Abstract
Purpose
Human epidermal growth factor receptor 2 (HER2, ERBB2)–activating mutations occur in 2% of lung cancers. We assessed the activity of ado-trastuzumab emtansine, a HER2-targeted antibody-drug conjugate, in a cohort of patients with HER2-mutant lung cancers as part of a phase II basket trial.
Patients and Methods
Patients received ado-trastuzumab emtansine at 3.6 mg/kg intravenously every 3 weeks until progression. The primary end point was overall response rate using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. A Simon two-stage optimal design was used. Other end points included progression-free survival and toxicity. HER2 testing was performed on tumor tissue by next generation sequencing, fluorescence in situ hybridization, immunohistochemistry, and protein mass spectrometry.
Results
We treated 18 patients with advanced HER2-mutant lung adenocarcinomas. The median number of prior systemic therapies was two (range, zero to four prior therapies). The partial response rate was 44% (95% CI, 22% to 69%), meeting the primary end point. Responses were seen in patients with HER2 exon 20 insertions and point mutations in the kinase, transmembrane, and extracellular domains. Concurrent HER2 amplification was observed in two patients. HER2 immunohistochemistry ranged from 0 to 2+ and did not predict response, and responders had low HER2 protein expression measured by mass spectrometry. The median progression-free survival was 5 months (95% CI, 3 to 9 months). Toxicities included grade 1 or 2 infusion reactions, thrombocytopenia, and elevated hepatic transaminases. No patient stopped therapy as a result of toxicity or died on study.
Conclusion
Ado-trastuzumab emtansine is an active agent in patients with HER2-mutant lung cancers. This is the first positive trial in this molecular subset of lung cancers. Further use and study of this agent are warranted.
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2, ERBB2)–activating mutations occur in 2% of lung cancers.1-3 These mutations are transforming in lung cancer models and result in kinase activation, conferring some in vitro sensitivity to trastuzumab.4,5 An earlier generation of studies of trastuzumab in lung cancers selected patients on the basis of HER2 protein expression by immunohistochemistry (IHC). Results were disappointing.6-11 The HER2 tyrosine kinase inhibitors dacomitinib, afatinib, and neratinib have produced some responses in patients with HER2-mutant lung cancers, but the low response rates of 0% to 19% stalled further development.12-15 Case series of trastuzumab in combination with chemotherapy produced higher response rates of up to 50% in patients with HER2-mutant lung cancers.16,17 However, it is not possible to ascertain the contribution of trastuzumab because it was always combined with active chemotherapeutic agents. Despite the plethora of agents targeting HER2 in patients with breast cancers, there is no approved targeted therapy for patients with HER2-mutant lung cancers.
Ado-trastuzumab emtansine (also known as T-DM1) is a HER2-targeted antibody-drug conjugate linking trastuzumab with the antimicrotubule agent emtansine and is an approved medicine for patients with HER2-amplified or -overexpressing metastatic breast cancers.18 Response to ado-trastuzumab emtansine has been reported in a patient with HER2-mutant lung cancer.19 We hypothesized that HER2-activating mutations in lung cancers, rather than IHC, can lead to cellular signal dependence on this pathway that may result in preferential binding and internalization of ado-trastuzumab emtansine to produce antitumor response. We report the primary results from a cohort of patients with HER2-mutant lung cancers treated as part of a phase II basket trial of ado-trastuzumab emtansine in patients with HER2-mutant or -amplified cancers.
PATIENTS AND METHODS
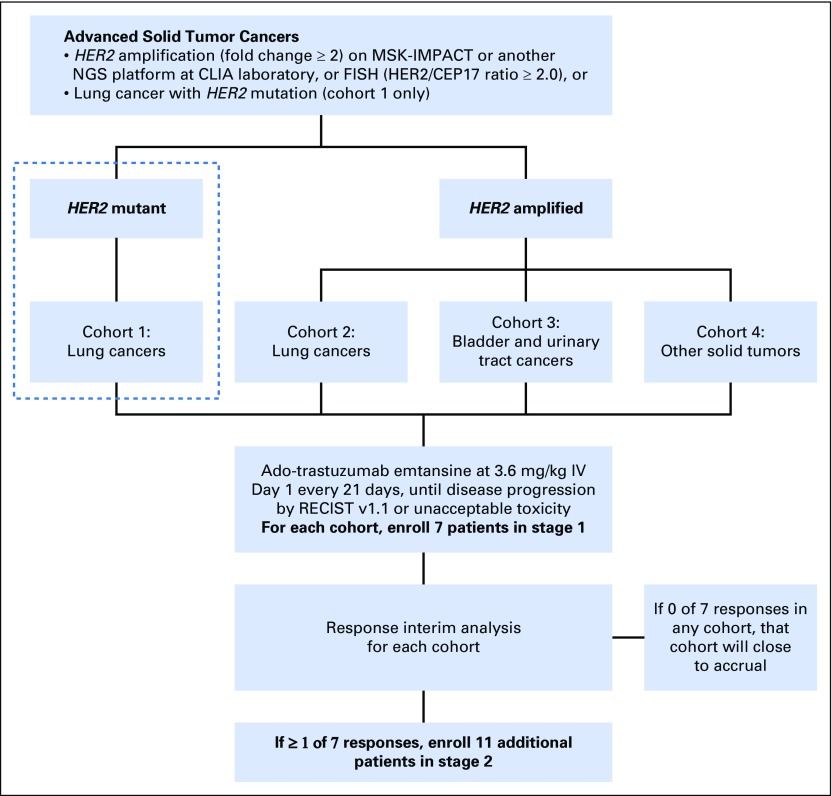
This was a single-center, investigator-sponsored, phase II basket trial of ado-trastuzumab emtansine at Memorial Sloan Kettering Cancer Center in New York (ClinicalTrials.gov identifier: NCT02675829). This study was approved by the Memorial Sloan Kettering Institutional Review Board, and informed consent was obtained from each patient in accordance with the precepts of the Helsinki Declaration. Patients with stage IV or recurrent HER2-mutant lung cancer were eligible for this cohort. Other cohorts of this basket trial not reported in this article included patients with HER2-amplified lung, bladder, and other solid tumors, as illustrated by the schema in Appendix Fig A1 (online only). HER2 mutation was identified through next-generation sequencing (NGS) at a Clinical Laboratory Improvement Amendments–certified laboratory,20,21 including exon 20 insYVMA, insGSP, or insTGT; single–base pair substitutions L755A, L755S, V777L, V659E, or S310F; or other likely activating mutations. Other inclusion criteria included age 18 years or older; Karnofsky performance status of ≥ 70%; measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; and adequate left ventricular, bone marrow, and hepatic function. Patients were eligible regardless of whether they were treatment naïve or had received prior anticancer or other HER2-targeted therapy, including trastuzumab.
All patients received ado-trastuzumab emtansine 3.6 mg/kg by intravenous infusion every 21 days until disease progression or unacceptable toxicity. Physical examination and safety assessments were performed every 3 weeks. Tumor assessments using contrast-enhanced computed tomography of the chest, abdomen, and pelvis were performed at baseline, week 6, and week 12, and then every 12 weeks thereafter until disease progression. Brain imaging was not routinely performed unless clinically indicated. Left ventricular ejection fraction measurements by echocardiography or multigated acquisition scan were performed at baseline and then every 3 months. The primary objective was the determination of overall response rate (ORR; complete plus partial response rate) of ado-trastuzumab emtansine according to RECIST version 1.1 as assessed by investigator. Secondary objectives included assessment of progression-free survival (PFS) and toxicity according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.1. An exploratory objective was to examine the molecular associations between HER2 mutations, HER2 amplification, and HER2 protein expression using different molecular diagnostic assays whenever archival tissue was available. NGS assessed HER2 mutation and amplification, fluorescence in situ hybridization (FISH) assessed HER2 amplification, and IHC and quantitative mass spectrometry assessed HER2 protein overexpression. FISH was performed using probe sets (PathVysion, Abbott, Chicago, IL; and HER2 IQFISH pharmDx, Dako, Carpinteria, CA) approved by the US Food and Drug Administration (FDA) and defined as HER2/CEP17 ratio ≥ 2.0.12,22 HER2 protein by IHC was assessed using the 4B5 Ventana antibody (Ventana, Tucson, AZ). Quantitative HER2 protein by selected reaction monitoring mass spectrometry on formalin-fixed, paraffin-embedded tissue was performed using methods previously validated in breast cancers, with ≥ 740 amol/μg as the cutoff for high HER2 expression.23
Statistical Considerations
For each cohort of the basket trial including HER2-mutant lung cancers, a Simon two-stage optimal design was used to determine whether ado-trastuzumab emtansine has sufficient activity to warrant further development in each cohort. Target accrual was a minimum of seven patients (stage 1) and a maximum of 18 patients (stage 1 and 2) in each cohort. The primary end point was best confirmed ORR per RECIST v1.1 as assessed by investigator. The Simon’s optimal two-stage design is used with a multiple testing adjusted type I error rate for each cohort. For a targeted agent where higher response rates are expected, a true ORR of ≤ 10% will be considered unacceptable (null hypothesis), whereas a true ORR of ≥ 40% will merit further study (alternative hypothesis). In the first stage, seven patients were accrued; if there were no responses observed at interim analysis of the seven patients in a particular cohort, the cohort would be closed. Otherwise, 11 additional patients would be accrued for a total of 18 patients. For the overall trial, the null hypothesis would be rejected for each cohort separately if at least five responses were observed in each cohort. This design controls type I error rate at 2.7% and generates 89% power for detecting active cohorts. The overall family-wise error rate at the study level was < 10%. The exact 95% CI for ORR was calculated using the Clopper-Pearson method. PFS time was estimated using the Kaplan-Meier method. Follow-up time was calculated from the start of treatment to the most recent patient follow-up assessment.
RESULTS
Patients
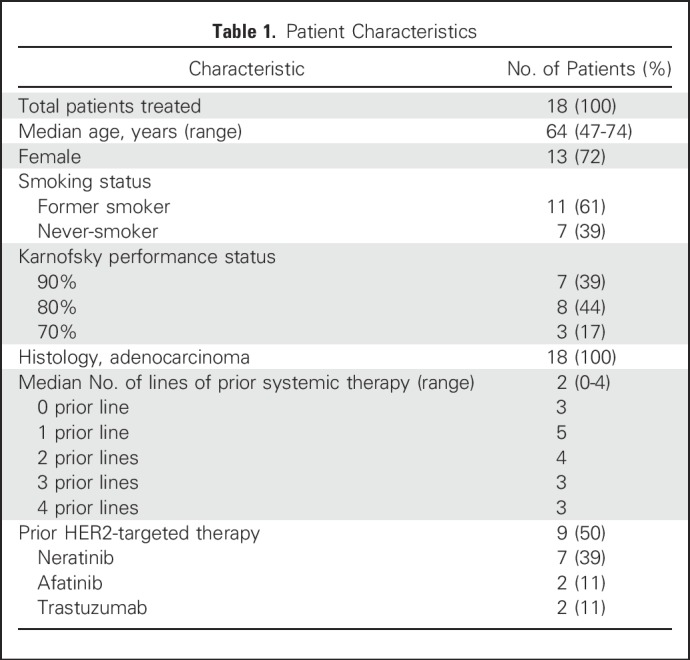
We accrued a cohort of 18 patients with metastatic HER2-mutant lung adenocarcinomas between March and December 2016. The median follow-up time was 10 months. Sixteen patients (89%) were identified through MSK-IMPACT (Memorial Sloan Kettering Cancer Center, New York, NY) NGS at Memorial Sloan Kettering Cancer Center.21 Patient characteristics are listed in Table 1. The median number of lines of prior systemic therapy was two (range, zero to four prior lines), and 50% of patients had received prior HER2-targeted therapy including neratinib, afatinib, and trastuzumab.
Table 1.
Patient Characteristics
Clinical Activity and Safety
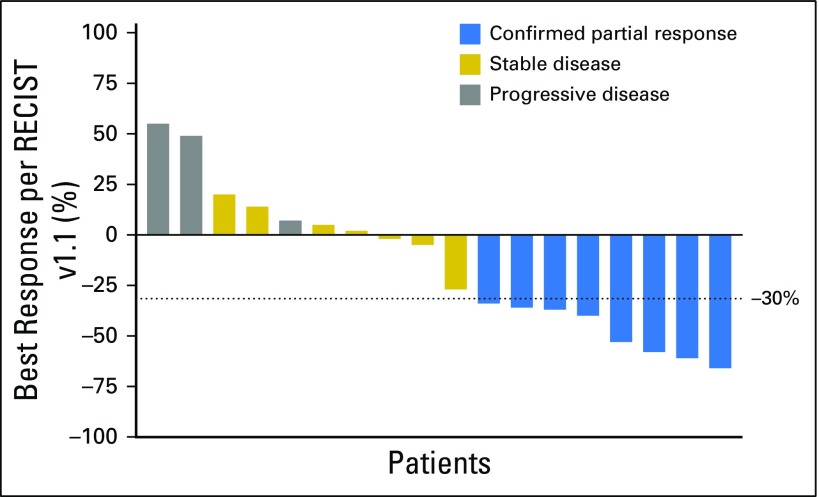
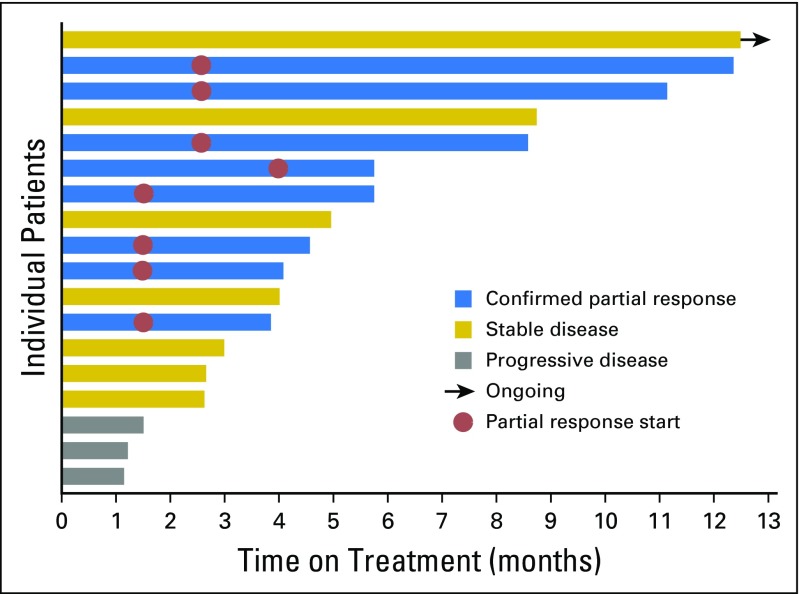
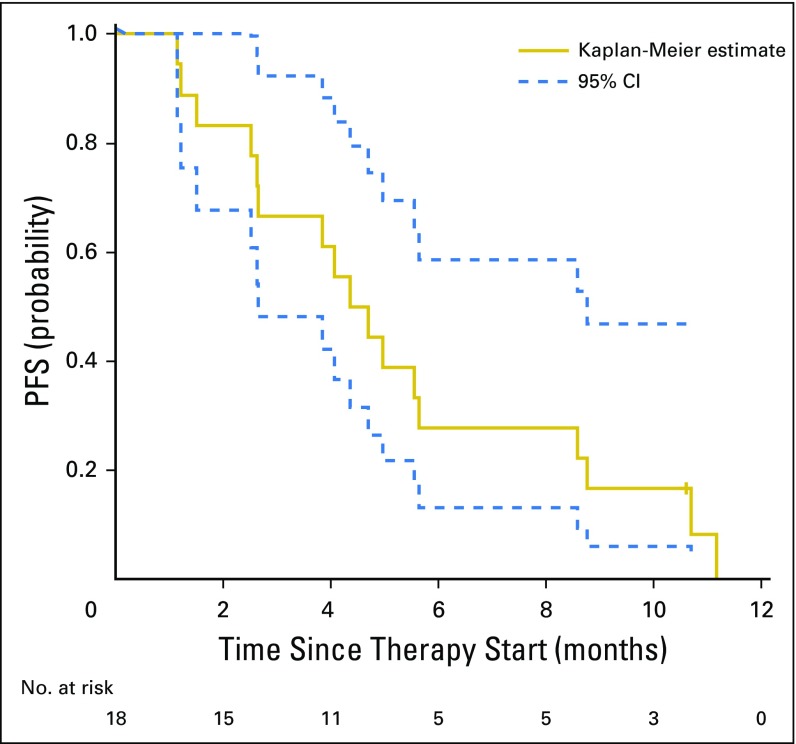
The ORR (all partial and confirmed responses) was 44% (95% CI, 22% to 69%), as summarized in Figure 1, thus rejecting the null hypothesis. Three (17%) of 18 patients had progression of disease as best response. The median PFS for all patients was 5 months (95% CI, 3 to 9 months), and median PFS for the responders was 6 months (95% CI, 4 months to not reached; Appendix Fig A2, online only). The longest PFS observed (11+ months) was in a patient with stable disease as best response with −27% tumor shrinkage (Fig 2). The median number of cycles of ado-trastuzumab emtansine administered was six (range, two to 19 cycles). The median duration of response was 4 months (range, 2 to 9 months). The median time to response from start of treatment was 2 months (range, 1 to 4 months). Of the eight patients with partial responses, two were previously untreated, and six were pretreated with two to four prior lines of systemic therapy, including four patients who received prior HER2-targeted therapy with neratinib and trastuzumab. One patient had previously responded to neratinib plus temsirolimus but did not respond to trastuzumab plus gemcitabine just before study entry. Three other patients had stable disease on prior neratinib, one of them immediately before study entry. Of the 15 patients who were pretreated with prior systemic therapy, six (40%) had responded to ado-trastuzumab emtansine. Only two patients had active untreated brain metastases at enrollment, but both patients had progression of disease systemically and in the CNS at first response assessment. Seven patients received prior anti–programmed cell death 1 immune checkpoint inhibitors, and none responded.
Fig 1.
Waterfall plot of best response. RECIST, Response Evaluation Criteria in Solid Tumors.
Fig 2.
Swimmers plot of progression-free survival.
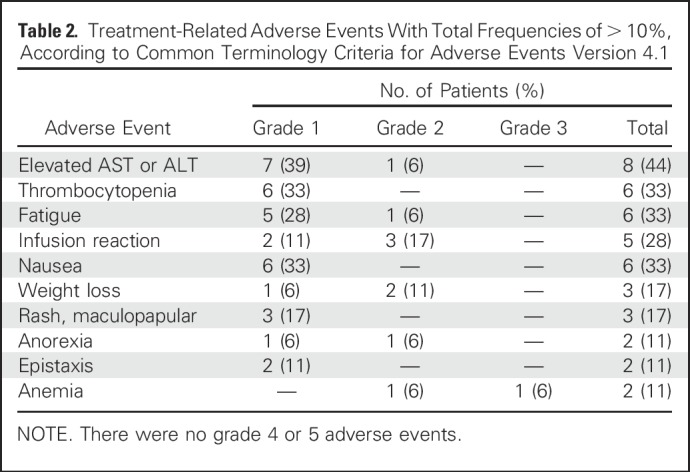
Treatment-related adverse events are listed in Table 2. They were mainly grade 1 or 2 events, including infusion reactions, thrombocytopenia, and elevations of hepatic transaminases. Infusion reactions characterized by mild rigors, chills, pruritus, and wheezing during treatment occurred in five (28%) of 18 patients. All resolved by slowing the infusion of ado-trastuzumab emtansine and administering antihistamines and did not preclude re-treatment. There were no deaths or grade 4 toxicities on study. There were no dose reductions or discontinuations as a result of treatment-related adverse effects.
Table 2.
Treatment-Related Adverse Events With Total Frequencies of > 10%, According to Common Terminology Criteria for Adverse Events Version 4.1
Biomarker Analyses
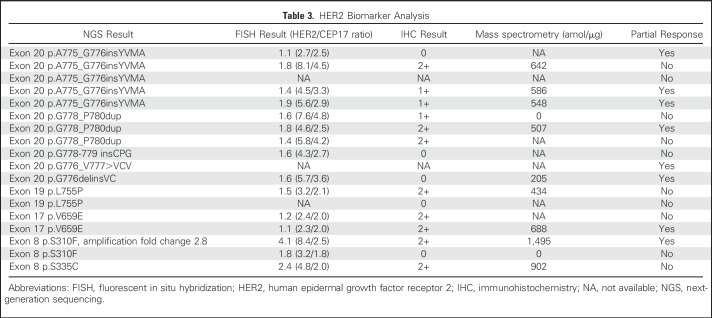
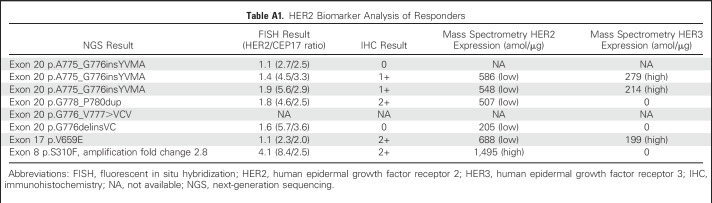
All 18 patients had HER2-activating mutations identified by NGS from their lung cancer tissue specimens. HER2 FISH was performed on archival specimens from 15 patients, and HER2 protein was assessed by IHC in 16 patients. The results are listed in Table 3. Of note, responders were seen across HER2 mutation subtypes, including exon 20 insertions and transmembrane and extracellular domain point mutations. Concurrent HER2 amplification was observed in two (11%) of 18 patients, both with extracellular domain mutations of S310F and S335C, and these two patients achieved partial response and stable disease, respectively. HER2 IHC ranged from 0 to 2+ among patients both with and without a partial response. There was no association between IHC and response to ado-trastuzumab emtansine. Quantitative mass spectrometry23 was performed on 11 patients. For the nine patients with HER2-mutant lung cancers without amplification, HER2 protein levels were low or nondetectable. The two patients with concurrent HER2 amplification showed high HER2 protein levels. Of the patients with partial responses, five of six tested by mass spectrometry showed low levels of HER2 protein, and the one responder with high HER2 protein had concurrent HER2 gene amplification. Three of six patients with partial responses showed increased HER3 expression (Appendix Table A1, online only).
Table 3.
HER2 Biomarker Analysis
DISCUSSION
In this phase II trial, ado-trastuzumab emtansine produced a 44% confirmed partial response rate and a median PFS of 5 months in a largely heavily pretreated population of patients with advanced HER2-mutant lung cancers; thus, this study met its primary end point. Furthermore, an additional 39% of patients achieved stable disease, including durable disease control for up to 11+ months. This is an important therapeutic progress in the context of more than a decade of negative clinical trials targeting HER2 in lung cancers, with clinical implications for the care of patients with HER2-mutant lung cancers today as well as shedding light upon the future of drug development in this area.
Analogous to the history of EGFR-targeted therapy, the initial development of HER2-targeted therapy in lung cancers focused on protein expression by IHC.24 This is in part driven by the experience in breast cancers that trastuzumab binding requires HER2 protein overexpression to elicit antitumor activity through inhibition of ligand-independent HER2 signaling, receptor internalization, and antibody-dependent cell-mediated cytotoxicity.25,26 HER2 IHC 3+ or HER2 amplification by FISH is much rarer in lung tumors than in breast cancers (2% v 20%, respectively). Consequently, clinical trials testing the activity of trastuzumab in lung cancers were conducted in tumors with lower levels of HER2 IHC positivity and/or not driven by HER2 signaling. The results of six phase II trials of trastuzumab in IHC HER2-positive lung cancers were uniformly negative,6-11,24 and more recent studies of ado-trastuzumab emtansine have again confirmed that HER2 IHC is not the ideal biomarker in lung cancers.27,28 Just as the discovery of EGFR mutations eventually led to a plethora of approved oncogene-targeted therapies transforming the care of patients around the world, HER2-activating mutations similarly show promise as a therapeutic target.
The low or undetectable levels of HER2 protein expression confirmed by mass spectrometry among HER2 mutants and responders to ado-trastuzumab emtansine suggest an alternative mechanism of trastuzumab binding and internalization of the antibody-drug conjugate other than through HER2 protein overexpression. An intriguing finding was the overexpression of HER3 protein in several responding patients, suggesting a potential role for dimerization with HER3 among HER2 mutants, possibly enabling preferential binding and internalization of trastuzumab through increased phosphorylation and receptor ubiquitination.29 The responses to ado-trastuzumab emtansine seen for the first time in transmembrane and extracellular domain HER2 mutations also lend support, as these mutations (V659E and S310F) are known to be activating dimers.5,30 Consequently ado-trastuzumab emtansine bound to mutant HER2 may be internalized into the cell at a higher rate compared with the wild-type receptor, regardless of the quantity of HER2 protein. This hypothesis requires further mechanistic studies for validation but may potentially open up a new therapeutic approach of targeting activating mutations with antibody-drug conjugates. Biomarker analysis also found concurrent HER2 amplification in 11% of HER2 mutants. This small overlap is consistent with other studies that, when taken together, suggest that HER2 mutation and amplification are largely separate therapeutic targets worthy of investigation in the laboratory and the clinic.22,31 The fact that both mutations and amplifications of HER2 can be readily identified by NGS speaks for its clinical utility and huge potential for therapeutic discovery.
Ado-trastuzumab emtansine was well tolerated in our patients with a comparable adverse effect profile to that seen in patients with HER2-amplified breast cancers, with the exception of a higher than expected rate of grade 1 or 2 infusion reactions compared with the experience in breast cancers as noted in the FDA label (28% v 1%, respectively).18,32 The reason for this difference in patients with lung cancers is unclear, although differences in coding definitions and a largely trastuzumab-naïve population may be contributing factors, and more data from our ongoing pan-cancer basket trial of ado-trastuzumab emtansine may provide insights. In all patients, infusion reactions were mild, never required drug discontinuation, and were easily managed by slowing infusions and administering antihistamines. Subsequent events were prevented by prophylactic antihistamine and acetaminophen administration.
Potential limitations of this trial are its small sample size and that it is a single-arm study from one institution. These potential shortcomings reflect the real-world challenges we face when studying less common yet complex molecular subsets of cancers. Traditional randomized phase II and III trial designs are impractical and potentially unnecessary. Basket trials and other novel approaches with efficient trial design strategies may represent solutions.33,34 Indeed, the FDA has recently approved the use of vemurafenib in BRAF V600E–mutant Erdheim-Chester disease on the basis of results of 22 patients from a basket trial.35,36 Multicenter collaboration is required to produce larger sample sizes34,35 and adds to the strength of the applicability of results. Because the patients treated here were heavily pretreated, including prior HER2-targeted therapies, and suffer from drug-resistant lung cancers, it is less likely that this cohort of patients with HER2-mutant lung cancers would be biased toward a more favorable outcome. The objective response rate of 44% is consistent with data from breast cancers; however, the median PFS of 5 months in individuals with HER2-mutant lung cancers is shorter than the 10-month PFS observed in patients with breast cancers.18
As NGS becomes more commonly used in the initial evaluation of lung cancers, more HER2 mutations will be uncovered and identified earlier in the clinical course. The recent FDA announcement that MSK-IMPACT and other NGS panels are considered approved platforms to detect molecular aberrations will further accelerate NGS use, the identification of HER2 alterations, and the need for therapies targeting HER2.37,38 The treatment of individuals with HER2-mutant lung cancers represents a critical medical need with poorer survival than patients with other oncogenic drivers.3,38 The current standard of care is chemotherapy, with pemetrexed providing the most benefit.39 Although the benefit from immune checkpoint inhibitors in patients with HER2 aberrations is yet to be defined, it is possible that response rates may be similarly low with HER2 as in other oncogene-driven cancers such as EGFR, ALK, and MET exon 14 mutants.40,41 Indeed, none of the seven patients in this study who received prior anti–programmed cell death 1 inhibitors achieved a response. Trials are needed to help choose best therapies, best combinations, and the sequence of targeted and non–HER2-targeted therapies. Should the results of ado-trastuzumab emtansine in this trial be replicated, it may represent a new standard treatment of these patients.
Ado-trastuzumab emtansine is an active agent in patients with HER2-mutant lung cancers. The success here, in contrast to the failure of earlier studies of HER2-targeted agents in patients with lung cancers with inadequate molecular selection, highlights the need for a comprehensive and precise genotyping both for care and trial eligibility. This first positive clinical trial in this molecular subset of lung cancers justifies further studies both of this agent and others in patients with HER2-mutant lung cancers.
Appendix
Fig A1.
Basket trial schema. CLIA, Clinical Laboratory Improvement Amendments; FISH, fluorescent in situ hybridization; IV, intravenous; NGS, next-generation sequencing; RECIST, Response Evaluation Criteria in Solid Tumors.
Fig A2.
Progression-free survival (PFS) of patients with HER2-mutant lung cancers (N = 18). The median PFS for all patients was 5 months (95% CI, 3 to 9 months), and median PFS for the responders was 6 months (95% CI, 4 months to not reached).
Table A1.
HER2 Biomarker Analysis of Responders
Footnotes
Supported by the Conquer Cancer Foundation, Genentech, and Comprehensive Cancer Center Core Grant No. P30 CA008748 at Memorial Sloan Kettering Cancer Center from the National Institutes of Health.
Presented, in part, at the 53rd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 2-6, 2017.
Clinical trial information: NCT02675829.
AUTHOR CONTRIBUTIONS
Conception and design: Bob T. Li, Ronglai Shen, Nick Pavlakis, Stephen Clarke, Gregory J. Riely, David B. Solit, José Baselga, Maurizio Scaltriti, Maria E. Arcila, Mark G. Kris
Administrative support: Bob T. Li, Zachary T. Olah, Edyta B. Brzostowski, José Baselga, Mark G. Kris
Provision of study materials or patients: Bob T. Li, Edyta B. Brzostowski, Gregory J. Riely, Alexander Drilon, Charles M. Rudin, Maurizio Scaltriti, Maria E. Arcila, Mark G. Kris
Collection and assembly of data: Bob T. Li, Darren Buonocore, Zachary T. Olah, Michelle S. Ginsberg, Gary A. Ulaner, Michael Offin, Daniel Feldman, Todd Hembrough, Fabiola Cecchi, Sarit Schwartz, Helen H. Won, Edyta B. Brzostowski, Gregory J. Riely, Alexander Drilon, Charles M. Rudin, Michael F. Berger, Maurizio Scaltriti, Maria E. Arcila, Mark G. Kris
Data analysis and interpretation: Bob T. Li, Ronglai Shen, Darren Buonocore, Zachary T. Olah, Ai Ni, Gary A. Ulaner, Michael Offin, Daniel Feldman, Todd Hembrough, Fabiola Cecchi, Sarit Schwartz, Nick Pavlakis, Stephen Clarke, Helen H. Won, Edyta B. Brzostowski, Gregory J. Riely, David B. Solit, David M. Hyman, Alexander Drilon, Charles M. Rudin, Michael F. Berger, José Baselga, Maurizio Scaltriti, Maria E. Arcila, Mark G. Kris
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Bob T. Li
Consulting or Advisory Role: Roche, Biosceptre International, Thermo Fisher Scientific, Mersana, Guardant Health
Research Funding: Genentech (Inst), Illumina (Inst), BioMed Valley Discoveries (Inst), AstraZeneca (Inst), GRAIL (Inst)
Ronglai Shen
No relationship to disclose
Darren Buonocore
Stock or Other Ownership: Merck, Novartis
Zachary T. Olah
No relationship to disclose
Ai Ni
No relationship to disclose
Michelle S. Ginsberg
No relationship to disclose
Gary A. Ulaner
Research Funding: Genentech, GE Healthcare, Novartis, Sanofi
Michael Offin
No relationship to disclose
Daniel Feldman
No relationship to disclose
Todd Hembrough
Employment: NantOmics
Leadership: NantOmics
Stock or Other Ownership: NantOmics, NantHealth
Patents, Royalties, Other Intellectual Property: Patents issued and pending as an employee of NantOmics
Fabiola Cecchi
Employment: NantOmics
Patents, Royalties, Other Intellectual Property: NantOmics
Travel, Accommodations, Expenses: NantOmics
Sarit Schwartz
Employment: NantOmics
Research Funding: NantOmics (Inst)
Patents, Royalties, Other Intellectual Property: NantOmics
Travel, Accommodations, Expenses: NantOmics
Nick Pavlakis
Consulting or Advisory Role: Pfizer, Novartis, Amgen, Boehringer Ingelheim, Roche, Takeda, AstraZeneca, Ipsen, Merck-KgA, Merck Sharp & Dohme, Bristol-Myers Squibb
Stephen Clarke
Consulting or Advisory Role: Merck, Ipsen, Bayer, AstraZeneca/MedImmune
Speakers' Bureau: Merck, Novartis/Ipsen
Helen H. Won
No relationship to disclose
Edyta B. Brzostowski
No relationship to disclose
Gregory J. Riely
Research Funding: Novartis (Inst), Genentech (Inst), Millennium (Inst), Pfizer (Inst), Infinity Pharmaceuticals (Inst), ARIAD Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Patent application submitted covering pulsatile use of erlotinib to treat or prevent brain metastases (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme
David B. Solit
Honoraria: Loxo, Pfizer
Consulting or Advisory Role: Pfizer, Loxo
David M. Hyman
Consulting or Advisory Role: Atara Biotherapeutics, Chugai Pharma, CytomX Therapeutics, Boehringer Ingelheim, AstraZeneca, Pfizer, Debiopharm Group, Genentech
Research Funding: AstraZeneca, Puma Biotechnology, Loxo
Alexander Drilon
Consulting or Advisory Role: ARIAD Pharmaceuticals
Charles M. Rudin
Consulting or Advisory Role: Bristol-Myers Squibb, Abbvie, Seattle Genetics, Harpoon Therapeutics, Genentech, AstraZeneca
Michael F. Berger
Research Funding: Illumina
José Baselga
Leadership: Infinity Pharmaceuticals, Varian Medical Systems, Bristol-Myers Squibb, GRAIL
Stock or Other Ownership: PMV Pharma, Juno Therapeutics, Infinity Pharmaceuticals, GRAIL, Varian Medical Systems, Bristol-Myers Squibb, Tango Therapeutics, Foghorn Therapeutics, Aura Biomedical, Apogen, Northern Biologics
Honoraria: PMV Pharma, Juno Therapeutics, Infinity Pharmaceuticals, GRAIL, Northern Biologics
Consulting or Advisory Role: Eli Lilly, Novartis
Research Funding: Roche/Genentech
Patents, Royalties, Other Intellectual Property: Combination therapy using PDK1 and PI3K inhibitors. Pending. Memorial Sloan Kettering (MSK) owned, listed as investigator. Jul 16 Use of phosphoinositide 3-kinase inhibitors for treatment of vascular malformations. Licensed. MSK owned, listed as investigator. Inhibition of KMT2D for the treatment of breast cancer. Pending. MSK owned, listed as investigator.
Travel, Accommodations, Expenses: Roche/Genentech, Daiichi, Bristol-Myers Squibb
Maurizio Scaltriti
Research Funding: Daiichi Sankyo (Inst), Puma Biotechnology
Maria E. Arcila
No relationship to disclose
Mark G. Kris
Consulting or Advisory Role: AstraZeneca
REFERENCES
- 1.Stephens P, Hunter C, Bignell G, et al. : Lung cancer: Intragenic ERBB2 kinase mutations in tumours. Nature 431:525-526, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network : Comprehensive molecular profiling of lung adenocarcinoma. Nature 511:543-550, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kris MG, Johnson BE, Berry LD, et al. : Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 311:1998-2006, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang SE, Narasanna A, Perez-Torres M, et al. : HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 10:25-38, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Greulich H, Kaplan B, Mertins P, et al. : Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci USA 109:14476-14481, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatzemeier U, Groth G, Butts C, et al. : Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol 15:19-27, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Lara PN, Jr, Laptalo L, Longmate J, et al. : Trastuzumab plus docetaxel in HER2/neu-positive non-small-cell lung cancer: A California Cancer Consortium screening and phase II trial. Clin Lung Cancer 5:231-236, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Krug LM, Miller VA, Patel J, et al. : Randomized phase II study of weekly docetaxel plus trastuzumab versus weekly paclitaxel plus trastuzumab in patients with previously untreated advanced nonsmall cell lung carcinoma. Cancer 104:2149-2155, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Zinner RG, Glisson BS, Fossella FV, et al. : Trastuzumab in combination with cisplatin and gemcitabine in patients with Her2-overexpressing, untreated, advanced non-small cell lung cancer: Report of a phase II trial and findings regarding optimal identification of patients with Her2-overexpressing disease. Lung Cancer 44:99-110, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Langer CJ, Stephenson P, Thor A, et al. : Trastuzumab in the treatment of advanced non-small-cell lung cancer: Is there a role? Focus on Eastern Cooperative Oncology Group study 2598. J Clin Oncol 22:1180-1187, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Clamon G, Herndon J, Kern J, et al. : Lack of trastuzumab activity in nonsmall cell lung carcinoma with overexpression of erb-B2: 39810: A phase II trial of Cancer and Leukemia Group B. Cancer 103:1670-1675, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Kris MG, Camidge DR, Giaccone G, et al. : Targeting HER2 aberrations as actionable drivers in lung cancers: Phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol 26:1421-1427, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li BT, Lee A, O’Toole S, et al. : HER2 insertion YVMA mutant lung cancer: Long natural history and response to afatinib. Lung Cancer 90:617-619, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besse B, Soria J-C, Yao B, et al. : Neratinib (N) with or without temsirolimus (TEM) in patients (PTS) with non-small cell lung cancer (NSCLC) carrying HER2 somatic mutations: An international randomized phase II study. Ann Oncol 25:LBA39_PR, 2014. (abstr) [Google Scholar]
- 15.Gandhi L, Besse B, Mazieres J, et al. : Neratinib temsirolimus in HER2-mutant lung cancers: An international, randomized phase II study. J Thorac Oncol 12:S358-S359, 2016 [Google Scholar]
- 16.Mazières J, Peters S, Lepage B, et al. : Lung cancer that harbors an HER2 mutation: Epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 31:1997-2003, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Mazières J, Barlesi F, Filleron T, et al. : Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: Results from the European EUHER2 cohort. Ann Oncol 27:281-286, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Verma S, Miles D, Gianni L, et al. : Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783-1791, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiler D, Diebold J, Strobel K, et al. : Rapid response to trastuzumab emtansine in a patient with HER2-driven lung cancer. J Thorac Oncol 10:e16-e17, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Cheng DT, Mitchell TN, Zehir A, et al. : Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17:251-264, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li BT, Ross DS, Aisner DL, et al. : HER2 amplification and HER2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol 11:414-419, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuciforo P, Thyparambil S, Aura C, et al. : High HER2 protein levels correlate with increased survival in breast cancer patients treated with anti-HER2 therapy. Mol Oncol 10:138-147, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricciardi GR, Russo A, Franchina T, et al. : NSCLC and HER2: Between lights and shadows. J Thorac Oncol 9:1750-1762, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Slamon DJ, Leyland-Jones B, Shak S, et al. : Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783-792, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Hudis CA: Trastuzumab: Mechanism of action and use in clinical practice. N Engl J Med 357:39-51, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Stinchcombe T, Stahel RA, Bubendorf L, et al. : Efficacy, safety, and biomarker results of trastuzumab emtansine (T-DM1) in patients (pts) with previously treated HER2-overexpressing locally advanced or metastatic non-small cell lung cancer (mNSCLC). J Clin Oncol 35, 2017. (suppl; abstr 8509) [DOI] [PubMed] [Google Scholar]
- 28.Hotta K, Aoe K, Kozuki T, et al. : A phase II study of trastuzumab emtansine in HER2-positive non-small-cell lung cancer. J Thorac Oncol 13:273-279, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Scaltriti M, Verma C, Guzman M, et al. : Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 28:803-814, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Serra V, Vivancos A, Puente XS, et al. : Clinical response to a lapatinib-based therapy for a Li-Fraumeni syndrome patient with a novel HER2V659E mutation. Cancer Discov 3:1238-1244, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Hyman DM, Piha-Paul SA, Won H, et al. : HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554:189-194, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krop IE, Beeram M, Modi S, et al. : Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 28:2698-2704, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Cunanan KM, Iasonos A, Shen R, et al. : An efficient basket trial design. Stat Med 36:1568-1579, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunanan KM, Gonen M, Shen R, et al. : Basket trials in oncology: A trade-off between complexity and efficiency. J Clin Oncol 35:271-273, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hyman DM, Puzanov I, Subbiah V, et al. : Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 373:726-736, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond EL, Subbiah V, Lockhart AC, et al. : Vemurafenib for BRAF V600-mutant Erdheim-Chester disease and Langerhans cell histiocytosis: Analysis of data from the histology-independent, phase 2, open-label VE-BASKET study. JAMA Oncol 4:384-388, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.US Food and Drug Administration : FDA unveils a streamlined path for the authorization of tumor profiling tests alongside its latest product action. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm585347.htm
- 38.Pillai RN, Behera M, Berry LD, et al. : HER2 mutations in lung adenocarcinomas: A report from the Lung Cancer Mutation Consortium. Cancer 123:4099-4105, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eng J, Hsu M, Chaft JE, et al. : Outcomes of chemotherapies and HER2 directed therapies in advanced HER2-mutant lung cancers. Lung Cancer 99:53-56, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gainor JF, Shaw AT, Sequist LV, et al. : EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: A retrospective analysis. Clin Cancer Res 22:4585-4593, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabari JK, Montecalvo J, Chen R, et al. : PD-L1 expression and response to immunotherapy in patients with MET exon 14-altered non-small cell lung cancers (NSCLC). J Clin Oncol 35, 2017. (suppl; abstr 8512) [DOI] [PMC free article] [PubMed] [Google Scholar]