Abstract
Background
Pauci-bacillary pulmonary tuberculosis (TB) can be delayed to diagnose and start anti-TB therapy, especially in immunocompromised patients. We therefore evaluated the clinical and radiologic features of these delayed cases.
Methods
Immunocompromised adult patients with pauci-bacillary pulmonary TB were retrospectively enrolled in a tertiary hospital in an intermediate–TB burden country over a 5-year period. We defined “missed TB” or “not-missed TB” patients as those who started anti-TB therapy after or before positive mycobacterial culture results, respectively.
Results
Of 258 patients, 134 (52%) were classified in the missed TB group, and 124 (48%) in the not-missed TB group. Positive results of molecular tests including MTB polymerase chain reaction and/or Xpert TB/RIF were only obtained in the not-missed TB group (54/106, 54%). The median diagnostic delay in the missed TB group was longer than in the other group (30 vs 6 days; P < .001). In the missed TB group, the most common working diagnoses were pneumonia (46, 34%) and lung metastasis of malignancy (40, 30%). Typical radiologic findings for TB, such as upper lobe predominance and centrilobular nodules with tree-in-bud appearance, were less common in the missed TB group than in the other group. Old age (odds ratio [OR], 1.03), solid organ transplant (OR, 3.46), solid tumor (OR, 3.83), and hematologic malignancy (OR, 4.04) were independently associated with missed TB.
Conclusions
Care is needed to differentiate pauci-bacillary TB, especially in immunocompromised patients with the mentioned risk factors, even without the usual radiologic features of TB. Additional rapid diagnostic tests to rule out pauci-bacillary TB are urgently needed.
Keywords: immunocompromised, pauci-bacillary, tuberculosis
Delayed diagnosis and treatment of tuberculosis (TB) may lead to transmission of TB and increase the severity, morbidity, and mortality of the disease [1]. For these reasons, early diagnosis and prompt effective therapy form the key elements of TB control programs [2]. Identification of Mycobacterium tuberculosis in respiratory specimens by microbiological culture is the gold standard for establishing a diagnosis of pulmonary TB, but it usually takes 2–8 weeks to acquire positive results for mycobacterial culture [3]. Therefore, clinicians usually start anti-TB therapy, with appropriate isolation, based on clinical manifestations, acid-fast bacilli (AFB) smear microscopy, rapid molecular tests (such as M. tuberculosis polymerase chain reaction [PCR] and Xpert TB/RIF), and radiographic features, before the results of mycobacterial culture are available. However, the sensitivity of AFB smear microscopy is only 34%–80% [4], and although the rapid molecular tests are more sensitive for diagnosing pulmonary TB, they still have limited sensitivity in pauci-bacillary pulmonary TB patients [5, 6]. Moreover, because the clinical manifestations and radiologic features of pulmonary TB are affected by the host immune response to M. tuberculosis and are variable, especially in immunocompromised hosts, it is difficult to diagnose pulmonary TB in immunocompromised patients [7–9].
Therefore, pauci-bacillary pulmonary TB in immunocompromised patients can lead to delayed diagnosis and treatment and to transmission to other immunocompromised patients. Furthermore, such patients have atypical symptoms and radiologic findings, so clinicians can miss the pulmonary TB until the results of microbiological culture are available [10–15]. We therefore evaluated the clinical characteristics and radiologic findings of immunocompromised patients who received delayed diagnosis and treatment of pauci-bacillary pulmonary TB in an intermediate–TB burden country.
METHODS
Study Population
This retrospective study was performed at the Asan Medical Center, a 2700-bed tertiary care teaching hospital in Seoul, South Korea, an intermediate–TB burden country (annual TB incidence in 2016, 63.2 per 100 000 population) [16]. All immunocompromised adult patients (aged ≥18 years) with pauci-bacillary TB were retrospectively enrolled from January 2012 to December 2016. We excluded extrapulmonary TB because we wished to focus on the clinical features and chest computed tomography (CT) findings in cases of delayed diagnosis and treatment of pauci-bacillary pulmonary TB. We also excluded patients who were not followed up after their first sputum AFB examination and patients who were transferred from other hospitals with positive results of AFB smear microscopy at those hospitals (Figure 1). Immunocompetent patients and those with smear-positive TB, who are less likely to be missed TB patients, were excluded from the analysis. The study protocol was approved by the Institutional Review Board of our hospital. Informed consent was waived because of the retrospective nature of this study.
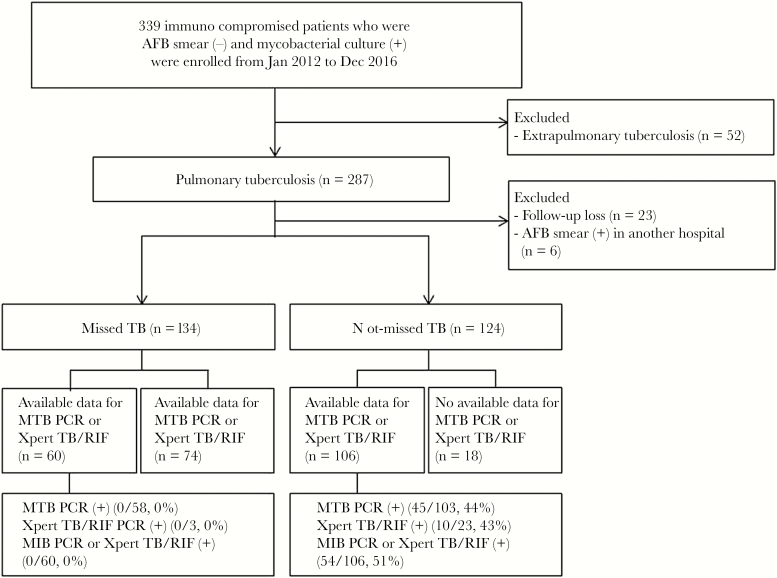
Figure 1.
Schematic flow chart of the study. Abbreviations: AFB, acid-fast bacillus; PCR, polymerase chain reaction; TB, tuberculosis.
Study Design and Definitions
Pauci-bacillary pulmonary TB was defined as pulmonary TB with negative AFB smear results and positive M. tuberculosis culture results from respiratory specimens. Immunocompromised patients were defined as patients with predisposing factors for TB, such as HIV infection, malignancy, liver cirrhosis, chronic renal failure, and transplantation status, or receipt of immunosuppressive treatment [10–15]. We defined “missed TB” patients as those who started anti-TB therapy after positive mycobacterial culture results were obtained and “not-missed TB” patients as those who started the therapy before obtaining the final mycobacterial culture results. In our hospital, we used both liquid and solid media for mycobacterial culture. Diagnostic delay was defined as the interval from mycobacterial test to initiation of anti-TB therapy. We defined “mycobacterial test” as the first AFB examination of respiratory specimens with positive results of mycobacterial culture. We compared the clinical characteristics and radiologic features of the “missed TB” group and the “not-missed TB” group. We also analyzed the working diagnoses of the patients in the missed TB group before the final diagnoses of pulmonary TB. We investigated the impact of empirical antibiotics exposure before diagnosing pulmonary TB. To select patients who were likely to have been exposed to antibiotics with substantial anti-TB activities when they had active TB, we chose the period of antibiotics exposure to be between 4 weeks before the date of mycobacterial tests with positive results and the date of initiation of anti-TB medication, as described in our previous study [17]. And we categorized long-term fluoroquinolone (FQ; at least 7 days’ exposure to FQ), short-term FQ (less than 7 days’ exposure to FQ), non-FQ (exposure to antibiotics other than FQ), and no empirical antibiotic use.
CT Evaluation
Two independent experienced radiologists (M.Y.K. and J.C.) who were unaware of the patients’ clinical characteristics and diagnoses reviewed the initial chest CT images from symptom onset. Final decisions were made by consensus. All images were viewed on the mediastinal (width, 450 HU; level, 50 HU) and lung window (width, 1500 HU; level, –700 HU) settings of the axial image on a picture archiving and communication system (PACS).
We used a glossary of CT imaging definitions to categorize pulmonary lesions [18]. Classifications of radiological patterns were as follows: centrilobular nodules with or without tree-in-bud appearance, bronchial wall thickening (or bronchiolar), macronodule, mass, airspace consolidation, ground-glass opacity, miliary nodules, lymphadenopathy, pleural effusion, and previous TB sequelae without activity. Centrilobular nodules were defined as small dot-like opacities in the center of a normal secondary pulmonary lobule. Tree-in-bud appearance was defined as clustered 2–4-mm nodular and linear branching centrilobular opacities with a lobular or segmented distribution. A macronodule was defined as a focal opacity with a diameter of >1 cm and ≤3 cm, and a mass was defined as an opacity >3 cm in diameter. Consolidation was defined as any exudate or other product of disease that replaced alveolar air, rendering the lung solid. Miliary nodules were defined as profuse, tiny, discrete, rounded pulmonary opacities (<3 mm in diameter) that were generally uniform in size and diffusely distributed throughout the lungs. A ground-glass opacity was defined as a hazy increased lung opacity with preserved margins of bronchioles and vessels [18].
Statistical Analysis
Categorical variables were compared using the χ2 or Fisher exact test, as appropriate, and continuous variables were compared using the Student t test or the Mann-Whitney U test, as appropriate. Risk factors associated with missed TB were investigated in univariate and multivariate analysis using logistic regression models. The final multivariate model was based on both the univariate analysis and the clinical relevance of potential risk factors. We included variables with P ≤ .20 in the final multivariate model. In addition, we also included clinically important risk factors, such as solid organ transplant, despite their having nonsignificant P values in univariate analysis. All tests of significance were 2-tailed, and a P value of less than .05 was considered statistically significant. Calculations were performed using SPSS for Windows, version 21K (SPSS Inc, Chicago, IL).
RESULTS
Patient Characteristics
During the study period, a total of 339 immunocompromised patients with pauci-bacillary TB were enrolled. Of these patients, 52 with extrapulmonary TB were excluded. Of the remaining 287 patients, 29 who were not followed up after their first sputum AFB examination (n = 23), or who had positive results of AFB smear microscopy in another hospital (n = 6), were also excluded. Finally, a total of 258 immunocompromised patients with pauci-bacillary pulmonary TB were analyzed. Of these patients, 134 (52%) were classified in the missed TB group, and the remaining 124 (48%) in the not-missed TB group (Figure 1). Of 106 rapid molecular test results, including M. tuberculosis PCR and/or Xpert TB/RIF, in the 124 patients with not-missed TB, 54 (51%) gave positive results, whereas all 60 molecular test results in the 134 patients with missed TB gave negative results (Figure 1). In addition, 13 (10%) of 134 patients in the missed TB group and 23 (19%) of 124 patients in the not-missed TB group underwent IFN-gamma releasing assay (IGRA), respectively (P = .04). Positive IGRA results were significantly lower in the missed TB group than in the not-missed TB group (Table 1).
Table 1.
Baseline Clinical Characteristics and Outcomes of the 258 Immunocompromised Patients With Pauci-Bacillary Pulmonary TB
| Missed TB (n = 134) | Not-Missed TB (n = 124) | P Value | |
|---|---|---|---|
| Age, median (IQR), y | 66.0 (56.0–75.0) | 60.5 (50.0–69.0) | .003 |
| Male gender | 99 (74) | 89 (72) | .70 |
| Initial clinical symptom or sign | |||
| Fever or febrile sense | 23 (17) | 24 (19) | .65 |
| Cough or sputum | 24 (18) | 29 (23) | .28 |
| Weight loss | 3 (2) | 6 (5) | .32 |
| Dyspnea | 19 (14) | 17 (14) | .91 |
| Other symptoms | 5 (4) | 3 (2) | .72 |
| Abnormal image of chest x-ray | 72 (54) | 54 (44) | .10 |
| Underlying disease | |||
| HIV | 0/116 (0) | 1/110 (1) | .49 |
| Hematologic malignancy | 12 (9) | 6 (5) | .20 |
| Solid tumor | 102 (76) | 70 (56) | <.001 |
| Bone marrow transplant | 2 (1) | 0 (0) | .499 |
| Solid organ transplant | 8 (6) | 8 (6) | .87 |
| Hemodialysis or peritoneal dialysis | 4 (3) | 6 (5) | .53 |
| Diabetes mellitus | 33 (25) | 33 (27) | .72 |
| Underlying condition | |||
| Neutropenia (ANC < 500/m3) | 4 (3) | 1 (1) | .21 |
| Steroid usea | 12 (9) | 6 (5) | .20 |
| Immunosuppressant useb | 14 (10) | 16 (13) | .54 |
| TNF-alpha blocker | 2 (1) | 5 (4) | .27 |
| Cytotoxic chemotherapy within 1 mo | 30 (22) | 15 (12) | .03 |
| Outpatient clinic | 7 (5) | 11 (9) | .25 |
| Previous history of tuberculosis | 20 (15) | 15 (12) | .51 |
| Previous history of tuberculosis treatment | 18/132 (14) | 13/122 (10) | .57 |
| Types of respiratory specimens | |||
| Expectorated sputum | 105 (78) | 83 (67) | .04 |
| Induced sputum | 9 (7) | 9 (7) | .87 |
| Bronchoalveolar lavage fluid | 20 (15) | 32 (26) | .03 |
| NAAT performed | 60 (45) | 106 (85) | <.001 |
| No. of M. tuberculosis PCRs requested | |||
| 1 | 42 | 67 | <.001 |
| 2 | 14 | 25 | .03 |
| 3 | 2 | 11 | .007 |
| No. of Xpert TB/RIFs requested | |||
| 1 | 3 | 20 | <.001 |
| 2 | 0 | 3 | .07 |
| Positive IGRA results | 4/13c (30) | 15/23c (65) | .047 |
| CT scan performed | 111 (83) | 119 (96) | .001 |
| Patient with concurrent extrapulmonary TB | |||
| Lymph node | 2 (1) | 6 (5) | .12 |
| Pleural | 8 (6) | 12 (10) | .27 |
| Pericardial | 0 (0) | 1 (1) | .48 |
| Intra-abdominal | 0 (0) | 9 (7) | <.001 |
| Genitourinary | 0 (0) | 1 (1) | .48 |
| Skeletal | 1 (1) | 0 (0) | >.99 |
| Central nervous system | 0 (0) | 1 (1) | .48 |
| Bone marrow | 0 (0) | 1 (1) | .48 |
| Disseminated | 3 (2) | 19 (15) | <.001 |
| Drug resistance | |||
| Isoniazid resistance | 10/112 (9) | 6/103 (6) | .39 |
| Rifampin resistance | 1/112 (1) | 2/103 (2) | .61 |
| Multidrug resistance | 2/112 (2) | 2/103 (2) | >.99 |
| Empirical antibiotics use | |||
| FQ use | 41 (31) | 30 (24) | .25 |
| Long-term FQ | 26 (19) | 11 (9) | .02 |
| Short-term FQ | 15 (11) | 19 (15) | .33 |
| Non-FQ use | 40 (30) | 39 (32) | .78 |
| Carbapenem | 7 (5) | 15 (12) | .048 |
| Linezolid | 0 | 0 | — |
| No empirical antibiotic use | 53 (39) | 55 (44) | .43 |
| Interval from mycobacterial test to initiation of anti-tuberculosis treatment, median (IQR), d | 30.0 (24.0–42.0) | 6.0 (1.0–14.0) | <.001 |
| Interval from mycobacterial test to positive results of mycobacterial culture on liquid or solid medium, median (IQR), d | 23.0 (19.0–29.0) | 20.0 (17.0–24.0) | <.001 |
| Interval from mycobacterial test to positive results of mycobacterial culture on liquid medium, median (IQR), d | 22.0 (18.0–26.0) | 20.0 (17.0–23.0) | .008 |
| Interval from mycobacterial test to positive results of mycobacterial culture on solid medium, median (IQR), d | 33.0 (28.0–41.0) | 31.0 (25.0–38.0) | .044 |
| ICU admission | 12 (9) | 12 (10) | .84 |
| Duration of ICU care, median (IQR), d | 9.5 (6.3–17.5) | 9.0 (4.5–71.3) | .98 |
| Outcome | |||
| Interval between death and acquisition of sputum mycobacterial culture, median (IQR), dd | 55.0 (17.0–104.0) | 85.0 (16.0–129.0) | .34 |
| 30-d mortality | 8 (6) | 8 (6) | .87 |
| 90-d mortality | 19 (14) | 13 (10) | .37 |
| 180-d mortality | 27 (20) | 23 (19) | .75 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AFB, acid fast bacilli; ANC, absolute neutrophil count; CT, computed tomography; FQ, fluoroquinolone; ICU, intensive care unit; IGRA, IFN-gamma releasing assay; IQR, interquartile range; NAAT, nucleic acid amplification test; PCR, polymerase chain reaction; TB, tuberculosis; TNF, tumor necrosis factor.
aCorticosteroid use is defined as the use of corticosteroids at a mean minimum dose of 0.3 mg/kg/d of prednisolone equivalent for ≥3 weeks.
bTreatment with immunosuppressants (eg, tacrolimus, cyclosporine, sirolimus, azathioprine, or mycophenolate mofetil) during the previous 90 days.
cNumber of patients with a positive test result/number of patients tested.
dPatients who died in the first 180 days.
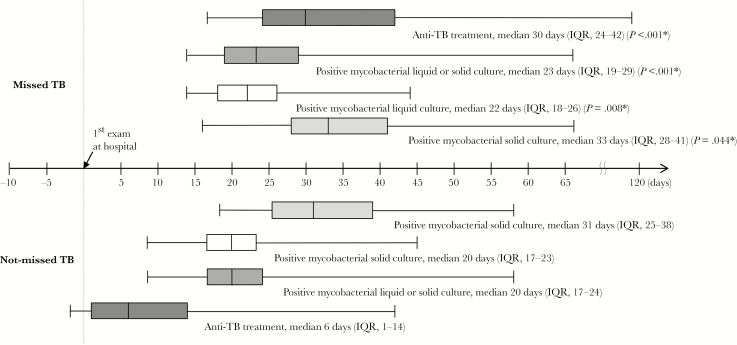
The baseline characteristics and clinical outcomes of all patients are shown in Table 1. The median interval from respiratory specimen collection for mycobacterial test to positive mycobacterial culture results on liquid or solid media in the missed TB group was (IQR) 23.0 (19.0–29.0) days, which was longer than in the not-missed TB group (20.0 [17.0–24.0] days; P < .001). The diagnostic delay was also significantly longer in the missed TB group (median [IQR], 30.0 [24.0–42.0] vs 6.0 [1.0–14.0] days; P < .001) (Figure 2). Patients in the missed TB group were also older (66.0 vs 60.5; P = .003). Abnormal images in chest radiography were the most common initial manifestations in both groups, and initial clinical symptoms, such as cough or sputum and weight loss, were similar in the 2 groups. Solid tumors (76% vs 56%; P < .001) and receipt of cytotoxic chemotherapy within 1 month (22% vs 12%; P = .03) were more common in the missed TB group. We analyzed FQ use including long-term FQ and short-term FQ, and non-FQ antibiotics including carbapenem and linezolid between the 2 groups (Table 1). Long-term FQ use was significantly higher in the missed TB group than in the not-missed TB group (P = .02), whereas carbapenem was significantly lower in the missed TB group than in the not-missed TB group (P = .048). There was no statistically significant difference between the groups in terms of other underlying diseases or conditions, and all-cause 30-day mortality was similar (6% vs 6%; P = .87) (Table 1).
Figure 2.
Box-and-whiskers plots showing the interval from mycobacterial test to initiation of antituberculosis treatment, and from mycobacterial test to positive results of mycobacterial culture, in the missed and not-missed tuberculosis groups. The boxes indicate lower and upper quartiles, the central lines indicate medians, and the ends of the whiskers indicate minima and maxima. Abbreviations: IQR, interquartile range; TB, tuberculosis.
Working Diagnoses in the Missed TB Group
The working diagnoses before pulmonary TB was identified are shown in Table 2. In the missed TB group, the most common working diagnoses were pneumonia (46 patients, 34%) (Figure 3) and lung metastasis of malignancy (40 patients, 30%) (Figure 4). Figures 3 and 4 show some of the CT images of patients with missed TB with atypical radiologic features of TB. Other cases were misdiagnosed with nontuberculous mycobacterium infection, inactive TB, or inflammatory lesions.
Table 2.
Working Diagnoses in Patients With Missed TB
| Working Diagnosis, No. (%) | |
|---|---|
| Possible pulmonary TB | 11a (8) |
| Pneumonia | 46 (34) |
| Bacterial | 6 |
| Viral | 6 |
| Fungal | 1 |
| Unknown | 33 |
| Lung metastasis of malignancy | 40 (30) |
| TB sequelae, inactive TB, LTBI | 16 (12) |
| NTM | 7 (5) |
| Inflammatory lesion | 5 (4) |
| Others | 9 (7) |
| Total | 134 |
Abbreviations: LTBI, latent tuberculosis infection; NTM, nontuberculous mycobacterium; TB, tuberculosis.
aThese 11 patients did not receive empirical anti-TB therapy until the results of mycobacterial cultures were available because they had minimal symptoms or they were reluctant to start the treatment immediately due to side effects of drugs.
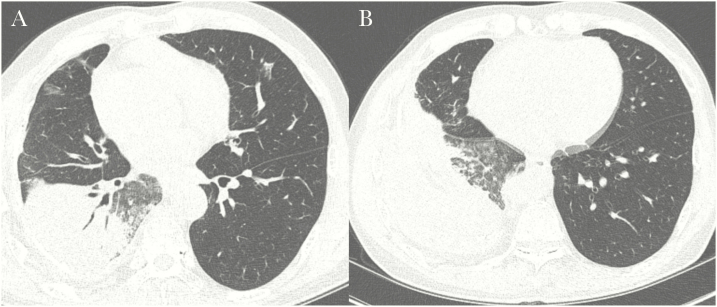
Figure 3.
A missed case of active pulmonary tuberculosis in a 58-year-old man with underlying liver cirrhosis and hepatocellular carcinoma. A, High-resolution computed tomography (CT) scan showing lobar consolidation with air-bronchogram and surrounding ground-glass opacities. Several patchy ground-glass opacities are evident in the right middle lobe and left upper lobe. B, Scan showing several ill-defined nodules in the right lower lobe. The CT findings were interpreted as nonspecific pneumonia, and the possibility of tuberculosis was neglected due to the lower lobe location and the main findings showing a consolidation with an air-bronchogram rather than discrete centrilobular nodules.
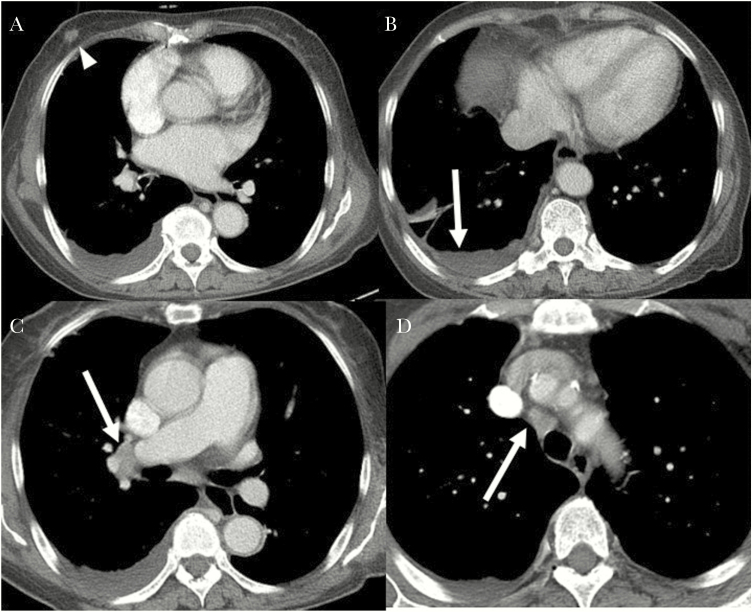
Figure 4.
A missed case of active pulmonary tuberculosis in a 68-year-old woman with primary myelofibrosis and recently diagnosed breast cancer. A, Contrast-enhanced chest computed tomography (CT) shows moderate right pleural effusion. The 1.3-cm nodule in the right breast was identified as breast cancer (arrowhead). B, There is mild enhancing smooth pleural thickening. C, D, Several borderline-to-enlarged right interlobar, hilar, and paratracheal lymph nodes are also visible. The CT findings were interpreted as malignant pleural effusion and possible metastatic lymph nodes.
CT Features of Missed TB and Not-Missed TB in Immunocompromised Patients
Of the 258 immunocompromised patients with pauci-bacillary pulmonary TB, 230 underwent chest CT scan at the time of symptom or sign onset. We compared the CT findings of 111 (83%) of 134 missed TB patients and 119 (96%) of 124 not-missed TB patients (Table 3).
Table 3.
Chest Computed Tomography Features of the 230 Immunocompromised Patients With Pauci-Bacillary Pulmonary TB
| Missed TB (n = 111a) | Not-Missed TB (n = 119a) | P Value | |
|---|---|---|---|
| Dominant lobe | |||
| Upper lobe | 62 (56) | 84 (71) | .02 |
| Middle or lower lobe | 38 (34) | 30 (25) | .13 |
| Centrilobular nodules (<1 cm) with segmental distribution | 92 (83) | 107 (90) | .12 |
| With tree-in-bud appearance | 44 (40) | 78 (66) | <.001 |
| Bronchial wall thickening (or bronchiolar) | 79 (71) | 91 (77) | .36 |
| Macronodules >1 cm and <3 cm | 58 (52) | 78 (66) | .04 |
| With cavity | 14 (13) | 30 (25) | .02 |
| With necrotic lower attenuation | 34 (31) | 38 (32) | .93 |
| Mass >3 cm | 3 (3) | 11 (9) | .04 |
| With cavity | 3 (3) | 7 (6) | .34 |
| With necrotic lower attenuation | 1 (1) | 7 (6) | .07 |
| Airspace consolidation | 39 (35) | 43 (36) | .87 |
| With cavity | 8 (7) | 15 (13) | .17 |
| With air bronchogram | 29 (26) | 34 (28) | .68 |
| With necrotic lower attenuation | 11 (10) | 28 (24) | .006 |
| Ground-glass opacity | 18 (16) | 31 (26) | .07 |
| Miliary nodules | 2 (2) | 11 (9) | .02 |
| Lymphadenopathy >1 cm short diameter | 10 (9) | 41 (35) | <.001 |
| With necrosis | 2 (2) | 12 (10) | .009 |
| Pleural effusion | 9 (8) | 35 (29) | <.001 |
| Previous TB sequelae | 63 (57) | 59 (50) | .28 |
Abbreviations: CT, computed tomography; TB, tuberculosis.
aTwenty-three of 134 missed TB patients and 5 of 124 not-missed TB patients were excluded because they did not undergo chest CT scan.
The dominant affected lobes of the lung were less frequently the upper lobes in the missed TB group than in the not-missed group (62% vs 71%; P = .02). Centrilobular nodules with tree-in-bud appearance (Supplementary Figures 1A–C, 2B), which is a typical finding of pulmonary TB, were also less common in the missed TB group (40% vs 66%; P < .001). Other less common findings in the missed TB group were macronodules (>1 cm and <3 cm) with and without cavities (P = .04 and P = .02, respectively) (Supplementary Figures 1A, 2A, B), masses (P = .04) (Supplementary Figure 3A), airspace consolidations with necrotic lower attenuations (P = .006) (Supplementary Figure 4A), miliary nodules (P = .02) (Supplementary Figure 4B), lymphadenopathy with and without necrosis (P < .001 or P = .009, respectively), and pleural effusion (P < .001).
Risk Factors Associated With Missed TB
The risk factors associated with missed TB are shown in Table 4. In univariate analysis, they were old age, solid tumor, and cytotoxic chemotherapy within 1 month (Table 4). Old age (odds ratio [OR], 1.03; 95% confidence interval [CI], 1.01–1.05; P = .005), solid organ transplant (OR, 3.46; 95% CI, 1.05–11.37; P = .041), solid tumor (OR, 3.83; 95% CI, 1.95–7.52; P < .001), and hematologic malignancy (OR, 4.04; 95% CI, 1.27–12.90; P = .02) were independently associated with missed TB in multivariate analysis (Table 4).
Table 4.
Risk Factors Associated With Missed TB
| Clinical Factor | Univariate Analysis | Multivariate Analysisa | ||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | |
| Age | 1.03 (1.01–1.05) | .003 | 1.03 (1.01–1.05) | .005 |
| Clinical symptoms or signs | ||||
| Cough or sputum | 0.72 (0.39–1.3) | .28 | — | |
| Weight loss | 0.45 (0.11–1.84) | .32 | — | |
| Abnormal image on chest x-ray | 1.51 (0.92–2.46) | .10 | — | |
| Underlying disease | ||||
| Hematologic malignancy | 1.93 (0.70–5.32) | .20 | 4.04 (1.27–12.90) | .02 |
| Solid tumor | 2.46 (1.44–4.19) | <.001 | 3.83 (1.95–7.52) | <.001 |
| Solid organ transplant | 0.92 (0.34–2.53) | .87 | 3.46 (1.05–11.37) | .04 |
| Underlying condition | ||||
| Neutropenia (ANC < 500/m3) | 3.79 (0.42–34.33) | .21 | — | |
| Steroid use | 1.93 (0.70–5.32) | .20 | — | |
| TNF-alpha blocker | 0.36 (0.07–1.89) | .27 | — | |
| Cytotoxic chemotherapy within 1 mo | 2.10 (1.07–4.12) | .03 | — |
Abbreviations: ANC, absolute neutrophil count; CI, confidence interval; OR, odds ratio; TB, tuberculosis; TNF, tumor necrosis factor.
aStatistically significant results were only presented in multivariate analysis.
DISCUSSION
Early diagnosis and treatment of TB are essential elements of TB control programs [2]. Previous studies generally analyzed the diagnostic and treatment delays associated with pulmonary TB from the aspect of socioeconomic and health care systems [19–21]. The clinical and radiologic characteristics of patients with pauci-bacillary pulmonary TB whose diagnosis and treatment are delayed have not previously been examined in detail, especially in immunocompromised patients. In this study, we found that the correct diagnosis of about half of the immunocompromised patients with pauci-bacillary pulmonary TB was missed until mycobacterial culture yielded positive results. The median diagnostic delay in the missed TB group was 30 days, 5 times longer than in the not-missed group. Transmission of TB to other patients during the period of diagnostic delay is a matter of concern [1]. Although smear-negative pulmonary TB is less infectious than smear-positive TB, pauci-bacillary pulmonary TB patients were found to be responsible for about 17% of all total TB transmissions [22]. Another study has shown that long-delayed diagnosis and treatment of pulmonary TB increase the severity of TB and the number of deaths [1]. Therefore, it is important to understand the clinical characteristics associated with diagnostic delay, as this could help clinicians to identify these patients early. We found that old age, solid organ transplants, and solid and hematologic malignancies were associated with missed TB, and that missed TB did not display typical CT findings of pulmonary TB.
In our study, rapid molecular tests, such as M. tuberculosis PCR or Xpert TB/RIF, were performed in 166 of 258 immunocompromised patients with pauci-bacillary pulmonary TB. M. tuberculosis PCR revealed positive results in 37 of 161 (23%) patients, and Xpert TB/RIF revealed positive results in 10 of 26 (38%) patients. Positive results of rapid molecular tests were only obtained in the not-missed TB group. The sensitivity of these tests in this study was lower than that previously reported [5, 6]. This could be explained by the fact that we only examined immunocompromised and smear-negative pulmonary TB patients. We also noted previously that the sensitivity of Xpert TB/RIF in BAL fluid in patients with extremely low pauci-bacillary TB was only 31% [23]. Therefore, early diagnosis of paucibacillary pulmonary TB relies heavily on CT findings. However, we showed above that the imaging findings in missed TB patients were less typical for pulmonary TB, and radiologic data were frequently misdiagnosed as pneumonia or metastases of other malignancies.
Radiologic manifestations are known to differ according to immune status [8, 9]. Atypical radiologic findings of pulmonary TB are more common in immunocompromised patients than immunocompetent ones [24], and as expected, we found that nonspecific CT findings were more common in the missed TB group. Further studies are needed of the possibility that the pathophysiology of extremely low pauci-bacillary TB in immunocompromised patients differs from that of standard TB. Our findings suggest that if a patient has clinical risk factors such as old age, solid organ transplants, or solid or hematologic malignancies, clinicians should keep in mind the possibility of TB even if there are no radiologic features suggestive of TB.
Our study has some limitations. First, it was retrospectively performed in a single large tertiary referral center running active clinical trials and employing the latest treatments for underlying diseases. Therefore, there could be a patient selection bias. Second, we could not separate diagnostic delay into patient delay and health care system delay [19], because data about onset of symptoms and exact times of first contact with a health care provider were limited. Third, rapid molecular tests were not performed in over half of the missed TB patients, so there could be some unmeasured bias that skews the performance of such tests in these patients [11]. In our hospital, the Xpert TB/RIF PCR test was introduced in August 2014, and this test has been conducted in selected patients with suspected tuberculosis due to the national insurance coverage criteria for this test (ie, suspected multidrug-resistant TB, life-threatening TB, and exposure to multidrug-resistant TB patients). Nevertheless, our findings do reflect real clinical practice in relation to suspicion of, and the delayed diagnosis of, TB in an intermediate–TB burden country. Fourth, only 1 patient with HIV was included in our study population. Therefore, our conclusions may not apply in countries with a high prevalence of HIV. Finally, we did not investigate other treatment outcomes, such as the time lag to isolate patients after admission to hospitals and additional exposure burden due to the delay of appropriate isolation. Further studies are needed to evaluate the clinical impact of delayed diagnosis and appropriate isolation of TB patients from other immunocompromised patients. Despite these limitations, our findings provide valuable evidence that clinicians can miss pauci-bacillary pulmonary TB in significant numbers of immunocompromised patients because they are frequently confronted with uncommon manifestations of pulmonary TB in countries with intermediate or high TB burden. It is worth noting that there were significant differences in further verification tests for TB, such as CT, IGRA, and requested respiratory specimen numbers between 2 groups, due to the different suspicion of TB by attending physicians. So, more liberal CT and IGRA tests with repeated respiratory specimen exams are warranted for the early diagnosis of TB in patients with suspicious TB. Furthermore, we find that the imaging findings in missed TB patients were less typical for pulmonary TB, and radiologic data were frequently misdiagnosed as pneumonia or metastases of other malignancies, which indicates that cautious interpretation is needed in this setting.
In conclusion, diagnosis and treatment of pauci-bacillary TB can be delayed until available mycobacterial culture results in about half of immunocompromised patients, especially in the elderly and those with solid organ transplants or hematologic or solid malignancies. Typical radiologic findings suggestive of pulmonary TB are less frequently encountered in missed TB patients. Therefore, care should be taken to differentiate pauci-bacillary TB, especially in immunocompromised patients with the mentioned risk factors. Therefore, before clinicians diagnose patients as having pneumonia or other malignancies of the lung, they need to consider the possibility of TB one more time. Routine evaluation for active TB with rapid molecular tests is needed in immunocompromised patients. Furthermore, additional rapid diagnostic tests to rule out pauci-bacillary TB are urgently needed.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by a grant from the National Research Foundation of Korea, funded by the Ministry of Science, ICT & Future Planning (grant NRF-2018R1D1A1A09082099) and the Asan Institute for Life Sciences (2018–7040).
Potential conflicts of interest. There are no potential conflicts of interest for any of the authors. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Virenfeldt J, Rudolf F, Camara C, et al. . Treatment delay affects clinical severity of tuberculosis: a longitudinal cohort study. BMJ Open 2014; 4:e004818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Global Tuberculosis Report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 3. Siddiqi K, Lambert ML, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis 2003; 3:288–96. [DOI] [PubMed] [Google Scholar]
- 4. Davies PD, Pai M. The diagnosis and misdiagnosis of tuberculosis. Int J Tuberc Lung Dis 2008; 12:1226–34. [PubMed] [Google Scholar]
- 5. American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000; 161:1376–95. [DOI] [PubMed] [Google Scholar]
- 6. Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014;1:Cd009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Crevel R, Ottenhoff TH, van der Meer JW. Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev 2002; 15:294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jones BE, Ryu R, Yang Z, et al. . Chest radiographic findings in patients with tuberculosis with recent or remote infection. Am J Respir Crit Care Med 1997; 156:1270–3. [DOI] [PubMed] [Google Scholar]
- 9. Geng E, Kreiswirth B, Burzynski J, Schluger NW. Clinical and radiographic correlates of primary and reactivation tuberculosis: a molecular epidemiology study. JAMA 2005; 293:2740–5. [DOI] [PubMed] [Google Scholar]
- 10. Johnson JL, Vjecha MJ, Okwera A, et al. . Impact of human immunodeficiency virus type-1 infection on the initial bacteriologic and radiographic manifestations of pulmonary tuberculosis in Uganda. Makerere University-Case Western Reserve University Research Collaboration. Int J Tuberc Lung Dis 1998; 2:397–404. [PubMed] [Google Scholar]
- 11. Kiyan E, Kilicaslan Z, Gurgan M, et al. . Clinical and radiographic features of pulmonary tuberculosis in non-AIDS immunocompromised patients. Int J Tuberc Lung Dis 2003; 7:764–70. [PubMed] [Google Scholar]
- 12. Muñoz P, Rodríguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis 2005; 40:581–7. [DOI] [PubMed] [Google Scholar]
- 13. Kamboj M, Sepkowitz KA. The risk of tuberculosis in patients with cancer. Clin Infect Dis 2006; 42:1592–5. [DOI] [PubMed] [Google Scholar]
- 14. Cho YJ, Lee SM, Yoo CG, et al. . Clinical characteristics of tuberculosis in patients with liver cirrhosis. Respirology 2007; 12:401–5. [DOI] [PubMed] [Google Scholar]
- 15. Kim SH, Song KH, Choi SJ, et al. . Diagnostic usefulness of a T-cell-based assay for extrapulmonary tuberculosis in immunocompromised patients. Am J Med 2009; 122:189–95. [DOI] [PubMed] [Google Scholar]
- 16. Korea Centers for Disease Control & Prevention. Annual Report on the Notified Tuberculosis in Korea, 2016. Osong, Republic of Korea: Korea Centers for Disease Control & Prevention; 2017. [Google Scholar]
- 17. Lee JY, Lee HJ, Kim YK, et al. . Impact of fluoroquinolone exposure prior to tuberculosis diagnosis on clinical outcomes in immunocompromised patients. Antimicrob Agents Chemother 2016; 60:4005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hansell DM, Bankier AA, MacMahon H, et al. . Fleischner society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization. Diagnostic and Treatment Delay in Tuberculosis. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 20. Li Y, Ehiri J, Tang S, et al. . Factors associated with patient, and diagnostic delays in Chinese TB patients: a systematic review and meta-analysis. BMC Med 2013; 11:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sreeramareddy CT, Qin ZZ, Satyanarayana S, et al. . Delays in diagnosis and treatment of pulmonary tuberculosis in India: a systematic review. Int J Tuberc Lung Dis 2014; 18:255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Behr MA, Warren SA, Salamon H, et al. . Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 1999; 353:444–9. [DOI] [PubMed] [Google Scholar]
- 23. Hong J, Lee SH, Ryu BH, et al. . Diagnostic usefulness of bronchoalveolar lavage fluid xpert MTB/RIF in pauci-bacillary pulmonary tuberculosis. Infect Dis (Lond) 2018; 50:725–7. [DOI] [PubMed] [Google Scholar]
- 24. Mathur M, Badhan RK, Kumari S, Kaur N, Gupta S. Radiological manifestations of pulmonary tuberculosis - a comparative study between immunocompromised and immunocompetent patients. J Clin Diagn Res 2017; 11:Tc06–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.