Abstract
Most fluorescent indicators for Mg2+ suffer from poor selectivity against other divalent cations, especially Ca2+, thus do not provide reliable information on cellular Mg2+ concentrations in processes in which such metals are involved. We report a new set of highly selective fluorescent indicators based on alkoxystyryl-functionalized BODIPY fluorophores decorated with a 4-oxo-4H-quinolizine-3-carboxylic acid metal binding moiety. The new sensors, MagQ1 and MagQ2, display absorption and emission maxima above 600 nm, with a 29-fold fluorescence enhancement and good quantum yields (Φ > 0.3) upon coordination of Mg2+ in aqueous buffer. Fluorescence response to Mg2+ is not affected by the presence of competing divalent cations typically present in the cellular milieu, and displays minimal pH dependence in the physiologically relevant range. The choice of alkoxy groups decorating the styryl BODIPY core does not influence the basic photophysical and metal binding properties of the compounds, but has a marked effect on their intracellular retention and thus in their applicability for detection of cellular Mg2+ by fluorescence imaging. In particular, we demonstrate the utility of a triethyleneglycol (TEG) functionalization tactic that endows MagQ2 with superior cellular retention in live cells by reducing active extrusion through organic anion transporters, which are thought to cause fast leakage of typical anionic dyes. With enhanced retention and excellent photophysical properties, MagQ2 can be applied in the detection of cellular Mg2+ influx without interference of high concentrations of Ca2+ akin to those involved in signaling.
Table of Contents Entry
New fluorescent sensors with excellent turn-on ratio and low energy excitation provide Mg2+ detection in live cells with high selectivity
Introduction
Tightly regulated levels of Mg2+ within mammalian cells are essential for maintaining numerous fundamental processes including enzyme activation,1 regulation of K+ and Ca2+ ion transport2–4 and stabilization of chromatin structure,5 among others. Recently, Mg2+ was shown to act as a second messenger coupled with T-cell receptors, activating a series of intracellular effectors in lymphocytes.6–7 To date, however, the involvement of Mg2+ in signaling and regulation of cellular activity remains mostly underexplored and somewhat controversial. This stands in contrast to the detailed knowledge on the role of Ca2+ in signaling, garnered over decades of research. The obvious disparity stems partly from the lack of sensors that can selectively detect transient changes of intracellular Mg2+ concentrations without interference from Ca2+.
Current commercially available fluorescent probes for cellular Mg2+ detection, including FURAPTRA8 (a.k.a. Mag-Fura-2)9 and Mag-fluo-4,9 are based on the o-aminophenol-N,N,O-triacetic acid (APTRA) metal binding group. This pentadentate chelator has been shown to form complexes with Mg2+ with a dissociation constant in the low millimolar range,10 which is optimal for achieving maximum sensitivity in the detection of typical cellular concentrations of “free” or “ionized” Mg2+ (0.5 mM – 1.0 mM).3 A significant drawback of APTRA, however, is the formation of tight complexes with other cations also present in biological samples, such as Ca2+ and Zn2+,9,11–15 that may cause significant interference in Mg2+ detection in systems with high levels of these metals. In particular, despite being much less abundant than Mg2+ in the intracellular milieu, Ca2+ ions involved in cellular signaling can rise to concentration ranges that are orders of magnitude higher than the typical basal levels, and may fall in the detection range of the sensors.16 Under these circumstances, APTRA-based sensors are likely to yield ambiguous results that hinder the study of Mg2+ in the context of Ca2+-mediated signaling processes.
Seeking to alleviate the interference from Ca2+ in the detection of cellular Mg2+, fluorescent indicators based on binding motifs with enhanced selectivity profiles have been long pursued. Farruggia et al reported the application of DCHQ series of fluorescent sensors, based on a diaza-18-crown-6 hydroxyquinoline motif, for the selective imaging of intracellular Mg2+. These sensors display a fluorescence enhancement upon Mg2+ binding, whereas other metals ions such as Ca2+ and Zn2+ only induce a minor response in concentration ranges that are well above biologically relevant levels.17 The affinity for Mg2+ of these sensors is quite high (Kd values of 44 and 73 μM for DCHQ1 and 2, respectively), hence they are thought to report total magnesium concentration based on their ability to outcompete natural intracellular chelators of the cation.17–18 The groups of Oka and Suzuki adopted the 4-oxo-4H-quinolizine-3-carboxylic acid Mg2+ binding motif19 and appended it to fluorophores such as fluorescein and rhodamine.20–21 The resulting Mg2+-selective turn-on fluorescent probes, i.e. the KMG series, have been shown to report Mg2+ dynamics in living cells successfully. Unfortunately, the fluorescein-based KMG 10420 only shows modest “turn-on” ratio and low fluorescence quantum yield (Φ = 0.02) upon chelation of Mg2+, which requires substantial loading of the probe and yields weak signal that may be affected by interference from cellular autofluorescence. The rhodamine-based analogue KMG 30121 displays improved fluorescence quantum yield (Φ = 0.15) upon Mg2+ binding, but the positively charged rhodamine accumulates in mitochondria, therefore restricting the use of the probe to these organelles and precluding the detection of Mg2+ in other cellular compartments, including the cytosol. More recently, Kikuchi and coworkers developed a new set of fluorescent probes, namely the MGQ series, designed for the detection of intracellular Mg2+ with high metal selectivity.22 The fluorescence “turn-off” response of these probes, however, is best suited for the detection of Mg2+ depletion and limits their general applicability. In this context, bright fluorescent indicators with high turn-on ratio and low-energy excitation, more generally applicable for detection of intracellular Mg2+ fluctuations, are critical but still missing tools for the study of cellular processes involving this metal cation. In this report, we address this deficiency and introduce a new set of highly selective, red-emitting turn-on fluorescent indicators for intracellular Mg2+. We illustrate their application in the detection of metal uptake in live cells, with no detectable interference from cellular Ca2+, and demonstrate a triethyleneglycol (TEG) functionalization tactic for enhanced intracellular retention valuable in fluorescence microscopy imaging experiments.
Results and discussion
Design and synthesis of BODIPY-based fluorescent indicators for Mg2+
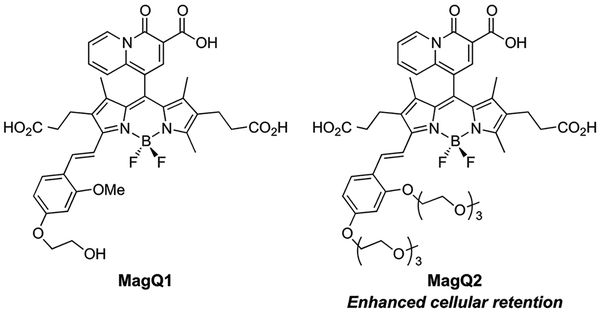
Our basic indicator design is comprised of two main components, namely a 4-oxo-4H-quinolizine-3-carboxylic acid19 serving as a Mg2+-selective recognition motif, and a red-emitting alkoxystyryl-functionalized boron-dipyrromethene (BODIPY) fluorophore that serves as reporter and translates the binding event into a fluorescence output (MagQ1, Figure 1). Fluorophores of the BODIPY family are remarkable for their brightness, photostability, and low sensitivity to pH fluctuations compared to common charged dyes such as fluorescein and rhodamine, thus have become increasingly popular in the design of sensors and tags for bioimaging applications.23–25 One other notable aspect of some BODIPY derivatives, however, is their limited water solubility and tendency to form aggregates that affect their optical properties.26–27 To ensure sufficient water solubility of our indicator and avoid potential accumulation in cellular lipophilic compartments, charged carboxypropionyl groups were incorporated onto the 2 and 6 position of the BODIPY core.
Figure 1.
Red-emitting BODIPY-based fluorescent sensors for Mg2+, MagQ1 and MagQ2
Photoinduced electron transfer (PeT) between the metal binding group and the BODIPY fluorophore is exploited here for modulation of the fluorescent output of the indicator, endowing it with metal-sensing capabilities. Specifically, Nagano and coworkers have shown that aryl substituents at the 8 position of 1,3,5,7-tetramethyl-BODIPY fluorophores have a marked influence on the fluorescence quantum yield of the dye, acting as either oxidants or reductants of the excited BODIPY and leading to quenching of the fluorescence emission.28–30 For our system, we anticipated that photoinduced reduction of the BODIPY by the quinolizine moiety would lead to formation of a non-emissive charge separated state resulting in low fluorescence output. Upon coordination of Mg2+, the driving force for PeT should decrease due to the electron-withdrawing effect of the metal ion on the Mg2+-recognition moiety, thus suppressing the quenching effect and leading to fluorescence enhancement of the indicator.31
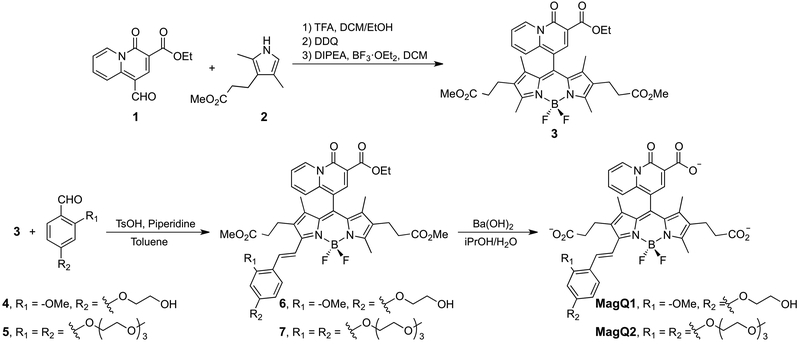
Synthesis of MagQ1 was achieved in two stages, as shown in Scheme 1. First, synthesis of BODIPY 3 was achieved via one-pot reaction starting from acid-catalyzed condensation of ethyl 1-formyl-4-oxo-4H-quinolizine-3-carboxylate19 (1) and pyrrole 232. The resulting intermediate was immediately oxidized by 1,3-dichloro-5,6-dicyano-benzoquinone (DDQ), and the fluorophore core was completed via treatment of BF3 OEt2 in the presence of Hünig’s base. The ester moieties in compound 3 can be hydrolyzed under basic conditions followed by neutralization to yield green-emitting BODIPY 3a, which displays Mg2+ sensing capabilities of its own. Nevertheless, the limited fluorescence enhancement and very low quantum yield displayed by this compound in aqueous solution (results not shown), discouraged us from pursuing it further in the context of imaging applications. During the course of our studies, Vendrell and coworkers reported a related BODIPY compound, devoid of the water-solubilizing carboxypropyl groups, and demonstrated similar Mg2+ sensing properties but only in aqueous/organic solvent mixtures.33
Scheme 1.
Synthesis of MagQ1 and MagQ2
In the second stage of the synthesis, the fluorophore core of BODIPY 3 was extended via Knoevenagel condensation with aldehyde 4, endowed with a glycol substituent to aid the water solubility of the resulting alkoxystyryl BODIPY 6. Final sensor
MagQ1 was obtained by hydrolysis of the ester substituents under basic conditions. Similar protocol was employed for the synthesis of triethylene glycol (TEG) substituted analogue MagQ2 (vide infra).
Photophysical and metal binding properties of Mg2+ indicators
Indicator MagQ1, with its styryl-functionalized BODIPY core, displays an absorption maximum at 600 nm (Figure S1) and fluorescence emission maximum at 635 nm in aqueous buffer at pH 7.0, 25 °C (Figure 2 and Table 1). The sensor is weakly fluorescent in the absence of Mg2+, with a quantum yield of Φ = 0.01. Addition of Mg2+, on the other hand, induces a significant increase of the fluorescence intensity (29-fold increase, Φ = 0.35 in the Mg2+ saturated form) with negligible spectral shift. These observations are consistent with a sensing mechanism involving modulation of PeT, which is expected to result in a fluorescence emission enhancement with little or no effect on the absorption profile. The photophysical properties of MagQ1, particularly the long wavelength excitation and emission maxima, combined with high brightness and good fluorescence enhancement for Mg2+ detection (summarized in Table 1), are very appealing for bioimaging applications in which reduced photodamage to the sample and minimal interference from autofluorescence are sought.
Figure 2.
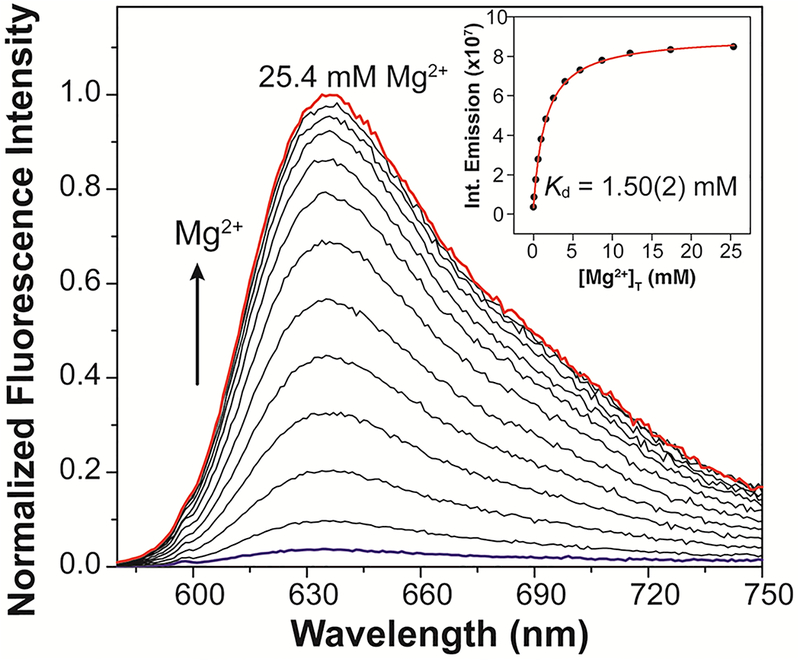
Fluorescence emission spectra of a 1.0 μM solution of MagQ1 treated with increasing concentrations of Mg2+. Titration conducted in 50 mM aqueous PIPES buffer, 100 mM KCl, pH 7.0, 25 °C. Excitation wavelength λex = 600 nm. Inset: non-linear fit (red line) of the integrated fluorescence emission (black circles) as a function of total magnesium concentration, [Mg2+]t, using a 1:1 metal-to-indicator binding model.
Table 1.
Photophysical properties of MagQ1 and MagQ2a
| Compound | λabs (nm) |
λexc (nm) |
λem (nm) |
Φb | Kd Mg2+ | Fmax/Fmin |
|---|---|---|---|---|---|---|
| MagQ1c | 600e | 600 | 635 | 0.0108(3) | ||
| MagQ1 + Mg2+d | 602 | 602 | 636 | 0.35(1) | 1.50(2) mM | 29 |
| MagQ2c | 600 | 600 | 634 | 0.0099(8) | ||
| MagQ2 + Mg2+d | 601 | 601 | 635 | 0.34(2) | 1.51(5) mM | 29 |
Data collected at a probe concentration of 1 μM in aqueous buffer (50 mM PIPES, 100 mM KCl, pH 7.0, 25 °C). Excitation wavelength λexc = 600 nm. Number in parenthesis correspond to uncertainty in the last significant figure
Relative quantum yield of fluorescence was determined using a cresyl violet solution in methanol as fluorescence
standard (Φ = 0.66).
Solution treated with 10 μM EDTA to remove adventitious metal.
Solution containing 200 mM Mg2+.
Molar absorptivity ε = 84000 ± 1000 M−1⋅cm−1.
The affinity of MagQ1 for Mg2+ was determined from non-linear fit of the integrated fluorescence emission of the indicator as a function of Mg2+ concentration (Figure 2, inset and Figure S2), using a 1:1 metal-to-indicator binding model. The Kd of the complex was found to be 1.50 ± 0.02 mM, a desirable dissociation constant to ensure optimal sensitivity for the detection of typical intracellular concentrations of this metal.
Studies by Levy and coworkers19 have revealed that the 4-oxo-4H-quinolizine-3-carboxylic acid motif, which on its own is somewhat fluorescent, is insensitive to chelatable Ca2+ even in millimolar concentrations that far exceed typical biological levels of this cation (~100 nM16). We anticipated that MagQ1 would share similar features and display good metal selectivity profile. Indeed, as shown in Figure 3A, whereas significant fluorescence enhancement is observed in the presence of a physiologically relevant concentration of Mg2+ (1 mM), almost no fluorescence turn-on is observed upon treatment with other divalent metal ions, including Ca2+ and the first row transition metal ions from Mn2+ to Zn2+ (10 μM). It should be noted that the exchangeable or chelatable pools of transition metal ions in the cellular milieu typically fall in concentration ranges that are much lower than those tested here, thus they are unlikely to induce a false fluorescence response in cellular imaging experiments. Furthermore, none of the tested metal ions, with the exception of Cu2+ in high concentrations, appears to interfere with the detection of Mg2+ in competition experiments (Figure 3B). These results indicate that MagQ1 should be suitable for detection of the target Mg2+ in the complex mixture of the cellular environment, where a variety of competing metal ions coexist.
Figure 3.
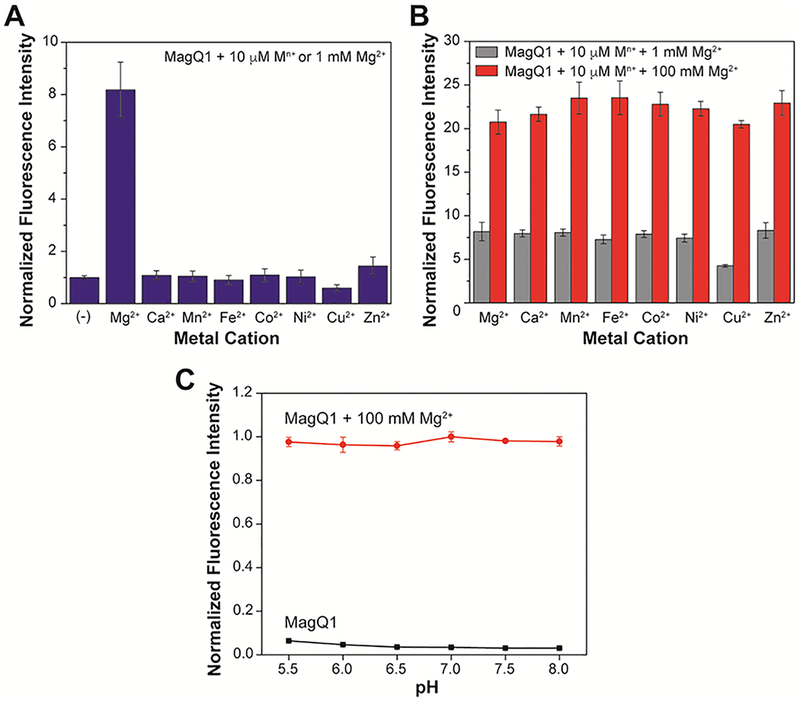
(A) Fluorescence response of 1.0 μM MagQ1 to physiological concentration of Mg2+ (1 mM) or to other divalent metal ions (10 μM) in aqueous buffer at 25 °C. (B) Fluorescence response of 1.0 μM MagQ1 to 1 mM or 100 mM of Mg2+ in the presence of competing divalent cations, showing the selectivity of the detection in 50 mM PIPES, 100 mM KCl, pH 7.0 buffer. (C) Fluorescence emission of a 1 μM solution of MagQ1 in aqueous buffer at pH ranging from 5.5 to 8.0 at 25 °C, in the absence (black squares) or presence (red circles) of 100 mM Mg2+. Excitation wavelength λex = 600 nm; emission wavelength λem = 635 nm. Error bars correspond to standard deviations on measurements conducted in triplicate.
Taking into consideration that intracellular pH varies among different subcellular compartments, ranging from ~5.0 in lysosomes34 to ~8.0 in mitochondria35, the effect of pH on the performance of MagQ1 for the detection of Mg2+ was investigated as well. As shown in Figure 3C, in the absence of Mg2+, the sensor remains weakly fluorescent over the whole pH range investigated. Treatment with the metal ion, on the other hand, leads to consistent turn on of the fluorescence emission, thus suggesting that natural pH variations among various cellular compartments will not affect the detection of Mg2+ with the new sensor.
Imaging of intracellular Mg2+ with MagQ1 in living cells
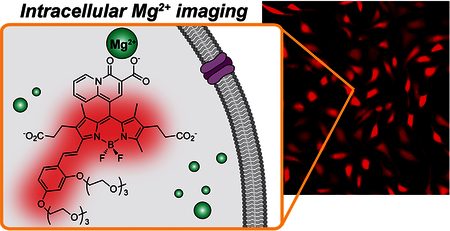
Encouraged by the optimal affinity and selectivity for Mg2+, combined with excellent tolerance to intracellular pH fluctuations and high turn-on ratio of the new sensor, we proceeded to evaluate MagQ1 for imaging Mg2+ in living cells. MagQ1 is negatively charged at pH 7 due to deprotonation of the carboxylic acid groups, thus has limited ability to diffuse across the cell membrane and enter the cells (results not shown). Hence, to enable cellular loading of the sensor, the free acid was converted into the neutral acetoxymethyl (AM) ester derivative,36 MagQ1-AM (Scheme S1), which is membrane permeable. Once internalized, intracellular esterases will cleave off the ester moieties, thus activating the pro-sensor for Mg2+ imaging. This non-invasive loading strategy has been widely used to deliver carboxylate-based fluorescent indicators into living cells, including various examples of Mg2+ indicators.8,20–21,37–40
As shown in Figure 4, MagQ1 enables imaging of endogenous Mg2+ in living HeLa cells, evidenced by the strong red fluorescence emission captured with a standard Texas Red filter set in a widefield fluorescence microscope. To verify the responsiveness of the new sensor to changes in intracellular Mg2+ levels, we artificially induced an influx of the cation into the cells by treatment with high concentration of exogenous MgCl2 and ionophore 4-bromo A23187 (Thermo Fisher Scientific). This ionophore is a non-fluorescent derivative of mobile ion-carrier A23187 (Calcimycin), which equilibrates Mg2+ and Ca2+ across the cell membrane driven by a concentration gradient of the cation.41–43 Under these cation influx conditions, the average fluorescence signal per cell originating from MagQ1 was significantly higher than that observed in control cells incubated either without the ionophore or in medium depleted of exogenous divalent ions (Figures 4B,C). These results confirm that the sensor is indeed responsive and able to report on changes in cellular Mg2+ (Figure 4C). Furthermore, the sensor is not toxic under the conditions typically employed for imaging, as demonstrated by cell viability studies conducted at concentrations up to ten-fold higher than those employed in our own imaging experiments (Figure S3).
Figure 4.
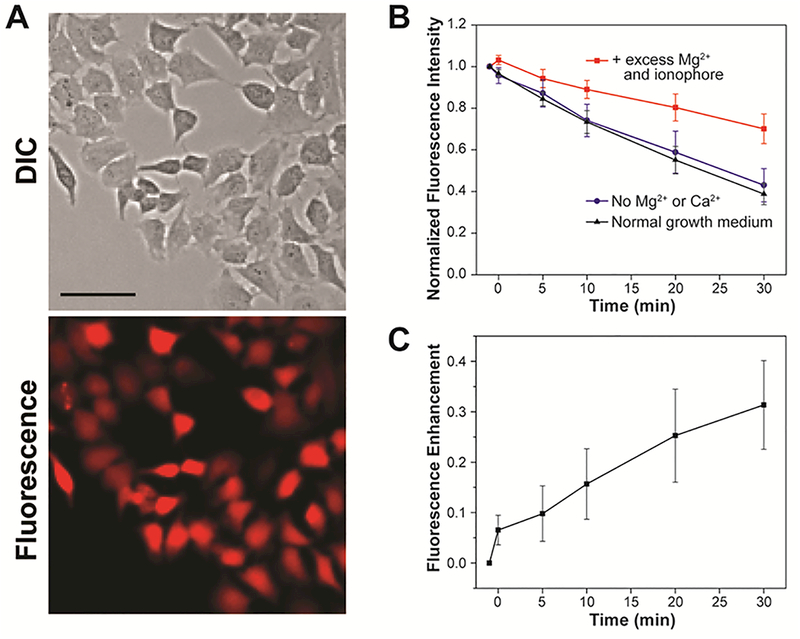
(A) Representative widefield fluorescence microscopy imaging of endogenous Mg2+ with MagQ1 in live HeLa cells. Scale bar = 50 μm. (B) Normalized average fluorescence per cell in samples imaged in normal growth medium (black triangles), in medium depleted from Ca2+ and Mg2+ (blue circles), and under supplementation with exogenous Mg2+ and ionophore (red squares). Error bars represent the SD, N = 20. (C) Net fluorescence enhancement caused by Mg2+ influx to cells treated with exogenous Mg2+ and ionophore. Error bars represent the SD, N = 20 cells.
To evaluate the potential for interference in Mg2+ detection caused by Ca2+ surges typically involved in cellular signaling, we also measured the fluorescence intensity of MagQ1 in cells treated with a high concentration of exogenous Ca2+ accompanied by the 4-bromo A23187 ionophore. The average fluorescence signal per cell obtained under these conditions showed no obvious enhancement with respect to control cells (Figure S4). These results are consistent with the metal selectivity profile displayed by the sensor in vitro, and clearly demonstrate the ability of MagQ1 to sense intracellular Mg2+ without interference from Ca2+ at elevated concentrations that are most relevant to signaling processes.
Despite the outstanding photophysical properties of MagQ1 and the encouraging results in Mg2+ detection in cellulo, an overall decrease in absolute fluorescence intensity over time was observed in all microscopy imaging experiments conducted, suggesting poor intracellular retention of the sensor under typical imaging conditions. This drawback is common for negatively charged small molecule sensors employed in cation detection,44 which are thought to be rapidly excreted through organic anion transporters (OAT, Figure 5A).45 For MagQ1, incubation at 25 °C (in the absence of a cation influx) led to loss of ~60% of the original signal over 30 min (Figures 4B and S5). Similar loss was observed upon incubation in media either supplemented with or depleted of Mg2+, indicating that the decrease in signal corresponds to dye leakage and not to a turn-off due to decrease in intracellular cation concentration. In contrast, treatment of the cells with 2.5 mM probenecid (p-[dipropylsulfamoyl]benzoic acid), an inhibitor of OAT, leads to full retention of the signal over the period of observation (Figure 5B). In combination, these results imply that the loss of signal does not correspond to photobleaching of the fluorophore but instead to its extrusion from the cell, and support the notion that the main mechanism of extrusion is some form of probenecid-sensitive active transport, and not simple diffusion.
Figure 5.
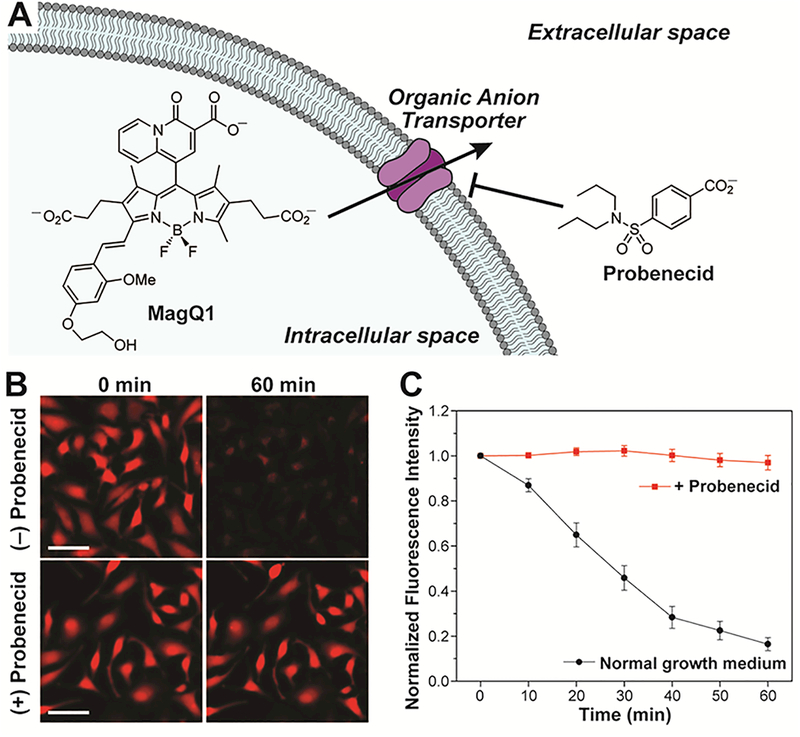
(A) Possible mechanism of cellular extrusion of anionic dyes by organic anion transporters. (B) Representative widefield microscopy images of live HeLa cells stained with MagQ1, imaged in normal growth medium (top) or medium treated with probenecid, an OAT inhibitor (bottom). Scale bar = 50 μm. (C) Normalized average fluorescence per cell in samples stained with MagQ1, imaged in normal growth medium (black squares), and medium treated with probenecid (red circles). Error bars represent the SD, N = 40.
Enhancing cellular retention: development of sensor MagQ2
To address the limited intracellular retention of MagQ1 while maintaining its attractive properties for Mg2+ sensing, we sought to investigate possible chemical modifications on the structure of the sensor that would possibly change the membrane permeability and interaction with biomolecules while imposing minimal electronic influence on either the metal-recognition moiety or the fluorophore. We concentrated on replacing the alkoxy substituents of the alkoxystyryl BODIPY and chose trethylene glycol (TEG) substituents, which would impart some of the attractive features of polyethylene glycol (PEG) conjugation seen in biomolecules and pharmaceuticals, such as reduction of interactions with (other) biomolecules,46–47 while maintaining the sensor in the small molecule regime suitable for passive cellular uptake.
TEG-functionalized analogue MagQ2 (Figure 1) was obtained by similar protocol to that developed for the synthesis of MagQ1 (Scheme 1), replacing aldehyde 4 by TEG-substituted benzaldehyde 5 in the Knoevenagel condensation step. Compared to its predecessor MagQ1, the new sensor MagQ2 inherited all the attractive photophysical properties, including low excitation energy, large turn-on ratio and high quantum yield. The compounds also display virtually identical Mg2+ binding and selectivity (Figures S6–9 and Table 1). In contrast, MagQ2 (loaded as the membrane-permeable acetoxymethyl ester derivative MagQ2-AM) displays a significantly improved cellular retention profile compared to MagQ1, with 75–80% of the average fluorescence signal maintained in living HeLa cells over 30 min of incubation in normal imaging medium (Figures 6 and S10). Such difference suggests that the TEG modification on the styryl-BODIPY core offers an effective tactic for enhancing the cellular retention of the sensor by reducing the interaction with the transporters responsible for the extrusion of organic anionic dyes. Moreover, the increased retention does not have an effect on cell viability (Figure S11), thus has an overall positive impact on the applicability of the sensor for live cell imaging purposes.
Figure 6.
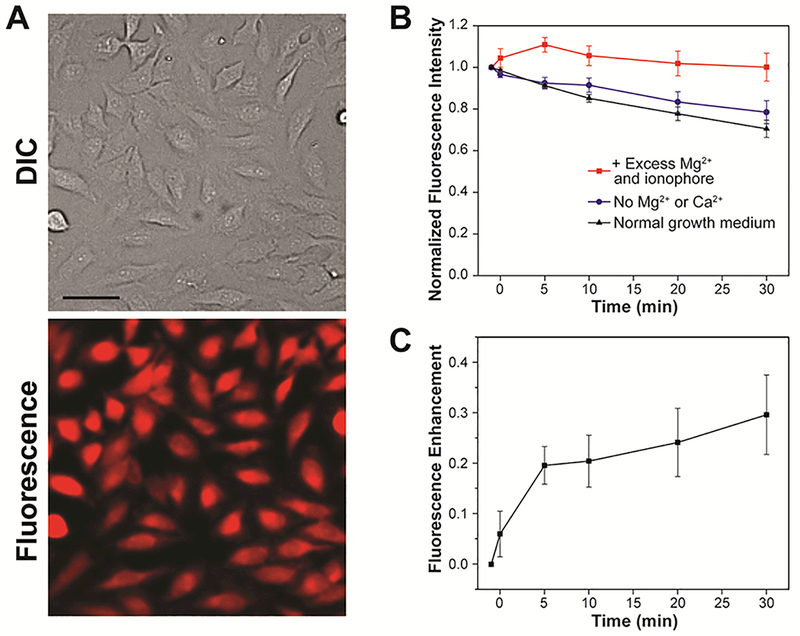
(A) Representative widefield fluorescence microscopy imaging of endogenous Mg2+ with MagQ2 in live HeLa cells. Scale bar = 50 μm. (B) Normalized average fluorescence per cell in samples imaged in normal growth medium (black triangles), in medium depleted from Ca2+ and Mg2+ (blue circles), and under supplementation with exogenous Mg2+ and ionophore (red squares). Error bars represent the SD, N = 20. (C) Net fluorescence enhancement caused by Mg2+ influx to cells treated with exogenous Mg2+ and ionophore. Error bars represent the SD, N = 20.
We then evaluated the fluorescence response of MagQ2 to intracellular changes in Mg2+ concentration in living HeLa cells by fluorescence microscopy. An influx of Mg2+ was generated by the same protocols applied for the evaluation of sensor MagQ1, treating with exogenous MgCl2 and an ionophore. As shown in Figures 6B and6C, a clear fluorescence enhancement of the sensor was captured within 5 min after the treatment, and the average fluorescence intensity per cell remained elevated compared to control samples during the 30 min period of observation. The results confirm that, like its non-TEGylated predecessor, MagQ2 is also able to report on changes in Mg2+ concentration in live cells. Yet with enhanced cellular retention, this sensor offers a new, valuable tool for fluorescence microscopy applications that may help address pressing questions in magnesium biology.
Conclusions
In summary, we have developed a new set of BODIPY-based fluorescent indicators, MagQ1 and MagQ2, for the detection of Mg2+ in live cells by fluorescence microscopy. The styryl-functionalized BODIPY core endows the new sensors with low energy excitation and emission, whereas the 4-oxo-4H-quinolizine-3-carboxylic acid metal binding moiety provides the molecules with strong fluorescence enhancement in response to Mg2+ both in vitro and in cellulo, with no interference from typical competing metal ions present in biological samples such as Ca2+ and Zn2+.
Despite their identical photophysical and metal-binding properties, MagQ1 and MagQ2 present very distinct cellular retention profiles that define their applicability in microscopy imaging experiments. Specifically, we demonstrated that functionalization of the BODIPY fluorophore with triethyleneglycol groups in MagQ2 has no significant impact on the basic photophysical and Mg2+ sensing properties of the compound compared to MagQ1, but endows it with superior retention in live cells by decreasing active extrusion that likely involves organic anion transporters. These results are significant in that they provide a first demonstration of a simple molecular functionalization strategy that alleviates a common mechanism of extrusion affecting not only many fluorescent sensors for cations, but also anionic organic dyes and drugs in general.
With low energy excitation, good Mg2+-induced fluorescence enhancement and excellent metal selectivity profile, the reported sensors offer new alternatives for the study of cellular magnesium biology by fluorescence techniques, and are particularly suited for the study of this cation in systems with elevated concentrations of competing metals such as in the context of calcium signaling.
Experimental Section
General materials and Synthetic Methods
Compounds 119, 232 and 548 and methyl 2-bromoacetate49 were synthesized using reported procedures. All other reagents were purchased from commercial sources and used as received. Solvents were purified and degassed by standard procedures. Analytical thin layer chromatography (TLC) was conducted on SorbTech polyester-backed 200 μm silica gel sheets. NMR spectra were acquired on Bruker Avance 400 and Avance III 600 MHz with triple resonance CTPCI-cryoprobehead spectrometers. 1H NMR chemical shifts are reported in ppm relative to SiMe4 (δ = 0) and were referenced internally with respect to residual protio impurity in the solvent (δ 7.26 for CDCl3, 2.05 for acetone-d6 and 2.50 for DMSO-d6).50 13C NMR chemical shifts are reported in ppm relative to SiMe4 (δ = 0) and were referenced internally with respect to the solvent signal (δ 77.16 for CDCl3, 29.84 for acetone-d6).50 19F NMR chemical shifts are reported relative to CFCl3 (δ = 0) and were referenced using C6H5CF3 in CDCl3 as external standard (δ = −63.72).51 Coupling constants are reported in Hz. High-resolution mass spectra (HRMS) were acquired on an Agilent 6224 Accurate-Mass TOF LC/MS using APCI or ESI ionization. Reverse-phased HPLC analyses were conducted on an Agilent 1260 system with UV-Vis detection, using a ZORBAX Eclipse Plus C18 reversed phase column (4.6×50 mm, 1.8 μm particle size) and a gradient of 10% to 100% acetonitrile/water (+ 0.1% trifluoroacetic acid) over 7 min. Purification of MagQ1, MagQ2, MagQ1-AM and MagQ2-AM was conducted on an Agilent 1260 system with UV-Vis detection, using a GRACE Vision HT C18 HL reverse phase column (10×150 mm, 5 μm particle size) and a gradient of 10% to 100% acetonitrile/water (+ 0.1% trifluoroacetic acid) over 22 min with a flow rate of 5 mL/min.
Synthesis of 3
A solution of 1 (0.10 g, 0.41 mmol) in a mixture of anhydrous dichloromethane (28 mL) and absolute ethanol (2 mL) was treated with 2 (0.18, 1.0 mmol) and trifluoroacetic acid (0.1 mL) under N2 atmosphere and stirred vigorously at R.T. overnight. DDQ (0.11 g, 0.49 mmol) was added to the resulting mixture and stirring was continued for 1 h. The solvent was evaporated under reduced pressure and the residue was dissolved in 30 mL of dichloromethane, treated with diisopropylethylamine (DIPEA, 1 mL), and stirred at R.T. for 30 min. BF3·OEt2 (1 mL) was then added and the solution was stirred for another 2 h. The resulting mixture was washed with water, followed by brine. All aqueous washes were combined and re-extracted with dichloromethane. The combined organic fractions were dried over Na2SO4 and evaporated under reduced pressure. The crude was purified by column chromatography (silica gel, 5:1 dichloromethane/acetone) to afford compound 3 as dark orange solid (0.17 g, 66% yield). The solid can be recrystallized from dichloromethane/hexane. 1H NMR (400 MHz, CDCl3, δ): 9.53 (d, 3J = 7.3 Hz, 1H), 8.27 (s, 1H), 7.65–7.60 (m, 2H), 7.30–7.27 (m, 1H), 4.41 (q, 3J = 7.1 Hz, 2H), 3.64 (s, 6H), 2.63–2.61 (m, 4H), 2.57 (s, 6H), 2.35–2.32 (m, 4H), 1.41 (t, 3J = 7.1 Hz, 3H), 1.35 (s, 6H). 13C{1H} NMR (150 MHz, CDCl3, δ): 173.0, 165.4, 155.8, 155.5, 144.4, 141.4, 138.7, 135.4, 134.0, 131.8, 130.3, 130.0, 123.3, 117.2, 107.5, 107.4, 61.4, 51.9, 34.3, 19.4, 14.6, 12.9, 12.5. 19F NMR (376 MHz, CDCl3, δ): −146.00 to −147.11 (AB quartet of quartets, δFA= −146.36, δFB = −146.75, 2JFF= 208.2 Hz, 1JFB= 32.1 Hz). HR-TOF-MS (m/z): [M+H]+ calcd for C33H36BF2N3O7, 636.2687; found 636.2698.
Synthesis of 4
A mixture of 4-hydroxy-2-methoxybenzaldehyde (0.30 g, 2 mmol) and K2CO3 (0.83 g, 6 mmol) was suspended in 10 mL of DMF. 2-Bromoethanol (0.21 mL, 3 mmol) was added to the suspension and the mixture was stirred at 80 °C overnight. The resulting mixture was cooled to R.T. and filtered. The filtrate was diluted with dichloromethane and washed with water, followed by brine. The organic fraction was dried over Na2SO4 and evaporated. The residue was purified by column chromatography (silica gel, 4:1 dichloromethane/ethyl acetate) to afford compound 4 as a pale pink solid (0.28 g, yield 72%). 1H NMR (600 MHz, CDCl3, δ): 10.28 (s, 1H), 7.80 (d, 3J = 8.6 Hz, 1H), 6.55 (dd, 3J = 8.6 Hz, 4J = 1.7 Hz, 1H), 6.48 (d, 4J = 2.2 Hz, 1H), 4.16 (t, 3J = 4.3 Hz, 2H), 4.01–3.98 (m, 2H), 3.89 (s, 3H), 2.15 (t, 3J = 6.1 Hz, 3H). 13C{1H} NMR (150 MHz, CDCl3, δ): 188.5, 165.3, 163.7, 130.9, 119.4, 106.3, 98.7, 69.7, 61.3, 55.8. HR-TOF-MS (m/z): [M+H]+ calcd for C10H12O4, 197.0808; found 197.0813.
Synthesis of 6
A mixture of 3 (50 mg, 79 μmol) and 4 (15 mg, 79 μmol) was suspended in toluene (30 mL) and treated with piperidine (0.5 mL). A few crystals of p-toluenesulfonic acid monohydrate were added and the suspension was refluxed overnight. The resulting mixture was cooled to R.T. and evaporated under reduced pressure. The crude was purified by column chromatography (silica gel, 2:1 to 1:1 dichloromethane/acetone) to afford 6 as a purple solid (9 mg, yield 14%). 1H NMR (600 MHz, CDCl3, δ): 9.54–9.53 (m, 1H), 8.29 (s, 1H), 7.70–7.60 (m, 5H), 7.30–7.27 (m, 1H), 6.57 (dd, 3J = 8.6 Hz, 4J = 2.3 Hz, 1H), 6.49 (d, 4J = 2.3 Hz, 1H), 4.42 (q, 3J = 7.1 Hz, 2H), 4.14 (t, 3J = 4.3 Hz, 2H), 4.00 (t, 3J = 4.6 Hz, 2H), 3.85 (s, 3H), 3.67 (s, 3H), 3.64 (s, 3H), 2.95–2.92 (s, 4H), 2.65–2.62 (m, 2H), 2.60 (s, 3H), 2.55–2.52 (m, 2H), 2.36–2.33 (m, 2H), 1.42 (t, 3J = 7.1 Hz, 3H), 1.39 (s, 3H), 1.36 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3, δ): 173.1, 173.0, 165.4, 161.1, 159.0, 155.5, 155.3, 152.6, 144.6, 141.6, 139.1, 137.9, 135.4, 133.0, 132.4, 132.2, 131.9, 130.34, 130.25, 130.1, 128.0, 123.4, 119.7, 117.3, 117.2, 107.8, 107.5, 106.2, 99.0, 69.5, 61.6, 61.3, 55.8, 51.9, 51.8, 34.3, 33.4, 20.9, 19.4, 14.6, 13.0, 12.5, 12.3. 19F NMR (376 MHz, CDCl3, δ): −142.65 to −143.68 (AB quartet of quartets, δFA = −143.02, δFB = −143.32, 2JFF= 179.9 Hz, 1JFB= 32.7 Hz). HR-TOF-MS (m/z): [M+H]+ calcd for C43H46BF2N3O10, 814.3317; found 814.3314.
Synthesis of 7
A mixture of 3 (50 mg, 79 μmol) and 5 (41 mg, 94 μmol) was suspended in toluene (25 mL) and treated with piperidine (0.5 mL). A few crystals of p-toluenesulfonic acid monohydrate were added and the suspension was refluxed overnight. The resulting mixture was cooled to R.T. and evaporated under reduced pressure. The crude was purified by column chromatography (silica gel, 2:1 to 1:1 dichloromethane/acetone) to afford 7 as a purple solid (11 mg, yield 13%). 1H NMR (600 MHz, acetone-d6, δ): 9.48–9.47 (m, 1H), 8.27 (s, 1H), 7.97–7.94 (m, 1H), 7.76–7.67 (m, 3H), 7.64–7.62 (m, 1H), 7.56–7.53 (m, 1H), 6.72–6.70 (m, 2H), 4.31 (q, 3J = 7.1 Hz, 2H), 4.26 (t, 3J = 4.7 Hz, 2H), 4.23 (t, 3J = 4.7 Hz, 2H), 3.92 (t, 3J = 5.0 Hz, 2H), 3.86–3.84 (m, 2H), 3.69–3.66 (m, 4H), 3.62–3.57 (m, 12H), 3.54–3.53 (m, 2H), 3.49–3.47 (m, 2H), 3.42–3.41 (m, 2H), 3.29 (s, 3H), 3.23 (s, 3H), 2.98 (t, 3J = 7.9 Hz, 2H), 2.69–2.67 (m, 2H), 2.62–2.59 (m, 5H), 2.42–2.39 (m, 2H), 1.51 (s, 3H), 1.48 (s, 3H), 1.33 (t, 3J = 7.1 Hz, 3H). 13C{1H} NMR (150 MHz, acetone-d6, δ): 173.3, 173.2, 165.7, 162.5, 159.3, 156.2, 155.3, 152.6, 145.7, 141.9, 140.1, 139.1, 137.5, 134.1, 133.7, 133.3, 132.3, 131.3, 130.9, 130.8, 128.3, 124.0, 120.0, 118.5, 118.1, 108.1, 107.3, 107.2, 100.7, 72.7, 72.6, 71.5, 71.34, 71.28, 71.23, 71.11, 71.08, 70.3, 70.2, 69.0, 68.7, 61.0, 58.80, 58.76, 51.8, 51.7, 34.5, 33.9, 21.4, 19.8, 14.7, 13.1, 12.7, 12.4. 19F NMR (376 MHz, acetone-d6, δ): −141.23 to −141.49 (q, 1JFB = 32.8 Hz). HR-TOF-MS (m/z): [M+H]+ calcd for C54H68BF2N3O15, 1048.4784; found 1048.4803.
Synthesis of MagQ1
A sample of 6 (8 mg, 9.8 μmol) was suspended in a mixture of isopropanol (0.5 mL) and water (0.5 mL). The suspension was treated with 0.5 mL of saturated aqueous Ba(OH)2 solution and stirred vigorously at R.T. The reaction was monitored to completion by HPLC (~8 h). The resulting mixture was cooled in an ice bath and acidified by addition of 1 M HCl solution. The precipitate was collected via filtration and purified by preparative reversed phase HPLC to afford the product as purple solid (1.6 mg, 22% yield). 1H NMR (600 MHz, DMSO-d6, δ): 9.48 (d, 3J = 7.2 Hz, 1H), 8.26 (s, 1H), 8.09–8.06 (m, 1H), 7.76–7.71 (m, 2H), 7.54–7.52 (m, 3H), 6.69 (dd, 3J = 8.6 Hz, 4J = 2.4 Hz, 1H), 6.63 (d, 4J = 2.4 Hz, 1H), 4.07 (t, 3J = 4.9 Hz, 2H), 3.84 (s, 3H), 3.74 (t, 3J = 4.7 Hz, 2H), 2.80–2.77 (m, 2H), 2.53–2.52 (m, 5H), 2.43–2.38 (m, 2H), 2.29–2.26 (m, 2H), 1.31 (s, 3H), 1.28 (s, 3H). HR-TOF-MS (m/z): [M-H]+ calcd for C39H38BF2N3O10, 756.2546; found 756.2547.
Synthesis of MagQ2
A sample of 7 (4 mg, 3.8 μmol) was dissolved in a mixture of isopropanol (0.2 mL) and water (0.2 mL). The solution was treated with 0.2 mL of saturated aqueous Ba(OH)2 solution and stirred vigorously at R.T. The reaction was monitored to completion by HPLC (~3 h). The resulting mixture cooled in an ice bath, neutralized by addition of 1 M HCl solution, and purified by preparative reversed phase HPLC to afford the product as purple solid (1.0 mg, 26% yield).1H NMR (600 MHz, DMSO-d6, δ): 9.47 (d, 3J = 6.6 Hz, 1H), 8.26 (s, 1H), 8.05 (br, 1H), 7.73–7.69 (m, 2H), 7.59–7.51 (m, 3H), 6.70–6.68 (m, 2H), 4.19–4.17 (m, 4H), 3.81 (t, 3J = 4.8 Hz, 2H), 3.77 (t, 3J = 4.6 Hz, 2H), 3.61–3.58 (m, 4H), 3.56–3.50 (m, 6H), 3.48–3.46 (m, 2H), 3.44–3.43 (m, 2H), 3.37–3.35 (m, 2H), 3.24 (s, 3H), 3.17 (s, 3H), 2.80 (t, 3J = 7.7 Hz, 2H), 2.53–2.52 (m, 5H), 2.42–2.38 (m, 2H), 2.28–2.25 (m, 2H), 1.31 (s, 3H), 1.28 (s, 3H). HR-TOF-MS (m/z): [M-H]- calcd for C50H60BF2N3O15, 990.4013; found 990.3998.
General procedure for the preparation of MagQ1-AM and MagQ2-AM
Following completion of the hydrolysis of compound 6 or 7, as described in the protocol for the preparation MagQ1 and MagQ2, respectively; the reaction was neutralized by 1 M HCl aqueous solution and then lyophilized. The residue was suspended in a mixture of DMF/Acetonitrile (1:2, v/v, 120 μL/mg of 6 or 7) and treated with K2CO3 powder (10 equiv), followed by addition of methyl 2-bromoacetate (15 equiv). The mixture was stirred vigorously at R.T. for 1 h, after which the reaction was complete by HPLC analysis. The resulting suspension was filtered and the filtrate was purified by preparative HPLC to afford the desired product MagQ1-AM (11% yield in two steps) or MagQ2-AM (14% yield in two steps). The product was dissolved in acetonitrile (concentration of the solutions was quantified using the molar absorptivity of compound 6 at 600 nm, ε600 = 62900 ± 900 M−1⋅cm−1, measured in the same solvent at 25 °C) and the resulting solution was divided into aliquots of 20 nmol and evaporated in a centrifugal evaporator. The solid aliquots were stored at −20 °C until use.
MagQ1-AM: HR-TOF-MS (m/z): [M+H]+ calcd for C48H50BF2N3O16, 974.3325; found 974.3339.
MagQ2-AM: HR-TOF-MS (m/z): [M+H]+ calcd for C59H72BF2N3O21, 1208.4792; found 1208.4793.
General Spectroscopic Methods
All aqueous solutions were prepared using de-ionized water having a resistivity of 18 MΩ.cm. Other solvents were supplied by commercial vendors and used as received. Organic buffers, high purity metal chlorides, high-purity 25% HCl, and high purity 45% KOH were purchased from Sigma Aldrich. Quantitative solutions of the sensors in their acid form were stored at −20 °C in 50 μL aliquots in DMSO, and thawed immediately before each experiment. Measurements at pH 7.0 were conducted in aqueous buffer containing 50 mM PIPES and 100 mM KCl. Buffers were treated with Chelex resin (Bio-Rad) according to the manufacturer’s protocol to remove adventitious metal ions unless otherwise noted. Measurements of pH were conducted using a Mettler Toledo FE20 with glass electrode. UV-visible absorption spectra were acquired on a Cary 100 spectrophotometer using quartz cuvettes (1 cm path length). Fluorescence spectra were acquired on a QuantaMaster 40 Photon Technology International spectrofluorometer equipped with xenon lamp source, emission and excitation monochromators, excitation correction unit, and PMT detector. Emission spectra were corrected for the detector wavelength-dependent response. The excitation spectra were corrected for the wavelength-dependent lamp intensity. Measurements were conducted at 25.0 ± 0.1 °C. Fluorescence quantum yields were determined using 0.1–1.0 μM solutions of the sensor in aqueous buffer at pH 7.0, exciting at the reported excitation maxima. Solutions of cresyl violet in methanol, with a reported quantum yield of 0.66 upon excitation at 610 nm52 were used as standards. Fluorescence emission spectra were integrated from 580 to 750 nm. Data for metal selectivity studies were collected on a FlexStation 3 Multi-Mode Microplate Reader from Molecular Devices. The sensors were excited at 600 nm and the fluorescence intensity was recorded at 635 nm.
Determination of metal dissociation constants
Fluorescence titrations with Mg2+ were conducted using 1 μM solutions of the sensor in aqueous buffer at pH 7.0. Magnesium was added from a 200 mM MgCl2 stock solution, to cover a range of 0–30 mM [Mg2+]t in the cuvette. Excess metal was employed to confirm the saturation value. Changes in fluorescence intensity were observed immediately upon mixing and were stable over time. For each titration, the metal stock solution was treated with an appropriate amount of the sensor to match the concentration in the cuvette and prevent sensor dilution throughout the experiment. Apparent Kd values for Mg2+ dissociation were obtained from non-linear fitting using equation S1, where Fmin is the integrated fluorescence emission of the sensor in the absence of metal and Fmax is the integrated fluorescence emission of the sensor at metal saturation. The approximation [Mg2+] ≈ [Mg2+]t was made.
Metal selectivity studies
Metal selectivity studies were performed using 1 μM solution of sensor MagQ1 or MagQ2 in aqueous buffer at pH 7.0, treated with either CaCl2, MnCl2, Fe(SO4)2, CoCl2, NiCl2, CuCl2, or ZnCl2 in aqueous buffer for a final concentration of 10 μM Mn+, or 10 μM Mn+ and 1 mM MgCl2, or 10 μM Mn+ and 100 mM MgCl2. Fluorescence emission of metal-free sensors was determined with solutions of MagQ1 or MagQ2 treated with 10 μM EDTA to sequester adventitious metal ions. All measurements were conducted in triplicate.
General cell culture and imaging protocols
HeLa cells cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37 °C in a 5% CO2 humidified atmosphere. Cells were seeded in 35 mm glass bottom cell culture dishes (MatTek) and allowed to grow to 50–70% confluence prior to fluorescence imaging. Fluorescence imaging experiments were performed on a Leica DMI6000B inverted fluorescence microscope equipped with a Hamamatsu ORCA-Flash 4.0 CCD camera, scanning stage, high- speed filter wheel for excitation filters and a mercury metal halide external light source. A Leica Texas Red filter cube was employed for the imaging. The microscope was operated with Leica LAS AF software. Image processing for fluorescence intensity determination was performed with Metamorph 7.7.0.0 software. Briefly, images were background subtracted and a threshold was applied to define cell boundaries. Results are presented as normalized average fluorescence intensities per cell. Error bars correspond to standard deviation (SD) on the normalized average fluorescence per cell, with N = total number of cells included in the analysis. All images were processed by the same protocol and are shown in the same scale. At least two biological replicas were conducted for each experiment.
Sensor loading and fluorescence imaging
Live HeLa cells were bathed in DMEM containing 1 μM of MagQ1-AM or MagQ2-AM in 0.01% Pluronic F-127 at R.T. for 30 min. The medium was then removed and the cells were rinsed with 2 mL of DMEM, followed by incubation in fresh DMEM at R.T. for 1 h to allow deesterification of the AM esters. The medium was then removed and cells were washed twice with HHBSS (20 mM HEPES-buffered HBSS containing 0.8 mM MgCl2, 1.8 mM CaCl2 and 2 g/L glucose) and bathed in 1 mL HHBSS for fluorescence imaging. After an initial image was collected, an additional 1 mL of HHBSS was added to the plate on the microscope stage and fluorescence images were captured over a period of 30 min.
To rule out the effect of dye leakage vs. possible signal loss due to metal depletion, a second set of experiments was performed as described above, using HHBSS without calcium and magnesium for the final washing and imaging.
Inhibition of extrusion by active transport
Live HeLa cells were bathed in DMEM containing 1 μM of MagQ1-AM or MagQ2-AM, 0.01% Pluronic F-127, and 2.5 mM probenecid at R.T. for 30 min. The medium was then removed and the cells were rinsed with 2 mL of DMEM, followed by incubation in fresh DMEM containing 2.5 mM probenecid at R.T. for 1 h to allow deesterification of the AM esters. The medium was then removed and cells were washed twice with HHBSS (20 mM HEPES-buffered HBSS containing 0.8 mM MgCl2, 1.8 mM CaCl2 and 2 g/L glucose) and bathed in 2 mL HHBSS with 2.5 mM probenecid for fluorescence imaging over a period of 1 h. Control experiments were performed using similar protocol, using media without probenecid for sensor loading and fluorescence imaging, as described in the general sensor loading and fluorescence imaging section.
Response to Mg2+ or Ca2+ influx
To verify that the sensors are responsive to Mg2+ influx, cells were loaded with 1 μM of MagQ1-AM or MagQ2-AM as described in the general sensor loading protocol, followed by washing and initial imaging in HHBSS without Ca2+ and Mg2+. Subsequently, 1 mL of HHBSS solution containing 10 μM of 4-bromo A23187 ionophore and 30 mM MgCl2 was added to the plate while on the microscope stage, and additional fluorescence images were captured over a period of 30 min.
A similar experiment was performed using 3 mM CaCl2, instead of the 30 mM MgCl2, to induce a cellular influx of Ca2+ and study the possible interference of this ion in the detection of endogenous Mg2+.
Cell viability studies
CellTiter-Glo® Luminescent Cell Viability Assay (Promega) was performed to assess the cytotoxicity of MagQ1 and MagQ2.
HeLa cells plated in a white opaque 96-well plate (20,000 cells per well) were bathed in DMEM (100 μL) containing various concentrations of MagQ1-AM or MagQ2-AM (1, 5, or 10 μM) with 0.01% Pluronic F-127 at R.T. for 30 min. The medium was then removed and the cells were incubated in fresh DMEM (100 μL) at R.T. for 1 h to allow deesterification of the AM esters, as described in the general cell loading procedure above. Cells were washed and treated with CellTiter-Glo® Reagent (Promega, 100 μL) according to the manufacturer’s protocol. Control experiments were performed in the same fashion, using DMSO as vehicle. Luminescence was read using a FlexStation 3 Multi-Mode Microplate Reader from Molecular Devices. Results shown are averages from eight replicas.
Supplementary Material
Acknowledgements
The authors thank the National Institutes of Health (R01CA217817) for financial support. The Bruker Avance 400 NMR spectrometer was acquired with support of the National Science Foundation under award number CHE-01162222. The CPTCI-cryoprobehead was acquired through the support of the National Institute of Health S10 grant underaward number OD016343. The authors wish to thank Jessica J. Gruskos for valuable discussions.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic Supplementary Information (ESI) available: supporting figures and schemes, additional experimental protocols and characterization data for new compounds. See DOI:10.1039/x0xx00000x
References
- 1.Cowan JA, BioMetals, 2002, 15, 225. [DOI] [PubMed] [Google Scholar]
- 2.White RE and Hartzell HC, Biochem. Pharmacol, 1989, 38, 859. [DOI] [PubMed] [Google Scholar]
- 3.Romani AM, Arch. Biochem. Biophys, 2011, 512, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Baaij JHF, Hoenderop JGJ and Bindels RJM, Physiol. Rev, 2014, 95, 1. [DOI] [PubMed] [Google Scholar]
- 5.Hartwig A, Mutat. Res, 2001, 475, 113. [DOI] [PubMed] [Google Scholar]
- 6.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC and Lenardo MJ, Nature, 2011, 475, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaigne-Delalande B, Li FY, O’Connor GM, Lukacs MJ, Jiang P, Zheng L, Shatzer A, Biancalana M, Pittaluga S, Matthews HF, Jancel TJ, Bleesing JJ, Marsh RA, Kuijpers TW, Nichols KE, Lucas CL, Nagpal S, Mehmet H, Su HC, Cohen JI, Uzel G and Lenardo MJ, Science, 2013, 341, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raju B, Murphy E, Levy LA, Hall RD and London RE, Am. J. Physiol, 1989, 256, C540. [DOI] [PubMed] [Google Scholar]
- 9.Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies, Life Technologies Corporation, 2010. [Google Scholar]
- 10.Brady M, Piombo SD, Hu C and Buccella D, Dalton Trans, 2016, 45, 12458. [DOI] [PubMed] [Google Scholar]
- 11.Brady M, Piombo SD, Hu C and Buccella D, Dalton Trans, 2016, 45, 12458. [DOI] [PubMed] [Google Scholar]
- 12.Basaric N, Baruah M, Qin W, Metten B, Smet M, Dehaen W and Boens N, Org. Biomol. Chem, 2005, 3, 2755. [DOI] [PubMed] [Google Scholar]
- 13.Simons TJB, J. Biochem. Bioph. Methods, 1993, 27, 25. [DOI] [PubMed] [Google Scholar]
- 14.Sensi SL, Canzoniero LMT, Yu SP, Ying HS, Koh J-Y, Kerchner GA and Choi DW, J. Neurosci, 1997, 17, 9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng C and Reynolds IJ, J. Neurochem, 1998, 71, 2401. [DOI] [PubMed] [Google Scholar]
- 16.Clapham DE, Cell, 2007, 131, 1047. [DOI] [PubMed] [Google Scholar]
- 17.Farruggia G, Iotti S, Prodi L, Montalti M, Zaccheroni N, Savage PB, Trapani V, Sale P and Wolf FI, J. Am. Chem. Soc, 2006, 128, 344. [DOI] [PubMed] [Google Scholar]
- 18.Farruggia G, Iotti S, Prodi L, Zaccheroni N, Montalti M, Savage P, Andreani G, Trapani V and Wolf F, Journal of Fluorescence, 2009, 19, 11. [DOI] [PubMed] [Google Scholar]
- 19.Otten PA, London RE and Levy LA, Bioconj. Chem, 2001, 12, 203. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu H, Iwasawa N, Citterio D, Suzuki Y, Kubota T, Tokuno K, Kitamura Y, Oka K and Suzuki K, J. Am. Chem. Soc, 2004, 126, 16353. [DOI] [PubMed] [Google Scholar]
- 21.Shindo Y, Fujii T, Komatsu H, Citterio D, Hotta K, Suzuki K and Oka K, PLoS One, 2011, 6, e23684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsui Y, Sadhu KK, Mizukami S and Kikuchi K, Chem. Commun, 2017, 53, 10644. [DOI] [PubMed] [Google Scholar]
- 23.Loudet A and Burgess K, Chem. Rev, 2007, 107, 4891. [DOI] [PubMed] [Google Scholar]
- 24.Boens N, Leen V and Dehaen W, Chem. Soc. Rev, 2012, 41, 1130. [DOI] [PubMed] [Google Scholar]
- 25.Hinkeldey B, Schmitt A and Jung G, ChemPhysChem, 2008, 9, 2019. [DOI] [PubMed] [Google Scholar]
- 26.Bergström F, Mikhalyov I, Hägglöf P, Wortmann R, Ny T and Johansson LBÅ, J. Am. Chem. Soc, 2002, 124, 196. [DOI] [PubMed] [Google Scholar]
- 27.Mikhalyov I, Gretskaya N, Bergström F and Johansson LBÅ, PCCP, 2002, 4, 5663. [Google Scholar]
- 28.Gabe Y, Urano Y, Kikuchi K, Kojima H and Nagano T, J. Am. Chem. Soc, 2004, 126, 3357. [DOI] [PubMed] [Google Scholar]
- 29.Ueno T, Urano Y, Kojima H and Nagano T, J. Am. Chem. Soc, 2006, 128, 10640. [DOI] [PubMed] [Google Scholar]
- 30.Sunahara H, Urano Y, Kojima H and Nagano T, J. Am. Chem. Soc, 2007, 129, 5597. [DOI] [PubMed] [Google Scholar]
- 31.Lin Q, Gruskos JJ and Buccella D, Org. Biomol. Chem, 2016, 14, 11381. [DOI] [PubMed] [Google Scholar]
- 32.Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M, Nagano T, Watanabe T, Hasegawa A, Choyke PL and Kobayashi H, Nat. Med, 2009, 15, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treadwell R, de Moliner F, Subiros-Funosas R, Hurd T, Knox K and Vendrell M, Org. Biomol. Chem, 2018, 16, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mindell JA, Annu. Rev. Physiol, 2012, 74, 69. [DOI] [PubMed] [Google Scholar]
- 35.Llopis J, McCaffery JM, Miyawaki A, Farquhar MG and Tsien RY, Proc. Natl. Acad. Sci. USA, 1998, 95, 6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsien RY, Nature, 1981, 290, 527. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y, Komatsu H, Ikeda T, Saito N, Araki S, Citterio D, Hisamoto H, Kitamura Y, Kubota T, Nakagawa J, Oka K and Suzuki K, Anal. Chem, 2002, 74, 1423. [DOI] [PubMed] [Google Scholar]
- 38.Afzal MS, Pitteloud J-P and Buccella D, Chem. Commun, 2014, 50, 11358. [DOI] [PubMed] [Google Scholar]
- 39.Zhang G, Gruskos JJ, Afzal MS and Buccella D, Chem. Sci, 2015, 6, 6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruskos JJ, Zhang G and Buccella D, J. Am. Chem. Soc, 2016, 138, 14639. [DOI] [PubMed] [Google Scholar]
- 41.Reed PW and Lardy HA, J. Biol. Chem, 1972, 247, 6970. [PubMed] [Google Scholar]
- 42.Abbott BJ, Fukuda DS, Dorman DE, Occolowitz JL, Debono M and Farhner L, Antimicrob. Agents Chemother, 1979, 16, 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deber CM, Tom-Kun J, Mack E and Grinstein S, Anal. Biochem, 1985, 146, 349. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi A, Camacho P, Lechleiter JD and Herman B, Physiol. Rev, 1999, 79, 1089. [DOI] [PubMed] [Google Scholar]
- 45.Divirgilio F, Steinberg TH, Swanson JA and Silverstein SC, J. Immunol, 1988, 140, 915. [PubMed] [Google Scholar]
- 46.Harris JM and Chess RB, Nat. Rev. Drug Discov, 2003, 2, 214. [DOI] [PubMed] [Google Scholar]
- 47.Roberts MJ, Bentley MD and Harris JM, Adv. Drug Del. Rev, 2012, 64, 116. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen CB, Johnsen M, Arnbjerg J, Pittelkow M, McIlroy SP, Ogilby PR and Jorgensen M, J. Org. Chem, 2005, 70, 7065. [DOI] [PubMed] [Google Scholar]
- 49.Grynkiewicz G, Poenie M and Tsien RY, J. Biol. Chem, 1985, 260, 3440. [PubMed] [Google Scholar]
- 50.Fulmer GR, Miller AJM, Sherden NH, Gottlieb HE, Nudelman A, Stoltz BM, Bercaw JE and Goldberg KI, Organometallics, 2010, 29, 2176. [Google Scholar]
- 51.Dungan CH and Wazer JRV, Compilation of Reported F19 NMR Chemical Shifts, 1951 to Mid-1967, Wiley-Interscience, 1970. [Google Scholar]
- 52.Shen J and Snook RD, Chem. Phys. Lett, 1989, 155, 583. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.