Ciliates divide by tandem duplication, a developmental process that remodels the parental cell into two daughters aligned head-to-tail. Here, Jiang et al. investigate the elo1-1 mutation in Tetrahymena that causes the division plan to form too close....
Keywords: Lats, Tetrahymena, pattern, ciliate, polarity
Abstract
In a single cell, ciliates maintain a complex pattern of cortical organelles that are arranged along the anteroposterior and circumferential axes. The underlying molecular mechanisms of intracellular pattern formation in ciliates are largely unknown. Ciliates divide by tandem duplication, a process that remodels the parental cell into two daughters aligned head-to-tail. In the elo1-1 mutant of Tetrahymena thermophila, the segmentation boundary/division plane forms too close to the posterior end of the parental cell, producing a large anterior and a small posterior daughter cell, respectively. We show that ELO1 encodes a Lats/NDR kinase that marks the posterior segment of the cell cortex, where the division plane does not form in the wild-type. Elo1 acts independently of CdaI, a Hippo/Mst kinase that marks the anterior half of the parental cell, and whose loss shifts the division plane anteriorly. We propose that, in Tetrahymena, two antagonistic Hippo circuits focus the segmentation boundary/division plane at the equatorial position, by excluding divisional morphogenesis from the cortical areas that are too close to cell ends.
CILIATES are among the most sophisticated cell types known. In a single large and polarized cell, ciliates assemble an amazingly complex set of cytoskeletal organelles that are anchored to the plasma membrane at specific positions along the anteroposterior and circumferential axes. The cell cortex—the outermost layer of the cytoplasm which is rich in cytoskeletal structures—is the likely carrier of positional information. For example, Stentor, a large ciliate that is amenable to microsurgery, can regenerate its cortical pattern after most of its endoplasm is removed. Furthermore, an endoplasmic mass can regenerate the entire pattern only if at least a small patch of cell cortex is left on the cell surface (Tartar 1956, 1961). The mechanisms that govern the formation, duplication, and regeneration of the cortical pattern in ciliates remain largely unknown, especially at the molecular level. Insights into the pattern formation in ciliates could uncover broader principles of how organelles are positioned inside the cell (Frankel 1989; Kirschner et al. 2000; Marshall 2011).
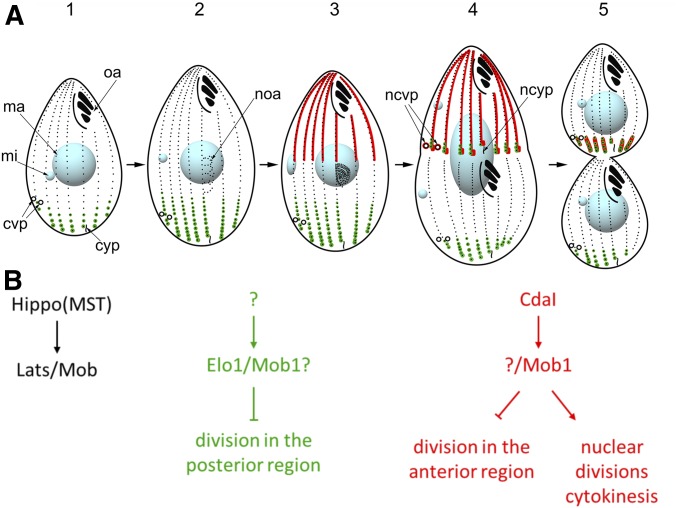
As compared to Stentor, Tetrahymena thermophila is a much smaller ciliate, which, however, can be studied by forward and reverse genetic approaches (reviewed in Ruehle et al. 2016). The main constituents of the cell cortex of Tetrahymena are ∼20 longitudinal rows of locomotory cilia (reviewed in Wloga and Frankel 2012). A single oral apparatus is located near the anterior cell end, while the contractile vacuole pores (CVPs) and the cytoproct (site of defecation) are positioned at different circumferential positions near the posterior cell end (Figure 1A).
Figure 1.
The course of cell division in Tetrahymena and its two Hippo signaling circuits. (A) The outline of the stages of cell division in Tetrahymena. The localization patterns of Elo1 and Mob1 (green, the two proteins perfectly colocalize) and CdaI (red) are shown. The Mob1 data are based on Tavares et al. (2012). The diagram is a modification of the one published in Jiang et al. (2017). mi, micronucleus; ma, macronucleus; oa, oral apparatus; noa, new oral apparatus (oral primordium); cvp, contractile vacuole pore; ncvp, new contractile vacuole pore; cyp, cytoproct; ncyp, new cytoproct. The following stages are marked: stage 1, interphase; 2, early oral primordium; 3, late oral primordium; 4, cortical subdivision and mitosis of the micronucleus; 5, cytokinesis and macronuclear amitosis. (B) The proposed composition and the activities of the two consecutive Hippo signaling circuits in Tetrahymena. Components of a generic Hippo circuit are shown on the left.
Ciliates divide by “tandem duplication,” a process of segmentation that reorganizes the parental cell into two daughters, which emerge aligned head to tail. The segmentation boundary that later becomes the fission line (we will refer to the positions of both structures collectively as the “division plane”) must develop at the proper latitude, to produce daughters of equal size, and its positioning operates in the context of the existing pattern of the parental cell.
Several studies have linked the Hippo kinase cascade to tandem duplication in ciliates, and specifically to the division plane positioning (Tavares et al. 2012; Slabodnick et al. 2014; Jiang et al. 2017). The Hippo kinase cascade is a highly conserved eukaryotic signaling pathway that generates diverse outcomes that regulate the animal organ size, cell proliferation, cell growth, cell cycle, cell fate determination, and mechanosensation. The conserved part of Hippo signaling consists of a triad of proteins: an upstream kinase Hippo/Mst, a downstream kinase Lats/NDR, and its activator/adapter Mob (reviewed in Yu et al. 2015). In Tetrahymena, a knockdown of an ortholog of Mob, Mob1, results in an excessively anterior division plane, and defects in segregation of nuclei and cytokinesis (Tavares et al. 2012). We recently showed that an identical phenotype is produced by a loss-of function of CdaI, an ortholog of Hippo/Mst kinases (Jiang et al. 2017).
Here we investigate elo1-1, a recessive loss-of-function mutation in Tetrahymena that results in an opposite phenotype: the division plane forms too close to the posterior cell end (Frankel 2008). Surprisingly, we find that the ELO1 gene encodes an ortholog of another conserved Hippo pathway component, a Lats/NDR kinase (Johnston et al. 1990; Xu et al. 1995; Hergovich 2013). Thus, in Tetrahymena, deficiencies in different core Hippo signaling proteins result in division plane displacements in opposite directions. While the effects of Elo1 and CdaI are antagonistic, our observations indicate that these proteins operate in two consecutive Hippo signaling circuits. Both proteins occupy cortical areas in which the divisional activities do not occur in the wild type. This and our earlier work (Jiang et al. 2017) reveal that cortical inhibition drives intracellular patterning in ciliates.
Materials and Methods
Tetrahymena strains
The T. thermophila strain IA388 elo1-1/elo1-1 (elo1-1, mt I) is homozygous for elo1-1, a mutation that shifts the division plane toward the posterior cell end (Frankel 2008). Strain IA237 cdaI-1/cdaI-1 (cdaI-1, mt II) is homozygous for cdaI-1, a temperature-sensitive mutation that shifts the division plane toward the anterior cell end (Frankel 2008). Strain CU427 chx1-1/chx1-1 (CHX1, cy-s, mt VI) was used for outcrosses and strain B*VII (cy-s, mt VII) was used for self-crosses by “short circuit genomic exclusion” (SCGE) (Bruns et al. 1976). Strain CU428 mpr1-1/mpr1-1 (MPR1, mp-s, mt VII) served as the wild type in phenotypic comparisons. All strains were obtained from the Tetrahymena Stock Center (Cornell University, Ithaca, NY). Cultures were grown in SPP medium (Gorovsky 1973) with antibiotics (SPPA, Gaertig et al. 2013) at 29 or 39°.
Identification of the elo1-1 causative mutation
We used the “allelic composition contrast analysis (ACCA)” as recently described (Jiang et al. 2017). IA388 was crossed to CU427, and a single F1 heterozygote (elo1-1/ELO1; chx1-1/CHX1) was propagated (for ∼8 days) to become sexually mature and sensitive to cycloheximide due to phenotypic assortment (Orias and Flacks 1975). A single F1 subclone was crossed to B*VII to induce SCGE and F2 clones were selected with 15 μg/ml cycloheximide; 31 independent F2 clones with the elo1-1 phenotype and 31 F2 clones with the wild-type phenotype were combined into separate elo1-1 and wild-type F2 pools, respectively. The pools were grown overnight, starved for 2 days in 60 mM Tris-HCl pH 7.5 at room temperature and total genomic DNA was extracted using the urea method (Gaertig et al. 1994).
The two F2 DNA pools were used to make genomic libraries using Illumina Truseq primer adapters and sequenced on an Illumina HiSeq X instrument, which generated paired-end reads of 150 bp length at ∼90× genome coverage. The MiModD suite of tools version 0.1.8 (https://sourceforge.net/projects/mimodd/) was used for ACCA-based variant mapping and identification as follows. For contrast mapping, the sequencing reads were aligned to the micronuclear reference genome (GenBank assembly accession GCA_000261185.1; Hamilton et al. 2016) and the aligned reads from both pools were used for joint multi-sample variant calling. The resulting variant call dataset was filtered for sites with ≥50× coverage for each of the two pools. Linkage scores contrasting the allelic composition of the mutant with that of the wild-type pool were computed for each retained variant site and the results plotted against genomic (micronuclear) coordinates. For variant identification, the same sequencing reads were aligned to the macronuclear reference genome (GenBank assembly accession GCA_000189635; (Eisen et al. 2006)) and the aligned reads subjected to variant calling as above.
Rescue and overexpression
To rescue the mutant phenotype of elo1-1, the predicted coding region of TTHERM_00035550, was placed in the BTU1 locus (Gaertig et al. 1999) under control of the cadmium-inducible MTT1 promoter (Shang et al. 2002) in the macronucleus using biolistic transformation and paromomycin selection based on a neo5 marker (Jiang et al. 2017). For overexpression in the wild-type background, the MTT1-GFP-TTHERM_00035550 transgene was integrated into the BTU1 locus in the CU428 strain. To induce overexpression, cells carrying the MTT1-GFP-TTHERM_00035550 transgene were exposed to cadmium chloride at 2.5 μg/ml for 3 hr.
Imaging by microscopy
To study the localization of the Elo1 protein under conditions of expression that are close to native, we attached a sequence encoding GFP to the 3′ end of the TTHERM_00035550 open reading frame (Stover et al. 2006) with a linked neo5. The tag was introduced into either the wild-type or elo1-1 background. In the elo1-1 cells, this led to expression of the Elo1-GFP mutant (G249V) protein. The transgene copy number was increased through phenotypic assortment, by growing transformant clones at increasing concentrations of paromomycin. The construction of the strain that expresses CdaI-GFP at the native locus was described (Jiang et al. 2017). The same targeting plasmid was used to make an elo1-1 strain that expresses CdaI-GFP.
Cells were analyzed by immunofluorescence (Gaertig et al. 2013) using the monoclonal anti-centrin 20H5 (EMD Milipore) and polyclonal anti-GFP (Rockland) antibodies, followed by secondary antibodies labeled with either CY3 or fluorescein (Rockland); DNA was stained with DAPI. The images were collected either on a Zeiss LSM 880 confocal microscope or a Zeiss ELYRA super resolution structured illumination (SR-SIM) microscope. The lengths of the emerging daughter cells were measured on the confocal images using NIH ImageJ (Schneider et al. 2012). The position of the division plane was quantified as the ratio of the length of the presumptive anterior daughter to the length of the presumptive posterior daughter (A/P ratio). The boundary between the daughters was either the plane of cortical subdivision or (in the early dividers) the plane immediately above the young oral primordium.
Structural analyses of Elo1 protein
The amino acid sequences of T. thermophila Elo1 (UniprotID: Q22MG5) and human Lats1 (UniprotID: O95835) were aligned using the default settings of MAFFT v7.058b (Katoh et al. 2002). A fragment of human Lats1 (residues 635–697) is present in the structure of Lats1-Mob1complex (PDBID: 5BRK) (Ni et al. 2015). Residues in Lats1 that are within 4 Å away from Mob1 were identified using PyMOL 1.7.6 (Grell et al. 2006). A homology-based model of Elo1 kinase domain was built using MODELER with the A chain of PDB structure 2F2U as template (Eswar et al. 2006; Yamaguchi et al. 2006). The G249V point mutation was introduced into the structure using the loopmodel class in MODELER. The models were visualized using PyMOL 1.7.6.
Data availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article and figures. Supplemental material available at Figshare: https://doi.org/10.6084/m9.figshare.7525193.
Results
The elo1-1 mutation causes the division plane to form at an excessively posterior position
The T. thermophila elo1-1 mutant clone was isolated in a genetic screen for cortical pattern mutants, following nitrosoguanidine mutagenesis (Frankel 2008). In the wild-type cell, the first sign of cell division is the new oral apparatus (oral primordium) that appears as a group of basal bodies near the right postoral ciliary row at a submedial position (Figure 1A stages 2 and 3, Figure 2A). At the subsequent stage of “cortical subdivision,” the ciliary rows become interrupted by gaps aligned around the cell’s equator (Figure 1A stage 4, Figure 2B). This is followed by the emergence of new cell ends on the two sides of the cortical subdivision, nuclear divisions and cytokinesis that produce two daughters of about equal size aligned as a tandem (Figure 1A stage 5, Figure 2C).
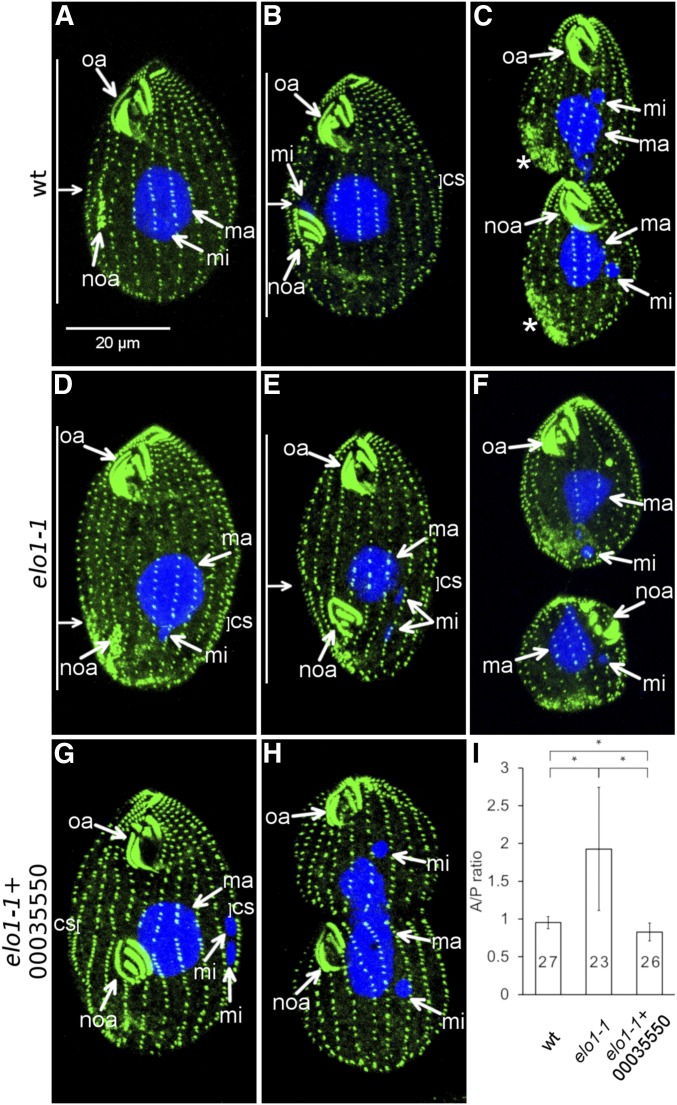
Figure 2.
elo1-1 shifts the division plane to the posterior cell end and expression of a wild-type THERM_00035550 protein rescues this defect. (A–F) The course of cell division in the wild type (A–C) and elo1-1 (D–F). The cells were labeled with the anti-centrin antibody (green) and DAPI (blue). The anti-centrin labels the basal bodies; in addition in some dividing cells these antibodies mark the vicinity of the contractile vacuoles [stars in (C)]. (A and D) Cells in the earliest stage of cell division (stage 1 in Figure 1A). Note that the oral primordium (noa) is sub-medial in the wild type (A) and excessively posterior in elo1-1 (D). (B and E) Cells during cortical subdivision (stage 4). (E). The micronucleus (mi) in the elo1-1 cell (E) is in a more advanced stage of mitosis as compared to the wild-type cell shown in (B). (C and F) Cells undergoing cytokinesis and amitosis of the macronucleus [the elo1-1 cell shown in (F) is in a more advanced stage]. (G–I) Introduction of a transgene expressing the GFP-TTHERM_00035550 rescues the unequal division in elo1-1 cells. (G and H) Dividing elo1-1 cells that carry the MTT1-GFP-THERM_0035550 transgene (grown without added cadmium ions). (I) The graph quantifies the position of the division plane as the ratio of the length of the presumptive anterior daughter to the length of the presumptive posterior daughter (the A/P ratio). Numbers of scored cells are indicated. Error bars are SD and asterisks show significant differences (two-sided t-test, P < 0.01). oa, oral apparatus; noa, new oral apparatus (primordium); ma, macronucleus; mi, micronucleus; cs, cortical subdivision. On the left side of (A, B, D, and E): the horizontal arrow indicates the position of the division plane and the vertical bars mark the lengths of the presumptive daughter cells.
In the elo1-1 cells, the oral primordium develops at an excessively posterior (but proper circumferential) position (Figure 2D). Subsequently, the elo1-1 mutants develop a cortical subdivision too close to the posterior cell end (Figure 2E) and cytokinesis produces a larger anterior and a smaller posterior daughter, respectively (Figure 2F). The position of the division plane was quantified as the ratio of the length of the presumptive anterior daughter to the length of the presumptive posterior daughter (the A/P ratio). The mean value of the A/P ratio was 0.95 ± 0.08 (n = 27) in the wild type, consistent with a nearly equal size of the two daughters, and 1.93 ± 0.8 (n = 23) in elo1-1, indicating that the anterior daughter is about twice the size of the posterior daughter (Figure 2I). Importantly, in elo1-1, the A/P ratio decreases as the division progresses (compare Figure 2, D and E and see Figure 5H for further details); despite this partial normalization, invariably the posterior daughter is smaller than the anterior daughter at the end of cytokinesis (Figure 2F). In the wild type, the micronucleus undergoes mitosis at the time of the onset of cortical subdivision (Figure 1A, stages 3–4); in anaphase, the elongated micronucleus is positioned posteriorly to the cortical subdivision (Figure 2B). The wild-type macronucleus undergoes amitosis by constricting at the time of cytokinesis (Figure 1A stages 4–5, Figure 2C). In the elo1-1 cells, the positions of both the dividing micronucleus and macronucleus were excessively posterior, but in a local agreement with the division plane position (Figure 2, E and F). In ciliates the positions of nuclei in the endoplasm are guided by cues from the cell cortex (Weisz 1951; de Terra 1973, 1975; Cohen and Beisson 1980; Gaertig and Cole 2000). Thus, the cortical guidance of nuclei is unaffected by elo1-1. Consequently, each elo1-1 daughter receives a complete set of nuclei despite the shift in the position of the division plane (Figure 2F). Some elo1-1 cells have abnormalities in the organization of the longitudinal ciliary rows that can be twisted or abnormally spaced apart (Figure 2, D–F). Overall, the most prominent and earliest defect in the elo1-1 cells is an excessively posterior initial specification of the division plane. Consequently, all events of cell division occur at excessively posterior positions, but otherwise appear unaffected.
Figure 5.
Elo1 and CdaI act in two consecutive Hippo circuits. (A–C) Addition of GFP to the C-terminus of CdaI confers a temperature-sensitive hypomorphic CdaI phenotype (cdaI-gfp). The cells are labeled with anti-centrin (green) and anti-GFP antibodies that detect CdaI-GFP (red). (A and B) A cdaI-gfp cell at a 29° (A) or 39° (B). Note the anterior shift of the division plane at 39°. (C) The A/P ratios of either wild-type or cdaI-gfp cells at 29° or 39° (3 hr exposure). Note a significant decrease in the A/P ratio in the cdaI-GFP cells at 39°. Numbers of total scored cells are indicated. Error bars are SD and the asterisks mark significant differences (two-sided t-test, P < 0.01). (D–F) Double mutant elo1-1_cdaI-gfp cells grown at 39°. Note that the division plane is excessively posterior at the earliest stage of cell division (D) and becomes nearly medial in the later stages (E and F). (G) A late phase elo1-1 cell at 39°. (H) The A/P ratios of elo1-1 or elo1-1_cdaI-gfp cells during either the early or late stage of cell division and either at 29 or 39° (3 hr). The horizontal dotted line represents the A/P ratio value of wild-type cells at 29° at the late phase of cell division. Note the rescue of the A/P ratio in the double mutant at 39°. Numbers of total scored cells are indicated. Error bars are SD and the asterisks mark significant differences (two-sided t-test, P < 0.01). On the left side of (A, B, and D–G): the horizontal arrow indicates the position of the division plane and the vertical bars mark the lengths of the presumptive daughter cells.
ELO1 encodes a Lats/NDR kinase
We used a comparative whole genome sequencing approach, ACCA (Jiang et al. 2017), to map the causal mutation for elo1-1 (see Materials and Methods). Briefly, we prepared pools of either wild-type or elo1-1 F2 clones (meiotic segregants) derived from a single F1 heterozygote. The pooled genomes were sequenced and the sequence reads were aligned to the micronuclear reference genome (Hamilton et al. 2016). For each sequence variant detected, a linkage score was calculated that reflected the degree of cosegregation of the alternate allele with elo1-1 and the corresponding cosegregation of the reference allele with the wild-type phenotype, respectively. The linkage scores were plotted along the length of the micronuclear chromosomes. This revealed a region of increased variant to phenotype linkage on the micronuclear chromosome 3 between 8 and 9 Mb (Figure 3A). Within this interval, a single sequence variant (a G to T substitution at the micronuclear position chr3:8620427) was supported by 100% alternate allele (T) reads in the pool of 31 mutant F2 clones and 100% reference allele (G) reads in the pool of 31 wild-type F2 clones. In the macronuclear genome, the corresponding base pair position is located in the coding region of TTHERM_00035550 gene that encodes a protein homologous to the Lats/NDR kinases, conserved components of the Hippo kinase cascade (Hergovich 2013). The predicted amino acid sequence of the conserved portion of TTHERM_00035550 protein that contains the kinase domain (positions 30–475) is 43% identical to the corresponding part of the Drosophila Trc (Geng et al. 2000), 39% identical to that of CBK1 of the budding yeast (Bidlingmaier et al. 2001), and 46% identical to that of the human NDR2 (Millward et al. 1995). Reciprocal BLASTp searches using the above mentioned Lats/NDR sequences against the predicted proteome of T. thermophila (Eisen et al. 2006) returned either TTHERM_00035550 or TTHERM_00283290 (a possible paralog) as top matches. Structural analyses further argue that the TTHERM_00035550 protein is a true Lats/NDR kinase (see below).
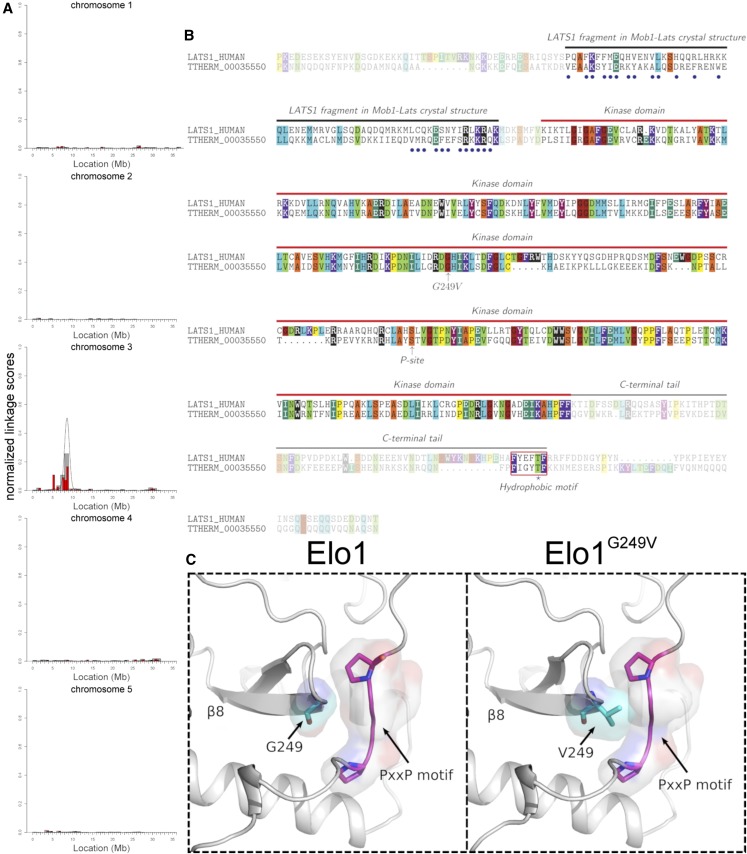
Figure 3.
The causal mutation for elo1-1 is in THERM_0035550 that encodes a Lats/NDR kinase. (A) The results of mapping of the causal DNA sequence variant for elo1-1 using ACAA (Jiang et al. 2017). Normalized linkage scores are shown along each of the five micronuclear chromosomes. Allelic cosegregation is increased on the left arm of chromosome 3. (B) An alignment of a portion of TTHERM_0035550/Elo1 and human Lats1 amino acid sequences. The blue circles mark the residues that are located within 4 Å from the Mob1 surface in the human Lats1-Mob1 complex (PDBID: 5BRK) (Gogl et al. 2015; Ni et al. 2015). Different colors represent different sizes of amino acid side chains as follows (in Å2): dark red, G 88.1; orange, A 118.2, S 129.8; yellow, C 146.1, P 146.8; teal, I 181.0, E 186.2; aqua blue, L 193.1, Q 193.2; H 202.5, M 203.3; violet, F 222.8, K 225.8; purple, Y 238.8; black, R 256.0, W 266.2. The red box highlights the hydrophobic motif (HM). The T-loop serine that undergoes autophosphorylation in the Lats/NDR kinases is marked as the “P-site.” (C) The 3D structure of an area of the Elo1 kinase domain showing a fragment of the β8 strand that carries G249 (mutated to V in elo1-1). The area is in proximity to the conserved PxxP motif in the C-terminal tail of AGC kinases.
The candidate causal mutation produces a G249V substitution. The elo1-1 allele is recessive (Frankel 2008). To test whether G249V in TTHERM_00035550 is the cause of elo1-1, we introduced a transgene expressing a wild-type TTHERM_00035550, as an N-terminal GFP fusion under the cadmium-inducible promoter MTT1 (Shang et al. 2002), into the elo1-1 cells. The dividing elo1-1 cells with GFP-TTHERM_00035550 had a nearly normal A/P ratio of 0.82 ± 0.12 (n = 26) (Figure 2, G–I); this rescue occurred without cadmium ions added, indicating that the basal level of expression under MTT1 was sufficient for complementation of the mutant phenotype. We conclude that the G249V substitution in TTHERM_00035550 is the cause of elo1-1, and, therefore, we named the TTHERM_00035550 gene ELO1.
A kinase classification algorithm (Talevich and Kannan 2013; McSkimming et al. 2017) confirmed that Elo1 belongs to the Lats/NDR subfamily of the AGC parent group of kinases. Lats/NDR kinases bind Mob—an adapter protein that is a part of the conserved core Hippo pathway (Luca and Winey 1998; Colman-Lerner et al. 2001; Weiss et al. 2002). An alignment of the amino acid sequences of Elo1 and human Lats1 (Figure 3B) revealed that the two proteins share sequence homology within a region that is N-terminal to the kinase domain, which, in Lats1, serves as a Mob-binding domain (Bothos et al. 2005). Several amino acids that, in the human Lats1, lie within 4 Å of the human Mob1 surface (Gógl et al. 2015; Ni et al. 2015), are conserved at the corresponding positions in Elo1 (blue circles in Figure 3B). Elo1 also has a conserved C-terminal hydrophobic motif (HM) with a threonine (Figure 3B) that, in the Lats/NDR kinases, is phosphorylated by an upstream kinase, Hippo/Mst (Stegert et al. 2005). Elo1 also has a serine, S303, in the T loop of the kinase domain (Figure 3B, “P-site”), which is homologous to serines in the well-studied Lats/NDR kinases (e.g., S281 in human NDR1 and S909 in human Lats1) that undergo autophosphorylation, which is required for the kinase activity (Tamaskovic et al. 2003; Bichsel et al. 2004).
G249 is located within the kinase domain (Figure 3B). To evaluate the impact of G249V, we used homology-based modeling to produce a possible 3D structure of the Elo1 kinase domain (Figure 3C). G249 is located at the beginning of β8 strand and therefore is not a part of the catalytic center. However, G249 is among the conserved AGC kinase group-specific amino acids (Kannan et al. 2007). AGC kinases are regulated by a C-terminal tail domain that is tethered to the kinase domain through a PxxP motif (Kannan et al. 2007), which is also conserved in Elo1 (Figure 3C). The 3D model of Elo1 suggests that G249 is in close contact with PxxP (Figure 3C). The PxxP motif is also the binding site for the SH3 domain (Jiang and Qiu 2003). It is possible that G249V alters the release of the C-terminal tail domain (which could affect the kinase activity) or perturbs regulatory interactions between Elo1 and SH3 domain-containing proteins.
Elo1 protein is enriched in the posterior cell cortex where the division plane does not form in the wild type
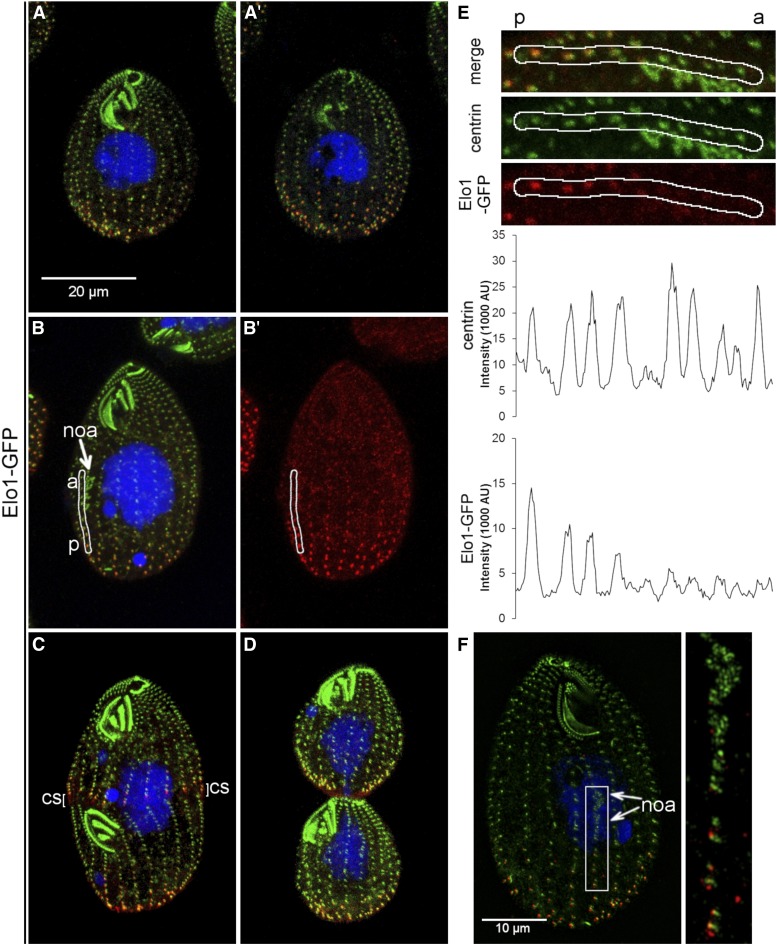
We added a green fluorescent protein (GFP) to the C-terminal end of Elo1, by engineering the ELO1 locus. During interphase, Elo1-GFP presented as dots near several most posterior basal bodies in each ciliary row, based on colocalization with centrin (Salisbury et al. 1988) (Figure 4, A and A’ and Supplemental Material, Figure S1, A–B’). The number of Elo1-GFP-positive basal bodies per row was similar on the ventral and dorsal cell side, indicating that distribution of Elo1 is polarized on the anteroposterior but not on the circumferential axis (Figure S1, A–B’). The posterior cortical zone of Elo1-GFP was present when the oral primordium appeared (Figure 4, B and B’). Along the ciliary row, the intensity of the Elo1-GFP dots decreased gradually toward the cell’s anterior end (Figure 4E). Based on the SR-SIM imaging, the most posterior basal bodies of the early oral primordium were located immediately anterior to the edge of the detectable Elo1-GFP (Figure 4F). During cortical subdivision, a new Elo1-GFP zone appeared at the posterior edge of the forming anterior daughter (Figure 4C and Figure S1, C–D’). At the time of cytokinesis, GFP-Elo1 marked the most posterior basal bodies equally strongly in the anterior and posterior daughter (Figure 4D and Figure S1, E–F’). The signal of the mutant protein, Elo1(G249V)-GFP, was weaker as compared to Elo1-GFP, indicating that G249V either decreases the levels of Elo1 or its ability to become enriched at the posterior cortex (the mean pixel intensity of Elo1-GFP over the five most posterior basal bodies was 4.6 times higher in the wild type as compared to elo1-1; see Figure S2 for details).
Figure 4.
Elo1-GFP is restricted to the posterior cortex. (A–D) Confocal immunofluorescence images of cells expressing Elo1-GFP under the native promoter, labeled with anti-GFP (red), anti-centrin (green) antibodies and DAPI (blue). The cells are in interphase [(A and A’) show the ventral and dorsal side of the same cell, respectively], early cell division with a young oral primordium ((B and B’) show all signals and just Elo1-GFP, respectively), cortical subdivision (C) and cytokinesis (D). (E) Images of a posterior portion of a single ciliary row extracted from the cell shown in (B and B’) (white outline), shown at a higher magnification. The images of Elo1-GFP (red), centrin (green) and both signals are shown. Below are corresponding intensity plots of the Elo1-GFP and centrin signals. Note multiple peaks of Elo1-GFP that correspond to the basal bodies. The signal intensity of Elo1-GFP decreases with the increased distance from the posterior cell end. a, anterior end of the section of ciliary row; p, posterior end of a section of the analyzed ciliary row. (F). An SR-SIM image of a dividing cell labeled with the anti-GFP (red) and anti-centrin (green) antibodies. The cell is in an early stage of development of the oral primordium. Note that the Elo1-GFP signal is visible along each ciliary row from the posterior cell end and to the bottom of the oral primordium. The inset shows a higher magnification of the area where the oral primordium forms. The two arrows mark the anterior and posterior boundaries of the oral primordium where the basal bodies are present on the left side of the stomatogenic ciliary row. noa, new oral apparatus; cs, cortical subdivision.
Elo1 (Lats/NDR) and CdaI (Hippo/Mst) act in two consecutive Hippo signaling circuits
In addition to Elo1, the medial position of the division plane depends on CdaI and Mob1, orthologs of the Hippo/Mst kinases and Mob proteins, respectively (Figure 1B). However, a loss of either CdaI or Mob1 moves the division plane closer to the anterior cell end (Tavares et al. 2012; Jiang et al. 2017). As described above, the effect of Elo1 is apparent at the earliest stage of divisional morphogenesis (the appearance of the oral primordium) (Figure 2). In contrast, in cells expressing a conditional mutant CdaI, the division plane is initially specified correctly and migrates in the anterior direction at the onset of cortical subdivision (Jiang et al. 2017). These observations suggest that Elo1 and CdaI are parts of two separate Hippo signaling circuits that act at consecutive stages of cell division. To test this idea further, we examined a double mutant. We took advantage of a serendipitous finding that adding GFP to an otherwise wild-type CdaI (cdaI-gfp allele) leads to a hypomorphic temperature-sensitive phenotype. At 29° the cdaI-gfp cells divide normally (Jiang et al. 2017; and Figure 5, A and C). At 39°, the cdaI-gfp cells had a mild (but significant) anterior displacement of the division plane (Figure 5, B and C), but lacked nuclear or cytokinesis defects, which frequently occur in the cells expressing the stronger allele, cdaI-1 (Jiang et al. 2017). To determine whether, and when, Elo1 and CdaI interact, we compared the A/P ratios between the elo1-1 and elo1-1_cdaI-gfp cells that were either in the early or late phase of cell division (based on the absence or presence of cortical subdivision, respectively). As already mentioned, in the elo1-1 single mutants (at both 29 and 39°), the A/P length ratio partially normalized as the division progresses (Figure 2, D and E and Figure 5H). For example, in the elo1-1 cells (at 39°), the A/P ratio decreased from 2.77 ± 0.7 (n = 14) in the early phase to 1.36 ± 0.12 (n = 15) in the late phase (Figure 5H). In the elo1-1_cdaI-gfp double mutant cells examined during the early phase, the A/P ratio was not significantly different from that of the elo1-1 single mutant cells either at 29 or 39°, indicating that CdaI does not influence the division plane position before the appearance of cortical subdivision (Figure 5, D and H). In the double mutant cells examined during the late phase at 39° (but not at 29°), the division plane significantly shifted anteriorly as compared to elo1-1 cells alone (Figure 5, E, F, and H). In the late phase at 39°, the A/P ratio was 1.00 ± 0.1 (n = 15) in elo1-1_cdaI-gfp as compared to 1.36 ± 0.12 in elo1-1 (n = 15). To summarize, the double mutant cells initially have the phenotype of elo1-1 and normalize during the later phase due to the effect of cdaI-gfp. Thus, CdaI and Elo1 act antagonistically but affect different stages of cell division (Figure 1B). Elo1 specifies the initial position of the division plane on the anteroposterior axis, while CdaI maintains the equatorial position of the division plane, and, as we showed earlier (Jiang et al. 2017), promotes nuclear divisions and cytokinesis.
Overexpression of Elo1 phenocopies a loss of CdaI
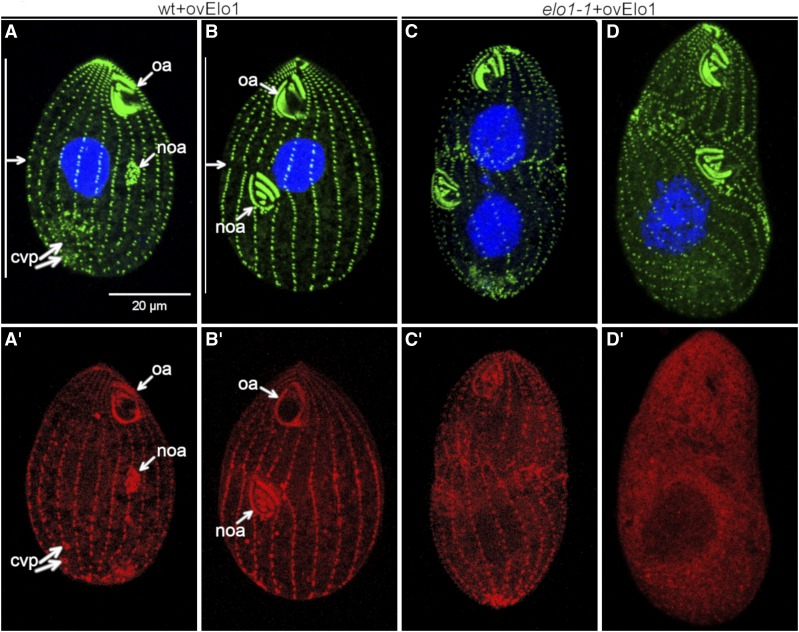
To test how important the levels of Elo1 are, we overexpressed GFP-Elo1 (using the cadmium-inducible MTT1 promoter) in a wild-type background. Cells overproducing GFP-Elo1 transgene had a mild anterior shift of the division plane (Figure 6, A and B, cf. Figure 2, A and B). This displacement was apparent already at the stage of early oral primordium (Figure 6A). In these overproducing cells, the GFP-Elo1 zone was greatly expanded to cover almost the entire cell, including the oral primordium and the old oral apparatus (Figure 6, A’–B’). Thus, upon overproduction, the zone of Elo1 expression is larger than what would be expected based solely on the mildly shifted position of the division plane. Likely, another factor limits the ability of Elo1 to mediate the exclusion of the divisional activities from the posterior cortex. The overproduced GFP-Elo1 highlighted the basal bodies, but also the old contractile vacuole pores (Figure 6, A’ and B’), suggesting that Elo1 binds to microtubules. We conclude that the size of the cortical domain of the emerging posterior daughter depends on the cellular levels of Elo1.
Figure 6.
Overexpression of Elo1in the wild-type background shifts the division plane anteriorly, and overexpression in the elo1-1 background phenocopies a loss of function of CdaI. (A–D’) Images of cells that overproduce GFP-Elo1 in the wild-type (A–B’) or elo1-1 (C–D’) background (induced by cadmium chloride for 3 hr at 2.5 μg/ml). For each cell the signals of anti-centrin and DAPI (A–D) and anti-GFP-Elo1 (A’–D’) are shown.
Next, we examined cells in which the MTT1-GFP-Elo1 transgene operated in the elo1-1 background. As mentioned earlier, without addition of cadmium ions, most of these cells had a normal equatorial cell division plane (Figure 2, G–I). Unexpectedly, in around one-third of the examined clones with an MTT1-GFP-Elo1 transgene in an elo1-1 background, induction of overexpression (by cadmium ions for 3 hr) caused a cell cycle arrest, nuclear segregation defects, and a strong anterior displacement of the division plane, which is a phenocopy of the strong CDAI loss-of-function allele, cdaI-1 (Jiang et al. 2017); in one clone examined by immunofluorescence, 85% (n = 47) of cells showed a cdaI-1 phenotype after 3 hr of exposure to cadmium ions (Figure 6, C–D’). Thus, in the elo1-1 background overproduced GFP-Elo1 could interfere with the function of CdaI. The simplest scenario is that an excessive GFP-Elo1 outcompetes a Lats/NDR kinase that acts downstream of CdaI, for binding to Mob1 (Figure 1B, and see Discussion). It remains unclear why this apparent interference with the proposed late Hippo circuit would occur only when GFP-Elo1 is overexpressed in the elo1-1 background. If the two circuits share the same Mob adapter, Mob1, one possibility is that GFP-Elo1, once loaded onto Mob1 as part of the proposed early Hippo circuit, is less likely than the wild-type Elo1 to be released from the Mob1 complex, to make room for the Lats/NDR kinase that operates in the proposed late Hippo circuit.
Discussion
It is now well established that ciliates utilize the Hippo kinase cascade to execute their unique mode of cell division by tandem duplication [this work and Tavares et al. (2012), Slabodnick et al. (2014), Jiang et al. (2017)]. Tavares and colleagues first linked the Hippo pathway to cell division in ciliates by showing that a knockdown of Mob1 in Tetrahymena results in an excessively anterior division plane and defects in cytokinesis and nuclear divisions (Tavares et al. 2012). A similar phenotype results from a loss of CdaI, a Hippo/Mst kinase (Jiang et al. 2017). Here we find that ELO1, whose loss of function displaces the division plane toward the posterior end (Frankel 2008), encodes another Hippo pathway component, a Lats/NDR kinase, a conserved binding partner of Mob adapters. We will argue that the opposite influences of different Hippo pathway proteins on the division plane position reflect the activities of two antagonistic and consecutive Hippo signaling circuits that may share the same Mob adapter, Mob1 (Figure 1B).
The early Hippo circuit involves Elo1
The elo1-1 mutation causes the oral primordium to appear at an excessively posterior position. Likely, Elo1 binds to a Mob adapter and acts downstream of an unknown Hippo/Mst kinase (Figure 1B). The Mob partner of Elo1 is likely Mob1, based on the virtually identical localization patterns of the two proteins (Tavares et al. 2012 and this study, Figure 1A). However, an inducible knockdown of Mob1 phenocopies a loss of CdaI (Tavares et al. 2012; Jiang et al. 2017) and not Elo1, consistent with Mob1 acting in the late Hippo circuit (see below). It is therefore possible that Mob1 is shared by the two Hippo circuits. When the depletion of Mob1 is induced in a growing population (Tavares et al. 2012), most cells are in interphase and may have a sufficient quantity of Mob1 and Elo1 already loaded onto the posterior cell cortex. Alternatively, Elo1 may bind another Mob adapter. Tetrahymena was believed to have only one gene encoding Mob, Mob1 (Tavares et al. 2012). However, we note that an unstudied protein TTHERM_001262898 is similar to Mob4, a subtype of Mob present in animal lineages (Trammell et al. 2008; Ye et al. 2009).
CdaI acts in the late Hippo circuit
During expression of conditional alleles of CdaI, the division plane is initially properly positioned (at the stage of oral primordium), and, at the subsequent stage (of cortical subdivision), shifts in an anterior direction (Jiang et al. 2017 and this study). This displacement does not appear to be merely a physical process; as the oral primordium moves, the sections of the ciliary rows between the old and new oral apparatus shorten, likely by resorption of their basal bodies (Frankel 2008; Jiang et al. 2017). Thus, the division plane migration appears to be a developmental respecification that changes the boundaries of the anterior and posterior daughter. In addition to the control of the division plane position, CdaI is also important for the completion of nuclear divisions and cytokinesis (Jiang et al. 2017).
The terminal phenotypes caused by CdaI and Mob1 loss of function are strikingly similar (Tavares et al. 2012; Jiang et al. 2017), arguing that the late Hippo switch involves CdaI, Mob1, and an unknown Lats/NDR kinase (Figure 1B). While the localization patterns of CdaI and Mob1 are mostly nonoverlapping, the two proteins colocalize at the posterior margin of the forming anterior daughter, and this exact stage of cell division is sensitive to the losses of either CdaI or Mob1 (Tavares et al. 2012; Jiang et al. 2017).
The unifying property of the two Hippo proteins studied here, Elo1 and CdaI, is that they both act by excluding the divisional activities from parts of the cell cortex. The components of Hippo signaling components studied thus far (Mob1, CdaI, and Elo1) are all asymmetrically localized along the anteroposterior axis, but uniformly distributed along the cell circumference. The unique environment of the cell extremities (such as the different plasma membrane curvatures) could provide cues for the polarization of Hippo proteins. In rod-shaped cells, including fission yeast and bacteria, cell ends are sources of protein gradients that confer spatial control over the cell cycle (reviewed in Kiekebusch and Thanbichler 2014). In the Tetrahymena con1-1 mutant, an altered shape of the posterior cell end correlates with a posteriorly displaced division plane (Doerder et al. 1975; Lynn 1977; Schäfer and Cleffmann 1982). On the other hand, in Tetrahymena whose posterior end was destroyed by cell–cell fusion, the oral primordium appears at a correct position and the nuclei are correctly guided with respect to the anteroposterior cell axis (Gaertig and Iftode 1989; Gaertig and Cole 2000). Thus, the mechanisms that generate the biased cortical localizations of Hippo pathway proteins along the anteroposterior axis remain unknown.
We note that Elo1 and CdaI are not needed for the determination of the different fates of cortical domains that form the daughter cells. Rather, these Hippo proteins regulate the sizes of cortical domains that differentiate into the two daughters. In animals, Hippo signaling regulates the size of body organs by influencing both the rate of cell proliferation as well as the growth rate and ultimately the size of individual cells in the organ (Neto-Silva et al. 2010; Tumaneng et al. 2012; Lloyd 2013). When Hippo signaling is active, the ciliate is in transition from a single-cell to a transient two-cell stage. Thus, in the broadest terms, the role of Hippo signaling in the control of cell size in a multicellular context might well be conserved between ciliates and animals. Further research of Hippo signaling in the model ciliates has potential for extending our understanding of the mechanisms of pattern formation and the control of cell size.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants 1021RR194369 (to J.G.) and 5RO1GM114409 (to N.K.), a bridge funding from the Office of the Vice-President for Research and the Department of Cellular Biology at the University of Georgia (to J.G.), the National Science Foundation grant MCB-1149106 (to N.K.), grants from Deutsche Forschungsgemeinschaft BIOSS CRC746 and CRC850 (to R.B.) and the National Science Centre, Poland Grant 2018/29/B/NZ3/02443 (to E.J.). We thank Muthugapatti K. Kandasamy [Biomedical Microscopy Core facility at University of Georgia, Athens (UGA)] for assistance with fluorescence microscopy.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.6084/m9.figshare.7525193.
Communicating editor: A. Gladfelter
Literature Cited
- Bichsel S. J., Tamaskovic R., Stegert M. R., Hemmings B. A., 2004. Mechanism of activation of NDR (nuclear Dbf2-related) protein kinase by the hMOB1 protein. J. Biol. Chem. 279: 35228–35235. 10.1074/jbc.M404542200 [DOI] [PubMed] [Google Scholar]
- Bidlingmaier S., Weiss E. L., Seidel C., Drubin D. G., Snyder M., 2001. The Cbk1p pathway is important for polarized cell growth and cell separation in Saccharomyces cerevisiae. Mol. Cell. Biol. 21: 2449–2462. 10.1128/MCB.21.7.2449-2462.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothos J., Tuttle R. L., Ottey M., Luca F. C., Halazonetis T. D., 2005. Human LATS1 is a mitotic exit network kinase. Cancer Res. 65: 6568–6575. 10.1158/0008-5472.CAN-05-0862 [DOI] [PubMed] [Google Scholar]
- Bruns P. J., Brussard T. B., Kavka A. B., 1976. Isolation of homozygous mutants after induced self-fertilization in Tetrahymena. Proc. Natl. Acad. Sci. USA 73: 3243–3247. 10.1073/pnas.73.9.3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Beisson J., 1980. Genetic analysis of the relationships between the cell surface and the nuclei in Paramecium tetraurella. Genetics 95: 797–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman-Lerner A., Chin T. E., Brent R., 2001. Yeast Cbk1 and Mob2 activate daughter-specific genetic programs to induce asymmetric cell fates. Cell 107: 739–750. 10.1016/S0092-8674(01)00596-7 [DOI] [PubMed] [Google Scholar]
- de Terra N., 1973. Further evidence for cortical control over replication of the macronucleus and basal bodies in Stentor. Dev. Biol. 32: 129–139. 10.1016/0012-1606(73)90225-X [DOI] [PubMed] [Google Scholar]
- de Terra N., 1975. Evidence for cell surface control of macronuclear DNA synthesis in Stentor. Nature 258: 300–303. 10.1038/258300a0 [DOI] [PubMed] [Google Scholar]
- Doerder F. P., Frankel J., Jenkins L. M., DeBault L. E., 1975. Form and pattern in ciliated protozoa: analysis of a genic mutant with altered cell shape in Tetrahymena pyriforms Syngen 1. J. Exp. Zool. 192: 237–258. 10.1002/jez.1401920214 [DOI] [PubMed] [Google Scholar]
- Eisen J. A., Coyne R. S., Wu M., Wu D., Thiagarajan M., et al. , 2006. Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol. 4: e286 10.1371/journal.pbio.0040286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswar N., Webb B., Marti-Renom M. A., Madhusudhan M. S., Eramian D., et al. , 2006. Comparative protein structure modeling using Modeller. Curr. Protoc. Bioinformatics Chapter 5: Unit-5.6 10.1002/0471250953.bi0506s15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel J., 1989. Pattern Formation. Ciliate Studies and Models. Oxford University Press, New York. [Google Scholar]
- Frankel J., 2008. What do genic mutations tell us about the structural patterning of a complex single-celled organism? Eukaryot. Cell 7: 1617–1639. 10.1128/EC.00161-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertig J., Cole E. S., 2000. The role of cortical geometry in the nuclear development of Tetrahymena thermophila. J. Eukaryot. Microbiol. 47: 590–596. 10.1111/j.1550-7408.2000.tb00095.x [DOI] [PubMed] [Google Scholar]
- Gaertig J., Iftode F., 1989. Rearrangement of the cytoskeleton and nuclear transfer in Tetrahymena thermophila cells fused by electric field. J. Cell Sci. 93: 691–703. [DOI] [PubMed] [Google Scholar]
- Gaertig J., Thatcher T. H., Gu L., Gorovsky M. A., 1994. Electroporation-mediated replacement of a positively and negatively selectable β-tubulin gene in Tetrahymena thermophila. Proc. Natl. Acad. Sci. USA 91: 4549–4553. 10.1073/pnas.91.10.4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertig J., Gao Y., Tishgarten T., Clark T. G., Dickerson H. W., 1999. Surface display of a parasite antigen in the ciliate Tetrahymena thermophila. Nat. Biotechnol. 17: 462–465. 10.1038/8638 [DOI] [PubMed] [Google Scholar]
- Gaertig J., Wloga D., Vasudevan K. K., Guha M., Dentler W. L., 2013. Discovery and functional evaluation of ciliary proteins in Tetrahymena thermophila. Methods Enzymol. 525: 265–284. 10.1016/B978-0-12-397944-5.00013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng W., He B., Wang M., Adler P. N., 2000. The tricornered gene, which is required for the integrity of epidermal cell extensions, encodes the Drosophila nuclear DBF2-related kinase. Genetics 156: 1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gógl G., Schneider K. D., Yeh B. J., Alam N., Nguyen Ba A. N., et al. , 2015. The structure of an NDR/LATS kinase-mob complex reveals a novel kinase-coactivator system and substrate docking mechanism. PLoS Biol. 13: e1002146 10.1371/journal.pbio.1002146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., 1973. Macro- and micronuclei of Tetrahymena pyriformis: a model system for studying the structure and function of eukaryotic nuclei. J. Protozool. 20: 19–25. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1550-7408.1973.tb05995.x [DOI] [PubMed] [Google Scholar]
- Grell L., Parkin C., Slatest L., Craig P. A., 2006. EZ-Viz, a tool for simplifying molecular viewing in PyMOL. Biochem. Mol. Biol. Educ. 34: 402–407. 10.1002/bmb.2006.494034062672 [DOI] [PubMed] [Google Scholar]
- Hamilton E. P., Kapusta A., Huvos P. E., Bidwell S. L., Zafar N., et al. , 2016. Structure of the germline genome of Tetrahymena thermophila and relationship to the massively rearranged somatic genome. eLife 5: e19090 10.7554/eLife.19090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A., 2013. Regulation and functions of mammalian LATS/NDR kinases: looking beyond canonical Hippo signalling. Cell Biosci. 3: 32 10.1186/2045-3701-3-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Qiu Y., 2003. Interaction between Src and a C-terminal proline-rich motif of Akt is required for Akt activation. J. Biol. Chem. 278: 15789–15793. 10.1074/jbc.M212525200 [DOI] [PubMed] [Google Scholar]
- Jiang Y. Y., Maier W., Baumeister R., Minevich G., Joachimiak E., et al. , 2017. The Hippo pathway maintains the equatorial division plane in the ciliate Tetrahymena. Genetics 206: 873–888. 10.1534/genetics.117.200766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. H., Eberly S. L., Chapman J. W., Araki H., Sugino A., 1990. The product of the Saccharomyces cerevisiae cell cycle gene DBF2 has homology with protein kinases and is periodically expressed in the cell cycle. Mol. Cell. Biol. 10: 1358–1366. 10.1128/MCB.10.4.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan N., Haste N., Taylor S. S., Neuwald A. F., 2007. The hallmark of AGC kinase functional divergence is its C-terminal tail, a cis-acting regulatory module. Proc. Natl. Acad. Sci. USA 104: 1272–1277 (corrigenda: Proc. Natl. Acad. Sci. USA 105: 9130 (2008)) 10.1073/pnas.0610251104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Misawa K., Kuma K., Miyata T., 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30: 3059–3066. 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiekebusch D., Thanbichler M., 2014. Spatiotemporal organization of microbial cells by protein concentration gradients. Trends Microbiol. 22: 65–73. 10.1016/j.tim.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Kirschner M., Gerhart J., Mitchison T., 2000. Molecular “vitalism”. Cell 100: 79–88. 10.1016/S0092-8674(00)81685-2 [DOI] [PubMed] [Google Scholar]
- Lloyd A. C., 2013. The regulation of cell size. Cell 154: 1194–1205. 10.1016/j.cell.2013.08.053 [DOI] [PubMed] [Google Scholar]
- Luca F. C., Winey M., 1998. MOB1, an essential yeast gene required for completion of mitosis and maintenance of ploidy. Mol. Biol. Cell 9: 29–46. 10.1091/mbc.9.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn D. H., 1977. Proportional control of organelle position by a mechanism which similarly monitors cell-size of wild-type and conical form-mutant Tetrahymena. J. Embryol. Exp. Morphol. 42: 261–274. http://dev.biologists.org/content/develop/42/1/261.full.pdf [Google Scholar]
- Marshall W. F., 2011. Origins of cellular geometry. BMC Biol. 9: 57 10.1186/1741-7007-9-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSkimming D. I., Rasheed K., Kannan N., 2017. Classifying kinase conformations using a machine learning approach. BMC Bioinformatics 18: 86 10.1186/s12859-017-1506-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward T., Cron P., Hemmings B. A., 1995. Molecular cloning and characterization of a conserved nuclear serine (threonine) protein kinase. Proc. Natl. Acad. Sci. USA 92: 5022–5026. 10.1073/pnas.92.11.5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto-Silva R. M., de Beco S., Johnston L. A., 2010. Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev. Cell 19: 507–520. 10.1016/j.devcel.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Zheng Y., Hara M., Pan D., Luo X., 2015. Structural basis for Mob1-dependent activation of the core Mst-Lats kinase cascade in Hippo signaling. Genes Dev. 29: 1416–1431. 10.1101/gad.264929.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orias E., Flacks M., 1975. Macronuclear genetics of Tetrahymena. I. Random distribution of macronuclear genecopies in T. pyriformis, syngen 1. Genetics 79: 187–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehle M. D., Orias E., Pearson C. G., 2016. Tetrahymena as a unicellular model eukaryote: genetic and genomic tools. Genetics 203: 649–665. 10.1534/genetics.114.169748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury J. L., Baron A. T., Sanders M. A., 1988. The centrin-based cytoskeleton of Chlamydomonas reinhardtii: distribution in interphase and mitotic cells. J. Cell Biol. 107: 635–641. 10.1083/jcb.107.2.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer E., Cleffmann G., 1982. Division and growth kinetics of the division mutant conical of Tetrahymena. A contribution to regulation of generation time. Exp. Cell Res. 137: 277–284. 10.1016/0014-4827(82)90028-3 [DOI] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S., Eliceiri K. W., 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Song X., Bowen J., Corstanje R., Gao Y., et al. , 2002. A robust inducible-repressible promoter greatly facilitates gene knockouts, conditional expression, and overexpression of homologous and heterologous genes in Tetrahymena thermophila. Proc. Natl. Acad. Sci. USA 99: 3734–3739. 10.1073/pnas.052016199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabodnick M. M., Ruby J. G., Dunn J. G., Feldman J. L., DeRisi J. L., et al. , 2014. The kinase regulator Mob1 acts as a patterning protein for Stentor morphogenesis. PLoS Biol. 12: e1001861 10.1371/journal.pbio.1001861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegert M. R., Hergovich A., Tamaskovic R., Bichsel S. J., Hemmings B. A., 2005. Regulation of NDR protein kinase by hydrophobic motif phosphorylation mediated by the mammalian Ste20-like kinase MST3. Mol. Cell. Biol. 25: 11019–11029. 10.1128/MCB.25.24.11019-11029.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover N. A., Krieger C. J., Binkley G., Dong Q., Fisk D. G., et al. , 2006. Tetrahymena genome database (TGD): a new genomic resource for Tetrahymena thermophila research. Nucleic Acids Res. 34: D500–D503. 10.1093/nar/gkj054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talevich E., Kannan N., 2013. Structural and evolutionary adaptation of rhoptry kinases and pseudokinases, a family of coccidian virulence factors. BMC Evol. Biol. 13: 117 10.1186/1471-2148-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaskovic R., Bichsel S. J., Rogniaux H., Stegert M. R., Hemmings B. A., 2003. Mechanism of Ca2+-mediated regulation of NDR protein kinase through autophosphorylation and phosphorylation by an upstream kinase. J. Biol. Chem. 278: 6710–6718. 10.1074/jbc.M210590200 [DOI] [PubMed] [Google Scholar]
- Tartar V., 1956. Pattern and substance in Stentor, in Cellular Mechanisms in Differentiation and Growth, edited by Rudnick D. Princeton University Press, Princeton, NJ. [Google Scholar]
- Tartar V., 1961. The Biology of Stentor. Pergamon Oress, Elmsford, NY. [Google Scholar]
- Tavares A., Goncalves J., Florindo C., Tavares A. A., Soares H., 2012. Mob1: defining cell polarity for proper cell division. J. Cell Sci. 125: 516–527. 10.1242/jcs.096610 [DOI] [PubMed] [Google Scholar]
- Trammell M. A., Mahoney N. M., Agard D. A., Vale R. D., 2008. Mob4 plays a role in spindle focusing in Drosophila S2 cells. J. Cell Sci. 121: 1284–1292. 10.1242/jcs.017210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumaneng K., Schlegelmilch K., Russell R. C., Yimlamai D., Basnet H., et al. , 2012. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat. Cell Biol. 14: 1322–1329. 10.1038/ncb2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. L., Kurischko C., Zhang C., Shokat K., Drubin D. G., et al. , 2002. The Saccharomyces cerevisiae Mob2p-Cbk1p kinase complex promotes polarized growth and acts with the mitotic exit network to facilitate daughter cell-specific localization of Ace2p transcription factor. J. Cell Biol. 158: 885–900. 10.1083/jcb.200203094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz P. B., 1951. An experimental analysis of morphogenesis in Stentor coeruleus. J. Exp. Zool. 116: 231–257. 10.1002/jez.1401160203 [DOI] [PubMed] [Google Scholar]
- Wloga D., Frankel J., 2012. From molecules to morphology: cellular organization of Tetrahymena thermophila. Methods Cell Biol. 109: 83–140. 10.1016/B978-0-12-385967-9.00005-0 [DOI] [PubMed] [Google Scholar]
- Xu T., Wang W., Zhang S., Stewart R. A., Yu W., 1995. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121: 1053–1063. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Kasa M., Amano M., Kaibuchi K., Hakoshima T., 2006. Molecular mechanism for the regulation of rho-kinase by dimerization and its inhibition by fasudil. Structure 14: 589–600. 10.1016/j.str.2005.11.024 [DOI] [PubMed] [Google Scholar]
- Ye X., Nikolaidis N., Nei M., Lai Z.-C., 2009. Evolution of the mob gene family. Open Cell Signal. J. 1: 1–11. 10.2174/1876390100901010001 [DOI] [Google Scholar]
- Yu F. X., Zhao B., Guan K. L., 2015. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163: 811–828. 10.1016/j.cell.2015.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article and figures. Supplemental material available at Figshare: https://doi.org/10.6084/m9.figshare.7525193.