Prior studies have suggested a role for the major glucose signaling Ras/ Protein Kinase A (PKA) pathway in kinetochore function and chromosome segregation, but with no clear mechanism. Here, Shah et al. show that PKA...
Keywords: Ras/PKA, Dam1 complex, Dam1p subunit, kinetochore, chromosome segregation, glucose signaling
Abstract
The Dam1 complex is an essential component of the outer kinetochore that mediates attachments between spindle microtubules and chromosomes. Dam1p, a subunit of the Dam1 complex, binds to microtubules and is regulated by Aurora B/Ipl1p phosphorylation. We find that overexpression of cAMP-dependent protein kinase (PKA) catalytic subunits (i.e., TPK1, TPK2, TPK3) is lethal in DAM1 mutants and increases the rate of chromosome loss in wild-type cells. Replacing an evolutionarily conserved PKA site (S31) in Dam1p with a nonphosphorylatable alanine suppressed the high-copy PKA dosage lethality in dam1-1. Consistent with Dam1p as a target of PKA, we find that in vitro PKA can directly phosphorylate S31 in Dam1p and we observed phosphorylation of S31 in Dam1p purified from asynchronously growing yeast cells. Cells carrying high-copy TPK2 or a Dam1p phospho-mimetic S31D mutant displayed a reduction in Dam1p localization at the kinetochore, suggesting that PKA phosphorylation plays a role in assembly and/or stability of the Dam1 complex. Furthermore, we observed spindle defects associated with S31 phosphorylation. Finally, we find that phosphorylation of Dam1p on S31 is reduced when glucose is limiting as well as during α-factor arrest, conditions that inhibit PKA activity. These observations suggest that the PKA site of Dam1p participates in regulating kinetochore activity. While PKA is a well-established effector of glucose signaling, our work shows for the first time that glucose-dependent PKA activity has an important function in chromosome segregation.
KINETOCHORES are multiprotein complexes assembled on centromeres that mediate attachments between chromosomes and microtubules (Kitagawa and Hieter 2001; Cheeseman and Desai 2008; Biggins 2013; Cheeseman 2014). The outer kinetochore complexes Dam1 (10 subunit complex) and Ndc80 (four subunit complex) establish persistent load-bearing attachments to the plus-ends of microtubules during anaphase (Akiyoshi et al. 2010; Lampert et al. 2010, 2013; Tien et al. 2010; Umbreit et al. 2014; Kim et al. 2017). The Dam1p and Ndc80p subunits of each complex physically bind to microtubules and are regulated by Aurora B kinase (Ipl1p in yeast) and Mps1p phosphorylation (Cheeseman et al. 2002; Shimogawa et al. 2006). We and others have reported genetic interactions between the nutrient signaling kinase, cyclic AMP (cAMP)–dependent protein kinase (PKA), and components of the kinetochore (Li et al. 2005; Magtanong et al. 2011; Ma et al. 2012). However, a molecular mechanism that explains the role PKA plays in kinetochore function and chromosome segregation remains unknown.
PKA is a tetrameric holoenzyme comprised of a regulatory (R) dimer bound to a catalytic (C) dimer and is part of the highly conserved family of AGC (PKA, cGMP-dependent protein kinase, and protein kinase C) ser/thr protein kinases (Toda et al. 1987a,b; Cameron et al. 1988; Knighton et al. 1993; Kim et al. 2005, 2007; Kennedy et al. 2009; Pearce et al. 2010; Rinaldi et al. 2010; Taylor et al. 2012; Freschi et al. 2013; Filteau et al. 2015). PKA is a well-established effector of nutrient signaling, in particular in response to glucose, and coordinates cell proliferation with glucose status (Zaman et al. 2009; Chepurny et al. 2010). In yeast, PKA is activated by Ras via cAMP. Intracellular glucose leads to activation of Ras, which in turn stimulates adenylate cyclase (AC) to generate cAMP from ATP (Broach 2012). Increases in cAMP levels activate PKA by binding to the inhibitory subunit, Bcy1p, which then dissociates from the Tpk catalytic subunits (Tpk1p, Tpk2p, Tpk3p), allowing them to phosphorylate their downstream targets (Thevelein and de Winde 1999).
Although PKA is best known for its role in glucose signaling, PKA in vertebrates and in yeast have also been shown to have mitotic functions. Several studies demonstrate that PKA can phosphorylate subunits of the Anaphase Promoting Complex (APC) and mutants in the APC display dosage sensitivity to PKA subunits (Yamashita et al. 1996; Kotani et al. 1998; Anghileri et al. 1999; Irniger et al. 2000; Bolte et al. 2003). Moreover, there are reports elucidating a pathway that reveals an important function for PKA in DNA damage checkpoint arrest via phosphorylation of Cdc20p, the APC activator (Searle and Sanchez 2004; Searle et al. 2004, 2011). Recently, it was shown that PKA can modulate the response to DNA damage by phosphorylating Mms21p, implicating a relationship between glycolysis and DNA damage response (Simpson-Lavy et al. 2015). Previous results from our laboratory indicate that deregulated PKA activity, as a result of decreased gene dosage of BCY1, gives rise to increased chromosome loss rates compared to wild-type cells (Magtanong et al. 2011). In addition, ectopic activation or inhibition of PKA can exacerbate or suppress temperature sensitivities of kinetochore mutants, suggesting that PKA modulates kinetochore activity (Li et al. 2005; Magtanong et al. 2011; Ma et al. 2012). The mechanism of how PKA activity can regulate kinetochore activity and alter chromosome segregation fidelity is not understood. Here, we show for the first time that PKA can directly phosphorylate Dam1p and influences kinetochore structure and function. We find that phosphorylation of Dam1p by PKA is associated with reduced localization of Dam1p to the kinetochore. Further, we demonstrate that modulating the Ras/PKA pathway by altering glucose and cAMP levels is associated with changes in phosphorylation of the PKA site within Dam1p. Together, the work reported here provides a mechanism that joins the major glucose signaling Ras/PKA pathway to kinetochore function and points to the possibility that chromosome segregation fidelity is influenced by glucose availability.
Materials and Methods
Yeast strains, techniques, and reagents
Please refer to Supplemental Material, Tables S1–S3 for a description of all yeast strains, plasmids, and oligonucleotides used. Yeast media and culturing conditions were based on methods previously described (Guthrie and Fink 1990). Yeast extract, peptone, dextrose (YPD) medium contained 1% yeast extract, 2% Bacto-peptone, and 2% glucose unless otherwise noted. Synthetic complete (SC) medium contained 0.17% yeast nitrogen base and 0.5% ammonium sulfate and amino acid mix, with appropriate drop out as noted. Transformations were performed by standard lithium acetate method (Gietz and Woods 2002). All yeast strains used were either in S288C or W303 backgrounds. Yeast dilution assays were performed at least three times with independent transformants. The concentration of the first spot of each row was normalized to an OD600 = 6.0 and then serial fivefold dilutions were spotted on plates as indicated.
Mutagenesis and epitope tagging
To construct nonphosphorylatable (S31A) and phospho-mimetic (S31D) mutants, we used a PCR strategy that incorporated changes in the S31 codon within the forward primers. A silent mutation that incorporates an XbaI restriction site proximal to the 31st codon was also incorporated into the forward primers and used as an initial screen to isolate transformants that incorporated the respective alanine or aspartate change. DAM1 that included a KanMX (DAM1::KanMX) marker in the 3′UTR was used as template. To generate the double phospho-mimetic S20DS31D mutation, a silent mutation that incorporates a ClaI restriction site proximal to the 20th codon was included in the forward primer. We used dam1-S31D::KanMX as a template to generate the PCR product to construct the S20DS31D mutant. Reverse primers included homologous 3′UTR sequences and annealed downstream of the KanMX marker. Refer to Table S3 for primer sequence details. For constructing S257A mutants, the forward primer carried the respective codon change to replace serine with alanine at the 257th position and candidates screened by sequencing. PCR products were used to transform BY4741 and candidates selected on YPD + G418 (200 μg/ml). Colony PCR was used to amplify the DAM1 gene from candidates and sequenced to confirm incorporation of the new codon.
For epitope tagging of dam1-S31A and dam1-S31D with GFP, we used pFA6a-GFP(S65T)-His3MX6 (JCB83) and followed the method as described (Longtine et al. 1998), using JCO127 and JCO154 primers. To tag SPC110p with mCherry, we used SPC110-mCherry::hphMX (JCY1034) as a template to generate a PCR product that contains mCherry at the C-terminal end of SPC110, using JCO269 and JCO271. PCR products were used to transform the following strains: Dam1p-GFP (JCY507), dam1p-S31A-GFP (JCY990), dam1p-S31D-GFP (JCY992), and dam1p-S20D31D-GFP (JCY1044); and transformants were selected on YPD + hygromycin (300 μg/ml). For epitope tagging of Dam1p with HBH at the C terminus, we used the pFA6a-HBH-hphMX4 plasmid as a template and JCO127 and JCO128 primers, following the method as described (Tagwerker et al. 2006). The following strains were transformed with linearized plasmid, pHIS3p:mTurquoise2-Tub1+3′ UTR::LEU2 (JCB352): Dam1p-GFP (JCY507), dam1p-S31A-GFP (JCY990), and dam1p-S31D-GFP (JCY992); to tag TUB1 with mTurquoise to visualize the spindle.
Chromosome loss assays
The chromosome transmission fidelity (CTF) assay was performed essentially as described (Choy et al. 2011). CTF strains carrying the indicated plasmids or in which the endogenous DAM1 was altered to carry S31A or S31D mutations were first grown in the appropriate synthetic media to select for the reporter chromosome fragment (CFIII-HIS3) and plasmids if needed. The next day, 1–10 dilutions were made in fresh selective media and grown for 3–4 hr before spreading 100–200 μl of cells on YPD plates at an OD600 of 0.0005. Plates were incubated at 30° for 3–4 days until colonies were a few millimeters in diameter. Plates were incubated at 4° for at least 2 days to enhance the development of the red pigment associated with CFIII loss (Hieter et al. 1985).
Purification of GST-Tpk fusion proteins from yeast
Yeast carrying a galactose inducible copy of GST-TPK1 (JCY588) or GST-TPK2 (JCY588) was grown overnight in Yeast extract, peptone YP 2% Raffinose liquid media at 30°. The next day, cells were washed twice with deionized water and fresh cultures were started with an OD600 of 0.4 in 40 ml of YP 3% galactose at 30° for 8–10 hr or until the OD600 reached ∼1. Cell pellets were resuspended in 700 µl lysis buffer (LB) [100 mM NaCl, 50 mM Tris, pH 7.4, 2 mM EDTA, 2 mM EGTA, 0.1% Triton X-100, 2 mM PMSF, 1× protease inhibitor cocktail (Roche), 1 mM DTT, and phosphatase inhibitors (50 mM NaF, 50 mM β-glycerophosphate)]. All subsequent steps were performed at 4°. Cells were lysed with glass beads for 15 min on, rest 5 min to dissipate heat in a vortexer and repeated for seven to eight times until sufficient lysis was achieved (90–95%, by visual inspection under microscope). Then, 20 µl of glutathione beads were washed twice with 250 µl of LB and beads were collected at low speed at 500 rpm for 2 min. Washed glutathione beads were resuspended in 10 µl of LB and incubated with lysate for 2 hr in a rotor. Beads were collected with centrifugation at low speed at 500 rpm for 2 min and washed two times with 300 µl of LB including 10 mM cAMP, and one time with 300 µl of 100 mM Tris HCL, pH 7.4, 10 mM cAMP. Finally, beads were resuspended in 10 µl of Tris HCL, pH 7.4, 10 mM cAMP. To confirm, we purified active GST-Tpk1p and GST-Tpk2p, we performed kinase assays using a PepTag Assay as described by the manufacturer (Promega, Madison, WI) (Figure S1B).
Recombinant Dam1 complex purification
The Dam1 complex is carried on a polycistronic vector, in which all 10 subunits of the Dam1 complex (gift from Trisha N. Davis, University of Washington) were expressed in BL21 Rosetta cells (Novagen). Purification of the complex was facilitated by a C-terminal His6X-tag on Spc34p, one of the subunits of the Dam1 complex. Transformed bacterial cells harboring the plasmid were grown till midlog phase expression of the complex was induced by addition of 0.2 M IPTG for 4 hr at 37°, pelleted, washed with 1× PBS/2 mM PMSF, snap-frozen using liquid nitrogen and stored at −80° until subsequent steps. Pellets were resuspended in 20 ml LB [50 mM NaPO4, 500 mM NaCl, pH 6.9, 2 mM PMSF, 5 mM imidazole, complete protease inhibitor cocktail without EDTA (Roche), and 1 μl benzonase nuclease (EMD Millipore)] and cells were lysed by French pressing twice with supplementation of 100 µl of 2 mM PMSF after each press. Cleared lysate was obtained by centrifuging at 14,000 rpm for 15 min in a SLC-1500 rotor (Sorvall RC6 plus). The supernatant was incubated with Ni-NTA beads (Thermo Scientific, Waltham, MA) for 2 hr in constant rotation, followed by three washes with wash buffer (50 mM NaPO4, 500 mM NaCl, and 8 mM imidazole) in a chromatography column (0.8 × 4 cm). Elution buffer (50 mM NaPO4, 500 mM NaCl, 400 mM imidazole, pH 6.9) was used to elute bound recombinant Dam1 complex and 1 ml fractions collected. Fractions containing all 10 subunits were pooled together, concentrated using 100 kDa MWCO concentrator (GE Healthcare). Pooled fractions were purified by size exclusion chromatography (Bio-Gel P-100 Media; Bio-Rad, Hercules, CA), concentrated, and stored in 5% glycerol at −80°.
PKA kinase assays
PKA kinase reactions were performed essentially as described (Zelter et al. 2015) with minor changes. Briefly, kinase reactions contained the following: 5× reaction buffer [100 mM Tris-HCl (pH 7.4), 50 mM MgCl2, 5 mM ATP], PKA activator 5× solution (5 μM cAMP in water), peptide protection (protease inhibitors) from Promega, and Dam1 complex (4 µM). Assembly of reactions were performed on ice, then incubated in a 30° water bath for 1 min. Purified GST-Tpk1p, GST-Tpk2p, or glutathione beads were added to the kinase reactions, incubated at room temperature for 30 min. Reactions were stopped by incubating the tubes at 95° for 10 min. 2× Laemmli buffer was added and samples loaded on a 4–12% gradient SDS-PAGE (GenScript). Gels were either transferred to nitrocellulose membrane and blotted with anti-RRXSPO- antibodies [Cell Signaling Technology; phospho-PKA substrate (RRXS*/T*) (100G7E) rabbit mAb, 1:5000] or stained with Coomassie blue.
Mass spectrometry
Coomassie-stained bands corresponding to Dam1p were excised from denaturing polyacrylamide gels, washed to remove SDS and stain, then digested with trypsin overnight at 37°. Peptides were extracted from gel pieces, dried, and redissolved in 20 μl of 2.5% acetonitrile, 0.1% formic acid. Digested peptides were separated using a Dionex Ultimate3000 RSLCnano LC system (Thermo Scientific) running a 1 hr gradient through a peptide CSH C18 column (0.075 × 250 mm, 130 Å, 1.7 µm; Waters Corp, Milford, MA) feeding into a Q-Exactive-High Field (QE-HF) mass spectrometer (Thermo Scientific). The MS/MS data were processed using Proteome Discoverer v2.2 (Thermo Scientific) connected to the search engine Mascot Server v2.6.1 (Matrix Science, London, UK) and the modification site localization tool ptmRS, formerly phosphoRS (Taus et al. 2011), to calculate the percentage certainty that a particular residue is phosphorylated and to integrate the area under the peak for each peptide measured. Areas from both unphosphorylated peptide and phosphorylated peptides were used to calculate the percentage of phosphorylated peptide for each site of interest as shown in Table S4.
Affinity purification and Western blot analysis
Denaturation conditions were used for purifications to improve binding affinity of the 6× His tag to cobalt beads and also to minimize phosphatase activity to preserve phosphorylated forms of Dam1p. Cells were grown overnight in YPD liquid media at 30°. The next day, fresh cultures were started with optical density OD600 of 0.1 in 500 ml YPD at 30° for 8–10 hr or until OD600 ∼1. Cells were then harvested, frozen in liquid nitrogen, and stored at −80°. Pellets were resuspended with 8 ml ice-cold denaturation buffer containing 100 mM NaH2PO4•H20, 10 mM Tris base, 8 M urea along with 400 mM NaCl, 10 mM imidazole, 2 mM Na3VO4, 1× protease inhibitor cocktail (Roche), and 2 mM PMSF. Cells were lysed using a bead beater for 30 sec on, 30 sec off, repeated for 10 min or until sufficient lysis was achieved (90% lysis-check efficiency by microscope). Lysates were centrifuged at 12,500 rpm for 15 min in a S34 rotor (Sorvall RC 6 plus) and supernatants were transferred to clean tubes. Cobalt beads (Thermo Scientific) (300 μl) were washed twice with 1 ml of denaturation buffer and beads were collected at low speed at 500 rpm for 2 min. Washed beads were then mixed with the lysate, and incubated on a rotating platform at 4° for 2 hr. Lysate and beads were then transferred onto a Bio-Rad Poly-Prep Chromatography Column (0.8 × 4 cm) with a reservoir and allow the column pack by gravity. Beads were then washed with 30 ml (3 times × 10 ml) wash buffer containing 800 mM NaCl, 20 mM imidazole, 2 mM Na3VO4, 1 mM DTT, 1× protease inhibitor cocktail (Roche), and 2 mM PMSF. During each wash, beads were incubated with these buffer for 10 min at 4°. Washed beads were then treated with 200 µl of 2× Laemmli buffer and heated to 90–100° for 10 min and frozen at −80°.
Protein samples were separated on a 4–12% gradient SDS-PAGE (GenScript) and blotted to nitrocellulose membrane. The following primary antibodies were used: anti-RRXSPO- antibody [Cell Signaling Technology; phospho-PKA substrate (RRXS*/T*) (100G7E) rabbit mAb, 1:5000], anti-His antibody (Proteintech; 6*His, His-Tag mouse monoclonal antibody, 1:5000), anti-GFP antibody (Proteintech; GFP tag mouse monoclonal antibody, catalog number; 66002-1-Ig), and anti-GAPDH antibody (Proteintech; GAPDH mouse monoclonal antibody, catalog number: 60004-1-Ig, 1:5000). Secondary antibodies used were anti-rabbit IgG [Invitrogen; goat anti-rabbit IgG (H+L) Cross-adsorbed secondary antibody, HRP catalog number: G-21234, 1:7000] and anti-mouse IgG [anti-mouse IgG (H+L) HRP conjugate, 1:7000; Promega]. Finally, a ChemiDoc imaging system was used to detect chemiluminescence signals from blots and band intensities were quantified using ImageJ software.
Protein extraction
Cells were grown overnight in 5 ml SC media or SC-uracil (SC-URA) (strains transformed with 2 µm pRS426 and pRS426-TPK2) at 25°. The next day, fresh cultures were started with OD600 of 0.1 in 20 ml SC or SC-URA at 34° until OD600 was closer to 0.8. Cells were then harvested, snap-frozen with liquid nitrogen, and stored at −80°. For total protein extract, Trichloroacetic acid was performed as described previously (Mishra et al. 2011) and protein concentration was determined using Pierce BCA protein assay kit (Thermo scientific).
Microscopy
Strains expressing GFP along with mCherry or mTurquoise were grown in SC media or SC-URA (for strains transformed with 2 µm pRS426 and pRS426-TPK2) overnight at 25°. The next day, fresh cultures were started with 1–10 dilution (10 ml) in the same media and grown for 4 hr at 34°. Then, 2 µl of cell suspension from the newly grown cultures was placed in a slide chamber with a 1% agarose pad containing SC or SC-URA media. Images were acquired on a Delta Vision Elite wide-field deconvolution microscope (Applied Precision/GE Healthcare, Issaquah, WA) equipped with Scientific CMOS Camera. Z-sections (0.2 µm slices) through the entire volume of cells were taken with an Olympus 60×/NA 1.4 and DAPI-FITC-mCherry-Cy5 channels were selected. Images were taken with FITC (0.8 sec exposure time) and mCherry (2.0 sec exposure time) filters. Foci of GFP and mCherry were quantified using Fiji software, and GFP intensity was normalized to mCherry. Only large budded cells with clearly separated GFP signals (GFP foci distances typically ranged from 1.3 to 4 μm) were quantified. For characterizing spindle morphology, CFP-YFP-mCherry channels were selected. Images were taken with CFP (0.8 sec exposure time) and YFP (1.0 sec) filters were used to image mTurquoise (CFP variant) and GFP, respectively. All the images that are shown are the maximum intensity projection from Z-section stacking.
Reagent and data availability
Strains and plasmids are available upon request. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7256039.
Results
High-copy TPK1, TPK2, and TPK3 antagonize Dam1p function and induces chromosome instability
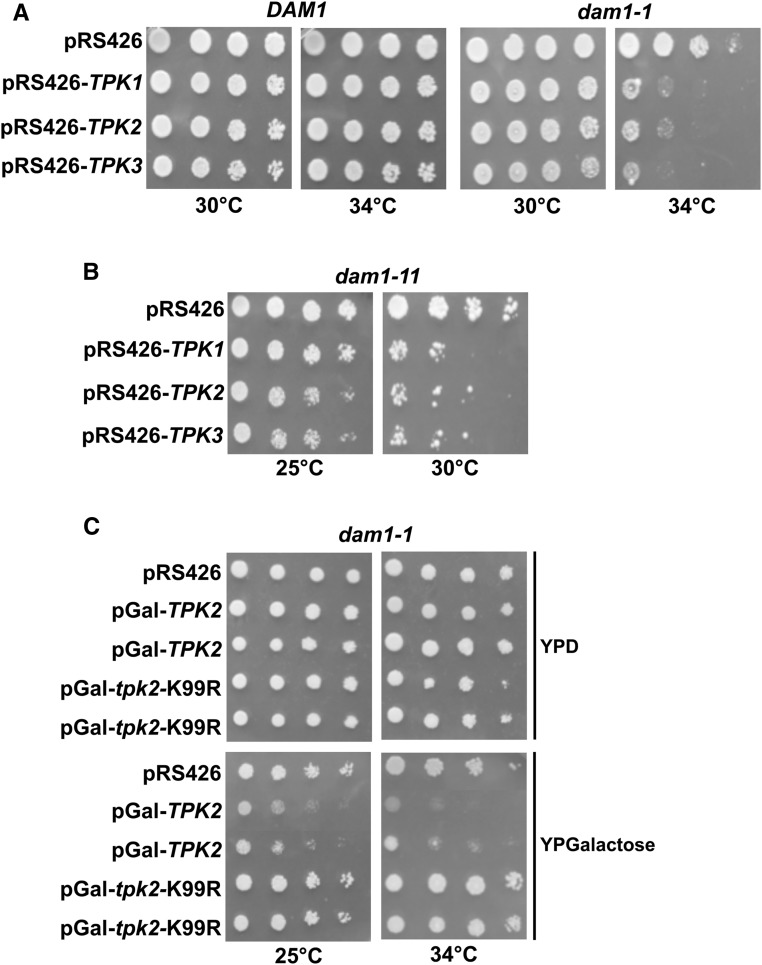
Work from our group and others have previously reported that mutations, which activate the Ras/PKA pathway confer increased rates of chromosome loss. (Li et al. 2005; Magtanong et al. 2011; Ma et al. 2012). Furthermore, activating mutations in the Ras/PKA pathway enhanced temperature sensitivity in outer kinetochore mutants. In particular, dam1-1 mutants have elevated temperature sensitivity when combined with a deletion in BCY1 or expressing a constitutively active allele of RAS2 (Li et al. 2005). These observations suggest that increased PKA activity antagonizes Dam1p. However, these studies did not directly test the catalytic subunits of PKA (i.e., Tpk1, Tpk2, Tpk3). While the three Tpks act on common substrates, there are functional differences between them as unique substrates have been identified for each Tpk (Robertson et al. 2000). We sought to test if a specific isoform of PKA interacted with DAM1, by introducing high-copy 2 μm vectors (pRS426) carrying TPK1, TPK2, or TPK3 genes into dam1-1 cells. Spot assays revealed that dam1-1 mutants were sensitive to all three high-copy TPKs as dam1-1 displayed a marked growth reduction when carrying pRS426-TPK1, pRS426-TPK2, and pRS426-TPK3 compared to carrying the empty vector pRS426 (Figure 1A). To determine if the dosage effect of high-copy TPKs is specific to the dam1-1 allele, we tested an additional allele of DAM1 (i.e., dam1-11). Similar to dam1-1, introduction of high-copy TPK1, TPK2, and TPK3 resulted in greater temperature sensitivity in dam1-11 (Figure 1B). To determine if the catalytic activity of PKA was necessary for the dosage lethality, we turned to testing a catalytically inactive mutant of Tpk2, tpk2-K99R (Pan and Heitman 2002). We expressed wild-type TPK2 or mutant tpk2-K99R from a galactose-inducible promoter in dam1-1 cells. We observed that similar to high-copy TPK2, when pGal-TPK2 was induced by growth on galactose there was a marked loss of growth (Figure 1C). However, cells expressing the inactive mutant, pGal-tpk2-K99R, grew similar to controls (Figure 1C), showing that catalytic activity is required for the dosage lethality. Furthermore, there was no discernible growth effect in wild-type cells carrying pGal-TPK2 when grown on galactose (Figure S1A). These results suggest that all three PKA isoforms can enhance temperature sensitivity in DAM1 mutants.
Figure 1.
High-copy TPKs enhance temperature sensitivity of DAM1 mutants, which depends on the catalytic activity of PKA. (A and B) Overexpression of PKA catalytic subunits (TPK1, TPK2, TPK3) increases the temperature sensitivity of dam1-1 and dam1-11 mutants (JCY124 and JCY125, respectively) but not DAM1 wild-type cells (JCY91). Fivefold serial dilutions of the indicated strains carrying high-copy (2µm) plasmids pRS426 (JCB63), pRS426-TPK1 (JCB58), pRS426-TPK2 (JCB59), or pRS426-TPK3 (JCB60) were spotted on SC-URA plates at 30° or 34° and allowed to grow for 3 days. (C) Fivefold serial dilutions of dam1-1 (JCY124) cells carrying vector alone (pRS426) or vector with a galactose inducible wild-type TPK2 (JCB31) or catalytically inactive tpk2-K99R mutant (JCB153) were spotted on YP 2% glucose (YPD) or YP 2% galactose (YPG) plates at 25° and 34° and allowed to grow for 4 days.
Previously, we reported that loss of one copy of BCY1, encoding the negative regulator of PKA, was associated with higher rates of chromosome loss (Magtanong et al. 2011). This observation predicts that increasing PKA activity contributes to higher rates of chromosome segregation errors. To test this possibility we measured chromosome loss in the presence of high-copy TPKs using the CTF assay. CTF strains carry a nonessential chromosome fragment that when lost result in white colonies with red sectors (Hieter et al. 1985). The frequency of red-white half-sectored colonies can be used to estimate the chromosome loss rate (Hieter et al. 1985; Zheng et al. 2011). We introduced pRS426-TPK1, pRS426-TPK2, and pRS426-TPK3 into a wild-type CTF strain and measured the frequency of red-white half-sectored colonies. We observed that all three high-copy TPKs resulted in ∼6- to 12-fold increase in chromosome loss when compared to empty vector control (Table 1). This result suggests that increased PKA activity leads to greater chromosome instability, consistent with our previous report showing that diploid yeast with a hemizygous deletion of BCY1 have increased rates of chromosome loss compared to wild-type cells (Magtanong et al. 2011; Choy et al. 2013). Together, these observations indicate that PKA antagonizes Dam1p activity and increases errors in chromosome segregation.
Table 1. Chromosome loss in cells carrying high-copy TPKs, dam1-S31A, and dam1-S31D.
| Genotype | Total colonies | Chromosome loss in first division (red/white half sectors) |
|---|---|---|
| 1pRS426 CFIII | 3544 | 8.47E−04 (3); 1 |
| 2pRS426-TPK1 CFIII | 2972 | 5.72E−03 (17); 6.8× higher vs. pRS426 |
| 3pRS426-TPK2 CFIII | 2516 | 9.94E−03 (25); 11.7× higher vs. pRS426 |
| 4pRS426-TPK3 CFIII | 2972 | 6.06E−03 (18); 7.1× higher vs. pRS426 |
| 5pRS426-TPK2 dam1-S31A CFIII | 4212 | 1.66E−03 (7); 1.9× higher vs. pRS426 |
| 6DAM1 CFIII | 5582 | 1.25E−03 (7); 1 |
| 7dam1-S31A CFIII | 6158 | 1.30E−03 (8); 1× higher vs. DAM1 |
| 8dam1-S31D CFIII | 7996 | 2.38E−03 (19); 2× higher vs. DAM1 |
Numbers correspond to the following strains: 1–4 and 6 are in JCY534, 5 and 7 are in JCY1000, 8 is in JCY611.
PKA phosphorylates Dam1p
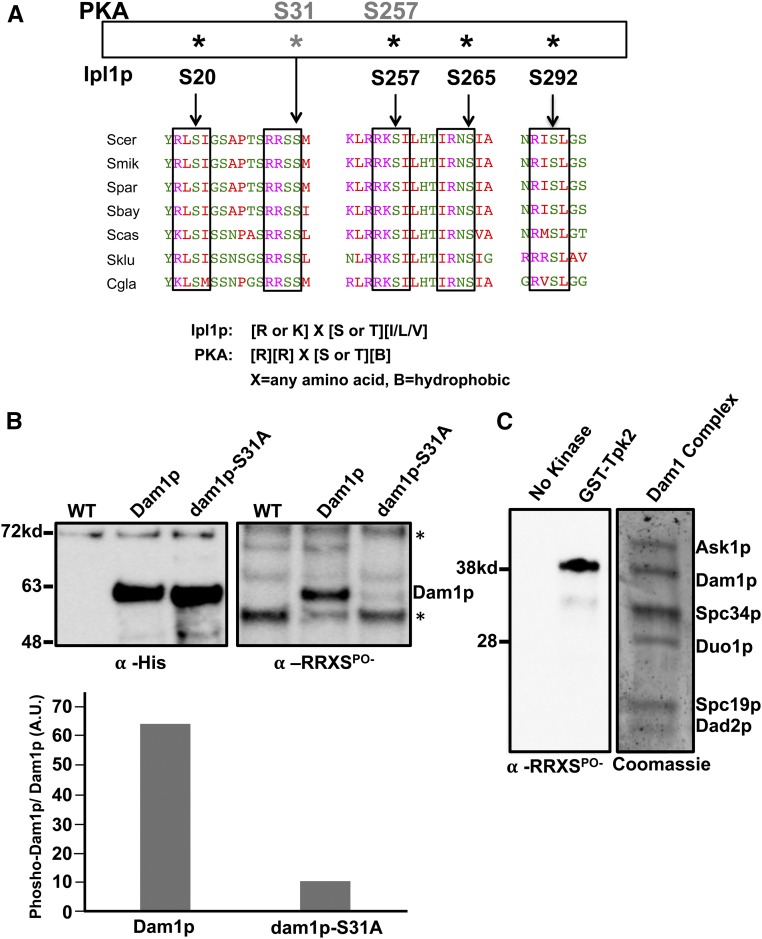
Our results show that PKA activity influences the accuracy of chromosome segregation, possibly by acting on Dam1p. A previous report to identify all possible PKA substrates in yeast showed that Dam1p contains two predicted PKA sites at position S31 (RRSS31M) and S257 (RRKS257I) that are evolutionarily conserved across species of budding yeast (Figure 2A) (Budovskaya et al. 2005). Position S257 is one of three sites in the C-terminal half of Dam1p phosphorylated by Ipl1p and proximal to residues that can be cross-linked to β-tubulin (Cheeseman et al. 2002; Zelter et al. 2015; Legal et al. 2016). On the other hand, Dam1p peptides carrying phosphorylated S31 have been observed in two independent global phosphoproteome reports (Soulard et al. 2010; Kanshin et al. 2015), yet there are no studies investigating the function of S31 nor is the kinase responsible for phosphorylating S31 known. Considering that S257 is an already well-established Ipl1p target, we first sought to confirm that Dam1p is phosphorylated on S31. Yeast expressing wild-type Dam1p or a nonphosphorylatable mutant dam1-S31A were fused to His6-Biotin-His6 (HBH) tags and used in denaturing affinity pull-down experiments (Tagwerker et al. 2006). Purified Dam1p or dam1-S31A from asynchronously growing cultures were analyzed by Western blotting. An anti-His tag antibody was used to detect total purified Dam1p and an antibody that recognizes phosphorylated serine/threonine within the consensus PKA site (anti-RRXSPO-) was used to measure levels of PKA phosphorylation on Dam1p. Nearly equal amounts of purified Dam1p and dam1-S31A were observed by anti-His antibody (Figure 2B, left). However, we observed a marked phosphorylation signal from the anti-RRXSPO- antibody blot of wild-type Dam1-HBH, which was reduced to near background levels in the dam1-S31A-HBH mutant, demonstrating that the signal from the wild-type Dam1-HBH depends largely on S31 (Figure 2B, right). The anti-RRXSPO- antibody likely recognizes S257 phosphorylation, as a faint signal is still visible in the dam1-S31A lane that is completely absent in the untagged control. Nonetheless, the large reduction in reactivity observed for the dam1-S31A suggests that the majority of the signal is due to S31 phosphorylation (Figure 2B, bottom graph). Therefore, these results confirm the prior phosphoproteome reports showing that the conserved S31 PKA site on Dam1p is phosphorylated in vivo.
Figure 2.
PKA phosphorylates Dam1p. (A) Alignment of the DAM1 region showing the relevant phosphorylation sites across multiple budding yeast species indicates that Dam1p contains evolutionary conserved PKA sites (S31, S257 is known to be an Ipl1p target) in addition to the well-studied Aurora B/Ipl1p sites. Alignments were performed using UniProt/Align. (B) Purified Dam1p-HBH (JCY986) and nonphosphorylatable dam1-S31A-HBH (JCY988) from asynchronously growing cells were analyzed by Western blot analysis. Blots were treated with anti-His tag antibody to measure total Dam1p and dam1p-S31A (left panel) or an antibody that recognizes phospho-serine/threonine within the consensus PKA site (anti-RRXSPO-antibody) to measure levels of phosphorylated Dam1p and dam1p-S31A (right panel). (C) In vitro kinase reactions were carried out with recombinant Dam1 complexes and purified GST-Tpk2 or no kinase control followed by western analysis with anti-RRXSPO-antibody to measure levels of phosphorylation of Dam1p. Samples from the Gst-Tpk2 reactions were also run in another lane and Coomassie-stained to determine which subunit was phosphorylated (right panel). HBH, 6X-histidine-biotin-6X- histidine tag fused to c-terminus of Dam1p or dam1p-S31A. * indicates nonspecific bands.
Although there is similarity between the consensus S31 PKA site and an Aurora B/Ipl1p site (R-R-X-S/T-B vs. R/K-X-S/T-[I/V/L], X = any amino acid, B = hydrophobic amino acid, respectively; Cheeseman et al. 2002; Budovskaya et al. 2005), previous studies have shown that Ipl1p phosphorylates S20, S257, S265, and S292 but no other sites in Dam1p (Cheeseman et al. 2002; Franck et al. 2007; Zelter et al. 2015), raising the possibility that this site may be targeted by PKA. Toward testing this hypothesis we performed kinase reactions using recombinantly assembled Dam1 complexes and purified yeast Tpk2p. The Dam1 complex can be expressed and self-assembles in bacteria using a 10-cistron containing plasmid (Miranda et al. 2005; Westermann et al. 2005; Franck et al. 2007). This offers the possibility of using a physiologically relevant substrate for our kinase assays rather than the Dam1p subunit alone. We performed in vitro kinase assays by incubating recombinant Dam1 complexes with GST-Tpk2p or no kinase control and subsequently analyzed the phospho-state of Dam1p by western analysis using the anti-RRXSPO- antibody. In control reactions without Gst-Tpk2p, there was an absence of phosphorylated Dam1p (Figure 2C). In contrast, the lane with sample from reactions containing Gst-Tpk2p clearly shows evidence of phosphorylated Dam1p, as the phosphorylation signal overlapped with where the Dam1p subunit migrates in a Coomassie-stained gel of the Dam1 complex (Figure 2C). We confirmed the Western blot analysis by subjecting the band containing Dam1p to mass spectrometry. The analysis revealed that Dam1p is phosphorylated at S31 and S257 (Table S4). We observed similar results with kinase reactions using Gst-Tpk1p (Table S4). These results demonstrate that PKA can directly phosphorylate Dam1p on S31 and S257 within the context of the Dam1 complex.
Replacing S31 but not S257 with a nonphosphorylatable alanine protects dam1-1 cells from high-copy TPK dosage lethality and high-copy TPK-induced chromosome instability
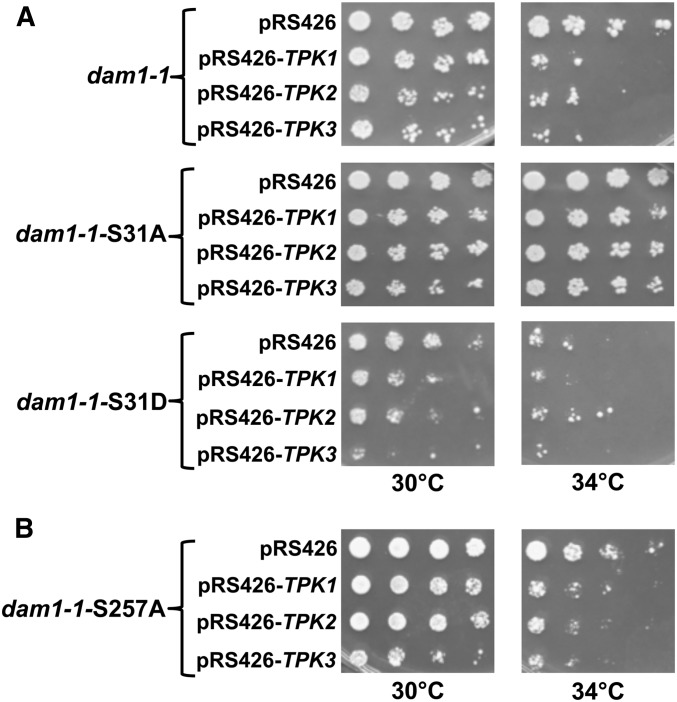
Our results suggest that phosphorylation of S31 or S257 in Dam1p might explain the genetic interactions between high-copy TPKs and dam1-1 as well as the associated increase in chromosome loss when cells overexpress TPKs (Figure 1 and Table 1). Considering that previous work has established S257 as an Ipl1p target (Cheeseman et al. 2002), we first tested the hypothesis that phosphorylation of Dam1p on S31 contributed to the lethality observed in dam1-1. To test this possibility we constructed dam1-1 mutants in which serine 31 was replaced with a nonphosphorylatable alanine (dam1-1-S31A). We then introduced high-copy TPK2 into each strain and performed growth assays. We observed that dosage lethality conferred by high-copy TPK2 was largely suppressed in dam1-1-S31A compared to dam1-1 (Figure 3A). In contrast, dam1-1-S31D mutants displayed greater temperature sensitivity even in the absence of high-copy TPK2 (Figure 3A). These results suggest that S31 is a PKA target in vivo.
Figure 3.
Replacing Serine 31 with a nonphosphorylatable alanine in dam1-1 suppresses high-copy TPK2 induced lethality and conversely, replacing serine 31 with a phospho-mimetic aspartate (JCY999) enhances dam1-1 temperature sensitivity. (A) Fivefold serial dilutions of the indicated strains were spotted onto SC-URA plates at 30° or 34° and allowed to grow for 3 days. (B) In contrast to dam1-1-S31A (JCY998), dam1-1-S257A (JCY575) does not suppress high-copy TPK2. Fivefold serial dilutions of the indicated strains were spotted onto SC-URA plates at 30° or 34° and allowed to grow for 3 days.
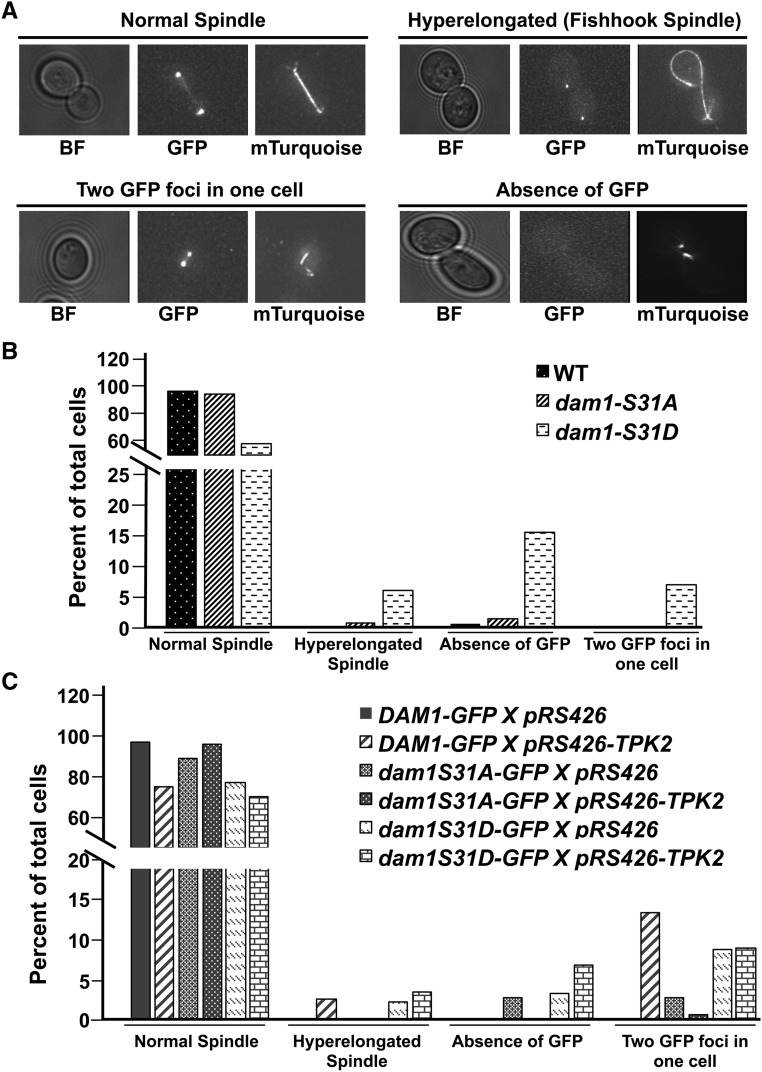
Previously, dam1-1 cells were reported to have abnormal spindle morphologies, including a complete loss of the spindle at nonpermissive temperatures (Cheeseman et al. 2001; Jones et al. 2001). To determine if the enhanced temperature sensitivity in dam1-1 conferred by the S31D mutation was associated with spindle defects, we introduced a Tub1p-mTurquoise fusion into the dam1-1 strains and imaged cells growing at permissive and semipermissive temperatures. Consistent with the greater loss in growth at semipermissive temperature, we observed an elevated frequency of cells with a complete absence of a spindle or with a hyper-elongated spindle in dam1-1-S31D mutants compared to dam1-1 mutants (Figure S2). At 34°, ∼60% of dam1-1-31D mutants were devoid of a spindle and ∼5% displayed broken spindles (Figure S2). In contrast, only ∼20% of dam1-1 mutants were devoid of a spindle at 34° and ∼3% displayed broken spindles (Figure S2). Together, these observations demonstrate that phosphorylation of S31 in dam1-1 enhances spindle abnormalities.
Although previous work showed that S257 is phosphorylated by Ipl1p, our in vitro kinase assays showed that PKA can phosphorylate S257 raising the possibility that this site might contribute to the overexpression phenotypes (Cheeseman et al. 2002). Therefore, we tested if replacing the S257 residue with alanine would protect dam1-1 mutants from high-copy TPK lethality. We observed nearly identical sensitivity to high-copy TPK2 between dam1-1 and dam1-1-S257A (Figure 3B). Together, these results show that the dosage lethality conferred by high-copy TPK2 in dam1-1 depends on S31.
To determine if the S31 site contributes to the increased chromosome loss rates associated with high-copy TPKs, we introduced an S31A mutation into DAM1 in the CTF strain and measured sectoring in the presence of high-copy TPK2 or vector alone. We observed a ∼sixfold reduction in half-sectors in dam1-S31A cells (1.66 × 10−3) compared to wild-type DAM1 cells (9.94 × 10−3) carrying high-copy TPK2 (Table 1). While the S31A mutation suppressed the elevated chromosome loss rate associated with high-copy TPK2, the basal chromosome loss rates of S31A (1.3 × 10−3) and wild-type DAM1 (1.23 × 10−3) were similar (Table 1). Taken together these data support the hypothesis that PKA targets S31 in Dam1p to regulate Dam1p function and contributes to chromosome segregation fidelity.
Phosphorylation of Dam1p reduces its localization to the kinetochore and is associated with spindle defects
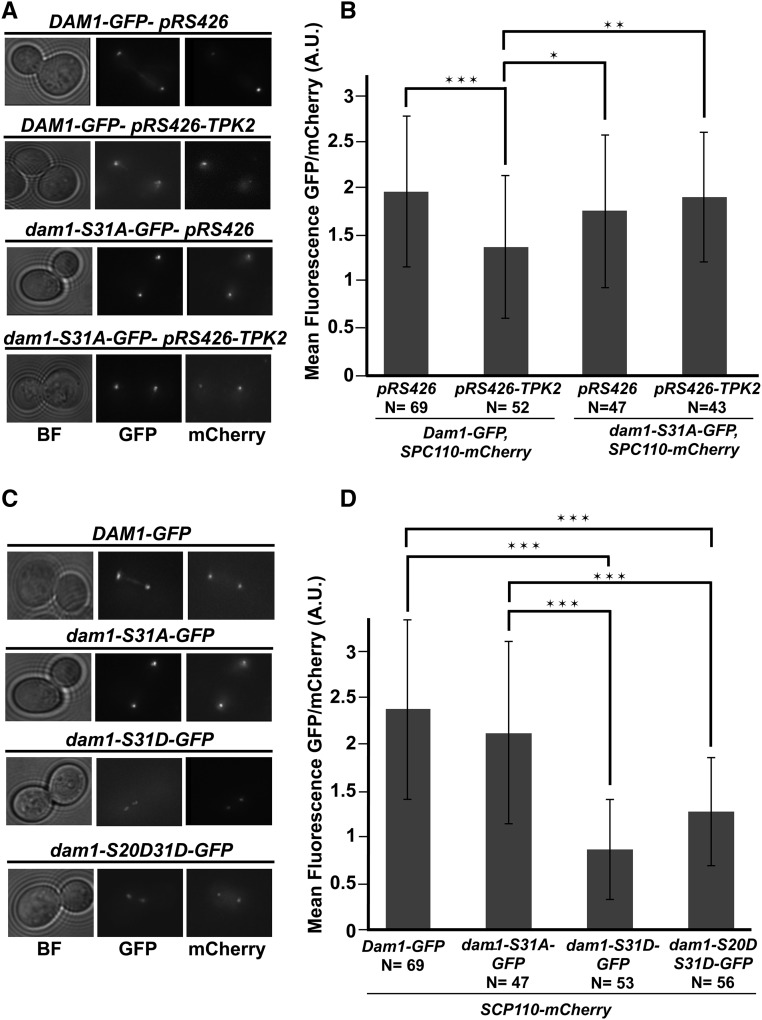
There are four serine residues on Dam1p that are phosphorylated by Ipl1p (Cheeseman et al. 2002). Three sites in the c-terminal portion of Dam1p, S257, S265, S292 directly contact microtubules and contribute to binding of microtubules (Westermann et al. 2005; Zelter et al. 2015; Legal et al. 2016). Phosphorylation of these three sites weaken binding between the Dam1 complex and plus-ends of microtubules (Zelter et al. 2015; Legal et al. 2016). The serine at position 20 has been proposed to participate in Dam1 oligomerization and phosphorylation of S20 may disrupt Dam1 oligomerization (Sarangapani et al. 2013; Umbreit et al. 2014; Zelter et al. 2015; Jenni and Harrison 2018). Considering the proximity of S31 to S20 we sought to determine if elevated PKA activity might alter the level of kinetochore associated Dam1p. Previously, a carboxy terminal GFP fusion to Dam1p was used to image Dam1p kinetochore localization (Jones et al. 2001). Therefore, we introduced high-copy TPK2 into a strain expressing Dam1p-GFP as its sole copy of Dam1p and quantified total fluorescence at the kinetochore from large budded cells with clearly separated GFP foci (i.e., anaphase cells). Cells also carried a SPC110-mCherry fusion at the endogenous locus that was used to normalize the GFP measurements. We found a ∼25% reduction in the ratio of Dam1p-GFP/Spc110p-mCherry at the kinetochore in cells carrying high-copy TPK2 compared to vector control (Figure 4, A and B). To test if the reduction in Dam1p-GFP depends on S31 we measured Dam1p-GFP levels in cells carrying dam1-S31A-GFP with high-copy TPK2. We observed that dam1p-S31A-GFP/Spc110p-mCherry levels remained nearly identical to vector-alone control (Figure 4, A and B). To determine if the overall levels of Dam1p-GFP were altered when TPK2 is overexpressed we performed Western blot analysis using whole-cell extracts. We observed a slight decrease in wild-type Dam1p-GFP when cells carried high-copy TPK2, and a marked decrease in levels of dam1p-S31A-GFP. Importantly, there was no further reduction in total dam1p-S31A-GFP in the presence of high-copy TPK2 (Figure S3A).
Figure 4.
Phosphorylation of S31 is associated with a reduction in Dam1p at the kinetochore. (A) Wild-type strains Dam1p-GFP, Spc110p-mCherry (JCY1041) and nonphosphorylatable dam1p-S31A-GFP, Spc110p-mCherry (JCY1042) strains carrying 2 µm empty vectors, pRS426 (JCB63), or high-copy TPK2, pRS426-TPK2 (JCB59) were grown in SC-URA and imaged. Images of GFP and mCherry are the maximum intensity projections of Z-sections of the stacks onto a single plane. (B) Measurements for the mean fluorescence intensity of Dam1p-GFP or dam1p-S31A-GFP normalized to Spc110p-mCherry are plotted. (C) Strains expressing wild-type Dam1p-GFP (JCY1041), Spc110p-mCherry, nonphosphorylatable dam1p-S31A-GFP (JCY1042), Spc110p-mCherry, single phospho-mimetic dam1p-S31D-GFP, Spc110p-mCherry (JCY1043), or double phospho-mimetic dam1p-S20DS31D-GFP (JCY1045) were grown in SC media and imaged. Images of GFP and mCherry are the maximum intensity projections of Z-sections of the stacks onto a single plane. (D) As in B, measurements for the mean fluorescence intensity of GFP normalized to Spc110p-mCherry are plotted. In all cases, fresh cultures were started with a 1:10 dilution from an overnight culture at 25° and allowed to grow for ∼3–4 hr at 34° before imaging by fluorescence microscopy. Z-stacks were taken with a DeltaVision Elite microscope (60× objective lens, 1.4 NA). Quantification of GFP foci normalized to mCherry were measured for large budded cells that have clearly separated GFP signals using Fiji software. Number of cells counted indicated by (N). Plotted is the mean intensity GFP/mCherry ratio (B and D) and error bars represent the SD. * P < 0.01, ** P < 0.001,*** P < 0.0001, as calculated using Student’s t-test. BF, bright field; GFP, Dam1p-GFP (and mutants); mCherry, Spc110p-mCherry.
If phosphorylation of S31 in Dam1p was responsible for the decreased levels of Dam1p-GFP, then introducing a phospho-mimetic S31D mutation into Dam1p might reproduce the effects of high-copy TPKs. Measurements of Dam1p-GFP with a S31D mutation showed that there was a ∼twofold decrease in the ratio of dam1p-S31D-GFP/Spc110p-mCherry intensity compared to wild-type Dam1p-GFP/Spc110p-mCherry (Figure 4, C and D). If the phosphorylation of S31 contributes to oligomerization of Dam1p similar to that proposed for S20, then a double phospho-mimetic might be predicted to have a greater decrease in localization to the kinetochore than the single S31D mutant. We constructed a dam1p-S20DS31D-GFP double phospho-mimetic mutant and observed a decrease in dam1p-S20DS31D-GFP/Spc110p-mCherry levels similar to that observed for the single dam1p-S31D-GFP mutant (Figure 4, C and D). To determine the overall levels of Dam1p-GFP, dam1p-S31A-GFP, dam1p-31D-GFP, and dam1p-S20DS30D-GFP, we performed Western blot analysis using whole cell extracts. We found that total dam1-S31D-GFP and dam1p-S20DS30D-GFP was greatly diminished compared to Dam1p-GFP (Figure S3B). A reduction in Dam1p would be predicted to weaken kinetochore microtubule attachments. Therefore, we tested if dam1-S31D and dam1-S20DS31D mutants display a greater sensitivity to the microtubule depolymerizing drug, benomyl. We observed a mild reduction in growth of dam1-S31D and dam1-S20DS31D cells in benomyl compared to wild-type and dam1-S31A cells (Figure S4A). Taken together, these results suggest that S31 phosphorylation contributes to Dam1p localization to the kinetochore.
Previous work has established a role for Dam1p in maintaining the stability of the spindle as well as localization of Dam1 complexes to the spindle (Jones et al. 1999, 2001; Cheeseman et al. 2001, 2002; He et al. 2001). We sought to determine if phosphorylation of S31 would lead to any spindle abnormalities by introducing a copy of TUB1 fused to the fluorescent protein, mTurquoise, to visualize the mitotic spindle (Markus et al. 2015). We observed ∼20% of the phospho-mimetic dam1p-S31D mutant displayed evidence of chromosome segregation defects (i.e., ∼15% with an absence of GFP and ∼5% with two GFP foci in the same cell), and another ∼7% displayed hyper-elongated spindles (Figure 5, A and B). In contrast, the nonphosphorylatable dam1p-S31A mutant displayed many fewer aberrant events (<3% displayed an absence of GFP or two GFP foci in the same cell, and <1% have hyper-elongated spindles) (Figure 5, A and B). We did not observe any defective phenotypes in wild-type Dam1p-GFP cells (Figure 5, A and B). When wild-type cells carried high-copy TPK2, there was an increase in the frequency of unbudded cells carrying two GFP foci (∼14%) and cells with hyper-elongated spindles (∼3%) compared to the vector control, which displayed none of these defects (Figure 5, A and C). However, there was a reduction in abnormal spindles in dam1p-S31A cells carrying high-copy TPK2 (0% of cells with a hype-relongated spindle) as well as a lower frequency of cells carrying two GFP foci (<4%) (Figure 5, A and C). On the other hand, the 31D phospho-mimetic displayed a higher frequency of cells without GFP or with two GFP foci, when carrying empty vector or high-copy TPK2 (∼3–6% and ∼10%, respectively) (Figure 5C). In addition, we also observed a higher frequency of hyper-elongated spindles in the 31D phospho-mimetic carrying empty vector or high-copy TPK2 (∼2% and ∼4%, respectively) (Figure 5C). Dam1p and other Dam1 complex subunits are reported to be localized along the length of the mitotic spindle (Hoffman et al. 1998; Cheeseman et al. 2001; He et al. 2001; Jones et al. 2001; Li et al. 2002). Twenty percent of wild-type Dam1p-GFP cells carrying high-copy TPK2 displayed a loss in Dam1p-GFP along the length of the spindle compared to vector alone (Figure S4B). We observed nearly double this number (∼38%) of spindles devoid of GFP in dam1p-S31A-GFP mutants (Figure S4B). In contrast, all spindles were devoid of dam1p-S31D-GFP (Figure S4B). Together, these results show that phosphorylation of Dam1p on S31 is associated with abnormal spindle morphologies and a loss of localization along the length of the mitotic spindle.
Figure 5.
Abnormal spindle morphology and chromosome segregation defects in dam1p-S31D phospho-mimetic mutants and in wild-type cells carrying high-copy TPK2. (A) Shown are representative images for four different phenotypes: “normal spindle,” “hyper-elongated spindle” (fishhook spindle), “two GFP foci” (refers to two GFP foci in one unbudded cell or in one cell of a large budded cell), and “absence of GFP” (refers to an absence of GFP in unbudded or large budded cells). All GFP and mTurquoise images shown are the maximum intensity projections of Z-sections of the stacks onto a single plane. Wild-type Dam1p-GFP, Tub1p-Turquoise (JCY1036); dam1p-S31A-GFP, Tub1p-Turquoise (JCY1036); and dam1p-S31D-GFP, Tub1p-Turquoise (JCY1037) strains were grown in SC media as described in Materials and Methods and imaged. Images that represent normal spindle are taken from Dam1p-GFP, Tub1p-Turquoise; hyper-elongated spindle is from dam1p-S31D-GFP, Tub1p-Turquoise; unbudded cell with two GFP foci is taken from Dam1p-GFP carrying high-copy TPK2; and absence of GFP is taken from dam1p-S31D-GFP. (B) Percent of total cells in four different categories were plotted. Number of cells counted are: Dam1p-GFP, n = 216; dam1p-S31A-GFP, n = 143; and dam1p-S31D-GFP, n = 193. (C) Wild-type Dam1p-GFP, Tub1p-Turquoise (JCY1036); dam1p-S31A-GFP, Tub1p-Turquoise (JCY1036); and dam1p-S31D-GFP, Tub1p-Turquoise (JCY1037) strains carrying 2 µm empty vector, pRS426 (JCB63), or high-copy TPK2, pRS426-TPK2 (JCB59) were grown in SC-URA as described in Materials and Methods and imaged. Percent of total cells are plotted. Numbers of cells counted are:. Dam1p-GFP pRS426, n = 121; Dam1p-GFP pRS426-TPK2, n = 76; dam1p-S31A-GFP pRS426, n = 69; dam1p-S31A-GFP pRS426-TPK2, n = 155; dam1p-S31D-GFP pRS426, n = 175; and dam1p-S31D-GFP pRS426-TPK2, n = 138. BF, bright field; GFP, Dam1p-GFP (and mutants); mTurquoise, Tub1p-mTurquoise.
Next, we tested if dam1-S31D was associated with greater chromosome loss by introducing a dam1-S31D mutation into the CTF strain. We observed a ∼twofold increase in frequency of half-sectored colonies compared to wild-type cells (Table 1). Taken together, these results are consistent with PKA phosphorylating Dam1p at S31, which influences Dam1p kinetochore localization and function.
Modulating PKA activity by glucose and α-factor leads to changes in S31 phosphorylation in Dam1p
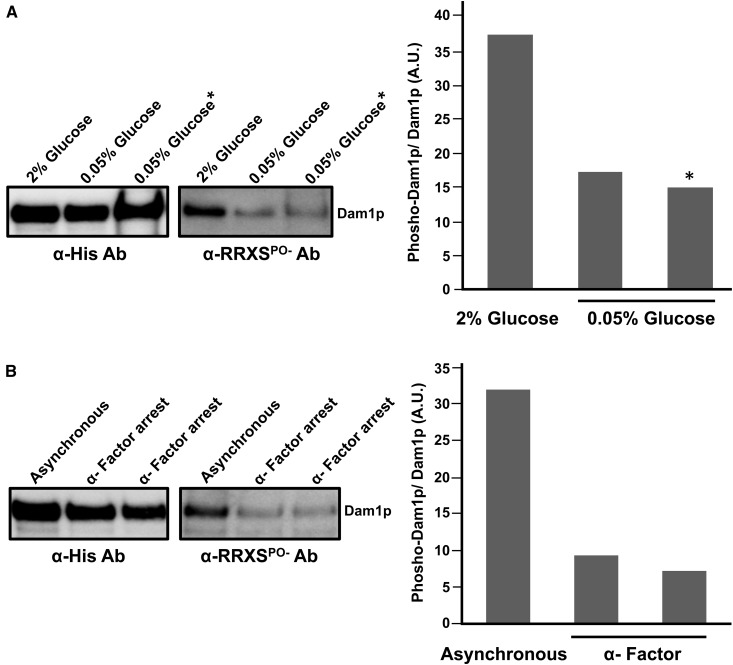
Considering that the Ras/PKA pathway regulates glucose signaling (Thevelein and de Winde 1999; Broach 2012), our results suggest the possibility that glucose availability might modulate Dam1p S31 phosphorylation. Previously, it was reported that low concentrations of glucose (0.05%) resulted in increased abundance and phosphorylation of Bcy1p at multiple sites that together lead to downregulation of PKA activity (Werner-Washburne et al. 1991; Budhwar et al. 2010). Therefore, we measured phosphorylation of S31 in Dam1p from cells grown in limiting glucose conditions. We grew cells carrying Dam1-HBH in rich media with 2% glucose before splitting the culture into two new cultures; one half was kept in 2% glucose and for the other half, we decreased the glucose concentration to 0.05%. After 4 hr of growth we collected cells and performed affinity purification for Dam1-HBH. We observed a marked reduction in S31 phosphorylation in Dam1p from cells growing on 0.05% glucose compared to 2% glucose (Figure 6A). We also grew cells strictly in 0.05% glucose and observed a similar reduction in Dam1-S31 phosphorylation (Figure 6A). These results support the hypothesis that phosphorylation of S31 in Dam1p is subject to glucose availability.
Figure 6.
Dam1p S31 phosphorylation is regulated by glucose and cAMP. (A) Overnight cultures of Dam1p-HBH (JCY986) were grown in YP 2% glucose. Fresh cultures from the overnight were prepared in YP 2% glucose and grown until midlog phase, then split into YP 2% glucose or into YP 0.05% glucose for 4 hr. Dam1p-HBH was purified from cells and analyzed (lanes 1 and 2). Alternatively, Dam1p-HBH was purified from asynchronously grown cultures of (JCY986) in YP 0.05% glucose started from an overnight culture grown in YP 0.05% glucose (*, lane 3). (B) Dam1p-HBH was purified from cells (JCY986) arrested with α-factor. Cultures were treated with α-factor and cells were collected only when >95% cells appeared to be arrested with a shmoo morphology. Two replicates of the α-factor arrest experiment is shown.
We used α-factor as an alternative approach to test if modulating the Ras/PKA pathway would result in a corresponding change in Dam1-S31 phosphorylation. Previously, it was shown that α-factor inhibits AC and downregulates PKA activity (Liao and Thorner 1980). Therefore, we predicted that α-factor–arrested cells would also lead to a reduction in S31 phosphorylation due to downregulated PKA activity. We treated cells carrying Dam1-HBH with α-factor followed by purification of Dam1-HBH. We found a marked reduction in S31 phosphorylation similar to what was observed in 0.05% glucose (Figure 6B). These results further support the hypothesis that the Ras/PKA pathway can regulate PKA mediated phosphorylation of S31 in Dam1p.
Discussion
Initial genetic observations suggested a role for PKA in kinetochore function and chromosome segregation (Dubacq et al. 2002; Li et al. 2005; Magtanong et al. 2011; Ma et al. 2012). However, whether the genetic interactions were due to direct phosphorylation of the kinetochore by PKA or an indirect function of PKA remained unknown. In this report, we show that PKA can directly phosphorylate S31 in Dam1p establishing a direct link between PKA and the kinetochore. Consistent with S31 as an in vivo target of PKA, we observed that the nonphosphorylatable S31A mutation suppressed the high-copy TPK2 lethality in dam1-1 mutants, the higher rate of chromosome loss in wild-type cells carrying high-copy TPK2, and the reduction in kinetochore localization of Dam1p associated with high-copy TPK2. On the other hand, the diminished Dam1p localization at the kinetochore was even greater in a phospho-mimetic (dam1-S31D) and reproduced the higher rates of chromosome loss as observed in cells with high-copy TPKs. Furthermore, we find that the level of S31 phosphorylation responds to glucose and cAMP levels, suggesting that nutrient signaling can modulate the activity of the kinetochore through PKA. To the best of our knowledge, this is the first report to describe a molecular mechanism that explains the genetic interactions between PKA and DAM1, as well as the higher rates of chromosome loss associated with deregulated PKA previously reported (Li et al. 2005; Magtanong et al. 2011; Ma et al. 2012). Further, our work points to the possibility that chromosome segregation might be influenced by glucose availability, which to our knowledge, has never been reported.
Dam1p is crucial in mediating end-on attachments between kinetochores and microtubules and is a major target of the Aurora B/Ipl1p kinase (Cheeseman et al. 2002; Westermann et al. 2005; Wang et al. 2007; Grishchuk et al. 2008; Lampert et al. 2010; Tien et al. 2010; Umbreit et al. 2014). There are four sites on Dam1p (S20, S257, S265, and S292) known to be phosphorylated by Ipl1p both in vivo and in vitro (Cheeseman et al. 2002; Franck et al. 2007; Zelter et al. 2015). In addition, Mps1p has been reported to phosphorylate Dam1p in vitro at multiple sites (S218 and S221) and to play an important role in coupling the kinetochore to plus-ends of microtubules (Shimogawa et al. 2006). The C-terminal half of Dam1p binds to microtubules and Ipl1p phosphorylation of S257, S265, and S292 contribute to weakening the interactions with microtubules to promote kinetochore-microtubule dissociation in correcting kinetochore-microtubule attachment errors (Pinsky et al. 2006). On the other hand, in vitro experiments suggest that S20 plays a role in oligomerization of the Dam1 complex. High-resolution cross-linking studies and optical tweezer experiments point to S20 as a site that may function in Dam1 complex oligomerization into multimeric (e.g., 16-mer ring) structures (Sarangapani et al. 2014; Umbreit et al. 2014; Zelter et al. 2015). Phosphorylation of S20 by Ipl1p also leads to kinetochore-microtubule dissociation and is hypothesized to do so by disrupting the Dam1 complex rather than by weakening kinetochore-microtubule binding interactions (Sarangapani et al. 2014). Recent cryo-EM studies also predict S20 as a key site in regulating Dam1 oligomeric interactions (Jenni and Harrison 2018). Considering the proximity of S31 to S20, we tested if S31 may function together with S20 in regulating Dam1 oligomeric interactions by generating a double phospho-mimetic, dam1p-S20DS31D. If S20 and S31 functioned in separate pathways to regulate Dam1 complex oligomerization, we expected to observe enhanced reduction of the double phospho-mimetic at the kinetochore and greater benomyl sensitivity compared to S31D alone. Instead we observed phenotypes that were similar between cells carrying the S20DS31D and single S31D phospho-mimetic. One possible explanation might be that S31D alters the Dam1 complex to a point that cannot be changed any further by S20D. Perhaps cells with S31D have partial ring structures and these are no longer susceptible to further dissociation by S20D. Another possibility might be that phosphorylation of one site depends on the phosphorylation of the other as reported for some proteins phosphorylated on multiple sites by different kinases (Rosasco-Nitcher et al. 2008; Randell et al. 2010). In this case, perhaps S20 phosphorylation depends on phosphorylation of S31. Therefore, dam1p-S31D will have a high likelihood of also carrying phosphorylated S20, so the double phospho-mimetic will have the same phenotype as an S31D alone. Additional work will be needed to understand the mechanism of how phosphorylation of S20 and S31 work to regulate Dam1p localization to the kinetochore.
If phosphorylation of S31 disrupts Dam1 complex oligomerization this could explain the reduction in Dam1p-GFP and possibly the loss of association to microtubules observed when cells overexpress TPK2 or express a dam1-S31D-GFP phospho-mimetic. Fewer Dam1 complexes would be predicted to weaken kinetochore-microtubule binding and lead to greater sensitivity to benomyl, and increased rates of chromosome loss, which is what we observed in cells that overexpress TPK2 or express a dam1-S31D phospho-mimetic. Alternatively, the reduction in dam1p-S31D-GFP might indicate that only levels of the Dam1p subunit are diminished at the kinetochore. In this scenario, Dam1 complexes are assembled at the kinetochore with weakened microtubule binding due to the reduced number of Dam1p subunits that would normally bind to microtubules. This alternative mechanism is highly unlikely since previous reports show that recombinant Dam1 complexes only partially or completely fail to assemble when Dam1p lacks its C-terminal 158 amino acids or with a dam1-1p temperature-sensitive mutant, respectively (Westermann et al. 2005). Additional studies will be required to clearly determine if there is a reduction in Dam1 complexes vs. a decrease in the Dam1p subunit alone. A third hypothesis for how S31 functions draws on studies showing that kinetochores can have two Dam1 rings that form around microtubules (Kim et al. 2017). It is possible that in cells with high-copy TPKs or dam1-S31D, the reduction in Dam1p reflects the presence of only a single Dam1 ring, which would also reduce kinetochore-microtubule interactions (Kim et al. 2017). Previous reports show that Aurora B phosphorylation plays an important role in assembly of kinetochore complexes and in the absence of phosphorylation, these subunits can be targeted for ubiquitin mediated degradation. Notably, Aurora B phosphorylation of Dsn1p contributes to assembly of the MIND complex (Akiyoshi et al. 2013a,b). In turn, nonphosphorylatable Dsn1p mutants, which are not efficiently assembled into the kinetochore, are targeted for ubiquitin-dependent proteolysis (Akiyoshi et al. 2013a,b). Drawing on this work, a fourth possible hypothesis for S31 function is that the cycle of S31 phosphorylation and dephosphorylation may contribute to Dam1p degradation. This hypothesis would help explain the strong reduction in total levels of dam1p-S31D and to a lesser extent dam1-S31A as revealed by Western analysis. The large reduction in total dam1p-S31D-GFP compared to wild-type Dam1p-GFP cells is consistent with also seeing less dam1p-31D-GFP at the kinetochore in our microscopy measurements. However, future studies will be required to understand if degradation is stimulated by S31 phosphorylation directly (i.e., possibly through ubiquitylation) or if phosphorylation of S31 causes a reduction in kinetochore assembly, leading to unassembled Dam1p subunits that are targeted for degradation. That we also observed a decrease in total levels of the nonphosphorylatable dam1p-S31A-GFP suggests a balance in nonphosphorylated and phosphorylated S31 determines its instability. Perhaps the phosphorylated state of S31 in different cell cycle stages act differently on Dam1p degradation mechanisms. While Western blot analysis revealed that total levels of nonphosphorylatable dam1p-S31A were less than wild-type levels, we observed nearly identical amounts of dam1p-S31A-GFP and wild-type Dam1p-GFP at the kinetochore by microscopy. This suggests that dam1p-S31A-GFP can efficiently assemble into the outer kinetochore. However, we observe a clear reduction in dam1p-S31A-GFP associated with the spindle compared to wild-type Dam1p-GFP, but not to the same extent as for dam1p-S31D-GFP, in which all cells were devoid of dam1p-S31D-GFP spindle association. It is possible that the reduction in the total levels of dam1p-S31A-GFP observed by Western blot analysis is a reflection of the pool of free dam1p-S31A-GFP that is not associated with the spindle. In contrast, dam1p-S31D-GFP might have defects in kinetochore and spindle association, leading to much higher levels of free dam1p-S31D-GFP, which is degraded. Future experiments will be necessary to explore how S31 phosphorylation might influence the total cellular levels of Dam1p.
Importantly, in all four mechanisms, kinetochore-microtubule attachments would be compromised because of defects in the integrity of the Dam1 complex and drive the phenotypes we report here that are associated with S31 phosphorylation. Moreover, we observed a mild sensitivity to benomyl in dam1p-S31D cells, a complete absence of dam1p-S31D-GFP localization to the spindle, which was also observed in cells carrying dam1p-S31A-GFP but to a much lesser degree (∼38%). The reduction in Dam1p localization to the spindle was reproduced in cells with wild-type Dam1p-GFP carrying high-copy TPK2. Taken together, S31 phosphorylation is not only important for localization to the kinetochore but also affects Dam1p binding to microtubules.
Previous studies reported the localization of Dam1p on the spindle (Hoffman et al. 1998; Cheeseman et al. 2001; He et al. 2001; Jones et al. 2001; Li et al. 2002) and showed that conditional DAM1 mutants developed both hyper-elongated and broken spindles (Jones et al. 1999; Cheeseman et al. 2001). We found an increase in similar spindle defects in wild-type cells with high-copy TPK and in S31D mutants. There is also a clear loss of Dam1p-GFP on spindles when TPK2 is overexpressed (∼20%) and to an even higher degree in the S31D phospho-mimetic (∼100%) (Figure S4B). The inability to detect Dam1-GFP on the spindle may also contribute to the chromosome loss defect and the abnormal spindle phenotypes (Figure 5). It is not clear what role the Dam1 complex plays at the spindle. There have been proposed functions that include stabilizing the microtubules possibly by cross-linking spindles (Jones et al. 1999, 2001; Cheeseman et al. 2001; He et al. 2001; Li et al. 2002). That we observe abnormal spindle morphologies in S31D and enhanced spindle defects in dam1-1 by introducing the S31D mutation suggests that phosphorylation of Dam1p on S31 contributes to the role the Dam1 complex plays in regulating microtubule activity and function.
Our results showing that PKA can phosphorylate Dam1p also suggests that PKA and Ipl1p may work together in regulating kinetochore activity and chromosome segregation fidelity. In mutants where PKA is unregulated and overexpressed, it may be possible that PKA is phosphorylating kinetochore sites that are typically regulated by Ipl1p considering that there is overlap in their consensus sequences. Alternatively, there may be additional PKA specific sites that are important for chromosome segregation in addition to kinetochore components. The results of chromosome loss assays are consistent with additional sites being targeted as the frequency of half-sectors was three to sixfold higher when cells carried high-copy TPKs compared to cells expressing the dam1-S31D phospho-mimetic. Addressing how the Ipl1p sites and S31 contribute to Dam1 complex assembly and function will be important for a more comprehensive understanding of what roles phosphorylation plays in regulating kinetochore structure and function.
Regulation of kinetochore assembly and activity by changes in cell physiology or extrinsic factors (e.g., nutrient or environmental stresses) is not well studied. Previously, it was reported that different carbon sources enhance or suppress the temperature sensitivity of kinetochore mutants (Ma et al. 2012). However, the mechanism underlying these responses is unknown but suggests that kinetochores respond to nutritional status. Our results reveal that at least with respect to glucose, PKA might act as the intermediate actor connecting kinetochore activity to glucose availability. We found that switching cells from 2 to 0.05% glucose is associated with a dramatic reduction in phosphorylation of Dam1p on S31, suggesting that PKA phosphorylation of Dam1p is regulated by glucose availability. In a complementary experiment we treated cells with α-factor, which inhibits AC and downregulates PKA activity (Liao and Thorner 1980). When cells were arrested with α-factor we observed a marked decrease in Dam1-S31 phosphorylation as well. Together these results support the idea that kinetochore function can be modulated directly by the Ras/PKA pathway. Additional work will be required to fully elucidate how glucose levels and possibly other physiological changes can regulate PKA-mediated phosphorylation of Dam1p. Although to the best of our knowledge, this is the first report showing that glucose can direct changes in the phosphorylation of Dam1p, there have been studies that reveal a link between the nutrient sensing Tor1 complex (TORC1) and Dam1p function. TORC1 is best studied for its role in nitrogen sensing/signaling, in particular via amino acids (e.g., glutamine), and integrating nutritional status with translation and cell growth and proliferation (Loewith and Hall 2011). Two studies show that mutations in TORC1 can suppress ipl1-2 temperature sensitivity (Tatchell et al. 2011; Robinson et al. 2012). The mechanism of suppression is through TORC1 control of nuclear levels of the Glc7 phosphatase that opposes Ipl1p function by dephosphorylating Dam1p (Tatchell et al. 2011; Robinson et al. 2012). This raises the possibility that TORC1 and PKA work together to integrate nutrient status with kinetochore activity to modulate chromosome segregation fidelity. The biological significance for a signaling mechanism that links nutrient status to chromosome segregation fidelity is not clear. Increases or decreases in chromosome segregation errors can lead to more or less karyotypic diversity. Considering that yeast live in fluctuating environments, the ability to “tune” chromosome segregation fidelity with respect to nutrient availability might offer greater ability to adapt and survive. Future studies of TORC1 and PKA will be needed to address these provocative possibilities.
Acknowledgments
We thank Sue Biggins, Trisha Davis, Charlie Boone, Joseph Hietman, Mark Winey, Peter Kaiser, and Munira Basrai for providing plasmids and strains. We are extremely grateful to Trisha Davis and Jae Ook Kim for purified Dam1 complexes for initial experiments. We thank Trisha Davis, Sue Biggins, and Ian Cheeseman for discussions and sharing unpublished data and Wei-Chun Au for assistance with experiments. We thank Sophie Alvarez and the Proteomics and Metabolomics Facility at the Center for Biotechnology, University of Nebraska-Lincoln for mass spectrometry work. We also thank members of the Choy laboratory for helpful discussions and Pam Tuma, John Golin, and Venigala Rao for sharing reagents and equipment. Finally, we thank two anonymous reviewers for their thoughtful comments and insights, which helped to improve this work. J.S.C. is grateful for support from the Litovitz Family Fund.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.7256039.
Communicating editor: J. Nickoloff
Literature Cited
- Akiyoshi B., Sarangapani K. K., Powers A. F., Nelson C. R., Reichow S. L., et al. , 2010. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 468: 576–579. 10.1038/nature09594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi B., Nelson C. R., Biggins S., 2013a The aurora B kinase promotes inner and outer kinetochore interactions in budding yeast. Genetics 194: 785–789. 10.1534/genetics.113.150839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi B., Nelson C. R., Duggan N., Ceto S., Ranish J. A., et al. , 2013b The Mub1/Ubr2 ubiquitin ligase complex regulates the conserved Dsn1 kinetochore protein. PLoS Genet. 9: e1003216 10.1371/journal.pgen.1003216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anghileri P., Branduardi P., Sternieri F., Monti P., Visintin R., et al. , 1999. Chromosome separation and exit from mitosis in budding yeast: dependence on growth revealed by cAMP-mediated inhibition. Exp. Cell Res. 250: 510–523. 10.1006/excr.1999.4531 [DOI] [PubMed] [Google Scholar]
- Biggins S., 2013. The composition, functions, and regulation of the budding yeast kinetochore. Genetics 194: 817–846. 10.1534/genetics.112.145276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte M., Dieckhoff P., Krause C., Braus G. H., Irniger S., 2003. Synergistic inhibition of APC/C by glucose and activated Ras proteins can be mediated by each of the Tpk1–3 proteins in Saccharomyces cerevisiae. Microbiology 149: 1205–1216. 10.1099/mic.0.26062-0 [DOI] [PubMed] [Google Scholar]
- Broach J. R., 2012. Nutritional control of growth and development in yeast. Genetics 192: 73–105. 10.1534/genetics.111.135731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhwar R., Lu A., Hirsch J. P., 2010. Nutrient control of yeast PKA activity involves opposing effects on phosphorylation of the Bcy1 regulatory subunit. Mol. Biol. Cell 21: 3749–3758. 10.1091/mbc.e10-05-0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budovskaya Y. V., Stephan J. S., Deminoff S. J., Herman P. K., 2005. An evolutionary proteomics approach identifies substrates of the cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 102: 13933–13938. 10.1073/pnas.0501046102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron S., Levin L., Zoller M., Wigler M., 1988. cAMP-independent control of sporulation, glycogen metabolism, and heat shock resistance in S. cerevisiae. Cell 53: 555–566. 10.1016/0092-8674(88)90572-7 [DOI] [PubMed] [Google Scholar]
- Cheeseman I. M., 2014. The kinetochore. Cold Spring Harb. Perspect. Biol. 6: a015826 10.1101/cshperspect.a015826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I. M., Desai A., 2008. Molecular architecture of the kinetochore-microtubule interface. Nat. Rev. Mol. Cell Biol. 9: 33–46. 10.1038/nrm2310 [DOI] [PubMed] [Google Scholar]
- Cheeseman I. M., Enquist-Newman M., Muller-Reichert T., Drubin D. G., Barnes G., 2001. Mitotic spindle integrity and kinetochore function linked by the Duo1p/Dam1p complex. J. Cell Biol. 152: 197–212. 10.1083/jcb.152.1.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I. M., Anderson S., Jwa M., Green E. M., Kang J., et al. , 2002. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111: 163–172. 10.1016/S0092-8674(02)00973-X [DOI] [PubMed] [Google Scholar]
- Chepurny O. G., Kelley G. G., Dzhura I., Leech C. A., Roe M. W., et al. , 2010. PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am. J. Physiol. Endocrinol. Metab. 298: E622–E633. 10.1152/ajpendo.00630.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy J. S., Acuna R., Au W. C., Basrai M. A., 2011. A role for histone H4K16 hypoacetylation in Saccharomyces cerevisiae kinetochore function. Genetics 189: 11–21. 10.1534/genetics.111.130781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy J. S., O’Toole E., Schuster B. M., Crisp M. J., Karpova T. S., et al. , 2013. Genome-wide haploinsufficiency screen reveals a novel role for gamma-TuSC in spindle organization and genome stability. Mol. Biol. Cell 24: 2753–2763. 10.1091/mbc.e12-12-0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubacq C., Guerois R., Courbeyrette R., Kitagawa K., Mann C., 2002. Sgt1p contributes to cyclic AMP pathway activity and physically interacts with the adenylyl cyclase Cyr1p/Cdc35p in budding yeast. Eukaryot. Cell 1: 568–582. 10.1128/EC.1.4.568-582.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filteau M., Diss G., Torres-Quiroz F., Dube A. K., Schraffl A., et al. , 2015. Systematic identification of signal integration by protein kinase A. Proc. Natl. Acad. Sci. USA 112: 4501–4506. 10.1073/pnas.1409938112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck A. D., Powers A. F., Gestaut D. R., Gonen T., Davis T. N., et al. , 2007. Tension applied through the Dam1 complex promotes microtubule elongation providing a direct mechanism for length control in mitosis. Nat. Cell Biol. 9: 832–837. 10.1038/ncb1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschi L., Torres-Quiroz F., Dube A. K., Landry C. R., 2013. qPCA: a scalable assay to measure the perturbation of protein-protein interactions in living cells. Mol. Biosyst. 9: 36–43. 10.1039/C2MB25265A [DOI] [PubMed] [Google Scholar]
- Gietz R. D., Woods R. A., 2002. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350: 87–96. 10.1016/S0076-6879(02)50957-5 [DOI] [PubMed] [Google Scholar]
- Grishchuk E. L., Efremov A. K., Volkov V. A., Spiridonov I. S., Gudimchuk N., et al. , 2008. The Dam1 ring binds microtubules strongly enough to be a processive as well as energy-efficient coupler for chromosome motion. Proc. Natl. Acad. Sci. USA 105: 15423–15428 (erratum: Proc. Natl. Acad. Sci. USA 105: 19562). 10.1073/pnas.0807859105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, C., and G. Fink (Editors), 1990 Guide to Yeast Genetics and Molecular Biology. Elsevier, Amsterdam. [Google Scholar]
- He X., Rines D. R., Espelin C. W., Sorger P. K., 2001. Molecular analysis of kinetochore-microtubule attachment in budding yeast. Cell 106: 195–206. 10.1016/S0092-8674(01)00438-X [DOI] [PubMed] [Google Scholar]
- Hieter P., Mann C., Snyder M., Davis R. W., 1985. Mitotic stability of yeast chromosomes: a colony color assay that measures nondisjunction and chromosome loss. Cell 40: 381–392. 10.1016/0092-8674(85)90152-7 [DOI] [PubMed] [Google Scholar]
- Hoffman C., Cheeseman I. M., Goode B. L., McDonald K. L., Barnes G., et al. , 1998. Saccharomyces cerevisiae Duo1p and Dam1p, novel proteins involved in mitotic spindle function. J. Cell Biol. 143: 1029–1040. 10.1083/jcb.143.4.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irniger S., Baumer M., Braus G. H., 2000. Glucose and ras activity influence the ubiquitin ligases APC/C and SCF in Saccharomyces cerevisiae. Genetics 154: 1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni S., Harrison S. C., 2018. Structure of the DASH/Dam1 complex shows its role at the yeast kinetochore-microtubule interface. Science 360: 552–558. 10.1126/science.aar6436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. H., Bachant J. B., Castillo A. R., Giddings T. H., Jr., Winey M., 1999. Yeast Dam1p is required to maintain spindle integrity during mitosis and interacts with the Mps1p kinase. Mol. Biol. Cell 10: 2377–2391. 10.1091/mbc.10.7.2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. H., He X., Giddings T. H., Winey M., 2001. Yeast Dam1p has a role at the kinetochore in assembly of the mitotic spindle. Proc. Natl. Acad. Sci. USA 98: 13675–13680. 10.1073/pnas.241417098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanshin E., Bergeron-Sandoval L. P., Isik S. S., Thibault P., Michnick S. W., 2015. A cell-signaling network temporally resolves specific vs. promiscuous phosphorylation. Cell Rep. 10: 1202–1214. 10.1016/j.celrep.2015.01.052 [DOI] [PubMed] [Google Scholar]
- Kennedy E. J., Yang J., Pillus L., Taylor S. S., Ghosh G., 2009. Identifying critical non-catalytic residues that modulate protein kinase A activity. PLoS One 4: e4746 10.1371/journal.pone.0004746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Xuong N. H., Taylor S. S., 2005. Crystal structure of a complex between the catalytic and regulatory (RIalpha) subunits of PKA. Science 307: 690–696. 10.1126/science.1104607 [DOI] [PubMed] [Google Scholar]
- Kim C., Cheng C. Y., Saldanha S. A., Taylor S. S., 2007. PKA-I holoenzyme structure reveals a mechanism for cAMP-dependent activation. Cell 130: 1032–1043. 10.1016/j.cell.2007.07.018 [DOI] [PubMed] [Google Scholar]
- Kim J. O., Zelter A., Umbreit N. T., Bollozos A., Riffle M., et al. , 2017. The Ndc80 complex bridges two Dam1 complex rings. eLife 6: e21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K., Hieter P., 2001. Evolutionary conservation between budding yeast and human kinetochores. Nat. Rev. Mol. Cell Biol. 2: 678–687. 10.1038/35089568 [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Bell S. M., Zheng J., Ten Eyck L. F., Xuong N. H., et al. , 1993. 2.0 A refined crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with a peptide inhibitor and detergent. Acta Crystallogr. D Biol. Crystallogr. 49: 357–361. 10.1107/S0907444993000502 [DOI] [PubMed] [Google Scholar]
- Kotani S., Tugendreich S., Fujii M., Jorgensen P. M., Watanabe N., et al. , 1998. PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol. Cell 1: 371–380. 10.1016/S1097-2765(00)80037-4 [DOI] [PubMed] [Google Scholar]
- Lampert F., Hornung P., Westermann S., 2010. The Dam1 complex confers microtubule plus end-tracking activity to the Ndc80 kinetochore complex. J. Cell Biol. 189: 641–649. 10.1083/jcb.200912021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert F., Mieck C., Alushin G. M., Nogales E., Westermann S., 2013. Molecular requirements for the formation of a kinetochore-microtubule interface by Dam1 and Ndc80 complexes. J. Cell Biol. 200: 21–30. 10.1083/jcb.201210091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legal T., Zou J., Sochaj A., Rappsilber J., Welburn J. P., 2016. Molecular architecture of the Dam1 complex-microtubule interaction. Open Biol. 6: 150237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. M., Li Y., Elledge S. J., 2005. Genetic analysis of the kinetochore DASH complex reveals an antagonistic relationship with the ras/protein kinase A pathway and a novel subunit required for Ask1 association. Mol. Cell. Biol. 25: 767–778. 10.1128/MCB.25.2.767-778.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Bachant J., Alcasabas A. A., Wang Y., Qin J., et al. , 2002. The mitotic spindle is required for loading of the DASH complex onto the kinetochore. Genes Dev. 16: 183–197. 10.1101/gad.959402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H., Thorner J., 1980. Yeast mating pheromone alpha factor inhibits adenylate cyclase. Proc. Natl. Acad. Sci. USA 77: 1898–1902. 10.1073/pnas.77.4.1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith R., Hall M. N., 2011. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 189: 1177–1201. 10.1534/genetics.111.133363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., III, Demarini D. J., Shah N. G., Wach A., et al. , 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Ma L., Ho K., Piggott N., Luo Z., Measday V., 2012. Interactions between the kinetochore complex and the protein kinase A pathway in Saccharomyces cerevisiae. G3 (Bethesda) 2: 831–841. 10.1534/g3.112.002675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magtanong L., Ho C. H., Barker S. L., Jiao W., Baryshnikova A., et al. , 2011. Dosage suppression genetic interaction networks enhance functional wiring diagrams of the cell. Nat. Biotechnol. 29: 505–511. 10.1038/nbt.1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus S. M., Omer S., Baranowski K., Lee W. L. 2015. Improved plasmids for fluorescent protein tagging of microtubules in Saccharomyces cerevisiae. Traffic 16: 773–786. 10.1111/tra.12276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda J. J., De Wulf P., Sorger P. K., Harrison S. C., 2005. The yeast DASH complex forms closed rings on microtubules. Nat. Struct. Mol. Biol. 12: 138–143. 10.1038/nsmb896 [DOI] [PubMed] [Google Scholar]
- Mishra P. K., Au W. C., Choy J. S., Kuich P. H., Baker R. E., et al. , 2011. Misregulation of Scm3p/HJURP causes chromosome instability in Saccharomyces cerevisiae and human cells. PLoS Genet. 7: e1002303 10.1371/journal.pgen.1002303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Heitman J., 2002. Protein kinase A operates a molecular switch that governs yeast pseudohyphal differentiation. Mol. Cell. Biol. 22: 3981–3993. 10.1128/MCB.22.12.3981-3993.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L. R., Komander D., Alessi D. R., 2010. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11: 9–22. 10.1038/nrm2822 [DOI] [PubMed] [Google Scholar]
- Pinsky B. A., Kung C., Shokat K. M., Biggins S., 2006. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat. Cell Biol. 8: 78–83 (erratum: Nat. Cell Biol. 8: 100). 10.1038/ncb1341 [DOI] [PubMed] [Google Scholar]
- Randell J. C., Fan A., Chan C., Francis L. I., Heller R. C., et al. , 2010. Mec1 is one of multiple kinases that prime the Mcm2–7 helicase for phosphorylation by Cdc7. Mol. Cell 40: 353–363. 10.1016/j.molcel.2010.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi J., Wu J., Yang J., Ralston C. Y., Sankaran B., et al. , 2010. Structure of yeast regulatory subunit: a glimpse into the evolution of PKA signaling. Structure 18: 1471–1482. 10.1016/j.str.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L. S., Causton H. C., Young R. A., Fink G. R., 2000. The yeast A kinases differentially regulate iron uptake and respiratory function. Proc. Natl. Acad. Sci. USA 97: 5984–5988. 10.1073/pnas.100113397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L. C., Phillips J., Brou L., Boswell E. P., Tatchell K., 2012. Suppressors of ipl1–2 in components of a Glc7 phosphatase complex, Cdc48 AAA ATPase, TORC1, and the kinetochore. G3 (Bethesda) 2: 1687–1701. 10.1534/g3.112.003814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosasco-Nitcher S. E., Lan W., Khorasanizadeh S., Stukenberg P. T., 2008. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science 319: 469–472. 10.1126/science.1148980 [DOI] [PubMed] [Google Scholar]
- Sarangapani K. K., Akiyoshi B., Duggan N. M., Biggins S., Asbury C. L., 2013. Phosphoregulation promotes release of kinetochores from dynamic microtubules via multiple mechanisms. Proc. Natl. Acad. Sci. USA 110: 7282–7287. 10.1073/pnas.1220700110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangapani K. K., Duro E., Deng Y., Alves Fde L., Ye Q., et al. , 2014. Sister kinetochores are mechanically fused during meiosis I in yeast. Science 346: 248–251. 10.1126/science.1256729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle J. S., Sanchez Y., 2004. Stopped for repairs: a new role for nutrient sensing pathways? Cell Cycle 3: 865–868. 10.4161/cc.3.7.980 [DOI] [PubMed] [Google Scholar]
- Searle J. S., Schollaert K. L., Wilkins B. J., Sanchez Y., 2004. The DNA damage checkpoint and PKA pathways converge on APC substrates and Cdc20 to regulate mitotic progression. Nat. Cell Biol. 6: 138–145. 10.1038/ncb1092 [DOI] [PubMed] [Google Scholar]
- Searle J. S., Wood M. D., Kaur M., Tobin D. V., Sanchez Y., 2011. Proteins in the nutrient-sensing and DNA damage checkpoint pathways cooperate to restrain mitotic progression following DNA damage. PLoS Genet. 7: e1002176 10.1371/journal.pgen.1002176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogawa M. M., Graczyk B., Gardner M. K., Francis S. E., White E. A., et al. , 2006. Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase. Curr. Biol. 16: 1489–1501. 10.1016/j.cub.2006.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Lavy K. J., Bronstein A., Kupiec M., Johnston M., 2015. Cross-talk between carbon metabolism and the DNA damage response in S. cerevisiae. Cell Rep. 12: 1865–1875. 10.1016/j.celrep.2015.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulard A., Cremonesi A., Moes S., Schutz F., Jeno P., et al. , 2010. The rapamycin-sensitive phosphoproteome reveals that TOR controls protein kinase A toward some but not all substrates. Mol. Biol. Cell 21: 3475–3486. 10.1091/mbc.e10-03-0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagwerker C., Zhang H., Wang X., Larsen L. S., Lathrop R. H., et al. , 2006. HB tag modules for PCR-based gene tagging and tandem affinity purification in Saccharomyces cerevisiae. Yeast 23: 623–632. 10.1002/yea.1380 [DOI] [PubMed] [Google Scholar]
- Tatchell K., Makrantoni V., Stark M. J., Robinson L. C., 2011. Temperature-sensitive ipl1–2/Aurora B mutation is suppressed by mutations in TOR complex 1 via the Glc7/PP1 phosphatase. Proc. Natl. Acad. Sci. USA 108: 3994–3999. 10.1073/pnas.1014406108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taus T., Kocher T., Pichler P., Paschke C., Schmidt A., et al. , 2011. Universal and confident phosphorylation site localization using phosphoRS. J. Proteome Res. 10: 5354–5362. 10.1021/pr200611n [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Ilouz R., Zhang P., Kornev A. P., 2012. Assembly of allosteric macromolecular switches: lessons from PKA. Nat. Rev. Mol. Cell Biol. 13: 646–658. 10.1038/nrm3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M., de Winde J. H., 1999. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33: 904–918. 10.1046/j.1365-2958.1999.01538.x [DOI] [PubMed] [Google Scholar]
- Tien J. F., Umbreit N. T., Gestaut D. R., Franck A. D., Cooper J., et al. , 2010. Cooperation of the Dam1 and Ndc80 kinetochore complexes enhances microtubule coupling and is regulated by aurora B. J. Cell Biol. 189: 713–723. 10.1083/jcb.200910142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Scott J. D., et al. , 1987a Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol. Cell. Biol. 7: 1371–1377. 10.1128/MCB.7.4.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Wigler M., 1987b Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell 50: 277–287. 10.1016/0092-8674(87)90223-6 [DOI] [PubMed] [Google Scholar]
- Umbreit N. T., Miller M. P., Tien J. F., Ortola J. C., Gui L., et al. , 2014. Kinetochores require oligomerization of Dam1 complex to maintain microtubule attachments against tension and promote biorientation. Nat. Commun. 5: 4951 10.1038/ncomms5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. W., Ramey V. H., Westermann S., Leschziner A. E., Welburn J. P., et al. , 2007. Architecture of the Dam1 kinetochore ring complex and implications for microtubule-driven assembly and force-coupling mechanisms. Nat. Struct. Mol. Biol. 14: 721–726. 10.1038/nsmb1274 [DOI] [PubMed] [Google Scholar]
- Werner-Washburne M., Brown D., Braun E., 1991. Bcy1, the regulatory subunit of cAMP-dependent protein kinase in yeast, is differentially modified in response to the physiological status of the cell. J. Biol. Chem. 266: 19704–19709. [PubMed] [Google Scholar]
- Westermann S., Avila-Sakar A., Wang H. W., Niederstrasser H., Wong J., et al. , 2005. Formation of a dynamic kinetochore- microtubule interface through assembly of the Dam1 ring complex. Mol. Cell 17: 277–290. 10.1016/j.molcel.2004.12.019 [DOI] [PubMed] [Google Scholar]
- Yamashita Y. M., Nakaseko Y., Samejima I., Kumada K., Yamada H., et al. , 1996. 20S cyclosome complex formation and proteolytic activity inhibited by the cAMP/PKA pathway. Nature 384: 276–279. 10.1038/384276a0 [DOI] [PubMed] [Google Scholar]
- Zaman S., Lippman S. I., Schneper L., Slonim N., Broach J. R., 2009. Glucose regulates transcription in yeast through a network of signaling pathways. Mol. Syst. Biol. 5: 245 10.1038/msb.2009.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelter A., Bonomi M., Kim J., Umbreit N. T., Hoopmann M. R., et al. , 2015. The molecular architecture of the Dam1 kinetochore complex is defined by cross-linking based structural modelling. Nat. Commun. 6: 8673 10.1038/ncomms9673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Epstein A., Klein H. L., 2011. Methods to study mitotic homologous recombination and genome stability. Methods Mol. Biol. 745: 3–13. 10.1007/978-1-61779-129-1_1 [DOI] [PubMed] [Google Scholar]