Abstract
Background
Community-acquired extended-spectrum beta-lactamase–producing Enterobacteriaceae (ESBL) infections are an evolving public health problem. Identifying predictive risk factors may improve patient management.
Methods
We identified 251 adult inpatients admitted to a 22-hospital system with an ESBL urinary tract infection (UTI) between 2001 and 2016. Cases were matched 1:1 with controls who had a UTI at admission with non-ESBL Enterobacteriaceae. Cases with a history of ESBL infections or hospitalization within 3 months of index admission were excluded. Univariate and multiple logistic regression were used to identify risk factors associated with ESBL UTIs.
Results
In univariate analysis, history of repeated UTIs, neurogenic bladder, urinary catheter presence at admission, and exposure to outpatient third-generation cephalosporins or fluoroquinolones within 3 months were associated with higher risk of ESBL UTIs. When controlling for severity of illness and comorbid conditions, history of repeated UTIs (adjusted odds ratio [aOR], 6.40; 95% confidence interval [CI], 3.42–12.66; P < .001), presence of urinary catheter at admission (aOR, 2.36; 95% CI, 1.15–4.98; P < .05), and prior antibiotic exposure (aOR, 7.98; 95% CI, 2.92–28.19; P < .001) remained associated with risk of ESBL infection.
Conclusions
Patients in the community with indwelling urinary catheters, history of recurrent UTIs, or recent antimicrobial use are at higher risk for de novo ESBL Enterobacteriaceae UTIs.
Keywords: antibiotic exposure, ESBL Enterobacteriaceae, urinary tract infection
Extended-spectrum beta-lactamase–producing Enterobacteriaceae (ESBLs) can hydrolyze penicillins, extended-spectrum cephalosporins, and monobactams [1, 2]. ESBL infections are associated with longer hospital stays, increased costs of care, and higher morbidity and mortality due to inappropriate antimicrobial therapy during initial phases of treatment [3–6]. Although typically associated with prior health care–related exposures, the prevalence of community-acquired ESBL infections is increasing [7, 8].
Prevalence of ESBL-associated infections varies depending upon local epidemiology and antibiotic prescribing practices, but has been rising over the past years. Studies in Spain and France have estimated the prevalence of ESBL-producing Enterobacteriaceae isolated from blood and urine cultures in hospitalized patients to be from 4% to 38% [9–11]. In the United States, the SENTRY Antimicrobial Surveillance Program reported that 7% of Klebsiella isolated from blood and urine cultures between 1997 and 2000 in 30 US hospitals tested positive for ESBL production [12]. In 2011–2013, the prevalence of ESBL-producing Klebsiella isolates increased to 15% in 79 US hospitals [12]. In 2013–2014, Talan et al. conducted a prospective study of adults seeking emergency care for pyelonephritis in “EMERGEncy ID NET,” a network of 10 university-affiliated urban emergency departments across the United States [13]. They estimated the prevalence of ESBL-producing Escherichia coli to be around 12%. In a recently published prospective observational study conducted across 5 US academic and community hospitals and associated clinics, Doi et al. found the prevalence of community-associated ESBL E. coli infections to be around 36% [14].
Despite rising prevalence, factors associated with community-acquired ESBL urinary tract infections (CA-ESBL UTIs) have not been well studied in the United States. We investigated the risk factors for ESBL urinary tract infections in adult patients admitted to a large United States health care system with a community-acquired infection. We hypothesized that patients admitted with a first episode of ESBL infection have specific risk factors compared with their age- and gender-matched controls. Early identification of risk factors may minimize ineffective empiric antibiotic therapy and improve clinical outcomes.
METHODS
Study Setting and Data Sources
We conducted a retrospective case-control study in adults admitted with a urinary tract infection from 2001 through 2016 to Intermountain Healthcare, a 22-hospital system extending from Southern Utah to Southern Idaho. Five are tertiary care teaching hospitals in urban settings; the remaining are smaller nonteaching hospitals in suburban and rural locations.
We identified patients with urine cultures growing >100 000 colony-forming units (CFU)/mL of ESBL-producing Enterobacteriaceae. Enterobacteriaceae were considered ESBL producers by our microbiology laboratory if they met the Clinical and Laboratory Standards Institute (CLSI) criteria for positive phenotypic combined-disc testing for ESBL production (for Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, and Proteus mirabilis isolates) and were also genotypically confirmed to be ESBL producers (in cases of Enterobacter, Citrobacter, and Serratia isolates to exclude phenotypic resistance due to inducible amp C enzyme production) [15]. To focus on community-acquired infections, we included only urine cultures collected 7 days before or within 48 hours after a patient’s index hospitalization. Patients with a previous urinary culture isolating Enterobacteriaceae that were either ESBL producers or were resistant to penicillins, third- or fourth-generation cephalosporins, and aztreonam were excluded. Although there is no clear definition of a health care–acquired ESBL infection, we excluded patients with a history of hospitalization for more than 24 hours within a 90-day period before the index hospitalization to minimize the odds of including patients with hospital-acquired infections. Prior antibiotic exposure was defined as any prior exposure to antibiotics for longer than 24 hours within a 3-month period preceding the index hospitalization [16, 17]. Repeated or recurrent urinary tract infections were defined as >3 episodes of urinary tract infections within 1 year preceding the index hospitalization [18, 19].
Most patients with symptomatic ESBL infections require inpatient hospitalization and intravenous carbapenem-based therapy. We decided to not include outpatients to minimize the number of patients with asymptomatic bacteriuria. Additionally, the majority of outpatient records did not have adequate data describing antibiotic exposures, symptoms, or indications for urine cultures. Inpatients with incomplete or unavailable medical records or asymptomatic bacteriuria were excluded. History of international travel was not recorded consistently in patient charts and was excluded from the final analysis. Pregnant or incarcerated patients were excluded from this study. Controls consisted of adult patients admitted with urine cultures positive for >100 000 CFU/mL of non-ESBL-producing Enterobacteriaceae collected 7 days before or within 48 hours of admission. Cases were matched 1:1 with controls based on age, gender, geographic region, and year of admission.
We obtained demographic and clinical data for study patients electronically using Intermountain Healthcare’s electronic data warehouse and electronic medical record. To capture missing or incomplete data, manual review of records was conducted by an Infectious Diseases–trained clinical fellow (D.G.). Manually collected data included severity and duration of symptoms, whether an indwelling urinary catheter was present at the time of admission, presence of an immunocompromising condition, history or renal stones/urinary obstruction, neurogenic bladder, prior urinary tract instrumentation, type and duration of antibiotic therapy, and whether appropriate antibiotic therapy was initiated (defined as the isolated organism having susceptibility to initially prescribed antimicrobial therapy). This study protocol was approved by the Intermountain Healthcare Institutional Review Board.
Statistical Analyses
We conducted univariate analysis using the chi-square or Fisher exact test for discrete outcomes. For continuous variables, the Student t test was used for normally distributed variables and Wilcoxon-Mann-Whitney U test for non–normally distributed variables. Data were presented as percentages for discrete variables and medians with interquartile ranges for continuous variables. We also calculated adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for target risk factors.
Risk factors with significant association to community-acquired ESBLs in the univariate analysis were included in a logistic regression model. We adjusted these results for severity of illness using Sequential Organ Failure Assessment (SOFA) and Charlson comorbidity scores [20, 21]. We defined statistical significance based on confidence intervals, and by P values <.05.
RESULTS
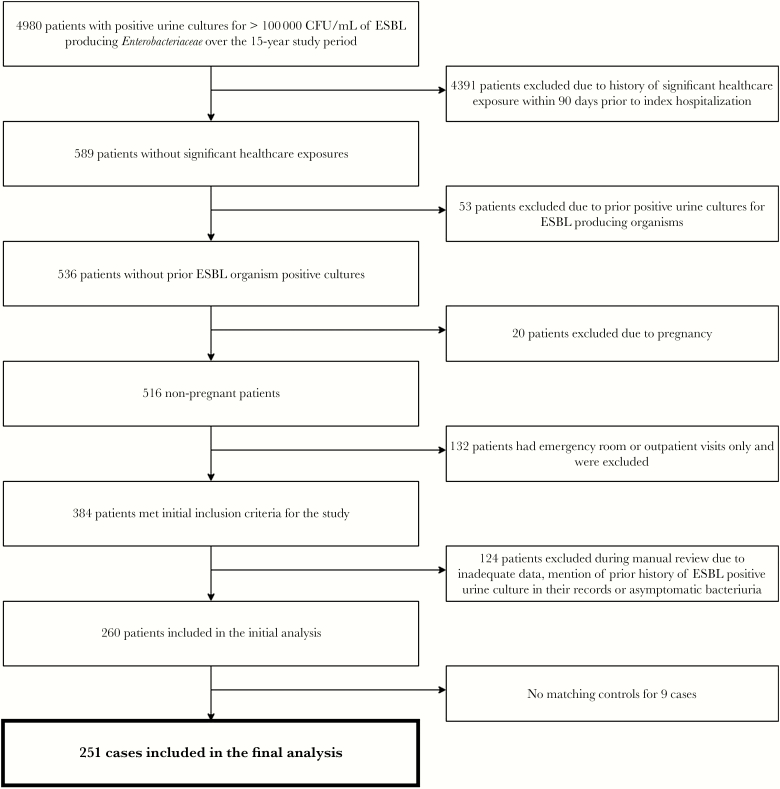
Over the 15-year study period, 4980 of 445 389 cultures of urine specimens collected from adult patients had >100 000 CFU/mL of ESBL-producing Enterobacteriaceae. Out of these, only 589 were from patients without a prior significant health care exposure within 90 days of index hospitalization. Further, after excluding 53 patients with prior cultures positive for ESBL-producing organisms, 20 pregnant patients, and 132 patients with emergency room or outpatient visits only, 384 patients met the inclusion criteria. One hundred twenty-four patients were excluded during manual review due to inadequate data, mention of a history of ESBL-positive cultures in patient records, or asymptomatic bacteriuria. After the above exclusions, 260 cases were included in the initial analysis (Figure 1). A total of 251 cases were included in the final analysis after exclusion of additional 9 patients due to lack of matching controls. Although controls were not matched to cases by the organism, the percentages of common isolates (E. coli and Klebsiella) were similar between cases and controls.
Figure 1.
Flow diagram of inclusion and exclusion of cases in final study analysis.
Common ESBL-producing organisms isolated in cultures were E. coli (74.5%), Klebsiella species (12.4%), Enterobacter cloacae (7.2%), and Citrobacter freundii (4%) (Table 1). Eighteen percent of cases in our study were bacteremic at the time of admission with the same organism that was isolated from their urine cultures.
Table 1.
Demographic and Clinical Characteristics of Cases and Controls Included in the Final Analysis
| Community-Acquired ESBL UTI (n = 251) | Community-Acquired Non-ESBL UTI (n = 251) | P Value | |
|---|---|---|---|
| Median age (IQR), y | 54 (39–64) | 53 (35–63) | .48 |
| Male, No. (%) | 77 (30.7) | 78 (31.1) | .99 |
| Caucasian, No. (%) | 228 (96.6) | 231 (97.5) | .99 |
| American Indian, No. (%) | 1 (0.4) | 0 (0.0) | |
| Asian/Pacific Islander, No. (%) | 3 (1.2) | 4 (1.6) | |
| Other/declined/unknown, No. (%) | 4 (1.6) | 2 (0.8) | |
| Transferred from a skilled nursing facility, No. (%) | 12 (4.8) | 6 (2.4) | .08 |
| Median SOFA score (IQR) |
4.0 (3.0–6.0) | 4.0 (3.0–6.0) | 1.0 |
| Median Charlson comorbidity index (IQR) | 5.0 (3.0–8.0) | 4.0 (3.0–7.0) | .48 |
| Median length of ICU stay (IQR), d | 1.34 (0.09–2.29) | 1.72 (0.14–3.01) | .20 |
| Escherichia coli, No. (%) | 187 (74.5) | 198 (78.9) | .24 |
| Klebsiella pneumoniae, No. (%) | 22 (8.8) | 31 (12.4) | .23 |
| Klebsiella oxytoca, No. (%) | 9 (3.6) | 23 (9.2) | .03 |
| Klebsiella ozaeanae, No. (%) | 0 (0) | 1 (0.04) | 1.00 |
| Citrobacter freundii, No. (%) | 10 (4.0) | 0 (0.0) | .003 |
| Enterobacter cloacae, No. (%) | 18 (7.2) | 0 (0.0) | <.001 |
| Enterobacter aerogenes, No. (%) | 3 (1.2) | 0 (0.0) | .24 |
| Morganella morganii, No. (%) | 2 (0.8) | 1 (0.4) | .96 |
| Proteus mirabilis, No. (%) | 6 (2.4) | 4 (1.6) | .69 |
| Proteus penneri, No. (%) | 1 (0.4) | 0 (0.0) | .98 |
| Proteus vulgaris, No. (%) | 1 (0.4) | 1 (0.4) | 1.00 |
| Serratia marcescens, No. (%) | 3 (1.2) | 0 (0.0) | .23 |
Abbreviations: ESBL, extended-spectrum beta-lactamase–producing Enterobacteriaceae; ICU, intensive care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment; UTI, urinary tract infection.
Multiple potential risk factors were included in univariate analyses (Table 2). However, presence of community-acquired ESBL UTI was found to be significantly associated only with history of recurrent UTIs (adjusted odds ratio [aOR], 9.51; 95% CI, 5.64–16.03; P < .001), presence of a urinary catheter at the time of admission (aOR, 4.3; 95% CI, 2.42–8.22; P < .001), neurogenic bladder (aOR, 3.1; 95% CI, 1.06–11.21; P = .05), and exposure to a third-generation cephalosporin (aOR, 15.89; 95% CI, 3.18–288.50; P < .01) or fluoroquinolone within 3 months before index hospitalization (aOR, 8.24; 95% CI, 3.47–24.31; P < .001). Cases were less likely to receive appropriate antibiotics at the time of admission (aOR, 0.019; 95% CI, 0.01–0.04; P < .001) or anytime during their hospitalization (aOR, 0.06; 95% CI, 0.02–0.14; P < .001) as compared with controls. Living in a nursing home or other extended care facility residence at the time of admission, presence of urosepsis, history of urinary tract instrumentation, prior stroke, history of diabetes mellitus type 1 or 2, or other immunocompromising condition at the time of admission did not reach statistical significance, although these were more frequently present in cases than controls.
Table 2.
List of Variables That Were Analyzed for Consideration as Potential Risk Factors in the Univariate and Multivariate Analysis
| • Duration and severity of symptoms |
| • Presence of a urinary catheter at the time of admission |
| • Presence of concomitant bacteremia |
| • Need for admission to ICU |
| • Duration of ICU stay |
| • Preexisting medical problems: diabetes mellitus, congestive heart failure, chronic liver disease, chronic kidney disease |
| • History of recurrent urinary tract infections (defined as >3 episodes of UTIs within the preceding year) |
| • History of renal stones/benign prostatic hypertrophy/urine outflow obstruction/urinary tract instrumentation or surgery/presence of neurogenic bladder |
| • History of stroke |
| • Travel outside North America within 3 months before index hospitalization |
| • Exposure to antibiotics within 3 months before index hospitalization |
| • Nursing home, long-term acute care facility, or other extended care facility residence at the time of admission |
Abbreviations: ICU, intensive care unit; UTI, urinary tract infection.
After controlling for severity of illness and comorbid conditions during multivariate analysis, history of repeated UTIs, presence of a urinary catheter at the time of admission, and antibiotic exposure within 3 months of admission remained independently associated with higher risk of community-acquired ESBL Enterobacteriaceae UTI (Table 3).
Table 3. .
Multivariate Logistic Regression Model Predicting the Risk Factors Associated With New Community-Acquired ESBL UTIs, Intermountain Healthcare 2001–2016 (n = 502)
| ESBL UTI, aOR (95% CI) | P Value | |
|---|---|---|
| History of repeated UTIs | 6.40 (3.42–12.66) | <.001 |
| Presence of a urinary catheter at the time of admission | 2.36 (1.15–4.98) | <.05 |
| Prior exposure to outpatient antibiotics within past 3 mo | 7.98 (2.92–28.19) | <.001 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; ESBL, extended-spectrum beta-lactamase–producing Enterobacteriaceae; UTI, urinary tract infection.
Cases with ESBL UTIs were more likely than controls to experience delay in receiving appropriate antibiotic therapy (aOR, 41.25; 95% CI, 15.22–151.41; P < .001). SOFA score >9 (aOR, 4.92; 95% CI, 1.55–17.47; P < .01) was also associated with delayed initiation of appropriate antibiotics. Charlson comorbidity score >4 was not associated (aOR, 0.83; 95% CI, 0.43–1.63; P = .59) with delay in appropriate antibiotic therapy. There was no statistically significant difference between the 2 groups in 30-day mortality, 90-day mortality, length of stay, or rate of intensive care unit admission.
DISCUSSION
Because rates of ESBL infections are increasing, several prior investigations have tried to identify risk factors for community-acquired ESBL infections. Tumbarello et al. conducted a case-control study identifying risk factors associated with community-acquired ESBL Enterobacteriaceae infections in Italy [22]. Six variables were independently associated with ESBL Enterobacteriaceae infection: prior hospitalization, history of transfer from another health care facility, Charlson comorbidity score ≥4, recent β-lactam and/or fluoroquinolone use, recent urinary catheterization, and age.
In another small retrospective study of risk factors for ESBL bloodstream infections (BSIs), 79% of ESBL BSIs were found to be community-associated [23]. The number of beta-lactam or fluoroquinolone courses completed by a patient over the past 3 months, prior infection or colonization with ESBL organisms within 12 months, and history of an outpatient procedure within 1 month of admission were found to be predictive of an ESBL BSI. Others have identified the following to contribute to risk of harboring an ESBL-producing organism: history of prior colonization or infection with ESBL Enterobacteriaceae, presence of chronic indwelling vascular hardware, age ≥43 years, recent hospitalization in an ESBL high-burden region, and ≥6 days of antibiotic exposure in the prior 6 months [24].
Our study found 3 risk factors that were independently associated with de novo community-acquired ESBL Enterobacteriaceae urinary tract infections: history of recurrent UTIs, presence of a urinary catheter at the time of admission, and exposure to outpatient antibiotics within 3 months before index hospitalization. Our study validates that antibiotic exposure before the hospitalization and presence of a urinary catheter at admission remain significant risk factors for acquiring a new ESBL urinary tract infection. Given the retrospective nature of this study, we did not have the ability to investigate the exact durations and doses of all outpatient antibiotics. In addition, a history of recurrent UTIs was also identified as an important risk factor. As we focused on only new-onset ESBL infections, we had already excluded patients with a history of prior ESBL infections, which is an established risk factor from most previous studies.
The strengths of this study are its large sample size, consistent case definitions, and exclusion of patients with asymptomatic bacteriurias. We included all urine cultures for patients admitted to Intermountain hospitals over a 15-year period. We used both an electronic database and manual review to collect data and defined strict inclusion and exclusion criteria. Manual data review allowed us to exclude cases with asymptomatic bacteriuria and prior ESBL infection(s).
Limitations included the retrospective design, which prevented us from reliably obtaining data on international travel or consumption of antibiotic-exposed livestock and poultry, both hypothesized to be associated with human ESBL Enterobacteriaceae strains [25–27]. We included only hospitalized patients, which prevents us from drawing conclusions about non-hospitalized ESBL infections. However, most patients with a symptomatic ESBL urinary tract infection require intravenous antibiotics like carbapenems and are often hospitalized. Without having access to culture data outside of the Intermountain Health Care system, we might have inadvertently included some patients with a history of ESBL infection. However, during manual chart review, we did exclude 12 patients whose medical chart documented a prior ESBL Enterobacteriaceae–positive culture outside of Intermountain Healthcare. Most ESBL isolates in our study were E. coli, Klebsiella pneumoniae, or K. oxytoca. There was no statistically significant difference among outcomes between infections caused by these species. Our study lacks the power to identify any differences in risk factors or outcomes for other less frequently encountered organisms. As we matched cases and controls based on age, gender, geographic region, and year of admission, our study may have missed any effect of differences in these key demographic factors on rates of ESBL infections between these two groups.
Due to the retrospective nature of this study, data on duration or reason for urinary catheterization were not available to us in most cases. It is possible that urinary catheterization may be a proxy for factors such as other underlying health issues or nursing home residence. A prospective investigation would need to be conducted to accurately study such associations.
This study complements prior studies and deepens our understanding of risk factors associated with ESBL urinary infections. Patients with ESBL and non-ESBL Enterobacteriaceae often have similar clinical symptomatology, making decisions about empiric antibiotic therapy difficult. Our results may help clinicians initiate effective antimicrobial therapy earlier, thereby improving clinical outcomes. These results may also be used to develop future risk prediction models for testing in prospective, controlled trials geared toward identifying risks and improving outcomes in patients with de novo ESBL urinary tract infections.
Acknowledgments
The authors would like to acknowledge the Members of the Divisions of Infectious Diseases, Intermountain Healthcare Murray, Utah; University of Utah Health, Salt Lake City, Utah; and Division of Pulmonary and Critical Care Medicine, Intermountain Healthcare, Murray, Utah.
Financial support. no funding was required for this study.
Potential conflicts of interest. The authors do not have an association that might pose a conflict of interest (eg, pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding). All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Present affiliation: Mercy Fairfield Hospital, Fairfield, Ohio
References
- 1. Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 2005; 18:657–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bush K, Fisher JF. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from gram-negative bacteria. Annu Rev Microbiol 2011; 65:455–78. [DOI] [PubMed] [Google Scholar]
- 3. Kim BN, Woo JH, Kim MN, et al. Clinical implications of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae bacteraemia. J Hosp Infect 2002; 52:99–106. [DOI] [PubMed] [Google Scholar]
- 4. Lautenbach E, Patel JB, Bilker WB, et al. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 2001; 32:1162–71. [DOI] [PubMed] [Google Scholar]
- 5. Peralta G, Sánchez MB, Garrido JC, et al. Impact of antibiotic resistance and of adequate empirical antibiotic treatment in the prognosis of patients with Escherichia coli bacteraemia. J Antimicrob Chemother 2007; 60:855–63. [DOI] [PubMed] [Google Scholar]
- 6. Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 2007; 60:913–20. [DOI] [PubMed] [Google Scholar]
- 7. Zhanel GG, Hisanaga TL, Laing NM, et al. ; NAUTICA Group Antibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents 2006; 27:468–75. [DOI] [PubMed] [Google Scholar]
- 8. Sanchez GV, Master RN, Karlowsky JA, Bordon JM. In vitro antimicrobial resistance of urinary Escherichia coli isolates among US outpatients from 2000 to 2010. Antimicrob Agents Chemother 2012; 56:2181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chervet D, Lortholary O, Zahar JR, et al. Antimicrobial resistance in community-acquired urinary tract infections in Paris in 2015. Med Mal Infect 2018;. 48(3):188–92. [DOI] [PubMed] [Google Scholar]
- 10. Zahar JR, Lesprit P, Ruckly S, et al. ; BacterCom Study Group Predominance of healthcare-associated cases among episodes of community-onset bacteraemia due to extended-spectrum β-lactamase-producing Enterobacteriaceae. Int J Antimicrob Agents 2017; 49:67–73. [DOI] [PubMed] [Google Scholar]
- 11. Artero A, Esparcia A, Alberola J, et al. Prospective cohort study of risk factors for extended-spectrum ß-lactamase-producing Escherichia coli urinary tract infections in elderly patients admitted to hospital. Int J Clin Pract 2017; 71(9). [DOI] [PubMed] [Google Scholar]
- 12. McDanel J, Schweizer M, Crabb V, et al. Incidence of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella infections in the United States: a systematic literature review. Infect Control Hosp Epidemiol 2017; 38:1209–15. [DOI] [PubMed] [Google Scholar]
- 13. Talan DA, Takhar SS, Krishnadasan A, et al. Fluoroquinolone-resistant and extended-spectrum β-lactamase-producing Escherichia coli infections in patients with pyelonephritis, United States(1). Emerg Infect Dis 2016; 22(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doi Y, Park YS, Rivera JI, et al. Community-associated extended-spectrum β-lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis 2013; 56:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. Twenty Second Informational Supplement Update. CLSI Document M100-S22 U. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 16. Nguyen ML, Toye B, Kanji S, Zvonar R. Risk factors for and outcomes of bacteremia caused by extended-spectrum ß-lactamase-producing Escherichia coli and Klebsiella species at a Canadian tertiary care hospital. Can J Hosp Pharm 2015; 68:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaya O, Akcam FZ, Gonen I, et al. Risk factors for bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli in a Turkish hospital. J Infect Dev Ctries 2013; 7:507–12. [DOI] [PubMed] [Google Scholar]
- 18. Epp A, Larochelle A; Urogynaecology Committee; Family Physicians Advisory Committee Recurrent urinary tract infection. J Obstet Gynaecol Can 2010; 32:1082–90. [DOI] [PubMed] [Google Scholar]
- 19. Dason S, Dason JT, Kapoor A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J 2011; 5:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–10. [DOI] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 22. Tumbarello M, Trecarichi EM, Bassetti M, et al. Identifying patients harboring extended-spectrum-beta-lactamase-producing Enterobacteriaceae on hospital admission: derivation and validation of a scoring system. Antimicrob Agents Chemother 2011; 55:3485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Augustine MR, Testerman TL, Justo JA, et al. Clinical risk score for prediction of extended-spectrum β-lactamase-producing Enterobacteriaceae in bloodstream isolates. Infect Control Hosp Epidemiol 2017; 38:266–72. [DOI] [PubMed] [Google Scholar]
- 24. Goodman KE, Lessler J, Cosgrove SE, et al. ; Antibacterial Resistance Leadership Group A clinical decision tree to predict whether a bacteremic patient is infected with an extended-spectrum β-lactamase-producing organism. Clin Infect Dis 2016; 63:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tängdén T, Cars O, Melhus A, Löwdin E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum beta-lactamases: a prospective study with Swedish volunteers. Antimicrob Agents Chemother 2010; 54:3564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woerther PL, Andremont A, Kantele A. Travel-acquired ESBL-producing Enterobacteriaceae: impact of colonization at individual and community level. J Travel Med 2017; 24:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leverstein-van Hall MA, Dierikx CM, Cohen Stuart J, et al. ; National ESBL Surveillance Group Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect 2011; 17:873–80. [DOI] [PubMed] [Google Scholar]