Abstract
Purpose
The 21-gene recurrence score (RS) breast cancer assay is clinically used to quantify risk of 10-year distant recurrence by category (low, < 18; intermediate, 18 to 30; high, ≥ 31) for treatment management among women diagnosed with hormone receptor–positive, human epidermal growth factor receptor 2–negative, lymph node–negative breast cancer. Although non-Hispanic black (NHB) women have worse prognosis compared with non-Hispanic white (NHW) women, the equivalency of 21-gene RS across racial groups remains unknown.
Patients and Methods
Using the Metropolitan Detroit Cancer Surveillance System, we identified women who were diagnosed with hormone receptor–positive, human epidermal growth factor receptor 2–negative, lymph node–negative invasive breast cancer between 2010 and 2014. Multinomial logistic regression was used to quantify racial differences in 21-gene RS category.
Results
We identified 2,216 women (1,824 NHW and 392 NHB) with invasive breast cancer who met clinical guidelines for and underwent 21-gene RS testing. The mean RS was significantly higher in NHBs compared with NHWs (19.3 v 17.0, respectively; P = .0003), where NHBs were more likely to present with high-risk tumors compared with NHWs (14.8% v 8.3%, respectively; P = .0004). These differences were limited to patients younger than 65 years at diagnosis, among whom NHBs had significantly higher RS compared with NHWs (20 to 49 years: 23.6 v 17.3, respectively; P < .001 and 50 to 64 years: 19.6 v 17.4, respectively; P = .023). NHBs remained more likely to have high-risk tumors compared with NHWs after adjusting for age, clinical stage, tumor grade, and histology (odds ratio [OR], 1.75; 95% CI, 1.18 to 2.59).
Conclusion
NHBs who met clinical criteria for 21-gene RS testing had tumors with higher estimated risks of distant recurrence compared with NHWs. Further study is needed to elucidate whether differences in recurrence are observed for these women, which would have clinical implications for 21-gene RS calibration and treatment recommendations in NHB patients.
INTRODUCTION
Accounting for approximately 30% of all new female cancer diagnoses in the United States, it is estimated that one in every eight women will develop breast cancer in her lifetime.1 Although breast cancer incidence rates have remained stable over the last decade, racial disparities in mortality have persisted. Across all breast cancer subtypes, non-Hispanic black (NHB) women disproportionately suffer from poorer prognoses and higher mortality rates compared with their non-Hispanic white (NHW) or Hispanic counterparts.1-3 These marked racial disparities in breast cancer survival outcomes partially reflect differences in socioeconomic status and access to care,4-8 but also capture differences in stage at diagnosis6,9,10 and hormone receptor (HR) status.11,12
In general, women with HR-positive breast cancer, which accounts for nearly 70% of all breast cancer diagnoses, demonstrate favorable responses to endocrine therapy and have better 5-year survival compared with patients with HR-negative disease. However, a pooled multisite analysis of > 10,000 women demonstrated a reversal in this effect 5 to 10 years after diagnosis, where women with HR-positive tumors had worse survival compared with those with HR-negative tumors.13 Furthermore, recent studies have shown that NHB women are nearly two-fold more likely to die specifically from HR-positive, human epidermal growth factor receptor 2 (HER2)–negative breast cancer than NHW women, particularly within the first 2 years of diagnosis, even when accounting for factors including tumor stage and other clinical characteristics.14-16 Although possible explanations for this disparity include delays in diagnosis and treatment initiation or less effective therapy among NHBs because of toxicity or underdosing, there is evidence for underlying racial differences in tumor biology among HR-positive, HER2-negative breast tumors.5,17 Advancement of our knowledge in the biologic and clinical diversity of breast cancer between NHBs and NHWs could lead to improvement in patient outcomes.
Both evidence-based and patient-centered decisions regarding clinical interventions contribute to improved access, quality of care, and patient prognosis.18-21 The 21-gene recurrence score (RS) breast cancer assay is a clinical decision-making tool for the management of adjuvant therapies, where patients with a high-risk RS benefit from the addition of chemotherapy to adjuvant endocrine therapy regimens.22-27 This test categorizes HR-positive, HER2-negative, and lymph node–negative disease on the basis of 10-year distant recurrence risk into low (< 18), intermediate (18 to 30), or high (≥ 31) risk groups.22,23,27 Clinical trials, including the randomized Trial Assigning IndividuaLized Options for Treatment (Rx) (TAILORx) trial, have demonstrated the cost-effective, predictive, and prognostic benefits of this assay.23,26,28,29 However, it is important to note that both risk RS guidelines and results from clinical trials have been reported from cohorts of NHW women. To date, studies conducted in hospitals within an urban setting, among Medicare beneficiaries, and in academic institutions have reported conflicting evidence on racial differences in uptake of 21-gene RS testing.30-34 Recent evidence from population-based studies demonstrated no differences by race in uptake of testing among patients with HR-positive/HER2-negative, node-negative disease, but did not provide any information on 21-gene RS results.30,35,36
Given these contradictory findings and the well-established disparities in breast cancer outcomes by race, an understanding of how 21-gene RS scoring and corresponding risk groups may differ for NHBs compared with NHWs is needed on the basis of current National Comprehensive Cancer Network (NCCN) guidelines. Delineating the patterns of 21-gene RS scoring among a racially diverse group of women who all underwent this genetic test may help to determine whether there are racial differences beyond accessibility and cancer care quality, potentially indicating underlying differences in tumor biology.18-21,37 We investigated racial differences in 21-gene RS among women diagnosed with HR-positive, HER2-negative, node-negative breast cancer using data from the population-based Metropolitan Detroit Cancer Surveillance System (MDCSS) registry.
PATIENTS AND METHODS
Study Design and Information Sources
We compared NHW and NHB female patients diagnosed with HR-positive, HER2-negative, lymph node–negative invasive breast cancer for whom Oncotype DX (21-gene RS; Genomic Health, Redwood City, CA) score results were available in the MDCSS catchment areas of Wayne, Oakland, and Macomb counties in Southeast Michigan. The MDCSS is a founding member of the SEER Program38 and has been continuously collecting population-based cancer data since 1973. This study was determined as non-human participant research by the Wayne State University Institutional Review Board.
Study Population
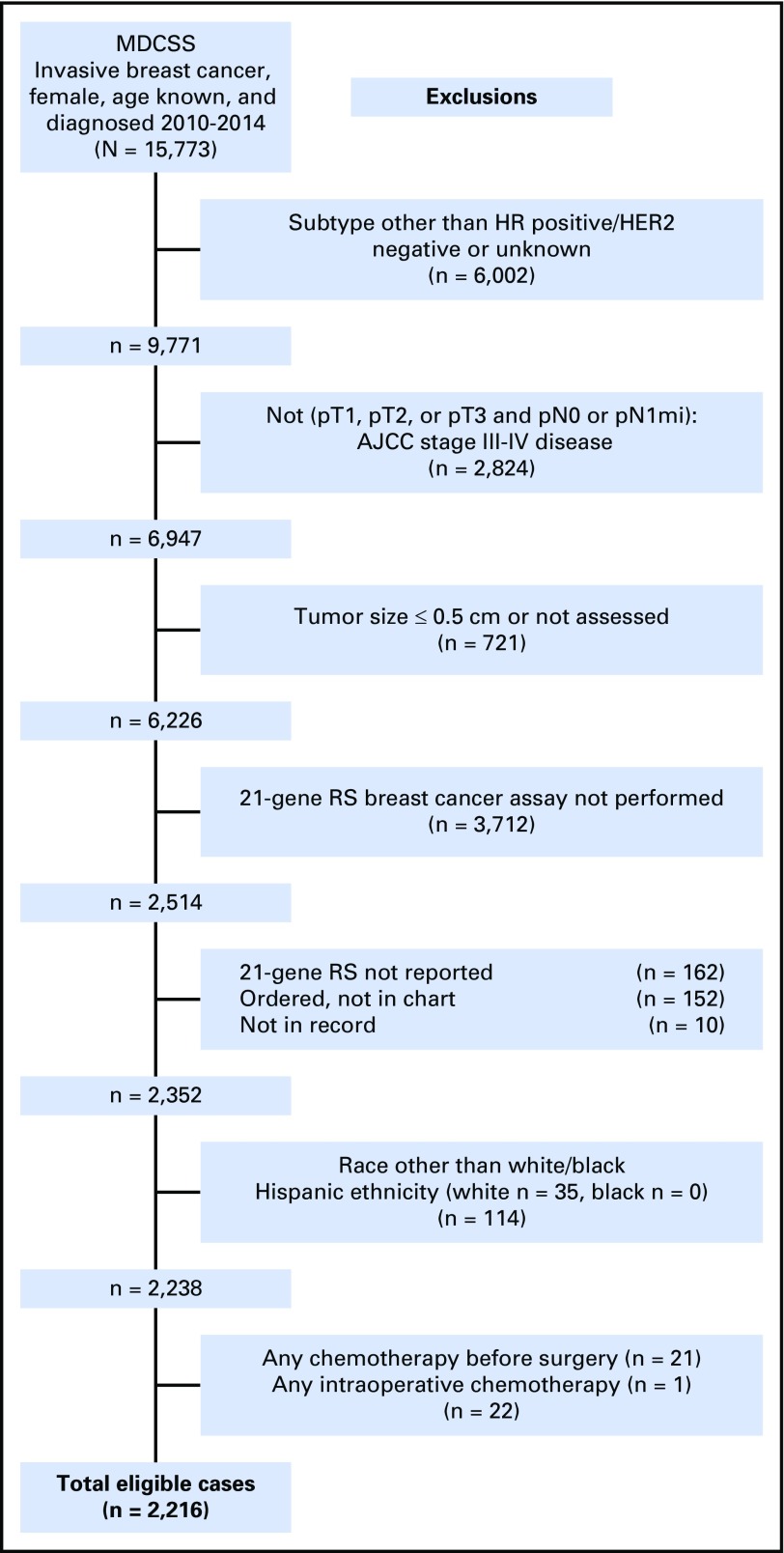
From the MDCSS database, we obtained de-identified data on 15,773 women (≥ 20 years of age) who were diagnosed with and underwent surgery for invasive breast cancer between 2010 and 2014. 2010 is the first year for which HER2 status data were available in the MDCSS database (Fig 1). We restricted our study sample to women who would have been recommended for 21-gene RS testing using NCCN guidelines (HR positive, HER2 negative, node negative, tumor size > 0.5 cm).24 After exclusions made on the basis of HR status, HER2 status, lymph node status, tumor size, 21-gene RS data availability, and race/ethnicity (Fig 1), our final cohort consisted of 2,242 women with 21-gene RS risk information who underwent surgery after diagnosis of HR-positive, HER2-negative, node-negative invasive breast cancer.
Fig 1.
Composition of study population with exclusion criteria. AJCC, American Joint Committee on Cancer; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; MDCSS, Metropolitan Detroit Cancer Surveillance System; RS, recurrence score.
MDCSS Database Linkage to Oncotype DX (21-gene RS) Test Results
Results of the 21-gene RS test were populated in the MDCSS database through linkage with Genomic Health, Inc. (GHI) Clinical Laboratory data. Linkage was performed by an honest broker, the SEER Database Management System developer, Information Management System, Inc. (IMS). Linkage variables included patient name, date of birth, social security number, sex, address, phone number, and medical record number(s). IMS used Registry Plus Link Plus software and an SAS algorithm to link the data. Link Plus is a probabilistic record linkage program developed by the Centers for Disease Control and Prevention (http://www.cdc.gov/cancer/npcr/tools/registryplus/lp.htm). To ensure quality control, a linkage evaluation study was conducted where a random sample of cases was selected for review by registrars to confirm correct classification of cases. Comparison of manually collected data to linkage data revealed RS discrepancies in 5% of cases across all SEER registries, which resulted in RS group misclassification in 2% of cases (n = 649,311 cancer cases across all SEER registries). Probable matches were submitted to the MDCSS database for review and final determination of match status. All matches were combined into a single file, which contained a unique GHI record number and corresponding masked identification number that were forwarded to GHI. GHI provided 21-gene RS test results (RS, scale 0 to 100; RS group, low/intermediate/high risk; month and year of test; or reason for no RS) to IMS using the masked identification number. IMS replaced the masked identity with a unique registry identifier for each patient and distributed the 21-gene RS variables to the MDCSS database. Follow-up for each case was current within 22 months of the annual submission date (November 1, 2015).
Clinical and Demographic Variables
Variables of interest included the 21-gene RS (low risk, < 18; intermediate risk, 18 to 30; high risk, ≥ 31), age at diagnosis, race/ethnicity, American Joint Committee on Cancer (AJCC) clinical stage, histology, tumor size and grade, breast cancer subtype, and HR status. International Classification of Diseases for Oncology site codes were used to define tumor location by primary site. For those women whose treatment data were recorded in the MDCSS database, first course of treatment was defined as receipt of therapy for a cancer diagnosis before disease progression or recurrence and included type of surgery (breast conserving v mastectomy), adjuvant chemotherapy, endocrine therapy, and radiation therapy.
Statistical Analysis
Univariable associations between demographic/clinical characteristics and 21-gene RS by race were examined using χ2 tests and t tests for categorical and continuous variables, respectively. Multinomial logistic regression was used to estimate odds ratios (OR) and 95% CIs to quantify associations among race, clinical/demographic factors, and 21-gene RS risk group, where the reference outcome category was low risk of distant recurrence. Associations between race and 21-gene RS (low, < 18; intermediate, 18 to 30; high, ≥ 31) were assessed in unadjusted and adjusted models. The adjusted model included age at diagnosis (20 to 49, 50 to 64, or ≥ 65 years), race/ethnicity (NHB or NHW), American Joint Committee on Cancer clinical stage (I or II), tumor size (> 0.5 to < 2 cm or 2 to 5 cm) and grade (low or high), and histology (ductal, lobular, ductal and lobular, or other) on the basis of patients’ records having complete information for these covariates. All data were analyzed using SAS version 9.4 statistical software (SAS Institute, Cary, NC). All statistical tests were two-sided, with a P value < .05 considered to be statistically significant.
RESULTS
A total of 6,226 breast cancer cases clinically eligible for 21-gene RS testing on the basis of NCCN guidelines (ie, those who underwent surgery after a diagnosis of HR-positive, HER2-negative, node-negative invasive breast cancer) were identified within the MDCSS database over the 5-year study period (Fig 1). Approximately 40% of these patients (n = 2,514) underwent 21-gene RS testing of their tumors. Uptake of 21-gene RS testing did not differ between NHW and NHB women, among whom 59.6% of NHWs (2,937 of 4,856 patients) and 61.6% of NHBs (673 of 1,089 patients) did not undergo 21-gene RS testing (P = .42). Subsequent analyses focused on the subset of this population who self-identified as NHW or NHB with reported 21-gene RS, which included 2,216 non-Hispanic women (1,824 NHW and 392 NHB patients).
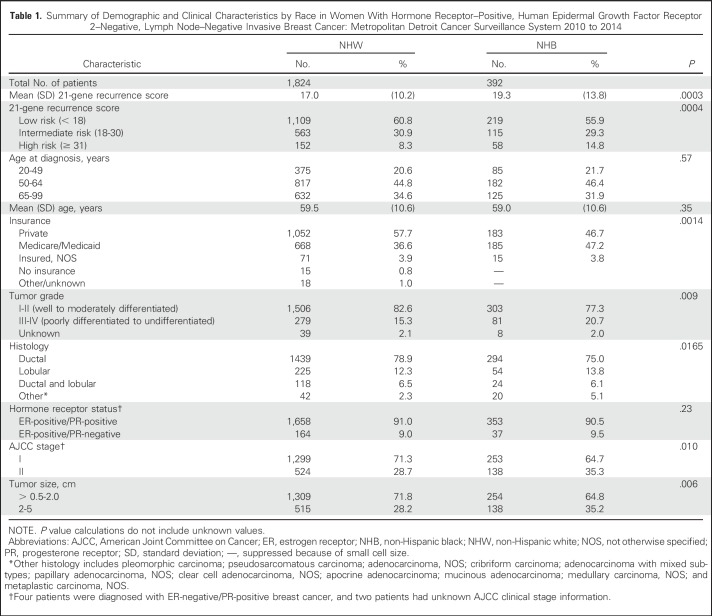
The mean 21-gene RS significantly differed by race. The mean RS for NHB women was classified into the intermediate-risk group per NCCN guidelines and was significantly higher compared with NHW women, for whom the mean RS was categorized as low risk of distant recurrence (19.3 v 17.0, respectively; P = .0003; Table 1). Nearly 10% of all patients (n = 210) with a 21-gene RS were classified into the high-risk group (score, 31 to 100). NHB women were more likely to present with tumors classified as high risk compared with NHW women (14.8% v 8.3%, respectively; P = .0004). The mean age at diagnosis did not differ by race; NHW and NHB individuals were diagnosed at the age of 59.5 and 59.0 years, respectively (Table 1). NHB women were more likely to have Medicaid/Medicare as their primary payment method at the time of diagnosis (NHB 47.2% v NHW 36.6%; P = .0014). Clinically, NHB patients presented with higher grade (NHB 20.7% v NHW 15.3%; P = .009) and more stage II tumors (NHB 35.3% v NHW 28.7%; P = .010) compared with NHW counterparts. Although the majority of breast cancers were infiltrating ductal carcinomas, NHB patients were also significantly less likely to be diagnosed with this histologic subtype compared with NHW patients (75.0% v 78.9%, respectively; P = .0016).
Table 1.
Summary of Demographic and Clinical Characteristics by Race in Women With Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative, Lymph Node–Negative Invasive Breast Cancer: Metropolitan Detroit Cancer Surveillance System 2010 to 2014
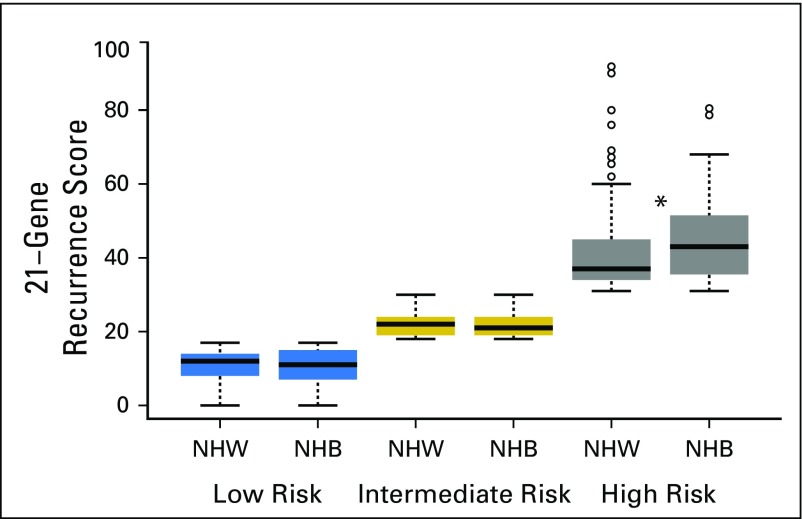
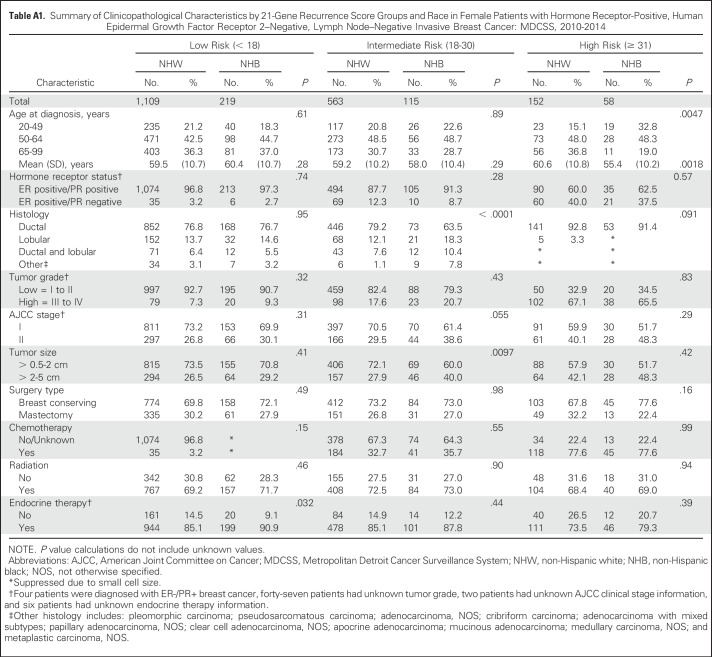
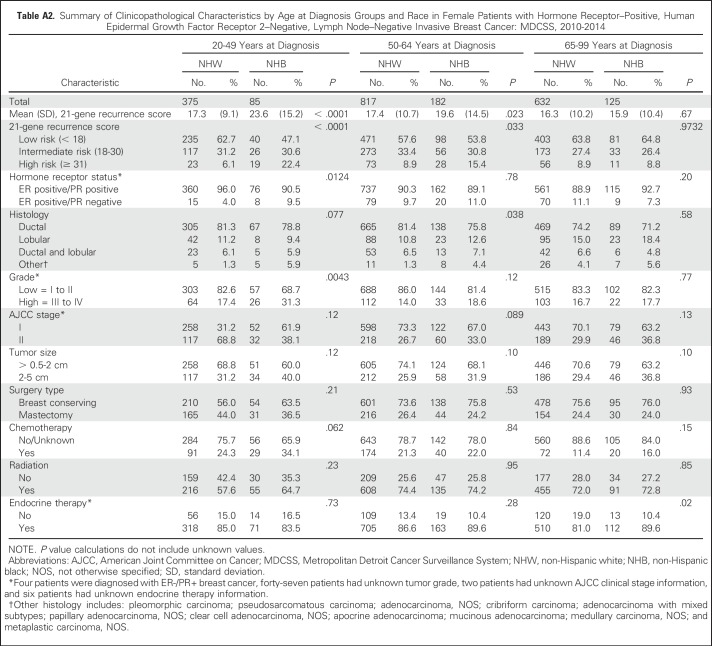
We next evaluated racial differences in 21-gene RS within 21-gene RS risk strata. No racial differences in mean 21-gene RS were observed among patients in the low- and intermediate-risk groups. However, the mean 21-gene RS was significantly higher for NHB compared with NHW women among patients in the high-risk group (45.8 v 41.4, respectively; P = .023; Fig 2). Stratification of 21-gene RS risk group by race and age at breast cancer diagnosis (Fig 3) helped to further define this difference. The mean age at diagnosis among women with high-risk recurrence scores was significantly lower among NHBs compared with NHWs (55.4 v 60.6 years, respectively; P = .0018; Appendix Table A1, online only). Among women in the high-risk category, 32.8% of NHB women were diagnosed with breast cancer age younger than of 50 years compared with 15.1% of NHW women (P = .0047). Furthermore, the observed racial difference in 21-gene RS was limited to women younger than 65 years of age. NHB women were significantly more likely to be in the high-risk group compared with NHW women in the 20 to 49–year age group (22.4% v 6.1%, respectively; P < .0001) and the 50 to 64–year age group (15.4% v 8.9%, respectively; P = .0331; Fig 3, Appendix Table A2, online only).
Fig 2.
Distributions of 21-gene recurrence score versus race. Boxplots of 21-gene recurrence scores are shown by race and recurrence score category. Blue: low risk, < 18; gold: intermediate risk, 18 to 30; gray: high risk, ≥ 31. The bold line within each boxplot represents the median 21-gene recurrence score, and the upper and lower bounds of the boxplot represent the 75th and 25th percentiles, respectively. Outer edges reflect the 10th (lower) and 90th (upper) percentiles, respectively, and outliers are denoted in circles. NHW, non-Hispanic white; NHB, non-Hispanic black. Asterisk denotes a statistically significant difference between NHB and NHW patients.
Fig 3.
Age patterns by race and 21-gene recurrence score. Proportions of women within 21-gene recurrence score categories are shown by age category and race. Blue: low risk, < 18; gold: intermediate risk, 18 to 30; gray: high risk, ≥ 31. NHB, non-Hispanic black; NHW, non-Hispanic white.
Racial differences in tumor and clinical characteristics were noted only among patients with intermediate risk or who were diagnosed between the ages of 50 and 64 years (Appendix Tables A1 and A2). NHBs were significantly more likely to have larger tumors and to present at advanced clinical stages compared with NHWs. Despite the potential for chemotherapy misclassification as the result of unknown therapy information, we noted expected trends in uptake of chemotherapy with decreasing age and by increasing 21-gene RS among all-comers (Appendix Tables A1 and A2). No racial or age differences in type of surgery, adjuvant chemotherapy, or radiation were observed in our study population. Differences in endocrine therapy were noted among patients 65 to 99 years of age or among patients with low-risk tumors. NHB patients were more likely to receive treatment compared with NHW patients (P = .0204 and P = .032, respectively; Appendix Tables A1 and A2).
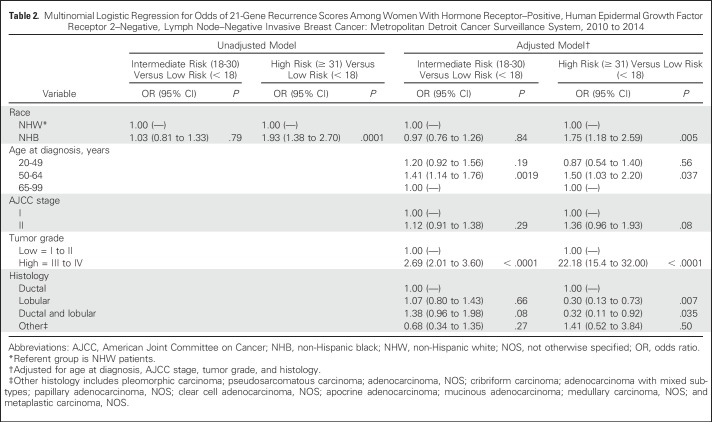
We next quantified the association between race and 21-gene RS group in both unadjusted and adjusted multivariable logistic regression models (Table 2). In the unadjusted model, NHB women were nearly twice as likely to have a high-risk 21-gene RS compared with NHW women (OR, 1.93; 95% CI, 1.38 to 2.70; P = .0001). This association remained after adjusting for age at diagnosis, clinical stage, tumor grade and histology; NHBs remained approximately 75% more likely to be classified into high-risk RS groups compared with NHWs (OR, 1.75; 95% CI, 1.18 to 2.59; P = .005). In the multivariable model, an age of 50 to 64 years at diagnosis and high tumor grade were also independent predictors of having a high-risk 21-gene RS. In contrast, a lobular or ductal and lobular carcinoma was inversely associated with a high-risk RS (Table 2). No association was found between race and intermediate-risk 21-gene RS, although an age of 50 to 64 years at diagnosis and high tumor grade were also independent predictors of having an intermediate-risk 21-gene RS.
Table 2.
Multinomial Logistic Regression for Odds of 21-Gene Recurrence Scores Among Women With Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative, Lymph Node–Negative Invasive Breast Cancer: Metropolitan Detroit Cancer Surveillance System, 2010 to 2014
DISCUSSION
Our study of 2,216 women who underwent 21-gene RS testing of their breast tumors revealed differences in risk RS by race. We noted differences in age at diagnosis and mean 21-gene RS between NHB and NHW patients with tumors classified as high risk. This finding is novel, given that our study is the first population-based evaluation of racial and age patterns among differences in 21-gene RS classification according to clinical guidelines.24
Differences in high-quality treatment and quality of care are factors that effect racial disparities in breast cancer prognosis and outcomes. Yet conflicting evidence has been reported for uptake of 21-gene RS testing by race. In 2012, Lund et al31 used hospital-based tumor registry data from three academic hospitals to describe racial differences in 21-gene RS testing, scores, and treatment, noting that African American women were twice as likely to be categorized as high risk compared with white counterparts; however, classification of risk groups remains inconsistent with NCCN standards.30-34 In contrast, more recent studies reported no racial variations in uptake of 21-gene RS testing among patients with breast cancer.30,39 Consistent with these recent findings, we observed no significant differences in uptake of 21-gene RS testing by race, although NHB patients were slightly less likely to have the assay performed. Reasons for patients to forego 21-gene RS testing are unknown, but could include patient preferences or tumor characteristics and clinically provided sufficient evidence-based information for an informed treatment decision.
Twenty one-gene RS testing is clinically indicated for adjuvant therapy decision making and management; patients classified as high risk (RS, 31 to 100) benefit from chemotherapy followed by endocrine therapy.22-27 In our study, we found that NHB women had larger, higher-grade breast tumors than NHW women, consistent with previous reports from the Carolina Breast Cancer Study.4,30 We also observed trends in increased uptake of chemotherapy by decreasing age and increasing RS group among all patients with breast cancer. Although we are limited in our ability to interpret these data because of potential misclassification as the result of missing chemotherapy data (ie, women classified as not having received chemotherapy when in reality those data were not recorded), this increased uptake with increasing 21-gene RS is consistent with previous reports.
Women diagnosed with young-onset (age at diagnosis < 40 years) breast cancer tend to present with higher-grade tumors and higher frequency of regional lymph node spread compared with older patients and tend to be associated with family history of disease.40 Indeed, previous studies have reported that germ-line mutation frequencies tend to be higher among NHB women compared with NHW women.41,42 Although we were unable to assess family history of cancer, we observed no differences in the overall mean age at diagnosis among NHB and NHW patients in our cohort. Notably, stratification by 21-gene RS groups by age showed that the majority of racial differences in 21-gene RS were observed for women younger than 65 years of age, particularly because NHB women were twice as likely to be diagnosed with breast cancer before the age of 50 years compared with their NHW counterparts.
The use of data from the population-based MDCSS, part of the NCI’s SEER program,38 is a strength of this study because it allowed pathologically-verified cases to be identified from standardized database entries that are continuously monitored for accuracy; this made our results more generalizable to the larger US population. We also used NCCN clinical guidelines for testing to define our inclusion criteria, ensuring that our results are clinically meaningful. However, one weakness of our study is that we cannot separate the effects of ancestry from the social dimensions of race on the observed differences in 21-gene RS from these data alone. Although African ancestry has been suggested to play a role in breast tumor aggressiveness,43,44 factors such as obesity-related inflammation45 and parity-related involution46 may reflect mechanisms by which race as a social construct could affect breast biology. It will be important to incorporate both genetic ancestry markers and hormonal, reproductive, and anthropometry data in future studies to better understand these racial differences. A second limitation is that chemotherapy uptake data may be missing because SEER data collection is conducted primarily at hospitals, radiation facilities, and laboratories rather than physician offices, where such treatment may be obtained. The duration of follow-up was also limited such that we were unable to evaluate recurrence status or mortality.
Among women who were diagnosed with HR-positive, HER2-negative, node-negative breast cancer and who were clinically eligible for 21-gene RS testing in metropolitan Detroit, NHB women are more likely to have tumors classified as having a high risk of distant recurrence compared with NHW women. Furthermore, among all-comers with 21-gene RS test results classified as high risk, NHB women were more likely to be diagnosed at a younger age than their NHW counterparts and had higher individual 21-gene RS. These data highlight the need for long-term studies to evaluate whether these racial differences in 21-gene RS translate to differences in distant recurrence rates. Studies such as these, as well as further studies of the molecular and clinical characteristics of these breast tumors, are needed to explore whether recurrence score classification should be tailored by race.
ACKNOWLEDGMENT
We thank Valentina Petkov for guidance using the 21-gene recurrence score assay data in the Metropolitan Detroit Cancer Surveillance System database.
Appendix
Table A1.
Summary of Clinicopathological Characteristics by 21-Gene Recurrence Score Groups and Race in Female Patients with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2–Negative, Lymph Node–Negative Invasive Breast Cancer: MDCSS, 2010-2014
Table A2.
Summary of Clinicopathological Characteristics by Age at Diagnosis Groups and Race in Female Patients with Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative, Lymph Node–Negative Invasive Breast Cancer: MDCSS, 2010-2014
Footnotes
Partially supported by the Komen for the Cure Graduate Training in Disparities Research Program (GTDR14299438) Fellowship to A.N.H. and M.L.C. for the design and conduct of the study and for the collection, management, analysis, and interpretation of data. Also partially supported by the Epidemiology Core, Health and Human Services contract HHSN261201300011I, and NIH Center Grant P30CA022453 to the Karmanos Cancer Institute at Wayne State University for conduct of the study.
AUTHOR CONTRIBUTIONS
Conception and design: Andreana N. Holowatyj, Michele L. Cote, David H. Gorski, Kristen S. Purrington
Collection and assembly of data: Andreana N. Holowatyj, Julie J. Ruterbusch, Ann G. Schwartz, Fawn D. Vigneau, Kristen S. Purrington
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Racial Differences in 21-Gene Recurrence Scores Among Patients With Hormone Receptor–Positive, Node-Negative Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Andreana N. Holowatyj
No relationship to disclose
Michele L. Cote
No relationship to disclose
Julie J. Ruterbusch
No relationship to disclose
Kristina Ghanem
No relationship to disclose
Ann G. Schwartz
No relationship to disclose
Fawn D. Vigneau
No relationship to disclose
David H. Gorski
No relationship to disclose
Kristen S. Purrington
No relationship to disclose
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2017. CA Cancer J Clin 67:7-30, 2017 [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Siegel RL, Sauer AG, et al. : Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin 66:290-308, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Roseland ME, Pressler ME, Lamerato LE, et al. : Racial differences in breast cancer survival in a large urban integrated health system. Cancer 121:3668-3675, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Carey LA, Perou CM, Livasy CA, et al. : Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295:2492-2502, 2006 [DOI] [PubMed] [Google Scholar]
- 5.D’Arcy M, Fleming J, Robinson WR, et al. : Race-associated biological differences among Luminal A breast tumors. Breast Cancer Res Treat 152:437-448, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg J, Chia YL, Plevritis S: The effect of age, race, tumor size, tumor grade, and disease stage on invasive ductal breast cancer survival in the U.S. SEER database. Breast Cancer Res Treat 89:47-54, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Sineshaw HM, Gaudet M, Ward EM, et al. : Association of race/ethnicity, socioeconomic status, and breast cancer subtypes in the National Cancer Data Base (2010-2011). Breast Cancer Res Treat 145:753-763, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Li CI, Malone KE, Daling JR: Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med 163:49-56, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Henson DE, Chu KC, Levine PH: Histologic grade, stage, and survival in breast carcinoma: Comparison of African American and Caucasian women. Cancer 98:908-917, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Robbins AS, Lerro CC, Barr RD: Insurance status and distant-stage disease at diagnosis among adolescent and young adult patients with cancer aged 15 to 39 years: National Cancer Data Base, 2004 through 2010. Cancer 120:1212-1219, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Holowatyj AN, Ruterbusch JJ, Ratnam M, et al. : HER2 status and disparities in luminal breast cancers. Cancer Med 5:2109-2116, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howlader N, Altekruse SF, Li CI, et al. : US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 106:dju055, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blows FM, Driver KE, Schmidt MK, et al. : Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: A collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 7:e1000279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma H, Lu Y, Malone KE, et al. : Mortality risk of black women and white women with invasive breast cancer by hormone receptors, HER2, and p53 status. BMC Cancer 13:225, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien KM, Cole SR, Tse CK, et al. : Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res 16:6100-6110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warner ET, Tamimi RM, Hughes ME, et al. : Racial and ethnic differences in breast cancer survival: Mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol 33:2254-2261, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huo D, Hu H, Rhie SK, et al. : Comparison of breast cancer molecular features and survival by African and European ancestry in The Cancer Genome Atlas. JAMA Oncol 10.1001/jamaoncol.2017.0595 [epub ahead of print on May 4, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill J, Watanabe-Galloway S, Shostrom V, et al. : Breast cancer survival among African-Americans living in the Midwest: Disparities and recommendations to decrease mortality. J Natl Black Nurses Assoc 26:8-14, 2015 [PubMed] [Google Scholar]
- 19.Chowdhury R, David N, Bogale A, et al. : Assessing the key attributes of low utilization of mammography screening and breast-self exam among African-American women. J Cancer 7:532-537, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortel M, Rauscher GH, Murphy AM, et al. : Racial and ethnic disparity in symptomatic breast cancer awareness despite a recent screen: The role of tumor biology and mammography facility characteristics. Cancer Epidemiol Biomarkers Prev 24:1599-1606, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George P, Chandwani S, Gabel M, et al. : Diagnosis and surgical delays in African American and white women with early-stage breast cancer. J Womens Health (Larchmt) 24:209-217, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paik S, Tang G, Shak S, et al. : Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726-3734, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Sparano JA, Gray RJ, Makower DF, et al. : Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373:2005-2014, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Comprehensive Cancer Network : Breast Cancer. Fort Washington, PA, NCCN Clinical Practice Guidelines in Oncology; 2015 [Google Scholar]
- 25.Mamounas EP, Liu Q, Paik S, et al. : 21-Gene recurrence score and locoregional recurrence in node-positive/ER-positive breast cancer treated with chemo-endocrine therapy. J Natl Cancer Inst 109:djw259, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albain KS, Barlow WE, Shak S, et al. : Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol 11:55-65, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paik S, Shak S, Tang G, et al. : A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817-2826, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Mamounas EP, Tang G, Fisher B, et al. : Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: Results from NSABP B-14 and NSABP B-20. J Clin Oncol 28:1677-1683, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dowsett M, Cuzick J, Wale C, et al. : Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study. J Clin Oncol 28:1829-1834, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Roberts MC, Weinberger M, Dusetzina SB, et al. : Racial variation in the uptake of Oncotype DX testing for early-stage breast cancer. J Clin Oncol 34:130-138, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund MJ, Mosunjac M, Davis KM, et al. : 21-Gene recurrence scores: Racial differences in testing, scores, treatment, and outcome. Cancer 118:788-796, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Guth AA, Fineberg S, Fei K, et al. : Utilization of Oncotype DX in an inner city population: Race or place? Int J Breast Cancer 2013:653805, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeFrank JT, Salz T, Reeder-Hayes K, et al. : Who gets genomic testing for breast cancer recurrence risk? Public Health Genomics 16:215-222, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassett MJ, Silver SM, Hughes ME, et al. : Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol 30:2218-2226, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis BA, Aminawung JA, Abu-Khalaf MM, et al. : Racial and ethnic disparities in Oncotype DX test receipt in a statewide population-based study. J Natl Compr Canc Netw 15:346-354, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Dinan MA, Mi X, Reed SD, et al. : Initial trends in the use of the 21-gene recurrence score assay for patients with breast cancer in the Medicare population, 2005-2009. JAMA Oncol 1:158-166, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Henry NL, Braun TM, Ali HY, et al. : Associations between use of the 21-gene recurrence score assay and chemotherapy regimen selection in a statewide registry. Cancer 123:948-956, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Howlader N NA, Krapcho M, Miller D, et al. : Cronin KA (eds). SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2014/
- 39.Guth AA, Chun Kim J, Schwartz S, et al. : The relationship of race, Oncotype DX, and Ki67 in a population highly screened for breast cancer. Breast J 23:177-181, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Anders CK, Hsu DS, Broadwater G, et al. : Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 26:3324-3330, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Armstrong K, Micco E, Carney A, et al. : Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA 293:1729-1736, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Jagsi R, Griffith KA, Kurian AW, et al. : Concerns about cancer risk and experiences with genetic testing in a diverse population of patients with breast cancer. J Clin Oncol 33:1584-1591, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deshmukh SK, Srivastava SK, Tyagi N, et al. : Emerging evidence for the role of differential tumor microenvironment in breast cancer racial disparity: A closer look at the surroundings. Carcinogenesis 38:757-765, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiagge E, Jibril AS, Chitale D, et al. : Comparative analysis of breast cancer phenotypes in African American, White American, and West versus East African patients: Correlation between African ancestry and triple-negative breast cancer. Ann Surg Oncol 23:3843-3849, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Vaysse C, Lømo J, Garred Ø, et al. : Inflammation of mammary adipose tissue occurs in overweight and obese patients exhibiting early-stage breast cancer. NPJ Breast Cancer 3:19, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ginsburg OM, Martin LJ, Boyd NF: Mammographic density, lobular involution, and risk of breast cancer. Br J Cancer 99:1369-1374, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]