A cohort study to assess LDCT screening for lung cancer targeting the general population demonstrated a reduction in lung cancer mortality and estimated the proportion of overdiagnosis.
Keywords: radiology-CT/MRI, epidemiol, lung-med
Abstract
Objectives
To evaluate the effectiveness of lung cancer screening using low-dose computed tomography for the general population, we conducted a retrospective cohort study of screening for participants among Hitachi residents.
Materials and Methods
Citizens aged 50–74 who underwent low-dose computed tomography screening at least once during 1998–2006 were defined as the computed tomography group, and those who underwent X-ray screening at least once during the same period, but did not receive low-dose computed tomography screening throughout the follow-up period, were defined as the XP group. We investigated the lung cancer incidence rate, mortality rate and all-cause mortality rate for both groups from the first lung cancer screening to the end of 2012.
Results
In the computed tomography group (17 935 residents; 9790 males and 8145 females), 273 cases of lung cancer (1.5%), 72 cases of lung cancer death (0.4%), and 885 cases of all-cause death (4.9%) were observed. On the other hand, 164 cases (1.1%) of lung cancer, 80 cases (0.5%) of lung cancer death and 1188 cases (7.6%) of all-cause death were observed in the XP group (15 548 residents; 6526 males and 9022 females). The hazard ratios of the computed tomography group to the XP group adjusted for gender, age and smoking history were 1.23 for lung cancer incidence rate, 0.49 for lung cancer mortality rate and 0.57 for all-cause mortality rate. Non-smokers and light smokers (<30 pack-years) had a significantly lower lung cancer mortality (0.41 and 0.21, respectively).
Conclusion
low-dose computed tomography screening for a population including non-smokers and light smokers may be effective.
Introduction
In Japan, 53 208 males and 21 170 females died of lung cancer in 2015, accounting for the highest cancer-related mortality rate among cancers (1). The most important risk factor for lung cancer is smoking. However, an increase in the incidence of lung cancer in non-smokers (2) and increased risk of mortality from passive-smoking-related lung cancer were recently reported (3). Furthermore, in Asian countries, including Japan, the incidence of lung cancer among non-smokers is higher than that in Europe/the USA (4). Thus, in Japan, countermeasures for lung cancer in non-smokers and light smokers are important.
According to the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) conducted in the USA, lung cancer screening using chest X-ray did not reduce the lung cancer mortality rate (5). On the other hand, in Japan, several case-control studies reported a decrease in the lung cancer mortality rate (6), and chest X-ray screening is conducted nationwide. However, a pooled analysis of these case-control studies in Japan revealed that the efficacy of chest X-ray screening lasted only 1 year (6), and a more powerful screening modality is awaited.
According to the results of the National Lung Screening Trial (NLST), lung cancer screening using low-dose computed tomography (LDCT screening) for heavy smokers reduced the lung cancer mortality by 20% (7). In the USA, LDCT screening is covered by Medicare (8), and in Europe, a statement was announced by a relevant society (9). Therefore, LDCT screening for heavy smokers may be increasingly applied in Western countries.
In Japan, LDCT screening has been employed since the 1990s (10–12). However, different from other countries, examinees included light smokers and non-smokers. As a result, a large number of stage I lung cancers were detected, and the survival rate of LDCT screening-detected lung cancer patients was high. As its effectiveness for light smokers/non-smokers remains to be clarified, an assessment study involving these subjects is necessary. Although Sagawa et al. started a randomized controlled trial (RCT) for non-smokers and light smokers to investigate the effectiveness of LDCT screening (13), over 10 years are needed for the results. As LDCT screening has been performed for many years in some areas of Japan, an effectiveness assessment study using previous data on LDCT screening should be conducted.
In Hitachi city (Ibaraki Prefecture, Japan), LDCT screening for people aged ≥50 years was introduced regardless of smoking status for employees, retired persons and their spouses in 1998, and for community dwellers or as a part of comprehensive health checkups in 2001. It was estimated in 2006 that ≥30% of targeted residents had undergone LDCT screening at least once by 2006. We previously conducted a chronological study to examine the lung cancer mortality rate in residents aged 50−75 years in Hitachi city and reported a 24% decrease in the lung cancer mortality rate 4−8 years after the introduction of LDCT screening in comparison with that for the Japanese population (14), but this chronological study had several limitations. Thus, the aim of this retrospective cohort study was to compare the mortality rate of those who underwent at least one LDCT screening with that of those who underwent chest X-ray screening.
Materials and methods
This study was primarily conducted in Hitachi city. Previous studies have reported the methods and results of LDCT screening in the Hitachi area (12,14,15). Briefly, LDCT screening was started for employees aged 50–69 years, those who had retired, and their spouses (Hitachi Health Care Center, Hitachi, Ltd.) in 1998. In 2001, it was successively introduced for residents aged ≥50 years as a regional cancer screening (Hitachi Medical Center) and for examinees aged ≥50 years during comprehensive health checkups (Hitachi General Health Center). At these institutions, screening was performed for males and females regardless of smoking status, i.e. non-smokers were included. We initially established follow-up principles for the management of detected pulmonary nodules, and the positive test rate on LDCT screening was maintained at a low value. The positive test rates on the first and repeat LDCT screening were 6.8 and 2.7%, respectively (12). A total of 98 264 LDCT scans were performed for residents in Hitachi city between 1998 and 2012 (occupational setting: 50 483, regional setting: 34 723 and comprehensive health checkups: 13 058). On the other hand, annual chest X-ray screening was recommended for all residents aged ≥40 years in Hitachi city regardless of gender or smoking status, and sputum cytology was additionally conducted for heavy smokers (≥30 pack-years). The positive test rate ranged from 1.6 to 2.6%. We were unable to obtain data for chest X-ray screening before 2000.
In this study, the ‘CT group’ was defined as residents who had undergone LDCT screening at least once between 1998 and 2006 and were aged 50–74 years at the first LDCT screening. The observation period began on the day of the first LDCT screening between 1998 and 2006. On the other hand, the ‘XP group’ was defined as residents who had undergone chest X-ray screening at least once between 2001 and 2006 and were aged 50–74 years at the first screening. However, there was an exclusion criterion for the XP group, i.e. ‘those who had undergone LDCT screening at least once during the entire study period (1998–2012)’ were excluded from the study because the influence of LDCT screening was regarded as extremely marked even in the follow-up period, and such residents were not considered for the XP group. The observation period for the XP group also began on the day of the first chest X-ray screening between 2001 and 2006.
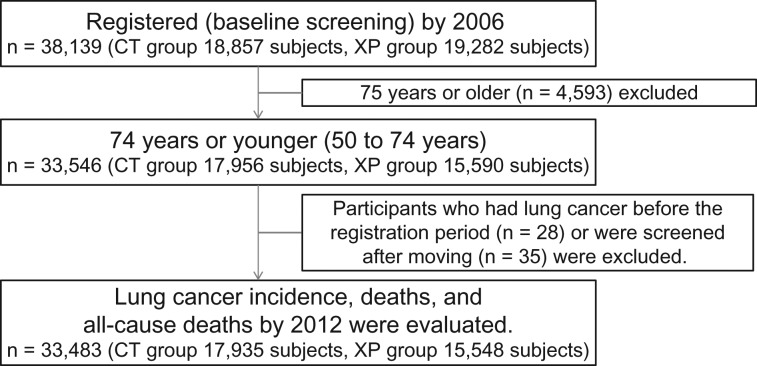
The process of data accumulation is described in the following (Fig. 1). The age at the first screening was defined as the age of the subject in this study. The total number of subjects was 38 139 (CT group: 18 857 subjects, XP group: 19 282 subjects). Excluding 4593 who were aged ≥75 years, 28 diagnosed with lung cancer before registration, and 35 who underwent screening after moving, 33 483 subjects (CT group: 17 935, XP group: 15 548) were finally analyzed.
Figure 1.
Data collection process.
We gathered death, cause of death and lung cancer incidence data until the end of 2012 from the Basic Resident Register of Hitachi city, regional cancer registration of Ibaraki Prefecture, and vital statistics reported by the Ministry of Health, Labour and Welfare. The observation period was from the day of the first CT/XP screening defined above to the day of event. In subjects without events, the final day of follow-up was set as 31 December 2012. When subjects moved to other areas during the follow-up period, follow-up was completed the day of moving. Even if they returned, follow-up was not resumed.
Regarding the pack-years of the subjects, we used the self-reported value on the day of the first screening. The pack-years values were classified into four categories: ‘0’, ‘0 < and < 30’, ‘30≤ and <50’ and ‘≥50’. For some subjects, a pack-years value of <30 was reported. We analyzed several scenarios for male with unknown pack-years category or unknown smoking history (pack-years 1/2, PYC1−4). Women with unknown pack-years category or smoking history data were considered to be non-smoking. Based on the smoking history of complete cases, we considered the pack-years 1 (PYC1) scenario, which assumed men with an unknown smoking frequency or smoking history to be in the ‘0< and 30’ pack-years category, to be the most realistic. Detailed methods and results of analysis based on multiple scenarios are shown in the supplement. The hazard ratios of LDCT screening for lung cancer incidence, lung cancer mortality and all-cause mortality in subgroups classified by gender/age/pack-years were calculated. In addition, we carried out the complete case analysis on sensitivity analysis.
Statistical analysis was carried out using R software for Windows (v.3.2.2; R Foundation for Statistical Computing) and the survival package. The baseline characteristics for the CT group and XP group were compared using the Student’s t-test for continuous variables and Fisher’s exact test for categorical variables. For the lung cancer incidence rate, lung cancer mortality rate and all-cause mortality rate of the CT group and XP group, the hazard ratios and their 95% confidence intervals were estimated with multivariate Cox proportional hazards models adjusted for gender, age and pack-years. A P value of 0.05 was regarded as significant.
The study was approved by the ethics review board of the hospital administration headquarters, Hitachi Ltd. (No. 2013–46).
Results
The characteristics of the subjects are shown in Table 1. The CT group consisted of 9790 males and 8145 females, and the XP group consisted of 6526 males and 9022 females. The gender composition of the two groups was significantly different. The mean age in the CT group was significantly younger than that in the XP group. In the CT group, the proportion of smokers among both males and females was significantly higher than that in the XP group. The mean screening cycles between 1998 and 2006 for males and females in the CT group were 3.0 ± 2.3 times and 2.0 ± 1.5 times, respectively.
Table 1.
The characteristics of the subjects
| Male | Female | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CT group | XP group | P | CT group | XP group | P | CT group | XP group | P | |
| Number of subjects | 9790 | 6526 | 8145 | 9022 | 17 935 | 15 548 | |||
| Mean age (SD) | 58.3 (6.9) | 62.0 (7.3) | <0.001 | 60.1 (6.6) | 61.2 (7.5) | <0.001 | 59.1 (6.8) | 61.6 (7.4) | <0.001 |
| Age group (%) | <0.001 | <0.001 | <0.001 | ||||||
| 50–55 | 3882 (39.6) | 1499 (23.0) | 2372 (29.1) | 2470 (27.4) | 6254 (34.8) | 3969 (25.5) | |||
| 56–63 | 3355 (34.3) | 1910 (29.3) | 3041 (37.4) | 2702 (29.9) | 6396 (35.7) | 4612 (29.7) | |||
| 64–74 | 2553 (26.1) | 3117 (47.7) | 2732 (33.5) | 3850 (42.7) | 5285 (29.5) | 6967 (44.8) | |||
| Smoking history (%) | <0.001 | <0.001 | <0.001 | ||||||
| Never | 2414 (24.7) | 1962 (30.1) | 7337 (90.1) | 8358 (92.7) | 9751 (54.4) | 10 320 (66.3) | |||
| Past | 1916 (19.6) | 1373 (21.0) | 49 (0.6) | 147 (1.6) | 1965 (11.0) | 1520 (9.8) | |||
| Current | 5213 (53.2) | 2830 (43.4) | 623 (7.6) | 288 (3.2) | 5836 (32.5) | 3118 (20.1) | |||
| Unknown | 247 (2.5) | 361 (5.5) | 136 (1.7) | 229 (2.5) | 383 (2.1) | 590 (3.8) | |||
| Pack-years 1 (%) | <0.001 | <0.001 | <0.001 | ||||||
| 0 | 2414 (24.7) | 1963 (30.1) | 7338 (90.0) | 8358 (92.7) | 9752 (54.3) | 10 321 (66.4) | |||
| 0< and <30 | 3649 (37.2) | 3097 (47.4) | 504 (6.2) | 298 (3.3) | 4153 (23.2) | 3395 (21.8) | |||
| 30≤ and <50 | 2740 (28.0) | 810 (12.4) | 137 (1.7) | 121 (1.3) | 2877 (16.0) | 931 (6.0) | |||
| 50≤ | 735 (7.5) | 284 (4.4) | 29 (0.4) | 12 (0.1) | 764 (4.3) | 296 (1.9) | |||
| Unknown | 252 (2.6) | 372 (5.7) | 137 (1.7) | 233 (2.6) | 389 (2.2) | 605 (3.9) | |||
| Pack-years 2 (%) | <0.001 | <0.001 | <0.001 | ||||||
| 0 | 2461 (25.1) | 3345 (51.2) | 7338 (90.0) | 8358 (92.7) | 9799 (54.6) | 11 703 (75.3) | |||
| 0< and <30 | 3602 (36.8) | 1715 (26.3) | 504 (6.2) | 298 (3.3) | 4106 (22.9) | 2013 (12.9) | |||
| 30≤ and <50 | 2740 (28.0) | 810 (12.4) | 137 (1.7) | 121 (1.3) | 2877 (16.0) | 931 (6.0) | |||
| 50≤ | 735 (7.5) | 284 (4.4) | 29 (0.4) | 12 (0.1) | 764 (4.3) | 296 (1.9) | |||
| Unknown | 252 (2.6) | 372 (5.7) | 137 (1.7) | 233 (2.6) | 389 (2.2) | 605 (3.9) | |||
The distribution of pack-years is also shown in Table 1. For males, ‘0< and <30’ (pack-years 1) or ‘0’ (pack-years 2) was adopted as an alternative category when only ‘a pack-years value of <30’ was obtained. For 994 subjects (CT group: 389, XP group: 605) for whom the pack-years value was not available (2.97% of all subjects), analyses were conducted using the alternative pack-years category with the four scenarios (PYC1−4) described in the supplement.
The follow-up period, number of lung cancer cases diagnosed, number of lung cancer deaths, number of cancer deaths excluding lung cancer and number of all-cause deaths excluding cancer in each group are shown in Table 2. In the CT group, the mean follow-up period was 9.85 ± 2.71 years, whereas it was 8.65 ± 2.09 years in the XP group. In the CT group, 273 subjects developed lung cancer, 72 died of lung cancer, 404 died of cancer other than lung cancer and 409 died from other cause. On the other hand, 164 subjects developed lung cancer, 80 died of lung cancer, 516 died of cancer other than lung cancer and 592 died from other cause in the XP group.
Table 2.
Follow-up period, lung cancer cases, deaths and all-cause deaths in each group
| Male | Female | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CT group | XP group | P | CT group | XP group | P | CT group | XP group | P | |
| Number of subjects | 9790 | 6526 | 8145 | 9022 | 17 935 | 15 548 | |||
| Mean follow-up days (SD) | 3783 (1 054) | 3201 (863) | <0.001 | 3367 (851) | 3 129 (681) | <0.001 | 3594 (989) | 3159 (763) | <0.001 |
| Number of lung cancer cases diagnosed (%) | 169 (1.7) | 111 (1.7) | 0.951 | 104 (1.3) | 53 (0.6) | <0.001 | 273 (1.5) | 164 (1.1) | <0.001 |
| Number of lung cancer deaths (%) | 63 (0.6) | 63 (1.0) | 0.028 | 9 (0.1) | 17 (0.2) | 0.24 | 72 (0.4) | 80 (0.5) | 0.142 |
| Number of cancer deaths, excluding lung cancer (%) | 287 (2.9) | 313 (4.8) | <0.001 | 117 (1.4) | 203 (2.3) | <0.001 | 404 (2.3) | 516 (3.3) | <0.001 |
| Number of all-cause deaths, excluding cancer (%) | 292 (3.0) | 380 (5.8) | <0.001 | 117 (1.4) | 212 (2.4) | <0.001 | 409 (2.3) | 592 (3.8) | <0.001 |
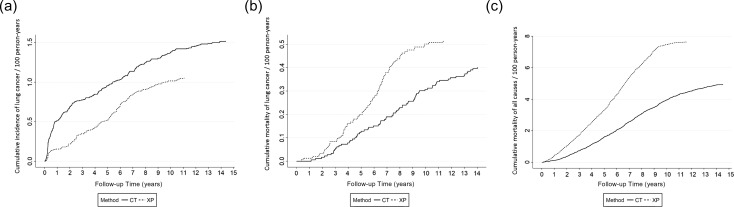
The cumulative incidence rate of lung cancer, lung cancer mortality rate and all-cause mortality rate in each group are shown in Fig. 2. The cumulative incidence rate of lung cancer in the CT group was higher than that in the XP group during the entire follow-up period. A large number of lung cancer lesions were detected in the CT group within 2 years after the first screening, causing a marked difference between the two groups. Subsequently, the incidence rate of lung cancer increased in the XP group 5−7 years after the first screening, reducing the difference between the two curves. The two groups then exhibited a similar increase (Fig. 2a) .
Figure 2.
Cumulative incidence, mortality of lung cancer, and all-cause mortality in both groups. a. Lung cancer incidence, b. Lung cancer mortality, c. All-cause mortality.
There was no marked difference in the cumulative lung cancer mortality rate (Fig. 2b) between the two groups within 2 years after the first screening. Subsequently, there was a decrease in the lung cancer mortality rate in the CT group after 3−4 years, and then there was a marked decrease 5−8 years after the first screening. On the other hand, the cumulative all-cause mortality rate in the CT group was lower than that in the XP group throughout the follow-up period, including during the first year (Fig. 2c). The slope of the curve was constant and was not affected by the interval from the first screening, differing from the cumulative incidence rate and lung cancer mortality rate.
The hazard ratios calculated by multivariate analysis adjusted for gender/age/pack-years in the ‘PYC1’ scenario of ‘pack-years 1’, which may be the most realistic, are shown in Table 3. The adjusted hazard ratios for the lung cancer incidence rate, lung cancer mortality rate and all-cause mortality rate in the CT group compared with those in the XP group were 1.23 (95% confidence interval: 1.00–1.51), 0.49 (0.34–0.70) and 0.57 (0.52–0.62), respectively. In the CT group, the lung cancer incidence rate was significantly higher than that in the XP group, whereas the lung cancer mortality rate and all-cause mortality rate were significantly lower. Regarding the other factors, the lung cancer incidence rate, lung cancer mortality rate and all-cause mortality rate increased with age or pack-years value. The lung cancer mortality rate and all-cause mortality rates were higher in males than in females.
Table 3.
Hazard ratios for lung cancer incidence, mortality and all-cause mortality according to the pack-years 1 and PYC1 scenarios.
| Multivariate analysisa | Lung cancer incidence | Lung cancer mortality | All-cause mortality | |||
|---|---|---|---|---|---|---|
| Method (ref: XP) | HRb | 95% C.I.c | HR | 95% C.I. | HR | 95% C.I. |
| CT | 1.23 | 1.00–1.51 | 0.49 | 0.34–0.70 | 0.57 | 0.52–0.62 |
| Gender (ref: Female) | ||||||
| Male | 1.09 | 0.83–1.45 | 2.12 | 1.20–3.76 | 1.89 | 1.67–2.13 |
| Age group (ref: 50–55) | ||||||
| 56–63 | 1.91 | 1.46–2.50 | 1.62 | 0.99–2.67 | 1.80 | 1.56–2.07 |
| 64–74 | 2.50 | 1.91–3.26 | 3.46 | 2.17–5.52 | 4.29 | 3.76–4.90 |
| Pack-years (ref: 0) | ||||||
| 0< and <30 | 1.38 | 1.02–1.86 | 1.80 | 1.04–3.16 | 1.10 | 0.98–1.25 |
| 30≤ and <50 | 2.42 | 1.76–3.32 | 5.69 | 3.30–9.80 | 1.36 | 1.18–1.58 |
| 50≤ | 3.63 | 2.46–5.67 | 6.57 | 3.43–12.60 | 1.70 | 1.39–2.07 |
aCox proportional hazards model, bHazard ratio, c95% Confidence interval.
The hazard ratios of analyses with respect to screening modalities (LDCT screening/X-ray screening) in the ‘PYC2-4’ scenarios are shown in Supplementary Table. The incidence of lung cancer, lung cancer mortality rate and all-cause mortality rate ranged from 1.20 to 1.27, 0.46 to 0.52 and 0.57 to 0.58, respectively, and were similar to those in the ‘PYC1’ scenario. In addition, the results of analyses of the ‘PYC1–4’ scenarios of ‘pack-years 2’ were similar. There was no notable difference between our results and those of the complete case analysis.
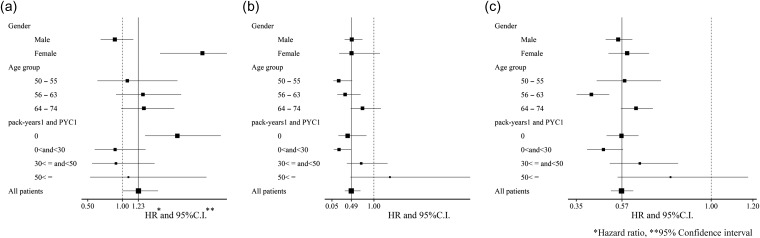
The hazard ratios of LDCT screening for the lung cancer incidence rate, lung cancer mortality rate and all-cause mortality rate in gender-/age-/pack-year-based subgroups with the ‘pack-years 1’ and ‘PYC1’ scenarios are shown in Fig. 3. As the number of subjects in the subgroups was limited, there were no significant differences in most subgroups. However, subgroup analyses revealed some differences in hazard ratios from the overall results, i.e. LDCT screening increased the lung cancer incidence rate in females (hazard ratio: 2.15) and non-smokers (1.79) and decreased the lung cancer mortality rate in younger subjects (0.20 in 50−55 years and 0.35 in 56−63 years) and non-/light smokers (0.41 in non-smokers, and 0.21 in 0< and <30 pack-years).
Figure 3.
Forest plots for lung cancer incidence, mortality, and all-cause mortality in several subgroups by LDCT screening (ref: XP) according to the pack-years1 and PYC1 scenarios. a. Lung cancer incidence, b. Lung cancer mortality, c. All-cause mortality.
Discussion
We reported the results of a cohort study involving residents in Hitachi city, Ibaraki Prefecture, Japan, where LDCT screening had been widely conducted for residents for many years. The risk of lung cancer death for residents who underwent LDCT screening decreased by 51% and the risk of lung cancer increased by 23% in comparison with those who underwent X-ray screening. On the other hand, the risk of all-cause death in the CT group decreased by 43%. This is the first cohort study regarding the effectiveness of LDCT screening for subjects including non-/light smokers.
Regarding the lung cancer incidence rate, the number of subjects diagnosed with lung cancer in the CT group was 1.23 times greater than that in the XP group. The detection capacity of low-dose CT for lung cancer may be much higher than that of chest X-ray. Moreover, most lesions detected by LDCT screening in Japan have been lung cancer measuring ≤20 mm in diameter (10–12), most of which were not detectable by X-ray screening. As indicated in the graph of the cumulative lung cancer incidence rate (Fig. 2a), more lung cancers were detected in the CT group than in the XP group within a follow-up period of ≤2 years, leading to a marked difference between the two groups. Subsequently, the lung cancer incidence rate increased in the XP group 5−7 years after the first screening, reducing the difference. This result suggests a delay in the detection of some lung cancer lesions in the XP group in comparison with early detection in the CT group. Such changes in the incidence rate related to the interval after the first screening were similar to the results of the NLST (7).
Regarding the lung cancer mortality rate, there was no marked difference between the two groups within 2 years after the first screening. The cumulative lung cancer mortality rate in the CT group was lower than that in the XP group after 3–4 years, but the relative difference decreased 5–8 years after the first screening (Fig. 2b). Ideally, cancer mortality should not decrease in the early period after the introduction of cancer screening due to the detection of curative and advanced cancers, and instead decreases in the later period. The timing of lung cancer mortality in our present study was consistent with this scenario and the results of the NLST (7). Furthermore, the lung cancer incidence rate in the XP group increased 5−7 years after the first screening and the lung cancer mortality rate in the XP group increased during the same period. These results demonstrated that some lung cancer lesions were detected several years later in the XP group as advanced cancer. These results are consistent with our previous chronological study that demonstrated a 24% reduction in the lung cancer mortality rate among residents in Hitachi city 4−8 years after the introduction of LDCT screening in comparison with the lung cancer mortality in Japan (14).
In subgroup analyses, LDCT screening was associated with an increase in the lung cancer incidence rate in females and non-smokers, and with a decrease in lung cancer mortality in younger subjects and non-/light smokers. This increase in the incidence rate is consistent with the large number of ground-glass nodules with a long doubling-time observed on LDCT screening in females and non-smokers. Regarding the lung cancer mortality rate, it should be noted that the hazard ratios were low even in non-/light smokers, and the possibility that LDCT screening is ineffective for non-/light smokers, as suggested in previous reports (17,19), was not indicated in the present study.
For cancer screening, overdiagnosis is always present to some extent. A previous study found that a maximum of 11.0% of all lung cancer patients undergoing LDCT screening were overdiagnosed in the NLST (16). During LDCT screening for non-/light smokers, a large number of lung cancer lesions with ground-glass nodules with a long doubling-time were detected (17–19); therefore, some investigators speculated that overdiagnosis markedly increases for non-/light smokers. In the present study, 55.1% of the subjects in the CT group and 67.8% of those in the XP group were non-smokers, and many lung cancer lesions were small-sized cancers or pure ground-glass nodules in the CT group (12,15). However, the incidence rate of lung cancer in the CT group was only 1.23-fold that in the XP group. When calculating overdiagnosis in accordance with the method described by Patz et al., a maximum of 18.7% of all lung cancer patients in the CT group were overdiagnosed. The value was higher than the 11.0% in the NLST but was lower than expected and not excessive in comparison with other cancer screening methods (20). Furthermore, due to the short observation period, it is unclear how many deaths will be due to lung cancer in the future. Thus, the true overdiagnosis rate in the CT group may be lower than this value.
In addition to overdiagnosis, the potential harm of LDCT screening, including radiation exposure, is of concern. In the present study, the all-cause mortality rate in the CT group was consistently lower than that in the XP group throughout the observation period from the beginning of the follow-up period, and the subsequent slopes of curves for the CT and XP groups were constant (Fig. 2c). This pattern is different from those of the lung cancer mortality rate, suggesting that there is no increase in mortality associated with LDCT screening. However, side effects due to radiation exposure do not appear until a long period of time has elapsed; therefore, we cannot conclude this issue with short-term observation.
There are several limitations in this study that must be discussed. First, we cannot deny the existence of self-selection bias, because the hazard ratio of all-cause mortality in the CT group as low as 0.57 cannot be explained with only a reduction in lung cancer mortality (hazard ratio 0.49). However, the CT group had more heavy smokers than the XP group. In addition, the lung cancer incidence rate in both groups was similar after the initial CT group distribution, as shown in Fig. 2b. We considered there to be no significant difference in the risk of lung cancer between the two groups.
Second, when discussing the effectiveness of cancer screening, the disadvantages should also be discussed. In this study, disadvantages other than overdiagnosis, such as false-positive cases, were not directly investigated, but we previously reported that a low positive rate was maintained by establishing a protocol for the management of pulmonary nodules at the start of LDCT screening in Hitachi city in 1998 to avoid excessively detailed examinations or invasive treatment (12). The disadvantages of LDCT screening should be further investigated in the future.
Third, data were complemented with insufficient pack-years data in this study; therefore, the validity must be confirmed. We conducted the multiple-imputation procedure for sensitivity analysis (10 imputation datasets were created, with two sets of variables used for complementation) and the complete case analysis with deficit-value-free data alone. As a result, the hazard ratios for lung cancer mortality were 0.49−0.50 using the multiple imputation, and those for all-cause mortality were 0.57, which were similar to the results of primary analyses. Based on the results of complete case analyses, the hazard ratios for lung cancer mortality were 0.45−0.47, and those of all-cause mortality were 0.57−0.58, which were also similar to the results of primary analyses. These results confirmed the validity of data complementation in primary analyses.
Fourth, the subjects were grouped into either the CT or XP group according to a screening history during a specified period without taking the interval from screening to diagnosis into account in the analyses. Consequently, both groups included subjects with a long interval from screening to diagnosis, for which the screening was unlikely to have contributed to the diagnosis of lung cancer. Therefore, the misclassification of screening status was inevitable in relation to the timing of the diagnosis of lung cancer.
Fifth, as considerably more lung cancers with a long doubling-time were assumed to be detected by LDCT screening in non-/light smokers than in heavy smokers, annual screening may have been excessive and disadvantageous for the subjects. The NELSON study suggested that annual LDCT screening was excessive for individuals with negative findings on the first screening (21). However, there are no reliable data for an adequate interval, and it was not clarified in this study. To present an adequate screening schedule in accordance with individual risks, a case-control study or model analysis may be helpful.
In conclusion, a cohort study including non-/light smokers with a mean follow-up of ≥9 years demonstrated that the incidence of lung cancer in residents in Hitachi city who underwent LDCT screening increased by 23% in comparison with that in those who underwent X-ray screening. On the other hand, the lung cancer and all-cause mortality rates decreased by 51 and 43%, respectively. Thus, lung cancer screening using low-dose CT for a population including non-/light smokers may be effective. Furthermore, no deterioration of the hazard ratio may be important even for subgroup analysis of non-/light smokers. The effectiveness of lung cancer screening for non-/light smokers using low-dose CT should be further investigated.
Supplementary Material
Acknowledgements
The authors thank Drs Toshimi Sairenchi, Fujiko Irie, Kazuya Sukegawa, Katsuyuki Endo, Hitachi city, Ibaraki Prefecture, Hitachi Health Insurance Society and all related staff who contributed to this screening program.
Funding
This study was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Health, Labour and Welfare; the Ministry of Education, Culture, Sports, Science and Technology; the National Cancer Center Research and Development Fund and by the Practical Research for Innovative Cancer Control of the Japan Agency for Medical Research and Development, AMED, Japan.
Conflict of interest statement
None declared.
References
- 1. Center for Cancer Control and Information Services , National Cancer Center, Japan. Available at: https://ganjoho.jp/reg_stat/statistics/stat/index.html (17 December 2017, date last accessed)
- 2. Cufari ME, Proli C, De Sousa P, et al. . Increasing frequency of non-smoking lung cancer: Presentation of patients with early disease to a tertiary institution in the UK. Eur J Cancer 2017;84:55–9. [DOI] [PubMed] [Google Scholar]
- 3. Hori M, Tanaka H, Wakai K, Sasazuki S, Katanoda K. Secondhand smoke exposure and risk of lung cancer in Japan: a systematic review and meta-analysis of epidemiologic studies. Jpn J Clin Oncol 2016;46:942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thun MJ, Hannan LM, Adams-Campbell LL, et al. . Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med 2008;5:e185. 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oken MM, Hocking WG, Kvale PA, et al. . Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011;306:1865–73. [DOI] [PubMed] [Google Scholar]
- 6. Sagawa M, Nakayama T, Tsukada H, et al. . The efficacy of lung cancer screening conducted in 1990s: four case-control studies in Japan. Lung Cancer 2003;41:29–36. [DOI] [PubMed] [Google Scholar]
- 7. National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. . Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chin J, Syrek Jensen T, Ashby L, Hermansen J, Hutter JD, Conway PH. Screening for lung cancer with low-dose CT-translating science into Medicare coverage policy. N Engl J Med 2015;372:2083–5. [DOI] [PubMed] [Google Scholar]
- 9. Oudkerk M, Devaraj A, Vliegenthart R, et al. . European position statement on lung cancer screening. Lancet Oncol 2017;18:e754–66. [DOI] [PubMed] [Google Scholar]
- 10. Sobue T, Moriyama N, Kaneko M, et al. . Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol 2002;20:911–20. [DOI] [PubMed] [Google Scholar]
- 11. Sone S, Li F, Yang ZG, et al. . Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br J Cancer 2001;84:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nawa T, Nakagawa T, Kusano S, Kawasaki Y, Sugawara Y, Nakata H. Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest 2002;122:15–20. [DOI] [PubMed] [Google Scholar]
- 13. Sagawa M, Nakayama T, Tanaka M, Sakuma T, Sobue T. JECS Study Group . A randomized controlled trial on the efficacy of thoracic CT screening for lung cancer in non-smokers and smokers of <30 pack-years aged 50–64 years (JECS Study): research design. Jpn J Clin Oncol 2012;42:1219–21. [DOI] [PubMed] [Google Scholar]
- 14. Nawa T, Nakagawa T, Mizoue T, et al. . A decrease in lung cancer mortality following the introduction of low-dose chest CT screening in Hitachi, Japan. Lung Cancer 2012;78:225–8. [DOI] [PubMed] [Google Scholar]
- 15. Nawa T, Nakagawa T, Mizoue T, et al. . Long-term prognosis of patients with lung cancer detected on low-dose chest computed tomography screening. Lung Cancer 2012;75:197–202. [DOI] [PubMed] [Google Scholar]
- 16. Patz EF Jr, Pinsky P, Gatsonis C, et al. . Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174:269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamashima C, Sobue T, Muramatsu Y, Saito H, Moriyama N, Kakizoe T. Comparison of observed and expected numbers of detected cancers in the research center for cancer prevention and screening program. Jpn J Clin Oncol 2006;36:301–8. [DOI] [PubMed] [Google Scholar]
- 18. Henschke CI, Salvatore M, Cham M, et al. . Baseline and annual repeat rounds of screening: implications for optimal regimens of screening. Eur Radiol 2017; 10.1007/s00330-017-5029-z. [DOI] [PubMed] [Google Scholar]
- 19. Kakinuma R, Noguchi M, Ashizawa K, et al. . Natural history of pulmonary subsolid nodules: a prospective multicenter study. J Thorac Oncol 2016;11:1012–28. [DOI] [PubMed] [Google Scholar]
- 20. Carter JL, Coletti RJ, Harris RP. Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods. BMJ 2015;350:g7773. 10.1136/bmj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yousaf-Khan U, van der Aalst C, de Jong PA, et al. . Risk stratification based on screening history: the NELSON lung cancer screening study. Thorax 2017;72:819–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.