Abstract
Purpose
Improvements in magnetic resonance imaging (MRI), total mesorectal excision (TME) surgery, and the use of (chemo)radiotherapy ([C]RT) have improved local control of rectal cancer; however, we have been unable to eradicate local recurrence (LR). Even in the face of TME and negative resection margins (R0), a significant proportion of patients with enlarged lateral lymph nodes (LLNs) suffer from lateral LR (LLR). Japanese studies suggest that the addition of an LLN dissection (LLND) could reduce LLR. This multicenter pooled analysis aims to ascertain whether LLNs actually pose a problem and whether LLND results in fewer LLRs.
Patients and Methods
Data from 1,216 consecutive patients with cT3/T4 rectal cancers up to 8 cm from the anal verge who underwent surgery in a 5-year period were collected. LLND was performed in 142 patients (12%). MRIs were re-evaluated with a standardized protocol to assess LLN features.
Results
On pretreatment MRI, 703 patients (58%) had visible LLN, and 192 (16%) had a short axis of at least 7 mm. One hundred eight patients developed LR (5-year LR rate, 10.0%), of which 59 (54%) were LLRs (5-year LLR rate, 5.5%). After multivariable analyses, LLNs with a short axis of at least 7 mm resulted in a significantly higher risk of LLR (hazard ratio, 2.060; P = .045) compared with LLNs of less than 7 mm. In patients with LLNs at least 7 mm, (C)RT plus TME plus LLND resulted in a 5-year LLR of 5.7%, which was significantly lower than that in patients who underwent (C)RT plus TME (5-year LLR, 19.5%; P = .042).
Conclusion
LLR is still a significant problem after (C)RT plus TME in LLNs with a short axis at least 7 mm on pretreatment MRI. The addition of LLND results in a significantly lower LLR rate.
INTRODUCTION
Diagnostic and treatment strategies for rectal cancer have dramatically changed in the last decades, with the universal acceptance of the total mesorectal excision (TME) technique1 and improved imaging with magnetic resonance imaging (MRI),2-4 which allows better selection of high-risk patient categories; however, developments in treatment have gone in different directions in the East and the West. In Western countries, several trials have been conducted that have demonstrated increased local control with preoperative (chemo)radiotherapy ([C]RT),5-8 resulting in the adoption of (C)RT followed by TME as the standard treatment of clinical stage II and III rectal cancer.9,10 In contrast, in the East (predominantly in Japan) a surgical approach without (C)RT that combines TME with prophylactic lateral lymph node dissection (LLND) has been the standard treatment in low cT3/4 rectal cancers,11,12 as it has been demonstrated in anatomic studies that advanced tumors below the peritoneal reflection are at a higher risk of spreading to lateral nodes.13-15 Standard strategies in both Eastern and Western countries have resulted in similar local recurrence (LR) rates,16 which has provided a rationale for Western surgeons to rely on (C)RT to sterilize the lateral compartment, alleviating fears of operative morbidity and long-term sexual and urinary dysfunction associated with LLND.17 This may be particularly relevant in the more obese Western patients in whom LLND is technically more difficult.
Recent evidence suggests, however, that in select cases, (C)RT is not sufficient to prevent lateral LR (LLR). Three Korean studies, in which no LLNDs were performed, demonstrated increased LLR rates in patients who underwent (C)RT with TME when enlarged lateral lymph nodes (LLNs) were identified on primary MRI, showing an almost linear relationship between nodal size and LLR.18-20 Moreover, a recent Oxford series showed similar 30% to 40% LLR rates in nodes greater than 10 mm in short axis on pretreatment imaging and concluded that size might be a better measure in LLNs than assessment of malignant features.21 Both the Korean and the Oxford studies demonstrated that more than 50% of all LRs were only in the lateral compartment and that even in the recurrent cases, approximately one half of the patients had no distant metastases at the time of recurrence diagnosis, which suggests that it was still localized disease.
Still, there is a debate over whether an LLND would prevent these LLRs from occurring or whether lateral nodal spread is more a sign of systemic disease.22,23 Several Japanese centers have begun offering indicated LLNDs in patients with enlarged (generally 7 mm or larger) LLNs after neoadjuvant (C)RT. This approach has shown excellent disease-free survival rates,24,25 which strongly suggests that lateral nodal disease is rather a local, than a distant issue. Eastern and Western management of LLNs is already showing signs of converging. Eastern surgeons are adopting neoadjuvant (C)RT with indicated LLNDs to prevent overtreatment,24,25 and Western surgeons are gradually recognizing that LLR is a significant issue in certain patients.21 This raises the challenge that if we wish to optimize treatment in this group of patients with rectal cancer—with acceptable morbidity—how do we select patients for LLND? Single-center studies do not provide sufficient patient numbers to be able to perform reliable statistics to formulate specific guidelines regarding lateral nodal disease, because it is relatively uncommon and only a proportion of these patients develop recurrence.
The current study is a multicenter pooled analysis of patients with low, locally advanced rectal cancer from referral centers in both Eastern and Western countries. The purpose of this study is to ascertain whether LLNs actually pose a problem after (C)RT plus TME and to study whether the addition of an indicated LLND results in fewer LLRs.
PATIENTS AND METHODS
Study Participants and Patient Selection
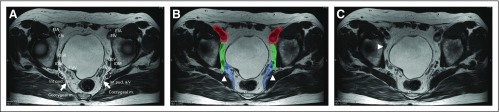
This study included patients from 12 hospitals in seven countries (Table 1). All participating hospitals were asked to collect the data and re-review MRIs of all consecutive patients who underwent operation for cT3/4 rectal cancer between January 2009 and December 2013 (Appendix Tables A1 and A2, online only). Only patients with low rectal cancers (within 8 cm of the anal verge on MRI) were included. Exclusion criteria were the absence of good-quality primary MRIs, the presence of distant metastases at initial staging, or a noncurative resection (R2 resection). Each center used a specific guideline with a color map atlas of the pelvis (Appendix Fig A1, online only) for re-evaluation of pretreatment and, if available, post-treatment MRIs by a local expert radiologist. Each center received institutional review board approval, according to local policies.
TABLE 1.
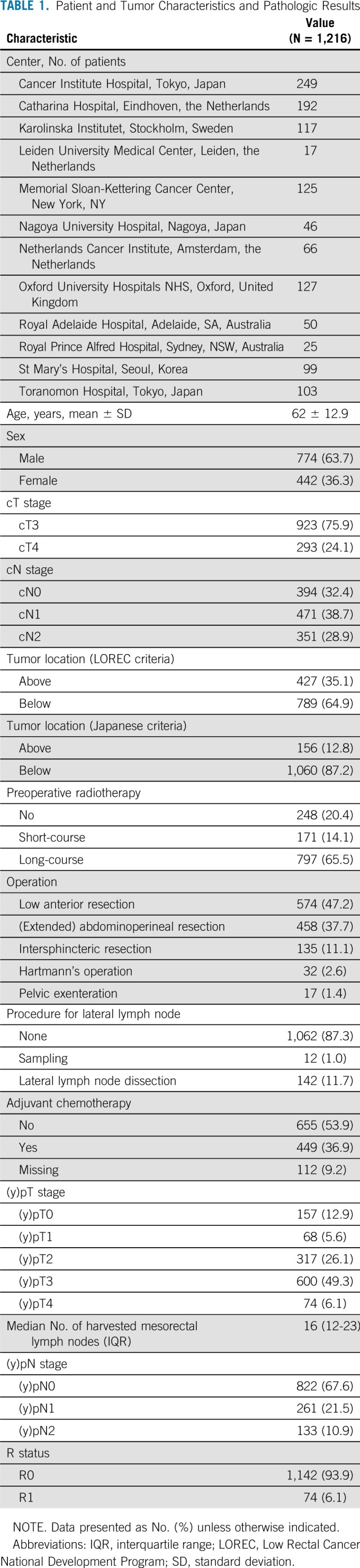
Patient and Tumor Characteristics and Pathologic Results
Re-Evaluation of MRIs
In addition to recording the standard TNM staging and circumferential resection margin assessment, radiologists were asked to specifically rereview two MRI features—the height of the tumor and LLN status. Tumor height was assessed according to both the English Low Rectal Cancer National Development Program (LOREC) and the Japanese definition of low rectal cancer. LOREC criteria26 defines low rectal cancer as a tumor of which the distal part is located below the origin of the levator muscles. In Japanese terminology, low rectal cancers are defined as those where the majority of the tumor is situated at or below the peritoneal reflection.12
Assessment of LLN status was based on the largest LLN identified on pretreatment MRI. Both long- and short-axis (LA and SA, respectively) size and site—internal iliac, external iliac, or obturator compartment—were recorded. The benign long-stretched nodes, located just behind the distal portion of the external iliac vein, were not included in the assessment (Appendix Fig A1C). Furthermore, the presence of malignant features—for example, internal heterogeneity or border irregularity—was also noted. Potential changes in the size and presence of malignant features of this same LLN after (C)RT were also evaluated for cases in which a restaging MRI was available. Shrinkage was defined as any reduction in SA size, whereas disappearance was defined as no visible node left in the compartment.
Preoperative Treatment
Treatment strategies for individual patients were determined during multidisciplinary team meetings in each hospital. All patients who received any type of neoadjuvant radiotherapy, with or without chemotherapy, were defined as having received. Each center provided information on the general radiation fields for cT3/4 rectal tumors situated within 8 cm of the anal verge. It was confirmed for each center that, in general, both the obturator and internal iliac compartments were located in the standard irradiated field for these types of tumors.
Surgical Resection
In most patients, LLNs were not resected. In a few cases, only sampling of a suspected LLN was performed. For the purposes of this study, a formal LLND was defined as the complete resection of lymphatic tissue from the lateral compartment, both from the internal iliac and the obturator area.11 Prophylactic LLND, which was the standard treatment of clinical stage II and III low rectal cancer in Japan, irrespective of LLN size, was performed in one Japanese hospital (Nagoya). The other two Japanese hospitals performed LLND selectively (indicated LLND) after neoadjuvant treatment in patients with LLNs equal or greater than 7 mm in the LA on primary imaging. Memorial Sloan Kettering Cancer Center and the Karolinska Institutet performed LLNDs for patients with suggestive findings on MRI after (C)RT, generally applying a size of equal or greater than 5 mm in combination with malignant features as an indication for LLND.
LR
Follow-up was performed according to local follow-up schemes. In patients with an LR, imaging was rereviewed and the site was categorized as one of the types—lateral, presacral, anastomotic site, anterior, or perineal—for which definitions have been previously described.16,27
Statistical Analyses
Statistical analyses were performed using Statistical Package for the Social Sciences for Windows, version 23 (SPSS, Chicago, IL). For median values, interquartile ranges (IQRs) were given. Individual variables were compared using t tests and χ2 tests. A P value < .05 was considered significant. Survival curves for LR, LLR, and distant recurrence (DR) rates, as well as cancer-specific survival (CSS), were calculated using the Kaplan-Meier method. To determine the risk factors, effects of covariates were analyzed using a univariable Cox proportional hazards regression model. Subsequently, a multivariable analysis using covariates with a significant effect (P < .10) was performed in which a P value of < .05 was considered significant. In this study, cN stage and (y)p-N stage always refer to mesorectal node stage.
RESULTS
Patients
Table 1 lists the patient and tumor characteristics and pathologic results of the total cohort; 1,216 patients with cT3/4 rectal cancer within 8 cm of the anal verge were included. Median follow-up duration after surgery was 56.5 months (IQR, 55.0 to 58.1 months).
Primary and Restaging MRI
At least one visible LLN was detected in 703 patients (57.8%) on initial MRI, 448 (63.7%) of which were located in the obturator compartment and 198 (28.2%) in the internal iliac compartment. The median size of the largest LLN was 7.0 mm in LA (IQR, 5.0 to 9.9 mm) and 5.0 mm in SA (IQR, 4.0 to 7.0 mm) before (C)RT. Malignant features were evident in 208 patients (17.1%) of the total cohort.
Of 968 patients who received (C)RT, 741 patients (76.5%) had a restaging MRI. LLN size significantly declined both in LA (P < .001) and in SA (P < .001), reducing median size to 5.0 mm (IQR, 3.0 to 7.1 mm) and 3.8 mm (IQR, 2.0 to 5.0 mm), respectively.
Surgery and Pathology
R0 resection was achieved in 1,142 patients (93.9%; Table 1). In 12 patients in whom LLN sampling was performed, nine (75.0%) were shown to have pathologic involvement. Mean harvest was two nodes (range, one to seven nodes). LLND was performed in 142 patients (11.7%) in five hospitals, which resulted in 35 patients (24.6%) with pathologically positive LLN. Mean harvest from the lateral compartment was 16 nodes (range, 0 to 62 nodes). In 87 patients who had restaging MRI after (C)RT and who underwent LLND, there was a similar rate of positive LLNs in the those with shrinkage of the nodes (P = .897); however, there was a trend toward lower LLN-positive rates if the nodes had disappeared completely (12.5% v 34.2% if still present; P = .211).
LR
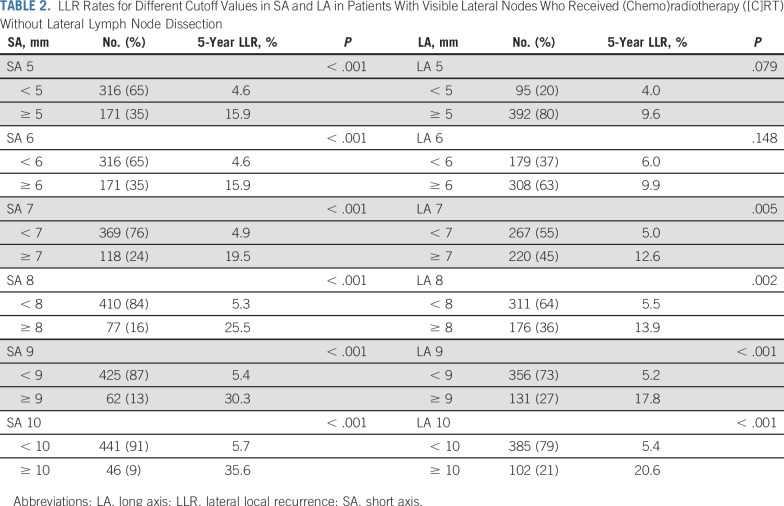
A total of 108 patients developed LR; 59 patients (54%) developed LR in the lateral compartment, 24 (22%) in the presacral site, 17 (16%) in the anastomotic site, four (4%) in the anterior, and four (4%) in the perineal site. Five-year general LR rate was 10.0% and the 5-year LLR rate was 5.5%. Table 2 lists LLR rates for different cutoff values in SA and LA in patients with visible LLNs who received (C)RT without LLND. An SA cutoff value of 7 mm was chosen as a future reference value, as the LLR rate of approximately 20% was considered too high.
TABLE 2.
LLR Rates for Different Cutoff Values in SA and LA in Patients With Visible Lateral Nodes Who Received (Chemo)radiotherapy ([C]RT) Without Lateral Lymph Node Dissection
SA Cutoff of 7 mm
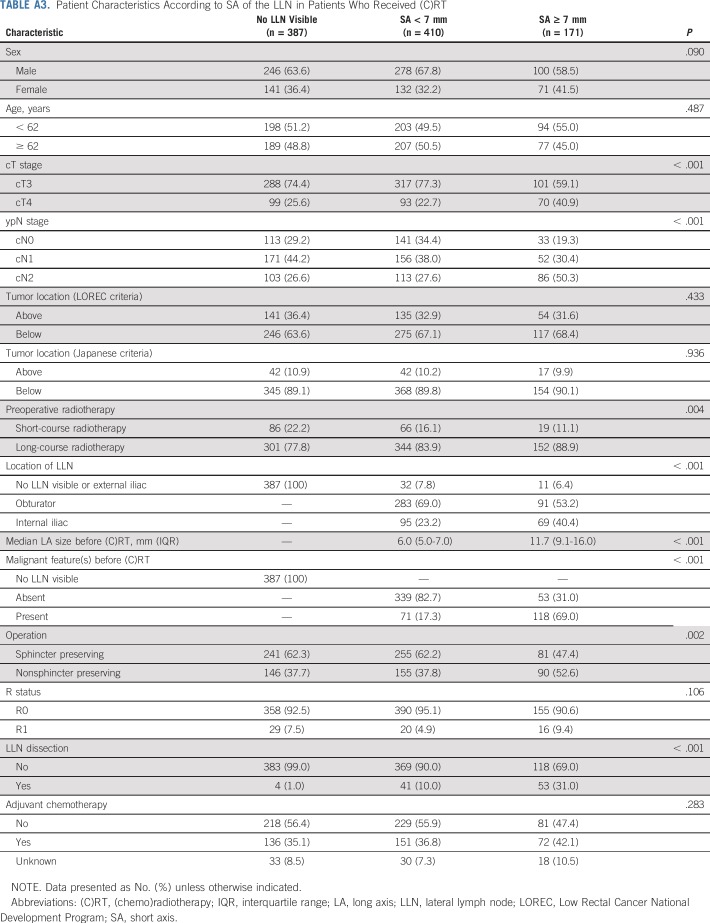
Of the 1,216 total patients, 192 (16%) had LLNs equal to or greater than 7 mm in SA on primary MRI. Appendix Table A3 (online only) lists the characteristics according to SA of the LLN in the patients who received (C)RT. Patients with LLNs ≥ 7 mm had more advanced cT stage and cN stage (P < .001) than did patients with smaller or absent nodes. Nodes in these patients also had a significantly higher rate of malignant features (P < .001) and were more often located in the internal iliac compartment (P < .001). R status was not significantly different between the three groups (P = .106).
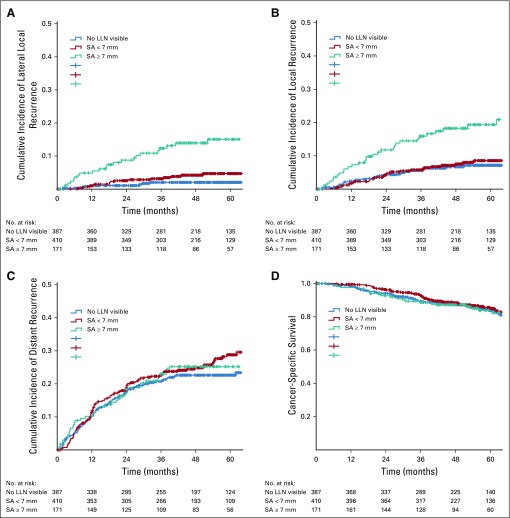
Patients with LLNs equal to or greater than 7 mm in SA had a significantly higher 5-year rates of LLR (15.0%) and LR (19.2%) than did patients with smaller LLNs, as shown in Figure 1 (P < .001). There were no significant differences in terms of DR and CSS (P = .540 and .756, respectively). There was no difference in 5-year rates for LLR and LR among patients with LLNs equal to or greater than 7 mm in SA between the short- versus long-course radiotherapy (P = .667 and .909, respectively).
FIG 1.
(A) Lateral local recurrence rate, (B) local recurrence, (C) distant recurrence, and (D) cancer-specific survival according to lateral lymph node (LLN) short axis (SA) size in patients who received (chemo)radiotherapy.
In patients without LLND who underwent restaging MRIs after (C)RT, there was no influence from the shrinkage or disappearance of LLNs with SA less than 7 mm on LLR (P = .482 and .305, respectively). In 96 patients without LLND with LLNs equal to or greater than 7 mm in SA and restaging MRIs, in five patients (5%) the nodes disappeared and in 74 (77%) there was shrinkage, whereas in 17 (18%) there was unchanged size or growth. LLR rates in the latter two categories were 17.3% and 25.4%, respectively (P = .440). In the five patients in whom nodes disappeared, there was no LLR but all cases were censored at 54 months.
Univariable and Multivariable Analyses
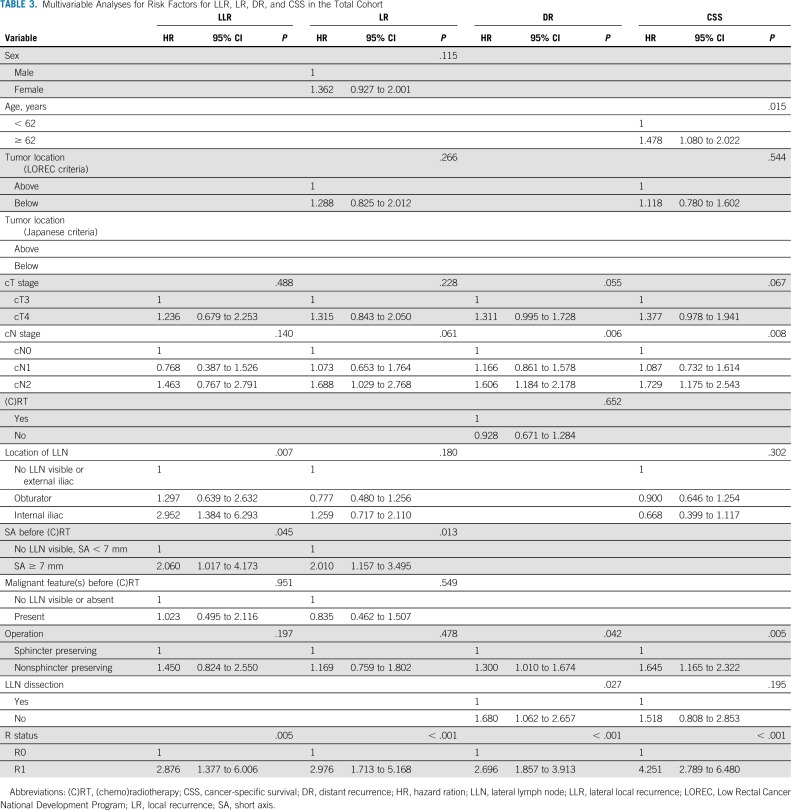
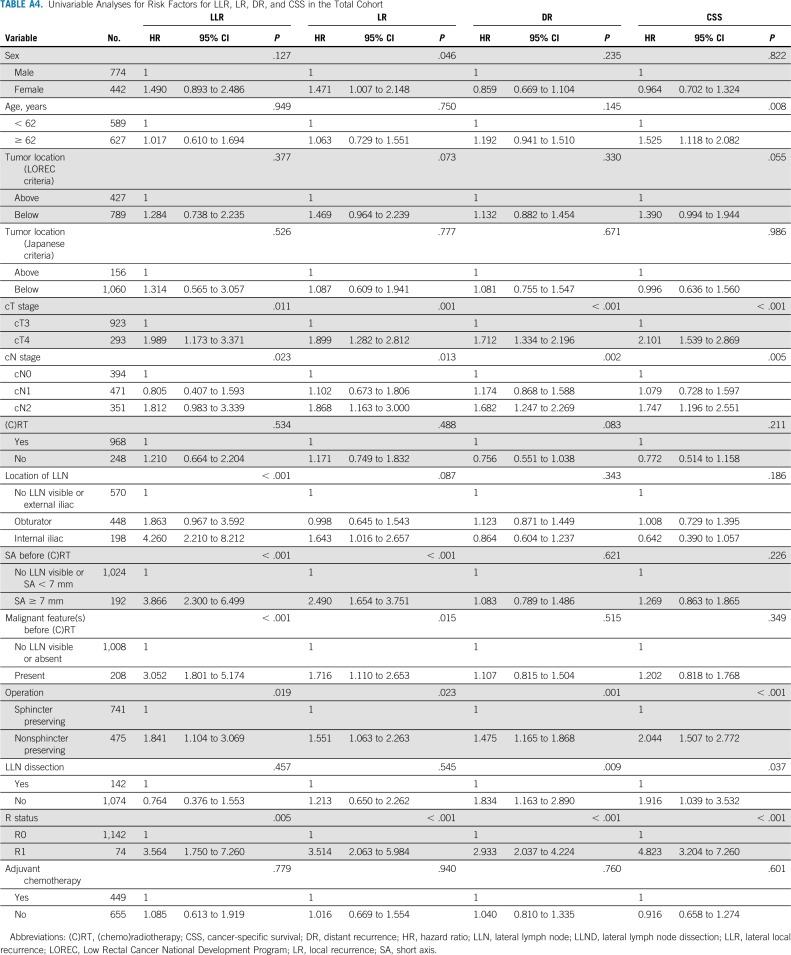
Appendix Table A4 (online only) and Table 3 show the uni- and multivariable analyses. Patients with LLNs equal to or greater than 7 mm in SA had a significantly higher risk of LR (hazard ratio [HR], 2.010; 95% CI, 1.157 to 3.495; P = .013) and LLR (HR, 2.060; 95% CI, 1.017 to 4.173; P = .045) compared with those with LLNs less than 7 mm in SA. In addition, LLNs in the internal iliac region were significantly associated with a higher risk of LLR (HR, 2.952; 95% CI, 1.384 to 6.293; P = .007). None of the LLN features were significantly associated with DR or CSS.
TABLE 3.
Multivariable Analyses for Risk Factors for LLR, LR, DR, and CSS in the Total Cohort
Effect of LLND
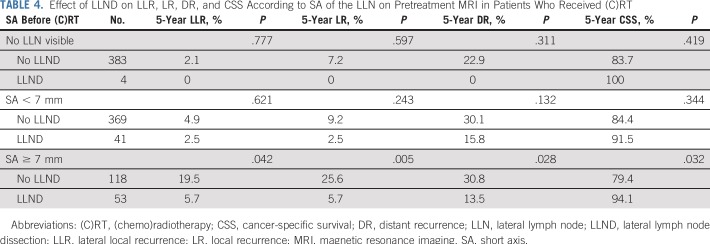
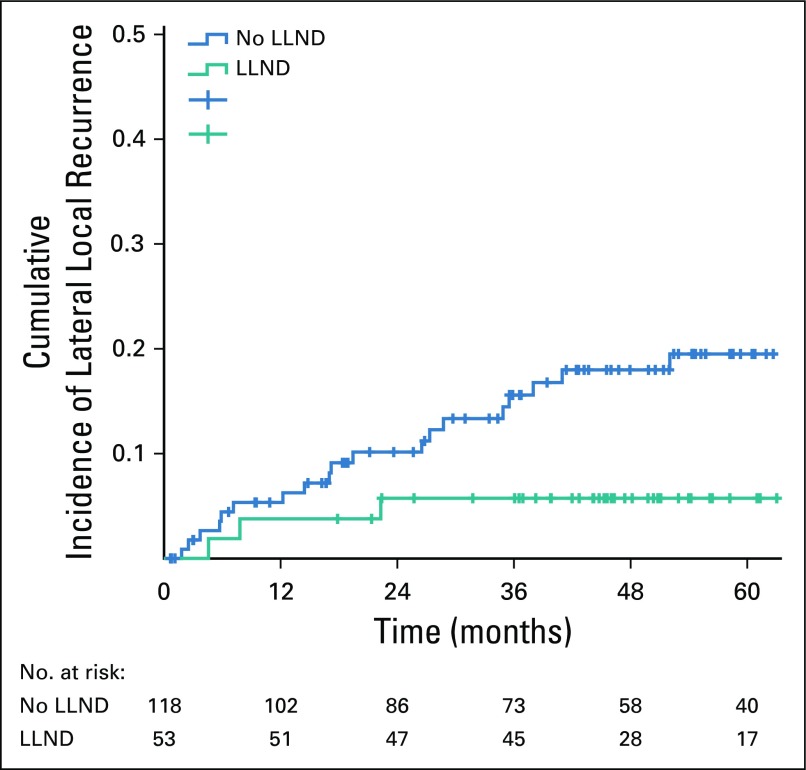
In 12 patients with LLN sampling, the 5-year LR rate was 51.1%. All LRs were located in the lateral compartment. Table 4 shows the effects of a formal LLND on LLR, LR, DR and CSS according to SA sizes of the LLN in patients who received (C)RT. After LLND, 5-year LLR and LR rates were significantly lower in patients with LLNs equal to or greater than 7 mm in SA (P = .042 and .0005, respectively) compared with patients who did not undergo LLND (Fig 2). In 27 (51%) of 53 patients who underwent LLND, pathologically positive LLNs were found.
TABLE 4.
Effect of LLND on LLR, LR, DR, and CSS According to SA of the LLN on Pretreatment MRI in Patients Who Received (C)RT
FIG 2.
Effect of lateral lymph node dissection (LLND) on lateral local recurrence in patients with a short axis ≥ 7 mm on pretreatment magnetic resonance imaging in patients who received (chemo)radiotherapy.
DISCUSSION
To our knowledge, this is the largest multi-institutional retrospective study of a cohort of patients with low cT3/4 rectal cancer who underwent operation in a 5-year period, with rereview of all MRIs on LLN features using a standardized protocol. In 1,216 total patients, results demonstrate that enlarged nodes result in high LR rates, despite (C)RT, with a 19.5% 5-year LLR rate in nodes with SA of equal to or greater than 7 mm after (C)RT plus TME. LLR is reduced to only 5.7% if (C)RT plus TME is combined with LLND in patients with similarly enlarged nodes in whom 51% pathologically proven LLN metastases were found.
Although this the largest cohort reported, to our knowledge, it is retrospective and multi-institutional, leading to heterogeneity in patients and treatments. Results therefore have to be interpreted with caution. LLND was performed in a subgroup of hospitals, so comparisons were across institutions. Acknowledging these limitations, we can conclude that lateral nodal disease imposes an undeniable problem if nodes are enlarged, irrespective of the exact cutoff value. Furthermore, LLN enlargement does not influence DR rate, which suggests that it is a local issue that must be addressed through targeted treatment in the pelvis rather simply representing a marker of poor prognosis and distant disease. Moreover, it can be stated that in patients in whom (C)RT and TME is combined with LLND, good local control can be achieved.
A cutoff value of 7 mm in SA on primary MRI was selected for several reasons. First, choosing SA rather than LA seemed logical as it is more reliable to standardize this measurement and reduce variation with different MRI protocols and reporting radiologists. In addition, concentrating on SA size redirected focus from the typically long-stretched benign lymph nodes that are often present in the pelvis to those with prognostic significance. Finally, analysis of the effect of SA indicated that a size equal to or greater than 7 mm was associated with an unacceptably high rate of LLR of approximately 20% compared with the more generally reported rate of 5% to 10%. However, this is a subjective measure and Table 2 can be used to adequately assess risk for separate LLN sizes.
There are several points for discussion with regard to optimizing the treatment of patients with enlarged LLN. First, this study demonstrates that limiting resection to the affected node(s) is of little benefit. More than one half of these patients will develop LR in the same compartment, which suggests that complete LLND is required. Second, this work focuses on lateral nodal features on primary MRI. There is a dilemma over the value of restaging MRI; shrinkage of enlarged LLNs reduces the risk of pathologic involvement, but does not significantly reduce the likelihood of recurrence after (C)RT plus TME in this series. However, in cases in which nodes disappear completely, there was no LR but the numbers were too small to state this with any certainty.
This study indicates that in patients with enlarged nodes, LLND after (C)RT can reduce the risk of LLR. This is an area of practice with a paucity of evidence. The randomized study from Japan, Mesorectal Excision With or Without Lateral Node Dissection for Clinical Stage II/III Lower Rectal Cancer: A Multicenter, Randomized Controlled, Noninferiority Trial (JCOG0212),28 considered patients with nodes up to 10 mm in SA who did not have any neoadjuvant treatment. Adding LLND to TME reduced rates of LLR from 12.6% to 7.4%, which is similar to the LLR rate in this study of approximately 5% to 6% in patients who received (C)RT plus TME with nodes with SA up to 10 mm (Table 2). However, the JCOG2012 study does not answer the question of how to deal with patients with larger nodes, as LLND is unlikely to be sufficient.
Is there enough evidence to convince Western surgeons that LLND should be added to their treatment in patients who are at risk for LLR, as defined by the presence of enlarged LLNs? Alternatively, should these patients receive more aggressive neoadjuvant treatment, perhaps starting with induction chemotherapy or increasing radiotherapy dose to the lateral compartment to induce a complete response of the nodes? This study suggests that lateral nodal disease is a local problem and that the addition of systemic chemotherapy will not suffice. Increasing the radiotherapy boost up to 60 Gy on pathologic nodes has already been established in gynecologic cancers and does not result in increased morbidity29; however, if nodes are resistant, performing an LLND after can be more hazardous. In addition, as the radiologic complete response rate of the nodes in this study was only 5%, the additional benefit of 60 Gy may be limited in terms of complete response. These important factors may only be resolved through a large prospective multicenter study, with high-volume referral centers for locally advanced rectal cancers, where expertise in optimal radiotherapy regimens and surgical expertise in LLND can be standardized and quality controlled. This may bring a convergence of practices in the East and West to eradicate LLR in the future.
Appendix
List of the Co-Authors of the Lateral Node Study Consortium
Co-authors (in alphabetical order): A. G. J. Aalbers, T. Aiba, T. Akiyoshi, R. G. H. Beets-Tan, M. Betts, I. M. Blazic, K. G. Brown, N. Campbell, M. H. Choi, M. J. Gollub, Y. Hanaoka, M. K. Kim, E. Meershoek-Klein-Kranenbarg, H. Kuroyanagi, M. Maas, A. Martling, J. Moore, G. A. Nieuwenhuijzen, S. N. Oh, S. Roodbeen, T. Sammour, D. Schaap, M. J. Solomon, M. Thomas, K. Tomizawa, M. E. van der Sande, C. Suzuki, M. J. M. van der Valk, T. Wells, and D. D. Won.
FIG A1.
An example slide of the color map atlas of the pelvis. (A) Atlas of the pelvis. (B) The obturator and internal iliac areas are divided by the lateral border of the main trunk of the internal iliac vessels. External iliac region (red); obturator region (green); internal iliac region (blue); internal iliac artery (arrow head). (C) Benign long-stretched node just behind distal portion of the external iliac vein that should not be included in the assessment (arrow head). EIA, external iliac artery; EIV, external iliac vein; ObV, obturator vein; IOM, internal obturator muscle; Inf VV, inferior vesical vein; Int pud. a/v, internal pudendal artery/vein; Coccygeal m., coccygeal muscle (arrow).
TABLE A1.
Participating Institutes and Investigators
TABLE A2.
Overview of Baseline Characteristics Per Institution
TABLE A3.
Patient Characteristics According to SA of the LLN in Patients Who Received (C)RT
TABLE A4.
Univariable Analyses for Risk Factors for LLR, LR, DR, and CSS in the Total Cohort
Footnotes
The Nagoya Surgical Support Organization financed the travel and living expenses of A.O. during a research fellowship in the Netherlands.
AUTHOR CONTRIBUTIONS
Conception and design: Atsushi Ogura, Tsuyoshi Konishi, Cornelis J.H. van de Velde, Geerard L. Beets, Harm J.T. Rutten, Miranda Kusters
Provision of study materials or patients: All authors
Collection and assembly of data: Atsushi Ogura, Tsuyoshi Konishi, Henrik Iversen, Shigeo Toda, In Kyu Lee, Hong Xiang Lee, Keisuke Uehara, Peter Lee, Cornelis J.H. van de Velde, Harm J.T. Rutten, Miranda Kusters
Data analysis and interpretation: Atsushi Ogura, Hein Putter, Miranda Kusters
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Julio Garcia-Aguilar
Consulting or Advisory Role: Medtronic
Hong Xiang Lee
Research Funding: Medtronic
No other potential conflicts of interest were reported.
REFERENCES
- 1.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 2.Beets-Tan RGH, Beets GL, Vliegen RFA, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357:497–504. doi: 10.1016/s0140-6736(00)04040-x. [DOI] [PubMed] [Google Scholar]
- 3.Taylor FG, Quirke P, Heald RJ, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34–43. doi: 10.1200/JCO.2012.45.3258. [DOI] [PubMed] [Google Scholar]
- 4.Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28:1465–1475. doi: 10.1007/s00330-017-5026-2. [Erratum: Eur Radiol 28:2711, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swedish Rectal Cancer Trial. Cedermark B, Dahlberg M, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 6.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 7.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 8.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 9.Benson AB, III, Venook AP, Bekaii-Saab T, et al. Rectal cancer, version 2.2015. J Natl Compr Canc Netw. 2015;13:719–728, quiz 728. doi: 10.6004/jnccn.2015.0087. [DOI] [PubMed] [Google Scholar]
- 10.Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv22–iv40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 11.Moriya Y, Sugihara K, Akasu T, et al. Nerve-sparing surgery with lateral node dissection for advanced lower rectal cancer. Eur J Cancer. 1995;31A:1229–1232. doi: 10.1016/0959-8049(95)00164-e. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1–34. doi: 10.1007/s10147-017-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi T, Ueno M, Azekura K, et al. Lateral node dissection and total mesorectal excision for rectal cancer. Dis Colon Rectum. 2000;43(suppl):S59–S68. doi: 10.1007/BF02237228. [DOI] [PubMed] [Google Scholar]
- 14.Ueno M, Oya M, Azekura K, et al. Incidence and prognostic significance of lateral lymph node metastasis in patients with advanced low rectal cancer. Br J Surg. 2005;92:756–763. doi: 10.1002/bjs.4975. [DOI] [PubMed] [Google Scholar]
- 15.Sugihara K, Kobayashi H, Kato T, et al. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum. 2006;49:1663–1672. doi: 10.1007/s10350-006-0714-z. [DOI] [PubMed] [Google Scholar]
- 16.Kusters M, Beets GL, van de Velde CJ, et al. A comparison between the treatment of low rectal cancer in Japan and the Netherlands, focusing on the patterns of local recurrence. Ann Surg. 2009;249:229–235. doi: 10.1097/SLA.0b013e318190a664. [DOI] [PubMed] [Google Scholar]
- 17.Georgiou P, Tan E, Gouvas N, et al. Extended lymphadenectomy versus conventional surgery for rectal cancer: A meta-analysis. Lancet Oncol. 2009;10:1053–1062. doi: 10.1016/S1470-2045(09)70224-4. [DOI] [PubMed] [Google Scholar]
- 18.Kim TH, Jeong SY, Choi DH, et al. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol. 2008;15:729–737. doi: 10.1245/s10434-007-9696-x. [DOI] [PubMed] [Google Scholar]
- 19.Kim TG, Park W, Choi DH, et al. Factors associated with lateral pelvic recurrence after curative resection following neoadjuvant chemoradiotherapy in rectal cancer patients. Int J Colorectal Dis. 2014;29:193–200. doi: 10.1007/s00384-013-1797-3. [DOI] [PubMed] [Google Scholar]
- 20.Kim MJ, Kim TH, Kim DY, et al. Can chemoradiation allow for omission of lateral pelvic node dissection for locally advanced rectal cancer? J Surg Oncol. 2015;111:459–464. doi: 10.1002/jso.23852. [DOI] [PubMed] [Google Scholar]
- 21.Kusters M, Slater A, Muirhead R, et al. What to do with lateral nodal disease in low locally advanced rectal cancer? A call for further reflection and research. Dis Colon Rectum. 2017;60:577–585. doi: 10.1097/DCR.0000000000000834. [DOI] [PubMed] [Google Scholar]
- 22.Yano H, Moran BJ. The incidence of lateral pelvic side-wall nodal involvement in low rectal cancer may be similar in Japan and the West. Br J Surg. 2008;95:33–49. doi: 10.1002/bjs.6061. [DOI] [PubMed] [Google Scholar]
- 23.Akiyoshi T, Watanabe T, Miyata S, et al. Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: Is it regional or distant disease? Ann Surg. 2012;255:1129–1134. doi: 10.1097/SLA.0b013e3182565d9d. [DOI] [PubMed] [Google Scholar]
- 24.Akiyoshi T, Ueno M, Matsueda K, et al. Selective lateral pelvic lymph node dissection in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy based on pretreatment imaging. Ann Surg Oncol. 2014;21:189–196. doi: 10.1245/s10434-013-3216-y. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda T, Sumi Y, Yamashita K, et al. Outcomes and prognostic factors of selective lateral pelvic lymph node dissection with preoperative chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis. 2018;33:367–374. doi: 10.1007/s00384-018-2974-1. [DOI] [PubMed] [Google Scholar]
- 26.Kusters M, Slater A, Betts M, et al. The treatment of all MRI-defined low rectal cancers in a single expert centre over a 5-year period: Is there room for improvement? Colorectal Dis. 2016;18:O397–O404. doi: 10.1111/codi.13409. [DOI] [PubMed] [Google Scholar]
- 27.Kusters M, Marijnen CA, van de Velde CJ, et al. Patterns of local recurrence in rectal cancer: A study of the Dutch TME trial. Eur J Surg Oncol. 2010;36:470–476. doi: 10.1016/j.ejso.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Fujita S, Mizusawa J, Kanemitsu Y, et al. Mesorectal excision with or without lateral lymph node dissection for clinical stage II/III lower rectal cancer (JCOG0212): A multicenter, randomized controlled, noninferiority trial. Ann Surg. 2017;266:201–207. doi: 10.1097/SLA.0000000000002212. [DOI] [PubMed] [Google Scholar]
- 29.Pötter R, Tanderup K, Kirisits C, et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol. 2018;9:48–60. doi: 10.1016/j.ctro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]