Abstract
Formation of adventitious roots in plants is a common response to hypoxia caused by flooding. In tobacco, after one week of root hypoxia treatment, plants produced twice as many adventitious roots as the aerated plants, but their maximum length was reduced. Hypoxia severely reduced net photosynthesis, transpiration rates, and photosynthetic light responses. Relative transcript abundance of the examined aquaporins in lateral roots was reduced by hypoxia, but in adventitious roots it remained unchanged. This apparent lack of an effect of root hypoxia on the aquaporin expression likely contributed to maintenance of high hydraulic conductance in adventitious roots. Lateral roots had lower porosity compared with adventitious roots and the expression of the ACS (1-aminocyclopropane-1-carboxylate synthase) gene was induced in hypoxic lateral roots, but not in adventitious roots, providing additional evidence that lateral roots were more affected by hypoxia compared with adventitious roots. ATP concentrations were markedly lower in both hypoxic lateral and adventitious roots compared with aerated roots, while the expression of fermentation-related genes, ADH1 (alcohol dehydrogenase 1) and PDC1 (pyruvate decarboxylase 1), was higher in lateral roots compared with adventitious roots. Since root porosity was greater in adventitious compared with lateral roots, the results suggest that the improved O2 delivery and stable root aquaporin expression in adventitious roots were likely the key factors helping flooded tobacco plants maintain high rates of root hydraulic conductance and, consequently, shoot gas exchange.
Introduction
Frequency of floods is predicted to increase globally due to the climate changes [1]. As O2 diffusion rate is extremely low in water compared to air, prolonged flooding can lead to O2 deprivation and reductions of growth and survival in terrestrial plant species [2]. Root O2 deficiency (hypoxia) limits respirational ATP synthesis and results in an energy crisis and toxicity due to a transition to glycolysis and fermentation [3]. Tobacco plants are susceptible to injury from flooding [4,5] with chlorosis and inhibition of leaf expansion reported in plants exposed for two days to waterlogging [6]. In field-grown tobacco, an inhibition of stem growth and a decrease of water potential occurred within six days following a flooding event [7].
Faced with hypoxia, plants need to reprogram transcription [8], and curtail energy-consuming processes such as DNA and protein synthesis and cell division [2]. Numerous hypoxia-responsive proteins and genes that have been identified by the application of proteomic approach and microarrays are associated with sugar metabolism, glycolysis, fermentation and hormonal regulation [9–12]. Alcohol dehydrogenase (ADH) and pyruvate decarboxylase (PDC) are the two key enzymes in fermentation and are commonly induced by hypoxia and anoxia in plants and fungi [13–15]. High activities of ADH and PDC are associated with improved survival of plants under low O2 conditions, mainly due to their involvement in alleviating energy crisis. [3]. However, the increase in fermentation may also lead to the accumulation of toxic end products, such as ethanol and acetaldehyde [8]. Genes encoding 1-Aminocyclopropane-1-Carboxylate (ACC) synthase (ACS) are also induced under O2 deficiency [16,17]. ACS catalyzes a regulatory step in endogenous ethylene synthesis, which is a major biochemical process in response to hypoxia [18].
Hydraulic adjustments are among the early responses of plants to flooding [19]. This is often manifested as wilting due to the loss of balance between water loss and uptake [20,21]. Aquaporins, including plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins, nodulin26-like intrinsic proteins, small basic intrinsic proteins, and the uncategorized intrinsic proteins, are membrane intrinsic proteins that can rapidly respond to various abiotic and biotic stresses including O2 deprivation [19,22]. The inhibition of aquaporin gating and root hydraulic conductivity in flooded plants may be regulated by cellular acidosis caused by the shift from respiration to fermentation [23], and depletion of ATP required for the phosphorylation of some aquaporins [20]. X-ray structure confirmed that the protonation of a conserved histidine residue under low pH during flooding resulted in the closed conformation of aquaporins [24]. In addition to regulating plant hydraulics, aquaporins are involved in the transport of other small molecules including CO2 [25,26], NH3 [27] and O2 [28]. Under flooding, transcription of Arabidopsis aquaporin NIP2;1 was induced [29], and shown to be a transporter of lactic acid that is produced by fermentation [30].
Plants vary in their flooding tolerance and adopt different survival strategies. These strategies include fast shoot elongation to escape from submergence [31] and development of suberized barriers in roots to reduce radial O2 loss [32,33]. Adventitious roots (ARs), are often induced in flooded plants. They may be placed close to the surface in some plants and usually have low gas diffusive resistance [34–36]. Six days of root hypoxia induced about twice as many ARs compared with aerated tobacco plants [37]. ARs promote internal diffusion of O2 from shoots to roots and elevate respiratory ATP production in roots [38]. Different types of biotic and abiotic stresses other than flooding can induce AR formation including wounding [39] and exogenous hormonal treatments [40]. Ethylene and other phytohormones have been implicated in the regulation of AR formation [21,37,41]. Different hormones may interact with each other in AR formation, but it still remains elusive how plants manage this complex regulation network in AR formation [42].
Formation of ARs has been highlighted as one of the most important adaptive traits under flooding in numerous species [43]. However, more evidence is still needed to evaluate the functional traits of ARs contributing to hypoxia tolerance. It was previously demonstrated that adventitious roots had higher hydraulic conductivity than similarly-sized lateral roots in flooded tamarack (Larix laricina) [44]. However, it remains unclear whether the high hydraulic conductivity of adventitious root is associated with aquaporin activities. In the present study, hydroponically grown tobacco plants were subjected to root hypoxia to shed more light on the processes in hypoxia-induced ARs that facilitate water transport. It was hypothesized that root hydraulics and ATP production under hypoxia can be enhanced by the formation of ARs. Root porosity, ATP contents and transcript profiles of hypoxia-responsive genes were compared between ARs and existing LRs. Transcription profiling of PIPs in ARs was also compared to LRs to examine potential significance of various PIPs in the responses of roots to hypoxia.
Materials and methods
Growth conditions and hypoxia treatment
Tobacco seeds were germinated and seedlings were grown in horticultural soil a controlled growth room with 18 h photoperiod, 22/18°C (day/night) temperature, 400 μmol m-2 s-1 photosynthetic photon flux density, 350 μmol mol−1 CO2 and 50% relative humidity. After 3 weeks of growth, plants were transferred to 40-L plastic tubs (~ 60×40×20 cm) containing 50% strength modified aerated Hoagland’s solution [45]. Thirty-two plants were randomly selected and grown in four tubs (8 plants in each tub). After one week, 16 plants in two tubs were subjected to hypoxia by flushing nitrogen gas (99.998%, Praxair, Danbury, CT, USA) through the solution to reach a dissolved O2 level of ~ 2 mg L-1 and then leaving the solution stagnant. The other 16 plants in two tubs were well-aerated with air pumps and served as control (dissolved O2 concentration of ~ 8 mg L-1).
Gas exchange and photosynthetic light responses
After two days and one week of treatments, gas exchange was measured with the Li-Cor LI-6400XT portable photosynthesis system equipped with the 2×3 cm2 red-blue light chamber (Li-Cor, Lincoln, NE, USA). Six plants in each treatment and three middle-position fully expanded leaves on each plant were randomly selected. Net photosynthetic rate (Pn), transpiration (E) and stomatal conductance (gs) were measured. Air flow rate was set to 400 μmol s-1, photosynthetic photon flux density (PPFD) was 400 μmol m-2 s-1, and reference CO2 concentration was 400 μmol mol-1. An automated program of LI-6400XT was used to determine photosynthetic light responses starting at PPFD of 1500 μmol m-2 s-1, followed by 1200, 1000, 800, 500, 300, 200, 100, 50, 20 and 0 μmol m-2 s-1. Three plants in each treatment were randomly selected and net photosynthesis was auto-logged when it reached a steady rate. A modified rectangular hyperbole model was employed to estimate light saturated Pn (Pm), light saturation point (Im) and light compensation point (Ic) [46].
Root hydraulic conductance (Kr)
A high–pressure flow meter (HPFM, Dynamax Incorporated, Houston, TX, USA) was used to measure tobacco root Kr as previously described [47,48]. Shoots were excised about 2 cm above the root collar, and the roots were connected to the HPFM. Roots were kept in treatment solutions during the measurements. Water was forced into roots at increasing pressures (0 to 0.5 MPa) and linear regression between applied pressure and flow rate was used to obtain a slope of the relationship which represented Kr.
Number, dry mass and maximum length of ARs
After one week of treatment, number of ARs was counted and the maximum length of ARs was measured with a ruler. Dry mass of ARs was determined after drying the roots in an oven at 85°C.
Root porosity
Root porosity in lateral (LR) and adventitious (AR) roots was estimated with the pycnometer method based on Archimedes’ principle [49] after one week of treatment using the following equation:
where Mw is mass of the water filled pycnometer, Mr is mass of roots, Mr+w is mass of pycnometer with roots and water and Mh is mass of pycnometer with homogenized roots [49].
Root ATP concentration
LRs and ARs were sampled and ground in liquid nitrogen after one week of hypoxia treatment. ATP concentration was determined with the ENLITEN ATP Assay Kit (Promega, Madison, WI, USA) by measuring bioluminescence and quantified with a standard curve using ATP standard (Promega). Luminescence signal was detected using a microplate reader (Fluostar Optima, BMG Labtech, Ortenberg, Germany) as previously described [28].
RNA transcription profiling
After two days and one week of treatment, LRs and ARs from 6 plants in each treatment were sampled. The samples were quickly frozen and kept in liquid nitrogen before being transferred to the –80°C freezer. The samples were ground with a mortar and pestle in liquid nitrogen. Total RNA was extracted with a Plant RNeasy extraction kit (Qiagen, Valencia, CA USA). First strand of cDNA was synthesized from 1μg total RNA using a Reverse Transcription Kit (Qiagen). Quantitative RT-PCR was employed to analyze relative RNA expression using the 2–ΔCt method. The relative transcript abundance of PIPs was normalized against geometric mean of the CT value of two reference genes, NtEF1-α (AF120093) and L25 (L18908) [50]. Transcripts levels of NtPIP1;1 (AF440271), PIP1;2 (= AQP1, AF024511), PIP1;3 (U62280), PIP1;4 (DQ914525) and PIP2;1 (AF440272) were analyzed. These aquaporins were selected because of their sequence availability in the public database and earlier studies showing their functional importance [25,28]. NtADH1 (alcohol dehydrogenase 1, X81853.1) and PDC1 (pyruvate decarboxylase 1, X81854.1) were selected as hypoxic indicators. Relative transcript abundance of ACS (X65982.1) was also determined since it encodes an enzyme catalyzing a rate-limiting step in ethylene synthesis [51]. Gene-specific primers are described in S1 Table.
Statistical analysis
Means (n = 3–6) and standard errors (SE) were calculated. Paired t-test was performed to compare AR formation and Kr between aerated and hypoxic roots (α = 0.05). In all other comparisons, one-way ANOVA followed by Tukey’s test was performed to compare means (α = 0.05). Three out of four replications of PDC1 relative transcript abundance of aerated LRs were too low to be detected and consequently only the relative transcript abundance of PDC1 of aerated ARs, hypoxic LRs and hypoxic ARs were compared in Tukey’s test.
Results
Leaf gas exchange and photosynthetic light responses
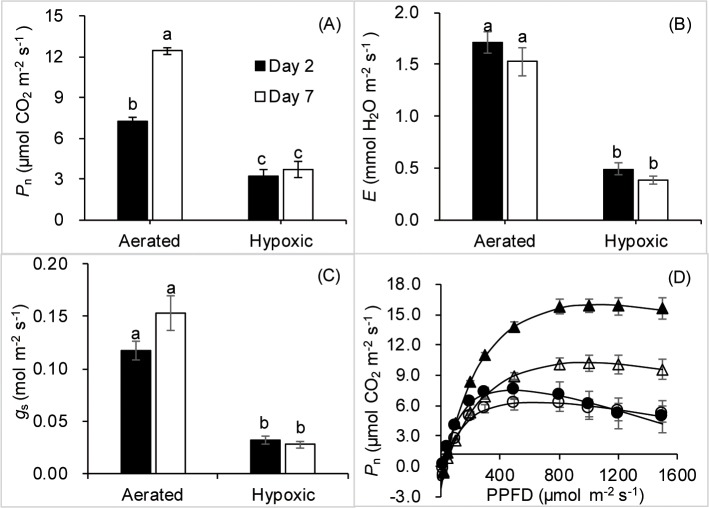
After one week of treatment, the leaves of hypoxic plants showed no signs of chlorosis or wilting. Pn of hypoxic plants decreased by over 50% and 70% compared with aerated plants after two days and one week of treatment, respectively (Fig 1A). Hypoxic plants also showed a decrease of more than 70% in E and gs on the two treatment days (Fig 1B and 1C).
Fig 1.
Net photosynthesis (Pn, A), transpiration rates (E, B), stomatal conductance (gs, C) and photosynthetic light responses (D) of well-aerated tobacco plants and plants subjected to root hypoxia treatment for two days and one week. Means ± SE (n = 5 or 6 for Pn, E and gs. and n = 3 for photosynthetic light responses) are shown. Different letters above the bars indicate statistically significant differences determined by the Tukey’s test after one-way ANOVA (P ≤ 0.05). Aerated after two days (open triangles), hypoxia after two days (open circles), aerated after one week (black triangles), and hypoxia after one week (black circles).
Hypoxia treatment profoundly affected photosynthetic light responses (Fig 1D). Both two days and one week of hypoxia treatments resulted in significant declines of Ic and Im (Table 1). Pm of hypoxic plants also showed a significant decrease compared with aerated plants after one week of treatment (Table 1).
Table 1. Comparison of estimated light-saturated photosynthesis (Pm, μmol CO2 m-2 s-1), light saturation point (Im, μmol m-2 s-1) and light compensation point (Ic, μmol m-2 s-1) in photosynthetic light responses of tobacco plants subjected to aeration and hypoxia treatment for two days and one week.
| Aerated DAY2 | Hypoxia DAY2 | Aerated DAY7 | Hypoxia DAY7 | |
|---|---|---|---|---|
| Pm | 11.47 ± 0.82 a | 7.39 ± 0.79 a | 17.85 ± 0.87 b | 8.76 ± 1.2 a |
| Im | 1045.99 ± 84.98 a | 683.54 ± 40.39 b | 1129.38 ± 52.12 a | 551.66 ± 103.49 b |
| Ic | 28.69 ± 2.55 a | 19.7 ± 0.11 b | 26.34 ± 1.94 a | 14.524 ± 3.68 b |
Means ± SE (n = 3) are shown. Different letters indicate statistically significant differences determined by the Tukey’s test after one-way ANOVA (P ≤ 0.05)
AR formation and root porosity
After one week of treatment, root mortality was observed in the lower part of hypoxic roots. Formation of ARs was induced by hypoxia around the stem base (Table 2). Hypoxic plants had over two-fold higher AR number compared with aerated plants. However, the maximum length of ARs in hypoxic plants was significantly lower compared with aerated plants (Table 2). Hypoxia treatment did not affect the total dry mass of ARs (Table 2).
Table 2. Number, maximum length, and dry mass of adventitious roots (ARs) in aerated and hypoxia treatment for one week.
| ARs | ||
|---|---|---|
| Aerated | Hypoxic | |
| Number | 14 ± 1.51 | 28.17 ± 2.27* |
| Maximum length | 5.77 ± 0.46* | 3.53 ± 0.18 |
| Dry mass | 0.059 ± 0.012 | 0.038 ± 0.007 |
Means ± SE are shown (n = 6 for number and maximum length, and n = 8 for dry mass of ARs). Asterisks indicate significance between aerated and hypoxic ARs determined by the t-test (P ≤ 0.05).
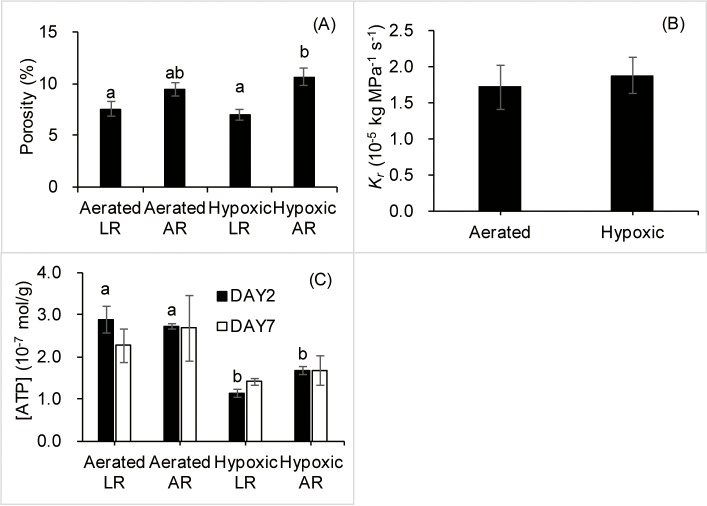
Root air space was estimated by the root porosity test after one week of treatment. Hypoxia lead to a 13% increase of porosity in ARs and a slight decrease of porosity in LRs (Fig 2A). The porosity of hypoxic ARs was higher by over 50% compared with hypoxic LRs. The porosity of aerated ARs was higher by over 25% compared with aerated LRs.
Fig 2.
Root porosity of adventitious (ARs) and lateral roots (LRs) (A) and hydraulic conductance (Kr, B) of tobacco plants subjected to aerated or root oxygen deprivation (hypoxia) treatments for one week, and ATP concentration of ARs and LRs (C) after two days and one week of treatment. Means ± SE (n = 4–6 for porosity test, n = 6 for Kr and n = 3 for ATP assay) are shown. Different letters above the bars indicate statistically significant differences determined by the Tukey’s test after one-way ANOVA (P ≤ 0.05).
Kr and root ATP concentration
There was no significant difference in Kr between hypoxic roots and aerated roots after one week of treatment (Fig 2B).
After two days of treatment, hypoxia resulted in a significant decrease of ATP concentration in both ARs and LRs (Fig 2C). ATP concentration of hypoxic LRs and ARs decreased by about 60% and 40%, respectively, compared with aerated roots (Fig 2C). After one week of treatment, no significant difference in ATP concentration between hypoxic and aerated roots was detected.
RNA expression profiling in roots
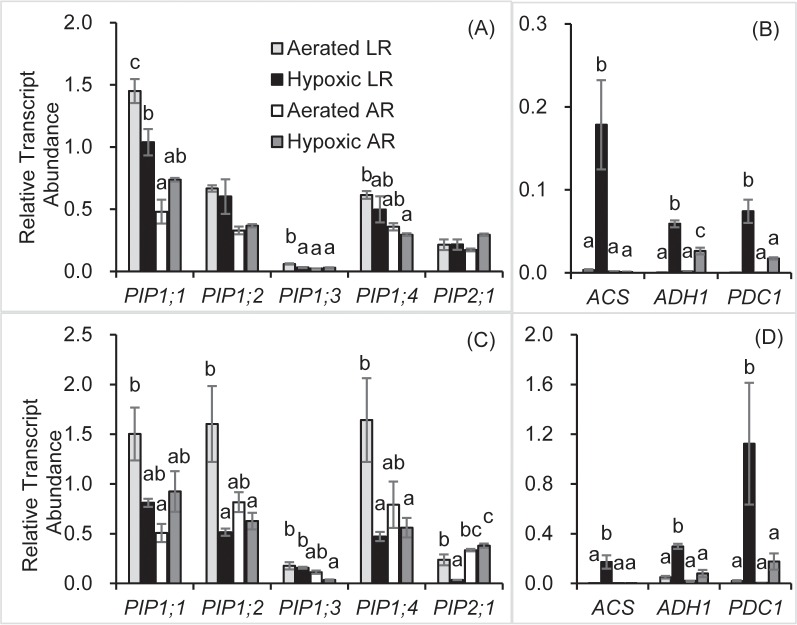
Hypoxia resulted in significant decreases of PIP1;1 and PIP1;3 relative transcript abundance in LRs after two days of treatment (Fig 3A). However, relative transcript abundance in ARs remained unchanged (Fig 3A). Relative transcript abundance of ACS, ADH1 and PDC1 in LRs were sharply induced by hypoxia (Fig 3B). Hypoxic ARs also showed significantly higher ADH1 relative transcript abundance compared with aerated ARs, however, ACS remained unchanged (Fig 3B).
Fig 3.
Relative transcript abundance of well-aerated tobacco plants and plants subjected to root hypoxia treatment for two days (A and B) and one week (C and D). Relative transcript abundance of plasma membrane intrinsic proteins (PIPs, A and C), 1-aminocyclopropane-1-carboxylate synthase (ACS), alcohol dehydrogenase 1 (ADH1) and pyruvate decarboxylase 1 (PDC1) (B and D) is shown. Means ± SE are shown (n = 4–6). PDC1 expression of LRs aerated after two days is not shown because of un-determined values. Different letters above the bars indicate statistically significant differences determined by the Tukey’s test after one-way ANOVA (P ≤ 0.05).
After one week of treatment, relative transcript abundance of PIP1;2, PIP1;4 and PIP2;1 in hypoxic LRs were significantly lower compared with the aerated LRs, whereas relative transcript abundance of PIP1;1 and PIP1;3 showed no change (Fig 3C). In contrast, hypoxia did not result in significant changes of PIP relative transcript abundance in ARs (Fig 3C). Relative transcript abundance of ACS, ADH1 and PDC1 exhibited similar trends as on day two (Fig 3D). A sharp increase of ACS, ADH1 and PDC1 expression was triggered in LRs by hypoxia (Fig 3D). Hypoxic ARs showed no changes in relative transcript abundance of the ACS, ADH1 and PDC1 compared with aerated ARs (Fig 3D).
Discussion
O2 deficiency is a challenging environmental factor that produces complex responses in plants. Following two days and one week of treatment, gas exchange in tobacco was sharply reduced by hypoxia. Hypoxia also increased the number of ARs but with a similar biomass as in aerated plants. ARs showed different response patterns to hypoxia compared with the LRs in terms of the transcript profiles of PIPs and hypoxia-responsive genes, which may partially contribute to maintaining Kr of hydroponically-grown tobacco plants.
Stomatal conductance, which is regulated by hydraulic and (or) chemical signals, is the main limiting factor for leaf carbon assimilation [52]. The decline in Pn after two days and one week of hypoxia was likely due to the stomatal closure, which was reflected by the decreased E. The decrease in stomatal opening, as demonstrated by lower gs, was likely responsible for the reductions in photosynthetic light responses, which showed that Pn of hypoxic plants was saturated at a significantly lower light intensity. Reduced gs of hypoxic leaves limits internal CO2 concentration used for carbon assimilation. Root hypoxia also reduced Ic, the light intensity at which the rate of total photosynthesis is balanced by the rate of respiration suggesting that root hypoxia inhibited leaf respiration in addition to photosynthesis. However, hypoxic plants maintained positive Pn at similar rates on days two and seven, which demonstrates relative tolerance of tobacco plants of hypoxic conditions.
One week of root hypoxia did not influence Kr in hydroponically grown tobacco. Effects of O2 deprivation on root hydraulics varies between plant species and experimental conditions. While some studies reported reduced Kr in response to O2 deficiency [53,54], other studies showed no effect [44,55]. In contrast to Kr, hypoxia resulted in a significant decrease of E in the present study. E and Kr are frequently strongly linked in plants [56], but this relationship may also be affected by other factors including leaf to root ratio [57]. Root growth is typically reduced more than leaf growth under hypoxic conditions [58]. In the present study, leaves of hypoxic plants showed no chlorosis and wilting, but root mortality was observed. Thus, even though Kr remained unchanged under hypoxic conditions, the increasing transpiration demand, as a result of leaf growth, may still lead to decreased E in the present study. The diurnal changes of root hydraulic conductivity were also found to be independent of transpiration in flooded Zea mays [59]. More likely, the signal triggering hydraulic adjustment either does not originate in the stomata or is impaired by the secondary changes caused by hypoxia.
It appears that the number of ARs, not its overall length and dry mass, was the overriding factor in maintaining root Kr in hypoxic tobacco plants. Even though root porosity was similar in aerated and hypoxia treatments, the number of ARs was increased by hypoxia. The formation of ARs is an important adaptation to low O2 conditions in some plants [2,34]. In the present study, hypoxia induced the formation of over twice as many ARs compared with aerated tobacco plants, but their length was reduced by hypoxia. Similar results were previously reported in tobacco and the authors concluded that the formation of short ARs cannot functionally replace the primary root system which contributed to the relative intolerance of tobacco to O2 deficiency [37]. However, in the present study, Kr in hypoxic plants showed no change compared with aerated plants. Additionally, Pn and E of hypoxic plants showed no further decrease after one week of hypoxia compared with two days of treatment. These results indicate that the ARs were effective in maintaining root water transport. The roles of hypoxia-induced ARs are not limited to replacing existing roots. ARs induced by hypoxia had higher porosity than the LRs and could conduct more air from shoots to roots and the rhizosphere (radial O2 loss, ROL). ROL of ARs may have a profound influence on rhizosphere aeration and nutrient availability in plants under O2 deficiency [60]. Thus, the numerous short ARs induced by hypoxia in this study could potentially affect tobacco rhizosphere aeration and contribute to the survival and functioning of the root system under hypoxia.
Decreased root hydraulic conductivity of anoxic Arabidopsis has been linked to aquaporin closure [23], and a structure-based protonation mechanism under O2 deprivation conditions has been demonstrated [23,24]. In addition to aquaporin gating analysis, aquaporin gene expression patterns can reveal the importance of various aquaporins in plant responses to O2 deficiency [19]. Although transcripts may not be translated under certain conditions and posttranslational regulation can modify the function of aquaporins, transcriptional responses can shed light on the translational potential of cells experiencing O2 deficiency [61]. Microarray analysis showed that aquaporin expression was downregulated in O2 deficient Arabidopsis [29] and Persea americana [62]. Here, the relative transcript abundance of PIPs in ARs was compared with LRs. After two days of hypoxia, relative transcript abundance of PIP1;1 and PIP1;3 decreased in LRs but showed no change in ARs. One week of hypoxia resulted in decreased relative transcript abundance of PIPs in LRs except PIP1;1 and PIP1;3, while hypoxic ARs showed unchanged expression of PIPs on day seven. These results indicate that the relative transcript abundance of PIPs in ARs was less affected by hypoxia, which may contribute to hydraulic adjustment. In addition to regulating water transport, PIPs impact other physiological processes. Tobacco PIP1;2 has been reported to be permeable to CO2 [25]. CO2 can accumulate in roots of plants growing in stagnant water due to its low diffusion rate in water and high concentrations of CO2 can cause cell acidification [60]. Thus, the down-regulation of PIP1;2 may lead to decreased efflux of CO2 in cells and intensify cell acidification. Interestingly, expression of PIP1;1 and PIP1;3 in LRs showed different responsive patterns compared with the other examined PIP genes under hypoxia. Further research is needed to shed more light on the biological roles of PIP1;1 and PIP1;3 in tobacco. Tobacco PIP1;3 has been shown to be potentially involved in the O2 transmembrane transport [28]. Although the experimental set-up of the two experiments are similar, the objective of this study was mainly comparing the differences between ARs and LRs under hypoxic conditions. However, unlike the present study, it was reported that the relative transcript abundance of tobacco PIP1;3 was upregulated by root hypoxia [28]. The reason for the difference may be that in the present study LRs and newly formed ARs were sampled separately rather than the whole root. Additionally, gene expression patterns in response to abiotic stresses may vary between different developmental stages [63]. Tobacco plants were exposed to root hypoxia three weeks after germination in the present study while plants were subjected to root hypoxia two weeks after germination in the study of Zwiazek et al. [28], which may also contribute to the differences in results. Since ARs were less affected by hypoxia than LRs in terms of PIP transcription, ARs can likely more actively participate in root water uptake under hypoxic conditions. In fact, ARs induced by hypoxia in Larix laricina were shown to have higher hydraulic conductivity than the existing roots [44]. However, it should be emphasized that both transcriptional and post-transcriptional regulation may affect aquaporin abundance and functions and several studies have shown the lack of correlation between aquaporin mRNA and protein abundance [64–66]. It remains to be determined whether the changes in transcript abundance observed in the present study are functionally significant.
Phytohormones, especially ethylene, are involved in the regulation network in response to O2 deficiency, including the initiation and regulation of ARs [67,68]. In this study, transcript profiling showed that more ACS transcripts were induced in hypoxic LRs than ARs, which could result in the accumulation of ACC in hypoxic LRs. ACC, an ethylene synthesis precursor, is induced in flooded roots while the conversion of ACC to ethylene needs the presence of O2 [69]. The transport of ACC from stressed roots to other tissues serves as a signal and causes the formation of ARs in aerated shoots [51]. Both ADH and PDC were frequently reported to be up-regulated in O2 deficient tissues [13,62]. In this study, transcripts of tobacco ADH1 and PDC1 were sharply up-regulated in hypoxic LRs, while ARs showed much lower expression. Despite the inefficiency of ethanolic fermentation, the activities of ADH and PDC are essential to plants under low O2 conditions to meet the energy demand [3]. However, the accumulation of end products of fermentation is toxic [3]. In the present study, two days of hypoxia resulted in a significant decline of ATP concentrations in both ARs and LRs. Although hypoxic ARs did not differ from hypoxic LRs in ATP concentration, hypoxic ARs maintained markedly lower levels of ADH and PDC transcripts compared with LRs. This suggests that the better aeration of ARs reduced their dependence on inefficient and toxic fermentation compared with LRs under low O2 conditions.
In conclusion, the formation of short ARs was induced by hypoxia in hydroponically grown tobacco plants. Although, the length of ARs was reduced in hypoxic plants, it appears that they were likely more effective in facilitating O2 diffusion into the roots and maintaining low level of fermentation and high Kr. PIP expression patterns likely reflect the metabolic status in roots and may also be related to gas transport. Stable PIP transcripts of ARs under hypoxic conditions may be indicative of an active role of ARs in root water transport under hypoxic conditions.
Supporting information
(XLSX)
(DOCX)
Abbreviations
- ACC
1-aminocyclopropane-1-carboxylate
- ACS
1-aminocyclopropane-1-carboxylate synthase
- ADH
alcohol dehydrogenase
- AR
adventitious root
- gs
stomatal conductance
- HPFM
high–pressure flow meter
- Ic
light compensation point
- Im
light saturation point
- Kr
root hydraulic conductance
- LR
lateral root
- PDC
pyruvate decarboxylase
- PIP
plasma membrane intrinsic protein
- Pm
light saturated net photosynthesis
- Pn
net photosynthetic rate
- PPFD
photosynthetic photon flux density
- ROL
radial oxygen loss
- Tr
transpiration rate
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery (NSERC.ca) Grant to JJZ. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hirabayashi Y, Mahendran R, Koirala S, Konoshima L, Yamazaki D, Watanabe S, et al. Global flood risk under climate change. Nat Clim Change. 2013;3: 816–821. [Google Scholar]
- 2.Bailey-Serres J, Voesenek LACJ. Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol. 2008;59: 313–339. 10.1146/annurev.arplant.59.032607.092752 [DOI] [PubMed] [Google Scholar]
- 3.Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48: 223–250. 10.1146/annurev.arplant.48.1.223 [DOI] [PubMed] [Google Scholar]
- 4.Kramer PJ, Jackson WT. Causes of injury to flooded tobacco plants. Plant Physiol. 1954;29: 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padakandla SR. Climate sensitivity of crop yields in the former state of Andhra Pradesh, India. Ecol Indic. 2016;70: 431–438. [Google Scholar]
- 6.Yu Q, Rengel Z. Waterlogging influences plant growth and activities of superoxide dismutases in narrow-leafed lupin and transgenic tobacco plants. J Plant Physiol. 1999;155: 431–438. [Google Scholar]
- 7.Hunt PG, Campbell RB, Sojka R, Parsons JE. Flooding-induced soil and plant ethylene field-grown tobacco. Plant Soil. 1981;439: 427–439. [Google Scholar]
- 8.Gibbs J, Greenway H. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol. 2003;30: 1–47. [DOI] [PubMed] [Google Scholar]
- 9.Sachs MM, Freeling M, Okimoto R. The anaerobic proteins of maize. Cell. 1980;20: 761–767. [DOI] [PubMed] [Google Scholar]
- 10.Chang WW, Huang L, Shen M, Webster C, Burlingame AL, Roberts JK. Patterns of protein synthesis and tolerance of anoxia in root tips of maize seedlings acclimated to a low-oxygen environment, and identification of proteins by mass spectrometry. Plant Physiol. 2000;122: 295–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SC, Mustroph A, Sasidharan R, Vashisht D, Pedersen O, Oosumi T, et al. Molecular characterization of the submergence response of the Arabidopsis thaliana ecotype Columbia. New Phytol. 2011;190: 457–471. 10.1111/j.1469-8137.2010.03590.x [DOI] [PubMed] [Google Scholar]
- 12.Gibbs DJ, Lee SC, Isa NM, Gramuglia S, Fukao T, Bassel GW, et al. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature. 2011;479: 415–418. 10.1038/nature10534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mugnai S, Marras AM, Mancuso S. Effect of hypoxic acclimation on anoxia tolerance in Vitis roots: response of metabolic activity and K+ fluxes. Plant Cell Physiol. 2011;52: 1107–1116. 10.1093/pcp/pcr061 [DOI] [PubMed] [Google Scholar]
- 14.Lee MO, Hwang JH, Lee DH, Hong CB. Gene expression profile for Nicotiana tababum in the early phase of flooding stress. J Plant Biol. 2007;50: 496–503. [Google Scholar]
- 15.Mustroph A, Lee SC, Oosumi T, Zanetti ME, Yang H, Ma K, et al. Cross-kingdom comparison of transcriptomic adjustments to low-oxygen stress highlights conserved and plant-specific responses. Plant Physiol. 2010;152: 1484–1500. 10.1104/pp.109.151845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mekhedov SL, Kende H. Submergence enhances expression of a gene encoding 1-aminocyclopropane-1-carboxylate oxidase in deepwater rice. Plant Cell Physiol. 1996;37: 531–537. [DOI] [PubMed] [Google Scholar]
- 17.Rieu I, Cristescu SM, Harren FJM, Huibers W, Voesenek LACJ, Mariani C, et al. RP-ACS1, a flooding-induced 1-aminocyclopropane-1-carboxylate synthase gene of Rumex palustris, is involved in rhythmic ethylene production. J Exp Bot. 2005;56: 841–849. 10.1093/jxb/eri078 [DOI] [PubMed] [Google Scholar]
- 18.Voesenek LACJ, Sasidharan R. Ethylene–and oxygen signalling–drive plant survival during flooding. Plant Biol. 2013;15: 426–435. 10.1111/plb.12014 [DOI] [PubMed] [Google Scholar]
- 19.Tan X, Xu H, Khan S, Equiza MA, Lee SH, Vaziriyeganeh M, et al. Plant water transport and aquaporins in oxygen-deprived environments. J Plant Physiol. 2018;227: 20–30. 10.1016/j.jplph.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 20.Kamaluddin M, Zwiazek JJ. Ethylene enhances water transport in hypoxic aspen. Plant Physiol. 2002;128: 962–969. 10.1104/pp.010791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islam MA, MacDonald SE, Zwiazek JJ. Responses of black spruce (Picea mariana) and tamarack (Larix laricina) to flooding and ethylene. Tree Physiol. 2003;23: 545–552. [DOI] [PubMed] [Google Scholar]
- 22.Maurel C, Boursiac Y, Luu DT, Santoni V, Shahzad Z, Verdoucq L. Aquaporins in plants. Physiol Rev. 2015;95: 1321–1358. 10.1152/physrev.00008.2015 [DOI] [PubMed] [Google Scholar]
- 23.Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu DT, et al. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature. 2003;425: 393–397. 10.1038/nature01853 [DOI] [PubMed] [Google Scholar]
- 24.Törnroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, et al. Structural mechanism of plant aquaporin gating. Nature. 2006;439: 688–694. 10.1038/nature04316 [DOI] [PubMed] [Google Scholar]
- 25.Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature. 2003;425: 734–737. 10.1038/nature02027 [DOI] [PubMed] [Google Scholar]
- 26.Navarro-Ródenas A, Xu H, Kemppainen M, Pardo AG, Zwiazek JJ. Laccaria bicolor aquaporin LbAQP1 is required for Hartig net development in trembling aspen (Populus tremuloides). Plant Cell Environ. 2015;38: 2475–2486. 10.1111/pce.12552 [DOI] [PubMed] [Google Scholar]
- 27.Jahn TP, Møller ALB, Zeuthen T, Holm LM, Klærke DA, Mohsin B, et al. Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett. 2004;574: 31–36. 10.1016/j.febslet.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 28.Zwiazek JJ, Xu H, Tan X, Navarro-Ródenas A, Morte A. Significance of oxygen transport through aquaporins. Sci Rep. 2017;7: 40411 10.1038/srep40411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Vantoai T, Moy LP, Bock G, Linford LD, Quackenbush J. Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Physiol. 2005;137: 1115–1129. 10.1104/pp.104.055475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi W-G, Roberts DM. Arabidopsis NIP2;1, a major intrinsic protein transporter of lactic acid induced by anoxic stress. J Biol Chem. 2007;282: 24209–24218. 10.1074/jbc.M700982200 [DOI] [PubMed] [Google Scholar]
- 31.Van der Sman A, Voesenek LACJ, Blom C, Harren F, Reuss J. The role of ethylene in shoot elongation with respect to survival and seed output of flooded Rumex maritimus L. plants. Funct Ecol. 1991;5: 304–313. [Google Scholar]
- 32.De Simone O, Haase K, Müller E, Junk WJ, Hartmann K, Schreiber L, et al. Apoplasmic barriers and oxygen transport properties of hypodermal cell walls in roots from four amazonian tree species. Plant Physiol. 2003;132: 206–217. 10.1104/pp.102.014902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abiko T, Kotula L, Shiono K, Malik AI, Colmer TD, Nakazono M. Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant Cell Environ. 2012;35: 1618–1630. 10.1111/j.1365-3040.2012.02513.x [DOI] [PubMed] [Google Scholar]
- 34.Calvo-Polanco M, Señorans J, Zwiazek JJ. Role of adventitious roots in water relations of tamarack (Larix laricina) seedlings exposed to flooding. BMC Plant Biol. 2012;12: 99 10.1186/1471-2229-12-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayi Q, Zeng B, Liu J, Li S, van Bodegom PM, Cornelissen JHC. Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants. Ann Bot. 2016;118: 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herzog M, Striker GG, Colmer TD, Pedersen O. Mechanisms of waterlogging tolerance in wheat—a review of root and shoot physiology. Plant Cell Environ. 2016;39: 1068–1086. 10.1111/pce.12676 [DOI] [PubMed] [Google Scholar]
- 37.McDonald MP, Visser EJW. A study of the interaction between auxin and ethylene in wild type and transgenic ethylene-insensitive tobacco during adventitious root formation induced by stagnant root zone conditions. Plant Biol. 2003;5: 550–556. [Google Scholar]
- 38.Bailey-Serres J. Flood adaptive traits and processes: an overview. New Phytol. 2015;206: 57–73. 10.1111/nph.13209 [DOI] [PubMed] [Google Scholar]
- 39.Ahkami AH, Lischewski S, Haensch KT, Porfirova S, Hofmann J, Rolletschek H, et al. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: involvement of wound response and primary metabolism. New Phytol. 2009;181: 613–625. 10.1111/j.1469-8137.2008.02704.x [DOI] [PubMed] [Google Scholar]
- 40.Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L. Nitric oxide is required for root organogenesis. Plant Physiol. 2002;129: 954–956. 10.1104/pp.004036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steffens B, Wang J, Sauter M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta. 2006;223: 604–612. 10.1007/s00425-005-0111-1 [DOI] [PubMed] [Google Scholar]
- 42.Bellini C, Pacurar DI, Perrone I. Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol. 2014;65: 639–66. 10.1146/annurev-arplant-050213-035645 [DOI] [PubMed] [Google Scholar]
- 43.Bailey-Serres J, Fukao T, Gibbs DJ, Holdsworth MJ, Lee SC, Licausi F, et al. Making sense of low oxygen sensing. Trends Plant Sci. 2012;17: 129–138. 10.1016/j.tplants.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 44.Islam MA, Macdonald SE. Ecophysiological adaptations of black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding. Trees-Struct Funct. 2004;18: 35–42. [Google Scholar]
- 45.Epstein E. Mineral nutrition of plants: principles and perspectives Wiley; 1972. [Google Scholar]
- 46.Ye ZP. A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Photosynthetica. 2007;45: 637–640. [Google Scholar]
- 47.Tyree MT, Patino S, Bennink J, Alexander J. Dynamic measurements of root hydraulic conductance using a high-pressure flowmeter in the laboratory and field. J Exp Bot. 1995;46: 83–94. [Google Scholar]
- 48.Lee SH, Calvo-Polanco M, Chung GC, Zwiazek JJ. Role of aquaporins in root water transport of ectomycorrhizal jack pine (Pinus banksiana) seedlings exposed to NaCl and fluoride. Plant Cell Environ. 2010;33: 769–80. 10.1111/j.1365-3040.2009.02103.x [DOI] [PubMed] [Google Scholar]
- 49.Jensen CR, Luxmoore RJ, Gundy SD Van, Stolzy LH. Root air space measurements by a pycnometer method. Agron J. 1969;61: 474–475. [Google Scholar]
- 50.Schmidt GW, Delaney SK. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol Genet Genomics. 2010;283: 233–241. 10.1007/s00438-010-0511-1 [DOI] [PubMed] [Google Scholar]
- 51.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1984;35: 155–189. [Google Scholar]
- 52.Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annu Rev Plant Physiol. 1982;33: 317–345. [Google Scholar]
- 53.Else MA, Coupland D, Dutton L, Jackson MB. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol Plant. 2001;111: 46–54. [Google Scholar]
- 54.Araki H. Water uptake of soybean (Glycine max L. Merr.) during exposure to O2 deficiency and field level CO2 concentration in the root zone. Field Crop Res. 2006;96: 98–105. [Google Scholar]
- 55.Jackson MB, Davies WJ, Else MA. Pressure-flow relationships, xylem solutes and root hydraulic conductance in flooded tomato plants. Ann Bot. 1996;77: 17–24. [Google Scholar]
- 56.Liu J, Equiza MA, Navarro-Rodenas A, Lee SH, Zwiazek JJ. Hydraulic adjustments in aspen (Populus tremuloides) seedlings following defoliation involve root and leaf aquaporins. Planta. 2014;240: 553–564. 10.1007/s00425-014-2106-2 [DOI] [PubMed] [Google Scholar]
- 57.Rodríguez-Gamir J, Intrigliolo DS, Primo-Millo E, Forner-Giner MA. Relationships between xylem anatomy, root hydraulic conductivity, leaf/root ratio and transpiration in citrus trees on different rootstocks. Physiol Plant. 2010;139: 159–169. 10.1111/j.1399-3054.2010.01351.x [DOI] [PubMed] [Google Scholar]
- 58.Kozlowski TT. Responses of woody plants to flooding and salinity. Tree Physiol. 1997;17: 490–490. [Google Scholar]
- 59.Else MA, Davies WJ, Malone M, Jackson MB. A negative hydraulic message from oxygen-deficient roots of tomato plants? Plant Physiol. 1995;109: 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colmer TD. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003;26: 17–36. [Google Scholar]
- 61.van Dongen JT, Licausi F. Oxygen sensing and signaling. Annu Rev Plant Biol. 2014;66: 345–367. [DOI] [PubMed] [Google Scholar]
- 62.Reeksting BJ, Olivier NA, van den Berg N. Transcriptome responses of an ungrafted Phytophthora root rot tolerant avocado (Persea americana) rootstock to flooding and Phytophthora cinnamomi. BMC Plant Biol; 2016;16: 205 10.1186/s12870-016-0893-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skirycz A, De Bodt S, Obata T, De Clercq I, Claeys H, De Rycke R, et al. Developmental stage specificity and the role of mitochondrial metabolism in the response of Arabidopsis leaves to prolonged mild osmotic stress. Plant Physiol; 2010; 152: 226–244. 10.1104/pp.109.148965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boursiac Y, Chen S, Luu DT, Sorieul M, Van Den Dries N, Maurel C, et al. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005;139: 790–805. 10.1104/pp.105.065029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aroca R, Amodeo G, Fernández-Illescas S, Herman EM, Chaumont F, Chrispeels MJ. The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol. 2005;137: 341–353. 10.1104/pp.104.051045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muries B, Faize M, Carvajal M, Martínez-Ballesta MDC. Identification and differential induction of the expression of aquaporins by salinity in broccoli plants. Mol Biosyst. 2011;7: 1322–1335. 10.1039/c0mb00285b [DOI] [PubMed] [Google Scholar]
- 67.Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2010;63: 551–562. 10.1111/j.1365-313X.2010.04262.x [DOI] [PubMed] [Google Scholar]
- 68.Voesenek LACJ, Bailey-Serres J. Flooding tolerance: O2 sensing and survival strategies. Curr Opin Plant Biol. 2013;16: 647–653. 10.1016/j.pbi.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 69.Bradford KJ, Yang SF. Xylem transport of 1-Aminocyclopropane-1-carboxylic acid, an ethylene precursor, in waterlogged tomato plants. Plant Physiol. 1980;65: 322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.