Abstract
Endocrine-disrupting chemicals (EDCs) can act upon a developing organism to change its endocrine health and behavior in adulthood. Beyond actions on the exposed individuals, transgenerational effects of several EDCs have been reported. This study assessed the combinatorial impact of EDC-altered maternal care and transgenerational inheritance on F3 male and female offspring. Pregnant rats were exposed to EDCs with different modes of action: the weakly estrogenic polychlorinated biphenyl (PCB) mixture Aroclor 1221, the anti-androgenic fungicide vinclozolin (VIN), or the vehicle (6% dimethylsulfoxide in sesame oil; VEH) during embryonic development. The F1 male and female offspring were bred through the paternal- or maternal-lineage with untreated partners to generate F2 offspring. This process was repeated through both maternal and paternal lineages to create the F3 generation. Maternal care of F2 dams toward their F3 offspring was altered in a lineage-dependent manner, particularly in PCB paternal-lineage animals. When F3 pups were recorded for ultrasonic vocalizations (USVs) following separation from the mother, the rate of neonatal USVs in F3 offspring were decreased in PCB paternal-lineage pups. In adulthood, anxiety-like behaviors of the F3 rats were tested, with only small effects of EDCs detected. These interactions of maternal behaviors and EDC effects across generations, especially via the paternal lineage, has implications for health and environmental responses in wildlife and humans.
Keywords: Endocrine disrupting chemicals (EDCs), transgenerational, Polychlorinated biphenyl (PCB), Aroclor 1221, vinclozolin, maternal behavior, ultrasonic vocalization, anxiety
1. Introduction
Adverse early life experiences often dictate an individual’s susceptibility to future stressors and disease in adulthood, and can increase propensity for behavioral and psychiatric disorders such as depression, anxiety, and schizophrenia (Shonkoff et al., 2009). A key player in this experience is parental care. For example, in humans, the detrimental effects of childhood poverty, among others, can be mitigated with appropriate parental care (Conger et al., 1994). In mammals, variations in maternal care in particular are associated with differences in offspring health outcomes (Champagne et al., 2003; Meaney, 2001), and can persistently program the function of the hypothalamic-pituitary-adrenal axis in response to stress (Macrì et al., 2008; Plotsky et al., 2005). Offspring of high-care rodent mothers have lower levels of anxiety-like responses (Champagne and Meaney, 2006), and high-care mothers beget high-care mothering in the next generation (Champagne and Meaney, 2001). Therefore, maternal behavior can serve as a nongenomic mode of transmission of traits and effects from one generation to the next (Francis et al., 1999).
The neuroendocrine regulation of maternal care involves the appropriate hormonal milieu, including estrogen and progesterone signaling (Numan and Sheehan, 1997). These signaling systems are established during perinatal development and are susceptible to endogenous or exogenous perturbation. Endocrine-disrupting chemicals (EDCs) are one such perturbation: exposures during critical periods early in life can affect neurodevelopment and interfere with hormone-sensitive behaviors (Gore et al., 2018). EDCs are ubiquitous in our environment and include legacy chemicals such as polychlorinated biphenyls (PCBs) that were banned decades ago in most countries, but persist in the environment due to their resistance to degradation. We are also exposed to contemporary-use EDCs including vinclozolin (VIN), a commercially available fungicide commonly used in viniculture (Schneider et al., 2011), as well as many other compounds such as bisphenols, phthalates, flame retardants, and pesticides.
Exposure of a dam during pregnancy (F0 generation) to EDCs and other environmental factors directly affects her children (F1 – exposed as the fetus) and grandchildren (F2 – exposed as developing germ cells within the F1 fetus). Beginning with the F3 generation, any effects of EDC inheritance must be imparted through context- or germline-dependent epigenetic modifications (Anway and Skinner, 2006; Crews et al., 2012; McCarrey, 2012; Wolstenholme et al., 2012; Xin et al., 2015). Multigenerational effects observed to date in the F3 generation include physiological, morphological, hormonal, and behavioral alterations (Anway and Skinner, 2008; Berger et al., 2016; Crews et al., 2007; Gillette et al., 2014; Kirchner et al., 2010; Steinberg et al., 2008; Xin et al., 2015). Our lab recently showed that Aroclor 1221 (A1221), a weakly estrogenic PCB mixture, had transgenerational physiological effects that were propagated in a maternal or paternal lineage-specific manner (Mennigen et al., 2018). Extensive work on VIN demonstrated its transgenerational inheritance of disruptions in behavior, physiology, and gene expression and DNA methylation in the brain (Anway and Skinner, 2008; Crews et al., 2007; Gillette et al., 2014; Gillette et al., 2018). These EDCs, in long use in our laboratory, were selected because they interfere with gonadal-hormone mediated effects on developmental brain sexual differentiation.
Few studies have investigated how EDCs interact with other environmental factors and contextual life stressors (Crews et al., 2014; Gillette et al., 2014; Johnson et al., 2015; Kundakovic et al., 2013; Rosenfeld, 2015). Here, we investigated the combinatorial effects of maternal behavior and EDC inheritance on the F3 generation female and male offspring during development and in adulthood. We further hypothesized that each EDC from different classes would lead to unique behavioral trajectories.
2. Methods
2.1. EDC Treatment
The PCB mixture Aroclor 1221 (AccuStandard, New Haven, CT, C-221N-50MG, 083-166) and vinclozolin (VIN; Chem Service Inc., West Chester, PA N-13745-250MG, 4054200) both at 1 mg/kg, were dissolved in vehicle (VEH; 6% dimethylsulfoxide, Sigma #D4540; Sigma, St Louis, Missouri) in sesame oil. Each dam received a daily intraperitoneal injection of the same compound from embryonic day (E) 8 to 18. This spans the period of germline demethylation and remethylation (Smallwood and Kelsey, 2012), as well as the beginning of the period of brain sexual differentiation (Lenz et al., 2012). Previous research has shown that these dosages of EDCs are relevant to circulating levels in humans and at or below the acceptable daily intake for human health (Alyea et al., 2014; Dickerson et al., 2011; Fitzgerald et al., 2008; Steinberg et al., 2007). The dosages were also selected to match previous work (Gillette et al., 2018; Krishnan et al., 2018).
2.2. Animal Husbandry & Physiological Measures
All animal protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) at The University of Texas at Austin. Sprague-Dawley rats were purchased from Harlan Laboratories (Houston, Texas & Indianapolis, Indiana), housed in a temperature- and humidity-controlled room with a 10:14 partially reversed light cycle (lights off at 11:30 a.m.) and maintained at 21-23°C. Animals were housed together (2-3 per cage, same sex) in polycarbonate cages (43 × 21 × 25 cm) with bedding and a PVC tube for enrichment. All rats were fed ad libitum with a low phytoestrogen diet (Harlan-Teklad, Indianapolis, Indiana).
In adulthood (3-4 months old), females were bred with adult male rats (~6 months old). Vaginal smears were checked on the morning after mating, and sperm-positive dams were singly housed. The day of mating was designated as embryonic day 0 (E0). Dams were weighed daily from E8 – E18 and injected i.p. with ~0.1 mL of VEH, PCB or VIN depending on their weight, ~2 hours prior to the onset of the dark cycle. Several days prior to their expected date of parturition, dams were provided with nesting material. On postnatal day 1 (P1), the day after birth, F1 litters were checked and culled to 10 pups of equal sex ratio by removing those with extreme (longest/shortest) anogenital index measures (Gillette et al., 2017). After weaning on P21, individuals were housed with same-sex littermates. In adulthood (~P80), at least two F1 males and two F1 females from each litter were bred with untreated stimulus animals (purchased from Harlan) using the same protocol (Figure 1a) to form the paternal and maternal lineages of the F2 generation. Adult paternal males and maternal females from the F2 generation were bred with untreated mates to produce the F3 generation. The primary focus of this experiment was the maternal behavior of the F2 females towards their F3 generational offspring, and F3 physiological and behavioral outcomes (Figure 1b).
Fig. 1.
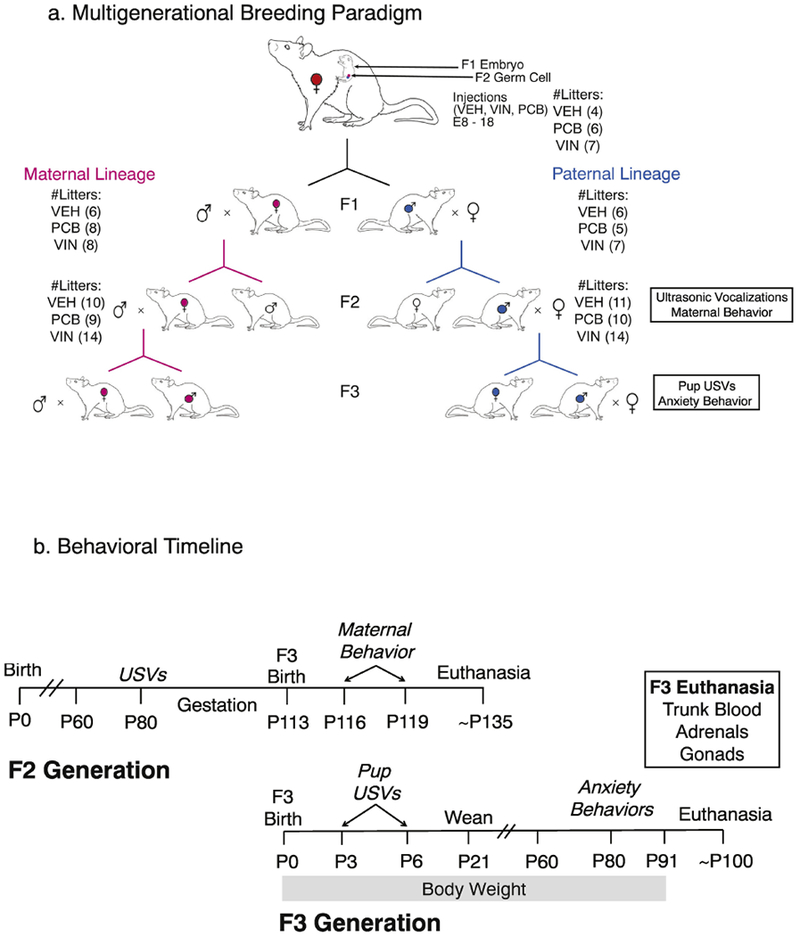
Breeding paradigm (a) and experimental timeline (b). Pregnant dams were exposed to vehicle (VEH), Aroclor 1221 (PCB) or vinclozolin (VIN) from E8-18. This exposures the F1 generation as fetuses, and the F2 generation as germ cells, with effects on the F3 individuals due to epigenetic (molecular or context-dependent) inheritance. (b) The experimental timeline for F2 mating and maternal behavior, and F3 developmental and behavioral outcomes, is shown.
The body weight and anogenital distance of each F3 individual was measured at birth and then weekly thereafter until P21. F3 pups were then weaned and re-housed (2-3 per cage) with same-sex littermates. To determine age of puberty, females were checked daily for vaginal opening beginning on P28, and males were checked for preputial separation beginning on P35. The individuals in each litter continued to be weighed weekly until P100. Behaviors conducted on these rats, and euthanasia, are described below.
2.3. Litter usage and sample size
The number of dams and litters is shown in Figure 1a. For birth outcomes and developmental milestones of the F3 generation, we used data from all five animals per sex per litter when available (Maternal females: VEH = 27, PCB = 20, VIN = 39; Maternal males: VEH = 35, PCB = 36, VIN = 63; Paternal females: VEH = 25, PCB = 20, VIN = 45; Paternal males: VEH = 43, PCB = 42, VIN = 68). For primary endpoints (behavior, physiology) of F3 animals, 2-3 males and females per litter were used. In all cases, we used litter as a covariate and determined that there were no litter effects in any of the physiological or behavioral endpoints measured.
2.4. Serum Hormones of the F3 rats
At euthanasia in adulthood, trunk blood was collected from F3 rats and centrifuged at 3000×g for 5 minutes. Serum was collected and stored at −80°C until analysis. Corticosterone was selected as a primary target based on its role as a stress hormone (Gillette et al., 2014), and estradiol (E2) was selected based on previous work highlighting its disruption by EDCs (Bell et al., 2016). Standards and samples for both assays were run in duplicate, and concentrations were calculated as an average of two duplicates from each individual. Serum corticosterone was measured in 10 μL serum from individual rats in an RIA (MP Biomedicals; Corticosterone 125I RIA, RCBK1720). The intra-assay coefficient of variation (CV) was 1.58% and assay sensitivity was 25 ng/mL. Serum E2 was measured in 200 μL serum from individual rats in an RIA (Beckman-Coulter, Estradiol 125I RIA, 170717C). The intra-assay CV was 2.3% and assay sensitivity was 5 pg/mL.
2.5. Adrenosomatic and Gonadosomatic Indices of the F3 rats
Adrenals and gonads were removed from all F3 carcasses at euthanasia. Once cleaned of fat and weighed, paired adrenals and gonads were normalized to the individual’s body weight and multiplied by 1000 and 100 respectively, to obtain the adrenosomatic (ASI) and gonadosomatic (GSI) indices (Crews et al., 2012; Reilly et al. 2015).
2.6. Behavioral Testing
2.6.1. F2 Maternal Behavior.
Dams were monitored for two 60-minute observation periods each on postnatal day 3 (P3) and 6 (P6) by trained individuals (~94% interrater reliability). Behaviors are described in Table 1. Observations occurred once during the light (8:30 a.m.) and once during the dark (12:30 p.m.) phase of the light/dark cycle. During each observation period, each mother was scored once every minute for the following behaviors: mother on the nest, licking/grooming pups, retrieving pups, and nursing pups. The mother’s posture was also observed: the arched-back position (outstretched legs to facilitate access to nipples), a blanket position (laying on the pups), or in a passive position [(lying on her back or her side); Figure 2a]. For the purpose of this experiment, any nursing position other than the active arched-back position was referred to as a passive nursing position. Note that the paternal lineage mothers are untreated stimulus females who were bred with F2 males exposed to EDCs, while maternal lineage mothers are F2 females who descended directly from EDC- or vehicle-treated progenitors and were mated with untreated stimulus males (Figure 1a). This is important, as the paternal lineage females have not been directly exposed to EDCs; rather, they are the recipients of courtship, mating behavior, and ejaculate of the EDC male descendants.
Table 1.
Maternal Care Behavior
| Behavior | Description |
|---|---|
| On Nest | Total time spent on the Nest |
| Time Nursing | Total time spent nursing in any position |
| Active Nursing | Nursing in an arched back, legs fully extended position |
| Passive Nursing | Nursing prone, supine, or on her side |
| Licking & Grooming (LG) | Total time spent licking & grooming the pups |
| Pup retrieval test | Time to retrieve the first pup after scattering across the nest |
Fig 2.
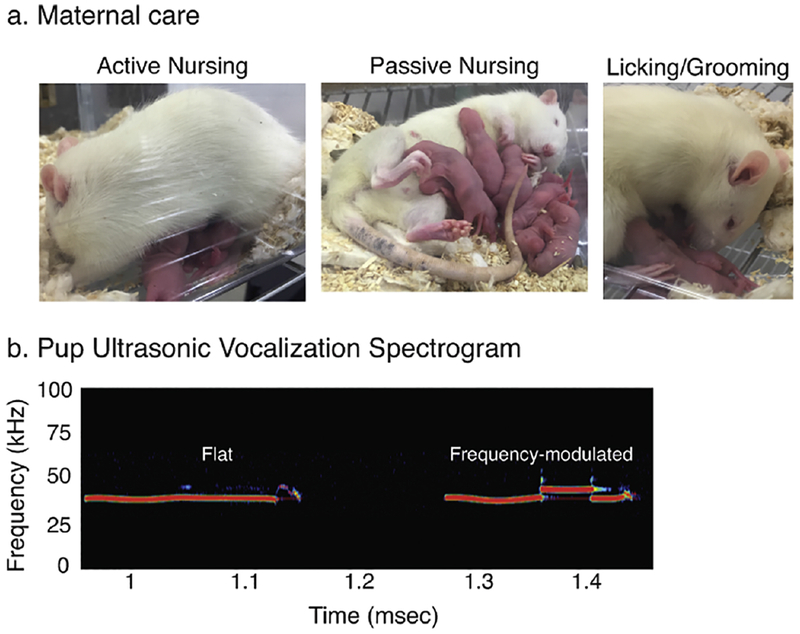
Representative images of (a) maternal care postures in rats such as active (arched back) nursing, passive nursing, and licking/grooming of pups, are shown. (b) A pup ultrasonic vocalization spectrogram is shown, demonstrating a flat call (left) and a frequency-modulated call (right).
2.6.2. F3 Pup Ultrasonic Vocalizations.
Pup ultrasonic vocalizations were recorded on P3 and P6, immediately after the period of maternal observation during the lights-on phase of the lighting cycle. For each session, one male and one female pup was randomly selected from the nest. They were rapidly transported (< 5 mins) to the ultrasonic vocalization (USV) room, where they were recorded for USVs for 5 mins while in isolation in clean mouse cages. USVs were recorded by a CM16/CMPA microphone (Avisoft, Berlin, Germany) suspended 10 cm above the floor of the cage. Calls were recorded with UltraSoundGate hardware and software and analyzed with SasLab Pro (Avisoft, Berlin, Germany). Recordings were played at 512 FFT-length and 75% overlap. Whistle tracking element separation, optimized to our apparatus and conditions, was used to automatically detect calls. The number of flat [<5 kHz change in frequency] and frequency-modulated [>5 kHz change in frequency] calls were counted, and a sample spectrogram is shown in Figure 2b for each call type. Spectrogram properties quantified for each call were duration, power (average amplitude), bandwidth, and mean frequency (pitch) as per (Bell et al., 2016; Krishnan et al., 2018).
2.6.3. Retrieval of F3 pups by their F2 dams.
Immediately after recording USVs from the pups, the dams were tested for latency to pup retrieval. First, the dam was removed from the cage for approximately 30 seconds. During this time, pups were scattered around the home cage. Once the dam was reintroduced to the home cage, she was timed for latency to retrieve the first pup.
2.6.4. F3 Adult Anxiety-like Behaviors.
In adulthood (P75-100), 2-3 F3 females and 2-3 F3 males from each litter were randomly selected for behavioral testing in the open field test and the light:dark box. The tests were performed at least 48h apart. Females were monitored for their estrous cycles, but were tested in the open field and light:dark box on a random day of their cycle. Cycle status was previously included as a covariate in statistical analysis in our lab, and had no effect on similar behavioral outcomes (Gillette et al., 2017). Behaviors from both tests were recorded using the AnyMaze automated tracking software.
Open Field Test of F3 adults.
The open field test measures aversion to explore brightly lit areas. The apparatus is a square arena, digitally divided into an inner (center) area, an outer area, and four corner areas. The outer area consisted of a 10 cm border around the center open-field. Each corner of the arena was 10 cm × 10 cm. The animals’ position, speed, and time immobile were recorded, as were total time in the corners and the center.
Light:Dark Box Test of F3 adults.
The Stoelting light:dark apparatus is a rectangular box bisected into two chambers, one clear and one opaque black Plexiglas (50 cm × 50 cm each), connected by a passageway. The light portion of the box was diffusely illuminated with a 60 w incandescent bulb ~3m from the top of the apparatus. The dark portion of the box was covered with a black Plexiglas transparent lid. Observation and video recording of the animal in the dark box was possible because the lid permitted red light to pass. To begin the light:dark box test, the animal was placed in the passageway, facing the light field, and tracked for 5 minutes. The time in each box, mean speed, and time immobile were recorded.
2.7. Statistical Analyses
Behavioral and physiological data were analyzed using a one-way analysis of variance (ANOVA) within each sex and lineage or lineage and day combination to test for main effects of treatment. For measures taken repeatedly over time in the same animals (body weight and anogenital distance) a repeated-measures statistic was used. Tukey’s HSD was used for post-hoc analyses, as it corrects for multiple statistical comparisons. The data were tested for normality and homoscedasticity using the Shapiro-Wilk and Bartlett’s test respectively. If the data failed these assumptions, they were transformed (log, square, or square-root) and analyzed with a similar factorial ANOVA. If the transformations did not resolve these issues, the data were analyzed using the non-parametric Kruskal-Wallis test (KW; given by the H statistic) to determine main effects of EDC treatment, lineage, and sex. Since this test does not determine an interaction effect, we conducted post-hoc analysis of any main effects by pairwise comparisons using the Steel-Dwass method. For ANOVAs, effect sizes for each factor were calculated as eta-squared (η2), which represents the proportion of variance that is accounted for by the factor being tested. An η2 of 0.14 or greater is considered large, whereas an η2 between 0.06 and 0.13 is considered medium. For KW tests, effect sizes were calculated as epsilon squared (ε2), using similar parameters as η2. An effect was considered significant at p < 0.05.
2.7.1. Correlation Matrices.
We analyzed the relationships between F2 and F3 generation behaviors and serum hormone levels using correlation matrices. Pearson’s r correlation coefficients are reported in Supplemental Table 1. Correlations were classified as strong, moderate or weak based on the correlation coefficient. Multiple comparisons were corrected using the Bonferroni method.
3. Results
Birth Outcomes and Development of the F3 Generation
3.1. Body weight and AGI of the F3 Generation
Body weight was analyzed in three distinct life phases, as in our previous studies (Gillette et al., 2017; Mennigen et al., 2018): prepuberty (P1 – P21), adolescence (P28 – P63), and adulthood (P70 – P98). Repeated measures analysis revealed significant differences in body weight in prepuberty in 3 of the 4 lineages, but these differences did not persist into adolescence or adulthood; effect sizes were small to medium (Figure 3). In the prepubertal phase, maternal lineage females differed by treatment (F2, 146 = 3.98, p = 0.02, η2 = 0.05), with VIN animals weighing significantly less than PCB (p = 0.01). Paternal lineage prepubertal females also differed by treatment (F2, 151 = 8.62, p < 0.0001, η2 = 0.11); PCB (p < 0.0001) and VIN (p = 0.002) descendants weighed less than their VEH counterparts. In prepubertal males, the maternal lineage also differed between treatment groups (F2, 140 = 3.74, p = 0.026, η2 = 0.05) in that PCB males weighed more than both VIN (p = 0.002) and VEH (p = 0.034) males.
Fig. 3.
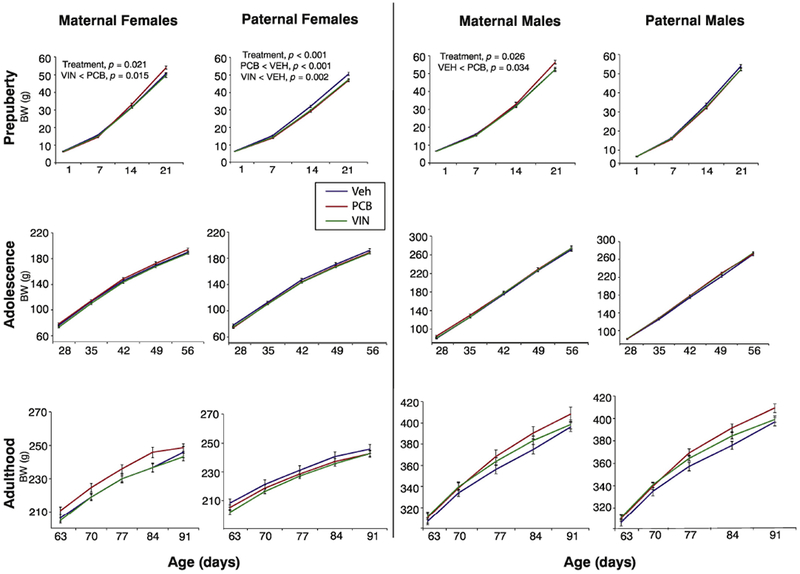
Body weight (g) of the F3 generation females and males is shown in each sex over three life phases: prepuberty (P1-21), adolescence (P28-56), and adulthood (P63-P91). Significant differences and p-values from repeated-measures ANOVAs are shown; differences indicated are for the entire time series. Data are mean ± SEM.
Anogenital index (AGI) was altered in maternal females (F2, 150 = 6.46, p = 0.002, η2 = 0.09) and paternal males (F2, 158 = 8.98, p < 0.0001, η2 = 0.11) in the first two weeks of life (P1, P7, P14; data not shown). In maternal females, both PCB (p = 0.002) and VIN (p = 0.03) animals had smaller AGIs than VEH; paternal PCB males had smaller AGIs than VEH (p = 0.03) and VIN (p < 0.0001) animals.
3.2. Age at Puberty in the F3 Generation
Age at puberty was determined based on vaginal opening and preputial separation for both females and males respectively. There were no treatment effects in F3 males (preputial separation at ~44 days of age in all groups), or in paternal-lineage females (vaginal opening at ~35 days of age). Maternal lineage females descended from ancestrally EDC groups experienced puberty ~2.5 days earlier than their VEH counterparts [VEH: 35.2 ± 0.3; PCB: 33.7 ± 0.3; VIN: 33.7 ± 0.3; (H(2, N=141) = 18.22, p < 0.0001, ε2 = 0.13)].
3.3. Adrenosomatic and Gonadosomatic Index in the F3 Generation
Adrenosomatic index (Figure 4a) differed between treatments in paternal lineage females (F2, 66 = 4.13, p = 0.020, η2 = 0.13), with VIN descendants having adrenals that were smaller than their PCB counterparts (p = 0.035). Paternal lineage males were significantly affected by treatment (F2, 66 = 3.47, p = 0.036, η2 = 0.10), but post-hoc analyses did not identify significant differences between specific groups.
Fig. 4.
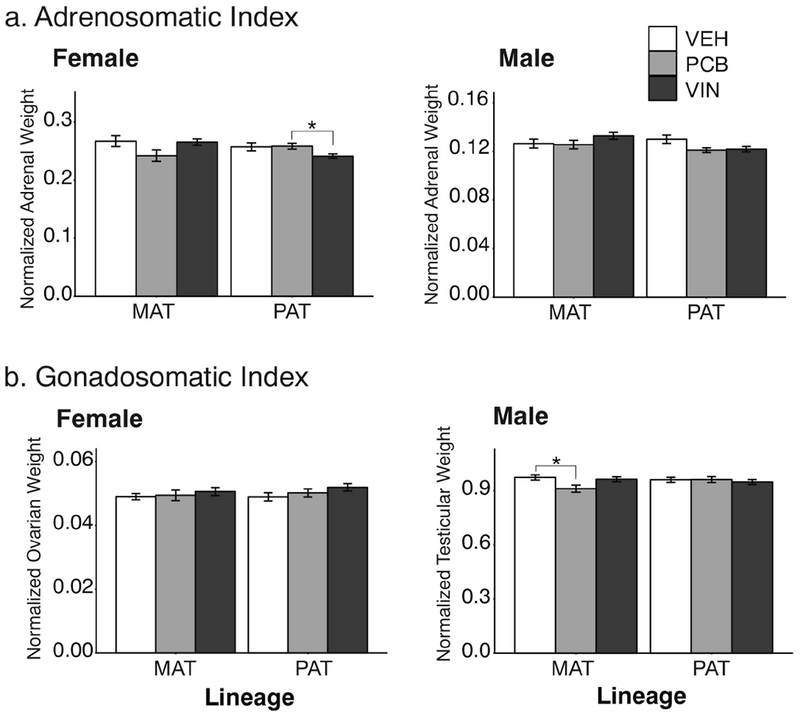
Adrenosomatic (a) and gonadosomatic (b) indices of F3 adults are shown. Significant effects of treatment are indicated as *,p < 0.05. Data are shown as mean ± SEM.
Ovarian index (Figure 4b) in females was unaffected by treatment in either lineage. Maternal lineage males showed significant treatment effects (F2, 67 = 3.12, p = 0.029, η2 = 0.11). Specifically, PCB descended males had smaller testes relative to their VEH controls (p = 0.039).
3.4. Serum Hormones in the F3 Generation
Although serum corticosterone concentrations (Figure 5a) did not significantly differ between treatment groups in the paternal lineage males, the effect size was large (F2, 24 = 3.22, p = 0.057, η2 = 0.27). Serum estradiol concentrations (Figure 5b) only differed between treatments in paternal lineage females (F2, 25 = 4.20, p = 0.026, η2 = 0.34), with significantly higher circulating estradiol levels in PCB then VEH descended females (p = 0.028).
Fig. 5.
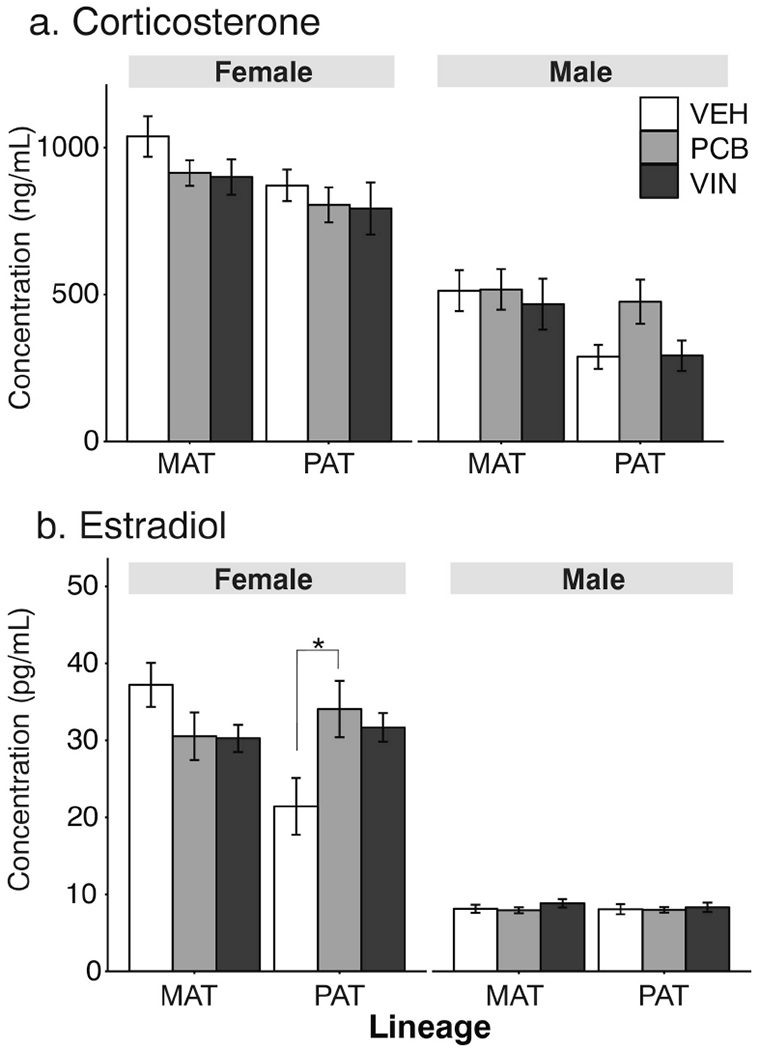
Serum corticosterone (a) and estradiol (b) concentrations of F3 adults are shown. Effects of treatment are indicated as *, p < 0.05. Data are shown as mean ± SEM.
Behaviors in F2 Dams and Their F3 Offspring
3.5. F2 Maternal Care
Maternal care was scored for one hour during the lights-on phase, and one hour during the lights-off phase, when pups were P3 and P6. Because no significant effects were observed on P6, we will only describe statistics for P3 maternal behaviors. Data are presented separately for the lights-on (Figure 6) and lights-off periods (Figure 7). During lights-on, the F2 maternal line females did not differ in the number of observations scored on the nest (Figure 6a). They also did not differ significantly when nursing their offspring although this had a large effect size (F2, 25 = 3.01, p = 0.067, η2 = 0.24; Figure 6b). Significant effects of treatment were detected for the number of observations of maternal females in the active nursing position [H(2, N=29) = 6.28, p = 0.045, ε2= 0.22; Figure 6c] with PCB < VEH (p < 0.05). No differences were found in the passive nursing position (Figure 6d). F2 maternal females also differed in numbers of observations of licking and grooming (LG) their pups (F2, 30 = 5.03, p = 0.013, η2 = 0.34), with VIN females greater than both PCB (p = 0.028) and VEH (p = 0.032) females (Figure 6e).
Fig. 6.
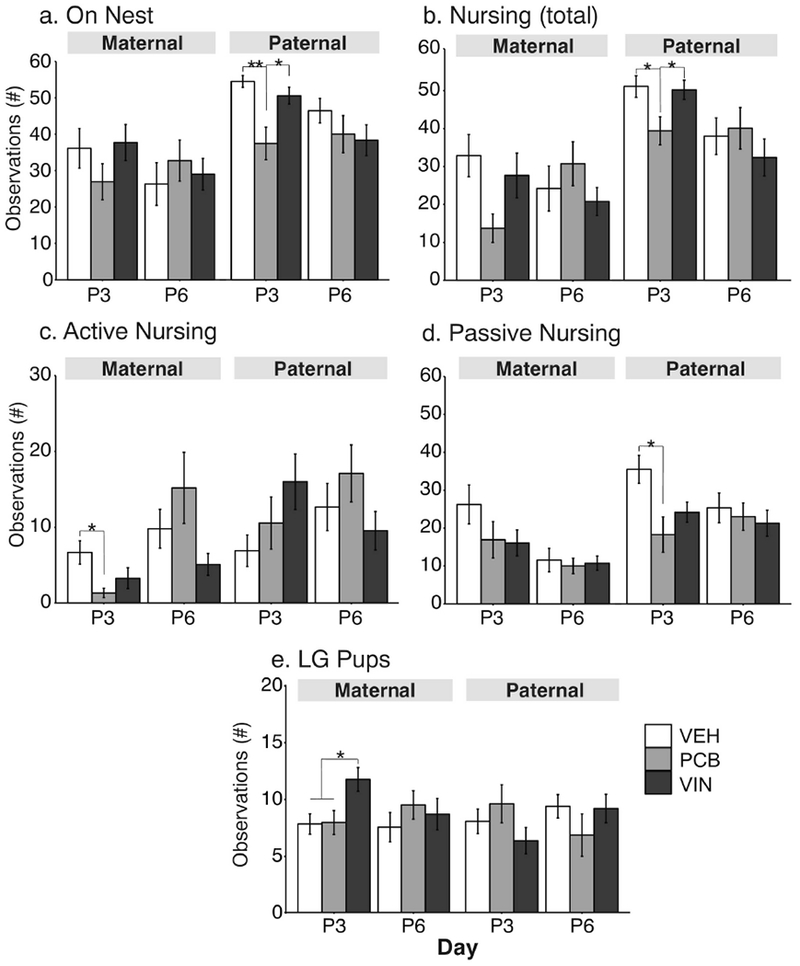
Maternal behavior results are shown during the lights-on phase at P3 and P6 for number of observations (a) on the nest, (b) total nursing, (c) active nursing, (d) passive nursing, and (e) licking/grooming (LG) pups. Effects of treatment are shown as *, p < 0.05; **, p < 0.01. Data are shown as mean ± SEM.
Fig. 7.
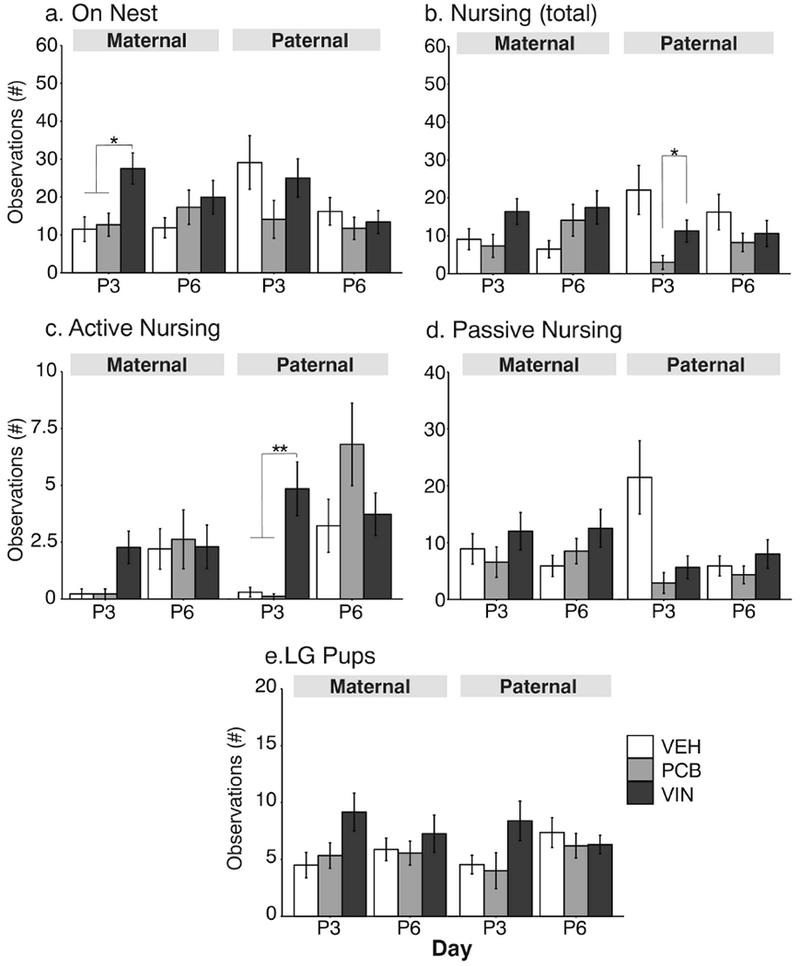
Maternal behavior results are shown during the lights-off phase at P3 and P6. See Figure 6 legend for details.
Some differences were also found for the paternal lineage F2 dams. It is notable that these were the untreated breeder females who mated with F2-lineage males. Paternal lineage females differed significantly in number of observations on the nest (F2, 30 = 8.12, p = 0.001, η2 = 0.54; Figure 6a) and total nursing (F2, 24 = 4.16, p = 0.02, η2 = 0.35; Figure 6b). For both of these behaviors, PCB females had fewer observations than their VEH (on nest: p = 0.001; LG: p = 0.04) and VIN (p = 0.01 and p = 0.04) counterparts. The number of observations passive nursing was also affected in paternal females (F2, 32 = 5.55, p = 0.008, η2 = 0.35), with PCB < VEH (Figure 6d, p = 0.007).
Maternal behavior during the dark phase (Figure 7) occurred at lower frequencies. Again, treatment differences were found only on P3. A significant effect of treatment was found in maternal females for numbers of observations on the nest (F2, 26 = 6.18, p = 0.006, η2= 0.48, Figure 7a), with VIN > VEH (p = 0.01) and VIN > PCB (p = 0.02). These females also differed in active nursing, with a large effect size [H(2, N=29) = 9.09, p = 0.01, ε2= 0.30, Fig 7c], although post-hoc analyses did not identify differences between specific groups. There were differences between maternal line females for LG pups (F2, 26 = 3.16, p = 0.05, η2 = 0.24, Figure 7e); although the effect size was large, post-hoc analyses were non-significant. In the paternal lineage, females differed significantly in numbers of observations nursing [H(2, N=28) = 7.77, p = 0.02, ε2 = 0.26], with PCB < VIN (p = 0.02, Figure 7b). These females also differed in observations of the active nursing position (H(2, N=29) = 16.52, p < 0.01, ε2= 0.50, Fig 7c], with VIN > VEH (p = 0.005) and VIN > PCB (p = 0.003). Passive nursing was significantly affected by treatment with a large effect size [H(2, N=28) = 6.28, p = 0.04, ε2= 0.20, Figure 7d], but post-hoc analyses could not resolve differences between treatment groups.
3.6. F3 Pup Retrieval Test by the F2 Dams
The time taken for the F2 dam to retrieve the first pup did not differ between treatment groups by lineage or day (p > 0.05 for all).
3.7. F3 Pup Ultrasonic Vocalizations
One male and one female pup per litter was scored for USV calls on P3 and P6. There were no sex differences between male and female pups in all aspects of their vocalizations (data not shown), therefore we analyzed pup vocalizations collapsed across sexes. Maternal lineage pups did not differ by treatment for call numbers or power (Figure 8a-c), but they differed in spectrographic properties of calls. On P6, call durations were significantly affected (F2, 46 = 4,32, p = 0.015, η2 = 0.13), with VIN < VEH (p < 0.05). Mean call frequency in the maternal lineage did not differ significantly on P6 (F2, 46 = 3.09, p = 0.054, η2 = 0.13; Figure 8e). Paternal lineage pups differed solely in the number of calls that they emitted (Figure 8c). On P6, compared to their VEH counterparts, PCB pups from the paternal lineage emitted significantly fewer total calls (F2, 51 = 4.56, p = 0.015, η2 = 0.18; Figure 8a) and frequency-modulated (FM) calls (F2, 51 = 3.30, p = 0.044, η2 = 0.13; Figure 8b), with no significant difference in flat calls (F2, 49 = 2.57, p = 0.087, η2 = 0.11; Figure 8c).
Fig. 8.
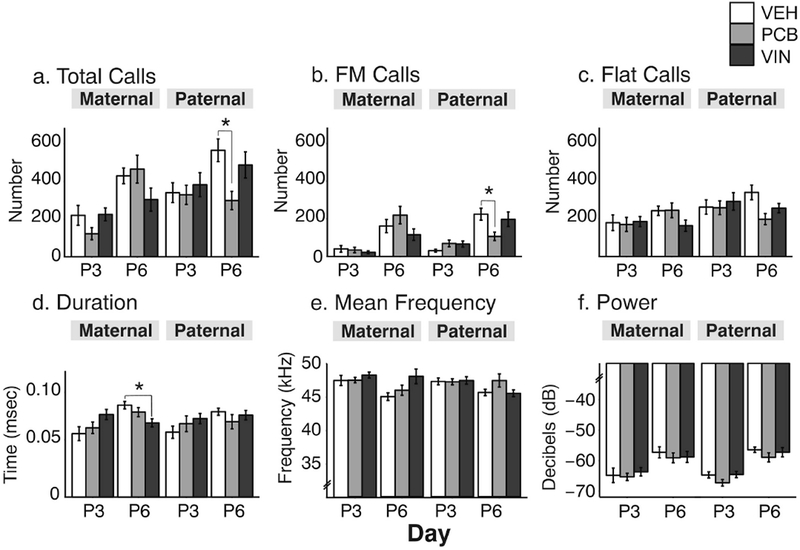
Pup ultrasonic vocalizations (USVs) numbers and properties were measured on P3 and P6. Data are shown for numbers of (a) total, (b) frequency-modulated (FM) and (c) flat calls, and for call (d) duration, (e) mean frequency, and (f) power. Effects of treatment are shown as *, p < 0.05. Data are shown as mean ± SEM.
3.8. F3 Adult Anxiety-like Behaviors
3.8.1. Open Field.
Both paternal- and maternal-lineage females differed by treatment in specific aspects of the open field test. For females, the time spent in the corners was not significantly affected (Figure 9a), but time spent in the center of the apparatus differed significantly (F2, 48 = 3,49, p = 0.036, η2 = 0.12, Figure 9b), with VIN < VEH (p < 0.05). Mean speed was also affected in maternal females (F2, 59 = 3.19, p = 0.048, η2 = 0.11, Figure 9c) with VIN faster than VEH rats (p < 0.05). Time immobile was unaffected in females (Figure 9d). For the paternal lineage females, there were no significant treatment effects on time spent in the corners or the center of the apparatus (Figure 9a, 9b), but there was a significant effect on speed (F2, 72 = 3.51, p = 0.026, η2 = 0.10), with paternal VIN females being significantly slower than PCB descendants (p < 0.05).
Fig. 9.
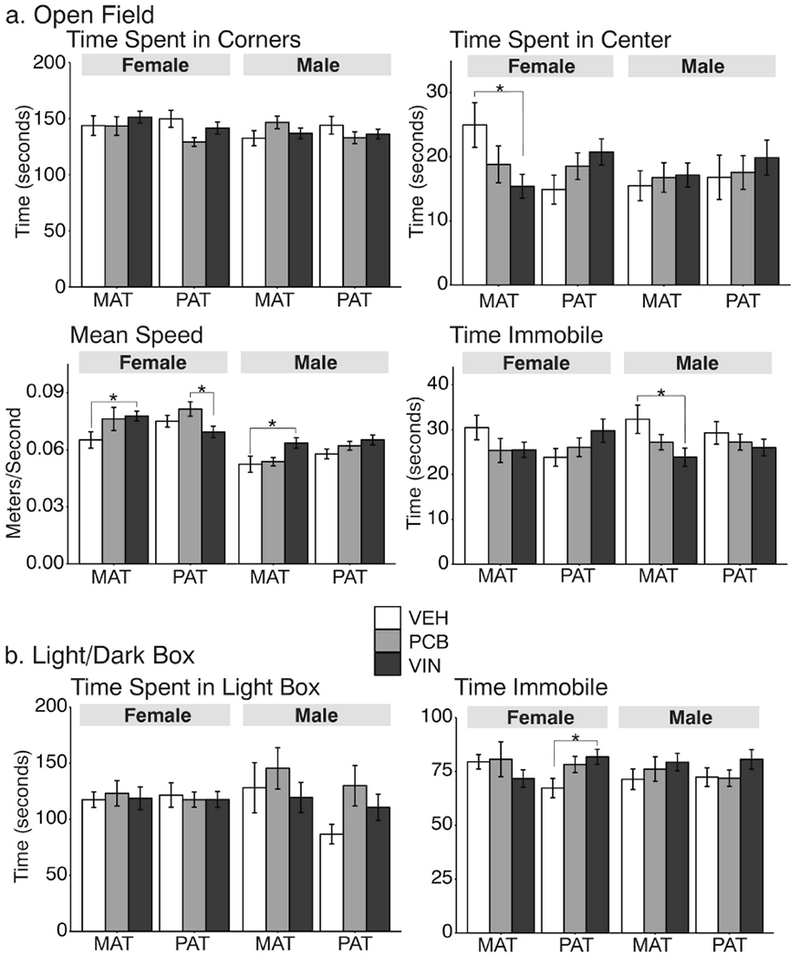
Anxiety-like behaviors were measured in the open field (OF) test (a) and the light:dark (LD) box (b). *,p < 0.05. Data are shown as mean ± SEM.
Among males, only the maternal line was affected: for speed (F2, 67 = 4,32, p = 0.034 η2 = 0.13, Figure 9c) with VIN faster than VEH (p < 0.05), and time spent immobile (F2, 66 = 3.36, p = 0.031, η2 = 0.10, Figure 9d), with VIN > VEH males (p < 0.05).
3.8.2. Light:Dark Box.
The only difference in the light:dark box test (Figure 9b) was in the time spent immobile in paternal lineage females, for which VIN females spent significantly more time immobile than VEH females (F2, 68 = 3.21, p = 0.036, η2 = 0.09).
3.9. Correlations
In a companion study (Krishnan et al., 2018) we found that in F2 adult animals, particularly males from the paternal lineage, there were effects of EDCs on numbers and acoustic properties of their USVs, as well as serum estradiol and testosterone concentrations. The F2 females from that study were observed for their maternal care in the current study; the maternal F2 females were those exposed in the germline to EDCs, and the paternal F2 females were untreated but bred to F2 males exposed in the germline to EDCs.
To relate F2 adult physiology (previous study) and maternal behaviors (this study) to those of their F3 offspring (this study), we conducted correlation analyses for F2 adult USVs, F2 maternal care, F3 pup USVs, F3 offspring anxiety-like behaviors, and F2 and F3 serum hormone concentrations. Pearson correlations were performed as: 1) F2 within-individual correlations, or 2) F2 individuals correlated with their own F3 offspring. The complete dataset is presented in Supplemental Table 1. In this results section, we focus on that subset of data for which correlations were strong and/or significant.
Correlations between adult USV properties and maternal care.
Strong, but nonsignificant correlations only occurred in paternal lineage F2 dams (Figure 10a & b). Within F2 paternal PCB animals, the duration of USVs was negatively correlated with the amount of time spent LG pups (r = −0.81; Figure 10a). In F2 VIN animals of the paternal lineage, the power of the USVs was positively correlated with both the time spent on the nest (r = 0.76; Figure 10b) and the time spent in the active nursing position (r = 0.71; data not shown). Mean bandwidth of F2 VEH adult USVs were positively correlated with the amount of time spent on the nest (r = 0.72; data not shown).
Fig. 10.
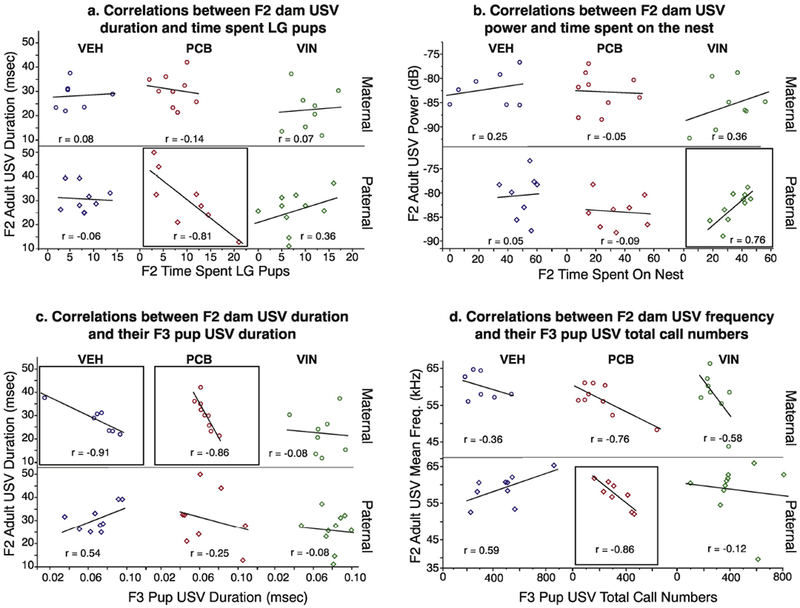
Within-litter Pearson r correlations are shown between F2 adult USV behaviors and their behavior towards their pups (a, b), and for USV characteristics between F2 adults and their F3 offspring (c, d). Correlations were determined, and data plotted, separately for each treatment group. Groups with strong correlations are boxed, although none of these reached significance.
F2 Adult USVs and F3 Offspring USVs.
The average duration of calls was strongly but non-significantly, negatively correlated between F2 adults and their F3 offspring in the maternal lineage VEH (r = −0.91) and PCB groups (r = −0.86; Figure 10c). However, no other treatment groups exhibited this correlation. The mean frequency of vocalizations emitted by paternal PCB F2 adults were strongly negatively correlated with total (r = −0.86) and flat calls (r = −0.88; Supplemental Table 1) emitted by their F3 offspring, although these relationships were non-significant as well (Figure 10d).
F2 Maternal Care and F3 Offspring USVs.
Strong correlations between F2 maternal care and F3 pup USVs only occurred on P3. In F2 maternal lineage VIN animals, the strong negative correlation between time spent LG pups and duration of F3 pup vocalizations (r = −0.87) was non-significant (Figure 11a). The time that F2 maternal lineage VEH animals spent in the active nursing posture was strongly inversely (but not significantly) correlated with the number of total (r = −0.91; Fig 11b), FM (r = −0.81; Supplemental Table 1) and flat calls (r = −0.80; Supplemental Table 1) emitted by their F3 offspring.
Fig. 11.
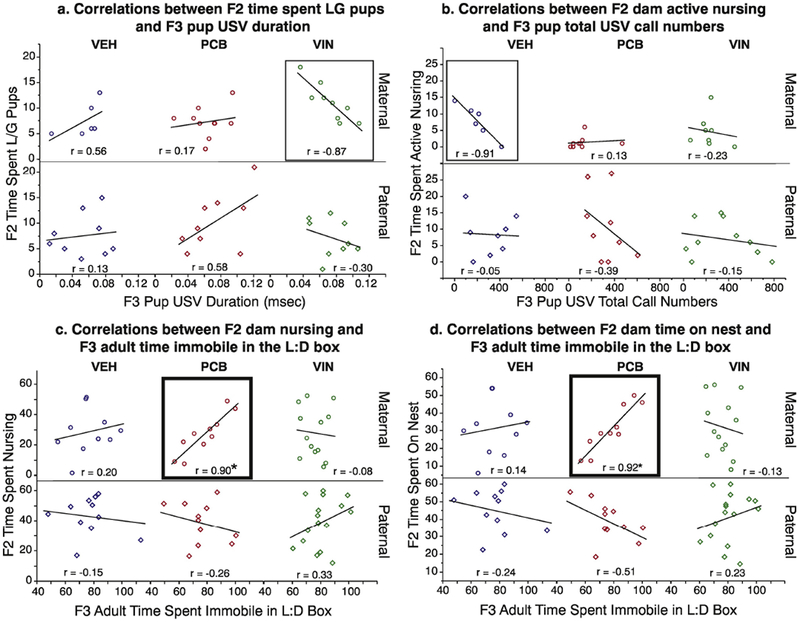
Within-litter Pearson r correlations are shown between F2 maternal care and F3 pup USV properties (a, b), and between F2 maternal care and F3 adult anxiety-like behaviors in the light:dark box (c, d). Groups with strong correlations are boxed, and those that are significant are in thicker boxes (*, p < 0.05).
F2 Maternal Care and F3 Adult Anxiety-like Behavior.
There was a strong, positive, and significant correlation between maternal care displayed by F2 maternal lineage PCB animals and their F3 offspring for light:dark box behavior. The time that F3 PCB animals spent immobile in the light:dark box apparatus was strongly correlated with the time that their F2 PCB parent spent nursing (r = 0.90; Figure 11c) and on the nest (r = 0.92; Figure 11d).
F2 and F3 Serum Hormone Levels.
Male and female serum corticosterone and estradiol concentrations were each correlated across generations. Only one significant correlation emerged: for the F2 VEH paternal dams, in which serum estradiol was significantly and strongly negatively correlation with estradiol concentrations in F3 VEH females (Supplemental Table 1).
4. Discussion
Research on transgenerational effects of EDCs is predicated on the assumption that EDC exposures cause germline changes, especially DNA methylation, that can be heritable (Anway and Skinner, 2008; Crews et al., 2007; Gillette et al., 2014; Gillette et al., 2018). However, it is equally important to consider the broader environmental context. For example, maternal care influences the epigenome of the offspring’s brain, leading to behavioral differences that may in turn be propagated to further generations (Champagne and Meaney, 2001). Our study investigated the potential for the experience of maternal care to interact with EDC inheritance (germline) to affect offspring physiology, developmental milestones, and anxiety-like behavior.
4.1. EDCs alter F2 maternal behavior in early F3 postnatal care
Maternal care is crucial to normal offspring physiological and psychosocial development. Rodent maternal care is susceptible to disruption by environmental stressors (Carini et al., 2013; Moussaoui et al., 2016; Nephew and Bridges, 2011), food restriction (Sabau and Ferkin, 2013), as well as stressors in their own early development (Del Cerro et al., 2015). To date, few studies have documented the effects of EDCs on maternal care in rodents (Johnson et al., 2015; Kougias et al., 2018; Rosenfeld, 2015), with divergent results. Dams consuming a high fat diet together with phthalate ingestion had increased licking and grooming behavior towards their offspring (Kougias et al., 2018). Studies in mice and rats showed that bisphenol A (BPA) decreased (Johnson et al., 2015; Seta et al., 2005), increased (Kundakovic et al., 2013), or did not alter (Boudalia et al., 2014) maternal care behaviors, depending on the duration and dosage of the exposure.
To our knowledge, our study is the first to examine how exposure of the F2 generation females to EDCs as germ cells (i.e., within the developing F1 embryos) alters those individuals’ maternal behavior as adults (maternal line), and whether an adult male with germ cell exposure may alter an unexposed females’ maternal care to her offspring (paternal line). In the maternal F2 lineage, PCB dams spent significantly less time in the active nursing position. This arched-back position allows for more efficient milk letdown from the mammary glands (Lonstein et al., 1998). Maternal F2 VIN females spent more time licking and grooming their pups, linked to lower stress responses in adult offspring (Champagne and Meaney, 2001).
In our paradigm, untreated females who mated with F2 PCB males (paternal line) had fewer observations on the nest and nursing, specifically in a passive nursing position on P3. These paternal line effects via altered maternal investment are noteworthy since these males previously exhibited changes in USV and mating behavior, potentially signaling diminished mate quality and reproductive potential. The transmission of effects to the F3 generation could also indicate a germline epigenetic change inherited in the offspring of the paternal lineage.
It is possible that the unexposed females displaying the maternal care might adjust their reproductive investment toward their offspring depending on such environmental factors that influence reproductive fitness, as postulated (Curley et al., 2011). For example, food restriction of male mice prior to natural mating caused females to alter their maternal investment in their offspring (Mashoodh et al., 2018). However, transfer of embryos fertilized by these same males did not produce any changes in the females’ maternal care, suggesting that the mating interaction, rather than the genetic and epigenetic material in the embryo, influenced the females’ behavior. Thus, we speculate that the legacy of EDC exposure in the male occurs via decreases in maternal behavior, especially since these females were not allowed to choose their reproductive partner. The reproductive compensation hypothesis suggests that females paired with unattractive males would increase their investment to mitigate any disadvantages that the offspring may inherit from their father (Sheppard et al., 2013). Our results suggest that other forms of mitigation could be taking place, given that females exposed to the paternal lineage males reduce the time they spend interacting with the offspring. Furthermore, we cannot eliminate the possibility that as-yet to be determined factors in the sperm/semen may convey information that leads to adjustments in maternal behavior, something that could be tested in cross-fostering experiments.
4.2. EDCs and F2 maternal care alter F3 pup USVs and developmental outcomes
Pup USVs have many functional similarities to human infant vocalizations. Just as human infant cries hasten maternal care and retrieval, so do pup USVs (Bowers et al., 2013; Hofer et al., 2002; Rosenblatt et al., 1979). Rodent pup USVs are believed to denote negative affective states such as distress, as they decrease with the administration of anxiolytic drugs like benzodiazepines (Miczek et al., 1995; Zimmerberg et al., 1994). Consistent with this, isolation from the mother leads to potentiation of USV calls from the pups, indicating a state of anxiety or distress (Hofer et al., 2002; Shair et al., 2015).
Unlike maternal care, which was solely altered on P3 in our study, pup USVs were almost exclusively altered on P6. At this age in the maternal lineage, call duration was significantly shorter in maternal VIN than VEH pups. Antidepressant drugs administered in early postnatal life reduce the duration of USVs (Hodgson et al., 2008), so the longer duration calls in the VIN pups in our paradigm could be representative of greater distress. Given the strong (albeit non-significant) correlation between maternal lineage LG behavior and pup USV duration in VIN animals (see Supplemental Table 1), it is possible that the duration of the offspring vocalizations on P3 drives the LG behavior to increase, resulting in lower levels of stress reactivity, and therefore shorter call lengths, in the offspring on P6. Further work is needed to test this hypothesis.
We also detected fewer calls from paternal PCB F3 pups than VEH after a period of separation, indicating either a baseline difference in the number of calls, or that these PCB pups experienced less distress when separated from their mother. Although the decline in calling could be a consequence of the decreased time that F2 PCB mothers spent on the nest and nursing on P3, other studies have shown that a decrease in maternal care behaviors resulted in greater anxiety-like behaviors in the offspring (Bowers et al., 2013; Shair et al., 2015). The F3 paternal lineage PCB pups, although devoid of direct exposure to EDCs, could inherit altered epigenetic marks via the mature sperm in the paternal germline as well (Probst et al., 2009), causing alterations in vocalization behavior of the offspring and subsequently altering the dam’s maternal care behaviors. Since offspring are not passive recipients of the care they receive, it is possible that the decline in their calling drives the reduction in maternal care, thereby influencing their own development (Curley et al., 2011).
4.3. EDC-altered F2 maternal care modulates F3 adult anxiety-like behavior
The impact of altered maternal care and prenatal environments on offspring anxiety-like behavior is well documented (Champagne et al., 2003; Champagne and Meaney, 2006; Weaver et al., 2006). Effects of ancestral EDC exposure in our paradigm were relatively modest, and not as strong as our pup USV results. In our study, regardless of sex or lineage, VIN animals differed in their anxiety-like behaviors; no significant differences were found with PCB animals. In the open field test, maternal lineage VIN females moved more quickly and spent less time in the center of the chamber than VEH females, suggesting higher stress reactivity. Maternal lineage VIN males also moved more quickly but spent less time immobile than VEH males, which suggests lower levels of stress reactivity.
Although F2 maternal care towards F3 offspring was altered in early postnatal development, the anxiety-like effects in F3 offspring did not persist into adulthood. Correlations between F2 maternal care and F3 adult offspring were few, but those that were found were significant – the time that PCB mothers spent on the nest and nursing was strongly, significantly, and positively correlated with the offspring’s time spent immobile in the light:dark box. This was unexpected, as greater nursing and nesting patterns traditionally result in less anxiety behavior in the adult offspring (Caldji et al., 1998; Champagne et al., 2003). However, the decreased care that we observed from paternal line PCB mothers could contribute to the physiological phenotype of higher corticosterone levels, lower estradiol levels, and smaller adrenals that we saw in our F3 PCB adult offspring. These outcomes indicate disruption of the hypothalamic-pituitary-adrenal axis, although our behavioral paradigms do not reflect the same. Future research utilizing more sensitive behavioral tests measuring anxiety- and depressive-like behaviors could better clarify potential behavioral alterations in stress reactivity in these individuals.
4.4. Conclusions and implications
The present data show the lineage-dependent impact of ancestral EDC exposure on F2 maternal care, as well as the combinatorial effects of transgenerational EDC exposure and altered maternal care on F3 physiology and behavior. In a companion study (Krishnan et al., 2018) we reported that F2 paternal lineage males exposed to PCBs were phenotypically different from their VEH counterparts. Although a single strong phenotype did not emerge in the F3 generation as it did in the F2, the alteration of F2 maternal behavior and F3 offspring outcomes in the paternal lineage PCB animals was particularly interesting.
Transgenerational transmission of EDC effects via the paternal line has previously been documented with high doses of VIN (Anway et al., 2005; Anway and Skinner, 2008), with F3 animals at increased risk for kidney disease, infertility and immune abnormalities at least four generations beyond the initial exposure. Behavioral work using this VIN model also found that females prefer males from non-EDC exposed lineages in a mate preference paradigm (Crews et al., 2007), indicating significant impacts of paternal lineage EDC transmission on reproductive success. Moreover, the individuals’ responses to stress during adolescence was altered in F3 animals of the VIN lineage (Crews et al., 2012), showing that the predisposition to a maladaptive stress response can be programmed generations earlier by EDC exposure.
Although our study establishes maternal care as a potential context-dependent mechanism by which EDC effects are propagated transgenerationally, it does not exclude germline-dependent mechanisms of action. This point is underscored by baseline differences in vehicle control groups of maternal and paternal lineages, notable for serum corticosterone concentrations in F3 males (Figure 5A), serum estradiol in F3 females (Figure 5B), and some maternal behaviors (Figures 6 and 7). We speculate that these lineage differences are due to differences in the timing of the original exposures to the EDCs, from gestational days 8-18 in the F1 generation. The developing germ cells (future F2 generation) undergo massive changes in epigenetic marks such as DNA methylation, with a complete erasure of marks in both sexes. However, the re-establishment of germline methylation marks is completed before birth in males, but not until puberty in females (Seisenberger et al., 2012) leading to maternal and paternal lineage differences in the F3 generation.
To tease apart context and germline dependent mechanisms, cross-fostering the F3 EDC lineage offspring with untreated mothers (and vice-versa) would be necessary. However, context-dependent mechanisms other than maternal care could also be involved in this transmission, such as intrauterine position, pre- and postnatal sex ratio of the litter, prenatal nutrition, etc. (de Medeiros et al., 2010; Lumey et al., 2011; Ryan and Vandenbergh, 2002).
The mechanisms for molecular epigenetic transmission of effects of EDCs across generations are incompletely understood (Skinner et al., 2013). Despite the waves of demethylation and remethylation that occur during germline development, some paternally methylated imprinted genes are exempt from demethylation and are transmitted to the next generation (Lane et al., 2003; Olek and Walter, 1997). To truly establish a role of epigenetic mechanisms in both paternal and maternal transmission of EDC effects, future studies must investigate the epigenetic modifications in the germ cells, the mechanism by which these modifications are inherited from one generation to the next, and the potential role of context in the phenotypic outcomes.
Supplementary Material
Highlights:
PCBs and vinclozolin are EDCs that cause developmental and transgenerational effects.
EDCs affect maternal behavior in the F2 generation, exposed as germ cells.
Ultrasonic vocalizations in the F3 pups were altered transgenerationally by EDCs.
Maternal behavior and EDC inheritance interact across generations.
Acknowledgements
We would like to thank Dr. Ross Gillette and Dr. Amanda Holley for partnership on rat colony maintenance.
Funding: This work was supported by the National Institutes of Health (Grant number RO1 ES023254).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alyea RA, Gollapudi BB, Rasoulpour RJ, 2014. Are we ready to consider transgenerational epigenetic effects in human health risk assessment? Environ. Mol. Mutagen 55, 292–298. doi: 10.1002/em.21831 [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK, 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469. doi: 10.1126/science.1108190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Skinner MK, 2008. Epigenetic programming of the germ line: effects of endocrine disruptors on the development of transgenerational disease. Reprod. Biomed. Online 16, 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Skinner MK, 2006. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology 147, S43–9. doi: 10.1210/en.2005-1058 [DOI] [PubMed] [Google Scholar]
- Bell MR, Thompson LM, Rodriguez K, Gore AC, 2016. Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 1. Sexually dimorphic effects on social and anxiety-like behaviors. Horm Behav 78, 168–177. doi: 10.1016/j.yhbeh.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou C, Flaws JA, 2016. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod. Toxicol 60, 39–52. doi: 10.1016/j.reprotox.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudalia S, Berges R, Chabanet C, Folia M, Decocq L, Pasquis B, Abdennebi-Najar L, Canivenc-Lavier MC, 2014. A multi-generational study on low-dose BPA exposure in Wistar rats: effects on maternal behavior, flavor intake and development. Neurotoxicol Teratol 41, 16–26. doi: 10.1016/j.ntt.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Bowers JM, Perez-Pouchoulen M, Edwards NS, McCarthy MM, 2013. Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J. Neurosci 33, 3276–3283. doi: 10.1523/JNEUROSCI.0425-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ, 1998. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA 95, 5335–5340. doi: 10.1073/pnas.95.9.5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carini LM, Murgatroyd CA, Nephew BC, 2013. Using chronic social stress to model postpartum depression in lactating rodents. J Vis Exp e50324–e50324. doi: 10.3791/50324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ, 2001. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog. Brain Res 133, 287–302. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ, 2003. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 79, 359–371. doi: 10.1016/S0031-9384(03)00149-5 [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ, 2006. Stress During Gestation Alters Postpartum Maternal Care and the Development of the Offspring in a Rodent Model. Biol Psychiatry 59, 1227–1235. doi: 10.1016/j.biopsych.2005.10.016 [DOI] [PubMed] [Google Scholar]
- Conger RD, Ge X, Elder GH, Lorenz FO, Simons RL, 1994. Economic stress, coercive family process, and developmental problems of adolescents. Child Dev 65, 541–561. [PubMed] [Google Scholar]
- Crews D, Gillette R, Miller-Crews I, Gore AC, Skinner MK, 2014. Nature, nurture and epigenetics. Mol Cell Endocrinol 398, 42–52. doi: 10.1016/j.mce.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK, 2012. Epigenetic transgenerational inheritance of altered stress responses. Proc. Natl. Acad. Sci. U.S.A 109, 9143–9148. doi: 10.1073/pnas.1118514109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK, 2007. Transgenerational epigenetic imprints on mate preference. Proc. Natl. Acad. Sci. U.S.A 104, 5942–5946. doi: 10.1073/pnas.0610410104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Mashoodh R, Champagne FA, 2011. Epigenetics and the origins of paternal effects. Horm Behav 59, 306–314. doi: 10.1016/j.yhbeh.2010.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medeiros CB, Rees SL, Llinas M, Fleming AS, Crews D, 2010. Deconstructing early life experiences: distinguishing the contributions of prenatal and postnatal factors to adult male sexual behavior in the rat. Psychol Sci 21, 1494–1501. doi: 10.1177/0956797610382122 [DOI] [PubMed] [Google Scholar]
- Del Cerro MCR, Ortega E, Gómez F, Segovia S, Pérez-Laso C, 2015. Environmental prenatal stress eliminates brain and maternal behavioral sex differences and alters hormone levels in female rats. Horm Behav 73, 142–147. doi: 10.1016/j.yhbeh.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC, 2011. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology 152, 581–594. doi: 10.1210/en.2010-1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, Belanger EE, Gomez MI, Cayo M, Mccaffrey RJ, Seegal RF, Jansing RL, Hwang S-A, Hicks HE, 2008. Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper Hudson River communities. Environ Health Perspect 116, 209–215. doi: 10.1289/ehp.10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ, 1999. Nongenomic Transmission Across Generations of Maternal Behavior and Stress Responses in the Rat. Science 286, 1155–1158. [DOI] [PubMed] [Google Scholar]
- Gillette R, Miller-Crews I, Nilsson EE, Skinner MK, Gore AC, Crews D, 2014. Sexually dimorphic effects of ancestral exposure to vinclozolin on stress reactivity in rats. Endocrinology 155, 3853–3866. doi: 10.1210/en.2014-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R, Reilly MP, Topper VY, Thompson LM, Crews D, Gore AC, 2017. Anxiety-like behaviors in adulthood are altered in male but not female rats exposed to low dosages of polychlorinated biphenyls in utero. Horm Behav 87, 8–15. doi: 10.1016/j.yhbeh.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R, Son MJ, Ton L, Gore AC, Crews D, 2018. Passing experiences on to future generations: endocrine disruptors and transgenerational inheritance of epimutations in brain and sperm. Epigenetics 13: 1106–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT, 2015. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev er20151093. doi: 10.1210/er.2015-1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Holley AM, Crews C, 2018. Mate choice, sexual selection, and endocrine-disrupting chemicals. Horm Behav 101, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson RA, Guthrie DH, Varty GB, 2008. Duration of ultrasonic vocalizations in the isolated rat pup as a behavioral measure: sensitivity to anxiolytic and antidepressant drugs. Pharmacol. Biochem. Behav 88, 341–348. doi: 10.1016/j.pbb.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Hofer MA, Shair HN, Brunelli SA, 2002. Ultrasonic vocalizations in rat and mouse pups. Curr Protoc Neurosci Chapter 8, Unit 8.14–8.14.16. doi: 10.1002/0471142301.ns0814s17 [DOI] [PubMed] [Google Scholar]
- Johnson SA, Javurek AB, Painter MS, Peritore MP, Ellersieck MR, Roberts RM, Rosenfeld CS, 2015. Disruption of parenting behaviors in california mice, a monogamous rodent species, by endocrine disrupting chemicals. PLoS ONE 10, e0126284. doi: 10.1371/journal.pone.0126284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner S, Kieu T, Chow C, Casey S, Blumberg B, 2010. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol. Endocrinol 24, 526–539. doi: 10.1210/me.2009-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kougias DG, Cortes LR, Moody L, Rhoads S, Pan Y-X, Juraska JM, 2018. Effects of Perinatal Exposure to Phthalates and a High-Fat Diet on Maternal Behavior and Pup Development and Social Play. Endocrinology 159, 1088–1105. doi: 10.1210/en.2017-03047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Mittal N, Thompson LM, Rodriguez-Santiago M, Duvauchelle CL, Crews D, Gore AC, 2018. Effects of the Endocrine-Disrupting Chemicals, Vinclozolin and Polychlorinated Biphenyls, on Physiological and Sociosexual Phenotypes in F2 Generation Sprague-Dawley Rats. Environ Health Perspect 126, 97005. doi: 10.1289/EHP3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA, 2013. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. U.S.A 110, 9956–9961. doi: 10.1073/pnas.1214056110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N, Dean W, Erhardt S, Hajkova P, Surani A, Walter J, Reik W, 2003. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis 35, 88–93. doi: 10.1002/gene.10168 [DOI] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, McCarthy MM, 2012. Sexual differentiation of the rodent brain: dogma and beyond. Front Neurosci 6, 26. doi: 10.3389/fnins.2012.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Stern JM, 1998. Functions of the caudal periaqueductal gray in lactating rats: kyphosis, lordosis, maternal aggression, and fearfulness. Behav. Neurosci 112, 1502–1518. [DOI] [PubMed] [Google Scholar]
- Lumey LH, Stein AD, Susser E, 2011. Prenatal famine and adult health. Annu Rev Public Health 32, 237–262. doi: 10.1146/annurev-publhealth-031210-101230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrì S, Chiarotti F, Würbel H, 2008. Maternal separation and maternal care act independently on the development of HPA responses in male rats. Behav. Brain Res 191, 227–234. doi: 10.1016/j.bbr.2008.03.031 [DOI] [PubMed] [Google Scholar]
- Mashoodh R, Habrylo IB, Gudsnuk KM, Pelle G, Champagne FA, 2018. Maternal modulation of paternal effects on offspring development. Proc. Biol. Sci 285, 20180118. doi: 10.1098/rspb.2018.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey JR, 2012. The epigenome as a target for heritable environmental disruptions of cellular function. Mol Cell Endocrinol 354, 9–15. doi: 10.1016/j.mce.2011.09.014 [DOI] [PubMed] [Google Scholar]
- Meaney MJ, 2001. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci 24, 1161–1192. doi: 10.1146/annurev.neuro.24.1.1161 [DOI] [PubMed] [Google Scholar]
- Mennigen JA, Thompson LM, Bell M, Tellez Santos M, Gore AC, 2018. Transgenerational effects of polychlorinated biphenyls: 1. Development and physiology across 3 generations of rats. Environ Health 17, 18. doi: 10.1186/s12940-018-0362-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Weerts EM, Vivian JA, Barros HM, 1995. Aggression, anxiety and vocalizations in animals: GABAA and 5-HT anxiolytics. Psychopharmacology 121, 38–56. [DOI] [PubMed] [Google Scholar]
- Moussaoui N, Larauche M, Biraud M, Molet J, Million M, Mayer E, Taché Y, 2016. Limited Nesting Stress Alters Maternal Behavior and In Vivo Intestinal Permeability in Male Wistar Pup Rats. PLoS ONE 11, e0155037. doi: 10.1371/journal.pone.0155037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS, 2011. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress 14, 677–684. doi: 10.3109/10253890.2011.605487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Sheehan TP, 1997. Neuroanatomical Circuitry for Mammalian Maternal Behavior. Ann N Y Acad Sci 1–25. [DOI] [PubMed] [Google Scholar]
- Olek A, Walter J, 1997. The pre-implantation ontogeny of the H19 methylation imprint. Nat. Genet 17, 275–276. doi: 10.1038/ng1197-275 [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ, 2005. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology 30, 2192–2204. doi: 10.1038/sj.npp.1300769 [DOI] [PubMed] [Google Scholar]
- Probst AV, Dunleavy E, Almouzni G, 2009. Epigenetic inheritance during the cell cycle. Nat. Rev. Mol. Cell Biol 10, 192–206. doi: 10.1038/nrm2640 [DOI] [PubMed] [Google Scholar]
- Reilly MP, Weeks CD, Topper VY, Thompson LM, Crews D, Gore AC, 2015. The effects of prenatal PCBs on adult social behavior in rats. Horm Behav 73, 47–55. doi: 10.1016/j.yhbeh.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JS, Siegel HI, Mayer AD, 1979. Progress in the Study of Maternal Behavior in the Rat: Hormonal, Nonhormonal, Sensory, and Developmental Aspects, in: Advances in the Study of Behavior. Elsevier, pp. 225–311. doi: 10.1016/S0065-3454(08)60096-0 [DOI] [Google Scholar]
- Rosenfeld CS, 2015. Bisphenol A and phthalate endocrine disruption of parental and social behaviors. Front Neurosci 1–15. doi: 10.3389/fnins.2015.00057/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG, 2002. Intrauterine position effects. Neurosci Biobehav Rev 26, 665–678. [DOI] [PubMed] [Google Scholar]
- Sabau RM, Ferkin MH, 2013. Food restriction affects the maternal behavior provided by female meadow voles (Microtus pennsylvanicus). J Mammal 94, 1068–1076. doi: 10.1644/13-MAMM-A-060.1 [DOI] [Google Scholar]
- Schneider S, Kaufmann W, Strauss V, van Ravenzwaay B, 2011. Vinclozolin: a feasibility and sensitivity study of the ILSI-HESI F1-extended one-generation rat reproduction protocol. Regul. Toxicol. Pharmacol 59, 91–100. doi: 10.1016/j.yrtph.2010.09.010 [DOI] [PubMed] [Google Scholar]
- Seisenberger S, Andrews S, Krueger F, Arand J, Walter J, Santos F, Popp C, Thienpont B, Dean W, Reik W, 2012. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell 48, 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seta Della, D., Minder I, Dessì-Fulgheri F, Farabollini F, 2005. Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats. Brain Res. Bull 65, 255–260. doi: 10.1016/j.brainresbull.2004.11.017 [DOI] [PubMed] [Google Scholar]
- Shair HN, Rupert DD, Rosko LM, Hofer MA, Myers MM, Welch MG, 2015. Effects of maternal deprivation and the duration of reunion time on rat pup ultrasonic vocalization responses to isolation: possible implications for human infant studies. Dev Psychobiol 57, 63–72. doi: 10.1002/dev.21258 [DOI] [PubMed] [Google Scholar]
- Sheppard JL, Clark RG, Devries JH, Brasher MG, 2013. Reproductive effort and success of wild female mallards: does male quality matter? Behav. Processes 100, 82–90. doi: 10.1016/j.beproc.2013.07.024 [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS, 2009. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA 301, 2252–2259. doi: 10.1001/jama.2009.754 [DOI] [PubMed] [Google Scholar]
- Skinner MK, Haque CG-BM, Nilsson E, Bhandari R, McCarrey JR, 2013. Environmentally Induced Transgenerational Epigenetic Reprogramming of Primordial Germ Cells and the Subsequent Germ Line. PLoS ONE 8, e66318–15. doi: 10.1371/journal.pone.0066318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood SBA, Kelsey G, 2012. De novo DNA methylation: a germ cell perspective. Trends Genet 28, 33–42. doi: 10.1016/j.tig.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Steinberg RM, Juenger TE, Gore AC, 2007. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav 51, 364–372. doi: 10.1016/j.yhbeh.2006.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Walker DM, Juenger TE, Woller MJ, Gore AC, 2008. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: development, reproductive physiology, and second generational effects. Biol Reprod 78, 1091–1101. doi: 10.1095/biolreprod.107.067249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver ICG, Meaney MJ, Szyf M, 2006. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA 103, 3480–3485. doi: 10.1073/pnas.0507526103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Edwards M, Shetty SRJ, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ, 2012. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology 153, 3828–3838. doi: 10.1210/en.2012-1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin F, Susiarjo M, Bartolomei MS, 2015. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Semin Cell Dev Biol 43, 66–75. doi: 10.1016/j.semcdb.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, Brunelli SA, Hofer MA, 1994. Reduction of rat pup ultrasonic vocalizations by the neuroactive steroid allopregnanolone. Pharmacol. Biochem. Behav 47, 735–738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


