Abstract
Rationale:
Mutations in the gene encoding the sarcomeric protein cardiac myosin-binding protein C (cMyBP-C) are a leading cause of hypertrophic cardiomyopathy (HCM). Mouse models targeting cMyBP-C and use of recombinant proteins have been effective in studying its roles in contractile function and disease. Surprisingly, while the N-terminus of cMyBP-C is important to regulate myofilament binding and contains many HCM mutations, an incorrect sequence, lacking the N-terminal 8 amino acids has been used in many studies.
Objectives:
To determine the N-terminal cMyBP-C sequences in ventricles and investigate the roles of species-specific differences in cMyBP-C on myofilament binding.
Methods and Results:
We determined cMyBP-C sequences in mouse and human by inspecting available sequence databases. N-terminal differences were confirmed using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Cosedimentation assays with actin or myosin were used to examine binding in mouse, human and chimeric fusion proteins of cMyBP-C. Time-resolved FRET (TR-FRET) with site-directed probes on cMyBP-C was employed to measure structural dynamics. LC-MS/MS supported the sequencing data that mouse cMyBP-C contains an eight-residue N-terminal extension (NTE) not found in human. Cosedimentation assays revealed that cardiac myosin binding was strongly influenced by the presence of the NTE, which reduced binding by 60%. 75% more human C0-C2 than mouse bound to myosin. Actin binding of mouse C0-C2 was not affected by the NTE. 50% more human C0-C2 than mouse bound to actin. TR-FRET indicates that the NTE did not significantly affect structural dynamics across domains C0 and C1.
Conclusions:
Our functional results are consistent with the idea that cardiac myosin binding of N-terminal cMyBP-C is reduced in the mouse protein due to the presence of the NTE, which is proposed to interfere with myosin regulatory light chain (RLC) binding. The NTE is a critical component of mouse cMyBP-C, and should be considered in extrapolation of studies to cMyBP-C and HCM mechanisms in human.
Keywords: cardiac myosin-binding protein C, NTE, myosin, regulation, myofilament, contractile proteins
1. Introduction.
Hypertrophic cardiomyopathy (HCM) is a leading cause of sudden death among young individuals and has a prevalence of greater than 1 in 500 in the general population[1]. HCM is characterized by unexplained left ventricular hypertrophy without dilation. Patients are prone to abnormalities such as diastolic dysfunction and arrhythmias that result in unpredictable heart failure at any age[2]. Mutations in myofilament proteins, most commonly in the genes encoding cardiac myosin-binding protein C (cMyBP-C) and β-myosin heavy chain[3, 4], are the initial cause of HCM. While progression of inherited HCM, from mutation to clinical phenotype is not well understood, it is currently thought that altered myofilament protein structure causes an initial insult of contractile dysfunction at the level of cross-bridge cycling, which over several decades, leads to progressive compensatory remodeling and eventual failure of the heart[5].
cMyBP-C is a thick filament-associated protein that is uniquely positioned in the sarcomere[6], capable of tuning contractile function by dynamic cross-bridge interactions. Mutations in cMyBP-C may result in altered contractility in HCM[7, 8]. While the exact location, modular crystal structure, and mechanisms of cMyBP-C are not yet known, researchers have routinely used mouse models, recombinant protein, and transgene approaches in studies of cMyBP-C in order to illuminate structural and functional roles. Recent studies have revealed that the N-terminus of cMyBP-C, particularly domains C0 through C2 (C0-C2: C0-P/A-C1-M-C2), is important for dynamically interacting with actin and myosin, and thereby in modulating contraction[9–13]. Phosphorylation of cMyBP-C by PKA at the M-domain, through β-adrenergic signaling, greatly reduces actin-myosin binding[14–17] probably by inducing a conformational change within C0-C2, as we, and others have shown[18, 19].
Future studies of cMyBP-C using mouse models and recombinant protein offer great promise for understanding the molecular underpinnings of this multifaceted myofilament protein function and associated human cardiac disease. However, it is unfortunate that the N-terminal residues of the mouse protein sequence currently remain undefined as it exists in the heart. An early mouse cMyBP-C cDNA sequence (e.g., GenBank: AF097333.1) is commonly used in studies of transgenic mice and recombinant proteins. It coincidentally starts at the same methionine as human (both translated protein sequences begin MPEP…). Importantly, this differs from the mouse genomic sequence (e.g., GenBank: AK146886.1 or AK146661.1) and numerous ESTs (e.g., GenBank: CA490691.1 or BY739189.1). The genomic sequence presumably provides the correct cMyBP-C protein sequence expressed in mouse myocardium. The cMyBP-C genomic and additional mouse ESTs contain an eight-residue N-terminal extension (NTE) of unknown importance preceding the starting methionine of the original mouse cDNA and human cMyBP-C sequences. In addition to the NTE, mouse and human sequences also vary significantly in the proline/alanine-rich linker (P/A) between C0 and C1 and this has been predicted to influence species-specific functions of cMyBP-C[9].
The aim of this study was to confirm the N-terminal sequence difference of cMyBP-C expressed in ventricles isolated from mouse or human (non-HCM) donors. We have then investigated, for the first time, the effects of species isoform differences on cMyBP-C N-terminal interactions with myofilaments. We also compared protein structural dynamics between the C0 and C1 domains (separated by the species-specific P/A) of the mouse (with and without its NTE) and human cMyBP-C. These findings aid in establishing a foundation of wild-type human and mouse characteristics of N-terminal cMyBP-C binding to myofilaments, providing mechanistic insight for determining perturbations to normal function due to HCM mutations and shedding light on species-specific differences in cMyBP-C interactions with myofilaments.
2. METHODS (See also: Online Supplement).
2.1. Isolation of ventricular mouse and human cMyBP-C by SDS-PAGE.
Mouse hearts were obtained from 3 commonly used WT strains (SVE/129, Black 6, and FVB/N, JAX Labs) by excision under euthanasia. Hearts were rinsed of blood in ice-cold Ringer’s solution containing 118 mM NaCl, 4.8 mM KCl, 2 mM NaH2PO4, 1.2 mM MgCl2, 25 mM HEPES, 11 mM glucose, and 0.5 mM CaCl2, pH 7.4, flash-frozen in liquid nitrogen, and stored at -80oC. Human ventricular tissue was from an explanted end-stage failing heart with dilated cardiomyopathy during heart transplant surgery, a kind gift from Dr. Zain Khalpey’s biobank at the University of Arizona. Cardiac myofibrils were prepared for electrophoresis as described previously[20], which is a modified protocol originally developed by Holroyode et al[21]. For human myofibrils cMyBP-C was extracted prior to SDS-PAGE, overnight at 22°C, with 10 mM EDTA 31 mM Na2HPO4, 124 mM NaH2PO4, pH 5.9[22, 23]. Full-length ventricular cMyBP-C samples from skinned cardiac myofibrils were separated by Coomassie stained SDS-PAGE for mass spectrometry analyses.
2.2. Mass Spectrometry acquisition and analyses.
Following SDS-PAGE, the gel bands suspected to be mouse or human cMyBP-C (~150 kD) were sent to Taplan Mass Spectrometry Facility at Harvard Medical School (Cambridge, MA) to identify protein peptide sequences. Sample identity was determined following in-gel digestion of the denatured band (trypsin for mouse; α-chymotrypsin for human), microcapillary LC/MS/MS analysis, protein database search and analysis, and web-based reporting of data[24].
2.3. Actin and myosin filament preparations.
Actin was prepared from rabbit skeletal muscle by extracting acetone powder in cold H2O as described in Prochniewicz [25] and Colson et al.[26] with minor modifications. Murine α-cardiac myosin was prepared as described in Wikman-Coffelt[27] and Uchida et al.[28] with minor modifications. See Online Supplement for details.
2.4. Recombinant C0-C2 preparations.
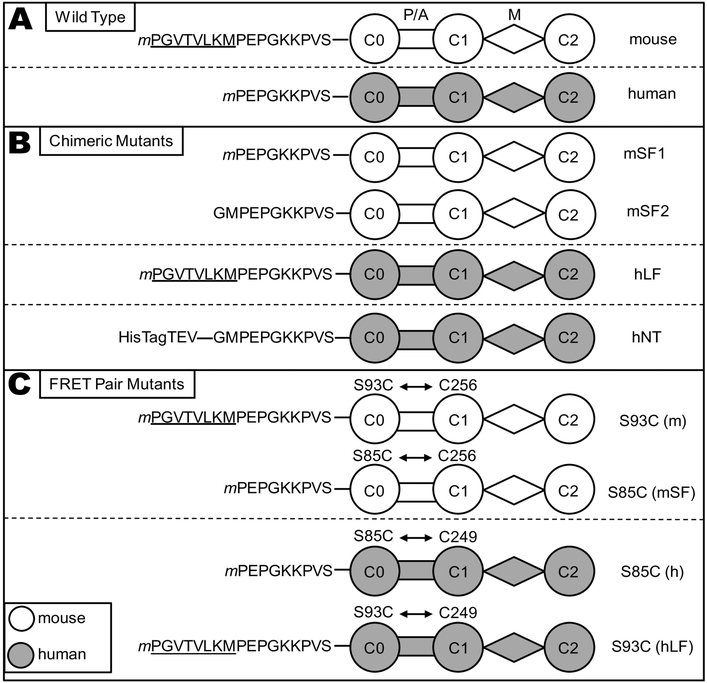
pET45b vectors encoding E. coli optimized codons for the C0-C2 portion of mouse and human cMyBP-C with N-terminal 6× His tag and TEV protease cleavage site were obtained from GenScript (Piscataway, NJ). In vitro mutagenesis was used to create versions where the TEV protease cleavage site and 6× His tags were located at the C-terminus. In addition to wild-type (WT) mouse and human C0-C2, four mutants were generated. Two mutants were used to test the importance of the mouse NTE for actin and myosin binding: mouse short form [mSF, lacking the 8 amino acid N-terminal extension (NTE) found in mouse but not human cMyBP-C] and human long form (hLF, containing the mouse NTE). Myosin binding experiments utilized mSF2 made as an N-terminal tagged fusion protein (following TEV protease cleavage to remove the tag the N-terminus begins GMPEP…) while actin binding and TR-FRET studies utilized mSF1 made as a C-terminal tagged fusion protein (N-terminus begins mPEP…). For TR-FRET studies four mutants were constructed: mouse S93C (previously S85C), human S85C, mSF1 S85C and hLF S93C. C0-C2 contains 5 native Cys; however, only a single Cys, mouse C256 (previously C248) and human C249, is readily labeled with thiol-reactive fluorescent probes under our labeling conditions (see Online Supplement). Thus, mutant C0-C2 for TR-FRET studies contained two reactive Cys to perform distance measurements between probes on C0 (S93C in mouse; S85C in human) and C1 (C256 in mouse; C249 in human) across the proline/alanine-rich linker (P/A) that is flexible and disordered[29, 30]. Mutations were engineered in the WT mouse/human C0-C2 using a Q5 Site-Directed Mutagenesis Kit (New England Bio Labs, Ipswich, MA). All sequences were confirmed by DNA sequencing (Eton Biosciences, San Diego, CA). For details of protein production and subsequent purification refer to the Online Supplement. TEV-cleaved C0-C2 was concentrated, dialyzed to 50/50 buffer (50 mM NaCl and 50 mM Tris pH 7.5) and stored at 4°C with 1 mM DTT added weekly and used for experiments within two weeks. DTT was not added to proteins to be labeled for TR-FRET.
2.5. TR-FRET-based distance measurements in C0-C2.
Mouse (S93C), human (S85C), mSF1 (S85C), and hLF (S93C) C0-C2 were labeled with donor/acceptor. Spectroscopic data acquisition for FRET experiments were performed at 25°C. TR-FRET waveforms were analyzed globally in model –independent and –dependent fittings. A single-Gaussian distance distribution, corresponding to the structural dynamics across the P/A from C0 to C1 was characterized by a center distance Rs and a full width at half-maximum Γs, was used to compare mouse and human structural dynamics. Labeling, spectroscopic data acquisition and FRET analyses are described in detail in the Online Supplement.
2.6. Cosedimentation assays.
Prior to actin or myosin binding, C0-C2 proteins were dialyzed into either actin binding buffer or myosin binding buffer and any insoluble proteins were removed by centrifugation for 30 minutes, 4°C, 120,000 rpm (513,000 x g) in a Beckman TLA-120.2 rotor in an Optima TLX ultracentrifuge (Beckman Coulter, Fullerton, CA). In the absence of actin or myosin, some sticking to centrifuge tubes of C0-C2 was observed. To reduce this, tubes were coated by incubating with 30 mg/ml BSA (4°C overnight or at room temperature for 2 hours) followed by rinsing with binding buffer. In all experiments identical samples were prepared in the absence of myosin or actin and the resulting background was subtracted from the cosedimenting values.
The affinity of native skeletal actin for C0-C2 was measured as in Rybakova et al.[31] and fully described in Online Supplement.
Binding of C0-C2 proteins to myosin was as described in Moos et al.[32]. Binding was done in myosin binding buffer (500 mM KCl, 0.5 mM DTT, 10 mM imidazole pH 7.0). A constant 0.78 μM of myosin (7.5 μg in 40 μl) was incubated with increasing amounts of C0-C2 for 20 minutes at room temperature. While vortexing, 160 μl of H2O was added to reduce the KCl to 100 mM and promote myosin filament formation. Following 30 minutes incubation on ice, myosin filaments and bound C0-C2 were collected by centrifugation (30 minutes, 4°C, 50,000 rpm (100,000 x g) in a TLA-100 rotor (Beckman). Pellets were rinsed with 200 μL of diluted myosin binding buffer (100 mM KCl, 2 mM imidazole pH 7) and then dissolved in 25 μl of SDS protein sample buffer (Invitrogen). C0-C2 and myosin were resolved by SDS-PAGE (4–12% NuPAGE Bis-Tris Gels with MOPS running buffer, Thermo Fischer Scientific), stained with Coomassie blue R-250, imaged using an Odyssey CLx, and quantitated using Image Studio (LI-Cor Biosciences).
2.7. Quantification of binding data.
The ratio of electrophoresis-separated band intensities of recombinant C0-C2 to actin or myosin in each lane loaded from the centrifuge tube of pelleted proteins was converted to a molar ratio (mole of cMyBP-C/mole of actin or myosin) using the known sizes of each protein. Staining intensity was linear over the amount of actin or myosin loaded in each cosedimentation assay.
Details of binding data fitting are described in the Online Supplement.
2.8. Statistics.
Sample means are from three or more independent experiments, and numbers of observations (n) are given in the figure legends. Each experiment was carried out using at least two independent protein preparations. Average data are provided as mean ± SE. Statistical significance was evaluated by use of an unpaired t-test.
3. RESULTS (See also: Online Supplement).
3.1. N-terminal sequence analysis of mouse and human cMyBP-C.
Examination of mouse genomic sequences (e.g., GenBank: AK146886.1, AK146661.1, NC_000068.7, AC016982.4) indicates that mouse cMyBP-C begins with 8 amino acids (the N-terminal extension, NTE) not present in human cMyBP-C. This extension is also found in a very closely related species the Ryukyu mouse (Mus caroli; XP_021006227.1) but not in the more distantly related shrew mouse (Mus pahari; XP_021048157.1) nor in rat (Ratus norvegicus; XP_006234565). This NTE is missing in an early mouse cMyBP-C cDNA sequence (e.g., GenBank: AF097333.1) that is commonly used in studies of transgenic mice and recombinant proteins. Failure to identify the upstream start methionine codon likely arose from an error in generation of the original cDNA resulting in a sequence lacking the NTE and beginning with the same methionine as human (both translated protein sequences begin MPEP). In addition to the genomic sequences establishing the existence of the NTE in mouse, the vast preponderance of cDNAs and ESTs from mouse contain the upstream start codon (e.g. GenBank: NM_008653.2, AK146886.1, AK146661.1, BC094945.1, CA490691.1, CA495773.1, CA495480.1, CA490571.1, CA490567.1, BY740080.1).
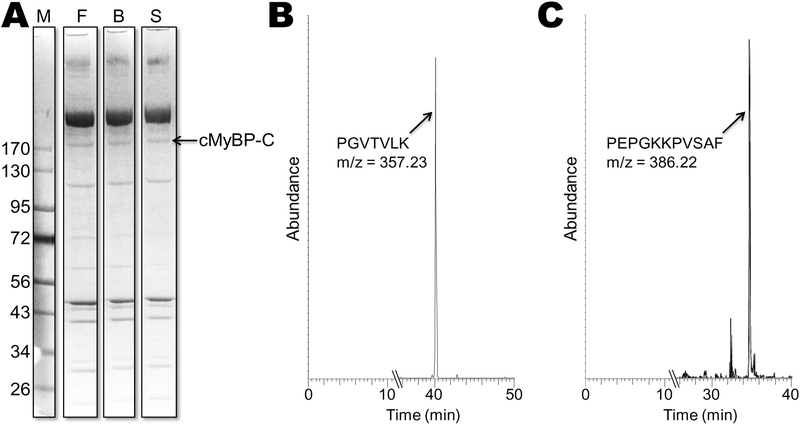
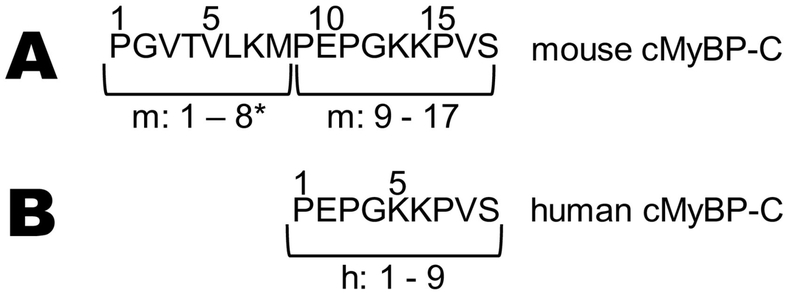
To confirm the addition of the 8 amino acids in mouse cMyBP-C, revealed from genomic and translated DNA databases, we examined the N-terminal cMyBP-C protein sequence expressed in ventricles of adult mouse and human samples. Proteins present in myofibrillar preparations from ventricles were separated by SDS-PAGE (Fig. 1A) and the band(s) corresponding to full-length cMyBP-C’s molecular weight ~150 kD were cut out of the gel and analyzed for amino acid sequence composition using mass spectrometry. Sequences of mouse and human ventricular samples were verified to be in agreement with genomic DNA on chromosome 11 of mouse and human cMyBP-C (NC_000077.6 and NC_000011.10, respectively) (Fig. 1B). As expected, LC-MS/MS confirmed that mouse cMyBP-C (n = 3 strains) contains an eight-residue N-terminal extension (NTE: mPGVTVLKM) that is not found in human cMyBP-C (Fig. 1C), preceding the canonical human N-terminal sequence mPEPGKKPV (Fig. 2). In addition, the starting methionine (m) of both mouse and human was removed by aminopeptidase following synthesis, leaving a non-acetylated proline as the first residue in ventricles of both species.
Fig. 1. Mass Spectrometry.
(A) Myofibrillar proteins from ventricle isolated from 3 mouse inbred strains (F, FVB/N; B, Black 6; and S, SVE129) separated by SDS-PAGE. The single cMyBP-C band was identified as running ~175 kD was prepared for LC-MS/MS analysis. (B) LC-MS/MS resulting total ion chromatogram of the trypsin peptide from the cMyBP-C band from one of the strains. This peptide peak (PGVTVLK; m/z = 357.23) was present in all 3 strains. (C) Ion chromatogram of the α-chymotrypsin peptide peak (PEPGKKPVSAF; m/z = 386.22) from cMyBP-C from a human ventricular sample.
Fig. 2. N-terminal sequences.
Amino acid sequences of (A) mouse and (B) human cMyBP-C N-termini prior to C0 (see PDB: 2K1M). Methionine residues were not present as starting residues in either group due to aminopeptidase-catalyzed methionine removal. Residues 1–8 of mouse cMyBP-C are the N-terminal extension (NTE)*.
We next confirmed that C0-C2 fusion proteins produced in E. coli contained the correct N-terminus. Mouse and human C0-C2 encoding protein sequences corresponding to the ventricular protein sequences were cloned into an E. coli expression vector that (pET45b) that included a C-terminal 6× His-tag to accommodate purification from bacterial cells. LC-MS/MS confirmed that in these C-terminal tagged human and mouse proteins the starting methionine is post-translationally removed, and each begins with their respective prolines. The recombinant C0-C2 forms used here are depicted in Fig. 3.
Fig. 3. C0-C2 constructs.
Schematic representation of the cMyBP-C “C0-C2” fragment constructs used in this study. (A) Wild Type C0-C2 constructs differ in their N-terminal sequence, with the mouse (white) isoform containing an N-terminal extension (NTE, underlined) relative to human (gray). All constructs, with the exception of mSF2 and hNT, (defined below) contain a TEV protease-cleavable C-terminal 6× His tag (extending from domain C2) for purification that is removed for functional and structural studies, (cleaved tags not shown). Starting methionine residues (m) were not present in the mature proteins. (B) Mouse short form (mSF1) has the 8-residue NTE removed from the N-terminus and used for actin binding and TR-FRET studies. A second mouse short form (mSF2) was constructed as an N-terminal tagged protein and the resulting protein after removal of the tag with TEV protease begins GMPEP (see methods) and used for myosin binding studies. Human long form (hLF) has the 8-residue mouse NTE added to the N-terminus. Human N-terminal tagged (hNT) C0-C2, used as an uncleaved control for myosin binding assays to non-specifically extend the N-terminus, contains a 6× His tag, 15 amino acids from the pET45 vector, and the TEV recognition sequence for a total of 28 amino acids at the amino terminus prior to GMPEP. (C) WT C0-C2 has a single endogenous Cys in domain C1 (C249 human, C256 mouse) that is readily labeled by thiol-reactive probes. A second Cys in C0 is introduced by site-directed mutagenesis (S85C in human; S93C in mouse) in order to measure FRET distances from C0 to C1. The S93C/S85C were also introduced into the mSF1/hLF chimeras for TR-FRET.
In addition to the NTE being absent in the original mouse C0-C2 cDNA (GenBank: AF097333.1) we also note variability in sequences used in previous experiments that differ from the wild type mouse sequence (NCBI NM_008653.2 and XM_011239341.2). See Online Supplement for details.
3.2. Reduced binding of mouse vs human C0-C2 to actin is not due to NTE.
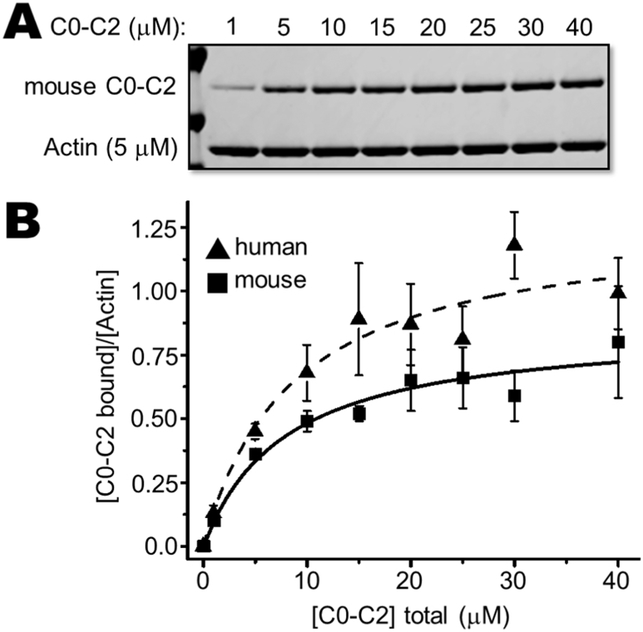
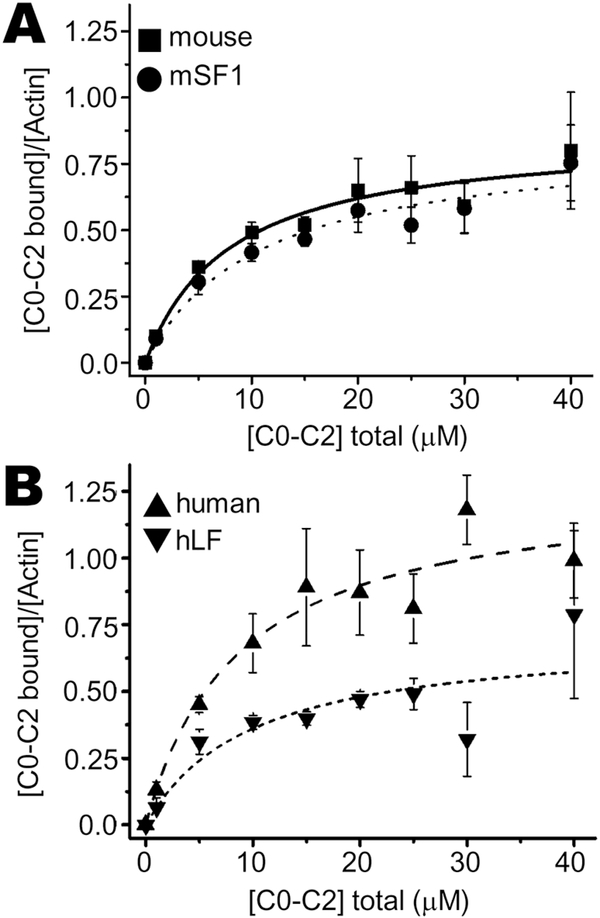
We quantified binding interactions between the cMyBP-C N-terminus C0-C2 (comprised of C0-P/A-C1-M-C2) and actin by measuring binding with F-actin using high speed cosedimentation assays. When mixed with actin filaments, C0-C2 pellets along with the F-actin (Fig. 4A). Binding was saturable at approximately a ~1:1 molar ratio of mouse C0-C2 bound to actin (Bmax = 0.86 ± 0.04) and a Kd of 7.9 ± 1.1 μM (Table 1). The apparent binding affinity of human C0-C2 (Kd of 8.6 ± 1.6 μM) to actin was indistinguishable from mouse C0-C2 binding (Table 1); however, human C0-C2 had a greater molar binding ratio than mouse (Fig. 4A, Table 1; Bmax = 1.28 ± 0.07 vs 0.86 ± 0.04, p < 0.02). Removal of the NTE from mouse C0-C2, mouse short form (mSF1), did not alter its binding to actin (Bmax = 0.85 ± 0.04, Kd of 10.7 ± 1.6 μM; Fig. 4B; Table 1). These data are consistent with earlier studies[16] that utilized mSF. Addition of the mouse NTE onto the human C0-C2 (hLF) interfered with actin binding (Fig. 5; Table 1).
Fig. 4. Actin cosedimentation assays: mouse versus human C0-C2.
Mouse cMyBP-C fragment of C0-C2 with its wild type NTE binding to actin filaments was determined by cosedimentation. (A) Representative high-speed cosedimentation binding experiment with increasing amounts of mouse C0-C2 and constant 5 μM actin. (B) Binding curves of wild type mouse and human C0-C2 fit to a quadratic equation (see Methods). Each experiment was done with independently prepared C0-C2 proteins (n = 4–6). See Table 1 for summary of actin binding results for apparent Kd and Bmax values.
Table 1:
Apparent dissociation constants (Kd) and molar binding ratios (Bmax) for binding of recombinant C0-C2 chimeric proteins to F-actin
| C0-C2 | Kd | Bmax |
|---|---|---|
| Mouse | 7.9 ± 1.1 | 0.86 ± 0.04* |
| human | 8.6 ± 1.6 | 1.28 ± 0.07 |
| mSF1 | 10.7 ± 1.6 | 0.85 ± 0.04* |
| hLF | 9.7 ± 1.0 | 0.72 ± 0.03**# |
Data represent mean ± S.E. Significant difference in Bmax relative to human C0-C2 (*p < 0.02; **p < 0.0003) or mouse C0-C2 (#p < 0.05).
Fig. 5. Actin cosedimentation assays: NTE chimeric mutants.
Chimeric NTE mutant cMyBP-C fragments of C0-C2 compared to wild type mouse or human C0-C2 binding to actin filaments was determined by cosedimentation. Cosedimentation binding experiment with increasing amounts of (A) mouse WT or mSF1 C0-C2, or (B) human WT or hLF C0-C2, and constant 5 μM actin. Binding curves fit to a quadratic equation (see Methods). Each experiment was done with independently prepared C0-C2 proteins (n = 4–6). See Table 1 for summary of actin binding results for chimeric C0-C2.
3.3. Mouse NTE strongly inhibits myosin filament binding.
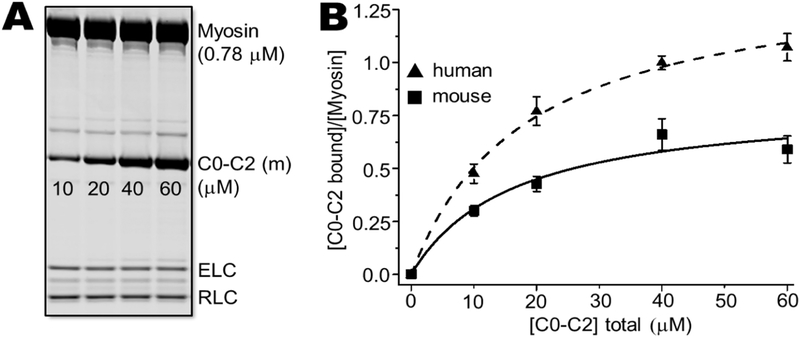
Mouse and human C0-C2 binding to myosin filaments have not been directly compared, to our knowledge. In cosedimentation assays, we found marked differences in binding between mouse and human C0-C2. Human C0-C2 bound myosin nearly twice as much (mol/mol) compared to mouse C0-C2 over five concentrations from 0 to 60 μM C0-C2 (Fig. 6). Curve fittings yields a 74% increase in binding for human versus mouse C0-C2 (mouse Bmax = 0.82 ± 0.05, human Bmax = 1.43 ± 0.02, p < 0.0001).
Fig. 6. Myosin cosedimentation assays: mouse versus human C0-C2.
Human and mouse C0-C2 (10–60 μM) were incubated with 0.78 μM cardiac myosin. Myosin filaments and bound C0-C2 were cosedimented and analyzed by SDS-PAGE. (A) SDS-PAGE gel showing for human C0-C2 binding. (B) Binding curves fit to a quadratic for mouse (squares) and human (triangles) C0-C2 binding to myosin. Each experiment was done with independently prepared C0-C2 proteins (n = 6).
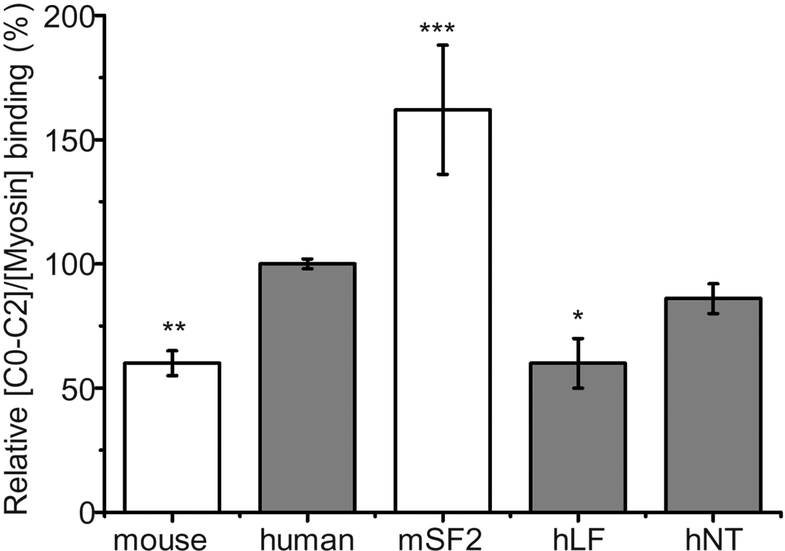
To determine if the difference in species isoform binding to myosin is due, in part, to the mouse NTE, we removed it from mouse C0-C2 and added it onto human C0-C2. We compared myosin cosedimentation of these variants with that exhibited by human C0-C2 at 40 μM C0-C2 added to 0.78 μM myosin (Fig. 7). At these concentrations, binding is near the beginning of saturation and there is a large separation between mouse and human binding (Fig. 6). Mouse C0-C2 binding is 60% that of the human isoform (Fig. 7; 8 separate experiments with different protein preps; 3–5 cosedimentations per experiment for a total of 33 samples each, p < 0.005). Removal of the NTE (mouse short form, mSF2) significantly increased the cosedimentation (p < 0.0001) to 162% that seen in human C0-C2 (3 experiments with different protein preps; 3–4 cosedimentations per experiment, 10–12 total samples each). Addition of the mouse NTE to human C0-C2 (human long form, hLF) reduced its cosedimentation (p < 0.007) to 60% wild type human C0-C2, a level that is the same as that seen with mouse C0-C2. An N-terminally tagged hC0-C2 containing 28 unrelated amino acids, hNT, did not significantly (p = 0.19) affect binding. These data suggest that the mouse NTE plays a major inhibitory role in reducing mouse cMyBP-C binding to myosin.
Fig. 7. Myosin cosedimentation assays: NTE chimeric mutants.
Myosin cosedimentation assays comparing mouse (white), human (gray) and N-terminal variant C0-C2 proteins of short form (no NTE) mouse C0-C2 (mSF2), long form (with the mouse NTE) human C0-C2 (hLF), and N-terminal tagged human C0-C2 (hNT) control. Mole fraction (relative to myosin) binding was determined for all forms at 40 μM C0-C2 and 0.78 μM mouse cardiac myosin. Values were normalized to the level of cosedimentation observed for human C0-C2 (see Methods). *p < 0.01, **p < 0.005, and ***p < 0.0001 indicate a significant difference as compared to wild type human C0-C2.
3.4. cMyBP-C FRET indicates conservation between species in the C0 to C1 spatial relationship.
Differences observed in mouse and human C0-C2 binding to myosin filaments might be the result of the NTE directly interfering with binding or alternatively the NTE could act indirectly by changing the spatial relationship between myosin binding sites in C0 and C1 as both of these domains in human cMyBP-C have been shown to interact with myosin [RLC and S2[10, 33]]. In addition to containing the unique NTE preceding C0, there is species variability in the P/A between C0 and C1 that could influence the distance between them and the activity of cMyBP-C[9]. We used time-resolved FRET (TR-FRET) to probe the spatial relationship of C0 to C1 in mouse and human proteins.
Intramolecular distance and dynamics measurements were made in engineered C0-C2 constructs with one probe in the C0 domain (S93C in mouse; S85C in human) and the other probe attached to the endogenous thiol-reactive Cys in domain C1 (C256 mouse; C249 human). These site pairs in mouse and human are located in analogous positions in each isoform, so differences in FRET lifetimes can be attributed to differences in the three-dimensional protein arrangement rather than differences in location of the probes within each protein.
Using IAEDANS donor and DDPM acceptor we employed FRET as a molecular ruler[34]. The presence (hLF) or absence (mSF1) of the NTE also had no effect on probes (Table 3, Fig. S1). Gaussian analysis of these FRET data gave a distance between probes centered around 2.9 nm for all chimeras. The full width at half-maximum Γs, a measure of the order/disorder was 1.6 nm (Table 3, Fig. S1).
Table 3:
Center distances and widths for C0-to-C1 TR-FRET in C0-C2
| C0-C2 | Center, R (nm) | Width, FWHM (nm) | |
|---|---|---|---|
| mouse | S93C | 2.8 ± 0.1 | 1.8 ± 0.1 |
| human | S85C | 2.9 ± 0.2 | 1.7 ± 0.3 |
| mSF | S85C | 3.0 ± 0.1 | 1.9 ± 0.1 |
| hLF | S93C | 3.0 ± 0.1 | 1.9 ± 0.2 |
Data represent mean ± S.E. No significant differences observed between centers or widths among chimeras.
4. DISCUSSION.
cMyBP-C is a crucial regulator of cardiac contractility and mutations in the gene encoding it are a leading genetic cause of inherited HCM. For this reason intensive work using mouse models exploring the functions of cMyBP-C is underway in several laboratories. Our studies suggest that there are significant differences between mouse and human cMyBP-C that need to be considered when translating results from mouse studies to the human protein. First, due to the presence of an upstream start codon the mouse protein contains 8 amino acids at its N-terminus that are not present in human cMyBP-C (Fig. 2) nor in mouse proteins used in many current studies both in vitro and in vivo. Second, the presence of this 8 amino acid NTE affects the interactions between cMyBP-C and myosin (Fig. 6). Third, we also present the first curves of human C0-C2 binding to actin (Fig. 4). Finally, in vitro molecular distances and dynamics between the first two Ig-like domains (C0 and C1) are similar among the mouse, mSF1, human, and hLF chimeras of cMyBP-C (Table 3, Fig. S1). One possibility consistent with these results is that the NTE interacts with myosin as an inhibitory peptide to reduce interactions at the C0-RLC interface.
Nucleotide sequences of numerous cDNAs and mouse genomic DNA indicate that the initial cDNA, which has been used for many, if not most, subsequent studies was missing 8 amino acids at its N-terminus. Instead it begins with a sequence identical to that found in the human protein. Mass spectroscopy analysis of the protein from 3 mouse strains and a human sample confirmed the presence of the 8 amino acid NTE in the mouse cMyBP-C protein. The predicted human N-terminus, lacking the NTE, was confirmed in human cardiac protein. While mass spectroscopy exhibits bias in the peptides it detects and thus might miss a potential mouse protein lacking the NTE or human protein containing it, these findings confirm the overwhelming genetic data. Mass spectroscopy also confirmed that in vivo the N-terminal methionine is removed leaving a non-acetylated proline as the first amino acid in both cases (Fig. 1B-C). Being non-acylated is expected to confer stability (a long half-life) on both mouse and human cMyBP-C proteins via the N-end-rule pathway[35].
Examination of other nucleotide sequences in available databases suggests that house mouse (Mus musculus) is not the only species to have additional sequences prior to the previously recognized start methionine. The closely related Mus caroli (Ryukyu or ricefield mouse) sequence contains the identical 8 amino acid NTE (XP_021006227.1) while more distantly related Mus pahari (shrewmouse) sequence (XP_021048157.1) apparently begins at the same initial methionine as is found human cMyBP-C. The hypothetical desert woodrat cMyBP-C sequence (OBS65447.1) appears to have 15 rather divergent amino acids preceding C0. Further investigation is warranted to determine whether the NTE’s in these particular small mammals have evolved to reduce myosin binding to accommodate high heart rates or tuning of other related features of the myocardium such as reduced myofilament sliding inhibition or viscoelastic load.
N-terminal domains of cMyBP-C, the “business end” of the molecule, are thought to regulate contractility through their interactions with actin and myosin and we have examined the effect of the NTE on these interactions. Binding of wild type (containing the NTE) mouse cMyBP-C to actin filaments in cosedimentation assays (Fig. 4A-B, Table 1) appears to be very similar to that observed in previous work that used mouse cMyBP-C without the NTE[16]. Comparison of mouse to human C0-C2 binding to actin using cosedimentation indicates similar affinities but species-specific differences may be important to actin binding as 50% more human protein was seen to bind F-actin (Fig. 4A-B, Table 1). Addition of the mouse NTE to the human protein significantly reduced its binding (Fig. 5, Table 1). More work would be required to explain the mechanism of this interference in this interspecies hybrid protein. We can speculate that the NTE may somehow interact with actin binding residues in C0 thereby reducing its ability to bind actin. We speculate that the mouse C0 domain evolved to accommodate the NTE so that these interactions do not occur resulting in no effect of the NTE on its ability to bind actin. We note that these studies address actin binding, whereas in the intact system actin is decorated with troponin and tropomyosin, which could substantially alter the binding affinities.
To examine the effect of the mouse NTE on the ability of cMyBP-C to interact with myosin, we first established binding curves for wild type mouse and human C0-C2 with mouse cardiac myosin and then proceeded to examination of mutations removing the NTE from mouse and adding it to human.
We find highly significant differences between mouse and human C0-C2 binding to and cosedimenting with mouse cardiac myosin. Mouse C0-C2 saturated binding at approximately half the levels of human C0-C2 (Fig. 6, Table 2). It appears that this difference may be directly mediated by the NTE as its removal from mouse C0-C2 increases myosin binding and its addition to human C0-C2 reduces binding to levels seen in the mouse protein. As we comment above for actin binding, negative results obtained from an interspecies chimeric protein, where mouse NTE has been added to a human protein not evolved to accommodate it, should be viewed with caution. However, the complementary positive results where removal of the NTE from mouse C0-C2 dramatically increases binding to myosin strongly suggests a direct role for the NTE. The lack of significant inhibition by an alternative 28 amino acids added to the human protein (hNT, Fig. 7) is consistent with a role for the specific amino acids in the NTE as opposed to steric hindrance of random amino acids at the N-terminus.
Table 2:
Apparent dissociation constants (Kd) and molar binding ratios (Bmax) for binding of recombinant C0-C2 chimeric proteins to myosin filaments
| C0-C2 | Kd | Bmax |
|---|---|---|
| mouse | 16.5 ± 3.0 | 0.82 ± 0.05*** |
| human | 18.2 ± 0.9 | 1.43 ± 0.02 |
Data represent mean ± S.E. Significant difference in Bmax relative to human C0-C2 (***p < 0.0001).
The balance of α versus β myosin isoform present in cardiac muscle is a major determinant of cross-bridge kinetics [36–38], which is further modulated by cMyBP-C [39]. It is important to note that mouse cardiac myosin, used here, is mainly the α-myosin isoform and further work will be needed to determine if these differences extend to β-myosin (the predominant form in human ventricular tissue). With this caveat in mind it is tempting to speculate that the reduced binding to myosin or actin by mouse cMyBP-C that we observe has evolved in response to the increased heart rate of mice over other mammals[40]. One possibility is that human cMyBP-C (within the C0-C2 portion) interacts with both RLC and S2, whereas mouse cMyBP-C interacts with only one site. This would result in reduced cMyBP-C interactions with the myofilament to accommodate high heart rates, whereas humans and other larger mammals lacking the inhibitory NTE would exhibit enhanced cMyBP-C-myofilament binding in diastole.
NMR chemical shift perturbations in C0 observed as it interacts with RLC and mini-HMM (a short portion of heavy meromyosin to which the RLCs bind, as in[10]) places one of the three interaction surfaces directly adjacent to the 9 amino acids preceding C0 in human cMyBP-C. These results suggest that it is the RLC binding interface that is most likely to be impaired by the NTE. The lack of evidence for simple steric hindrance suggests that residues of the NTE might interfere with C0-RLC interaction surfaces by folding back onto C0 Ig-like domain binding residues necessary for RLC binding, thereby masking them. Whatever the mechanism, NTE inhibition, if it acts through RLC it would extend to situations where mouse cMyBP-C interacts with either α or β-myosin as both contain the same RLC isoform.
In addition to the mouse NTE, other amino acids attached to the amino terminus may have effects on myosin interactions and care should be taken when attaching additional amino acids to the N-terminus of cMyBP-C proteins. Our findings of N-terminal function using chimeric proteins are also consistent with the idea that NTE is a major regulator of cMyBP-C binding to myosin, and this knowledge could even be exploited for development of cMyBP-C based therapies to tune contractility in cardiac disorders. We note that HCM mutations in C0 have been reported to reduce binding the RLC, which could act to release inhibition of myosin heads by cMyBP-C resulting in hypercontractility in the disease pathology.
We envision that cMyBP-C interaction with actin and myosin can be modulated by species-specific amino acid differences directly by changing residues responsible for contacts or indirectly by changing distances and dynamics between interaction surfaces. To initiate the assessment of species-specific domain relationships we have compared the distances and dynamics between sites on C0 and C1 in mouse and human cMyBP-C. As both of these domains contain proposed myosin interaction sites, the distance and degree of order/disorder between them are predicted to influence myofilament interactions[30]. No detectable differences in the spatial relationship of C0 to C1 were observed for the mouse and human proteins or the mSF1 and hLF mutants (Table 3, Fig. S1). This lack of structural variability among the 4 chimeras is consistent with the NTE interacting with the adjacent C0 binding site for myosin.
It remains unknown whether these changes in binding that we observe in solution significantly impact functional interactions in the sarcomere. Spatial relationships between N-terminal domains when bound to actin and/or myosin will likely be different from those we have measured for isolated C0-C2 proteins in solution. Importantly, in vivo cMyBP-C interacts with thin filaments not bare actin.
Previous in vitro studies of mSF cMyBP-C remain important and valid. The NTE does not appear to affect the overall unbound N-terminal structure as indicated by no significant difference in distances between sites on C0 and C1 (Fig. 5A, Table 1). Actin binding is un-altered by the presence or absence of the NTE in the mouse protein. A major effect on myosin binding is seen and studies of mSF and myosin interactions would be subject to new interpretations. However, most studies of myosin binding have used human cMyBP-C [10, 15].
Finally, the NTE’s affect on in vitro binding and in vivo contractile kinetics may be modulated by phosphorylation of cMyBP-C in the motif region between C1 and C2. When cMyBP-C is not phosphorylated, as in our work here, the balance between actin versus myosin binding is different in mSF and mouse wild type cMyBP-C due to the strong effect of the NTE on myosin binding and the absence of an effect on actin binding. These differences may contribute to species-specific differences between human and mouse responses to the phosphorylation state of cMyBP-C.
5. CONCLUSIONS.
The major finding of our study is that mouse cMyBP-C has an 8 amino acid NTE that is not found in the human protein and this greatly reduces its binding to myosin (Fig. 7). These results are consistent with the idea that the NTE, rather than other species-specific sequence variations, is a regulator of myosin binding possibly mediated through interactions with the RLC. It remains that other sequence differences between humans and mice, besides the NTE, could possibly play a role in this inhibitory myosin-binding mechanism of cMyBP-C (Fig. 6, Table 2). In contrast to these robust myosin-binding effects, we did not detect any differences in actin binding affinity or TR-FRET distance analyses due to the NTE.
Importantly, we also observed that human N-terminal cMyBP-C bound both myosin and actin filaments with a significantly greater molar occupancy than mouse. Here, the NTE plays a role in myosin but not actin binding. Thus, both the NTE and other species-specific sequences play roles in increased binding stoichiometry of actomyosin in humans as compared to mouse.
It is clear that the N-terminal extensions, domains, and linkers of cMyBP-C confer plasticity, and tunability of cardiac performance. They therefore offer promise for devising preventative and/or curative strategies for cardiomyopathy and other cardiac disorders. While wild-type (i.e., containing the NTE) sequences should be used for studying mouse cMyBP-C, studies performed with mouse cMyBP-C lacking the NTE conceivably hold enhanced impact in the broader context of understanding the human cMyBP-C due to interactions with myosin that have been humanized. Future work will need to resolve whether removal of the NTE has been fortuitous or has complicated study of mechanisms of human cardiac function and disease.
Supplementary Material
Highlights.
Mouse cMyBP-C contains an 8 residue N-terminal extension (NTE) not found in human.
Mouse and human N-terminal sequences of cMyBP-C are confirmed by mass spectrometry.
cMyBP-C binding to cardiac myosin is strongly reduced by the presence of the NTE.
Human N-terminal cMyBP-C may bind myosin at two sites and mouse cMyBP-C at mainly one site.
cMyBP-C biochemical and biophysical properties provide key mechanistic insight.
Acknowledgments:
We thank Dr. Henk Granzier at the University of Arizona for his critical review of the manuscript. We give special thanks to Alfred Gallegos for his assistance in the isolation of mouse hearts. LC-MS/MS experiments were performed at Taplin Mass Spectrometry Facility at Harvard Medical School.
Sources of Funding: This work was supported in part by an NIH grant R00HL122397 to B.A.C., and a University of Arizona Sarver Heart Center “Novel Research Project Award in the Area of Cardiovascular Disease and Medicine” to B.A.C.
Non-standard Abbreviations and Acronyms:
- cMyBP-C
cardiac myosin-binding protein C
- HCM
hypertrophic cardiomyopathy
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- NTE
N-terminal extension
- TR-FRET
time-resolved fluorescence/Förster resonance energy transfer
- C0-C2
N-terminal fragment of cMyBP-C comprised of C0-P/A-C1-M-C2 domains and linkers
- P/A
proline/alanine-rich linker between domains C0 and C1; ESTs, expressed sequence tags
- hWT
wild-type human C0-C2
- mWT
wild-type mouse C0-C2
- hLF
long form of human C0-C2
- hNT
N-terminal tagged human C0-C2
- mSF
short form of mouse C0-C2
- DDPM
N-(4-(dimethylamino)-3,5-dinitrophenyl)-maleimide
- IAEDANS
(1,5-IAEDANS, 5-((((2-Iodoacetyl)amino)ethyl)amino) Naphthalene-1-Sulfonic Acid)
- Rs
distance (Å)
- R0
Förster distance of donor-acceptor pair at which the energy transfer efficiency is 50%, Γs, full width at half maximum
- F
actin, filamentous actin
- DTT
dithiothreitol
- Kd
dissociation constant
- Bmax
maximum molar binding ratio
- m
starting methionine that is post-translationally removed
- M
domain, phosphorylatable linker between C1 and C2
- RLC
myosin regulatory light chain
- S2
myosin subfragment-2
- WT
wild-type
Footnotes
Disclosures: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Semsarian C, Ingles J, Maron MS, Maron BJ, New perspectives on the prevalence of hypertrophic cardiomyopathy, J Am Coll Cardiol 65(12) (2015) 1249–54. [DOI] [PubMed] [Google Scholar]
- [2].Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW, G. American College of Cardiology Foundation/American Heart Association Task Force on Practice, 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons, J Am Coll Cardiol 58(25) (2011) e212–60. [DOI] [PubMed] [Google Scholar]
- [3].Theis JL, Bos JM, Theis JD, Miller DV, Dearani JA, Schaff HV, Gersh BJ, Ommen SR, Moss RL, Ackerman MJ, Expression patterns of cardiac myofilament proteins: genomic and protein analysis of surgical myectomy tissue from patients with obstructive hypertrophic cardiomyopathy, Circ Heart Fail 2(4) (2009) 325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xu Q, Dewey S, Nguyen S, Gomes AV, Malignant and benign mutations in familial cardiomyopathies: insights into mutations linked to complex cardiovascular phenotypes, J Mol Cell Cardiol 48(5) (2010) 899–909. [DOI] [PubMed] [Google Scholar]
- [5].Tardiff JC, Carrier L, Bers DM, Poggesi C, Ferrantini C, Coppini R, Maier LS, Ashrafian H, Huke S, van der Velden J, Targets for therapy in sarcomeric cardiomyopathies, Cardiovasc Res 105(4) (2015) 457–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Luther PK, Winkler H, Taylor K, Zoghbi ME, Craig R, Padron R, Squire JM, Liu J, Direct visualization of myosin-binding protein C bridging myosin and actin filaments in intact muscle, Proc Natl Acad Sci U S A 108(28) (2011) 11423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stelzer JE, Patel JR, Walker JW, Moss RL, Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation, Circ Res 101(5) (2007) 503–11. [DOI] [PubMed] [Google Scholar]
- [8].Harris SP, Bartley CR, Hacker TA, McDonald KS, Douglas PS, Greaser ML, Powers PA, Moss RL, Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice, Circ Res 90(5) (2002) 594–601. [DOI] [PubMed] [Google Scholar]
- [9].Shaffer JF, Harris SP, Species-specific differences in the Pro-Ala rich region of cardiac myosin binding protein-C, J Muscle Res Cell Motil 30(7–8) (2009) 303–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ratti J, Rostkova E, Gautel M, Pfuhl M, Structure and interactions of myosin-binding protein C domain C0: cardiac-specific regulation of myosin at its neck?, J Biol Chem 286(14) (2011) 12650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].De Lange WJ, Grimes AC, Hegge LF, Spring AM, Brost TM, Ralphe JC, E258K HCM-causing mutation in cardiac MyBP-C reduces contractile force and accelerates twitch kinetics by disrupting the cMyBP-C and myosin S2 interaction, The Journal of General Physiology 142(3) (2013) 241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kampourakis T, Yan Z, Gautel M, Sun YB, Irving M, Myosin binding protein-C activates thin filaments and inhibits thick filaments in heart muscle cells, Proceedings of the National Academy of Sciences of the United States of America 111(52) (2014) 18763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Harris SP, Belknap B, Van Sciver RE, White HD, Galkin VE, C0 and C1 N-terminal Ig domains of myosin binding protein C exert different effects on thin filament activation, Proc Natl Acad Sci U S A 113(6) (2016) 1558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gautel M, Zuffardi O, Freiburg A, Labeit S, Phosphorylation switches specific for the cardiac isoform of myosin binding protein-C: a modulator of cardiac contraction?, EMBO J 14(9) (1995) 1952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gruen M, Gautel M, Mutations in beta-myosin S2 that cause familial hypertrophic cardiomyopathy (FHC) abolish the interaction with the regulatory domain of myosin-binding protein-C, J Mol Biol 286(3) (1999) 933–49. [DOI] [PubMed] [Google Scholar]
- [16].Shaffer JF, Kensler RW, Harris SP, The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner, J Biol Chem 284(18) (2009) 12318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Weith AE, Previs MJ, Hoeprich GJ, Previs SB, Gulick J, Robbins J, Warshaw DM, The extent of cardiac myosin binding protein-C phosphorylation modulates actomyosin function in a graded manner, Journal of Muscle Research and Cell Motility 33(6) (2012) 449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Colson BA, Thompson AR, Espinoza-Fonseca LM, Thomas DD, Site-directed spectroscopy of cardiac myosin-binding protein C reveals effects of phosphorylation on protein structural dynamics, Proc Natl Acad Sci U S A 113(12) (2016) 3233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Previs MJ, Mun JY, Michalek AJ, Previs SB, Gulick J, Robbins J, Warshaw DM, Craig R, Phosphorylation and calcium antagonistically tune myosin-binding protein C’s structure and function, Proc Natl Acad Sci U S A 113(12) (2016) 3239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Colson BA, Patel JR, Chen PP, Bekyarova T, Abdalla MI, Tong CW, Fitzsimons DP, Irving TC, Moss RL, Myosin binding protein-C phosphorylation is the principal mediator of protein kinase A effects on thick filament structure in myocardium, J Mol Cell Cardiol 53(5) (2012) 609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Holroyde MJ, Small DA, Howe E, Solaro RJ, Isolation of cardiac myofibrils and myosin light chains with in vivo levels of light chain phosphorylation, Biochim Biophys Acta 587(4) (1979) 628–37. [DOI] [PubMed] [Google Scholar]
- [22].Offer G, Moos C, Starr R, A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization, J Mol Biol 74(4) (1973) 653–76. [DOI] [PubMed] [Google Scholar]
- [23].Hartzell HC, Glass DB, Phosphorylation of purified cardiac muscle C-protein by purified cAMP-dependent and endogenous Ca2+-calmodulin-dependent protein kinases, J Biol Chem 259(24) (1984) 15587–96. [PubMed] [Google Scholar]
- [24].Licklider LJ, Thoreen CC, Peng J, Gygi SP, Automation of nanoscale microcapillary liquid chromatography-tandem mass spectrometry with a vented column, Anal Chem 74(13) (2002) 3076–83. [DOI] [PubMed] [Google Scholar]
- [25].Prochniewicz E, Walseth TF, Thomas DD, Structural dynamics of actin during active interaction with myosin: different effects of weakly and strongly bound myosin heads, Biochemistry 43(33) (2004) 10642–52. [DOI] [PubMed] [Google Scholar]
- [26].Colson BA, Rybakova IN, Prochniewicz E, Moss RL, Thomas DD, Cardiac myosin binding protein-C restricts intrafilament torsional dynamics of actin in a phosphorylation-dependent manner, Proc Natl Acad Sci U S A 109(50) (2012) 20437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wikman-Coffelt J, Zelis R, Fenner C, Mason DT, Myosin chains of myocardial tissue. I. Purification and immunological properties of myosin heavy chains, Biochem Biophys Res Commun 51(4) (1973) 1097–104. [DOI] [PubMed] [Google Scholar]
- [28].Uchida K, Murakami U, Hiratsuka T, Purification of cardiac myosin from rat heart Proteolytic cleavage and its inhibition, J Biochem 82(2) (1977) 469–76. [PubMed] [Google Scholar]
- [29].Lu Y, Kwan AH, Trewhella J, Jeffries CM, The C0C1 fragment of human cardiac myosin binding protein C has common binding determinants for both actin and myosin, J Mol Biol 413(5) (2011) 908–13. [DOI] [PubMed] [Google Scholar]
- [30].Jeffries CM, Lu Y, Hynson RM, Taylor JE, Ballesteros M, Kwan AH, Trewhella J, Human cardiac myosin binding protein C: structural flexibility within an extended modular architecture, J Mol Biol 414(5) (2011) 735–48. [DOI] [PubMed] [Google Scholar]
- [31].Rybakova IN, Greaser ML, Moss RL, Myosin binding protein C interaction with actin: characterization and mapping of the binding site, J Biol Chem 286(3) (2011) 2008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Moos C, Offer G, Starr R, Bennett P, Interaction of C-protein with myosin, myosin rod and light meromyosin, J Mol Biol 97(1) (1975) 1–9. [DOI] [PubMed] [Google Scholar]
- [33].Ababou A, Rostkova E, Mistry S, Le Masurier C, Gautel M, Pfuhl M, Myosin binding protein C positioned to play a key role in regulation of muscle contraction: structure and interactions of domain C1, J Mol Biol 384(3) (2008) 615–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stryer L, Fluorescence energy transfer as a spectroscopic ruler, Annu Rev Biochem 47 (1978) 819–46. [DOI] [PubMed] [Google Scholar]
- [35].Varshavsky A, The N-end rule pathway and regulation by proteolysis, Protein Sci 20(8) (2011) 1298–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Locher MR, Razumova MV, Stelzer JE, Norman HS, Moss RL, Effects of low-level α-myosin heavy chain expression on contractile kinetics in porcine myocardium, Am J Physiol Heart Circ Physiol 300(3) (2011) H869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Locher MR, Razumova MV, Stelzer JE, Norman HS, Patel JR, Moss RL, Determination of rate constants for turnover of myosin isoforms in rat myocardium: implications for in vivo contractile kinetics, Am J Physiol Heart Circ Physiol 297(1) (2009) H247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rundell VL, Manaves V, Martin AF, de Tombe PP, Impact of beta-myosin heavy chain isoform expression on cross-bridge cycling kinetics, Am J Physiol Heart Circ Physiol 288(2) (2005) H896–903. [DOI] [PubMed] [Google Scholar]
- [39].Tanner BC, Wang Y, Robbins J, Palmer BM, Kinetics of cardiac myosin isoforms in mouse myocardium are affected differently by presence of myosin binding protein-C, Journal of Muscle Research and Cell Motility 35(5–6) (2014) 267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Spector WS, Handbook of biological data, Saunders, Philadelphia, 1956. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.