time point but not significant (p = 0.051) at 26 weeks postpartum. Although this association does not imply cause and effect, any instability in the pelvic joints from pregnancy and childbirth, or impaired transfer of muscle force during sustained activity, may contribute to decreased performance of functional mobility. Lower levels of physical activity during pregnancy and immediately after childbirth, shown with accelerometry and self-reported questionnaire, may also have contributed to greater fatigability, as suggested by the significant associations and lower distances on the six-minute walk. Physical activity was also associated with the fatigability of the trunk flexor muscles tested with the intermittent, isometric fatiguing exercise protocol in both healthy men and women51 and postpartum women.28 Lower body fat was also associated with decreased fatigability. These findings support the importance of individualized, prescribed exercise to increase muscle mass/endurance, increase cardiovascular fitness, and decrease body fat in the postpartum period.
The large fatigability of relatively high-functioning postpartum women highlights the need to evaluate fatigability in postpartum women, especially those with clinically significant pain syndromes and musculoskeletal impairments (such as incontinence and pelvic organ prolapse). The profound deficits in strength and fatigability observed in postpartum women at timepoints that extend beyond standard maternity leave (6–12 weeks) also highlights the importance of evaluating musculoskeletal readiness for return to occupations that require performance of repetitive, sustained or strenuous physical activity in order to decrease injury risk. Furthermore, the long-term implications of impaired fatigability of the core musculature is not currently known and warrants further investigation to determine if the increased fatigability observed in this study contributes to health conditions later in life, such as incontinence, low back pain, and pelvic organ prolapse.
Limitations.
In our cohort of women, the nulligravid and postpartum groups were statistically different in age; however, age and fatigability (time to task failure) were not associated. Furthermore, age related differences in musculoskeletal function typically do not present until after age 50 years, and studies of limb muscles show that older women tend to be less fatigable than younger women for isometric tasks.52 However, our data demonstrate that the postpartum women are more fatigable and older than nulligravid women. Although postpartum women are older, there is evidence that this 6-year difference in mean age among this young cohort does not impact muscular performance, including fatigability.
Furthermore, to minimize abdominal muscle activation prior to performing the ASLR fatigue task, IRD was measured at rest without an active head lift. Further research assessing the relationship between IRD with active head lift and fatigability are needed.
Finally, longitudinal comparisons were completed in a subgroup only and the primary analyses were cross-sectional. However, results from the longitudinal analyses supported the results from the cross-sectional analyses. Future research with a larger sample size that could be tested longitudinally would help to confirm these findings. More research is also needed to determine the impact of rehabilitation on fatigability of the lumbopelvic stabilizing muscles in all postpartum women.
Conclusions
Postpartum women demonstrate significant deficits in fatigability of the lumbopelvic stabilizing muscles up to 26 weeks postpartum, with no difference in fatigability between delivery types (vaginal vs Cesarean). Abdominal muscle strength, body fat, physical activity, and inter-recti distance may be contributing factors to fatigability of the lumbopelvic stabilizing muscles. Future research is needed to establish inter-rater reliability of the ASLR Fatigue Task. Further investigation is also needed for: the association of IRD, measured with head lift, and fatigability; fatigability of women (both nulligravid and postpartum) with clinically significant impairments; and the impact of rehabilitation on abdominal muscle fatigability in postpartum women.
Supplementary Material
Grant Support:
Women’s Health Research Program Grant, Department of Obstetrics & Gynecology, Medical College of Wisconsin
American Dissertation Fellowship, American Association of University Women
National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Numbers UL1TR001436 and 1TL1TR001437
This study protocol was approved by the Institutional Review Boards at Marquette University and the Medical College of Wisconsin, and the Office of Clinical Research and Innovative Care Compliance at Froedtert Hospital.
REFERENCES
- 1.Parker M, Millar A. Diastasis Rectus Abdominis and Lumbo-Pelvic Pain and Dysfunction-Are They Related? Journal of Women’s Health Physical Therapy. 2008;32(1):15–22. [Google Scholar]
- 2.Sjodahl J, Gutke A, Oberg B. Predictors for long-term disability in women with persistent postpartum pelvic girdle pain. European Spine Journal. 2013;22(7):1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn S, Youngblut J. Predictors of women’s postpartum health status in the first 3 months after childbirth. Asian Nursing Research. 2007;1(2):136–146. [DOI] [PubMed] [Google Scholar]
- 4.MacLennan A, Taylor A, Wilson D, Wilson D. The prevalance of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. British Journal of Obstetrics and Gynaecology. 2000;107:1460–1470. [DOI] [PubMed] [Google Scholar]
- 5.Gutke A, Ostgaard H, Oberg B. Association between muscle function and low back pain in relation to pregnancy. Journal of Rehabilitation Medicine. 2008;40:304–311. [DOI] [PubMed] [Google Scholar]
- 6.Chearskul S. Endocrine aspects of pregnancy. Siriraj Medical Journal. 2006;58(8):977–979. [Google Scholar]
- 7.Boissonnault JS, Blaschak MJ. Incidence of diastasis recti abdominis during the childbearing year. Phys Ther. 1988;68(7):1082–1086. [DOI] [PubMed] [Google Scholar]
- 8.Gilleard WL, Brown JMM. Structure and Function of the Abdominal Muscles in Primigravid Subjects During Pregnancy and the Immediate Postbirth Period. Journal of the American Physical Therapy Association. 1996;76(7):750–762. [DOI] [PubMed] [Google Scholar]
- 9.Coldron Y, Stokes MJ, Newham DJ, Cook K. Postpartum characteristics of rectus abdominis on ultrasound imaging. Manual therapy. 2008;13(2):112–121. [DOI] [PubMed] [Google Scholar]
- 10.Brown SHM, McGill SM. Transmission of Muscularly Generated Force and Stiffness Between Layers of the Rat Abdominal Wall. Spine. 2009;34(2):E70–E75. [DOI] [PubMed] [Google Scholar]
- 11.Ashton-Miller JA, Delancey JO. On the biomechanics of vaginal birth and common sequelae. Annu Rev Biomed Eng. 2009;11:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilstrap LC III, Cunningham FG, Vandorsten JP. Operative Obstetrics. Second ed: McGraw-Hill Education/Medical; 2002. [Google Scholar]
- 13.Gutke A, Lundberg M, Ostgaard HC, Oberg B. Impact of postpartum lumbopelvic pain on disability, pain intensity, health-related quality of life, activity level, kinesiophobia, and depressive symptoms. European Spine Journal. 2011;20(3):440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodges PW, Kaigle Holm A, Holm S, et al. Intervertebral stiffness of the spine is increased by evoked contraction of transversus abdominis and the diaphragm: in vivo porcine studies. Spine (Phila Pa 1976). 2003;28(23):2594–2601. [DOI] [PubMed] [Google Scholar]
- 15.Pool-Goudzwaard A, van Dijke GH, van Gurp M, Mulder P, Snijders C, Stoeckart R. Contribution of pelvic floor muscles to stiffness of the pelvic ring. Clinical Biomechanics. 2004;19(6):564–571. [DOI] [PubMed] [Google Scholar]
- 16.Pool-Goudzwaard AL, Slieker ten Hove MC, Vierhout ME, et al. Relations between pregnancy-related low back pain, pelvic floor activity and pelvic floor dysfunction. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(6):468–474. [DOI] [PubMed] [Google Scholar]
- 17.Hodges PW, Moseley GL. Pain and motor control of the lumbopelvic region: effect and possible mechanisms. J Electromyogr Kinesiol. 2003;13(4):361–370. [DOI] [PubMed] [Google Scholar]
- 18.Gildea JE, Hides JA, Hodges PW. Size and symmetry of trunk muscles in ballet dancers with and without low back pain. J Orthop Sports Phys Ther. 2013;43(8):525–533. [DOI] [PubMed] [Google Scholar]
- 19.Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain - A motor control evaluation of transversus abdominis. Spine. 1996;21(22):2640–2650. [DOI] [PubMed] [Google Scholar]
- 20.Hungerford B, Gilleard W, Hodges PW. Evidence of Altered Lumbopelvic Muscle Recruitment in the Presence of Sacroiliac Joint Pain. Spine. 2003;28(14):1593–1600. [PubMed] [Google Scholar]
- 21.Mens J, Vleeming A, Snijders C, Koes B, Stam H. Validity of the active straight leg raise test for measuring disease severity in patients with posterior pelvic pain after pregnancy. Spine. 2002;27(2):196–200. [DOI] [PubMed] [Google Scholar]
- 22.Liebenson C, Karpowics A, Brown S, Howarth S, McGill S. The active straight leg raise test and lumbar spine stability. American Academy of Physical Medicine and Rehabilitation. 2009;1:530–535. [DOI] [PubMed] [Google Scholar]
- 23.Mens JM, Vleeming A, Snijders CJ, Stam HJ, Ginai AZ. The active straight leg raising test and mobility of the pelvic joints. Eur Spine J. 1999;8(6):468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mens JMA, in ‘t Veld YHH, Pool-Goudzwaard A. The Active Straight Leg Raise test in lumbopelvic pain during pregnancy. Manual therapy. 2012;17(4):364–368. [DOI] [PubMed] [Google Scholar]
- 25.Borders N. After the Afterbirth: A Critical Review of Postpartum Health Relative to Method of Delivery. The Journal of Midwifery & Women’s Health. 2006;51(4):242–248. [DOI] [PubMed] [Google Scholar]
- 26.Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586(1):11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter SK, Duchateau J, Enoka RM. Muscle fatigue and the mechanisms of task failure. Exerc Sport Sci Rev. 2004;32(2):44–49. [DOI] [PubMed] [Google Scholar]
- 28.Deering RE, Cruz MO, Senefeld JW, Pashibin T, Eickmeyer S, Hunter SK. Impaired Trunk Flexor Strength, Fatigability, and Steadiness in Postpartum Women. Medicine & Science in Sports & Exercise In Press 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hislop HJ, Montgomery J. Daniels and Worthingham’s Muscle Testing: Techniques of Manual Examination. 7th ed. Philadelphia, Pennslyvania: Saunders; 2002. [Google Scholar]
- 30.Mens JM, Vleeming A, Snijders CJ, Koes BW, Stam HJ. Reliability and validity of the active straight leg raise test in posterior pelvic pain since pregnancy. Spine (Phila Pa 1976). 2001;26(10):1167–1171. [DOI] [PubMed] [Google Scholar]
- 31.Vleeming A, Albert HB, Ostgaard HC, Sturesson B, Stuge B. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17(6):794–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross RM, Murthy JN, Wollak ID, Jackson AS. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med. 2010;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kriska AM, Knowler WC, LaPorte RE, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13(4):401–411. [DOI] [PubMed] [Google Scholar]
- 34.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. [DOI] [PubMed] [Google Scholar]
- 35.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–197. [DOI] [PubMed] [Google Scholar]
- 36.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–273. [PubMed] [Google Scholar]
- 37.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25(22):2940–2952; discussion 2952. [DOI] [PubMed] [Google Scholar]
- 38.Stuge B, Garratt A, Krogstad Jenssen H, Grotle M. The pelvic girdle questionnaire: a condition-specific instrument for assessing activity limitations and symptoms in people with pelvic girdle pain. Phys Ther. 2011;91(7):1096–1108. [DOI] [PubMed] [Google Scholar]
- 39.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157–168. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment. 1995;7(4):524–532. [Google Scholar]
- 41.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185(6):1388–1395. [DOI] [PubMed] [Google Scholar]
- 42.Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60(4):573–578. [DOI] [PubMed] [Google Scholar]
- 43.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. [DOI] [PubMed] [Google Scholar]
- 44.Beer GM, Schuster A, Seifert B, Manestar M, Mihic-Probst D, Weber SA. The Normal Width of the Linea Alba in Nulliparous Women. Clinical Anatomy. 2009;22(6):706–711. [DOI] [PubMed] [Google Scholar]
- 45.Teyhen DS, Gill NW, Whittaker JL, Henry SM, Hides JA, Hodges P. Rehabilitative ultrasound imaging of the abdominal muscles. J Orthop Sports Phys Ther. 2007;37(8):450–466. [DOI] [PubMed] [Google Scholar]
- 46.Hunter SK. Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiol (Oxf). 2014;210(4):768–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunter SK, Critchlow A, Shin IS, Enoka RM. Men are more fatigable than strength-matched women when performing intermittent submaximal contractions. J Appl Physiol (1985). 2004;96(6):2125–2132. [DOI] [PubMed] [Google Scholar]
- 48.Hunter SK, Critchlow A, Shin IS, Enoka RM. Fatigability of the elbow flexor muscles for a sustained submaximal contraction is similar in men and women matched for strength. J Appl Physiol (1985). 2004;96(1):195–202. [DOI] [PubMed] [Google Scholar]
- 49.Keller-Ross ML, Pereira HM, Pruse J, et al. Stressor-induced increase in muscle fatigability of young men and women is predicted by strength but not voluntary activation. J Appl Physiol (1985). 2014;116(7):767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bost B. Should elective cesarean birth be offered at term as an alternative to labor and delivery for prevention of complications, including symptomatic pelvic prolapse, as well as stress urinary and fecal incontinence? Obstetrics & Gynecology. 2000;95(4). [Google Scholar]
- 51.Deering R, Senefeld J, Pashibin T, Neumann DA, Hunter S. Muscle Function and Fatigability of Trunk Flexors in Males and Females. Biology of Sex Differences. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunter SK, Pereira HM, Keenan KG. The Aging Neuromuscular System and Motor Performance. J Appl Physiol (1985). 2016: 10.1152/japplphysiol.00475.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


