Abstract
In most species, size homeostasis appears to be exerted in late G1 phase as cells commit to division, called Start in yeast and the Restriction Point in metazoans. This size threshold couples cell growth to division, and, thereby, establishes long-term size homeostasis. Our former investigations have shown that hundreds of genes markedly altered cell size under homeostatic growth conditions in the opportunistic yeast Candida albicans, but surprisingly only few of these overlapped with size control genes in the budding yeast Saccharomyces cerevisiae. Here, we investigated one of the divergent potent size regulators in C. albicans, the Myb-like HTH transcription factor Dot6. Our data demonstrated that Dot6 is a negative regulator of Start, and also acts as a transcriptional activator of ribosome biogenesis (Ribi) genes. Genetic epistasis uncovered that Dot6 interacted with the master transcriptional regulator of the G1 machinery, SBF complex, but not with the Ribi and cell size regulators Sch9, Sfp1, and p38/Hog1. Dot6 was required for carbon-source modulation of cell size, and it is regulated at the level of nuclear localization by the TOR pathway. Our findings support a model where Dot6 acts as a hub that integrates growth cues directly via the TOR pathway to control the commitment to mitotic division at G1.
Keywords: cell size, ribosome biogenesis, cell growth, cell division, transcriptional rewiring
IN a eukaryotic organism, cell size homeostasis is maintained through a balanced coordination between cell growth and division. In the last half century, a major focus of cell biology has been the study of cell division, but how eukaryotic cells couple growth to division to maintain a homeostatic size remains poorly understood. In most eukaryotic organisms, reaching a critical cell size appears to be crucial for commitment to cell division in late G1 phase, called Start in yeast and the Restriction Point in metazoans (Turner et al. 2012). Start is dynamically regulated by nutrient status, pheromones, and stress, and facilitates adaptation to changing environmental conditions in microorganisms to maximize their fitness (Lenski and Travisano 1994; Kafri et al. 2016).
Different genome-wide genetic analyses have been accomplished in different model organisms to uncover the genetic determinism of Start and cell size control in eukaryotes. Screens of Saccharomyces cerevisiae mutants has identified many ribosome biogenesis (Ribi) genes as small size mutants (whi) (Jorgensen et al. 2002; Dungrawala et al. 2012; Soifer and Barkai 2014), and revealed two master regulators of Ribi gene expression—the transcription factor Sfp1 and the AGC family kinase Sch9—as the smallest mutants (Jorgensen et al. 2004). These observations lead to the hypothesis that the rate of ribosome biogenesis is a critical element of the metric that dictates cell size (Jorgensen and Tyers 2004; Schmoller and Skotheim 2015). Sfp1 and Sch9 are critical effectors of the TOR pathway and form part of a dynamic, nutrient-responsive network that controls the expression of Ribi genes and ribosomal protein genes (Jorgensen et al. 2004; Marion et al. 2004; Urban et al. 2007; Lempiäinen et al. 2009). Sch9 is phosphorylated and activated by TOR, and, in turn, inactivates a cohort of transcriptional repressors of RP genes called Dot6, Tod6, and Stb3 (Huber et al. 2011).
Candida albicans is a diploid ascomycete yeast that is an important commensal and opportunistic pathogen in humans. While C. albicans and S. cerevisiae colonize different niches, common biological features are shared between the two yeasts, including the morphological trait of budding, and core cell cycle and growth regulatory mechanisms (Berman 2006; Côte et al. 2009). C. albicans has served as an important evolutionary milestone with which to assess evolutionary conservation of biological mechanism, and recent evidence suggests a surprising extent of rewiring of central signaling, transcriptional, and metabolic networks as compared to S. cerevisiae (Lavoie et al. 2009; Blankenship et al. 2010; Li and Johnson 2010; Sandai et al. 2012). To assess the conservation of the size control network, we performed recently a quantitative genome-wide analysis of a systematic collection of gene deletion strains in C. albicans (Sellam et al. 2016; Chaillot et al. 2017). Our screens uncovered that cell size in C. albicans is a complex trait that depends on diverse biological processes such as ribosome biogenesis, mitochondrial functions, cell cycle control, and metabolism. In addition to conserved mechanisms and regulators previously identified in S. cerevisiae and metazoans, we uncovered many novel regulatory circuits that govern critical cell size at Start specifically in C. albicans. In particular, we delineated a novel stress-independent function of the p38/HOG MAPK pathway as a critical regulator of both growth and division, and poised to exert these functions in a nutrient-sensitive manner (Sellam et al. 2016). Interestingly, some of the size genes identified were required for fungal virulence, suggesting that cell size homeostasis may be elemental to C. albicans fitness inside the host.
An unexpectedly potent negative Start regulator that emerges from our systematic screen was Dot6, which encodes a Myb-like HTH transcription factor that binds to the PAC (Polymerase A and C) motif GATGAG (Enfert and Hube 2007; Zhu et al. 2009; Sellam et al. 2016; Chaillot et al. 2017). dot6 was among the smallest mutant identified by our screen. C. albicans Dot6 is the ortholog of two redundant transcriptional repressors of rRNA and Ribi gene expression called Dot6 and Tod6 in S. cerevisiae, which are antagonized by Sch9, and which cause only a minor large-size phenotype when deleted together (Huber et al. 2011). Here, we show that the C. albicans Dot6 is a potent size regulator that governs critical cell size at Start, and, in an opposite role to that in S. cerevisiae, Dot6 acts as a transcriptional activator of Ribi genes. We also showed that the TOR pathway relays nutrient-dependent signal for size control to the Start machinery via Dot6. Genetic interactions with deletions of different known Start regulators revealed epistatic interaction with the master transcriptional regulator of the G1-S transition, SBF complex (Swi4-Swi6), but not with SCH9, SFP1, or HOG1. These data emphasize the evolutionary divergence between C. albicans and S. cerevisiae, and consolidate the role of Tor1-Dot6 network as a key cell size control mechanism in C. albicans.
Materials and Methods
Growth conditions and strains
The strains used in this study are listed in Supplemental Material, Table S1. C. albicans strains were generated and propagated using standard yeast genetics methods. For general propagation and maintenance conditions, the strains were cultured at 30° in yeast-peptone-dextrose (YPD) medium supplemented with uridine (2% Bacto-peptone, 1% yeast extract, 2% dextrose, and 50 µg/ml uridine) or in Synthetic Complete medium (SC; 0.67% yeast nitrogen base with ammonium sulfate, 2% glucose, and 0.079% complete supplement mixture). To assess the size of hyphal cells, both wild type (WT) (SFY87) and dot6 mutant cells were grown at 37° in YPD supplemented with 10% fetal bovine serum (FBS) for 3 hr.
The DOT6-Δ[1555–1803] truncated mutant was generated by inserting a STOP codon using CRISPR-Cas9 mutagenesis system (Vyas et al. 2015). Guide RNA (gRNA) was generated by annealing the Dot6-gRNA-Top and Dot6-gRNA-Bottom primers. Repair template was created using Dot6-STOP-Top and Dot6-STOP-Bottom primers (Table S2). The C. albicans SC5314 strain was cotransformed with the linearized plasmid pV1093 containing Dot6-gRNA with the repair template using lithium acetate transformation procedure and selected in Nourseothricin (Jena Bioscience). DOT6 truncation was confirmed by sequencing.
For the complementation assay in S. cerevisiae, the complete ORF of C. albicans DOT6 was amplified using XbaI-Dot6Ca-F and HindIII-Dot6Ca-R primers, and the resulting PCR fragments were cloned into the yeast pAG415GPD-ccdB plasmid (Susan Lindquist laboratory). The S. cerevisiae WT (Y2092) and dot6tod6 (Y3707) strains were then transformed with either the empty pAG415GPD-ccdB or pAG415GPD-CaDOT6-ccdB plasmids using a standard lithium-acetate-based procedure (Chen et al. 1992).
Cell size assessment
Cell size distributions were obtained using the Z2-Coulter Counter (Beckman, Fullerton, CA). C. albicans cells were grown overnight in YPD at 30°, diluted 1000-fold into fresh YPD or SC media and grown for 4 hr at 30° to an early log phase density of 5 × 106–107 cells/ml. A fraction of 100 µl of log phase culture was diluted in 10 ml of Isoton II electrolyte solution, sonicated three times for 10 sec, and the distribution measured at least three times on a Z2-Coulter Counter. Size distributions were normalized to cell counts in each of 256 size bins, and size is reported as the peak median value for the distribution. Data analysis and clustering of size distributions were performed using custom R scripts (Sellam et al. 2016).
Start characterization
The critical cell size at Start was determined by plotting budding index as a function of size in synchronous G1 phase fractions obtained using a JE-5.0 elutriation rotor with a 40 ml chamber in a J6-Mi centrifuge (Beckman) as described previously (Tyers et al. 1993). C. albicans G1 phase cells were released in fresh YPD medium, and fractions were harvested at intervals of 10 mins to monitor bud index. For the dot6 mutant and the WT strains, additional size fractions were collected to assess transcript levels of the RNR1, PCL2, and ACT1 using qPCR (quantitative real time PCR) as cells progressed through G1 phase at progressively larger sizes.
Growth assays
C. albicans cells were resuspended in fresh SC at an OD600 of 0.05. A total volume of 99 μl cells was added to each well of a flat-bottom 96-well plate in addition to 1 μl of the corresponding stock solution of either rapamycin or cycloheximide (Sigma). Growth assay curves were performed in triplicate in 96-well plate format using a Sunrise plate-reader (Tecan) at 30° under constant agitation.
In vivo GFP reporter assays
The GFP reporter assay was performed by replacing the ORF of PNO1 (Orf19.7618) by GFP coding sequence (Schaub et al. 2006) in its actual chromatin environment. pPNO1-GFP-F1/PNO1-GFP-R1 and pPNO1mut-GFP-F1/PNO1-GFP-R1 primer pairs were used to generate pPNO1-GFP and pPNO1-mut-GFP PCR cassettes that contains an intact (GATGAG) and a shuffled (ATGGAG) PAC motif, respectively. These cassettes were integrated into the WT (SN152) strain and isogenic strain deleted for DOT6 (DSY4169-B). GFP fluorescence was quantitatively assessed by flow cytometry (BD FACSCanto) using 106 exponentially growing cells. Each sample was measured three times.
Cellular localization of Dot6
A DOT6/dot6 heterozygous strain was GFP-tagged in vivo at the C-terminal region with a GFP-Arg4 PCR product as previously described (Gola et al. 2003). Transformants were selected on SC minus Arginine plates, and correct integration of the GFP tag was checked by PCR and sequencing (Table S2). Live-cell microscopy of Dot6-GFP was performed with a Leica DMI6000B inverted confocal microscope (Leica) and a C9100-13 camera CCD camera (Hamamatsu). The effect of TOR activity on Dot6-GFP localization was assessed as following: cells grown on SC medium were exposed to rapamycin (100 ng/ml) for 30 min, washed once with PBS buffer, and immediately visualized. C. albicans vacuoles were stained using the CellTracker Blue CMAC dye (ThermoFisher) following the manufacturer’s recommended procedure.
Size genetic epistasis
dot6 mutant was subjected to epistatic analysis with deletions of known Start regulators (Sellam et al. 2016) (Table S1). Gene deletion was performed as previously described (Gola et al. 2003). The complete set of primers used to generate deletion cassettes and to confirm gene deletions are listed in Table S2. Size distribution of at least, two independent double mutants were determined. Epistasis was noted only if size distributions of a single and double mutant overlapped.
Microarray transcriptional profiling
Overnight cultures of dot6 mutant and WT strains were diluted to an OD600 of 0.1 in 1 liter fresh YPD-uridine medium, grown at 30° to an OD600 of 0.8, and separated into size fractions using the Beckman JE-5.0 elutriation system at 16°. A total of 108 unbudded G1 phase cells were harvested, released into fresh YPD medium, and grown for 10 min prior to harvesting by centrifugation and storage at −80°. Total RNA was extracted using an RNAeasy purification kit (Qiagen) and glass bead lysis in a Biospec Mini 24 bead-beater. Total RNA was eluted, and assessed for integrity on an Agilent 2100 Bioanalyzer prior to cDNA labeling, microarray hybridization, and analysis (Sellam et al. 2009). The GSEA PreRanked tool (http://www.broadinstitute.org/gsea/) was used to determine statistical significance of correlations between the transcriptome of the dot6 mutant with a ranked gene list or GO biological process terms as described by Sellam et al. (2014). Data were visualized using the Cytoscape (Saito et al. 2012) and EnrichmentMap plugin (Merico et al. 2010).
Expression analysis by qPCR
For qPCR experiments, cell cultures and RNA extractions were performed as described for the microarray experiment. cDNA was synthesized from 1 µg of total RNA using the SuperScipt III Reverse Transcription kit (ThermoFisher). The mixture was incubated at 25° for 10 min, 37° for 120 min, and 85° for 5 min; 2 U/µl of RNAse H (NEB) was then added to remove RNA and samples were incubated at 37° for 20 min. qPCR was performed using an iQ5 96-well PCR system (Bio-Rad) for 40 amplification cycles with QuantiTect SYBR Green PCR master mix (Qiagen). The reactions were incubated at 50° for 2 min, 95° for 2 min, and cycled 40 times at 95°, 15 sec; 56°, 30 sec; 72°, 1 min. Fold-enrichment of each tested transcript was estimated using the comparative ΔΔCt method as described by Guillemette et al. (2004). To evaluate the gene expression level, the results were normalized using Ct values obtained from Actin (ACT1, C1_13700W_A). Primer sequences used for this analysis are summarized in Table S2.
Western blot analysis
A DOT6/dot6 heterozygous strain was Myc-tagged in vivo at the C-terminal region with a Myc-Arg4 PCR product as previously described (Lavoie et al. 2008). Transformants were selected on SC minus Arginine plates, and correct integration of the Myc-tag was checked by PCR and sequencing. The C. albicans Dot6-Myc strain was grown to midlog phase in SC medium. Cells at a final OD600 of 1 were treated with 100 ng/ml rapamycin and incubated for 15, 30, 60, or 120 min at 30°. Cells were harvested by centrifugation and lysed by bead beating in IP150 buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM Mg, 0.1% Nonidet P-40] supplemented with Complete Mini protease inhibitor mixture tablet (Roche Applied Science) and 1 mM phenylmethyl-sulfonyl fluoride (PMSF). The lysates were then cleared by centrifugation, and protein concentration was estimated using the DC protein quantification assay (Bio-Rad); 60 μg of total protein was boiled with SDS-PAGE loading buffer and resolved by 4–20% gradient SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane and analyzed by Western blotting using either 910E mouse c-Myc (1:200; Santa Cruz) or beta actin (1:5000; GenScript) antibodies.
Data availability
Strains and plasmids are available upon request. Supplemental files contain two figures (Figures S1 and S2) and five tables (Tables S1–S5) and are available at FigShare (DOI: 10.6084/m9.figshare.7008170). Gene expression data are available at GEO with the accession number GSE119089. Supplemental material available at Figshare: https://doi.org/10.6084/m9.figshare.7008170.v3.
Results
Dot6 is a negative regulator of start in C. albicans
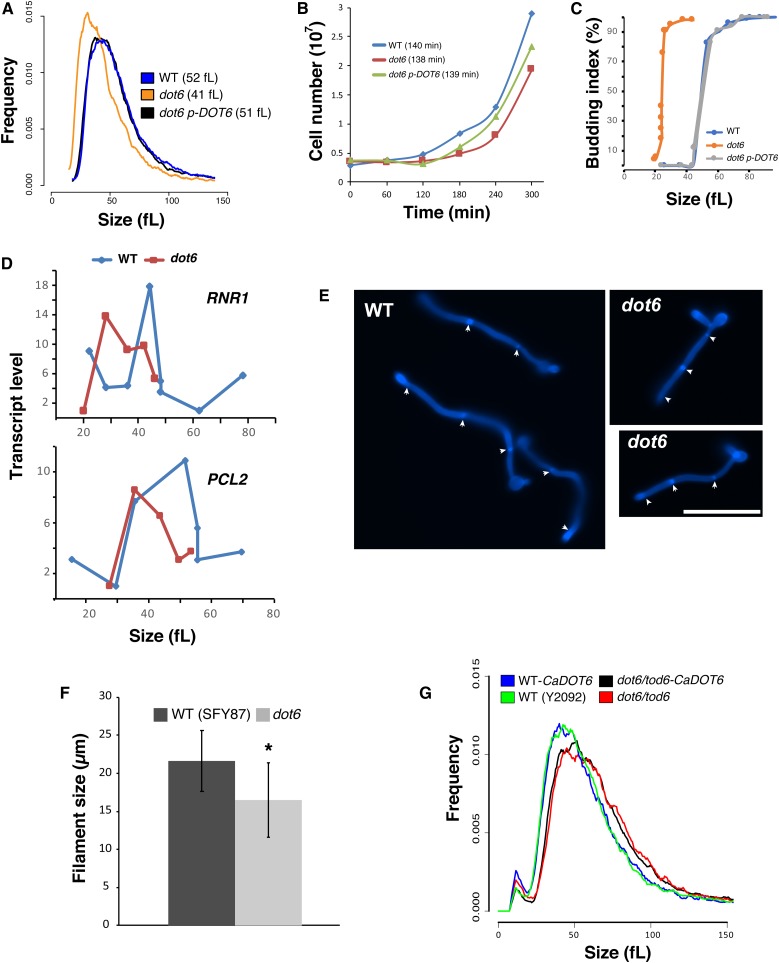
We have previously shown that the transcription factor Dot6 was required for cell size control in C. albicans (Sellam et al. 2016). A dot6 mutant had a median size that was 21% (41fL) smaller than its congenic parental (52fL) or the complemented strains (51fL) (Figure 1A). Inactivation of DOT6 resulted in only a minor growth defect with a doubling time comparable to the WT and the complemented strains during the log phase, suggesting that the size reduction of dot6 is not a growth rate-associated phenotype (Figure 1B). To ascertain that this effect was mediated at Start, we evaluated two hallmarks of Start, namely bud emergence and the onset of SBF-dependent transcription, as a function of cell size in synchronous G1 phase cells obtained by elutriation. As assessed by median size of cultures for which 90% of cells had a visible bud, the dot6 mutant passed Start after growth to 26fL, whereas a parental WT control culture became 90% budded at a much larger size of 61fL (Figure 1C). Importantly, in the same experiment, the onset G1/S transcription was accelerated in the dot6 strain as judged by the peak in expression of the two representative G1-transcripts, the ribonucleotide reductase large subunit, RNR1, and the cyclin PCL2 (Figure 1D). These results unequivocally demonstrated that Dot6 regulates the cell size threshold at Start in C. albicans.
Figure 1.
Dot6 is required for Start onset and cell size homeostasis. (A) Size distributions of the WT (SFY87), dot6 mutant, and the revertant strains. The median sizes of each strain are indicated in parentheses. (B) Growth of the WT (SFY87), dot6 mutant, and the revertant (dot6 p-DOT6) strains in YPD medium at 30° as determined by cell counts using the Z2-Coulter Counter. Results are the mean of three replicates. Doubling-times during the exponential phase of the growth for each strain are indicated in parentheses. (C and D) Start characterization of dot6. (C) Elutriated G1 phase daughter cells were released into fresh YPD medium and assessed for bud emergence as a function of size and G1/S transcription (D). RNR1 and PCL2 transcript levels were assessed by quantitative real-time PCR and normalized to ACT1 levels. (E and F) Dot6 is required for size homeostasis of hyphal cells. Fluorescence micrographs of both WT (SFY87) and dot6 mutant on YPD supplemented with 10% fetal bovine serum (FBS) at 37° for 3 hr and stained with calcofluor white (E). Bar, 10 µm. (F) Length of at least 20 hyphal cells of both WT (SFY87) and dot6 mutant. Bars represent the means ± SEs of the means. * P < 0.0003 using a two-tailed t-test. (G) C. albicans DOT6 (CaDOT6) failed to complement size defect of the S. cerevisiae dot6 tod6 double mutant. Size distributions of the S. cerevisiae WT (Y2092) and dot6 tod6 (Y3707) strains expressing, or not, CaDOT6.
The effect of DOT6 inactivation was also assessed on the size of C. albicans cells growing as invasive hyphae. While dot6 mutant was able to undergo the yeast-to-hyphae transition, the size of hyphal cells was significantly reduced as compared to the WT strain (Figure 1, E and F).
Opposite to C. albicans, inactivation of both DOT6 and its paralog TOD6 in S. cerevisiae resulted in a slight size increase (Huber et al. 2011). To test if the C. albicans Dot6 was functional in S. cerevisiae, we expressed CaDOT6 in the double mutant dot6tod6 of the budding yeast. The obtained transformants had a large size distribution comparable to dot6tod6, suggesting that the C. albicans Dot6 is not functional in S. cerevisiae (Figure 1G). These data, together with the contrasting size phenotype of mutants in both yeasts, suggest that S. cerevisiae Dot6/Tod6 and C. albicans Dot6 are functionally divergent.
Dot6 interacts genetically with the SBF transcription factor complex
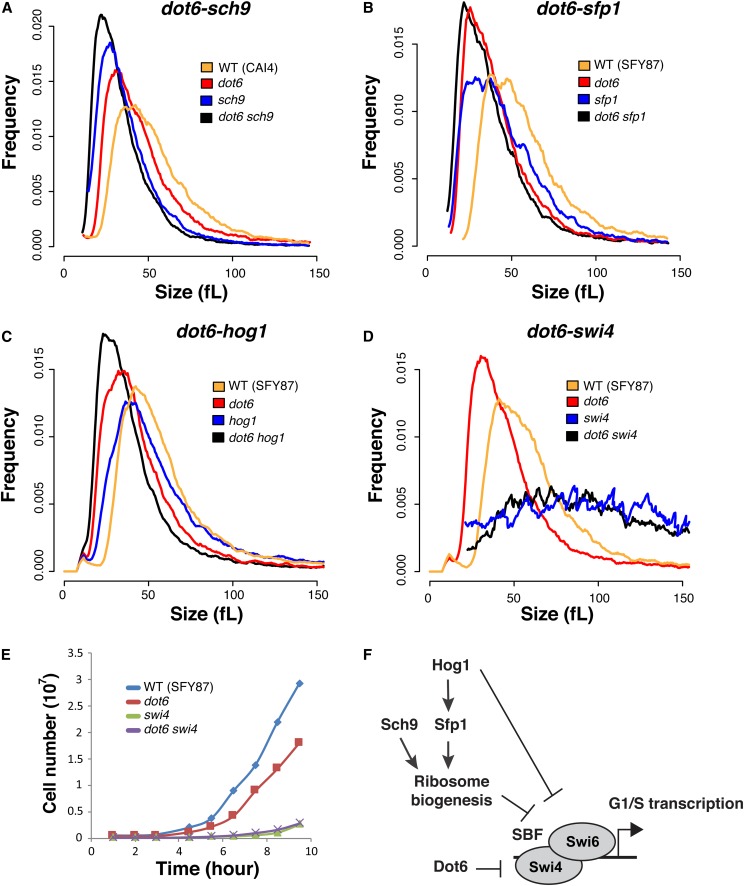
As cell size is a quantitative value, absolute changes in size between single and double mutants can be used to reveal genetic interactions between different genes to construct a cell size genetic interaction network (Jorgensen et al. 2002; Costanzo et al. 2004; de Bruin et al. 2004). To elucidate connections between Dot6 and previously identified Start regulators in C. albicans (Sellam et al. 2016), both DOT6 alleles were deleted in different small size mutants including sch9, sfp1, and hog1, as well as the SBF large size mutant, swi4. Inactivating DOT6 in either sch9, sfp1, or hog1 resulted in cells with smaller size as compared to their congenic strains suggesting that Dot6, Sfp1, Sch9, and the p38 kinase Hog1 act in different Start pathways (Figure 2, A–C). Furthermore, inactivation of DOT6 in the swi4 mutant resulted in a large size comparable to that of swi4 mutant, indicating that Dot6 acts via SBF complex to control Start (Figure 2D). SWI4 deletion is also epistatic to DOT6 regarding the growth rate in liquid YPD medium, confirming that both Dot6 and Swi4 act in a common pathway (Figure 2E). Given the absence of epistatic interaction between Dot6 and the known conserved Ribi and size regulators Sch9, Sfp1, and Hog1, our data uncovered a novel uncharacterized pathway that control the critical cell size threshold in C. albicans (Figure 2F).
Figure 2.
DOT6 size epistasis. Evaluation of size epistasis between dot6 and different potent Start mutations. DOT6 was inactivated in sch9 (A), sfp1 (B), hog1 (C), and swi4 (D) mutants, and the resulted double mutant strains were analyzed for cell size distribution. (E) SWI4 deletion is epistatic to DOT6 regarding the growth rate. Cells were grown in YPD medium at 30° under agitation, and cells were counted using the Z2-Coulter Counter. Results are the mean of three replicates. (F) Summary of DOT6 genetic interactions with the C. albicans Start machinery.
Dot6 is a positive regulator of ribosome biogenesis genes
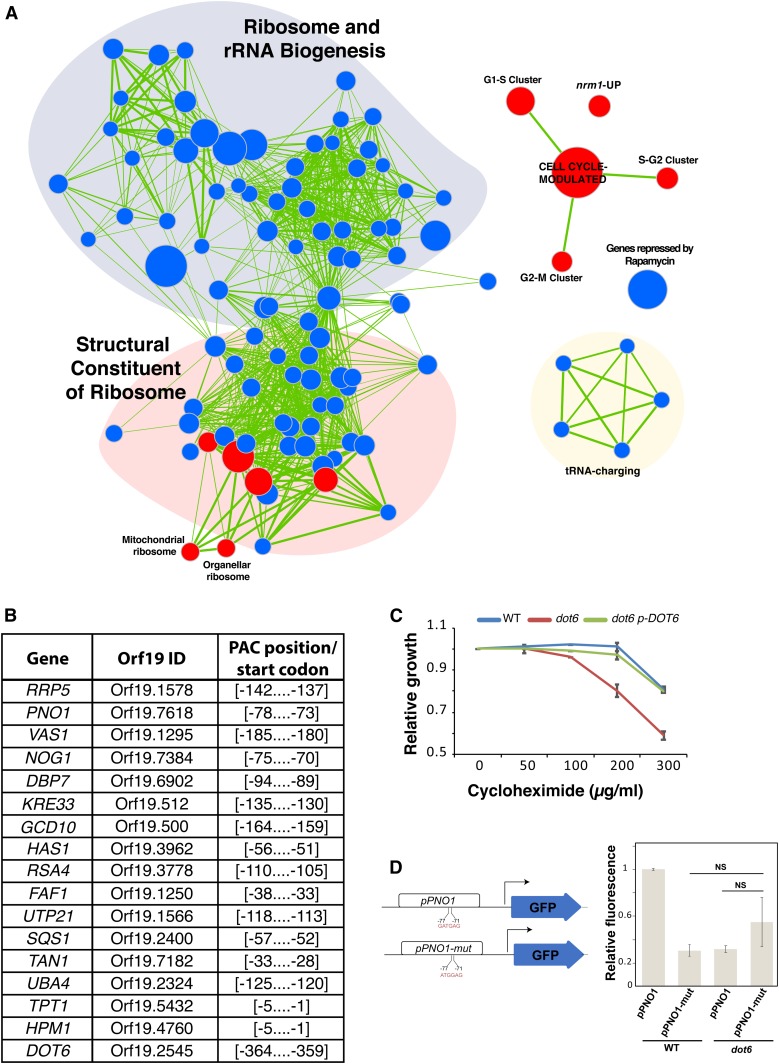
Dot6 and its paralog Tod6 are both Myb-like transcription factors that repress Ribi genes in the budding yeast (Lippman and Broach 2009; Huber et al. 2011). To investigate the role of Dot6 in Start control in C. albicans, we performed genome-wide transcriptional profiling by microarray. G1-cells of both dot6 mutants and the parental WT strain were collected by centrifugal elutriation and their transcriptomes were characterized. Gene Set Enrichment Analysis (GSEA) was used to correlate the dot6 transcript profile with C. albicans genome annotations and gene lists from other transcriptional profiles experiments (Subramanian et al. 2005; Sellam et al. 2012) (Table S3). dot6 mutant was unable to activate properly genes with functions mainly associated with protein translation, including ribosome biogenesis and structural constituents of the ribosome (Figure 3A). This suggest that, in contrast to the role of its ortholog in S. cerevisiae, Dot6 in C. albicans is an activator of Ribi. Analysis of the promoter region of the transcript downregulated in dot6 (transcript with 1.5-fold reduction using 5% FDR—Tables S4 and S5) showed the occurrence of the PAC motif bound by Dot6 in all promoters of genes related to Ribi (Figure 3B). Furthermore, transcripts downregulated in dot6 exhibited correlation with the set of genes downregulated in the presence of the TOR complex inhibitor, rapamycin (Bastidas et al. 2009). This suggest that the evolutionary conserved Ribi transcription control by TOR is mediated fully or partially through Dot6. In support of the role of Dot6 in transcriptional control of Ribi genes, and, thus, translation, a dot6 mutant exhibited an increased sensitivity to the protein translation inhibitor cycloheximide as compared to WT and revertant strains (Figure 3C).
Figure 3.
Dot6 is a positive regulator of ribosome biogenesis genes. (A) GSEA analysis of differentially expressed genes in a dot6 mutant relative to the WT strain (SFY87). Cells were synchronized in G1 phase by centrifugal elutriation, released in fresh SC medium for 10 min, and analyzed for gene expression profiles by DNA microarrays. Correlations of dot6 upregulated (red circles) and downregulated (blue circles) transcripts are shown for biological processes, gene lists in different C. albicans mutants and experiments. The diameter of the circle reflects the number of modulated gene transcripts in each gene set. Known functional connections between related processes are indicated (green lines). Images were generated in Cytoscape with the Enrichment Map plug-in. (B) Occurrence of the PAC motif in the promoters of Dot6-modulated Ribi genes. The 400 bp sequence upstream the start codon of downregulated genes in dot6 (transcript with 1.5-fold reduction using 5% FDR) were scanned for the GATGAG motif. (C) Effect of the translation inhibitor cycloheximide on the growth of the WT (SFY87), dot6 mutant, and the revertant (dot6 p-DOT6) strains. Strains were grown on in SC medium at 30° for 24 hr. Relative growth was calculated as fraction of OD600 of cycloheximide-treated cells relatively to the nontreated controls. Results are the mean of three replicates. (D) GFP reporter assay to confirm that the transcription at the PNO1 (Orf19.7618) locus is driven by the Dot6 PAC-binding element. The pPNO1-GFP reporter strain was constructed by replacing one copy of the PNO1 ORF by the GFP ORF. Mutation in the PAC motif of the pPNO1-mut strain was introduced by PCR using the forward primer pPNO1mut-GFP-F. GFP fluorescence was measured by flow cytometry, and results are presented as relative mean GFP fluorescence as compared to pPNO1-GFP construct in the WT strain. Bars show the means ± SEM. NS, not significant (P > 0.15).
The transcriptional programs characterizing the cell cycle G1/S transition in C. albicans (Côte et al. 2009) were hyperactivated in a dot6 mutant, which further supports the role of Dot6 as a negative regulator of G1/S transcription and Start (Figure 3A). Interestingly, dot6-upregulated transcripts showed a significant correlation with those activated in the deletion mutant of the negative regulator of Start in C. albicans, Nrm1 (Ofir et al. 2012; Sellam et al. 2016).
We used a GFP reporter assay to validate the role of the Dot6-binding PAC elements in Ribi transcriptional regulation. We mutated the PAC motif of the PNO1 genes encoding an essential protein required for rRNA processing (Tone and Toh 2002), and replaced the PNO1 ORF by GFP. GFP activity was reduced by 70% when the PAC motif was mutated (pPNO1-mut) as compared to the intact WT pPNO1-GFP construct (Figure 3D). A similar trend was observed when pPNO1-GFP was expressed in the dot6 mutant, reinforcing the fact that Dot6 recognize the PAC motif in C. albicans.
Dot6 localization and stability is regulated by the TOR signaling pathway
TOR is a central signaling circuit that controls cellular growth in response to environmental nutrient status and stress in eukaryotes. In S. cerevisiae, the transcription factor Sfp1 and the AGC kinase Sch9 are critical effectors of the TOR pathway and form part of a dynamic, nutrient-responsive network that controls the expression of Ribi genes, ribosomal protein genes and cell size (Jorgensen et al. 2004; Urban et al. 2007; Lempiäinen et al. 2009). In S. cerevisiae, both sch9 and sfp1 mutants are impervious to carbon source effects on Start (Jorgensen et al. 2004). In C. albicans, while sfp1 and sch9 mutants have the expected small size phenotype (Sellam et al. 2016), they still retain the ability to respond to carbon source shifts, unlike their S. cerevisiae counterparts (Figure S1). This suggests that the Sfp1-Sch9 regulatory circuit had rewired, and is unlikely to rely on the nutrient status of the cell to Start control in C. albicans.
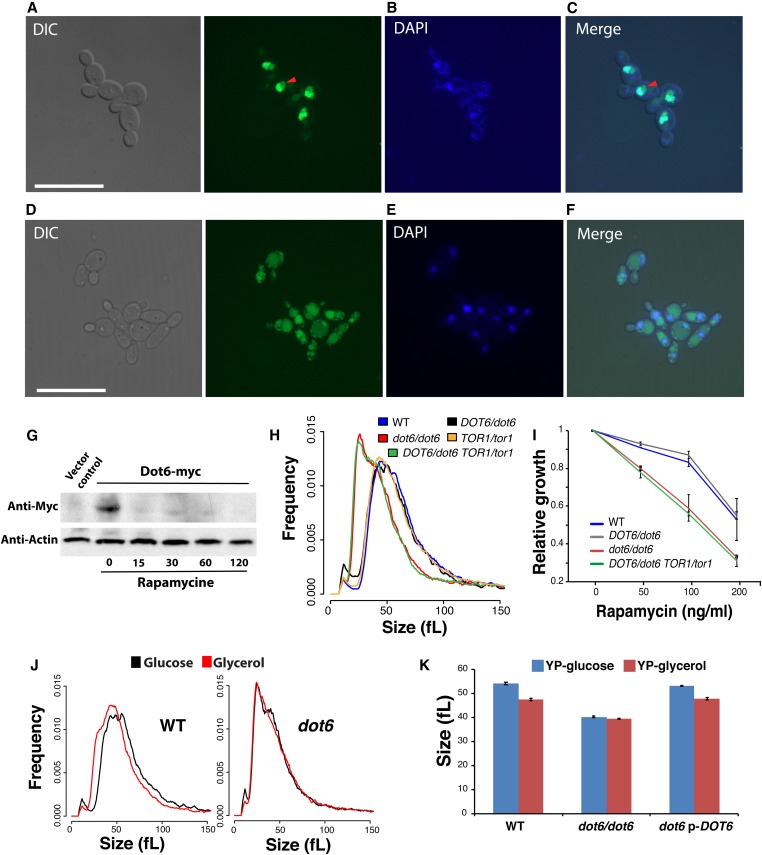
To assess whether the nutrient-sensitive TOR pathway communicates the nutrient status to Dot6, we first tested whether altering TOR activity by rapamycin could alter the subcellular localization of the Dot6-GFP fusion. In the absence of rapamycin, Dot6-GFP was localized exclusively in the nucleus, in agreement with its role as a transcriptional activator under a nutrient-rich environment (Figure 4, A–C). A weak GFP signal was also observed in the nucleolus and the vacuole. When cells were treated with rapamycin, Dot6-GFP was rapidly relocalized to the vacuole, and only a small fraction remain in the nucleus (Figure 4, D–F). The vacuolar localization of the Dot6-GFP was confirmed by its colocalization with the CellTracker Blue-stained vacuoles (Figure S2). These data suggest that the TOR pathway regulates the transcriptional function of Dot6. We also exanimated the effect of modulating TOR activity on the protein level of Dot6 using western blot. The level of Dot6 was significantly reduced in cells exposed to rapamycin, suggesting that, in addition to the control of Dot6 cellular localization, the TOR pathway also modulates the stability of Dot6 (Figure 4G).
Figure 4.
Dot6 localization is regulated by the TOR signaling pathway. (A–F) Dot6-GFP fluorescence was visualized using confocal microscopy in cells treated (D–F) or not (A–C) with the TOR pathway inhibitor, rapamycin. Exponentially grown cells in SC medium were treated with 100 ng/ml rapamycin for 1 hr. Nuclear and mitochondrial DNA were demarcated by DAPI staining (B and E). Red arrows indicate Dot6-GFP florescence in nucleolar regions. Bar, 5 µm. (G) Level of Dot6 in exponentially grown cells in SC medium treated or not with 100 ng/ml rapamycin for 15, 30, 60, and 120 min. Vector control corresponds to the untagged strain. (H and I) DOT6 and TOR1 genetic interaction for cell size and growth in the presence of rapamycin based on complex haploinsufficiency concept. (H) Size distributions of the WT (SN250), the heterozygous (DOT6/dot6), and homozygous (dot6/dot6) dot6 mutants, the heterozygous TOR1/tor1 strain and the double heterozygous mutant TOR1/tor1 DOT6/dot6. (I) DOT6 is epistatic to TOR1 with respect to their sensitivity toward rapamycin. Strains were grown on in SC medium at 30° for 24 hr. Relative growth was calculated as fraction of OD600 of rapamycin-treated cells relatively to the nontreated controls. Results are the mean of three replicates. (J and K) Dot6 is required for carbon-source modulation of cell size. (J) Cell size distribution of the WT and dot6 mutant strains grown in medium with either glucose or glycerol as the sole source of carbon. (K) Median size of the WT (SFY87), dot6 mutant and the revertant strains growing in synthetic glucose or glycerol medium. Results are the mean of three independent replicates.
To assess whether the control of Dot6 activity by TOR impacts the cell size of C. albicans, we examined genetic interactions between TOR1 and DOT6 by size epistasis. As TOR1 is an essential gene in C. albicans, we first tried to delete one allele in dot6 homozygous mutant. However, all attempts to generate such mutant were unsuccessful, suggesting a haplo-essentiality of TOR1 in dot6 mutant background. Subsequently, we analyzed genetic interaction of TOR1 and DOT6 using the complex haploinsufficiency (CHI) concept by deleting one allele of each gene, and measured the size distribution of the obtained mutant. While both DOT6/dot6 and TOR1/Tor1 mutants had no discernable size defect, the TOR1/tor1 DOT6/dot6 strain exhibited a cell size distribution similar to that of dot6/dot6, suggesting that DOT6 is epistatic to TOR1 (Figure 4H). Similarly, DOT6 was also epistatic to TOR1 with respect to their sensitivity toward rapamycin (Figure 4I). These data demonstrate that the TOR pathway controls cell size through Dot6.
Dot6 is required for carbon-source modulation of cell size
The effect of different carbon sources on the size distribution of WT and the dot6 mutant was assessed. While the cell size of WT and the revertant strains was reduced by 12% (47.6 ± 0.5 fL) when grown under the poor carbon source, glycerol, as compared to glucose (54.2 ± 0.5 fL), dot6 size remain unchanged regardless of carbon source (Figure 4, J and K). A similar finding was obtained when comparing cells growing on the nonfermentable carbon source, ethanol (data not shown). These results demonstrate that the transcription factor Dot6 is required for nutrient modulation of cell size.
To check whether Dot6 localization is modulated by carbon sources, the subcellular localization of the Dot6-GFP fusion was tested in cells that grew in poor (glycerol), or in the absence of, carbon sources. Neither the absence nor the quality of the carbon source altered the nuclear localization of Dot6 (data not shown). This suggests that Dot6 governs the carbon-source modulation of cell size through a mechanism that is independent of its cellular relocalization.
The CTG-clade specific acidic domain of Dot6 is required for size control in response to nonfermentable carbon sources
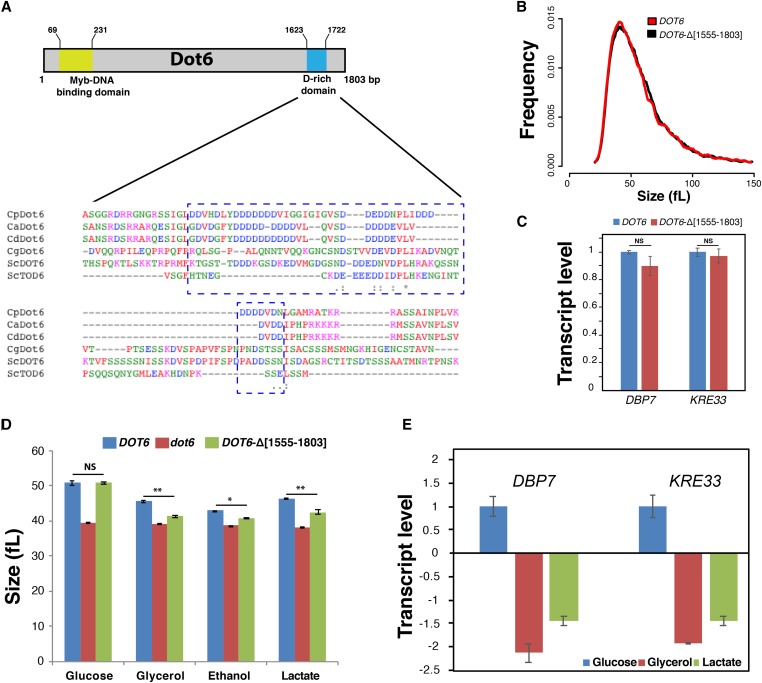
Our analysis unexpectedly reveals that Dot6 switched between activator and repressor transcriptional regulator of Ribi between C. albicans and S. cerevisiae, respectively. Sequence examination of C. albicans Dot6 protein revealed a C-terminal aspartate-rich domain that is similar to acidic activation domains of transcriptional activators. This Dot6 D-rich domain was found specifically in C. albicans and other related species of the CTG clade, and was absent in Dot6 orthologs in S. cerevisiae and other ascomycetes (Figure 5A). To check whether the presence of this acidic domain correlates with its function as transcriptional activator in C. albicans, we deleted this D-rich domain using the CRISPR-Cas9 mutagenesis tool. Size distribution of the truncated DOT6-Δ[1555–1803] strain was indistinguishable from that of the WT parental strain when cells grew on YP with glucose (YPD) (Figure 5B) or other fermentable sugars (YP-fructose, YP-galactose, YP-sucrose, and YP-mannose; data not shown). The ability of DOT6-Δ[1555–1803] to activate two Ribi transcripts (DBP7 and KRE33) in YP-glucose was preserved, which suggests that this domain is dispensable for the size control and gene expression activation functions in response to fermentable carbon sources (Figure 5C). When C. albicans cells were grown on nonfermentable carbon sources such as glycerol, ethanol, or lactate, the DOT6-Δ[1555–1803] mutant exhibited a reduced size as compared to the WT strain (Figure 5D). The two Ribi transcripts DBP7 and KRE33 were downregulated in the DOT6-Δ[1555–1803] mutant as compared to WT when cells utilized either glycerol or lactate as carbon sources (Figure 5E). This suggest that the D-rich domain of Dot6 is required to activate Ribi genes and adjust cell size under conditions of respiratory growth.
Figure 5.
The CTG-clade specific acidic domain of Dot6 is required for size control in response to nonfermentable carbon sources. (A) The C-terminal D-rich domain of Dot6 is conserved in the CTG clade species C. albicans (Ca), C. parapsilosis (Cp) and C. dubliniensis (Cd), but not in S. cerevisiae (Sc) and C. glabrata (Cg). Identical residues are indicated with asterisks. Conserved and semiconserved substitutions are denoted by colons and periods, respectively. (B) Cell size distribution of the WT (SC5314) and the truncated DOT6-Δ[1555–1803] strains. (C) Transcript levels of Ribi genes, including DBP7 and KRE33, were evaluated in both WT (SC5314) and the truncated DOT6-Δ[1555–1803] strains. Transcript levels were calculated using the comparative CT method using the ACT1 gene as a reference. Results are the mean of three replicates. For each transcript, fold changes in the WT and the truncated strains were not statistically significant (t-test). NS, not significant. (D) Median size of the WT (SC5314), dot6, and the truncated DOT6-Δ[1555–1803] strains growing in YP-glucose, YP-glycerol, YP-ethanol, and YP-lactate media. Results are the mean of three independent replicates. * P < 0.02; ** P < 0.01; (E) Transcript levels of Ribi genes, DBP7, and KRE33 in both WT (SC5314) and the truncated DOT6-Δ[1555–1803] strains growing in fermentable (glucose) and nonfermentable carbon sources (glycerol and lactate). Values represent transcript levels of DBP7 and KRE33 in cells growing in glycerol or lactate normalized to that of cells growing in glucose. Results are the mean of three replicates.
Discussion
Although both C. albicans and S. cerevisiae share the core cell cycle and growth regulatory machineries, our previous investigations uncovered a limited overlap of the cell size phenome between the two fungi (Sellam et al. 2016; Chaillot et al. 2017). This finding is corroborated by recent evidence showing an extensive degree of rewiring and plasticity of both transcriptional regulatory circuits and signaling pathways across many cellular and metabolic processes between the two yeasts (Homann et al. 2009; Lavoie et al. 2009, 2010; Blankenship et al. 2010; Li and Johnson 2010; Childers et al. 2016). The plasticity of the global size network underscores the evolutionary impact of cell size as an adaptive mechanism to optimize fitness. Indeed, many size genes in C. albicans were linked to virulence, which suggests that cell size is an important biological trait that contributes to the adaptation of fungal pathogens to their different niches (Sellam et al. 2016; Chaillot et al. 2017). So far, the requirement of Dot6 for the fitness of C. albicans inside its host has not yet been tested; however, inactivation of DOT6 led to the alteration of sensitivity toward antifungals (Vandeputte et al. 2012). Moreover, while dot6 mutant was able to form invasive filaments, the size of hyphae cells was significantly reduced, which might impact the invasiveness of host tissues and organs (Figure 1, E and F). This reinforces the fact that control of cell size homeostasis is an important attribute for C. albicans to persist inside its host.
We found that Dot6 is a major regulator of cell size in C. albicans as compared to S. cerevisiae, emphasizing an evolutionary drift regarding the contribution of this transcription factor in size modulation. The potency of the C. albicans Dot6 in size control could be attributed to different facts. First, and in contrast to its role in S. cerevisiae, Dot6 is an activator of Ribi genes. This might explain the small size of dot6 in C. albicans given the fact that inactivation of transcriptional activators of Ribi genes such as Sfp1 and Sch9 in either S. cerevisiae or C. albicans led to the acceleration of Start, and, consequently, to a whi phenotype (Jorgensen et al. 2002; Dungrawala et al. 2012; Soifer and Barkai 2014; Sellam et al. 2016; Chaillot et al. 2017). Second, in C. albicans, Dot6 had an expanded genetic connectivity with both the critical SBF complex, which controls the G1/S transition, and also with the TOR growth and Ribi machineries, which might explain the influential role of Dot6 in size control.
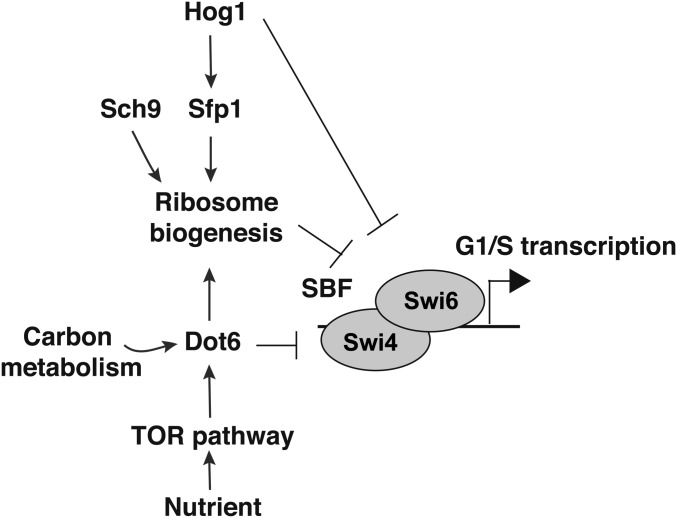
Our findings support a model whereby Dot6 acts as a hub that might integrate directly growth cues via the TOR pathway to control the commitment to mitotic division at G1. This regulatory behavior is similar to the p38/HOG1 pathway that controls the Ribi regulon through the master transcriptional regulator, Sfp1, and acts upstream of the SBF transcription factor complex to control division (Sellam et al. 2016). Meanwhile, our genetic interaction analysis showed that the dot6 hog1 double mutant had an additive small size phenotype, suggesting that both Dot6 and Hog1 act in parallel. This finding emphasizes that, in C. albicans, multiple signals are integrated at the level of G1 machinery to optimize adaptation to different conditions. Contrary to the p38/HOG pathway, Dot6 was required for size adjustment in response to glycerol, suggesting that this transcription factor provides a nexus for integrating carbon nutrient status to the ribosome synthesis and Start machineries (Figure 6).
Figure 6.
Schematic model of connections between Dot6 and Start control machinery in C. albicans.
Compared to other hemi-ascomycetes, Candida species of the CTG-clade possess a Dot6 with a C-terminal D-rich domain that resembles the acidic activation domains found in many transcriptional activators. We have shown that deletion of the D-rich domain had no impact on C. albicans cells size or the transcription of Ribi genes when cells grew in media with fermentable carbon sources. However, in the presence of a nonfermentable carbon source, the D-rich domain was required for both size homeostasis and Ribi transcription. These data suggest that the D-rich domain of Dot6 might function as a transcriptional activation domain of Ribi genes to promote growth, and, consequently, set a homeostatic cell size when C. albicans cells undergo respiratory growth. Previous investigations had shown that D-rich domains play multiple roles in gene transcription regulation through DNA mimicry to modulate mRNA processing and the activity of the general transcription machinery (Chou and Wang 2015). For instance, the D-rich domain of Taf1 exerts an inhibitory effect on transcription by competing with the TFIIA complex in binding TBP (TATA-box binding protein). For Dot6, the D-rich domain might behave similarly by competing with other transcriptional regulators that coordinate the transcription of Ribi with respiratory metabolism. A plausible interpretation of the small size of the truncated DOT6-Δ[1555–1803] strain is that Ribi promoters are modulated by a transcriptional repressor under respiratory growth, and, in the absence of the D-rich competitor domain, Ribi are repressed, which, in turn, might lead to Start acceleration and the whi phenotype.
How Dot6 switches its function from a transcriptional Ribi repressor in S. cerevisiae to an activator in C. albicans is an intriguing question. Under respiratory growth conditions, this might be explained by the fact that the potential D-rich activation domain was lost in S. cerevisiae, as discussed above. However, under fermentative growth, the D-rich domain was dispensable for size control and Ribi activation. For their repressive activity at the Ribi gene promoters in S. cerevisiae, both Dot6 and Tod6 recruit the histone deacetylase Rpd3L to establish a repressive chromatin state (Huber et al. 2011). Instead of a repressive chromatin-modifying complex, C. albicans Dot6 might recruit an activator that might impose its Ribi-activating function. However, so far, no such chromatin-modifying activator complex has been identified in C. albicans. Future studies are needed to characterize the contribution of chromatin remodelling and modification complexes to Ribi transcription in this opportunistic yeast.
Acknowledgments
We are grateful to James Broach (Princeton University), Robbie Loewith (Université de Genève), Julia Köhler (Harvard Medical School), and Dominique Sanglard [Le Centre Hospitalier Universitaire Vaudois (CHUV)-Université Lausanne] for providing strains. We would like to thank Christian Landry and Aléxandre Dubé [Université Laval-L’Institut de biologie intégrative et des systèmes (IBIS)] for sharing the pAG415GPD-ccdB plasmids. This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (#06625), the Canadian Foundation for Innovation and the Fonds de Recherche du Québec-Santé. J.C. was supported by a Université Laval Faculty of Medicine and Centre Hospitalier Universitaire de Québec (CHUQ) foundation Ph.D. scholarships. A.S. was supported by a Fonds de Recherche du Québec-Santé (FRQS) J1 salary award.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6953003.
Communicating editor: A. Gladfelter
Literature Cited
- Bastidas R. J., Heitman J., Cardenas M. E., 2009. The protein kinase Tor1 regulates adhesin gene expression in Candida albicans. PLoS Pathog. 5: e1000294 10.1371/journal.ppat.1000294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J., 2006. Morphogenesis and cell cycle progression in Candida albicans. Curr. Opin. Microbiol. 9: 595–601. 10.1016/j.mib.2006.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship J. R., Fanning S., Hamaker J. J., Mitchell A. P., 2010. An extensive circuitry for cell wall regulation in Candida albicans. PLoS Pathog. 6: e1000752 10.1371/journal.ppat.1000752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillot J., Cook M. A., Corbeil J., Sellam A., 2017. Genome-wide screen for haploinsufficient cell size genes in the opportunistic yeast Candida albicans. G3 (Bethesda) 7: 355–360. 10.1534/g3.116.037986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. C., Yang B. C., Kuo T. T., 1992. One-step transformation of yeast in stationary phase. Curr. Genet. 21: 83–84. 10.1007/BF00318659 [DOI] [PubMed] [Google Scholar]
- Childers D. S., Raziunaite I., Mol Avelar G., Mackie J., Budge S., et al. , 2016. The rewiring of ubiquitination targets in a pathogenic yeast promotes metabolic flexibility, host colonization and virulence. PLoS Pathog. 12: e1005566 10.1371/journal.ppat.1005566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. C., Wang A. H., 2015. Structural D/E-rich repeats play multiple roles especially in gene regulation through DNA/RNA mimicry. Mol. Biosyst. 11: 2144–2151. 10.1039/C5MB00206K [DOI] [PubMed] [Google Scholar]
- Costanzo M., Nishikawa J. L., Tang X., Millman J. S., Schub O., et al. , 2004. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell 117: 899–913. 10.1016/j.cell.2004.05.024 [DOI] [PubMed] [Google Scholar]
- Côte P., Hogues H., Whiteway M., 2009. Transcriptional analysis of the Candida albicans cell cycle. Mol. Biol. Cell 20: 3363–3373. 10.1091/mbc.e09-03-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin R. A., McDonald W. H., Kalashnikova T. I., Yates J., III, Wittenberg C., 2004. Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell 117: 887–898. 10.1016/j.cell.2004.05.025 [DOI] [PubMed] [Google Scholar]
- Dungrawala H., Hua H., Wright J., Abraham L., Kasemsri T., et al. , 2012. Identification of new cell size control genes in S. cerevisiae. Cell Div. 7: 24 10.1186/1747-1028-7-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enfert C., Hube B., 2007. Candida: Comparative and Functional Genomics. Caister Academic Press, Wymondham, Norfolk, UK. [Google Scholar]
- Gola S., Martin R., Walther A., Dunkler A., Wendland J., 2003. New modules for PCR-based gene targeting in Candida albicans: rapid and efficient gene targeting using 100 bp of flanking homology region. Yeast 20: 1339–1347. 10.1002/yea.1044 [DOI] [PubMed] [Google Scholar]
- Guillemette T., Sellam A., Simoneau P., 2004. Analysis of a nonribosomal peptide synthetase gene from Alternaria brassicae and flanking genomic sequences. Curr. Genet. 45: 214–224. 10.1007/s00294-003-0479-z [DOI] [PubMed] [Google Scholar]
- Homann O. R., Dea J., Noble S. M., Johnson A. D., 2009. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 5: e1000783 10.1371/journal.pgen.1000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A., French S. L., Tekotte H., Yerlikaya S., Stahl M., et al. , 2011. Sch9 regulates ribosome biogenesis via Stb3, Dot6 and Tod6 and the histone deacetylase complex RPD3L. EMBO J. 30: 3052–3064. 10.1038/emboj.2011.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P., Tyers M., 2004. How cells coordinate growth and division. Curr. Biol. 14: R1014–R1027. 10.1016/j.cub.2004.11.027 [DOI] [PubMed] [Google Scholar]
- Jorgensen P., Nishikawa J. L., Breitkreutz B. J., Tyers M., 2002. Systematic identification of pathways that couple cell growth and division in yeast. Science 297: 395–400. 10.1126/science.1070850 [DOI] [PubMed] [Google Scholar]
- Jorgensen P., Rupes I., Sharom J. R., Schneper L., Broach J. R., et al. , 2004. A dynamic transcriptional network communicates growth potential to ribosome synthesis and critical cell size. Genes Dev. 18: 2491–2505. 10.1101/gad.1228804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafri M., Metzl-Raz E., Jona G., Barkai N., 2016. The cost of protein production. Cell Rep. 14: 22–31. 10.1016/j.celrep.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie H., Sellam A., Askew C., Nantel A., Whiteway M., 2008. A toolbox for epitope-tagging and genome-wide location analysis in Candida albicans. BMC Genomics 9: 578 10.1186/1471-2164-9-578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie H., Hogues H., Whiteway M., 2009. Rearrangements of the transcriptional regulatory networks of metabolic pathways in fungi. Curr. Opin. Microbiol. 12: 655–663. 10.1016/j.mib.2009.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie H., Hogues H., Mallick J., Sellam A., Nantel A., et al. , 2010. Evolutionary tinkering with conserved components of a transcriptional regulatory network. PLoS Biol. 8: e1000329 10.1371/journal.pbio.1000329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempiäinen H., Uotila A., Urban J., Dohnal I., Ammerer G., et al. , 2009. Sfp1 interaction with TORC1 and Mrs6 reveals feedback regulation on TOR signaling. Mol. Cell 33: 704–716. 10.1016/j.molcel.2009.01.034 [DOI] [PubMed] [Google Scholar]
- Lenski R. E., Travisano M., 1994. Dynamics of adaptation and diversification: a 10,000-generation experiment with bacterial populations. Proc. Natl. Acad. Sci. USA 91: 6808–6814. 10.1073/pnas.91.15.6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Johnson A. D., 2010. Evolution of transcription networks–lessons from yeasts. Curr. Biol. 20: R746–R753. 10.1016/j.cub.2010.06.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman S. I., Broach J. R., 2009. Protein kinase A and TORC1 activate genes for ribosomal biogenesis by inactivating repressors encoded by Dot6 and its homolog Tod6. Proc. Natl. Acad. Sci. USA 106: 19928–19933. 10.1073/pnas.0907027106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion R. M., Regev A., Segal E., Barash Y., Koller D., et al. , 2004. Sfp1 is a stress- and nutrient-sensitive regulator of ribosomal protein gene expression. Proc. Natl. Acad. Sci. USA 101: 14315–14322. 10.1073/pnas.0405353101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merico D., Isserlin R., Stueker O., Emili A., Bader G. D., 2010. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One 5: e13984 10.1371/journal.pone.0013984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir A., Hofmann K., Weindling E., Gildor T., Barker K. S., et al. , 2012. Role of a Candida albicans Nrm1/Whi5 homologue in cell cycle gene expression and DNA replication stress response. Mol. Microbiol. 84: 778–794. 10.1111/j.1365-2958.2012.08056.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito R., Smoot M. E., Ono K., Ruscheinski J., Wang P. L., et al. , 2012. A travel guide to Cytoscape plugins. Nat. Methods 9: 1069–1076. 10.1038/nmeth.2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandai D., Yin Z., Selway L., Stead D., Walker J., et al. , 2012. The evolutionary rewiring of ubiquitination targets has reprogrammed the regulation of carbon assimilation in the pathogenic yeast Candida albicans. MBio 3: pii: e00495-12 10.1128/mBio.00495-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub Y., Dunkler A., Walther A., Wendland J., 2006. New pFA-cassettes for PCR-based gene manipulation in Candida albicans. J. Basic Microbiol. 46: 416–429. 10.1002/jobm.200510133 [DOI] [PubMed] [Google Scholar]
- Schmoller K. M., Skotheim J. M., 2015. The biosynthetic basis of cell size control. Trends Cell Biol. 25: 793–802. 10.1016/j.tcb.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellam A., Tebbji F., Nantel A., 2009. Role of Ndt80p in sterol metabolism regulation and azole resistance in Candida albicans. Eukaryot. Cell 8: 1174–1183. 10.1128/EC.00074-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellam A., Tebbji F., Whiteway M., Nantel A., 2012. A novel role for the transcription factor Cwt1p as a negative regulator of nitrosative stress in Candida albicans. PLoS One 7: e43956 10.1371/journal.pone.0043956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellam A., van het Hoog M., Tebbji F., Beaurepaire C., Whiteway M., et al. , 2014. Modeling the transcriptional regulatory network that controls the early hypoxic response in Candida albicans. Eukaryot. Cell 13: 675–690. 10.1128/EC.00292-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellam A., Chaillot J., Mallick J., Tebbji F., Richard Albert J., et al. , 2016. A systematic cell size screen uncovers coupling of growth to division by the p38/HOG network in Candida albicans. bioRxiv. https//.org/10.1101/094144
- Soifer I., Barkai N., 2014. Systematic identification of cell size regulators in budding yeast. Mol. Syst. Biol. 10: 761 10.15252/msb.20145345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., et al. , 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102: 15545–15550. 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone Y., Toh E. A., 2002. Nob1p is required for biogenesis of the 26S proteasome and degraded upon its maturation in Saccharomyces cerevisiae. Genes Dev. 16: 3142–3157. 10.1101/gad.1025602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. J., Ewald J. C., Skotheim J. M., 2012. Cell size control in yeast. Curr. Biol. 22: R350–R359. 10.1016/j.cub.2012.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyers M., Tokiwa G., Futcher B., 1993. Comparison of the Saccharomyces cerevisiae G1 cyclins: Cln3 may be an upstream activator of Cln1, Cln2 and other cyclins. EMBO J. 12: 1955–1968. 10.1002/j.1460-2075.1993.tb05845.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J., Soulard A., Huber A., Lippman S., Mukhopadhyay D., et al. , 2007. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol. Cell 26: 663–674. 10.1016/j.molcel.2007.04.020 [DOI] [PubMed] [Google Scholar]
- Vandeputte P., Pradervand S., Ischer F., Coste A. T., Ferrari S., et al. , 2012. Identification and functional characterization of Rca1, a transcription factor involved in both antifungal susceptibility and host response in Candida albicans. Eukaryot. Cell 11: 916–931. 10.1128/EC.00134-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas V. K., Barrasa M. I., Fink G. R., 2015. A Candida albicans CRISPR system permits genetic engineering of essential genes and gene families. Sci. Adv. 1: e1500248 10.1126/sciadv.1500248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Byers K. J., McCord R. P., Shi Z., Berger M. F., et al. , 2009. High-resolution DNA-binding specificity analysis of yeast transcription factors. Genome Res. 19: 556–566. 10.1101/gr.090233.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. Supplemental files contain two figures (Figures S1 and S2) and five tables (Tables S1–S5) and are available at FigShare (DOI: 10.6084/m9.figshare.7008170). Gene expression data are available at GEO with the accession number GSE119089. Supplemental material available at Figshare: https://doi.org/10.6084/m9.figshare.7008170.v3.