Abstract
The development of cells for regenerative therapy has encountered many pitfalls on its path to clinical translation. In cardiology, clinical studies of heart-targeted cell therapies began two decades ago, yet progress towards reaching an approved product has been slow. In this Perspective, I provide an overview of recent cardiac cell therapies, with a focus on the hurdles limiting the translation of cell products from research laboratories to clinical practice. By focusing on heart failure as a target indication, I argue that strategies for overcoming limitations in clinical translation require an increasing emphasis on mechanism-supported efficacy, rather than on phenomenological observations. As research progresses from cells to paracrine mechanisms to defined factors, identifying those defined factors that are involved in achieving superior therapeutic efficacy will better inform the use of cells as therapeutic candidates. The next generation of cell-free biologics may provide the benefits of cell therapy without the intrinsic limitations of whole-cell products.
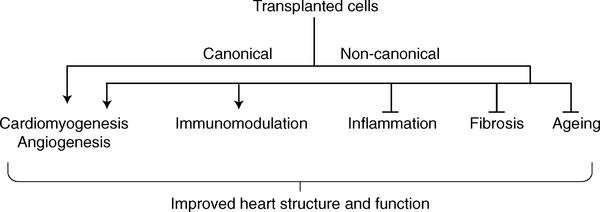
Despite major advances in pharmacology and device therapy, heart disease — specifically heart failure, the deadliest form — remains an increasing major public health challenge1. The dominant form of injury to the human heart is ischaemic: throm-bosis of a coronary artery leads to heart-tissue necrosis — a process commonly known as myocardial infarction. In adult mammals, the default response to myocardial infarction is scar formation, but neonatal mammals can regenerate the myocardium for a few days after birth. One goal of regenerative cardiology, which could in principle be achieved through cell therapies, is to take advantage of this developmental programme to convert the fibrotic response to a regenerative one in patients with myocardial infarction2 (Fig. 1). The canonical approach to this objective posits that transplanted stem cells or progenitor cells will engraft, proliferate and differentiate into new healthy tissue. Conversely, transplanted cells may also activate beneficial, non-canonical mechanisms, including triggering anti-fibrotic and anti-inflammatory processes that potentiate the overall healing response. Therefore, cell therapy has the potential to be a game changer in the treatment of heart failure, as none of the treatments approved for this indication to date reverse the pathology at a fundamental level3. The possibility of regenerating sufficient healthy myocardium to enable stabilization, or even regression, of heart failure has great allure. However, although conceptually appealing, the promise of cell therapy is so far unfulfilled.
Fig. 1 |. Biological processes modulated by cell therapy.
The direct progeny of transplanted cells can generate new heart muscle and blood vessels by canonical mechanisms. Yet other biological processes may be stimulated or suppressed via non-canonical (indirect) mechanisms of cell action.
The state of the art
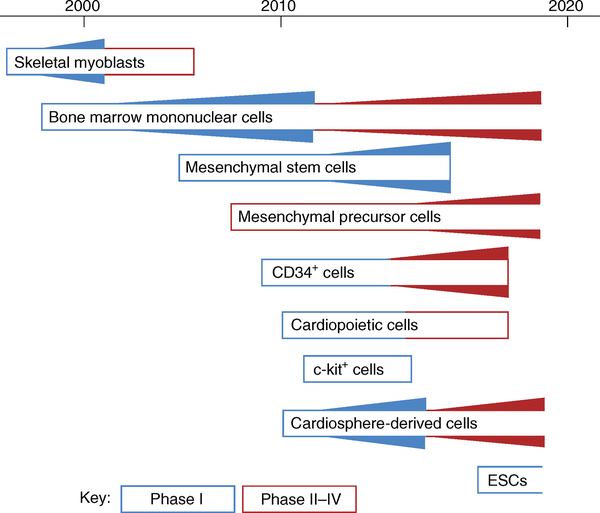
Multiple cell therapy approaches for heart disease have been tested in a clinical setting over the years (Fig. 2). The first systematic efforts in cardiac regeneration, which occurred by the turn of the millenium4, were based on the much earlier finding that autologous skeletal myoblasts can engraft and proliferate when transplanted into the heart5. Skeletal muscle, unlike cardiac muscle, is not coupled to the surrounding syncytium, nor does it beat spontaneously. Nevertheless, the hope was that the transplant would trigger the formation of new contractile units within the myocardium to boost contraction. The research and development programme followed a logical sequence, starting with small animal models6, continuing to more realistic preclinical models7 and, ultimately, running patient trials. Clinical testing of surgically implanted skeletal myoblasts in patients with heart failure showed hints of efficacy but also enhanced arrhythmogenesis8; consequently, development efforts for this cell type seem to have been abandoned.
Fig. 2 |. Clinical testing of cell therapies for heart disease.
Cell types that are actively being studied are depicted as boxes with an open righthand edge. Cell types in fully enclosed boxes represent programmes that no longer seem in active clinical development since the time of the last reported trial. The thickness of the triangles is roughly proportional to the number of trials conducted at each time point; phase-I trials are depicted in blue, and phase-II and later trials in red. ESCs, embryonic stem cells.
As the skeletal myoblast approach was being tested, a less methodical translational programme unfolded around the study of bone-marrow-derived cells for acute myocardial infarction (AMI). In 2001, researchers made the extraordinary claim that “locally delivered bone marrow cells can generate de novo myocardium, ameliorating the outcome of coronary artery disease”9. This discovery in a mouse model of AMI was subsequently discredited10, but despite this clinical studies followed almost immediately11. The general rationale for the therapy was as follows: patients presenting with AMI underwent routine clinical care, consisting of percutaneous coronary intervention to re-open the occluded coronary artery; afterwards (typically 1–14 days after the AMI), bone marrow aspiration was performed, and autologous bone marrow mononuclear cells were isolated and delivered by intracoronary infusion into the injured region of the heart. Several thousand such patients underwent the procedure12. The general treatment scheme has proven to be quite safe, but overall efficacy remains uncertain. With the possible exception of the ongoing BAMI trial13, no study has been adequately powered to assess hard clinical outcomes such as rehospitalization and mortality. Meta-analyses resulted in conflicting conclusions — some showed improvement14, but others revealed no clear benefit12,15. Overall, no convincing evidence of heart regeneration has been presented in any of the clinical studies involving bone marrow mononuclear cells.
While trials of bone marrow cells were proceeding, experimentation began with second-generation cell products. Some of these, notably bone-marrow-derived mesenchymal stem cells (MSCs)16, were adapted from other indications for which they had been therapeutic candidates for some time. Variants of MSCs that have reached clinical testing for heart failure include cardiopoietic cells (autologous MSCs fortified in vitro17) and antigen-selected mesenchymal precursor cells18. Regular MSCs have been used primarily in small, uncontrolled trials19, yet cardiopoietic cells20 and mesenchymal precursor cells21 have been tested more rigorously. Other cell sources have also been the focus of research: for instance, endothelial progenitor cells and autologous circulating bone-marrow-derived cells selected on the basis of CD34 expression seem to be effective in mitigating refractory angina22. Furthermore, alternative strategies were explored for recruiting and augmenting innate regenerative responses in the heart. In this context, cardiac progenitor cells seemed particularly attractive, owing to their potential to engraft, proliferate and differentiate into new myocardium. Heart cells selected for expression of the c-kit surface antigen were proposed to work by these canonical mechanisms23, which motivated the SCIPIO trial of autologous c-kit-selected heart cells24. Unfortunately, once again the background science supporting this trial was later called into question25, and the SCIPIO trial itself triggered an editorial expression of concern in the Lancet26.
The cell types used in these studies were, at best, adult progenitor cells and not pluripotent stem cells. Although pluripotent cells are more likely to work via the originally hypothesized canonical mechanisms, they have experienced a glacially slow translational-development trajectory. Since 1998, human pluripotent cells have been known to produce heart tissue in vitro and in vivo27. Nevertheless, clinically relevant studies injecting pluripotent-cell-derived cardiomyocytes (or cardiomyocyte precursors) in large-animal AMI models have uncovered safety concerns in the form of severe ventricular arrhythmias, which were detected frequently28,29. Such arrhythmias could be fatal in humans; therefore, clinical studies with intramyocardially injected pluripotent-cell-derived cardiomyocytes will be difficult to justify until this complication is avoided. Still, some researchers have leapfrogged further study and transplanted embryonic stem-cell-derived cardiomyocyte sheets onto the surface of the heart (that is, epicardially) in several patients with advanced heart failure. The cases reported to date have revealed no apparent safety concerns30. However, unlike cells that are injected into the heart muscle itself, epicardially administered cells are unlikely to work by engrafting and proliferating to create new heart muscle31, thus falling short of the conceptual attractiveness of pluripotency — that is, the ability of stem cells to self-renew, with potential differentiation into functional cardiomyocytes.
Several major clinical trials of cell therapy for heart disease are currently recruiting (Table 1). At the moment, only three cell types seem to be in active commercial development as cell-based therapies with a view to ultimate product registration for cardiac indications: autologous bone marrow mononuclear cells (CardiAMP trial, NCT02438306) and allogeneic mesenchymal precursor cells (DREAM HF-1 trial, NCT02032004) are both in phase-III testing for heart failure with reduced ejection fraction, and allogeneic cardiosphere-derived cells (CDCs) are being developed for various specialized types of heart failure, notably the cardiomyopathy associated with Duchenne muscular dystrophy (HOPE-2 trial, NCT03406780).
Table 1 |.
Clinical trials of cell therapy for heart disease
| Trial name | Indication | Design; sponsor; phase | Number of patients projected | Delivery method |
|---|---|---|---|---|
| Bone marrow mononuclear cells | ||||
| REPEAT (NCT01693042) | Chronic post-infarction heart failure | Autologous cells, single versus dual infusion; academic; phase II–III | 676 | Intracoronary |
| CardiAMP Heart Failure Trial (NCT02438306) | Chronic post-infarction heart failure | Autologous cells, randomized with sham control; BioCardia; phase III | 250 | Transendocardial intramyocardial delivery |
| Andalucia DCM Trial (NCT02033278) | Dilated cardiomyopathy | Autologous cells, DBPCT; academic; phase II-III | 51 | Intracoronary |
| BAMI (NCT01569178) | Acute myocardial infarction | Autologous cells, randomized to cell infusion or standard care; academic; phase III | 350 | Intracoronary |
| Mesenchymal cells | ||||
| DREAM HF-1 (NCT02032004) | Chronic heart failure with reduced ejection fraction | Allogeneic MPCs, randomized with sham control; Mesoblast; phase III | 600 | Transendocardial intramyocardial delivery |
| Cardiosphere-derived cells | ||||
| HOPE-2 (NCT03406780) | Cardiomyopathy and skeletal myopathy of Duchenne muscular dystrophy | Allogeneic, multiple repetitive doses, DBPCT; Capricor; phase III | 80 | Intravenous |
| Combinations | ||||
| CONCERT-HF (NCT02501811) | Ischaemic cardiomyopathy | Autologous bone marrow MSCs and/or autologous c-kit-selected heart cells, DBPCT; academic; phase II | 144 | Transendocardial intramyocardial delivery |
Actively recruiting multicentre trials were taken from https://clinicaltrials.gov (accessed 22 February 2018). Each trial is listed with its National Clinical Trial identifier. DBPCT, double-blind placebo- controlled trial; MPC, mesenchymal precursor cell; MSC, mesenchymal stem cell.
Barriers to translation
The field of cardiac cell therapy has not lacked ideas, many of which have undergone extensive preclinical testing to establish proof of concept. How then, can one rationalize the inability, to date, to translate any of those concepts to approved products? Various obstacles to translation, depicted schematically in Fig. 3, are discussed in detail below.
Fig. 3 |. Obstacles in the translation of cell therapy, from proof of concept through to product approval.
The process is akin to navigating a maze, with obstacles including legacy concerns introduced by a history of unsound scientific practices and relentless hype; the transition from small-scale phase-I manufacturing to commercial-scale manufacturing introduces the potential for product drift, lack of reproducibility of key product characteristics, difficulties establishing mechanistically based potency assays, and concerns related to safety.
Legacy obstacles.
Multiple translational failures, compounded by allegations of scientific irregularities surrounding reports of bone-marrow-derived cells and c-kit-selected heart cells, have poisoned the public perception of cardiac cell therapy. Clinical studies have often proceeded with little information regarding cell dosing, a parameter that is rarely optimized in translational studies prior to clinical application. Likewise, the risk–benefit ratio of the various delivery options receives little attention before clinical studies are initiated. This gloomy perspective has been compounded by hype — expectations are raised only to be dashed later, creating a state of confusion that undermines the entire field. As a case in point, a recent Editorial32, published in Nature Biotechnology, that targeted cardiologists’ hype33,34 over a trial of CD34-selected cells for AMI35 ended up as a wholesale indictment of cardiac cell therapy. Given these considerations, it is no wonder that the investment climate for cardiac cell therapy is lukewarm at best, and that companies with significant heart-regeneration programmes tend to fare poorly in the public markets. Without commercial interest, it is impossible to move from carefully curated proof-of-concept academic studies to larger placebo-controlled trials and, eventually, to approved products.
Manufacturing challenges.
Although there is ample evidence for the efficacy of cardiac cell therapy in preclinical models36, the fact that cells are fragile living entities that can be difficult to manufacture and to handle precludes the application of standard manufacturing processes37. Cellular therapeutic candidates generally originate in academic laboratories, where they are made to research-grade standards and tested pre-clinically without much attention to scalability or regulatory compliance. First-in-human studies require relatively modest manufacturing process optimization and, because they are designed to treat only a handful of patients, the scalability, cost and reproducibility of the cell product are secondary concerns. Only a few cell sources have progressed beyond phase-I clinical trials of cell therapies for cardiovascular indications, and the most perilous pitfalls lie in the steps that follow those first trials. As a product undergoes further development, the typical translational sequence requires discovery scientists to hand over to biotechnology companies or to contract manufacturing operations. There, an oft-heard mantra is ‘good management trumps good science’: cost of goods sold, process development and quality control — terms well-known in the pharmaceutical industry by their acronyms (COGS, PD and QC, respectively), but alien to the discovery scientist — begin to dominate manufacturing priorities. Unlike small molecules, whose synthesis and standards for purity and formulation are well-established, cells are alive and dynamic, evolving and adapting in response to the environment. Consequently, maintaining efficacy is a continuing challenge, especially for cell types that undergo senescence (or lose potency) after multiple passages following isolation from source tissues38. Importantly, the optimization of manufacturing processes (for instance, a switch from monolayer culture to suspension culture) introduces changes that may seem innocuous, but can fundamentally alter the final product, often in unpredictable ways39. Cryopreservation of allogeneic cells for increased shelf-life further alters the product, with potential diminished efficacy after thawing. In the extreme, the therapeutic candidates can stray so far from their intended formulations that they lose disease-modifying properties. The risk of product drift is influenced by the prevailing corporate culture; without strong scientifically based management, bioactivity takes a back seat to process expediency, potentially derailing a cell product40.
Reproducibility and efficacy.
A major concern for commercial biomedical products is consistency. To conform to regulatory standards, a product sold today must be comparable in identity, potency and composition to the same product manufactured and sold years from now. Sequential quality-control procedures must therefore be put in place at various steps in the manufacturing process to validate the equivalence of all cell banks. There is no guide book for how this should be done, as no allogeneic cell therapy products have been approved in the United States (with the exception of GINTUIT, an allogeneic keratinocyte and fibroblast product approved by the US Food and Drug Administration in 2012 for topical use in dental applications). It seems reasonable to presume that the more attention paid to product uniformity and quality, the greater the risk of product drift. A related, significant concern in commercial product development is diminished efficacy, which should be tackled by the establishment and validation of assays that measure potency. This can be a challenging endeavour, as cell therapy has such diverse targets that it can be difficult to pinpoint a single molecule or pathway as being particularly essential for therapeutic efficacy. In vivo models, which prevail at the discovery stage, have the virtue of providing integrative, functional readouts, but they suffer from biological variability, long duration, high cost and subjectivity of interpretation. Reductionist models in vitro, on the other hand, can fall short in terms of disease relevance. Rapid, reliable, indication-relevant potency assays are highly desirable but rarely implemented. As an example of such an assay, secretion of tumour necrosis factor-α receptor serves as a potency marker for Prochymal, an allogeneic MSC product approved in Canada for graft-versus-host disease (this receptor binds a pro-inflammatory cytokine that is linked mechanistically to graft-versus-host disease41). Insofar as cells are viewed as black boxes, progress towards better potency assays will be elusive. Therefore, detailed mechanistic dissection is a route to identifying determinants of efficacy that, in turn, can reveal markers of potency.
Safety.
Cell therapies carry unique safety-related concerns. These can generally be categorized as either related to delivery strategies or as specific to the cells themselves. Delivery methods used to date in the clinic vary from innocuous to highly invasive, with accordingly escalating risk. When targeting the heart, intravenous infusion (the least extreme method), has been assumed to be generally inferior, owing to the lack of direct administration to the heart itself; however, intravenous infusion is poised for a resurgence, given the increasing evidence that cells can exert long-distance effects mediated by exosomes (as reviewed below).
Most clinical trials to date, however, have presumed that direct delivery is required. Catheters have frequently been used to infuse cells into the coronary circulation, with a favourable safety profile comparable to that of routine coronary angiography (which results in serious complications in approximately 1 in 500 patients42). The coronary route has the advantage that cells are administered into a natural space (the coronary arterial lumen), where they can lodge and eventually extravasate43; no artificial space is created, nor is the heart disrupted mechanically. The recent demonstration that stop-flow vessel occlusion is not required for efficacy has further simplified intracoronary delivery44,45. The use of experimental transendocardial intramyocardial injection catheters is the riskiest method by far, and is only supported by a paucity of reliable data46. In this method, the goal is to introduce remotely deployed needles into multiple sites in the heart, thereby enabling the injection of the cell product into the myocardium itself. Along with the creation of an unnatural space, and tissue disruption, the method has a considerable delivery-related mortality, driven by a high risk (from approximately 1 in 25 (ref. 22) to 1 in 80 (ref. 47)) of iatrogenic cardiac perforation when applied to patients. Nevertheless, several clinical trials continue to use intramyocardial injection (of the seven ongoing trials listed in Table 1, three are using this poorly validated method). In addition, open-chest surgery has been used to inject cells directly into the heart48, or to apply cell-containing sheets or gels to the surface of the heart (epicardial administration30,49). Surgical delivery methods are so invasive, however, that they are justifiable only in patients with other indications for cardiac surgery.
When considering risks associated with the cells themselves, the risk of neoplasm is ever-present with proliferating transplanted cells. Pluripotent cells can form tumours in vivo; therefore, pre-differentiation to the desired lineage, and verification of absence of lingering pluripotent cells, are vital precautions in contemplating their clinical application50. This risk is more than theoretical, as tumours have been reported in several patients treated in commercial stem cell clinics51–53. Allogeneic adult stem cells, which are likely to be cleared immunologically over time54, may offer an added measure of safety in this regard, but immune rejection may occur so quickly that it undermines the ability of allogeneic cells to exert paracrine mechanisms of action, ultimately limiting efficacy55. Another potential safety concern with transplanted cells is the chance for progressive sensitization to foreign antigens, which might lead, at least in principle, to faster clearance of allogeneic cells in repeat-dosing protocols55. This may become a problem in regimens that depend on multiple cell doses, applied sequentially over time, as is likely to be required for sustained efficacy in diseases with a progressive underlying pathology such as Duchenne muscular dystrophy. Crossmatching — that is, determining compatibility between donor and recipient — may mitigate the theoretical concern of sensitization, if it turns out to be genuine56.
Another safety consideration, unique to cardiac applications of cell therapy, is the potential for enhanced arrhythmogenesis. The heart functions as an electrical syncytium in which the transmission of electrical impulses relies on the efficient coupling between excitable myocardial cells. Transplanted cells can disrupt the electrical impulses, either by creating uncoupled or poorly coupled islands of new tissue that block conduction (thus potentially functioning as parasystolic foci favouring abnormal automaticity), or by coupling with surrounding myocardium and influencing local activation and repolarization57. Skeletal myoblasts worsen arrhythmia by the first mechanism (conduction block), an effect that may be mitigated by the use of epicardial cell sheets58. By contrast, immature cardiomyocytes derived from pluripotent cells cause arrhythmias by coupling with surrounding mature heart cells, leading to regional inhomogeneities that facilitate re-entry or triggered activity57. In this context, non-integrating adult heart cells, working by paracrine mechanisms, may have a safety advantage over engrafting cell types.
Mechanistic insights for overcoming translational barriers
Much of the work in the development of cell therapies to date has been driven more by doctrine than by a deep mechanistic understanding of precisely how cells exert therapeutic benefits. This may underlie the general failure to advance preclinical findings and small-scale clinical trials. For cell therapies to succeed in the clinic, they need to be centred on the underlying biological mechanisms. Mechanistic insights are leading the field away from the cells themselves and refocusing on the cells’ paracrine functions, and will ultimately zone in on defined factors. Understanding the defined factors, in turn, can inform the clever use of cells as therapeutic candidates. The development trajectory of CDCs for heart failure, for instance, exemplifies how mechanistic discoveries can feed-back on and focus the translational process of cell therapies. Such a trajectory is only possible by discarding doctrine and following the data.
The promise of CDCs.
In 2004, my colleagues and I began to develop CDCs as therapeutic candidates for heart failure, and in 2007 we reported a manufacturing process for CDCs and their properties59 (Fig. 4). Since then, about 160 papers have been published using this cell type from over 45 independent laboratories worldwide, confirming the cells’ therapeutic bioactivity60. CDCs uniformly express CD105 and are negative for CD45 and other haematopoietic markers; they qualify as cardiac progenitor cells, being of intrinsic cardiac origin61, multipotent and clonogenic62. On the basis of these features, the phase-I clinical trial CADUCEUS (NCT00893360) used autologous cells63 with the presumption that transplanted cells would generate new tissue that would be rejected immunologically if it were not a perfect match to the host. CADUCEUS showed autologous CDCs to be safe, and yielded hints of regenerative efficacy. Three other trials of autologous CDCs have been conducted independently in Japan, in infants with hypoplastic left heart syndrome and in adult heart failure, with no apparent safety concerns and multiple signs of efficacy49,64,65.
Fig. 4 |. CDC properties.
The approach for specimen processing for CDC growth and expansion. Figure adapted from ref. 59, Wolters Kluwer Health, Inc.
From autologous to allogeneic cells.
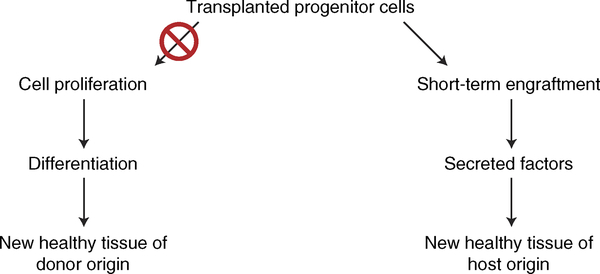
There is extensive evidence that cell therapy works indirectly, even with bona fide progenitor cells (Fig. 5). Whereas this is true in general, the evidence is particularly strong for CDCs. Few (< 1% of injected) cells are detectable 3–4 weeks after transplantation, but functional and structural benefits persist for at least 6 months54,66. During the approximate 2 weeks in which appreciable numbers of transplanted CDCs persist in the tissue, the cells indirectly induce cardiomyogenesis in the host myocardium67. Notably, the bioactivity of CDCs is not limited to fostering regeneration; the cells also induce antifibrotic, anti-inflammatory and immunomodulatory effects68, harnessing all of the benefits (identified in Fig. 1) that are critical to improve heart structure and function. Attempts to pinpoint the key paracrine signals initially focused on growth factors and chemokines, such as SDF-1 (ref. 66).
Fig. 5 |. Changes from canonical to indirect (paracrine) mechanisms of action of cell therapy.
The realization that transplanted cells do not linger in the heart for long, yet exert lasting benefits, motivated the testing of cells from unrelated donors without immunosuppression. Preclinical studies showed that allogeneic CDCs are safe, and that they are as effective as autologous CDCs54,69, even with repeated dosing70. This has led to three completed (DYNAMIC71; ALLSTAR, NCT01458405; and HOPE-Duchenne, NCT02485938) and three ongoing (ALPHA for pulmonary hypertension, NCT03145298; Regress-HFpEF for heart failure with reduced ejection fraction, NCT02941705; and HOPE-2 for Duchenne muscular dystrophy, NCT03406780) trials of allogeneic CDCs. The emphasis of cell therapy research shifted in 2014, when exosomes were first implicated as the mediators of CDC benefits72.
The promise of exosomes.
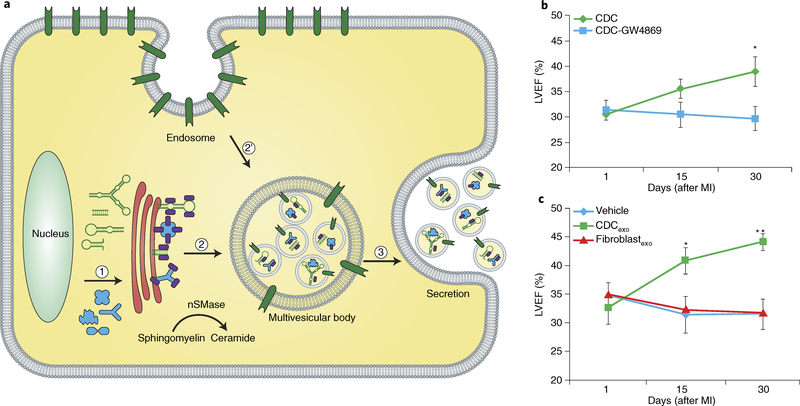
Serum-free medium conditioned by CDCs contains extracellular vesicles, including exosomes, as gauged by particle size and payload (Fig. 6a). Exosomes are products of the ceramide-requiring endolysosomal pathway, and arise from the fusion of surface membrane invaginations (endosomes) and products of the Golgi apparatus to create multivesicular bodies73. Accordingly, inhibitors of ceramide synthesis, such as GW4869, block the secretion of exosomes. These nanoparticles are secreted not only by CDCs but by all other eukaryotic cells examined to date, and contain rich repertoires of bioactive nucleic acids and proteins that can be taken up by, and transferred to, neighbouring or distant cells. In a mouse model of AMI, blocking exosome production renders CDCs ineffective (Fig. 6b), whereas CDC exosomes (CDCexo) can reproduce the benefits of the parent CDCs (Fig. 6c)72. Thus, exosomes are necessary and sufficient for CDC efficacy. The concepts described here for heart-derived progenitor cells have since been generalized to all other cell types under consideration as therapeutic candidates for heart disease; even pluripotent-stem-cell products are now acknowledged to act largely via exosome secretion74–76. Many, if not most, of the effects of exosomes are mediated by their RNA contents, specifically miRs and other non-coding RNAs (ncRNAs)77. Among ncRNAs, non-miR Y RNA fragments are richly expressed in CDCexo and modulate target cell gene expression77. The emerging concept for the mechanism of action of CDCexo relies on progenitor cells secreting exosomes that transfer payloads into target cells, inducing transcriptomic and phenotypic changes that underlie the benefits of cell therapy. As suggested by the data (Fig. 6c), exosomes may themselves be attractive next-generation cell-free therapeutic candidates.
Fig. 6 |. Exosome biology and evidence of exosome efficacy.
a, Schematic of exosome biogenesis and release. (1) Exosome payload, including proteins and RNA, are synthesized in the nucleus and matured in the endoplasmic reticulum. (2) Sorting occurs in the trans-Golgi system (shown in red), where exosome signals are labelled with ceramide and with trafficking proteins that mediate budding into the endosome. (2’) The endosome itself is a product of invagination of the plasma membrane. Intraluminal vesicles form by fusion with the endosome, and acquire surface-marker profiles that are conserved. The markers include tetraspanins and heat-shock proteins, as well as markers specific to the parent cell. (3) The multivesicular body progressively acidifies, which leads to fusion with the plasma membrane and the release of intraluminal vesicles (exosomes). nSMase, neutral sphingomyelinase. b, Blockade of CDC exosome biosynthesis with the small molecule GW4869 undermines CDC efficacy in improving heart function after myocardial infarction (MI). c, CDC exosomes mimic the functional benefits of CDCs after MI. LVEF, left ventricular ejection fraction. Panels b and c adapted from ref. 72, Elsevier.
Will exosomes supplant cells entirely? Probably not. Cells have been in therapeutic development for decades, whereas purified exosomes have undergone very limited testing in humans, and never for cardiac indications78. The challenge is in identifying the situations in which cells are preferable to exosomes. Exogenously delivered exosomes are still poorly understood in terms of biodistribution, delivery and bioactivity in vivo; they can travel near and far79, although uptake is stronger and most predictable with local administration within tissue. Perhaps unsurprisingly, cells may turn out to be the best vehicles to deliver exosomes and their contents to target tissues in certain situations. AMI is one indication in which cells seem to be more efficacious than exosomes, at least when both products are administered via the intracoronary route80, as cells lodge within the microvasculature and function as sustained-release vehicles for exosomes, whereas administered exosomes pass quickly through the coronary vessels into the rest of the body, diluting their efficacy.
Another instance in which cells trump exosomes arises when cells actually engraft meaningfully within the tissue and work canonically. Pluripotent-cell-derived cardiomyocytes or cardiomyocyte precursors have been shown to be capable of long-term engraftment81, although the efficiency of the process may be forbiddingly low. Engraftment and differentiation of transplanted cells also exacerbate the risk of arrhythmia, a potentially lethal complication28,29. Disease targets in which pluripotent-cell-derived cardiomyocytes may be preferable include structural defects in the heart associated with localized deficiency of muscle tissue, such as ventricular septal defects or ventricular aneurysms. Here, cell patches grown ex vivo and implanted surgically or percutaneously may find focused application. Such patches may also function adequately without requiring full electromechanical integration with surrounding heart tissue, conceivably minimizing the risk of arrhythmia. From an electrophysiological perspective, the most hazardous applications of pluripotent-cell-derived cardiomyocytes are those involving intramyocardial delivery of dispersed cells into regions of infarcted tissue, where risk may well outweigh benefit57.
From cells to exosomes to soluble factors
Going forward, it is important to identify specific situations in which cell therapy may be preferable to therapy using downstream mediators; otherwise, cell therapy is arguably an interim technology. Once it became clear that some effects of cell therapies are indirect, the goal logically shifted to understanding and optimizing the mediators, so they themselves could be used as therapeutic candidates. As reviewed previously78, exosomes offer the potential to overcome key limitations of cell therapy. Potential advantages over cells include product stability82, even with repeated freeze– thaw cycles83, and immune tolerance (human CDCexo are effective in non-immunosuppressed mice84, rats77 and pigs80, even following repeated administration84). Exosomes are efficacious after systemic delivery because they are relatively non-immunogenic84–86, and their dosing is not limited by microvascular plugging or by loss of transplanted cell viability77. Moreover, there are multiple approaches to enhancing exosome efficacy, including genetic engineering of the parent cells87. Commercial product development for exosomes is, however, still in its infancy, so expectations for the pace of progress should be cautious.
When the exploration of cell therapy applications for heart disease began, there was no inkling that exosomes were involved. Now that their role is clear, the focus has moved to pinpointing the factors within exosomes that are vital to conferring efficacy in any given setting. In AMI, for example, certain miRs (miR-146a, ref. 72; and miR-181b, ref. 88) and a Y RNA fragment77 have been implicated as exosomal contents that are central for cardioprotection (Fig. 7). One implication of these findings is clear: the defined factors themselves, individually or in combination, can become new therapeutic candidates. Another implication of these observations is the potential to use mechanistic insights to feed-back on the development process of cell therapies towards their improvement. For instance, mechanistically based potency assays could be developed around the quantification of the identified factors in exosomes secreted by a cell therapy candidate. Thus, mechanistic insight, instead of simply leading us away from cells towards exosomes and defined factors, should lead to improvements in the quality, reproducibility and efficacy of parent-cell-based therapeutic approaches. Nevertheless, major gaps remain regarding the comprehension of mechanisms, and additional fundamental research is necessary to refine and inform the development of rational and robust potency assays for cell therapy.
Fig. 7 |. Defined exosome contents implicated in cdc-mediated cardioprotection.
Left: biogenesis and secretion of exosomes. Right: exosome contents. lncRNA, long non-coding RNA; miRNA, microRNA; IL-10, interleukin 10; PKCδ, protein kinase C isoform; TRAF-6, tumour necrosis factor receptor- associated factor 6. Figure adapted from ref. 78, Elsevier.
Outlook
The mechanistic deconstruction of cell therapy has led the way from autologous cell therapy, which was based on canonical doctrine, to exosomes and their contents as next-generation therapies. This dramatic evolution has played out over less than a decade, with CDCs offering a case study of the key mechanistic discoveries: (1) in 2004, the isolation of CDCs from the human heart, for use in autologous therapy; (2) in 2014, the recognition of durable therapeutic benefits despite cell transience — this led to the allogeneic paradigm in 2012; (3) in 2014, the identification of exosomes as mediators, which has implications for cell-free therapeutics; and (4) since 2014, the mining of exosome contents has helped identify defined factors that may usher in next-generation therapeutics. The identification of exosomes as mediators resulted from the recognition of the equivalent benefits of autologous cell sources and allogeneic cell sources. The mining of exosome contents, in turn, pinpointed certain ncRNA species as defined factors worthy of attention in their own right.
Despite the progression away from cells towards defined factors, cell-based therapies retain their potential for application in defined indications. For adult cell therapy applications, enhancing and capitalizing on the indirect effects of cells will be central to maximizing their efficacy. For pluripotent-derived cells, the emphasis should logically be on improving engraftment and maximizing the adult phenotype to mitigate arrhythmic risk in applications to treat myocardial infarction. Beyond their roles as candidates for novel therapeutic approaches, cells have already begun to pay off as discovery platforms; in the process of investigating mechanisms, the potential value of exosomes and their contents has come into focus. Recognition of the critical bioactive secreted factors in any given setting provides a mechanistic rationale for the development of new potency assays. Such assays, in turn, have the potential to improve cell therapy by providing reliable beacons of disease-modifying bioactivity, as checks and balances against the product drift that can be introduced by commercially optimized manufacturing processes and quality-control efforts.
Acknowledgements
This work is supported by grants from the National Institutes of Health, the California Institute for Regenerative Medicine, the United States Department of Defense, and Coalition Duchenne. I thank A. Ibrahim for creating a first draft of Fig. 6a, and L. Marbán for a critical reading of the manuscript and for helpful suggestions.
Footnotes
Competing interests
E.M. holds founder’s equity in, and serves as unpaid scientific advisor to, Capricor Inc.
References
- 1.Roth GA et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol 70, 1–25 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eschenhagen T et al. Cardiomyocyte regeneration: a consensus statement. Circulation 136, 680–686 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinescu KK, Uriel N, Mann DL & Burkhoff D Left ventricular assist device-induced reverse remodeling: it’s not just about myocardial recovery. Expert Rev. Med. Devices 14, 15–26 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menasche P et al. Autologous skeletal myoblast transplantation for cardiac insufficiency. First Clin. Case. Arch. Mal. Coeur. Vaiss 94, 180–182 (2001). [PubMed] [Google Scholar]
- 5.Voronov RA Experimental study of the regenerative potentialities of the cardiac and somatic musculatures. Arkh. Anat. Gistol. Embriol 69, 35–40 (1975). [PubMed] [Google Scholar]
- 6.Koh GY, Klug MG, Soonpaa MH & Field LJ Differentiation and long-term survival of C2C12 myoblast grafts in heart. J. Clin. Invest 92, 1548–1554 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor DA et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat. Med 4, 929–933 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Menasche P et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation 117, 1189–1200 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Orlic D et al. Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Chien KR Stem cells: lost in translation. Nature 428, 607–608 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Strauer BE et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 106, 1913–1918 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Fisher SA, Doree C, Mathur A, Taggart DP & Martin-Rendon E Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst. Rev 12, CD007888 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathur A et al. The consensus of the Task Force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for the treatment of acute myocardial infarction and heart failure: update 2016. Eur. Heart J 38, 2930–2935 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeevanantham V, Afzal MR, Zuba-Surma EK & Dawn B Clinical trials of cardiac repair with adult bone marrow-derived cells. Methods Mol. Biol 1036, 179–205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowbar AN et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ 348, g2688 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang LT et al. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J. Biomed. Sci 23, 76 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behfar A et al. Guided stem cell cardiopoiesis: discovery and translation. J. Mol. Cell. Cardiol 45, 523–529 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Psaltis PJ et al. Enrichment for STRO-1 expression enhances the cardiovascular paracrine activity of human bone marrow-derived mesenchymal cell populations. J. Cell. Physiol 223, 530–540 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Golpanian S, Wolf A, Hatzistergos KE & Hare JM Rebuilding the damaged heart: mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiol. Rev 96, 1127–1168 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartunek J et al. Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial design. Eur. J. Heart Fail 18, 160–168 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perin EC et al. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ. Res 117, 576–584 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Povsic TJ et al. The RENEW trial: efficacy and safety of intramyocardial autologous CD34+ cell administration in patients with refractory angina. JACC Cardiovasc. Interv 9, 1576–1585 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Beltrami AP et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Bolli R et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.van Berlo JH et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Lancet Editors. Expression of concern: the SCIPIO trial. Lancet 383, 1279 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson JA et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Chong JJ et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiba Y et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Menasche P et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol 71, 429–438 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Menasche P The future of stem cells: should we keep the “stem” and skip the “cells”? J. Thorac. Cardiovasc. Surg 152, 345–349 (2016). [DOI] [PubMed] [Google Scholar]
- 32.The Editor. A futile cycle in cell therapy. Nat. Biotechnol 35, 291 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Bolli R Repeated cell therapy: a paradigm shift whose time has come. Circ. Res 120, 1072–1074 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tompkins BA, Natsumeda M, Balkan W & Hare JM What is the future of cell-based therapy for acute myocardial infarction. Circ. Res 120, 252–255 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quyyumi AA et al. PreSERVE-AMI: a randomized, double-blind, placebo-controlled clinical trial of intracoronary administration of autologous CD34+ cells in patients with left ventricular dysfunction post STEMI. Circ. Res 120, 324–331 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zwetsloot PP et al. Cardiac stem cell treatment in myocardial infarction: a systematic review and meta-analysis of preclinical studies. Circ. Res 118, 1223–1232 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Dodson BP & Levine AD Challenges in the translation and commercialization of cell therapies. BMC Biotechnol. 15, 70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell A et al. Concise review: process development considerations for cell therapy. Stem Cells Transl. Med 4, 1155–1163 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karnieli O Cell therapy: early process development and optimization of the manufacturing process are critical to ensure viability of the product, quality, consistency and cost efficiency. J. Commer. Biotechnol 10.5912/jcb695 (2015). [DOI] [Google Scholar]
- 40.Szymczak MM, Friedman RL, Uppoor R & Yacobi A Detection, measurement, and control in pharma manufacturing. Pharm. Technol 35, 70–76 (2011). [Google Scholar]
- 41.Bravery CA et al. Potency assay development for cellular therapy products: an ISCT review of the requirements and experiences in the industry. Cytotherapy 15, 9–19 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Johnston N, Schenck-Gustafsson K & Lagerqvist B Are we using cardiovascular medications and coronary angiography appropriately in men and women with chest pain? Eur. Heart J 32, 1331–1336 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Cheng K et al. Brief report: mechanism of extravasation of infused stem cells. Stem Cells 30, 2835–2842 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki G et al. Global intracoronary infusion of allogeneic cardiosphere-derived cells improves ventricular function and stimulates endogenous myocyte regeneration throughout the heart in swine with hibernating myocardium. PLoS ONE 9, e113009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tseliou E et al. Widespread myocardial delivery of heart-derived stem cells by nonocclusive triple-vessel intracoronary infusion in porcine ischemic cardiomyopathy: superior attenuation of adverse remodeling documented by magnetic resonance imaging and histology. PLoS ONE 11, e0144523 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golpanian S et al. Concise review: review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl. Med 5, 186–191 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Losordo DW et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ. Res 109, 428–436 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh H Cell therapy trials in congenital heart disease. Circ. Res 120, 1353–1366 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Takehara N et al. The ALCADIA (Autologous Human Cardiac-Derived Stem Cell to Treat Ischemic Cardiomyopathy) trial. Circulation 126, 2776–2799 (2012). [Google Scholar]
- 50.Fox IJ et al. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science 345, 1247391 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amariglio N et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 6, e1000029 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berkowitz AL et al. Glioproliferative lesion of the spinal cord as a complication of “stem-cell tourism”. N. Engl. J. Med 375, 196–198 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Thirabanjasak D, Tantiwongse K & Thorner PS Angiomyeloproliferative lesions following autologous stem cell therapy. J. Am. Soc. Nephrol 21, 1218–1222 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malliaras K et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation 125, 100–112 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schu S et al. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med 16, 2094–2103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Daccak R & Charron D Allogenic benefit in stem cell therapy: cardiac repair and regeneration. Tissue Antigens 86, 155–162 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Smith RR, Barile L, Messina E & Marban E Stem cells in the heart: what’s the buzz all about? Part 2: arrhythmic risks and clinical studies. Heart Rhythm. 5, 880–887 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyagawa S et al. Phase I clinical trial of autologous stem cell–sheet transplantation therapy for treating cardiomyopathy. J. Am. Heart Assoc 6, e003918 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith RR et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115, 896–908 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Sanz-Ruiz R & Fernández-Avilés F Autologous and allogeneic cardiac stem cell therapy for cardiovascular diseases. Pharmacol. Res 127, 92–100 (2018). [DOI] [PubMed] [Google Scholar]
- 61.White AJ et al. Intrinsic cardiac origin of human cardiosphere-derived cells. Eur. Heart J 34, 68–75 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Davis DR et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS ONE 4, e7195 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Makkar RR et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishigami S et al. Intracoronary cardiac progenitor cells in single ventricle physiology: the PERSEUS (cardiac progenitor cell infusion to treat univentricular heart disease) randomized phase 2 trial. Circ. Res 120, 1162–1173 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Tarui S et al. Transcoronary infusion of cardiac progenitor cells in hypoplastic left heart syndrome: three-year follow-up of the Transcoronary Infusion of Cardiac Progenitor Cells in Patients with Single-Ventricle Physiology (TICAP) trial. J. Thorac. Cardiovasc. Surg 150, 1198–1207 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Chimenti I et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res 106, 971–980 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malliaras K et al. Stimulation of endogenous cardioblasts by exogenous cell therapy after myocardial infarction. EMBO Mol. Med 6, 760–777 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanz-Ruiz R & Fernandez-Aviles F Autologous and allogeneic cardiac stem cell therapy for cardiovascular diseases. Pharmacol. Res 127, 92–100 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Malliaras K et al. Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation 128, 2764–2775 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reich H et al. Repeated transplantation of allogeneic cardiosphere-derived cells boosts therapeutic benefits without immune sensitization in a rat model of myocardial infarction. J. Heart Lung Transplant 35, 1348–1357 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Chakravarty T et al. TCT-820 multivessel intracoronary infusion of allogeneic cardiosphere derived cells in dilated cardiomyopathy: long term outcomes of the Dilated Cardiomyopathy Intervention with Allogeneic Myocardially-Regenerative Cells (DYNAMIC trial). J. Am. Coll. Cardiol 68, B332 (2016). [Google Scholar]
- 72.Ibrahim AG, Cheng K & Marban E Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2, 606–619 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ibrahim A & Marban E Exosomes: fundamental biology and roles in cardiovascular physiology. Annu. Rev. Physiol 78, 67–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int. J. Cardiol 192, 61–69 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kervadec A et al. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J. Heart Lung Transplant 35, 795–807 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Khan M et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res 117, 52–64 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cambier L et al. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol. Med 9, 337–352 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marban E The secret life of exosomes: what bees can teach us about next-generation therapeutics. J. Am. Coll. Cardiol 71, 193–200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aminzadeh MA et al. Exosome-mediated benefits of cell therapy in mouse and human models of Duchenne muscular dystrophy. Stem Cell Rep. 10, 942–955 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gallet R et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J> 38, 201–211 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Funakoshi S et al. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep 6, 19111 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akers JC et al. Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer Biomark. 17, 125–132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bosch S et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep 6, 36162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aminzadeh MA et al. Reversal of cardiac and skeletal manifestations of Duchenne muscular dystrophy by cardiosphere-derived cells and their exosomes in mdx dystrophic mice and in human Duchenne cardiomyocytes. Preprint at https://www.biorxiv.org/content/early/2017/04/20/128900 (2017).
- 85.Chen KH et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 7, 74537–74556 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vandergriff AC et al. Intravenous cardiac stem cell-derived exosomes ameliorate cardiac dysfunction in doxorubicin induced dilated cardiomyopathy. Stem Cells Int. 2015, 960926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Conlan RS, Pisano S, Oliveira MI, Ferrari M & Mendes Pinto I Exosomes as reconfigurable therapeutic systems. Trends Mol. Med 23, 636–650 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Couto G et al. Exosomal MicroRNA transfer into macrophages mediates cellular postconditioning. Circulation 136, 200–214 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aminzadeh MA et al. Therapeutic efficacy of cardiosphere-derived cells in a transgenic mouse model of non-ischaemic dilated cardiomyopathy. Eur. Heart J 36, 751–762 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tseliou E et al. Cardiospheres reverse adverse remodeling in chronic rat myocardial infarction: roles of soluble endoglin and TGF-β signaling. Basic Res. Cardiol 109, 443 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Lauden L et al. Allogenicity of human cardiac stem/progenitor cells orchestrated by programmed death ligand 1. Circ. Res 112, 451–464 (2013). [DOI] [PubMed] [Google Scholar]
- 92.Marban E Breakthroughs in cell therapy for heart disease: focus on cardiosphere-derived cells. Mayo Clin. Proc 89, 850–858 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]