Abstract
Purpose
Although the life expectancy of patients with follicular lymphoma (FL) has increased, little is known of their causes of death (CODs) in the rituximab era.
Patients and Methods
We pooled two cohorts of newly diagnosed patients with FL grade 1-3A. Patients were enrolled between 2001 and 2013 in two French referral institutions (N = 734; median follow-up 89 months) and 2002 and 2012 in the University of Iowa and Mayo Clinic Specialized Program of Research Excellence (SPORE; N = 920; median follow-up 84 months). COD was classified as being a result of lymphoma, other malignancy, treatment related, or all other causes.
Results
Ten-year overall survival was comparable in the French (80%) and US (77%) cohorts. We were able to classify COD in 248 (88%) of 283 decedents. In the overall cohort, lymphoma was the most common COD, with a cumulative incidence of 10.3% at 10 years, followed by treatment-related mortality (3.0%), other malignancy (2.9%), other causes (2.2%), and unknown (3.0%). The 10-year cumulative incidence of death as a result of lymphoma or treatment was higher than death as a result of all other causes for each age group (including patients ≥ 70 years of age at diagnosis [25.4% v 16.6%]) Follicular Lymphoma International Prognostic Index score 3 to 5 (27.4% v 5.2%), but not Follicular Lymphoma International Prognostic Index score 0 to 1 (4.0% v 3.7%); for patients who failed to achieve event-free survival within 24 months from diagnosis (36.1% v 7.0%), but not for patients who achieved event-free survival within 24 months of diagnosis (6.7% v 5.7%); and for patients with a history of transformed FL (45.9% v 4.7%), but not among patients without (8.1% v 6.2%). Overall, 77 of 140 deaths as a result of lymphoma occurred in patients whose FL transformed after diagnosis.
Conclusion
Despite the improvement in overall survival in patients with FL in the rituximab era, their leading COD remains lymphoma, especially after disease transformation. Treatment-related mortality also represents a concern, which supports the need for less-toxic therapies.
INTRODUCTION
Follicular lymphoma (FL) is the most frequent indolent lymphoma.1,2 Despite recent progress in understanding the development of this disease,3,4 its clinical heterogeneity remains poorly understood. Some patients have an indolent evolution over several decades, whereas others show a rather aggressive clinical course, generally accompanied by a histologic transformation5 and poor prognosis.6,7 In the last two decades, life expectancy of patients with FL has markedly improved with the introduction of anti-CD20 therapies.8-12 For patients with symptomatic disease at diagnosis, standard of care includes immunochemotherapy (IC).13 In patients who are asymptomatic, treatment options are still debated.14,15 Despite these improvements, most physicians consider FL to be incurable, with a continuous pattern of relapse.16 Furthermore, patients with FL who are treated upfront with IC who are event free (no death, relapse, or retreatment) within 24 months of diagnosis (EFS24) have a subsequent survival that is comparable to the age- and sex-matched background population,17 whereas those who experience an event before 24 months have a more aggressive course with poor outcomes.17,18 In this context, long-term, treatment-related adverse effects and toxicity may become important issues.
In the rituximab era, deaths as a result of lymphoma in patients with FL have decreased at the population level,9 but there are limited data on the precise causes of death (CODs). For instance, when analyzing the long-term outcome of 281 patients with FL over different time periods, a significant improvement in cause-specific survival, but not in overall survival (OS), was found,19 which suggests that treatment-related toxicities may occur. Another report demonstrated that lymphoma and complications of treatments remained the leading COD in younger patients treated in a randomized trial12; however, it is important to note that survival is a function of lymphoma aggressiveness as well as of competing risks of mortality, which are lower for younger patients but are substantially increased with age. To provide a better characterization of CODs in the modern treatment era according to patient and disease characteristics, we conducted a pooled analysis of two independent cohorts of patients with FL from France and the United States.
PATIENTS AND METHODS
This study was conducted according to the Hospices Civils de Lyon, Centre Léon Bérard, University of Iowa, and Mayo Clinic institutional ethics guidelines and in accordance with the Declaration of Helsinki. The French cohort consisted of 734 consecutive, newly diagnosed or referred patients with grade 1 to 3A FL who were treated at the Centre Hospitalier Lyon Sud and Centre Léon Bérard between 2001 and 2013. The US cohort consisted of 920 consecutive, newly diagnosed patients with grade 1 to 3A FL who consented and were enrolled between 2002 and 2012 in the University of Iowa and Mayo Clinic Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource.20 In both cohorts, all pathology, including histologic transformations, were reviewed by study hematopathologists, and patient management, including staging, treatment(s), and clinical surveillance, was per the treating physician. Loss to follow-up was 0% in the French cohort and less than 1% in the SPORE cohort.
Disease progression, retreatment, transformation, and death events were verified via extensive review of medical records. COD was uniformly coded (by C.S., H.G., and G.S. for the French cohort; and C.A.T., T.M.H., and B.K.L. for the US cohort) as a result of lymphoma (progression or transformation), treatment, unrelated cancer, other causes, or unknown. Treatment-related mortality (TRM) was broadly defined and further classified into infection, cardiac, secondary myelodysplastic syndrome/acute myeloid leukemia (MDS/AML), or other. TRM was considered when death occurred either during treatment without evidence of disease refractoriness or shortly after treatment without evidence of progressive disease, or when death occurred much later but was unambiguously documented in the medical record as a result of treatment. If COD was unclear, the case was discussed between investigators and classified by consensus. FL transformation status was usually confirmed by biopsy; however, in some cases, diagnosis of transformation was based on previously described clinical criteria.5,21
Event-free survival (EFS) was defined as the time since diagnosis until progression, relapse, retreatment, or death as a result of any cause. On the basis of our prior publication,17 we defined early failure in patients treated with IC as EFS24 and for all other initial management strategies (including observation) as EFS at 12 months (EFS12). OS was defined as the time since the date of diagnosis to the date of death (any cause) or last known follow-up for patients still alive. COD was first categorized into the five major groupings (lymphoma, treatment, unrelated cancer, other causes, or unknown) and subsequently collapsed into three groupings: lymphoma-related deaths, defined as lymphoma or treatment related; deaths unrelated to lymphoma, which included other malignancies and other causes; and unknown. All-cause OS was determined via Kaplan-Meier method. Cumulative incidence for the competing risks of COD and tests of equality for COD between groups were calculated using the cuminc function from the cmprsk package in R.22 For early event analysis, time to death was defined as the time since the 12- or 24-month time point for patients who achieved EFS12/EFS24 and time since the first event for patients who did not achieve EFS12/EFS24. For COD by transformation analysis, which was based only on the US cohort, time to death was defined using the time since the date of transformation in the set of patients with transformation. CODs in patients without transformation were analyzed using the time to death since diagnosis in all patients from diagnosis, with censoring at the date of transformation in the set of patients who experienced transformation before death. All analyses were performed using SAS (SAS/STAT User’s Guide, Version 9.4; SAS Institute, Cary, NC) and R (version 3.2.3).
RESULTS
Descriptive Characteristics
Baseline characteristics, treatments, and summary outcome data are described for each cohort and the pooled cohort of 1,654 patients (Table 1). French and US cohorts were similar in age structure, sex distribution, Ann Arbor stage, and Follicular Lymphoma International Prognostic Index (FLIPI) score. IC was the most common initial therapy in the French cohort (67%), whereas in the US cohort IC (38%) and watch and wait (36%) were the most common initial therapies.
TABLE 1.
Characteristics of the French, US, and Pooled Cohorts

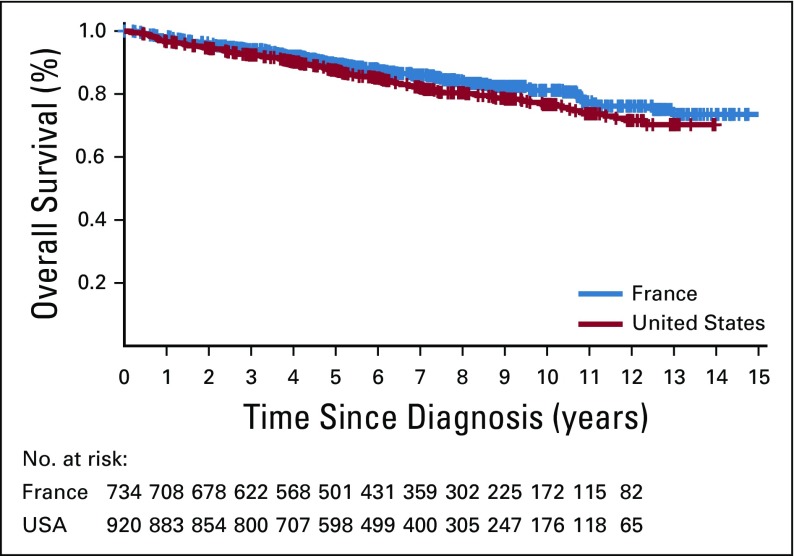
In the French cohort, after a median follow-up of 89 months for living patients, there were 113 deaths. In the US cohort, after a median follow-up of 84 months for living patients, there were 170 deaths. Ten-year OS was comparable in the French (79.8%) and US (76.6%) cohorts (Appendix Fig A1, online only), as was EFS24 (69.9% in each cohort; Table 1).
Cumulative Incidence and Distribution of CODs
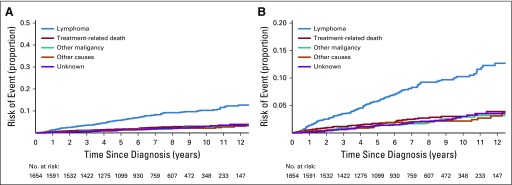
We were able to classify COD in 248 (88%) of 283 decedents. As shown in Figure 1, death as a result of lymphoma was the most common COD, with a cumulative incidence of 10.3% at 10 years (95% CI, 8.6% to 12.2%), followed by TRM (3.0%; 95% CI, 2.2% to 4.1%), other malignancy (2.9%; 95% CI, 2.0% to 4.2%), other causes (2.2%; 95% CI, 1.5% to 3.1%), and unknown cause (3.0%; 95% CI, 2.1% to 4.4%).
FIG 1.
Cumulative incidence for the competing risks of cause of death in the pooled cohort. (A) Cumulative incidence by cause of death. (B) Cumulative incidence by cause of death with y-axis rescaled.
The cumulative incidence for each of these causes demonstrated a monotonic (essentially linear) increase over the first decade after diagnosis of FL. Table 2 summarizes the CODs. Among 248 decedents with a known COD, 57% were a result of lymphoma, 17% were treatment related, 13% were a result of other malignancies, and 13% were a result of other causes (provided in Appendix Table A1, online only). Whereas these distributions were broadly similar in the two cohorts, there was a higher percentage of deaths attributed to lymphoma in the French (65%) versus the US (50%) cohort, and a lower percentage of deaths attributed to other causes in the French (7%) versus the US (18%) cohort. However, outcomes were similar on the basis of the more valid comparison using cumulative incidence with competing risk of death; the 10-year cumulative incidence of death as a result of lymphoma was 14.2% (95% CI, 11.4% to 17.7%) in the French cohort and 12.5% (95% CI, 10.2% to 15.4%) in the US cohort, whereas the 10-year cumulative incidence of death as a result of other causes was 3.7% (95% CI, 2.3% to 6.1%) and 6.1% (95% CI, 4.4% to 8.4%), respectively.
TABLE 2.
Causes of Death

Of the 140 deaths as a result of lymphoma, 77 (55%) occurred in patients whose FL had transformed at some point in the disease history. Of the 42 deaths as a result of TRM, 20 (48%) were because of infection, 12 (29%) because of MDS/AML, six (14%) because of cardiotoxicity, and four (9%) because of other treatment-related causes.
COD Pattern by Demographic and Clinical Characteristics
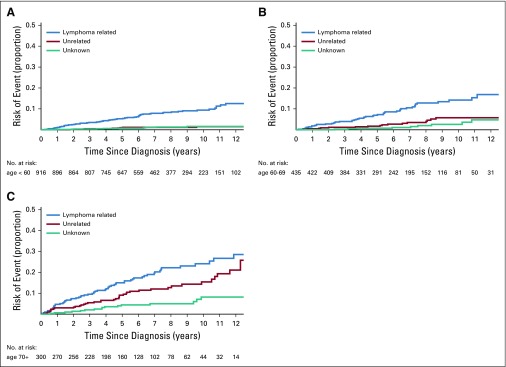
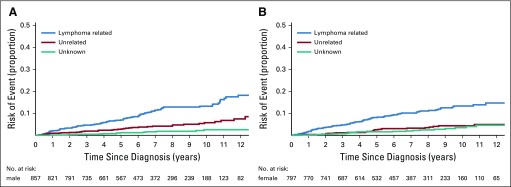
We next modeled the cumulative incidence for each COD category for key subgroups defined on demographic and clinical characteristics. To increase interpretability, we grouped COD as lymphoma-related deaths (lymphoma or treatment related), deaths unrelated to lymphoma (other malignancies and other causes), and deaths as a result of unknown causes. We also estimated the 5- and 10-year cumulative incidence along with 95% CIs for lymphoma-related and non–lymphoma-related deaths by subgroup and conducted a formal statistical test for differences in cumulative incidence by subgroup (Table 3). There was no difference in the cumulative incidence of lymphoma-related (P = .62) or non–lymphoma-related (P = .20) deaths by sex (Appendix Fig A2, online only). As expected, the cumulative incidence of lymphoma-related (P < .001) and non–lymphoma-related (P < .001) deaths increased with age at diagnosis. Of note, in all age groups, the cumulative incidence in lymphoma-related deaths were higher than that of non–lymphoma-related deaths (Fig 2). For example, the 10-year cumulative incidence of lymphoma-related deaths was 9.4% in patients who were diagnosed at ≥ 60 years, 14.2% for patients diagnosed at age 61 to 70 years, and 25.4% for patients diagnosed at older than age 70 years. The respective 10-year cumulative incidence of non–lymphoma-related mortality was 1.5%, 5.8%, and 16.6%, respectively.
TABLE 3.
Five- and 10-Year Risk of Death With 95% CIs for Lymphoma and Non–Lymphoma-Related Deaths, Pooled French and US Cohorts
FIG 2.
Cumulative incidence for the competing risks of cause of death. (A) Cumulative incidence by cause of death for patients age ≤ 60 years. (B) Cumulative incidence by cause of death for patients age 61 to 70 years. (C) Cumulative incidence by cause of death for patients age > 70 years.
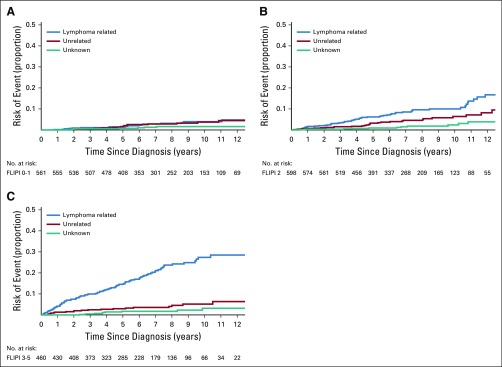
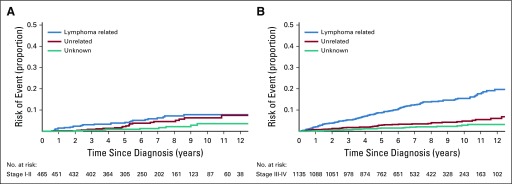
The cumulative incidence of lymphoma-related mortality increased more rapidly with time since diagnosis in patients with stage III to IV disease compared with stage I to II disease (Appendix Fig A3, online only), with a 10-year cumulative incidence of 15.5% for stage III to IV compared with 7.8% for stage I to II disease (P < .001). In contrast, there was no difference in the cumulative incidence of non–lymphoma-related deaths by stage (P = .58). Similarly, the cumulative incidence of lymphoma-related mortality increased more rapidly with a higher FLIPI score (Fig 3), with a 10-year cumulative incidence of 4.0% for FLIPI 0 to 1, 10.0% for FLIPI 2, and 27.4% for FLIPI 3 to 5 (P < .001), but there was no difference in the cumulative incidence of non–lymphoma-related deaths by FLIPI score (P = .15). Of note, in patients with FLIPI 0 to 1 disease, the 10-year cumulative incidence of non–lymphoma-related deaths (3.7%) was similar to that of lymphoma-related deaths (4.0%).
FIG 3.
Cumulative incidence for the competing risks of cause of death according to Follicular Lymphoma International Prognostic Index (FLIPI) score. (A) Cumulative incidence by cause of death for patients with FLIPI score 0 to 1. (B) Cumulative incidence by cause of death for patients with FLIPI score 2. (C) Cumulative incidence by cause of death for patients with FLIPI score 3 to 5.
COD Pattern by Early Event and Transformation Status
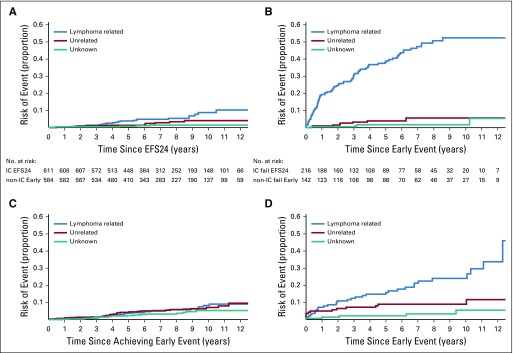
Among patients who achieved EFS24, the subsequent cumulative incidences of lymphoma- and non–lymphoma-related mortality were similar, with a 10-year cumulative incidence since the EFS24 time point of 6.7% for lymphoma-related mortality and 5.7% for non–lymphoma-related mortality (Table 3). In contrast, for patients who did not achieve EFS24, the subsequent cumulative incidence of lymphoma-related mortality was greater than that of non–lymphoma-related mortality, with a 10-year cumulative incidence from an event of 36.1% for lymphoma-related mortality and 7.0% for non–lymphoma-related mortality. The higher cumulative incidence of lymphoma-related (36.1% v 6.7%; P < .001) and non–lymphoma-related (5.7% v 7.0%; P = .0052) mortality in patients who did not achieve EFS24 compared with patients who achieved EFS24 were both statistically significant. Stratifying the analysis to EFS24 for patients who were initially treated with IC and to EFS12 for patients who were initially treated with all other approaches (non–IC treated), the subsequent cumulative incidence of lymphoma- and non–lymphoma-related mortality were similar (Appendix Fig A4A, online only), with a 10-year cumulative incidence from the EFS24 (IC treated) or EFS12 (non–IC treated) time points of 8.8% and 8.2% for lymphoma-related mortality and 4.1% and 7.1%, respectively, for non–lymphoma-related mortality. In contrast, for patients who did not achieve EFS24 (for IC treated) or EFS12 (for non-IC treated), the subsequent cumulative incidence of lymphoma-related mortality was greater than that of non–lymphoma-related mortality (Appendix Fig A4B), with a 10-year cumulative incidence from an event of 52.4% and 24.0% for lymphoma-related mortality and 5.6% and 9.0% for non–lymphoma-related mortality, respectively. There were too few events in the non–IC-treated group to provide estimates by specific type of initial management—that is, observation versus rituximab monotherapy versus other.
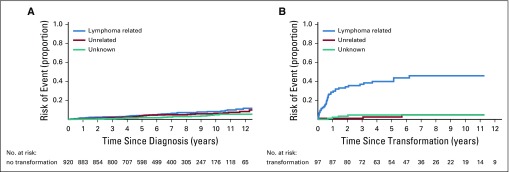
For the US cohort, we had data on the transformation status for all patients. Among patients without transformation, the cumulative incidence of lymphoma- and non–lymphoma-related mortality were similar (Appendix Fig A5A, online only), with a 10-year cumulative incidence since diagnosis of 8.1% for lymphoma-related mortality and 6.2% for non–lymphoma-related mortality. In contrast, the subsequent cumulative incidence of lymphoma-related mortality after transformation was greater than that of non–lymphoma-related mortality (Appendix Fig A5B), with a 10-year cumulative incidence since transformation of 45.9% for lymphoma-related mortality and 4.7% for non–lymphoma-related mortality.
DISCUSSION
There are several clinically relevant conclusions from our pooled analysis of two cohort studies of patients with FL who were diagnosed and treated in the rituximab era. First, OS was approximately 80% at 10 years since diagnosis in both US and French cohorts, which is comparable to the long-term outcome of patients included in the SWOG12 (ClinicalTrials.gov identifier: NCT000006721) or PRIMA (Clinicaltrials.gov identifier: NCT00140582) trials.13 Second, lymphoma was the leading COD in the first decade since diagnosis, with a cumulative risk of mortality as a result of lymphoma of 10.3% at 10 years, which increased to 13.3% when combined with TRM. In contrast, a cumulative risk of mortality as a result of non–lymphoma-related causes was only 5.1% at 10 years. Third, lymphoma-related mortality was the major COD in all age groups, even for patients older than age 70 years. Fourth, whereas FLIPI score was strongly associated with lymphoma-related mortality, it was not associated with non–lymphoma-related mortality. Fifth, the 10-year cumulative risk of lymphoma-related mortality in patients who did not achieve EFS24 was 36.1%. Finally, the cumulative risk of lymphoma-related mortality after transformation was 45.9%.
The strengths of the current study include the use of two independent cohorts, review and classification of all deaths, availability of clinical data, and use of a competing risk analysis to model cumulative incidence. The survival rate in these French and US reference centers were comparable to the OS reported in the literature,7,23 which suggests minimal bias and good external validity. However, these cohorts were not population based and future work should evaluate these findings in this setting. A limitation of this real-world study is that management strategies (eg, surveillance, retreatment, and repeat biopsy) were not protocol driven but were at the discretion of the managing physician, and the effect of this on COD is unknown. We also did not have systematic prospective data collection on long-term toxicity, which could potentially underestimate TRM. Indeed, is it likely that not all deaths related to chronic toxicities (ie, pulmonary fibrosis from alkylators, or peripheral vascular disease from radiation) were captured. Other limitations include missing data for some deaths and a lack of racial or ethnic diversity. Finally, we only had data on the first decade of deaths in the rituximab era, and longer follow-up is needed to understand late deaths in this cancer with a long natural history.
Whereas recent reports have demonstrated an improvement in excess mortality rate among patients with FL since the introduction of immunotherapy in combination with chemotherapy,9,24 the leading COD remains lymphoma. Indeed, our findings corroborate the long-term follow-up of the younger cohorts of patients with symptomatic disease included in the SWOG trial.12 Mounier at al9 have reported that the excess mortality rate was higher in the elderly population and in those with stage III to IV disease, which is in agreement with our findings that showed that stage was strongly associated with lymphoma-related death and that lymphoma remained the principal COD in patients older than age 70 years. As expected and reported,25,26 we also observed a more important rate of non–lymphoma-related death among patients ≥ 70 years of age. A recent24 population-based study that included 961 patients with FL (with a short 55-month median follow-up) also reported that the higher standardized mortality ratio for FL (standardized mortality ratio, including all causes of death) was associated with FLIPI score and EFS12/EFS24 failures. Of note, in the current study, women tended to have a higher standardized mortality ratio than men but a similar global OS in contrast to that reported in the National LymphoCare Study27 and the Swedish Lymphoma Registry study28, both of which demonstrated lower lymphoma-related mortality for women.27,28 In our study, the cumulative incidence of lymphoma-related deaths by sex were identical, whereas a nonsignificant trend toward a lower rate of non–lymphoma-related deaths was observed in women. Of note, of the 140 lymphoma deaths, 77 (55%) were related to transformation. Indeed, among patients without a transformation during their disease history, the cumulative incidence of death related to lymphoma is equivalent to that for deaths unrelated to lymphoma (4.0% v 3.5% at 5 years and 8.1% v 6.2% at 10 years for deaths related to lymphoma v deaths unrelated to lymphoma, respectively). This important notion that transformation in FL is the major cause of lymphoma-related death should provide a framework for future specific approaches.
In our series, 17% of deaths (n = 42) were attributed to treatment, and these were most commonly a result of infections (n = 20) and secondary MDS/AML (n = 12). Others have reported a significant incidence of fatal secondary myeloid neoplasia in patients who received radioimmunotherapy or a fludarabine-mitoxantrone–based regimen12; however, little is known regarding other long-term, treatment-related fatal complications, such as infections or cardiac deaths, especially in patients with indolent lymphomas. Finally, the risk of nonmyeloid neoplasia (and myeloid secondary neoplasia) in survivors of lymphoma is well known and seems to be higher in patients with FL than in those with diffuse large B-cell lymphoma.29 Long-term outcome data of clinical trials, registry data, and additional studies are needed to have a better estimate of the long-term fatalities directly or indirectly associated with specific treatments.
In conclusion, we provide a comprehensive description of the pattern of deaths observed in patients with FL in the rituximab era. Despite a favorable (80%) 10-year OS, lymphoma represents the leading COD in the first decade after diagnosis. This is particularly true for patients who present with a high FLIPI score, for those with transformed disease, and for those who did not achieve EFS12/EFS24. Deaths related to treatment seem to also be a significant burden and new, less-toxic treatment options need to be investigated. Given the adverse outcomes associated with early treatment failure (EFS24) and histologic transformation, efforts that focus on clinical or biologic models to identify at-risk patients and the development of new therapeutic approaches are an important challenge in FL.
Appendix
FIG A1.
Overall survival since diagnosis.
FIG A2.
Cumulative incidence for the competing risks of cause of death according to sex. (A) Cumulative incidence by cause of death for male patients. (B) Cumulative incidence by cause of death for female patients.
FIG A3.
Cumulative incidence for the competing risks of cause of death according to disease stage. (A) Cumulative incidence by cause of death for patients with stage I and II disease. (B) Cumulative incidence by cause of death for patients with stage III and IV disease.
FIG A4.
Cumulative incidence for the competing risks of cause of death. (A) Patients treated upfront with immunochemotherapy (IC) who achieved event-free survival within 24 months of diagnosis (EFS24). (B) Patients treated upfront with IC who did not achieve EFS24 diagnosis. (C) Patients not treated with up-front IC (non-IC treated) who achieved EFS12/early event achieve non−IC treated. (D) Patients not treated with up-front IC (non-IC treated) who did not achieve EFS12 (early event)/early event fail non−IC treated.
FIG A5.
Cumulative incidence for the competing risks of cause of death. (A) US cohort patients without follicular lymphoma transformation/US cohort no transformation (B) US cohort patients with follicular lymphoma transformation/US cohort transformation.
TABLE A1.
Description of Other Causes of Death

Footnotes
Presented at the 34th International Conference on Machine Learning, Sydney, NSW, Australia, August 6-11, 2017. Presented at the International Conference on Malignant Lymphoma, ICML, Lugano, Switzerland, June 14-17, 2017
Supported by National Institutes of Health Grants No. P50-CA97274 and U01-CA195568.
AUTHOR CONTRIBUTIONS
Conception and design: Clémentine Sarkozy, Matthew J. Maurer, James R. Cerhan, Gilles A. Salles
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: Clémentine Sarkozy, Matthew J. Maurer, James R. Cerhan, Gilles A. Salles, Hervé Ghesquières, Emmanuel Bachy, Brian K. Link, Thomas M. Habermann
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cause of Death in Follicular Lymphoma in the First Decade of the Rituximab Era: A Pooled Analysis of French and US Cohorts
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Clémentine Sarkozy
Honoraria: Genentech, Celgene
Research Funding: Genentech (Inst), Takeda
Travel, Accommodations, Expenses: Takeda
Matthew J. Maurer
Consulting or Advisory Role: MorphoSys
Research Funding: Kite Pharma (Inst), Celgene (Inst), NanoString Technologies (Inst)
Brian K. Link
Consulting or Advisory Role: Genentech, AbbVie, Gilead Sciences, Celgene
Research Funding: Genentech (Inst), Pharmacyclics (Inst), Janssen Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Genentech, Celgene
Hervé Ghesquieres
Honoraria: Gilead Sciences
Consulting or Advisory Role: Gilead Sciences, Celgene
Travel, Accommodations, Expenses: Gilead Sciences, Amgen, Roche
Andrew L. Feldman
Consulting or Advisory Role: Infinity Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Inventor of technology for which Mayo Clinic holds an unlicensed patent or has submitted a patent application (Inst)
Stephen M. Ansell
Honoraria: WebMD, Research to Practice
Research Funding: Bristol-Myers Squibb (Inst), Seattle Genetics (Inst), Affirmed Therapeutics (Inst), Regeneron (Inst), Pfizer (Inst), LAM Therapeutics (Inst), Trillium Therapeutics (Inst)
Emmanuel Bachy
Honoraria: Gilead Sciences, Roche, Amgen, Janssen-Cilag
Consulting or Advisory Role: Roche
Travel, Accommodations, Expenses: Janssen-Cilag
James R. Cerhan
Consulting or Advisory Role: Janssen Pharmaceuticals
Research Funding: NanoString Technologies, Celgene
Gilles Salles
Honoraria: Genentech, Amgen, Janssen Pharmaceuticals, Bristol-Myers Squibb, Celgene, Servier, Gilead Sciences, Novartis
Consulting or Advisory Role: Genentech, Gilead Sciences, Janssen Pharmaceuticals, Celgene, Novartis, Merck, Novimmune, Pfizer, Acerta Pharma, Kite Pharma, Bristol-Myers Squibb, Servier
Research Funding: Genentech (Inst), Gilead Sciences, Celgene, Genentech, Merck
Travel, Accommodations, Expenses: Genentech, Sanofi
No other potential conflicts of interest were reported.
REFERENCES
- 1.Campo E, Swerdlow SH, Harris NL, et al. : The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 117:5019-5032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teras LR, DeSantis CE, Cerhan JR, et al. : 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 10.3322/caac.21357 [epub ahead of print on September 12, 2016] [DOI] [PubMed] [Google Scholar]
- 3.Roulland S, Faroudi M, Mamessier E, et al. : Early steps of follicular lymphoma pathogenesis. Adv Immunol 111:1-46, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Kridel R, Chan FC, Mottok A, et al. : Histological transformation and progression in follicular lymphoma: A clonal evolution study. PLoS Med 13:e1002197, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Link BK, Maurer MJ, Nowakowski GS, et al. : Rates and outcomes of follicular lymphoma transformation in the immunochemotherapy era: A report from the University of Iowa/MayoClinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J Clin Oncol 31:3272-3278, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casulo C, Nastoupil L, Fowler NH, et al. : Unmet needs in the first-line treatment of follicular lymphoma. Ann Oncol 28:2094-2106, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner-Johnston ND, Link BK, Byrtek M, et al. : Outcomes of transformed follicular lymphoma in the modern era: A report from the National LymphoCare Study (NLCS). Blood 126:851-857, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salles G, Ghesquières H: Current and future management of follicular lymphoma. Int J Hematol 96:544-551, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Mounier M, Bossard N, Belot A, et al. : Trends in excess mortality in follicular lymphoma at a population level. Eur J Haematol 94:120-129, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Tan D, Horning SJ, Hoppe RT, et al. : Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: The Stanford University experience. Blood 122:981-987, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sant M, Minicozzi P, Mounier M, et al. : Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: Results of EUROCARE-5, a population-based study. Lancet Oncol 15:931-942, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Shadman M, Li H, Rimsza L, et al. : Continued excellent outcomes in previously untreated patients with follicular lymphoma after treatment with CHOP plus rituximab or CHOP plus 131I-tositumomab: Long-term follow-up of phase III randomized study SWOG-S0016. J Clin Oncol 36:697-703, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salles G, Seymour JF, Offner F, et al. : Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet 377:42-51, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Solal-Céligny P, Bellei M, Marcheselli L, et al. : Watchful waiting in low-tumor burden follicular lymphoma in the rituximab era: Results of an F2-study database. J Clin Oncol 30:3848-3853, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Ardeshna KM, Qian W, Smith P, et al. : Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: An open-label randomised phase 3 trial. Lancet Oncol 15:424-435, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Haines IE: Should the paradigm shift for low-grade follicular non-Hodgkin lymphoma be toward less therapy? J Clin Oncol 31:1797-1798, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Maurer MJ, Bachy E, Ghesquières H, et al. : Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol 91:1096-1101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casulo C, Byrtek M, Dawson KL, et al. : Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: An analysis from the National LymphoCare study. J Clin Oncol 33:2516-2522, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conconi A, Motta M, Bertoni F, et al. : Patterns of survival of follicular lymphomas at a single institution through three decades. Leuk Lymphoma 51:1028-1034, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Cerhan JR, Link BK, Habermann TM, et al. : Cohort profile: The lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource (MER) cohort study. Int J Epidemiol 46:1753-1754i, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Tourah AJ, Gill KK, Chhanabhai M, et al. : Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol 26:5165-5169, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 23.Provencio M, Sabín P, Gomez-Codina J, et al. : Impact of treatment in long-term survival patients with follicular lymphoma: A Spanish Lymphoma Oncology Group registry. PLoS One 12:e0177204, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provencio M, Royuela A, Torrente M, et al. : Prognostic value of event-free survival at 12 and 24 months and long-term mortality for non-Hodgkin follicular lymphoma patients: A study report from the Spanish Lymphoma Oncology Group. Cancer 123:3709-3716, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Maartense E, Le Cessie S, Kluin-Nelemans HC, et al. : Age-related differences among patients with follicular lymphoma and the importance of prognostic scoring systems: Analysis from a population-based non-Hodgkin’s lymphoma registry. Ann Oncol 13:1275-1284, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Alig S, Jurinovic V, Pastore A, et al. : Impact of age on genetics and treatment efficacy in follicular lymphoma. Haematologica 103:e364-e367, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabhan C, Zhou X, Day BM, et al. : Disease, treatment, and outcome differences between men and women with follicular lymphoma in the United States. Am J Hematol 91:770-775, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Junlén HR, Peterson S, Kimby E, et al. : Follicular lymphoma in Sweden: Nationwide improved survival in the rituximab era, particularly in elderly women—A Swedish Lymphoma Registry study. Leukemia 29:668-676, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Morton LM, Curtis RE, Linet MS, et al. : Second malignancy risks after non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: Differences by lymphoma subtype. J Clin Oncol 28:4935-4944, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]