Abstract
Cyclic arginine-glycine-aspartate (RGD) peptides and their derivatives have been intensively studied as tumor targeting probes. One major drawback, however, is their short blood circulation half-lives, which greatly compromises their targeting efficacy. To address this issue, a cyclic peptide, c(RGDyK), and an organic dye (IRDye800 or Cy5.5) were covalently conjugated onto human serum albumin (HSA). The conjugates were subjected to in vitro cell staining, in vivo near-infrared fluorescence (NIRF) imaging, ex vivo NIRF imaging, and histologic studies to evaluate their feasibility as tumor imaging probes. As a control, RAD peptide was also coupled with HSA and labeled with IRDye800 for in vivo imaging. The HSA-RGD-IRDye800 exhibited integrin αvβ3–specific binding in cell staining experiment. In vivo NIRF imaging showed higher tumor accumulation and tumor to background contrast of HSA-RGD-IRDye800 over RGD-IRDye800. The integrin specificity of HSA-RGD-IRDye800 is confirmed by both successful inhibition of tumor uptake in the presence of c(RGDyK) and the inability to accumulate in integrin-positive tumors by RAD-HSA-IRDye800. Histologic examination revealed initial tumor vascular binding and eventually both tumor vasculature and tumor cell integrin binding in vivo. In summary, we successfully developed an RGD-based protein conjugate with prolonged circulation half-life for NIRF imaging of tumor integrin αvβ3 expression. The success of this study may be generalizable for other peptide-based probes to be conjugated with HSA for prolonged tumor contrast and improved pharmacokinetics.
INTEGRIN αVβ3 is a cell adhesion receptor that is highly expressed on most of the solid tumor vasculature1–3 and, in certain cases, the tumor cells themselves.4 This virtue and the fact that the receptor has high affinity with proteins and peptides containing the arginine-glycine-aspartate (RGD) sequence make it an ideal tumor indicator. Specifically, in the field of molecular imaging, RGD peptide conjugates could be used as tracers, which will specifically accumulate at tumor areas owing to the RGD-integrin association. This strategy has been used in many types of imaging modalities, including positron emission tomography (PET),5–9 single-photon emission computed tomography (SPECT),10–12 magnetic resonance imaging (MRI),13–15 ultrasonography,16 and near-infrared fluorescence (NIRF).17–19 The application of appropriate RGD peptide probes for diagnosis and treatment response monitoring has been summarized in several recent review articles.20–24
One of the main limitations of this approach, however, is the short circulation time of RGD peptides. As small peptide probes, the majority of the injected RGD probes are subjected to rapid renal and/or hepatobiliary clearance, resulting in a transient circulation time and hence suboptimal targeting efficacy. One strategy to overcome this issue is to increase the targeting affinity between the RGD peptide tracer and the integrin receptor, with the intention of homing more agents to the areas of interest within a limited period of time. Toward this end, there has been a transition from using linear RGD to cyclized RGD.25,26 More recently, several groups reported that RGD multimerization could greatly enhance the integrin binding affinity. In particular, RGD octamer has a binding affinity 27 times that of the monomer,27 which greatly improved the tumor targeting efficacy. However, the synthesis of multimeric RGD peptides is technically challenging, and these high-affinity RGD multimers with high tumor affinity and accumulation also have a relatively high background, thus compromising their clinical translatability.
Another approach is to conjugate RGD peptides to nanoparticles such as quantum dots, iron oxide nanoparticles, and carbon nanotubes. This measure could save RGD from the fate of renal excretion but could, on the other hand, enhance reticular endothelial system uptake, which does not necessarily elongate the circulation time.15,19,28,29 There have been reports regarding achieving prolonged RGD blood half-lives by PEGylation30,31 and polymer engraftment,32 which helps attenuate enzymatic degradation and fast renal clearance. This is done, however, at the expense of decreased binding affinity. In this work, we evaluated the feasibility of using RGD-human serum albumin (HSA) conjugate as the probe for tumor imaging. HSA is a 65 kDa protein that is abundant in the circulation system. Owing to its long circulation virtue, it has been used as a carrier for drug delivery, with several of them already approved by the Food and Drug Administration and several others in the process of advanced clinical trials.33 The employment of HSA in molecular imaging, in particular, in association with RGD to achieve a strengthened targeting probe, has not been carefully investigated.
Materials and Methods
Cell Culture and Animal Model
Human glioblastoma cancer cell line U87MG was purchased from the American Type Culture Collection (ATCC) and cultured with ATCC-formulated Eagle’s Minimum Essential Medium in a cell culture incubator. Athymic nude mice were purchased from Harlan Laboratories (Indianapolis, IN). Approximately 5 × 106 U87MG cells were subcutaneously inoculated, and the in vivo imaging was carried out 3 weeks after the inoculation on the front flank when the tumor size reached 100 mm3. All animal work was conducted following a protocol approved by the Stanford University Administrative Panel on Laboratory Animal Care.
Dye Labeling and RGD Coupling
The bioconjugation was adapted from our previous publication.29 In brief, HSA was dissolved in borate buffer (50 mM, pH 8.5) at a concentration of 2 mg/mL and IRDye800-NHS ester or Cy5.5-NHS ester in dimethyl sulfoxide (2 mg/mL) was added at a 1:2 HAS to dye molar ratio. The labeling was proceeded in dark at 4°C for 2 hours. Afterward, the conjugates were purified through PD-10 column, and products were dispersed in borate buffer. Approximately 100 aliquots of N-succinimidyl 4-maleimidobutyrate (TCI America, Portland, OR) was added and the coupling was allowed to go for 1 hour at 4°C. The mixture was subjected to PD-10 column for purification, and the buffer was exchanged to phosphate-buffered saline (PBS) (pH 7.4). Thiolated RGD peptide, c(RGDy(ε-acetylthiol)K) (RGD-SH),34 was then added, and the reaction was proceeded in dark overnight at 4°C. The resulted solution was purified through PD-10 column with borate buffer (50 mM, pH 8.5) and redispersed in PBS buffer. As a control, thiolated RAD peptide, c(RADy(ε-acetylthiol)K) (RAD-SH) was also coupled with HSA-IRDye800. The IRDye800 concentration was determined by measuring absorption at 780 nm, and the HSA concentration was measured by Bradford assay. The number of dye molecules per protein was thus calculated to be [dye]/[HSA]. The number of RGD or RAD peptides per HSA protein molecule was determined by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry.
In Vitro Fluorescence Cell Staining
Approximately 1 × 105 U87MG cells were seeded in glass-bottom culture chambers (MatTek, Ashland, MA) the day before staining. Freshly made paraformaldehyde (4%) was used to fix the cells at room temperature for 15 minutes. After fixation, the cells were rinsed with PBS three times and then incubated with 0.1 μM HSA-RGD-Cy5.5, HSA-Cy5.5, or Cy5.5 only in integrin binding buffer (20 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 1 mmol/L MnCl2, 0.1% bovine serum albumin) for 1 hour at room temperature. After three rinse cycles with PBS, the chamber was removed and the slides were mounted with mounting medium containing DAPI. Fluorescence pictures were taken under an Anxiovert 200M inverted epifluorescence microscope (Carl Zeiss, Germany) using DAPI and Cy5.5 filter setting separately. Merged pictures were made using the image processing software MetaMorph (Molecular Devices, Sunnyvale, CA).
Circulation Half-Life Measurement
To measure the half-life of HSA-RGD-IRDye800 and RGD-IRDye800, nude mice were injected in the tail vein with 0.5 nmol IRDye800 equivalent of fluorescent probes and a blood sample (approximately 3–5 μL) was taken from the tail vein of the mice at different time points postinjection, and the photoluminescence intensity of the blood samples was measured by the IVIS 200 imaging system (Caliper Lifesciences, Hopkinton, MA). The circulation half-life was calculated by a method fitting the data to a one-phase exponential decay equation (GraphPad Prism, GraphPad Software Inc., La Jolla, CA).
In Vivo and Ex Vivo NIRF Imaging
Mice were divided into five groups (HSA-RGD-IRDye800, HSA-RGD-IRDye800 + RGD block, HSA-RAD-IRDye800, RGD-IRDye800, and IRDye800) (n = 4/group). All mice were intravenously injected with probes containing 0.5 nmol IRDye800. After injection, NIRF in vivo imaging was conducted using the Maestro Fluorescence Imaging System (CRI, Woburn, MA; excitation = 735 nm, band length = 20 nm, emission = 780 nm long pass) at 1-, 4-, and 24-hour time points postinjection. Quantification of tumor to background was done with Maestro 2.40 software. Another four mice injected with HSA-RGD-IRDye800 (0.5 nmol IRDye800/mouse) were also scanned with a Pearl Imager (Li-Cor, Lincoln, NE), which uses diffusive lasers specifically designed for IRDye680 and IRDye800. The 800 nm channel was used to image HSA-RGD-IRDye800-injected mice.
At 24 hours after the injection of HSA-RGD-IRDye800 and RGD-IRDye800, tumor mice were sacrificed. Tumors and major organs were collected and subjected to ex vivo NIRF imaging with an IVIS 200 imaging system. The tissues were weighted to normalize NIRF signals.
Histologic Analysis
U87MG tumor–bearing mice were injected with 1 nmol HSA-RGD-Cy5.5. At the 3- or 24-hour time point, the mice were injected with 200 μg fluorescien isothiocyanate (FITC)-labeled tomato lectin (Thermal Fisher Scientific, Rockford, IL). The mice were then sacrificed 10 minutes later, and the tumors were collected and made into frozen tissue block. These tumor specimens were subsequently sectioned with a thickness of 40 μm. Fluorescence pictures were taken under a microscope using FITC and Cy5.5 filter settings separately. Merged pictures were made using MetaMorph.
Results
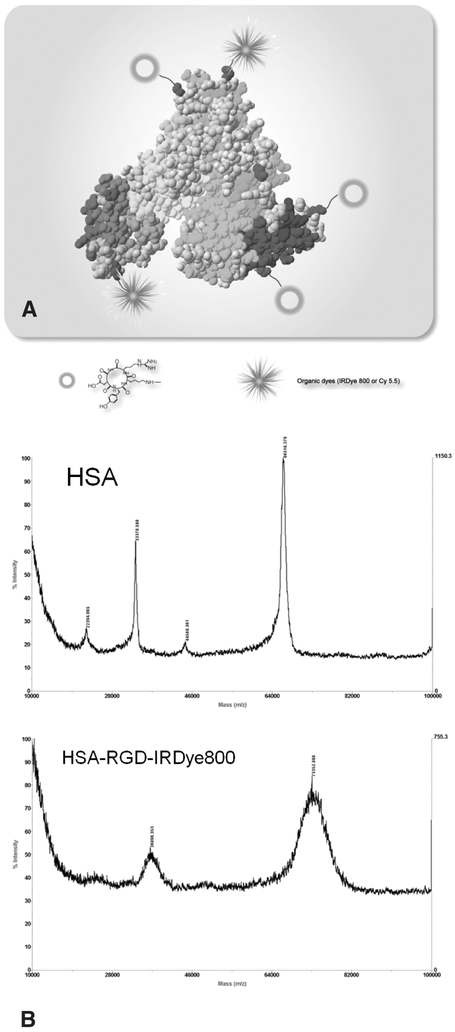
The multiple amine groups on the HSA surface allow biofunctionalization. In our study, a cyclic peptide, c(RGDyK), was covalently immobilized onto the HSA, and an organic dye, either IRDye800 or Cy5.5, was applied to make the HSA optically active (Figure 1A). The number of dye molecules per HSA protein was estimated to be around 1.1. The MALDI-TOF mass spectroscopy showed the molecular weight of HSA protein as 66.52 kDa and the average molecular weight of HSA-RGD-IRDye800 as 73.35 kDa (Figure 1B). The number of RGD peptides per HSA protein is thus calculated to be around 9.
Figure 1.
A, Schematic structure of HSA-RGD-Dye conjugate. B, MALDI-TOF mass spectra of human serum albumin (HSA) (molecular weight = 66.52 kDa) and HSA-RGD-IRDye800 (molecular weight = 73.35 kDa). The average number of cyclic RGD peptide per HSA is calculated to be 9.
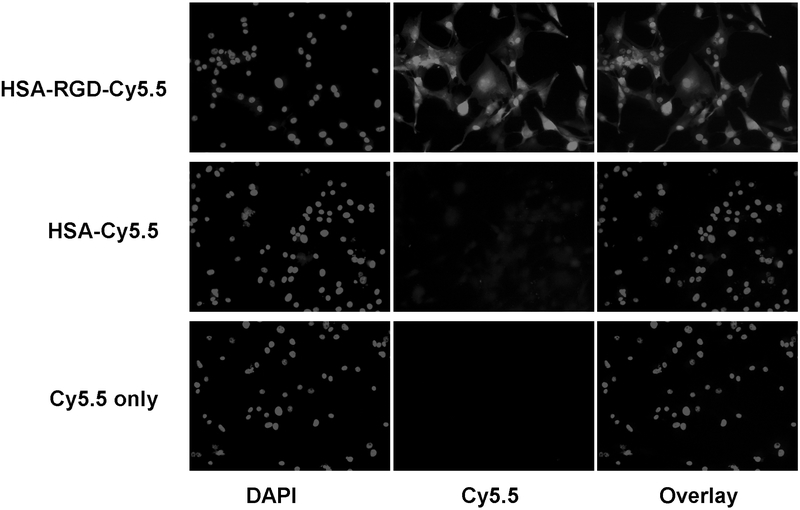
We first assessed the targeting specificity of HSA-RGD-Cy5.5 in vitro by incubating the probe with highly integrin αvβ3–expressing U87MG cells. We used Cy5.5 instead of IRDye800 conjugates for cell staining because the emission wavelength of IRDye800 (806 nm) is beyond the optimal detection capacity of our inverted epifluorescence microscope. Our staining results showed that HSA-RGD-Cy5.5 binds well to U87MG cells. HSA-Cy5.5 showed very weak cell staining owing to the low nonspecific absorption onto the cell surface. As a control, Cy5.5 alone showed virtually no binding to U87MG cells. We have previously shown that RGD-Cy5.5 binds to U87MG cells in an integrin-specific manner.17 The results shown in Figure 2 confirm that the targeting of HSA-RGD-Cy5.5 is mediated by RGD–integrin interaction.
Figure 2.
Cell staining of U87MG cells with Cy5.5 conjugates. Positive cell staining is possible only for HSA-RGD-Cy5.5 but not HSA-Cy5.5 or Cy5.5, confirming the integrin specificity of HSA-RGD-Cy5.5 (total magnification ×200).
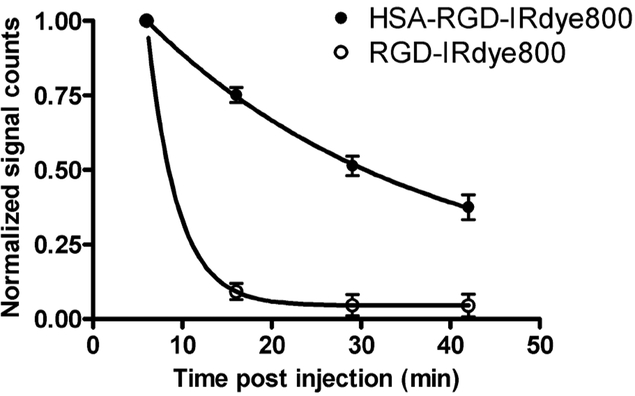
After verifying that HSA-RGD-Cy5.5 is able to bind to integrin αvβ3 in vitro, we proceeded to test HSA-RGD-IRDye800 in living subjects. IRDye800 is advantageous over Cy5.5 for in vivo imaging owing to its optimal emission wavelength around 800 nm, where autofluorescence is minimal.35 Nude mice bearing subcutaneous U87MG tumors (3 weeks postinoculation of 5 × 106 cells on the front flank when the tumor size reached 6 to 10 mm in diameter) were divided into five groups (n = 4/group), and each group of animals received intravenous injection of HSA-RGD-IRDye800, HSA-RGD-IRDye800 in the presence of a blocking dose of c(RGDyK) (10 mg/kg), HSA-RAD-IRDye800, RGD-IRDye800, or IRDye800 (0.5 nmol of IRDye800 equivalent per mouse). The mice were then imaged at multiple time points postinjection using the Maestro in vivo imaging system. After image acquisition, spectral unmixing yielded the pseudocolor images of the pure spectrum of IRDye800. Representative coronal images are shown in Figure 3A. The decrease in fluorescence signals of both HSA-RGD-IRDye800 and RGD-IRDye800 in the blood circulation was followed by one-phase exponential decay, with the circulation half-life for HSA-RGD-IRDye800 being 21 minutes, whereas the half-life for RGD-IRDye800 was only 2.3 minutes (Figure 4). The short circulation half-lives of RGD-IRDye800 and IRDye800 were also reflected by rapid renal clearance and the very minimal whole-body signal at late time points (see Figure 3). The optical imaging results after injection of HSA-RGD-IRDye800 were also acquired using a Pearl Imager, which uses diffusive laser excitation. The sagittal images from the Pearl Imager gave better tumor contrast (Figure 3B) compared with the CRI Maestro instrument using white light plus an appropriate filter as the excitation source.
Figure 3.
In vivo imaging of U87MG tumor mice with IRDye800 conjugates. A, Representative coronal images acquired from the CRI Maestro in vivo imaging system at 1, 4, and 24 hours after intravenous injection of IRDye800, RGD-IRDye800, HSA-RGD-IRDye800, and HSA-RGD-IRDye800 in the presence of c(RGDyK) (10 mg/g) and HSA-RAD-IRDye800 (0.5 nmol equivalent of IRDye800 per mouse, n = 4/group). All images were acquired and processed under the same conditions. Total fluorescence signals normalized by exposure time. B, Representative sagittal images acquired from the Li-Cor Pearl Imager using near-infrared laser illumination at 1, 4, and 24 hours after intravenous injection of HSA-RGD-IRDye800 (0.5 nmol equivalent of IRDye800 per mouse, n = 4).
Figure 4.
Circulation half-lives of RGD-IRDye800 (○) and HSA-RGD-IRDye800 (●). The decrease in fluorescence signals of both probes in the blood circulation followed by one-phase exponential decay, with the half-life for HSA-RGD-IRDye800 being 21 minutes and for RGD-IRDye800 being 2.3 minutes.
Despite the short circulation half-life, RGD-IRDye800 was able to accumulate in U87MG tumors with contrast up to 24 hours postinjection (tumor to contralateral background ratio of 1.22 ± 0.15). This observation correlated well with our previous reports on Cy5.5- and Cy7-labeled cyclic RGD peptides.17,18,36,37 By contrast, conjugation of HSA increased the blood half-life of the NIRF probes by almost 10-fold (see Figure 4). The prolonged circulation permits better tumor signal and higher tumor contrast. The tumor background ratios of HSA-RGD-IRDye800 based on the Maestro setting were 2.32 ± 0.12 at 1 hours, 3.63 ± 0.18 at 4 hours, and 5.67 ± 0.20 at 24 hours, respectively. The absolute tumor signal of HSA-RGD-IRDye800 is 6.1 times higher than that of RGD-IRDye800 at the 24-hour time point. HSA-RAD-IRDye800 showed minimal tumor uptake compared with HSA-RGD-IRDye800 because of no binding to integrin. The tumor uptake of HSA-RGD-IRDye800 can also be effectively inhibited in the presence of c(RGDyK) (10 mg/kg), further confirming the integrin specificity of HSA-RGD-IRDye800 in vivo.
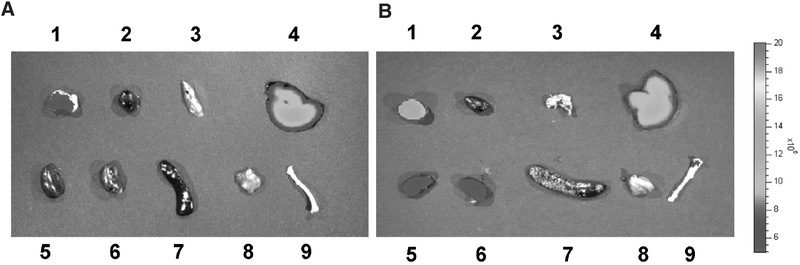
To avoid any deviation caused by tissue penetration limitation of fluorophores, tumor and major organs were collected at the 4-hour time point and were subjected immediately to the NIRF imaging. Figure 5 clearly shows better tumor targeting efficacy of HSA-RGD-IRDye800 over the RGD-IRDye800, with the tumor to muscle contrast being 4.3 times higher than the later one. Both HSA-RGD-IRDye800 and RGD-IRDye800 were found to accumulate in the kidneys, which might be due to the renal clearance and integrin αvβ3–specific accumulations in kidneys.27 A higher liver retention of HSA-RGD-IRDye800 over RGD-IRDye800 was observed, but the tumor to liver ratio of HSA-RGD-IRDye800 was still significantly higher than RGD-IRDye800.
Figure 5.
Ex vivo near-infrared fluorescence imaging of tumor and major organs and tissues at 4 hours postinjection of (A) RGD-IRDye800 and (B) HSA-RGD-IRDye800 using the Caliper Lifesciences IVIS 200 imaging system. 1 = tumor; 2 = heart; 3 = lung; 4 = liver; 5 = left kidney; 6 = right kidney; 7 = spleen; 8 = muscle; 9 = bone.
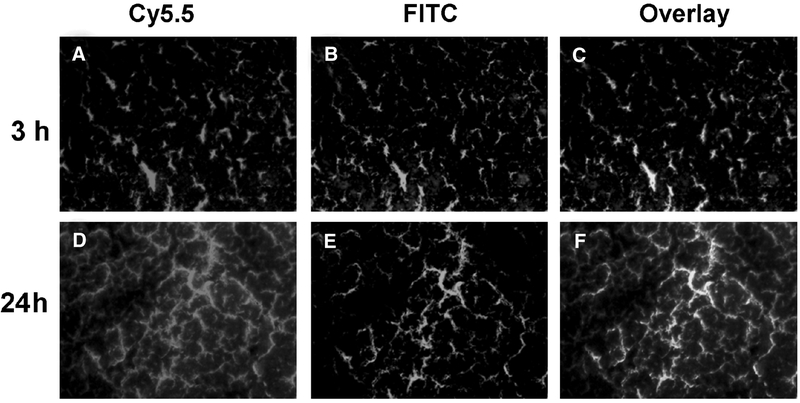
To further characterize the cellular distribution of HSA-RGD-Dye, we injected the animals with HSA-RGD-Cy5.5. After distribution of the dye conjugate for 3 or 24 hours, the mice were then injected in the tail vein with FITC-labeled tomato lectin and sacrificed 10 minutes after the injection.18 At the 3-hour time point, it could be seen that most of the HSA-RGD-Cy5.5 conjugates stayed within the blood vessels, including those already immobilized through RGD–integrin interaction and those still in the circulation (Figure 6). With time, more and more probes gradually extravasated from the blood vessels, as shown by the diffusive distribution pattern of HSA-RGD-Cy5.5 at the 24-hour time point.
Figure 6.
Regional localization of HSA-RGD-Cy5.5: (A, D) red = Cy5.5, (B, E) green = fluorescein isothiocyanate (FITC), (C, F) orange = overlay. U87MG tumor–bearing mice were injected with 1 nmol of HSA-RGD-Cy5.5 (A–C, 3 hours; D–F, 24 hours) followed by tomato lectin conjugated to FITC for in vivo dual staining of tumor vasculature to determine HSA-RGD localization. Cy5.5 (red) fluorescence staining of tumor vasculature and surrounding tumor cells and FITC (green) staining of tumor vasculature were acquired separately and then overlaid (orange) using MetaMorph imaging processing software.
Discussion
The main objective of this study is to examine if the conjugation of HSA can help prolong the circulation time of RGD peptide so as to achieve better tumor targeting efficacy. HSA functions in this context as a monomeric polymer featuring low immunogenicity, high biocompatibility, and excellent biodegradability. Furthermore, renal filtration of probes is substantially inhibited by the high molecular size of albumin, enabling prolonged exposure of the target cells to the conjugates.
We controlled the number of dye molecules per HSA protein to be around 1 to avoid potential quenching. We also tested different RGD to HSA reaction ratios and found that a higher RGD to HSA ratio resulted in a better tumor to background ratio (data not shown). The highest number of RGD per HSA via a poly(ethylene glycol) linker was reported by Temming and colleagues to be around 13.38 After IRDye800 or Cy5.5 dye coupling, we were able to reach 9 RGD peptides per HSA without the presence of a flexible PEG linker. The accumulation of the HSA-RGD-dye conjugate in the tumor area can be contributed by three parts: the probes that are specifically bound to the endothelial cell surface through RGD and αvβ3 interaction, the probes that are present in the tumor blood circulation, and those that actually diffused out of the tumor vessels and attached to U87MG tumor cells. As shown in Figure 6, A to C, most of the HSA-RGD-IRDye800 conjugates stayed in the blood vessel, with little signal in the interstitial space at the 3-hour time point, at which most of the optical signal comes from endothelial integrin binding and tumor bloodstream retention. Both RGD blocking of HSA-RGD-IRDye800 and HSA-RAD-IRDye800 control experiments with abrogated integrin targeting showed low tumor contrast at early time points (see Figure 3), presumably coming from the enhanced permeability and retention effect of macromolecules in the tumor tissue.39 The tumor vessel signal remained strong at late time points, but optical signal was also found out of the blood vessels (Figure 6D–F), which was not due to the diffusion of HSA conjugates into the extracellular space but, rather, tumor cell binding as the Cy5.5 signal cannot be washed off by perfusion (data not shown). There should be little to no blood retention of the dye conjugate as the circulation half-life of HSA-RGD-Cy5.5 is about 20 minutes. There should be less than 0.1% of injected dose remaining in the blood at the 24-hour time point, at which the contrast should be mostly from tumor vascular and tumor cell integrin binding. Future studies may include integrin staining overlay with the optical signal to further confirm integrin specificity and the trafficking of the HSA conjugates.
It is worth noting that although HSA alone showed very low adsorption to U87MG tumor cells in vitro, it might be possible that HSA molecules could be passively trapped at the tumor site and contribute to the probe enrichment. It is also known that tumor accumulation with albumin carriers is facilitated through binding to a cell surface, 60 kDa glycoprotein receptor (albondin), as well as binding to SPARC (secreted protein acid and rich in cysteine) in a variety of cancers.40–42 Successful blocking of HSA-RGD-IRDye800 by RGD peptide excluded this possibility, which might be due to the bioconjugation of peptides and fluorescent dyes that abrogated the albondin and SPARC binding. Another point is that although we only assessed the feasibility of using HSA-RGD conjugates as probes in optical imaging, the conjugate can be linked with isotopes for radionuclide imaging and RGD-modified albumins may be suitable carriers for cell-selective delivery of therapeutics.
In summary, we successfully developed an RGD-based macromolecular probe with prolonged half-life time in the blood for NIRF imaging of tumor integrin αvβ3 expression. The success of this study may be generalizable for other peptides or small molecule–based probes to be conjugated with HSA for prolonged tumor contrast and improved pharmacokinetics.
Acknowledgment
Financial disclosure of authors: This work was supported, in part, by the National Cancer Institute (NCI R01 CA119053, R21 CA121842, P50 CA114747, and U54 CA119367).
Footnotes
Financial disclosure of reviewers: None reported.
References
- 1.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science 1994;264:569–71. [DOI] [PubMed] [Google Scholar]
- 2.Friedlander M, Brooks PC, Shaffer RW, et al. Definition of two angiogenic pathways by distinct αv integrins. Science 1995;270:1500–2. [DOI] [PubMed] [Google Scholar]
- 3.Hynes RO. A reevaluation of integrins as regulators of angiogenesis. Nat Med 2002;8:918–21. [DOI] [PubMed] [Google Scholar]
- 4.Garanger E, Boturyn D, Dumy P. Tumor targeting with RGD peptide ligands—design of new molecular conjugates for imaging and therapy of cancers. Anticancer Agents Med Chem 2007;7:552–8. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Park R, Shahinian AH, et al. 18F-Labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol 2004;31:179–89. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Hou Y, Tohme M, et al. Pegylated Arg-Gly-Asp peptide: 64Cu labeling and PET imaging of brain tumor αvβ3-integrin expression. J Nucl Med 2004;45:1776–83. [PubMed] [Google Scholar]
- 7.Chen X, Tohme M, Park R, et al. Micro-PET imaging of αvβ3-integrin expression with 18F-labeled dimeric RGD peptide. Mol Imaging 2004;3:96–104. [DOI] [PubMed] [Google Scholar]
- 8.Wu Z, Li ZB, Chen K, et al. MicroPET of tumor integrin αvβ3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4). J Nucl Med 2007;48:1536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li ZB, Chen K, Chen X. 68Ga-labeled multimeric RGD peptides for microPET imaging of integrin αvβ3 expression. Eur J Nucl Med Mol Imaging 2008;35:1100–8. [DOI] [PubMed] [Google Scholar]
- 10.Liu S Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging. Mol Pharm 2006;3:472–87. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Hsieh WY, Jiang Y, et al. Evaluation of a 99mTc-labeled cyclic RGD tetramer for noninvasive imaging integrin αvβ3-positive breast cancer. Bioconjug Chem 2007;18:438–46. [DOI] [PubMed] [Google Scholar]
- 12.Lee BC, Sung HJ, Kim JS, et al. Synthesis of Tc-99m labeled glucosamino-Asp-cyclic(Arg-Gly-Asp-d-Phe-Lys) as a potential angiogenesis imaging agent. Bioorg Med Chem 2007;15:7755–64. [DOI] [PubMed] [Google Scholar]
- 13.Ke T, Jeong EK, Wang X, et al. RGD targeted poly(Lglutamic acid)-cystamine-(Gd-DO3A) conjugate for detecting angiogenesis biomarker αvβ3 integrin with MRT, mapping. Int J Nanomed 2007;2:191–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Persigehl T, Matuszewski L, Kessler T, et al. Prediction of antiangiogenic treatment efficacy by iron oxide enhanced parametric magnetic resonance imaging. Invest Radiol 2007;42:791–6. [DOI] [PubMed] [Google Scholar]
- 15.Lee HY, Li Z, Chen K, et al. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med 2008;49:1371–9. [DOI] [PubMed] [Google Scholar]
- 16.Borden MA, Zhang H, Gillies RJ, et al. A stimulus-responsive contrast agent for ultrasound molecular imaging. Biomaterials 2008;29:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Conti PS, Moats RA. In vivo near-infrared fluorescence imaging of integrin αvβ3 in brain tumor xenografts. Cancer Res 2004;64:8009–14. [DOI] [PubMed] [Google Scholar]
- 18.Hsu AR, Hou LC, Veeravagu A, et al. In vivo near-infrared fluorescence imaging of integrin αvβ3 in an orthotopic glioblastoma model. Mol Imaging Biol 2006;8:315–23. [DOI] [PubMed] [Google Scholar]
- 19.Cai W, Chen K, Li ZB, et al. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J Nucl Med 2007;48:1862–70. [DOI] [PubMed] [Google Scholar]
- 20.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med 2008;49 Suppl 2:113S–28S. [DOI] [PubMed] [Google Scholar]
- 21.Chen X Multimodality imaging of tumor integrin αvβ3 expression. Mini Rev Med Chem 2006;6:227–34. [DOI] [PubMed] [Google Scholar]
- 22.Cai W, Niu G, Chen X. Imaging of integrins as biomarkers for tumor angiogenesis. Curr Pharm Des 2008;14:2943–73. [DOI] [PubMed] [Google Scholar]
- 23.Beer AJ, Schwaiger M. Imaging of integrin αvβ3 expression. Cancer Metastasis Rev 2008;27:631–44. [DOI] [PubMed] [Google Scholar]
- 24.Haubner R αvβ3-Integrin imaging: a new approach to characterise angiogenesis? Eur J Nucl Med Mol Imaging 2006;33 Suppl 1:54–63. [DOI] [PubMed] [Google Scholar]
- 25.Bogdanowich-Knipp SJ, Chakrabarti S, Williams TD, et al. Solution stability of linear vs. cyclic RGD peptides. J Pept Res 1999;53:530–41. [DOI] [PubMed] [Google Scholar]
- 26.Marastoni M, Salvadori S, Scaranari V, et al. Synthesis and activity of new linear and cyclic peptide T derivatives. Arzneimittelforschung 1994;44:1073–6. [PubMed] [Google Scholar]
- 27.Li ZB, Cai W, Cao Q, et al. 64Cu-labeled tetrameric and octameric RGD peptides for small-animal PET of tumor αvβ3 integrin expression. J Nucl Med 2007;48:1162–71. [DOI] [PubMed] [Google Scholar]
- 28.Cai W, Chen X. Preparation of peptide-conjugated quantum dots for tumor vasculature-targeted imaging. Nat Protoc 2008;3:89–96. [DOI] [PubMed] [Google Scholar]
- 29.Cai W, Shin DW, Chen K, et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett 2006;6:669–76. [DOI] [PubMed] [Google Scholar]
- 30.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov 2003;2:214–21. [DOI] [PubMed] [Google Scholar]
- 31.Eto Y, Gao JQ, Sekiguchi F, et al. PEGylated adenovirus vectors containing RGD peptides on the tip of PEG show high transduction efficiency and antibody evasion ability. J Gene Med 2005;7:604–12. [DOI] [PubMed] [Google Scholar]
- 32.Mitra A, Mulholland J, Nan A, et al. Targeting tumor angiogenic vasculature using polymer-RGD conjugates. J Control Release 2005;102:191–201. [DOI] [PubMed] [Google Scholar]
- 33.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother 2006;7:1041–53. [DOI] [PubMed] [Google Scholar]
- 34.Xiong Z, Cheng Z, Zhang X, et al. Imaging chemically modified adenovirus for targeting tumors expressing integrin αvβ3 in living mice with mutant herpes simplex virus type 1 thymidine kinase PET reporter gene. J Nucl Med 2006;47:130–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Chen K, Niu G, Chen X. Site-specifically biotinylated VEGF121 for near-infrared fluorescence imaging of tumor angiogenesis. Mol Pharm 2009;6:285–94. [DOI] [PubMed] [Google Scholar]
- 36.Cheng Z, Wu Y, Xiong Z, et al. Near-infrared fluorescent RGD peptides for optical imaging of integrin αvβ3 expression in living mice. Bioconjug Chem 2005;16:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y, Cai W, Chen X. Near-infrared fluorescence imaging of tumor integrin αvβ3 expression with Cy7-labeled RGD multimers. Mol Imaging Biol 2006;8:226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Temming K, Meyer DL, Zabinski R, et al. Evaluation of RGD-targeted albumin carriers for specific delivery of auristatin E to tumor blood vessels. Bioconjug Chem 2006;17:1385–94. [DOI] [PubMed] [Google Scholar]
- 39.Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 2000;65:271–84. [DOI] [PubMed] [Google Scholar]
- 40.Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophorbased paclitaxel. Clin Cancer Res 2006;12:1317–24. [DOI] [PubMed] [Google Scholar]
- 41.John TA, Vogel SM, Tiruppathi C, et al. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol 2003;284:L187–96. [DOI] [PubMed] [Google Scholar]
- 42.Porter PL, Sage EH, Lane TF, et al. Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem 1995;43:791–800. [DOI] [PubMed] [Google Scholar]