Abstract
Inflammation contributes to the evolution of hypoxic-ischemic (HI) brain injury. High-mobility group box-1 (HMGB1) is a nuclear protein that is translocated from the nucleus and released after ischemia in adult rodents and thereby initiates inflammatory responses. However, there is very little information regarding the effects of HI on HMGB1 in immature brains. To investigate the effects of HI on HMGB1 in the term-equivalent fetal brain, ovine fetuses at 127 days gestation were studied after 30 minutes of carotid occlusion. Groups were sham-control and ischemia with 48 hours and ischemia with 72 hours of reperfusion. By immunohistochemistry, HMGB1 was found to be localized primarily in cell nuclei and partially in cytoplasmic compartments in the cerebral cortex of controls. Ischemia increased the area fraction of neuronal cells with cytoplasmic HMGB1 staining, and Western immunoblot revealed that cytosolic HMGB1 expression increased after ischemia (p < 0.05) and decreased in nuclei in ischemic versus the sham-control brains (p < 0.05). These data indicate that HMGB1 translocates from the nuclear to cytosolic compartments after ischemic brain injury in fetal sheep. This translocation may enable the action of HMGB1 as a proinflammatory cytokine that contributes to HI injury in the developing brain.
Keywords: Cytokine, Fetus, HMGB1, Inflammation, Ischemia, Sheep, Translocation.
INTRODUCTION
Hypoxia-ischemia is one of the leading causes of significant morbidity, mortality, and long-term neurological morbidities during the perinatal period ( 1 ). Neonates exposed to hypoxic-ischemic (HI) injury have increased risk of poor neurological and neurodevelopmental outcomes, including various learning deficits and disabilities ( 2–5 ). Abnormal neuroimaging findings are highly predictive of adverse outcomes in infants after HI brain injury ( 6 ). In particular, diffuse cortical gray matter damage has been associated with adverse outcomes in infants ( 6 ). Characterization of mechanism(s) underlying cellular abnormalities in the brain in response to hypoxia-ischemia is essential for developing novel strategies to attenuate perinatal brain injury ( 2 ). Inflammation is a key factor contributing to the evolving damage after neonatal HI brain injury ( 7 , 8 ). Markers of inflammation in both blood and brain have been consistently observed after HI brain injury ( 9 , 10 ).
The high-mobility group box 1 (HMGB1), previously known as HMG-1 or amphoterin, a nonhistone nuclear DNA-binding protein, has recently been identified as a proinflammatory cytokine upon extracellular release from necrotic tissue or activated immune cells ( 11 , 12 ). HMGB1 maintains DNA structure within cell nuclei ( 13 ), facilitates DNA transcription ( 14 , 15 ), and repairs damaged DNA ( 16 ); it can also function as an immune-stimulatory signal that augments inflammatory processes ( 17–19 ).
Early translocation of HMGB1 has been detected in the brain of adult rodents after stroke-related ischemic injury ( 20–22 ); serum levels of HMGB1 are also increased after the initial onset of symptoms in adult patients with stroke ( 23 ). Furthermore, in vitro studies have shown that oxygen/glucose deprivation and exogenous glutamate initiate the release of HMGB1 in primary neuronal cultures ( 24 ). Although the pathogenesis and underlying cascades of events in ischemic injury are similar between age groups, the effects of ischemia on neuropathological outcomes in neonates and adults have been shown to be variable ( 25–28 ). There is a paucity of information characterizing the effects of hypoxia-ischemia on changes in HMGB1 expression in the immature brain.
Thus far, a single report has examined the expression of HMGB1 in the brains of neonatal rodents exposed to hypoxia-ischemia with or without exposure to hyperoxia ( 29 ). In that study, nuclear HMGB1 levels were observed to be lower after resuscitation of hypoxia-ischemia–exposed neonatal rodents with 100% oxygen compared with those exposed to hypoxia-ischemia alone, suggesting that excessive oxygen exposure augments brain injury in part by increasing the nuclear release of HMGB1 ( 29 ). In addition, a recent clinical report demonstrated higher HMGB1 concentrations in umbilical cord blood of infants after unscheduled cesarean sections and vaginal deliveries compared with infants delivered after planned and controlled normal deliveries ( 30 ). These findings suggest that elevated HMGB1 concentrations in cord blood could be a sensitive marker of untoward perinatal events ( 30 ) and support the contention that HMGB1 could be an important indicator of injury during the perinatal period. However, there is very limited information regarding the expression patterns of HMGB1 after hypoxia-ischemia in the immature brain. Because changes in HMGB1 expression are important after injury in the adult brain ( 20–22 ), we sought to fill this knowledge gap by examining the patterns of change in HMGB1 using the well-studied in utero fetal-sheep model of brain ischemia ( 31–34 ). We examined patterns of expression of HMGB1 in the fetal-sheep model because of its similarities to the human fetal brain with regards to completion of neurogenesis, white matter development, onset of cerebral sulcation, and onset of cortical-evoked potentials ( 31 , 33 , 35–37 ). Moreover, maturation of the ovine brain at 127 days of gestation has similarities to that of the term-equivalent human infant ( 31 , 33 ).
The objective of the present study was to characterize the presence and localization of HMGB1 in the ovine fetal brain after ischemia-reperfusion. Given that cerebral cortical damage represents an important component of brain injury in infants after HI encephalopathy ( 6 ), we focused on changes in HMGB1 expression in the cerebral cortex of the fetal sheep using immunohistochemical localization and Western immunoblot methodology.
MATERIALS AND METHODS
Surgical Procedures and Study Design
This study was conducted after approval by the Institutional Animal Care and Use Committees of Brown University and Women & Infants Hospital of Rhode Island and according to the National Institutes of Health guidelines for use of experimental animals.
Surgery was performed on ewes between 118 and 121 days of gestation, as previously described in detail ( 34 , 38 , 39 ). Briefly, the ewes were administered halothane (1%–2%) and oxygen for anesthesia, the uterus was exposed, and the fetus was operated on via hysterotomy ( 40 ). Polyvinyl catheters were placed into a fetal brachial artery for blood sampling, heart rate, and blood pressure monitoring for the previous studies ( 34 ). An amniotic fluid catheter was placed to measure amniotic fluid pressure as a referent for fetal arterial blood pressure. The carotid arteries were exposed and both the vertebral-occipital anastomoses and lingual arteries were ligated in order to restrict blood flow from the vertebral and noncerebral blood vessels, respectively ( 33 ). Two inflatable 4-mm vascular occluders (In Vivo Metric, Healdsburg, CA) were then placed around each carotid artery, along with a perivascular flow probe (Transonic System, Inc., Ithaca, NY), caudal to the occluders. The ewes were permitted 5–8 days to recover from surgery.
The fetal sheep were randomly assigned to 3 groups: 30 minutes of ischemia followed by 48 hours of reperfusion (I/R-48), 30 minutes of ischemia followed by 72 hours of reperfusion (I/R-72), or control sham-operated nonischemic fetal sheep (Sham). Brain ischemia was induced by inflating the carotid artery occluders with 0.9% NaCl for 30 minutes. Thereafter, cerebral reperfusion began by deflating the occluders. The occluders were not inflated in the control sham group.
The physiological measurements of the fetal sheep have been previously reported ( 34 ). The baseline pH, PCO 2 , PO 2 , blood pressure, heart rate, and hematocrit values were within normal physiologic ranges. Significant differences in the measurements were not detected between the sham-operated and ischemia-exposed fetal sheep before or after ischemia ( 34 ).
Brain Removal and Pathological Processing
At the end of the reperfusion periods, a hysterotomy was performed on the ewes after intravenous injection of pentobarbital (15–20 mg/kg), the fetus was withdrawn, and the ewe and fetus were killed (100–200 mg/kg pentobarbital). The fetal brains were rapidly removed ( 34 ). One section of the frontal cortex was immediately frozen in liquid nitrogen and stored at −80 °C for later analysis. Samples were obtained in alternating fashion from the right or left frontal cerebral cortex of each fetal brain within each study group. The remainder of the brain was placed in 10% formalin. In the current study, we used coronal sections from our previous report, which were taken at the level of the posterior medial eminence that we termed “slice 3” ( 34 ). Brains from another group of sham-operated fetal sheep at similar gestational ages that had been perfused in situ with 10% formalin and fixed with 10% formalin were also provided by our collaborators (J.O.D., G.W., L.B., and A.J.G.) and included as a second set of control fetal sheep ( 41 ). The additional sham-operated group that had been perfused in utero were included in order to compare the results to our fetal sheep that were not perfused in utero because the earlier work in adult rodents had used an in vivo perfusion procedure at the time of death ( 20–22 ).
The preservation and histological techniques were very similar for the fetal sheep brains obtained from University of Auckland, Auckland, New Zealand, and those from the studies at Brown University, Providence, Rhode Island. The fixed brains in both laboratories were paraffin embedded and remained as intact blocks until the sections were cut and slides prepared specifically for use in the current studies. In the case of the slides from New Zealand, slides were freshly cut from the paraffin blocks of brains collected during the preceding season for this study. Serial sections were prepared after discarding the first section to obtain fresh tissue for the current study. The slides were stained in the laboratory at Brown University. Similarly, slides were prepared from the paraffin blocks from the previous studies ( 34 ). Although there could be trivial, nonmeasured differences in the cutting and mounting procedures between the University of Auckland and Brown University laboratories that might account for the slight differences in the immunohistochemical outcomes, the basic slide preparation procedures were similar.
Immunostaining
Paraffin-embedded 8-µm-thick sections from the Sham, I/R-48, and I/R-72 groups were heated for 1 hour at 60 °C before deparaffinization in xylene (Thermo Fisher Scientific, Waltham, MA) and rehydration in 100%, 95%, and 75% ethanol (Thermo Fisher Scientific). After washing in Tris-buffered saline with 0.025% Triton X-100 (TBS-T), sections were incubated with 0.3% H 2 O 2 for 10 minutes and washed with TBS-T. An antigen retrieval procedure was performed in a 0.1 M sodium citric acid (Sigma-Aldrich, St Louis, MO) buffer (pH 6.0, containing 0.05% Tween-20) by heating the slides in an autoclave at 120 °C for 10 minutes ( 22 ). Thereafter, the sections were washed with TBS-T and immersed for 2 hours in TBS solution containing 5% normal goat serum (Vector Laboratories, Burlingame, CA) and 1% bovine serum albumin (Sigma-Aldrich, Inc.) in order to block nonspecific binding. Sections were then incubated with mouse anti-HMGB1 monoclonal antibody ([mAb] R&D systems, Minneapolis, MN) for 1 hour at room temperature and, thereafter, washed with TBS-T. A horseradish peroxidase-labeled goat anti-mouse antibody was then added to the slides and incubated at room temperature for an additional 1 hour. After carefully washing with TBS-T, a 3,3′-diaminobenzidine (DAB) substrate kit (BD PharMingen, San Diego, CA) was applied to the slides and incubated in the dark for 3–5 minutes. Sections were placed in deionized water to stop the reaction; dehydrated in 70%, 90%, and 100% ethanol; cleared in xylene; and mounted.
Mouse anti-HMGB1 mAb (R&D systems) was mixed with a rabbit anti-NeuN Ab (Abcam, Cambridge, MA) for immunofluorescent staining to specifically identify HMGB1 translocation in neuronal nuclei. Sections were incubated with a mixture of the corresponding secondary antibodies: Alexa-555-conjugated goat anti-mouse IgG (Invitrogen, Grand Island, NY) and Alexa-488-conjugated goat anti-rabbit IgG (Invitrogen). The secondary antibody incubation lasted for 1 hour at room temperature before the sections were mounted with VECTASHIELD Hardset mounting medium with DAPI (Vector).
Microscopy and Quantification
Stained slides were observed under a Zeiss Axio Imager M2 imaging system (Carl Zeiss, Inc., Jena, Germany). Stereo Investigator 10.0 software by MBF Bioscience (MicroBrightField, Inc., Williston, VT) was used to trace the outline of the coronal sections that were visualized by DAB. One section of the sheep brain at the level of the posterior medial eminence of each animal was used for quantification. The entire cerebral cortex section of the brain was examined for brain regions containing cells with HMGB1-positive staining within the cytoplasmic compartment that were then considered as cells exhibiting HMGB1 translocation from the nucleus to the cytoplasm. Examination of the brain under the stereomicroscope revealed that the cells with HMGB1 translocation were not evenly distributed over the entire cerebral cortex. Therefore, we quantified the amount of cells with HMGB1 translocation by using the area fraction, which enabled analysis of the entire cerebral cortex of the fetal sheep brain. An outer contour outline was made of the entire coronal sheep brain section on each slide. This outermost contour line delineated the entire area of the brain section. Areas containing cells with HMGB1 translocation were then considered areas exhibiting cytoplasmic HMGB1 translocation. These areas were then circumscribed using new contour lines. The area fraction of cells containing cytoplasmic HMGB1 staining was calculated as the percent of the entire brain area. In a subset of control sham and ischemia-exposed fetal sheep, cell counting within the areas containing cells with HMGB1 translocation, which were contoured, revealed that more than 66% of the total number of cells exhibited HMGB1 translocation from the nucleus to the cytoplasm. In contrast, the adjacent areas containing cells that did not exhibit HMGB1 translocation, which were not contoured, exhibited less than 6% of cells with translocation (p < 0.001). Quantification of the area fraction was based upon immunohistochemical staining visualized by DAB. The study was conducted in a double-blind manner with an independent third-party coding and labeling slides for the 2 ischemia-reperfusion groups and the sham (I/R-48, I/R-72, and Sham) group. Investigators (J.Z. and D.K.) performing microscopy and statistical analysis did not have knowledge of treatment groups.
Protein Extraction of Cytoplasmic and Nuclear Fraction
Frozen samples from the frontal cerebral cortex of each fetus were subjected to protein extraction separately for the nuclear and cytosolic protein fractions using density gradient ultracentrifugation ( 42 , 43 ). Approximately 0.3 g of cerebral cortex was cut from the frozen samples of cerebral cortex and homogenized in buffer A solution (0.32 M sucrose, 10 mM Tris-HCl buffer, and 3 mM MgCl 2 , pH 6.8), which contained a 1% complete protease inhibitor cocktail (Roche, Nutley, NJ). The solution was then centrifuged at 850 g for 10 minutes at 4 °C. The pellet was assigned as pellet #1 and saved for the nuclear-fraction preparation. The supernatant was collected in a new ultracentrifuge tube as supernatant #1 and centrifuged for 1 hour at 40,000 g in a Beckman ultracentrifuge (Beckman, Indianapolis, IN), creating pellet #2 and supernatant #2. Supernatant #2 was saved for the cytosolic preparation. Pellet #1 was combined with 6.7 mL of buffer A and 28.3 mL of buffer B (2.4 M sucrose, 10 mM Tris-HCL buffer, and 1 mM MgCl 2 , pH 6.8) and centrifuged for 1 hour at 4 °C at 53,000 g , resulting in pellet #3 and supernatant #3. Pellet #3 was then resuspended in buffer A and centrifuged again at 850 g for 10 minutes to remove the 2.4 M sucrose. This pellet was stored in buffer A as the nuclear fraction, aliquoted, and frozen at -80°. Finally, supernatant #2 was centrifuged at 100,000 g for 1 hour, and the resultant supernatant was aliquoted and used as the cytosolic fraction ( 42–44 ).
Western Immunoblot
Two micrograms of either cytosolic or nuclear protein, prepared in a denaturing sample buffer, were fractionated using ready-to-use 4%–12% BIS TRIS SDS-polyacrylamide gel (Invitrogen) electrophoresis. After electrophoresis, proteins on the gel were transferred onto polyvinylidene diflouride membrane (0.2 µm, Bio-Rad Laboratories, Hercules, CA) using a semidry transfer technique. Membranes were blocked with 10% skim milk (Bio-Rad) prepared in TBS-T for 1 hour at room temperature and washed 4 times in TBS-T. The membranes were then probed overnight at 4 °C with rat anti-HMGB1 mAb conjugated to peroxidase using a peroxidase labeling kit-NH2 (Dojindo Molecular Technologies, Inc., Rockville, MD) and incubated with enhanced chemiluminescence solution (Amersham Pharmacia Biotech, Piscataway, NJ) before exposure to autoradiography film (Daigger, Vernon Hills, IL). Vinculin was probed with a mouse anti-vinculin antibody (Thermo Fisher Scientific) and a peroxidase-conjugated goat anti-mouse IgG (Zymax, Arlington, VA) as an internal loading control. Contamination of nuclear proteins within the cytosolic fraction was tested with a nuclear envelope protein, lamin B, using a mouse anti-lamin B antibody (Lifespan Biosciences, Seattle, WA), followed by the incubation with the same goat anti-mouse IgG (Zymax) as was used for vinculin.
All experimental samples were normalized to a protein extract obtained from a homogenate pool from the cerebral cortex of 1 adult sheep. These samples served as internal-control samples. The use of the internal-control standard allows for comparisons among large groups of study subjects examined on multiple immunoblots and facilitates accurate quantification of the Western immunoblots, as previously described ( 44–47 ). Vinculin expression was also used as a loading control to ensure that equal amounts of protein were applied to each lane. The optical densities of HMGB1 proteins were expressed as a ratio to the internal control values, thus facilitating normalized comparisons among the different experimental groups and immunoblots. Band intensities were analyzed with Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD). All experimental samples were normalized to the average of 3 internal control samples on each immunoblot. The final values represented the ratio of the experimental to average internal-control sample densitometry values obtained from at least 3 different immunoblots ( 44–47 ).
Glucose-6-Phosphate Dehydrogenase Assay
Glucose-6-phosphate dehydrogenase (G6PDH) is a cytosolic specific enzyme that catalyzes the conversion of glucose-6-phosphate (G6P) to 6-phosphoglucono-d-lactone (6-PG), the first step, and rate-limiting step, of the pentose phosphate pathway. To test for contamination of cytosolic proteins in the nuclear fraction, the enzyme activity of G6PDH in the extraction of the cytosolic and nuclear fractions was tested using a G6PDH assay kit (Sigma, MAK015-1) following the manufacturer’s protocol ( 48 ).
Statistical Analysis
Results are expressed as mean ± SD. One-way ANOVA was used to examine differences between groups for Western immunoblotting. If a significant effect of group was found, the Fisher least significant difference test was utilized to identify specific differences among the groups. Because data for the area fraction of cells containing HMGB1 translocation did not fit a normal distribution, data were log-transformed before ANOVA. Correlational analysis was used to compare the concentration of G6PDH to the quantity of nuclear HMGB1 and also to compare our previously reported pathological injury scores ( 34 ) with the area fraction of cells with cytosolic HMGB1 in the current report. A value of p < 0.05 was considered significant.
RESULTS
HMGB1 Staining in the Sham-Operated Fetal Sheep Brain
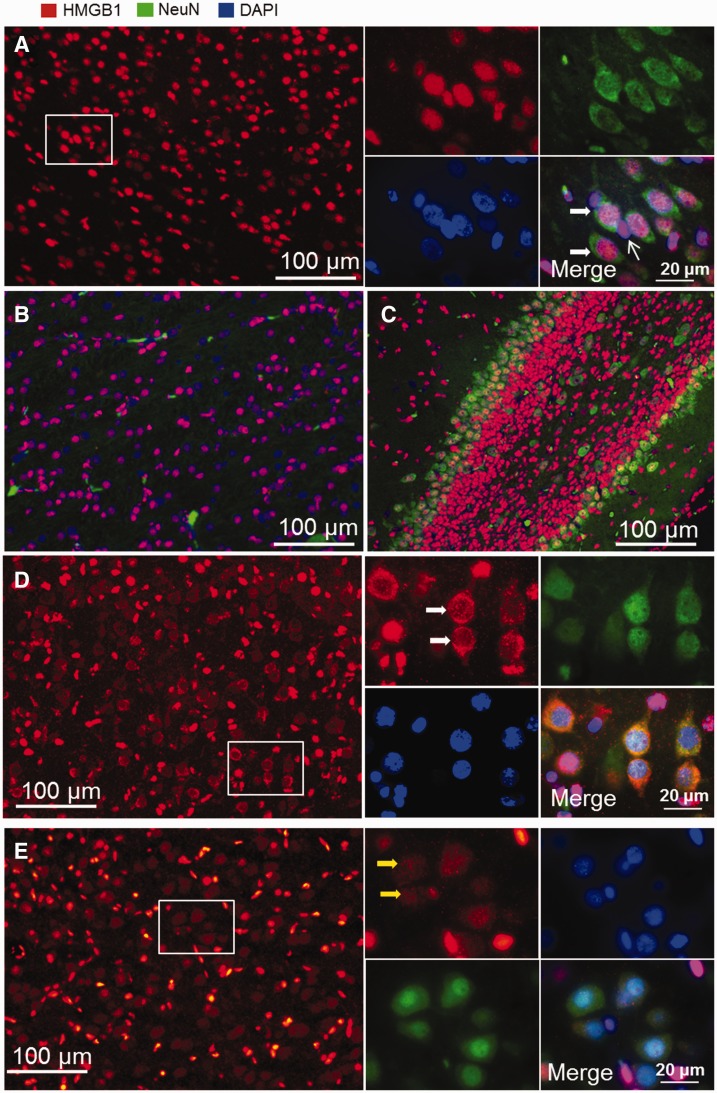
Immunofluorescent staining of whole coronal brain sections revealed that sections from the sham-operated fetal sheep exhibited HMGB1 staining that was mostly confined to the nuclear compartment, which colocalized with the DAPI-positive nuclear staining within the cerebral cortex ( Fig. 1A ), white matter ( Fig. 1B ), and hippocampus ( Fig. 1C ). Double staining for HMGB1 and the neuronal marker NeuN revealed that HMGB1 was located in both neuronal (wide arrows) and non-neuronal cells (narrow arrows) in the fetal brain ( Fig. 1A insert). However, HMGB1 was also localized to the cytoplasmic compartment in a few cerebral cortical regions in the deep sulci of the parietal cortex, as shown by decreased nuclear HMGB1 staining along with increased cytoplasmic HMGB1 staining ( Fig. 1D ).
FIGURE 1.
Immunofluorescent staining of HMGB1 in sham-operated fetal sheep brain. (A) HMGB1 staining is positive in the cell nuclei in cerebral cortex. Double staining for HMGB1 and NeuN ( A , insert) shows that HMGB1 localizes to nuclei in both neuronal (wide arrows) and non-neuronal cells (narrow arrow). (B, C) Nuclear localization of HMGB1 was also observed in white matter (B) and hippocampus (C) . (D) Cells with cytoplasmic staining for HMGB1 were detected in the cerebral cortex of the sham-operated fetal sheep brain (insert). Double staining for HMGB1 and NeuN indicates neurons with cytoplasmic staining for HMGB1 (white arrows). (E) Neuronal cells (yellow arrows) with cytoplasmic staining for HMGB1 were also detected in normal fetal brains that had been perfused with formalin before brain collection (red, HMGB1; green, neurons/NeuN; blue, nucleus/DAPI).
In our previous studies, the brain sections of the fetal sheep (as shown in Fig. 1A–D ) were rapidly removed and immersion formalin fixed and were not perfused in situ ( 34 ). In contrast, previous work in control adult rodents demonstrating predominant localization of HMGB1 to the nucleus was performed after in vivo perfusion fixation ( 22 , 49 ). Hence, we also used brain sections from sham-operated fetal sheep that were exposed to an in situ perfusion fixation procedure ( 41 ) in order to identify any effect of in situ perfusion versus immersion fixation on the expression of HMGB1. Figure 1E contains a representative section from a sham-operated fetal sheep brain that was obtained after in situ perfusion similar to the procedure that had been used in adult rodents ( 22 , 49 ). Cytoplasmic staining of HMGB1 was also detected in neuronal cells in the cerebral cortex in sections from the in situ–perfused fetal sheep ( Fig. 1E inset). The staining patterns of the cells with cytoplasmic HMGB1 show differences between the immersion-fixed and in situ–perfused fetal sheep brains ( Fig. 1E vs. Fig. 1D insets) in that the HMGB1 cytoplasmic staining in the nonperfused brain appeared to show more condensed staining in the cytoplasm along with depletion of HMGB1 staining within the nuclei ( Fig. 1D white arrows). In contrast, the sections from the in situ–perfused fetal brain exhibited a more diffuse pattern with similar densities of HMGB1 staining in both the cytoplasmic and nuclear compartments ( Fig. 1E insert, yellow arrows). Consequently, cytoplasmic HMGB1 staining detected in both the perfused and nonperfused fetal sheep brains may indicate normal background HMGB1 translocation from the nuclear to cytoplasmic compartments in some areas of the cerebral cortex in the sham-control sheep.
HMGB1 Staining in the Fetal Brain After Ischemic Injury
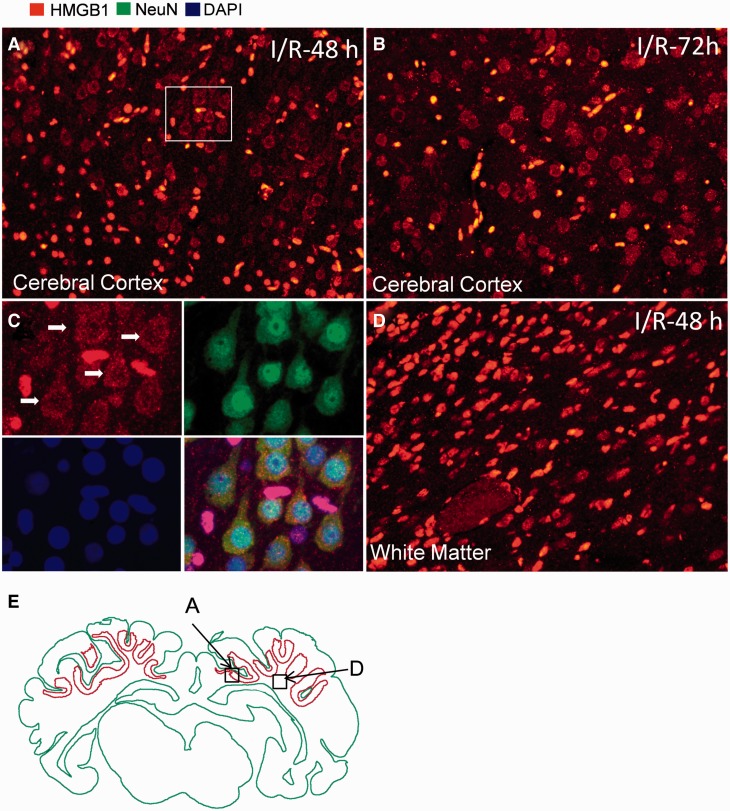
Immunofluorescence staining of HMGB1 in the fetal sheep brain exposed to ischemia and reperfusion demonstrated both nuclear (data not shown) and cytoplasmic staining of HMGB1 after 48 and 72 hours of reperfusion ( Fig. 2A, B ). Double staining for HMGB1 and NeuN indicated that the cytoplasmic staining of HMGB1 was mostly located in neuronal cells ( Fig. 2C ). Examination of the entire coronal fetal sheep brain by stereomicroscopy revealed that the cells with HMGB1 translocation were not evenly distributed over the entire cerebral cortex. Rather, they were condensed mainly within the deep sulci of layers 2–5 in the medial parietal cerebral cortex. In Figure 2E , the green outline delineates outline of major overall brain structures and the red outline delineates areas of cytoplasmic HMGB1 staining. Morphological changes of HMGB1 staining were not observed in the white matter of the fetal sheep exposed to ischemia-reperfusion ( Fig. 2D ).
FIGURE 2.
Immunofluorescent staining of HMGB1 in fetal sheep brain with ischemic brain injury. (A, B) Cytoplasmic staining for HMGB1 was observed in fetal brains 48 hours ( A , I/R-48 h) and 72 hours ( B , I/R-72 h) after ischemia reperfusion. (C) Higher magnification view of the insert in (A) shows double immunohistochemical staining for HMGB1 and NeuN, indicating that cells with cytoplasmic staining of HMGB1 were detected mainly in neurons. (D) Cytoplasmic staining for HMGB1 was not detected in the white matter of fetal brains 48 hours after ischemia reperfusion. (E) Brain regions with cytoplasmic staining for HMGB1 were contoured (red) in the cerebral cortex. Boxes in (E) indicate the regions where the images of (A) and (D) were obtained.
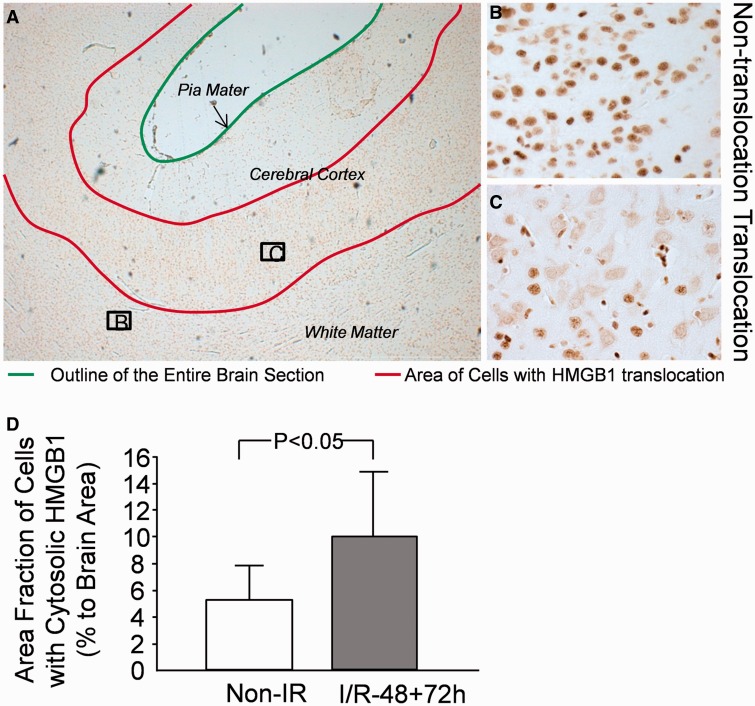
To quantify cytoplasmic staining of HMGB1, areas with cytoplasmic HMGB1 staining in all brain sections were contoured, and the area of the cellular translocation was determined as the percent of the total area of each coronal brain section examined ( Fig. 3A ). As shown in Figure 3B , areas expressing HMGB1 in the nucleus but not in the cytoplasm were identified as areas that did not exhibit translocation and, therefore, were not contoured. In contrast, areas expressing HMGB1 in the cytoplasm ( Fig. 3C ) were considered as cells with HMGB1 translocation and were contoured. In this study, we used slice number 3 from our former work ( 34 ). In that study, the neuropathological injury scores in slice number 3 did not differ between the I/R-48 and I/R-72 groups ( 34 ). Therefore, we combined the findings from the 2 ischemic reperfusion groups. Figure 3D contains a graphical representation of the area fraction of the regions containing HMGB1 translocation as a ratio to the total area of the whole coronal brain sections in the sham-control group and in the combined ischemia-reperfusion groups. There was a 2-fold increase in the area fraction of the regions with cells demonstrating HMGB1 translocation ( Fig. 3D , p < 0.04) in the brain sections of the ischemia-reperfusion groups versus sham controls. There was no significant correlation between our previously reported pathological injury scores ( 34 ) and the area fraction of cells expressing cytoplasmic HMGB1 (r = 0.34, n = 13, p = 0.31).
FIGURE 3.
Quantification of cells with cytoplasmic staining for HMGB1 in fetal sheep brain. (A) Immunohistochemical staining of HMGB1 in fetal sheep brain with ischemic injury (48 hours after ischemia). Green line indicates the brain surface; red lines indicate brain regions with cytoplasmic staining of HMGB1. (B) Cells with nuclear staining for HMGB1 contoured within green lines. (C) Cells with cytoplasmic staining for HMGB1 contoured in red lines. (D) Area fraction of cells with cytoplasmic staining for HMGB1 as a ratio of the total brain area; n = 3 in the control group without ischemia (Non-IR); n = 13 in the combined group with ischemia-reperfusion (I/R-48 + 72 h). Values are mean ± SD. *p < 0.05 vs. Non-IR.
Western Immunoblots
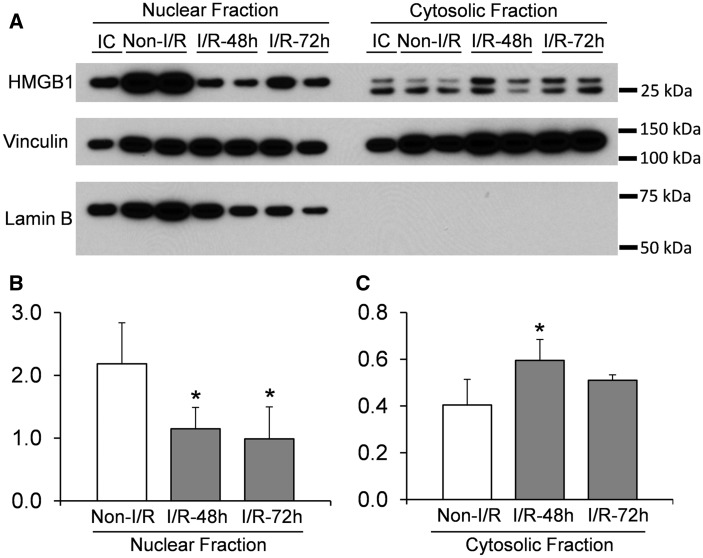
Translocation of HMGB1 in fetal brain after ischemic brain injury was further confirmed by Western immunoblot analysis using the nuclear and cytosolic fractions of the cerebral cortex in each group to determine HMGB1 expression ( Fig. 4 ). The HMGB1 protein from the nuclear fraction was detected as a single band at approximately 28 kDa. In contrast, HMGB1 in the cytosolic fraction exhibited 2 bands detected at 28 and 25 kDa ( Fig. 4A ). Moreover, ischemia-reperfusion did not appear to change the distribution of the 2 bands ( Fig. 4A ). Quantitative analysis of the densities of the HMGB1 bands demonstrated a decrease in the amount of HMGB1 in the nucleus 48 and 72 hours after ischemia. In contrast, there was an increase in HMGB1 expression in the cytosolic fraction 48 hours after ischemia ( Fig. 4B ), but no difference was detected in the cytosolic HMGB1 at 72 hours after ischemia compared with the sham group ( Fig. 4C ).
FIGURE 4.
Western immunoblot detection of HMGB1 in nuclear and cytosolic fractions extracted from cerebral cortex of ovine fetuses. (A) Representative images of Western immunoblot show HMGB1 detection in nuclear and cytosolic fractions. Vinculin was used as a loading control. Lamin B was detected to indicate that there was no contamination of the nuclear fraction in the cytosolic fractions. (B, C) Optical density analysis of HMGB1 bands in sham (Sham, n = 5), ischemia-reperfusion 48 h (I/R-48 h, n = 4), and ischemia-reperfusion 72 h (I/R-72 h, n = 5) animals in nuclear and cytosolic fractions, respectively. Values are mean ± SD; *p < 0.05 vs. Sham.
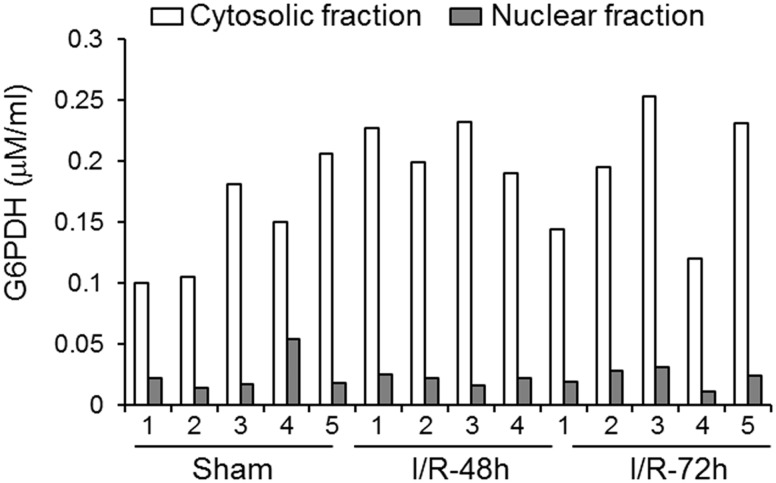
Lamin B, a nuclear envelope marker, was used to verify the purity of the cytosolic fraction. Lamin B was detected in all the nuclear samples but not in the cytosolic fractions, confirming a lack of contamination in the cytosolic fraction ( Fig. 4A ). In addition, a G6PDH assay also was used to verify the purity of the nuclear fraction. The cytosolic enzyme G6PDH was localized predominately to the cytosolic fraction within each sample used for the Western immunoblot ( Fig. 5 ). There were extremely low levels of G6PDH detected in the nuclear fraction compared with the cytosolic fraction. In addition, the concentration of G6PDH did not correlate with the amount of nuclear HMGB1 (r = 0.13, n = 14, p = 0.67). These findings indicate minimal contamination of the nuclear fraction by the cytosolic fraction, which would not affect the HMGB1 expression detected in the nuclear fraction.
FIGURE 5.
G6PDH assay in the cytosolic and nuclear fractions in each sample used for the Western immunoblot detection of HMGB1 in sham (Sham, n = 5), ischemia-reperfusion 48 h (I/R-48 h, n = 4), and ischemia-reperfusion 72 h (I/R-72 h, n = 5) animals in nuclear and cytosolic fractions. Numbers in x-axis indicate animal number in each group.
DISCUSSION
Translocation and release of HMGB1 after ischemic brain injury has been reported previously in models of stroke in adult rodents ( 22 , 49 ). The current study is the first to report changes in the localization and expression of HMGB1 in the immature brain after ischemia using the sheep model of fetal brain ischemia ( 33 , 34 ). The results showed that HMGB1 was widely expressed in cellular nuclei in the brain of the sham-operated animals along with a minor population of cells that expressed HMGB1 in the cytoplasm. In contrast, increased neuronal cytoplasmic HMGB1 staining was apparent, particularly in the deep sulci of the cerebral cortex after exposure of the fetal sheep to brain ischemia. This finding was detected by quantification of immunohistochemical staining and confirmed by Western immunoblot. Our findings in the fetal sheep brain are consistent with those in adult rodents after ischemic injury and suggest alterations in HMGB1 expression and localization after ischemia in the fetal brain that could contribute to ischemia-related inflammation ( 8 , 20–22 ).
Previous work in the normal developing mouse brain has reported 3 different cellular phenotypes of HMGB1 expression on embryonic day 16 (E16): (i) approximately 50% of cells express HMGB1 in nuclei, (ii) 1% of cells express HMGB1 in cytoplasm, and (iii) approximately 50% of the cells do not express HMGB1 ( 50 ). These findings are to some extent similar to the nuclear pattern of HMGB1 expression that we observed in nonischemic fetal sheep; that is, HMGB1 was localized to the nucleus in the majority of cells. Nonetheless, 5% of the cells in brain sections from the sham-operated fetal sheep expressed HMGB1 in the cytoplasmic compartment ( Figs. 1, 3 ). The observation that HMGB1 is positive in the majority of cells differs from the findings in the E16 mouse embryos that state only 50% of cells were positive for HMGB1 ( 50 ). However, differences between our findings in the sham-operated fetal sheep and the mouse embryo at E16 could be related to species and maturational differences ( 51 ).
The fetal sheep brains used for immunohistochemical quantification of HMGB1 translocation in this study were derived from brain samples that were immersion formalin fixed after brain removal ( Figs. 1A–D, 2, 3 ) ( 34 ). However, we also included an example of a brain from an in situ formalin-perfused fetal sheep at the same gestational age as the nonperfused fetal sheep and observed cytoplasmic staining of HMGB1 in the cerebral cortex ( Fig. 1E ). Although an in situ perfusion procedure should result in more optimal brain preservation than immersion fixation, this procedure requires approximately 10–20 minutes from the time of maternal death to the initiation of the in situ perfusion ( 41 ). In the adult, HMGB1 is thought to be a sensitive marker of cellular injury ( 52–54 ). Accordingly, both immersion fixation and in situ perfusion could have been associated with some degree of cellular injury during the fixation processes; that is, slight delays in fixation of individual brain cells could have resulted in injury-induced HMGB1 translocation even in the sham fetal brains. This contention is also supported by the finding that the cells containing the greatest amount of HMGB1 translocation were detected mostly in the deep sulci of the cerebral cortex in both sham and ischemic-reperfusion groups ( Fig. 2 ), which is an area that has been shown to be sensitive to ischemic injury ( 55 , 56 ). Nonetheless, the fetal sheep in both the sham-operated and ischemia-reperfusion groups were identically treated by immersion fixation ( 34 ). The higher amounts of cytoplasmic HMGB1 staining in the ischemic-reperfusion compared with the sham group suggest that ischemic injury promotes cytoplasmic HMGB1 expression and, consequently, its translocation from the nuclear to cytoplasmic compartment.
Although we attempted to identify cells with near-total loss of HMGB1 in the fetal sheep brain, this phenomenon was far more variable in the fetal sheep brains after global ischemia induced by bilateral carotid than after localized middle cerebral artery occlusion in the adult rodent brain ( 20 , 22 ). It is also important to note that carotid occlusion in the fetal sheep brain results in global brain injury involving both the cortex and white matter ( 34 ), with no ischemic core lesion as detected after stroke in the adult rodent brain ( 20 , 22 ). Consequently, it is not surprising that near loss of HMGB1 staining was not as apparent after ischemia in the fetal sheep brain as after stroke in the adult rodent brain ( 20 ). Therefore, we reported translocation of HMGB1 into the cytosol, rather than near-complete loss of HMGB1 from cells.
The differences between our findings in the fetal sheep and those in the adult mice and rats after stroke may also be related to maturational differences ( 26 , 27 ). In addition, we did not find a significant correlation between the pathological injury scores from our previous report ( 34 ) and area fraction of cells expressing cytoplasmic HMGB1. The lack of correlation could be related to the necessarily limited sample size in this large animal model or to the specific timing of the analysis. Moreover, we examined subcellular localization of HMGB1, whereas in the previous report, we had measured overall histopathological injury rather than specific subcellular injury; this difference could also account for the lack of apparent correlation.
In the present study, we focused on changes in HMGB1 in the cerebral cortex because damage to this area of the brain is associated with multiple long-term morbidities ( 6 ). The current study demonstrated alternations in HMGB1 mainly in the cerebral cortex, suggesting that HMGB1 may be a sensitive marker for cerebral cortical ischemic injury. Moreover, we did not observe apparent HMGB1 translocation in the white matter of the sham control or fetal sheep exposed to ischemia ( Figs. 1B, 2D ). The data are consistent with previous findings in adult rodents showing that HMGB1 translocation occurs mainly in neurons ( 20 , 22 ). In contrast, the major cell types are oligodendrocytes and astrocytes in the white matter. We speculate that there could be a differential sensitivity of the neurons and oligodendrocytes to ischemia-reperfusion injury and/or differences in the spatial heterogeneity of oligodendrocyte sensitivity to ischemia ( 57 ).
The results from immunohistochemical quantification were supported by Western immunoblot analysis. There was a significant increase in cytosolic HMGB1 in conjunction with reductions in nuclear HMGB1, which further supports the contention that ischemia enhances HMGB1 translocation from the nuclei to the cytosol in the ovine fetus ( Fig. 4 ). The protein extraction methods that we utilized to obtain the cytosolic fractions of cerebral cortex do not distinguish between the cellular cytosolic fraction and soluble proteins contained within the extracellular space, however. Therefore, the cytosolic HMGB1 detected by Western immunoblot could have also included HMGB1 in the extracellular space. This possibility might explain the relatively high cytosolic expression of HMGB1 detected even in the sham-operated animals ( Fig. 4 ).
The Western immunoblot analysis revealed that 2 bands of HMGB1 protein were detected using our specific rat anti-bovine HMGB1 mAb ( 22 ) in the cytosolic fraction of the cerebral cortex, whereas only 1 band was detected in the nuclear fraction in both the sham and ischemic-reperfusion groups ( Fig. 4 ). The upper band of HMGB1 in the cytosolic fraction was located at the same position on the immunoblot as the nuclear fraction with a molecular weight of approximately 28 kDa. The lower band of HMGB1 in the cytosolic fraction was approximately 25 kDa ( Fig. 4A ), which suggests the possibility of structural changes in HMGB1 during its translocation. This concept is supported by recent evidence suggesting that the redox state of HMGB1 is critical in determining which HMGB1 receptor is activated ( 58 , 59 ). Three isoforms of HMGB1 that have been identified include the fully reduced HMGB1, disulfide-HMGB1, and oxidized HMGB1 ( 59–62 ). Only the disulfide-HMGB1 possesses cytokine-inducing activity among the 3 isoforms ( 59–62 ). Our findings are also consistent with a previous report showing a shift in the HMGB1 Western immunoblot bands after oxidation in ischemic rat liver ( 63 ). Consistent with our findings, the reported molecular weights of HMGB1 were 28 and 26 kDa ( 62 ). Therefore, we speculate that the lower band of HMGB1 in the cytosolic fraction ( Fig. 4 ) could represent the oxidized form of HMGB1. However, 2 bands were observed in the cytosolic fractions of both the sham and ischemic reperfusion groups, suggesting the possibility that the process of translocation itself could have been responsible for the expression of the 2 bands in the cytosolic fraction in the ovine fetus. Nonetheless, our results suggest that HMGB1 can also be translocated in the fetal brain after ischemic brain injury and could contribute to brain inflammation similarly to our previous findings in adult rats after ischemic brain injury ( 22 ).
The mechanism for the translocation of HMGB1 from the nucleus to the cytoplasm cannot be discerned from this study; however, previous work has suggested that mechanisms of HMGB1 translocation from the nucleus to the cytoplasm include posttranslational modifications. Acetylation of the lysine residues in HMGB1 neutralizes the basic charge of HMGB1, facilitating translocation to the cytoplasm ( 64 ). The proinflammatory signals of HMGB1 after ischemic brain injury activate calcium signaling through calmodulin, NF-κB, and mitogen-activated protein kinases, which can cause acetylation ( 65 ). Alternatively, tumor necrosis factor can initiate phosphorylation at the serine residues of HMGB1, which can also neutralize its negative charge, inducing HMGB1 secretion ( 66 ). Therefore, at least 2 known residues and 2 distinct mechanisms could have been responsible for the HMGB1 cytoplasmic localization that we observed after ischemia. Although the mechanisms of translocation in the sham and ischemic fetal sheep brain cannot be determined by our study, we speculate that HMGB1 might be posttranslationally modified via acetylation and/or phosphorylation, resulting in its translocation into the cytoplasm.
The only approved treatment for HI encephalopathy in the newborn is hypothermia, which is only partially protective ( 3 , 67 , 68 ). Inflammation with increases in proinflammatory cytokines is thought to be an important component of evolving HI brain injury after reperfusion ( 69–71 ). The findings of our study suggest that HMGB1 constitutes an important component of the postischemic inflammatory milieu in the brain during development. Therefore, future studies are needed to employ potential anti-inflammatory strategies to augment the partial neuroprotective effects of hypothermia ( 22 , 49 ).
We conclude that HMGB1 is translocated from the nucleus to the cytoplasmic compartment after ischemic brain injury in fetal sheep. We speculate that the translocation of HMGB1 may play an important role in facilitating the action of HMGB1 release, which could be a potentially critical proinflammatory cytokine that accentuates HI brain injury in the developing brain. The results from the current study also suggest that HMGB1 inhibition could represent a potential therapeutic strategy for the treatment of neonatal HI injury.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Richard Tucker, BA, in the Department of Pediatrics of the Women & Infants Hospital of Rhode Island for the support of the data analysis and statistics.
REFERENCES
- 1. Jacobs S, Hunt R, Tarnow-Mordi W , et al. . Cooling for newborns with hypoxic ischaemic encephalopathy . Cochrane Database Syst Rev 2007. ; 4 : CD003311. [DOI] [PubMed] [Google Scholar]
- 2. Perrone S, Stazzoni G, Tataranno ML , et al. . New pharmacologic and therapeutic approaches for hypoxic-ischemic encephalopathy in the newborn . J Matern Fetal Neonatal Med 2012. ; 25(Suppl 1) : 83 – 8 [DOI] [PubMed] [Google Scholar]
- 3. Shankaran S, Laptook AR, McDonald SA , et al. . Temperature profile and outcomes of neonates undergoing whole body hypothermia for neonatal hypoxic-ischemic encephalopathy . Pediatr Crit Care Med 2012. ; 13 : 53 – 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shankaran S, Pappas A, McDonald SA , et al. . Childhood outcomes after hypothermia for neonatal encephalopathy . N Engl J Med 2012. ; 366 : 2085 – 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stephens BE, Liu J, Lester B , et al. . Neurobehavioral assessment predicts motor outcome in preterm infants . J Pediatr 2010. ; 156 : 366 – 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leijser LM, Vein AA, Liauw L , et al. . Prediction of short-term neurological outcome in full-term neonates with hypoxic-ischaemic encephalopathy based on combined use of electroencephalogram and neuro-imaging . Neuropediatrics 2007. ; 38 : 219 – 27 [DOI] [PubMed] [Google Scholar]
- 7. McAdams RM, Juul SE. The role of cytokines and inflammatory cells in perinatal brain injury . Neurol Res Int 2012. ; 2012 : 561494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferriero DM. Neonatal brain injury . N Engl J Med 2004. ; 351 : 1985 – 95 [DOI] [PubMed] [Google Scholar]
- 9. Liu T, Clark RK, McDonnell PC , et al. . Tumor necrosis factor-alpha expression in ischemic neurons . Stroke 1994. ; 25 : 1481 – 8 [DOI] [PubMed] [Google Scholar]
- 10. Saito K, Suyama K, Nishida K , et al. . Early increases in TNF-alpha, IL-6 and IL-1 beta levels following transient cerebral ischemia in gerbil brain . Neurosci Lett 1996. ; 206 : 149 – 52 [DOI] [PubMed] [Google Scholar]
- 11. Wang H, Bloom O, Zhang M , et al. . HMG-1 as a late mediator of endotoxin lethality in mice . Science 1999. ; 285 : 248 – 51 [DOI] [PubMed] [Google Scholar]
- 12. Yang H, Wang H, Czura CJ , et al. . The cytokine activity of HMGB1 . J Leukoc Biol 2005. ; 78 : 1 – 8 [DOI] [PubMed] [Google Scholar]
- 13. Catez F, Yang H, Tracey KJ , et al. . Network of dynamic interactions between histone H1 and high-mobility-group proteins in chromatin . Mol Cell Biol 2004. ; 24 : 4321 – 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calogero S, Grassi F, Aguzzi A , et al. . The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice . Nat Genet 1999. ; 22 : 276 – 80 [DOI] [PubMed] [Google Scholar]
- 15. Bianchi ME. Significant (re)location: How to use chromatin and/or abundant proteins as messages of life and death . Trends Cell Biol 2004. ; 14 : 287 – 93 [DOI] [PubMed] [Google Scholar]
- 16. Lange SS, Vasquez KM. HMGB1: The jack-of-all-trades protein is a master DNA repair mechanic . Mol Carcinog 2009. ; 48 : 571 – 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation . Nature 2002. ; 418 : 191 – 5 [DOI] [PubMed] [Google Scholar]
- 18. Rovere-Querini P, Capobianco A, Scaffidi P , et al. . HMGB1 is an endogenous immune adjuvant released by necrotic cells . EMBO Rep 2004. ; 5 : 825 – 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: Friend and foe . Cytokine Growth Factor Rev 2006. ; 17 : 189 – 201 [DOI] [PubMed] [Google Scholar]
- 20. Qiu J, Nishimura M, Wang Y , et al. . Early release of HMGB-1 from neurons after the onset of brain ischemia . J Cereb Blood Flow Metab 2008. ; 28 : 927 – 38 [DOI] [PubMed] [Google Scholar]
- 21. Kim SW, Lim CM, Kim JB , et al. . Extracellular HMGB1 released by NMDA treatment confers neuronal apoptosis via RAGE-p38 MAPK/ERK signaling pathway . Neurotox Res 2011. ; 20 : 159 – 69 [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Takahashi HK, Liu K , et al. . Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats . Stroke 2011. ; 42 : 1420 – 8 [DOI] [PubMed] [Google Scholar]
- 23. Goldstein RS, Gallowitsch-Puerta M, Yang L , et al. . Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia . Shock 2006. ; 25 : 571 – 4 [DOI] [PubMed] [Google Scholar]
- 24. Faraco G, Fossati S, Bianchi ME , et al. . High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo . J Neurochem 2007. ; 103 : 590 – 603 [DOI] [PubMed] [Google Scholar]
- 25. Yager JY, Thornhill JA. The effect of age on susceptibility to hypoxic-ischemic brain damage . Neurosci Biobehav Rev 1997. ; 21 : 167 – 74 [DOI] [PubMed] [Google Scholar]
- 26. Vexler ZS, Ferriero DM. Molecular and biochemical mechanisms of perinatal brain injury . Semin Neonatol 2001. ; 6 : 99 – 108 [DOI] [PubMed] [Google Scholar]
- 27. Vexler ZS, Tang XN, Yenari MA. Inflammation in adult and neonatal stroke . Clin Neurosci Res 2006. ; 6 : 293 – 313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vexler ZS, Yenari MA. Does inflammation after stroke affect the developing brain differently than adult brain? Dev Neurosci 2009. ; 31 : 378 – 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gill MB, Bockhorst K, Narayana P , et al. . Bax shuttling after neonatal hypoxia-ischemia: Hyperoxia effects . J Neurosci Res 2008. ; 86 : 3584 – 604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakamura T, Yamada S, Yoshioka T. Measurement of plasma concentration of high mobility group box1 (HMGB1) in early neonates and evaluation of its usefulness . Clin Chim Acta 2012. ; 413 : 237 – 9 [DOI] [PubMed] [Google Scholar]
- 31. Back SA, Riddle A, Hohimer AR. Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury . J Child Neurol 2006. ; 21 : 582 – 9 [DOI] [PubMed] [Google Scholar]
- 32. Elitt CM, Sadowska GB, Stopa EG , et al. . Effects of antenatal steroids on ischemic brain injury in near-term ovine fetuses . Early Hum Dev 2003. ; 73 : 1 – 15 [DOI] [PubMed] [Google Scholar]
- 33. Gunn AJ, Gunn TR, de Haan HH , et al. . Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs . J Clin Invest 1997. ; 99 : 248 – 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petersson KH, Pinar H, Stopa EG , et al. . White matter injury after cerebral ischemia in ovine fetuses . Pediatr Res 2002. ; 51 : 768 – 76 [DOI] [PubMed] [Google Scholar]
- 35. Bernhard CG, Kolmodin GM, Meyerson BA. On the prenatal development of function and structure in the somesthetic cortex of the sheep . Prog Brain Res 1967. ; 26 : 60 – 77 [DOI] [PubMed] [Google Scholar]
- 36. Barlow RM. The foetal sheep: Morphogenesis of the nervous system and histochemical aspects of myelination . J Comp Neurol 1969. ; 135 : 249 – 62 [DOI] [PubMed] [Google Scholar]
- 37. Cook CJ, Williams C, Gluckman PD. Brainstem auditory evoked potentials in the fetal sheep, in utero . J Dev Physiol 1987. ; 9 : 429 – 39 [PubMed] [Google Scholar]
- 38. Chen X, Threlkeld SW, Cummings EE , et al. . Ischemia-reperfusion impairs blood-brain barrier function and alters tight junction protein expression in the ovine fetus . Neuroscience 2012. ; 226 : 89 – 100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang J, Sadowska GB, Chen X , et al. . Anti-IL-6 neutralizing antibody modulates blood-brain barrier function in the ovine fetus . Faseb J 2015. ; 29 : 1739 – 53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stonestreet BS, Patlak CS, Pettigrew KD , et al. . Ontogeny of blood-brain barrier function in ovine fetuses, lambs, and adults . Am J Physiol 1996. ; 271 : R1594 – 601 [DOI] [PubMed] [Google Scholar]
- 41. Guan J, Bennet L, George S , et al. . Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep . J Cereb Blood Flow Metab 2001. ; 21 : 493. [DOI] [PubMed] [Google Scholar]
- 42. Giufrida AM, Cox D, Mathias AP. RNA polymerade activity in various classes of nuclei from different regions of rat brain during postnatal development . J Neurochem 1975. ; 24 : 749 – 55 [PubMed] [Google Scholar]
- 43. Katsetos CD, Spandou E, Legido A , et al. . Acute hypoxia-induced alterations of calbindin-D28k immunoreactivity in cerebellar Purkinje cells of the guinea pig fetus at term . J Neuropathol Exp Neurol 2001. ; 60 : 470 – 82 [DOI] [PubMed] [Google Scholar]
- 44. Spasova MS, Sadowska GB, Threlkeld SW , et al. . Ontogeny of inter-alpha inhibitor proteins in ovine brain and somatic tissues . Exp Biol Med 2014. ; 239 : 724 – 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ron NP, Kazianis JA, Padbury JF , et al. . Ontogeny and the effects of corticosteroid pretreatment on aquaporin water channels in the ovine cerebral cortex . Reprod Fertil Dev 2005. ; 17 : 535 – 42 [DOI] [PubMed] [Google Scholar]
- 46. Sadowska GB, Malaeb SN, Stonestreet BS. Maternal glucocorticoid exposure alters tight junction protein expression in the brain of fetal sheep . Am J Physiol Heart Circ Physiol 2010. ; 298 : H179 – 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sadowska GB, Threlkeld SW, Flangini A , et al. . Ontogeny and the effects of in utero brain ischemia on interleukin-1beta and interleukin-6 protein expression in ovine cerebral cortex and white matter . Int J Dev Neurosci 2012. ; 30 : 457 – 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boylan JM, Gruppuso PA. Insulin receptor substrate-1 is present in hepatocyte nuclei from intact rats . Endocrinology 2002. ; 143 : 4178 – 83 [DOI] [PubMed] [Google Scholar]
- 49. Liu K, Mori S, Takahashi HK , et al. . Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats . Faseb J 2007. ; 21 : 3904 – 16 [DOI] [PubMed] [Google Scholar]
- 50. Guazzi S, Strangio A, Franzi AT , et al. . HMGB1, an architectural chromatin protein and extracellular signalling factor, has a spatially and temporally restricted expression pattern in mouse brain . Gene Expr Patterns 2003. ; 3 : 29 – 33 [DOI] [PubMed] [Google Scholar]
- 51. Dobbing J, Sands J. Comparative aspects of the brain growth spurt . Early Hum Dev 1979. ; 3 : 79 – 83 [DOI] [PubMed] [Google Scholar]
- 52. Bell CW, Jiang W, Reich CF, 3rd , et al. . The extracellular release of HMGB1 during apoptotic cell death . Am J Physiol Cell Physiol 2006. ; 291 : C1318 – 25 [DOI] [PubMed] [Google Scholar]
- 53. Berthelot F, Fattoum L, Casulli S , et al. . The effect of HMGB1, a damage-associated molecular pattern molecule, on polymorphonuclear neutrophil migration depends on its concentration . J Innate Immun 2012. ; 4 : 41 – 58 [DOI] [PubMed] [Google Scholar]
- 54. Hayakawa K, Qiu J, Lo EH. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke . Ann N Y Acad Sci 2010. ; 1207 : 50 – 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Torvik A. The pathogenesis of watershed infarcts in the brain . Stroke 1984. ; 15 : 221 – 3 [DOI] [PubMed] [Google Scholar]
- 56. Momjian-Mayor I, Baron JC. The pathophysiology of watershed infarction in internal carotid artery disease: Review of cerebral perfusion studies . Stroke 2005. ; 36 : 567 – 77 [DOI] [PubMed] [Google Scholar]
- 57. Riddle A, Luo NL, Manese M , et al. . Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury . J Neurosci 2006. ; 26 : 3045 – 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tang D, Kang R, Cheh CW , et al. . HMGB1 release and redox regulates autophagy and apoptosis in cancer cells . Oncogene 2010. ; 29 : 5299 – 310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang H, Lundback P, Ottosson L , et al. . Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1) . Mol Med 2012. ; 18 : 250 – 9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60. Janko C, Filipovic M, Munoz LE , et al. . Redox modulation of HMGB1-related signaling . Antioxid Redox Signal 2014. ; 20 : 1075 – 85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Antoine DJ, Harris HE, Andersson U , et al. . A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins . Mol Med 2014. ; 20 : 135 – 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Venereau E, Casalgrandi M, Schiraldi M , et al. . Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release . J Exp Med 2012. ; 209 : 1519 – 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu A, Fang H, Dirsch O , et al. . Oxidation of HMGB1 causes attenuation of its pro-inflammatory activity and occurs during liver ischemia and reperfusion . PLoS One 2012. ; 7 : e35379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bonaldi T, Talamo F, Scaffidi P , et al. . Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion . Embo J 2003. ; 22 : 5551 – 60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gardella S, Andrei C, Ferrera D , et al. . The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway . EMBO Rep 2002. ; 3 : 995 – 1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion . J Immunol 2006. ; 177 : 7889 – 97 [DOI] [PubMed] [Google Scholar]
- 67. Gluckman PD, Gunn AJ, Wyatt JS. Hypothermia for neonates with hypoxic-ischemic encephalopathy . N Engl J Med 2006. ; 354 : 1643 – 5 author reply -5 [PubMed] [Google Scholar]
- 68. Shankaran S, Laptook AR, Ehrenkranz RA , et al. . Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy . N Engl J Med 2005. ; 353 : 1574 – 84 [DOI] [PubMed] [Google Scholar]
- 69. Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: Role of tumor necrosis factor-alpha . Cerebrovasc Brain Metab Rev 1994. ; 6 : 341 – 60 [PubMed] [Google Scholar]
- 70. Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets . Ann N Y Acad Sci 1997. ; 825 : 179 – 93 [DOI] [PubMed] [Google Scholar]
- 71. Silverstein FS, Barks JD, Hagan P , et al. . Cytokines and perinatal brain injury . Neurochem Int 1997. ; 30 : 375 – 83 [DOI] [PubMed] [Google Scholar]