Abstract
An urgent need to deliver therapeutics across the blood-brain barrier (BBB) underlies a paucity of effective therapies currently available for treatment of degenerative, infectious, traumatic, chemical and metabolic disorders of the nervous system. With an eye towards achieving this goal, an in vitro BBB model was employed to simulate biodegradable polyanhydride nanoparticle-based drug delivery to the brain. Using a combination of confocal microscopy, flow cytometry, and high performance liquid chromatography, we examined the potential of polyanhydride nanoparticles containing the anti-oxidant, mito-apocynin, to be internalized and then transferred from monocytes to human brain microvascular endothelial cells. The efficacy of this nanoparticle-based delivery platform was demonstrated by neuronal protection against oxidative stress. Taken together, this polyanhydride nanoparticle-based delivery system holds promise for enhancing neuroprotection by facilitating drug transport across the BBB.
Keywords: nanoparticles, blood-brain barrier, cell-mediated transcytosis, polyanhydrides, mito-apocynin
INTRODUCTION
Age-associated neurodegenerative diseases such as Alzheimer’s and Parkinson’s Disease (AD and PD) and stroke are a current and future world-wide health problem.1 The economic burden associated with medical management, interpersonal and family needs and loss of productivity will all increase in the years ahead.2 This makes the development of effective therapies that slow or halt nervous system disease progression an immediate need. Regrettably, currently available drugs only manage disease symptoms and do not slow disease progression.
One target to combat disease resides in affecting mitochondrial function. Indeed, mitochondrial dysfunction heralds neurodegeneration, leading to accumulation of reactive oxygen and nitrogen species and cell injuries and loss due to oxidative stress.3 While there has been some success in the development of antioxidant drugs their efficacy is limited by deficits in drug metabolism, bioavailability and pharmacodynamics. These reduce the therapeutic index of the drug, leading to unabated cytotoxicity. One example of a compound that could be effective if facilitated delivery was realized is mito-apocynin (mAPO). Indeed, while mAPO can reduce oxidative stress and affect neuronal injuries in mouse models of degenerative and infectious diseases of the nervous system4, 5, it has known action limitations based on site delivery.6 A notable limiting factor that reduces drug efficacy for neuroprotection resides in its poor penetration across the blood-brain barrier (BBB).7
To this end, a spectrum of classes of polymeric nanoparticles (NP) have been developed with the potential to enhance drug delivery across the BBB.7–10 Among these are biodegradable polyanhydride NPs being studied to improve drug and vaccine delivery to areas of neural injury. Such NPs can provide sustained delivery of anesthetics, chemotherapeutics, insulin, analgesics, antibiotics, vaccine antigens, antibodies and proteins.11–21 Polyanhydride NPs possess excellent biocompatibility22–24 and their surface erosion mechanism facilitates their utilization as drug and vaccine carriers.25 Recent work performed in our own laboratories has shown that mAPO encapsulated in polyanhydride NPs can be effectively internalized by neurons and provide protection against hydrogen peroxide (H2O2)-associated oxidative stress.26 However, the specific abilities of polyanhydride NPs to efficiently cross the BBB is not yet known.
In this current report, we investigated the uptake of polyanhydride NPs by primary human brain microvascular endothelial cells (HBMEC), the major BBB constituent, primary human monocytes, and a rat mesencephalic N27 neuronal cell line. The effects of monocyte-endothelial cell interactions on NP uptake and cell-to-cell transfer were investigated and the data showed robust monocyte-facilitated uptake in HBMEC. mAPO-containing NPs were internalized by a neuronal cell line, and this correlated with enhanced protection against oxidative stress. These findings support our prior observations performed in primary cortical neurons and neurons differentiated from human mesencephalic cells.26 These data provide compelling evidence for the future development of polyanhydride NP-based nanomedicines for treatment of neurodegenerative disorders.
MATERIALS AND METHODS
Polyanhydride synthesis
Synthesis of sebacic anhydride (SA) and 1,6-bis(p-carboxyphenoxy)hexane (CPH) pre-polymers and copolymers was performed as previously described.27, 28 The resulting copolymer of 20 mol percent CPH and 80 mol percent SA (i.e., 20:80 CPH:SA) was characterized using 1H nuclear magnetic resonance spectroscopy (VXR-300, Varian, Palo Alto, CA) to verify copolymer composition and molecular weight. All properties of the synthesized copolymers were within expected ranges.27, 28
Mito-apocynin synthesis
Mito-apocynin (mAPO) was kindly provided by the Kalyanaraman laboratory at the Medical College of Wisconsin. Briefly, acetylvanillic acid chloride was synthesized from first mixing acetylvanillic acid with thionyl chloride, followed by dissolution of the mixture in methylene chloride (MeCl2) and aminoethyltriphenylphosphonium bromide and pyridine.29, 30 The acetylated mAPO was purified on a silica gel column followed by removal of the acetyl protective group. The final product was purified and characterized by high performance liquid chromatography (HPLC) and liquid chromatography mass spectrometry (LC-MS).30
CdSe-ZnS core-shell quantum dot (QD) synthesis
Cadmium selenide QDs were synthesized following a standard air-free hot-injection reaction procedure modified from Pu et al.31 and ZnS shell growth was achieved using a procedure modified from Talapin et al.32 Briefly, cadmium oxide (0.25 mmol, 32 mg) was dissolved in 2 mL of 1-octadecene (ODE) and 218 μL oleic acid (OA) in a 4 mL glass vial. A 0.4 M stock solution of selenium in ODE was prepared by sonicating 384 mg of selenium in 12.190 mL ODE using bath sonication. A Zn:S precursor solution was prepared by dissolving zinc chloride (0.19 mmol, 26 mg) and 51.43 μL of bis(trimethyldisilyl) sulfide in 1.436 mL of trioctylphosphine. To synthesize CdSe QDs, the solution of CdO in ODE and OA was heated to 240°C on an aluminum reaction block in a N2-filled glovebox. 310 μL of Se in ODE was injected into the reaction vial and the vial was removed from heat. To synthesize CdSe-ZnS core-shell QDs, the crude solution of CdSe was cooled slightly to 220°C and the aforementioned Zn:S precursor solution was added dropwise to the CdSe solution under vigorous stirring. The resultant QDs (λem = 550 nm) were washed by precipitating the QDs from the reaction solution with methanol and twice more with ethanol. Finally, the QDs were dispersed in methylene chloride at an appropriate concentration.
Polyanhydride NP synthesis
Rhodamine B (Sigma, St. Louis, MO) or mAPO or QDs were incorporated into the core of 20:80 CPH:SA NPs by anti-solvent nano-encapsulation.33 Briefly, the synthesized polymer (100 mg) and rhodamine B (5 mg), mAPO (0.2 mg), or QDs (1 mg) (5%, 0.2%, and 1% agent loading, respectively) were dispersed into 4–5 mL of MeCl2 and sonicated for 30–60s with a probe sonicator (Sonics and Materials, Newtown, CT). The solution was poured into 1 L pentane (Fisher Scientific), and the NPs were immediately recovered by vacuum filtration. The NP morphology and primary NP size were determined using scanning electron microscopy (SEM; Quanta 250 FE-SEM, FEI, Hillsboro, OR). ImageJ 1.43u software (National Institutes of Health, Bethesda, MD) was utilized to quantify primary NP sizes and size distribution.
Human monocyte isolation and culture
Monocytes were obtained from HIV-1, HIV-2 and hepatitis B seronegative donor leukopaks, separated by countercurrent centrifugal elutriation, and characterized as previously described.34, 35 Freshly elutriated monocytes were re-suspended in Dulbecco’s Modified Eagles Media containing 2 mM L-glutamine (Invitrogen, Carlsbad, CA), 10% heat-inactivated human serum, 100 μg/mL gentamicin, and 10 μg/mL ciprofloxacin. All reagents were prescreened for endotoxin (<10 pg/mL, Associates of Cape Cod, Woods Hole, MA) and mycoplasma contamination (Gen-probe II, Gen-probe, San Diego, CA).
HBMEC and neuronal cell line cultures
Primary HBMEC were isolated from brain tissue obtained during surgical removal of epileptogenic cerebral cortex in adult patients as we previously described.35–38 Routine evaluation by immunostaining for von-Willebrand factor, Ulex europaeus lectin and CD31 (all antibodies were from Abcam, Cambridge, MA) demonstrated that cells were >99% pure. Freshly isolated cells were cultured on collagen-coated culture plates as we previously described and cells at passage 2 to 4 were used in this study.35, 36, 38
A rat mesencephalic neuronal cell line (N27) was used for cytotoxicity, efficacy, and intracellular localization studies. Cells were thawed from liquid nitrogen and immediately added to 10% Roswell Park Memorial Institute (RPMI) media: RPMI-1640 containing 10% fetal bovine serum (FBS), 2 mM L-glutamine, 50 IU/mL penicillin, and 50 μg/mL streptomycin. Cells were washed by centrifugation for 5 min at 1,000 rcf; cell pellets were re-suspended in 15 mL of 10% RPMI and plated onto a T75 flask. Culture media was changed after overnight (~16 h) incubation, and subsequently changed every 2 days until passaging. Upon reaching 70% confluence, N27 cells were passaged by trypsinization using 0.25% trypsin/ 0.038% ethylenediaminetetraacetic acid (TE) in culture media. Trypsinized cells were resuspended in culture media, counted using a Vi-Cell XR instrument (Beckman Coulter, Brea, CA), and plated in T-75 flasks (one million cells per flask). For cultures in 96-, 24-, or 6-well plates, cells were plated at approximately 5,000 cells/well, 20,000 cells/well, or 80,000 cells/well, respectively. After 24 h, media was aspirated and the appropriate treatment was added to cells in 2% FBS-containing complete RPMI (2% RPMI).
Cytotoxicity assays
Monocytes were seeded at a density of 6.25 × 105 cells/mL and HBMEC cultured to confluence. For all cell experiments, NPs were suspended in one mL of working media, and sonicated for 30 s. In case of poor suspension quality (i.e., if NPs displayed aggregation), 0.1% PVA was added as a surfactant. This step was repeated up to 0.4% PVA, if necessary. To determine any potential toxic effects of NPs on cells, monocytes and HBMEC were treated with NPs at concentrations of 1 to 500 μg/mL for 48 h at 37°C and 5% CO2. Following loading of each NP formulation, cells were washed with serum-free culture media to remove excess drugs and cytotoxicity was assessed over 24 h using alamarBlue™ assay (Life Technologies).39, 40 Each experimental condition was evaluated in triplicate.
To test NP neurotoxicity, N27 cells were grown to 70% confluence in 96-well plates in 10% RPMI. NP stock suspensions were sonicated in 2% RPMI for 60 s using a bath sonicator. Old media on cells was aspirated and replaced by 2% RPMI containing NPs. After 24 h, 10 μL MTS dye was added to each well; cells in dye-containing medium were then incubated for 1 h at 37 °C in a CO2 incubator, and optical densities (OD) quantified by spectrophotometry using SpectraMax 190 (Molecular Devices, Sunnyvale, CA); with OD readings at 490 nm, and background corrections at 670 nm. Each experimental condition was evaluated in triplicate.
Endothelial cell-monocyte NP agent transfers
Primary HBMEC were cultured to confluence as previously described.41 For endothelial cell-monocyte communication, freshly elutriated human monocytes were loaded with 250 μg/mL 5% rhodamine-encapsulated NPs (Rho:NPs) for 48 h. Following NP loading, monocytes were washed three times with PBS to remove any free NPs. Monocytes were then co-cultured with endothelial cells for 2 h and HBMEC monolayers were washed 5 times with PBS to remove residual monocytes.
Immunofluorescence and confocal microscopy
For HBMEC, confluent cells cultured on glass coverslips were fluorescently labeled using the Vybrant 1,1 ´dioctadecyl- 3,3,3 ´,3 ´-tetramethylindodicarbocyanine perchlorate (DiO) cell-labeling solution (excitation 484 nm; emission 501 nm) as we previously described.39 DiO-labeled HBMEC were co-cultured for 2 h with Rho:NP-loaded monocytes. Following endothelial cell-monocyte co-culture, HBMEC monolayers were washed 5 times with PBS to remove monocytes, fixed, mounted in Prolong Gold antifade reagent containing DAPI (for nuclear staining) (Life Technologies, Grand Island, NY) and analyzed by fluorescence or confocal microscopy as we previously described.39 To determine the localization of NPs in endothelial cells, the triple labeled cell samples were examined under a Zeiss LSM 710 confocal laser scanning microscope using Zeiss Zen software.
For confocal microscopy of the N27 cells, the cells were grown to 70% confluence in 24-well plates on poly(D-lysine)-coated glass coverslips in 10% RPMI. NP stock suspensions were sonicated in 2% RPMI for 60 s using a bath sonicator. Cells were treated with 30 μg/mL 1% QD-loaded NPs (QD:NPs) in 2% RPMI for 24 h. Media was then aspirated and cells were washed in pre-warmed (37°C) (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) buffered saline solution (HBSS). HBSS was aspirated and the cells were incubated with HBSS containing 1/7,000 MitoTracker® Red, and 1/5,000 Hoechst dyes for 8 min. HBSS was aspirated and the cells were then washed twice with room temperature PBS and fixed by incubation with room temperature 4% paraformaldehyde (PFA) for 15 min. The coverslips were mounted onto SuperFrost® Slides (Sigma) for confocal microscopy (Leica SP5 X MP confocal/multiphoton microscope). Each experimental condition was evaluated in triplicate.
Fluorescence-activated cell sorting (FACS)
For FACS quantification of NP uptake by monocytes or HBMEC, the cells were exposed to 250 μg/mL 5% Rho:NPs for 48 h, and washed five times with PBS to remove free NPs. Monocytes and HBMEC were then fixed by incubation in 1% PFA for 20 min, washed, resuspended in PBS and analyzed by FACS, using a FACScan flow cytometer (BD Bioscience, San Jose, CA). The mean fluorescence channel – mean number of rhodamine positive cells was derived using CellQuest software (BD Bioscience). To determine the levels of NPs in monocytes and HBMEC following monocyte-endothelial cell communication, monocytes loaded with 250 μg/mL Rho:NPs were co-cultured for 2 h with unloaded HBMEC, then washed 5 times to separate monocytes from endothelial cells. HBMEC and monocytes recovered from co-cultures were then fixed by incubation in 1% PFA for 20 min, washed, resuspended in PBS and analyzed by FACS. Unloaded monocytes were used for gating when analyzing monocytes samples, while unloaded HBMEC were used for gating when analyzing HBMEC from direct loading or co-cultured experiments. The mean fluorescence channels – mean number of rhodamine positive cells was derived using CellQuest software (BD Bioscience). Each experimental condition was performed in duplicate.
For FACS quantification of NP uptake by neuronal cells, N27 cells were seeded onto a 6-well plate.26 After 24 h, cells were treated with 30 μg/mL of 1% QD:NPs in 2% RPMI for 24 h. The media was removed and the cells were washed once with PBS (Invitrogen) to remove excess QD:NPs. Cells were fixed in 4% PFA and stored at 4 °C overnight before reading. Internalization of QD:NPs by N27 cells was quantified via flow cytometry using a 633 nm laser (FACSCanto, BD Biosciences, San Jose, CA). Each experimental condition was performed in triplicate. Additionally, to account for QD release due to NP degradation which would result in cells “false positive” for NPs in the N27 cells, a released QD control (background) was subtracted from each treatment group as described previously.42 QD:NPs were allowed to incubate in 2% RPMI for 12 h, then centrifuged at 15,000 rcf for 10 min. Cell supernatants containing the released QDs were added to N27 cells for 24 h to account for any fluorescence caused by the uptake of released QDs as opposed to internalization of QD:NPs. Unloaded N27 cells were used as gating controls. Each experimental condition was evaluated in triplicate.
High Performance Liquid Chromatography (HPLC)
Freshly elutriated human monocytes and HBMEC were loaded with either 250 μg/mL of 0.2% mAPO-encapsulated NPs (MA:NP) (= 0.5 μg/mL mAPO), or 125 μg/mL of free mAPO for 48 h as described above, were washed five times with PBS to remove free NPs and harvested and pelleted by centrifugation. For monocyte-endothelial cell co-cultures, freshly elutriated human monocytes were loaded with either 0.2% mAPO-encapsulated 250 μg/mL of MA:NP, or 125 μg/mL of free mAPO for 48 h, washed five times with PBS to remove free NPs, and co-cultured with HBMEC for 2 h. Following co-cultures, monocytes and HBMEC were harvested separately and pelleted by centrifugation. Controls consisted of unloaded cells, with and without co-cultures. Controls included untreated HBMEC and HBMEC treated for 2 h with conditioned media from NP-free monocytes. HBMEC were then harvested and pelleted by centrifugation.
For HPLC, each cell pellet was sonicated in 20 μL of 0.5 M sodium hydroxide and incubated for 30 min at 23oC. Each sample was then mixed with 180 μL of methanol, centrifuged at 16,000 rcf for 10 min at 4oC; and 70 μL transferred to columns and analyzed by HPLC (mobile phase: 60% of 0.1% trifluoroacetic acid (TFA) in HPLC H2O, 40% of 0.1% TFA in acetonitrile; flow rate: 1.0 mL/min; wavelength for detection: 262 nm). A standard curve of 0 to 6 μg/mL of mAPO was used for quantitation of NPs in samples.
Cleaved caspase-3 quantification
N27 cells were grown to 70% confluence in 6-well plates in 10% RPMI. NP stock suspensions were sonicated in 2% RPMI for ~60 s using a bath sonicator. Old media was aspirated and cells were treated with either 5 μg/mL free mAPO or 30 μg/mL 0.2% mAPO-encapsulated MA:NPs (= 0.06 μg/mL mAPO) in 2% RPMI. At 18 h of treatment, cells were challenged with 100 μM H2O2. After 6 h of H2O2 challenge, supernatant from each well was centrifuged in individual microcentrifuge tubes at 1,000 rcf for 5 min to collect floating cells. Adherent cells were detached by aspirating media and adding 0.5 mL TE for 2 min. TE was neutralized by adding 1.0 mL 2% RPMI, and cells were centrifuged at 1,000 rcf for 5 min in corresponding tubes. Supernatant was aspirated and cells were rinsed with ice-cold PBS, followed by centrifugation at 1,000 rcf for 5 min. Supernatant was aspirated and 250 μL caspase buffer (50 mM HEPES, 10% sucrose, 0.1% 3-[(3-Cholamidopropyl)-dimethylammonio]-1-propanesulfonate, 1 mM 2,2’,2”,2”’-(Ethane-1,2-diyldinitrilo)tetraacetic acid 10 mM dithiothreitol) was added to each sample, vortexing to lyse cells. Samples were incubated at 37 °C for 20 min on a shaker. Samples were then centrifuged at 13,200 rcf for 5 min before transferring 190 μL supernatant from each sample to a 96 well plate. Ten microliters of caspase-3 substrate was added to each sample; samples were incubated at 37 °C for one hour on a shaker and the fluorescence was quantified using a 96-well plate reader (ex: 380 nm, em: 460 nm). A “NP only” control was used for the MA:NP treatment group to remove auto-fluorescent signal associated with NPs. The experiments were performed in triplicate.
RESULTS
Neurotoxicity of polyanhydride NPs
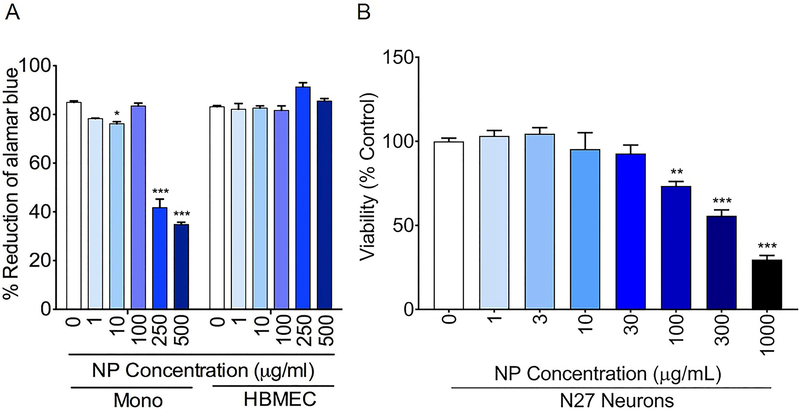
The potential neurotoxicity of NPs was evaluated in human monocytes and HBMEC, with each experimental condition tested in triplicate (Fig. 1A). After 48 h of treatment with NPs, cytotoxicity was monitored for 24 h; NPs were not cytotoxic in monocytes at concentrations between 1 to 100 μg/mL but showed reductions in monocyte viability at concentrations of 250 μg/mL or higher at 24 h. HBMEC were not affected by NPs. Therefore, the HBMEC internalization experiments, both direct and in co-culture with monocytes, were performed at a NP concentration of 250 μg/mL. In N27 cells, NPs were not cytotoxic at concentrations of 30 μg/mL or lower but showed cytotoxicity at concentrations of 100 μg/mL or above (Fig. 1B). Therefore, experiments in these cells were performed at a NP concentration of 30 μg/mL.
Figure 1.
(A) Effect of NPs on the viability of human monocytes and HBMEC. Cells from human donors were isolated and cultured as described in the Methods, loaded with NP (1 to 500 μg/mL) for 48 h and toxicity was assessed over 24 h by the alamarBlue™ assay. Representative toxicity data at 24 h are shown. * p<0.05, *** p<0.001 compared to untreated controls. (B) Effect of NPs on the viability of N27 neurons. After incubation of NPs (1 to 1000 μg/mL) with N27 cells for 24 h, toxicity was assessed for 1 h by the MTS cytotoxicity assay. ** p<0.01, *** p<0.001 compared to untreated controls.
NP transfer from monocytes to HBMEC
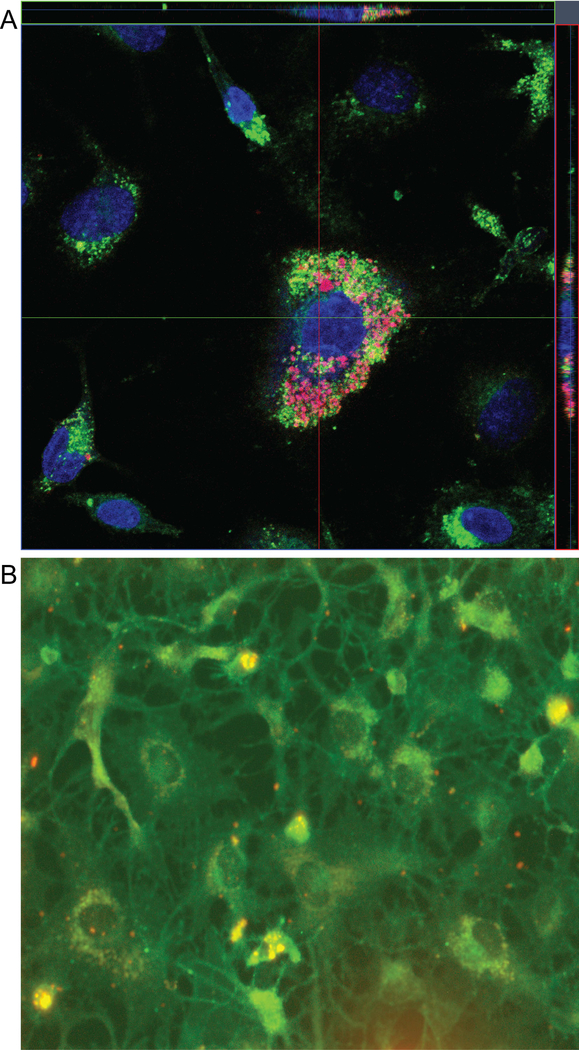
HBMEC, the major component of the BBB, is regularly in direct contact with circulating blood and separates the systemic circulation from brain tissues. We have previously shown uptake of polyanhydride NPs by a human monocyte cell line.33 We previously showed that dendritic cells and macrophages effectively internalized 20:80 CPH:SA NPs, primarily through phagocytosis.43–45 To determine whether circulating monocytes containing NPs can transfer them to cells of the brain endothelium, HBMEC were co-cultured with human monocytes containing 250 μg/mL Rho:NPs and the cell-to-cell transfer of NPs was investigated by confocal microscopy, fluorescence microscopy and flow cytometry.
Following 2 h of co-culture, transfer of NPs from monocytes to HBMEC readily occurred. Confocal microscopic examination showed NPs in and around the HBMEC (Fig. 2A). Furthermore, analyses using XZ or YZ line scan mode of the Zeiss LSM 710 confocal imaging demonstrated cytoplasmic and nuclear localization of NPs in HBMEC (Fig. 2A). Independent validation of NP transfer to HBMEC was performed by fluorescence microscopy. Following 2 h of co-culture of HBMEC with NP-loaded monocytes, NPs entered the endothelial cells (Fig. 2B).
Figure 2.
NPs are efficiently internalized by HBMEC following co-culture with 250 μg/mL 5% Rho:NP-loaded monocytes. (A) Monocytes loaded with Rho:NP were co-cultured with HBMEC labeled with DiO (green), and Rho:NP uptake following HBMEC-monocyte communication was visualized by confocal microscopy. Representative image shows uptake of Rho:NPs by primary HBMEC. (B) Representative immunofluorescence image showing HBMEC uptake of Rho:NP following co-culture with Rho:NP-loaded monocytes.
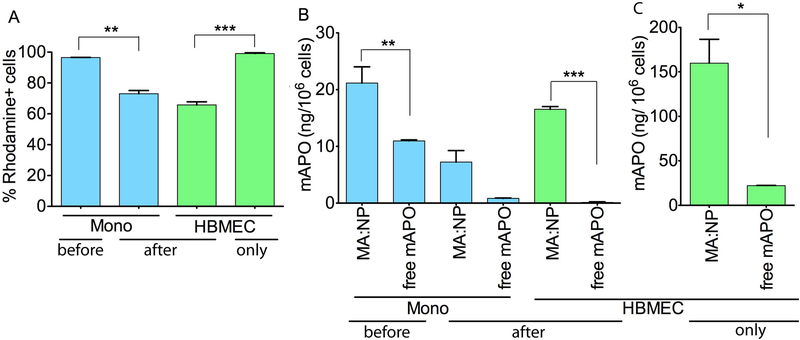
Monocytes containing NP co-cultured with HBMEC also showed NP transfer to HBMEC as measured by FACS. Efficient monocyte uptake of Rho:NPs (Fig. 3A) with up to 96% of monocytes positive for rhodamine after 48 h is shown. Co-culture of Rho:NP-loaded monocytes with HBMEC was associated with transfer of NPs to HBMEC (Fig. 3A). The transfer was associated with decreased levels of NPs in the monocytes (Fig. 3A). Co-culture of monocytes loaded with Rho:NPs with HBMEC decreased the percentage of rhodamine-positive monocytes by 23% (from 96 to 73%) (Fig. 3A, P<0.01). Finally, direct incubation of NPs with endothelial cells showed efficient NP internalization by the endothelial cells. When HBMEC were treated directly with Rho:NPs, 100% of the cells were positive for rhodamine (Fig. 3A).
Figure 3.
(A) FACS quantification of 5% Rho:NP direct uptake by primary human monocytes, cell-to-cell transfer of Rho:NPs following co-culture of HBMEC with Rho:NP-loaded monocytes, and direct uptake by HBMEC. “before”: NP levels in monocytes before monocyte-HBMEC co-culture. “after”: NPs levels in monocytes and HBMEC after monocyte-HBMEC co-culture. “only”: NP levels in HBMEC after direct incubation, i.e., without any prior treatment of monocytes and co-culture. (B) HPLC quantification of 0.2% MA:NPs and free-mAPO direct uptake by primary human monocytes, cell-to-cell transfer of MA:NPs following co-culture of HBMEC with MA:NP-loaded monocytes, and (C) direct uptake by HBMEC. Experiments were performed with 250 μg/mL of 0.2% MA:NPs or 125 μg/mL of free mAPO. Data represents mean ± SEM of three replicates (*p<0.05, **p<0.01, ***p<0.001 compared to free mAPO).
HPLC quantification of mAPO
We used HPLC to quantify mAPO levels in monocytes loaded with 250 μg/mL 0.2% mAPO-encapsulated MA:NP (= 0.5 μg/mL mAPO) before and after cell co-cultivation. Unloaded monocytes and monocytes loaded with 125 μg/mL free mAPO were used as controls. The mAPO levels in MA:NP-loaded monocytes were approximately two-fold higher than mAPO levels in free mAPO-loaded monocytes (Fig. 3B, P<0.01), however this two-fold increase was observed even when the dose of mAPO administered from MA:NPs was 0.4% of that from free mAPO. Co-culture of HBMEC with monocytes loaded with MA:NPs or free mAPO decreased mAPO levels in monocytes by 66 and 92% respectively (Fig. 3B).
Additionally, we used HPLC to quantify mAPO levels in HBMEC directly loaded with 250 μg/mL of 0.2% mAPO-encapsulated MA:NPs (= 0.5 μg/mL mAPO) as well as mAPO levels in HBMEC co-cultured with MA:NP-loaded monocytes. Untreated HBMEC and HBMEC loaded with 125 μg/mL of free mAPO were used as controls. Co-culture of HBMEC with monocytes loaded with MA:NPs resulted in mAPO transfer to HBMEC and higher transfer occurred in co-cultures with MA:NP-loaded monocytes compared to co-cultures with monocytes loaded with free mAPO (Fig. 3B). While mAPO was detected in HBMEC co-cultured with MA:NP-loaded monocytes, no detectable levels of mAPO were observed in HBMEC co-cultured with monocytes loaded with free mAPO (Fig. 3B). The levels of mAPO in 0.2% mAPO-encapsulated MA:NP-loaded HBMEC were more than seven-fold higher than mAPO levels in HBMEC loeaded with free mAPO (Fig. 3C, P<0.05).
Neuroprotection
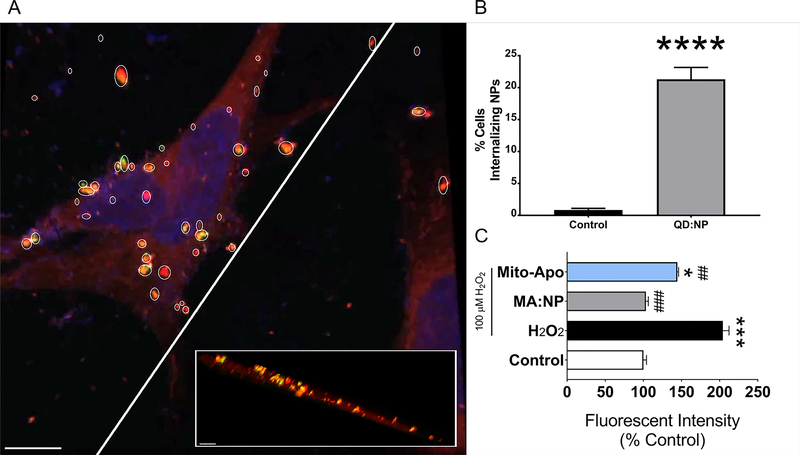
N27 internalization of QD:NPs was evaluated using confocal microscopy and flow cytometry. Confocal microscopy of cells incubated with QD:NPs for 24 h confirmed internalization of both formulations by showing NPs in the plane of the cells in both top-down and cross-sectional views (Fig. 4A). Additionally, flow cytometric analyses indicated that 21% of cells internalized QD:NPs (Fig. 4B). Protection by MA:NP or free mAPO-loaded N27 cells was evaluated after H2O2 challenge. After a 6 h H2O2 challenge, treating the cells with 0.2% mAPO-encapsulated MA:NPs provided optimal protection against oxidative stress when compared to free mAPO (Fig. 4C, P<0.001).
Figure 4.
NP internalization and protection in N27 neurons. (A) Confocal microscopy of 30 μg/mL 1% QD:NPs (yellow)-loaded N27 cells in 2% RPMI for 24 h before staining for mitochondria (red; MitoTracker® red dye) and nucleus (blue; hoechst) and fixing. The cross-sectional perspective is shown by the slice in the main image. Scale bar: 10 μm. Inset scale bar: 5 μm. (B) Flow cytometric analysis of N27 cells. Cells were incubated with 30 μg/mL 1% QD:NPs for 24 h in 2% RPMI. Cells were then collected, washed once with PBS to remove excess NPs and fixed with 4% PFA. Fluorescence was measured using a 633 nm laser, using unloaded cells for gating. **** p<0.0001 with respect to control. (C) Protection of cells treated with 30 μg/mL 0.2% MA:NPs against oxidative stress using a caspase-3 assay. After incubating cells for 18 h with either 30 μg/mL MA:NPs or 5 μg/mL (=10 μM) mAPO, media was replaced with 2% RPMI containing 100 μM H2O2 as well as the same concentration of MA:NPs or free mAPO, and incubated for 6 h. After the 6 h challenge, cells were collected and lysed using a caspase-3 buffer; supernatants from lysate were incubated with caspase-3 substrate for 1 h and fluorescence was quantified (em: 380, ex: 460 nm) using a 96-well plate reader. * p<0.05 with respect to control, *** p<0.001 with respect to control, ## p<0.01 with respect to H2O2, ### p<0.001 with respect to H2O2.
DISCUSSION
There is an immediate need to design delivery vehicles that can cross the BBB and deliver payloads to areas of neuronal injury, infection, and degeneration.46 Biodegradable polyanhydride NPs represent an attractive platform in this regard, based on their high biocompatibility24, 47 and extensive use in drug and vaccine delivery.48–50 Furthermore, carriers based on polyanhydrides (i.e., the Gliadel® wafer) have been approved by the U.S. FDA for use in humans to treat glioblastoma.51 However, the ability of polyanhydride NPs to cross the BBB has not been investigated. In this work, two methods of transport of the NPs across the BBB were investigated: direct NP interaction with the HBMEC and monocyte-mediated transfer to HBMEC. While uptake of NPs in HBMEC does not directly assess the ability to cross the BBB, previous work on nanotherapeutics using this in vitro model showed that enhanced HBMEC uptake correlated with higher in vivo drug concentrations in the brain.39 In addition, internalization of mAPO-containing polyanhydride NPs by a neuronal cell line and protection against oxidative stress was investigated to evaluate the efficacy of the nanomedicine treatment. In the internalization studies, we encapsulated two different types of fluorescent markers, rhodamine and QDs, in order to avoid overlap in emission by the various markers used to study intracellular domains and to demonstrate the capabilities of our polyanhydride NPs to encapsulate different types of imaging agents.
Polyanhydride NPs have previously been shown to be non-toxic to multiple cell types.23, 24, 26 In this work we established that polyanhydride NP concentrations of 250 μg/mL or lower resulted in limited cytotoxicity to primary human monocytes and no cytotoxicity to primary HBMEC, as shown in Fig. 1A. We also showed limited to no neurotoxicity with this formulation in N27 cells at concentrations up to 30 μg/mL, as shown in Fig. 1B. The cytotoxicity data in this work provides a wide administration window with respect to concentration for further studies with polyanhydride NPs for delivery of therapeutic cargo across the BBB and into neuronal cells. Based on these findings, we tested the transfer of 250 μg/mL NPs from monocytes to HBMEC, and internalization of 30 μg/mL NPs into N27 cells.
Previous work with 20:80 CPH:SA polyanhydride NPs demonstrated high levels of NP internalization by a human monocyte cell line.33 The polyanhydride NP formulation used in these studies, 20:80 CPH:SA, showed superior cellular internalization by antigen presenting cells compared to other polyanhydride NP formulations and the mechanism of internalization was shown to be primarily via phagocytosis.43 When the polyanhydride NPs were incubated directly with HBMEC, we observed enhanced internalization of these NPs resulting in dye positive and high drug concentrations, as shown in Fig. 3. Entry of NPs into HBMEC would indicate an increased likelihood of the NPs passing through the BBB. This suggests that polyanhydride NPs are capable of achieving endothelial transcytosis.7 In work with poly(butyl cyanoacrylate) NPs, it was shown that polysorbate surfactant and apolipoproteins on the surface of these NPs enhanced the transport of drug across the BBB.52 In previous work, we observed that apolipoproteins were among the serum proteins that adsorbed to the surface of polyanhydride particles upon incubation with serum.53–55 This would indicate that the NPs with adsorbed serum proteins can readily associate with HBMEC, and suggest that such interactions may enable transport across the BBB via receptor-mediated transcytosis.
Polyanhydride NPs have shown dose sparing in vaccine and drug formulations, leading to lower amounts of antigen/drug providing similar results to much higher amounts of free antigen/drug.23, 56, 57 In this work we observed significantly higher concentrations of mAPO in HBMEC treated with MA:NP compared to free mAPO by both monocyte-mediated and direct internalization mechanisms, as shown in Fig. 3. These higher drug concentrations were observed even with the total mass of mAPO encapsulated in the NPs being 0.4% of the mass of free mAPO administered. This suggests the dose sparing capabilities of polyanhydride NPs may play a significant role in enabling effective drug delivery across the BBB.
In previous studies, mAPO treated transgenic mice showed less motor coordination loss, demonstrating its therapeutic efficacy.4, 5 The current studies show that encapsulation of mAPO in polyanhydride NPs can increase the availability of the mAPO at the BBB interface by both direct NP- and cell-mediated interactions with the HBMEC, as shown in Fig. 3. Other recent work from our laboratories showed enhanced protection of primary mouse neuronal cells with FA-modified mAPO-loaded 20:80 CPH:SA NPs.26
When considering CNS therapeutic delivery vehicles such as these polyanhydride NPs, it is imperative that the vehicle is effective at each stage of delivery, i.e., at the BBB and at the neuronal level.58 Therefore, we also investigated internalization and protection capabilities of this NP formulation in N27 neurons. As shown in Fig. 4, internalization of NPs by N27 neurons led to enhanced protection of mAPO against oxidative stress with the total mass of mAPO encapsulated in the MA-NPs being 1.2% of the mass of free mAPO administered; these results are in agreement with our previous work using both primary cortical neurons and lund human mesencephalic cells differentiated to a dopaminergic neuron-like phenotype, strengthening the promise of this formulation for CNS delivery.26 Collectively, these studies demonstrate that polyanhydride NP-based delivery systems show promise for enhancing the effectiveness of therapeutics that require transport across the BBB and for efficacious drug delivery to neurons.
CONCLUSIONS
This work affirms the excellent biocompatibility and lack of toxicity of polyanhydride NPs with different cell systems. We have shown internalization of polyanhydride NPs by primary human monocytes and efficient transfer of the NPs by monocytes to primary HBMEC in vitro. This work also demonstrated that these NPs could be internalized directly by primary HBMEC. Finally, we observed that not only are these drug NPs internalized by HBMEC, they are also bioavailable compared to free drug alone. In neurons they showed a high level of cellular internalization that correlated with enhanced therapeutic anti-oxidative efficacy when compared to native drug. The results of this work indicate that polyanhydride NPs show promise as a drug delivery platform to enhance the effectiveness of therapeutics that require blood to brain transport.
Table 1.
20:80 CPH:SA nanoparticle characterization. Scale bar: 500 nm.
| Structure of 20:80 CPH:SA copolymer | ||
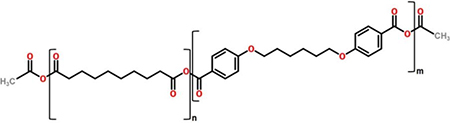 | ||
| SEM photomicrograph | Geometric diameter (nm) | Zeta potential (mV) |
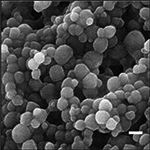 |
410 ± 21 | −21.5 ± 0.7 |
ACKNOWLEDGMENTS
The authors acknowledge financial support from the US Army Medical Research and Materiel Command (Grant No. W81XWH-11–1-0700) and the Iowa State University Nanovaccine Institute. The authors acknowledge financial support from the National Institute of Health grants R01 MH081780, R01 MH094160, P01DA028555, R01 AG043530, P30 MH062261, R01 MH115860, R01 NS034249, and R01 NS036126. R.D.N acknowledges the National Science Foundation Graduate Research Fellowship Program (Grant No. DGE1247194). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. B.N. acknowledges the Vlasta Klima Balloun Faculty Chair. Ms. Sangya Singh’s technical assistance is appreciated.
REFERENCES
- 1.Tofaris GK and Schapira AH, Neurodegenerative diseases in the era of targeted therapeutics: how to handle a tangled issue. Mol Cell Neurosci, 2015. 66(A): p. 1–2 doi: 10.1016/j.mcn.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 2.2017 Alzheimer’s disease facts and figures https://www.alz.org/documents_custom/2017-facts-and-figurespdf 2017. February 27, 2018].
- 3.Johri A and Beal MF, Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther, 2012. 342(3): p. 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh A, Langley MR, Harischandra D, Neal ML, Jin H, Anantharam A, Joseph J, Brenza TM, Narasimhan B, Kanthasamy A, Kalyanaraman B, and Kanthasamy AG, Mitoapocynin treatment protects against neuroinflammation and dopaminergic neurodegeneration in a preclinical animal model of Parkinson’s Disease. J Neuroimmune Pharmacol, 2016. 11(2): p. 259–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langley M, Ghosh A, Charli A, Sarkar S, Ay M, Luo J, Zuelonka J, Brenza TM, Bennett B, Jin H, Ghaisas S, Schlichtmann B, Kim D, Anantharam V, Kanthasamy A, Narasimhan B, Kalyanaraman B, and Kanthasamy AG, Mito-apocynin prevents mitochondrial dysfunction, microglial activation, oxidative damage and progressive neurodegeneration in MitoPark transgenic mice. Antioxidant Redox Signal, 2017. 27(14): p. 1048–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dranka BP, Gifford A, McAllister D, Zielonka J, Joseph J, O’Hara CL, Stucky CL, Kanthasamy AG, and Kalyanaraman B, A novel mitochondrially-targeted apocynin derivative prevents hyposmia and loss of motor function in the leucine-rich repeat kinase 2 (LRRK2R1441G) transgenic mouse model of Parkinson’s disease. Neurosci Lett, 2014. 583: p. 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallapragada SK, Brenza TM, McMillan JM, Narasimhan B, Sakaguchi DS, Sharma AD, Zbarska S, and Gendelman HE, Enabling nanomaterial, nanofabrication and cellular technologies for nanoneuromedicines. Nanomedicine (N. Y., NY, U. S.), 2015. 11: p. 715–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu K, Shi Y, Jiang W, Han J, Huang S, and Jiang X, Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: Preparation, characterization and efficacy in Parkinson’s disease. Int J Pharm, 2011. 415: p. 273–283. [DOI] [PubMed] [Google Scholar]
- 9.Tosi G, Costantino L, Rivasi F, Ruozi B, Leo E, Vergoni AV, Tacchi R, Bertolini A, Vandelli MA, and Fomi F, Targeting the central nervous system: In vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123. J Contr Rel, 2007. 122: p. 1–9. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Tsibouklis J, Weng T, Zhang B, Yin G, Feng G, Cui Y, Savina IN, Mikhalovska LI, Sandeman SR, Howel CA, and Mikhalovsky SV, Nano carriers for drug transport across the blood–brain barrier. Journal of Drug Targeting, 2017. 25(1): p. 17–28. [DOI] [PubMed] [Google Scholar]
- 11.Park E-S, Maniar M, and Shah JC, Biodegradable Polyanhydride Devices of Cefazolin Sodium, Bupivacaine, and Taxol for Local Drug Delivery: Preparation, and Kinetics and Mechanism of In Vitro Release. J. Controlled Release, 1998. 52(1–2): p. 179–189. [DOI] [PubMed] [Google Scholar]
- 12.Storm PB, Moriarity JL, Tyler B, Burger PC, Brem H, and Weingart J, Polymer Delivery of Camptothecin Against 9L Gliosarcoma: Release, Distribution, and Efficacy. J. Neuro-Oncol, 2002. 56: p. 209–217. [DOI] [PubMed] [Google Scholar]
- 13.Masters DB, Berde CB, Dutta S, Turek T, and Langer R, Sustained Local Anesthetic Release from Bioerodible Polymer Matrices: A Potential Method for Prolonged Regional Anesthesia. Pharm. Res, 1993. 10(10): p. 1527–1532. [DOI] [PubMed] [Google Scholar]
- 14.Carino GP, Jacob JS, and Mathiowitz E, Nanosphere based oral insulin delivery. J Contr Rel, 2000. 65(1–2): p. 261–269. [DOI] [PubMed] [Google Scholar]
- 15.Erdmann L and Uhrich KE, Synthesis and degradation characteristics of salicylic acid-derived poly(anhydride-esters). Biomaterials, 2000. 21: p. 1941–1946. [DOI] [PubMed] [Google Scholar]
- 16.Deng J-S, Meisters M, Li L, Setesak J, Claycomb L, Tian Y, Stephens D, and Widman M, The development of an injection-molding process for a polyanhydride implant containing gentamicin sulfate. PDA J Pharm Sci Technol, 2002. 56(2): p. 65–77. [PubMed] [Google Scholar]
- 17.Weiner AA, Bock EA, Gipson ME, and Shastri VP, Photocrosslinked Anhydride Systems for Long-term Protein Release. Biomaterials, 2008. 29(15): p. 2400–2407. [DOI] [PubMed] [Google Scholar]
- 18.Determan AS, Trewyn BG, Lin VSY, Nilsen-Hamilton M, and Narasimhan B, Encapsulation, stabilization, and release of BSA-FITC from polyanhydride microspheres. J Contr Rel, 2004. 100(1): p. 97–109. [DOI] [PubMed] [Google Scholar]
- 19.Torres MP, Determan AS, Anderson GL, Mallapragada SK, and Narasimhan B, Amphiphilic polyanhydrides for protein stabilization and release. Biomaterials, 2007. 28(1): p. 108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrillo-Conde BR, Schiltz E, Yu J, Minion FC, Phillips GJ, Wannemuehler MJ, and Narasimhan B, Encapsulation into amphiphilic polyanhydride microparticles stabilizes Yersinia pestis antigens. Acta Biomater, 2010. 6(8): p. 3110–3119. [DOI] [PubMed] [Google Scholar]
- 21.Carrillo-Conde BR, Darling RJ, Seiler SJ, Ramer-Tait AE, Wannemuehler MJ, and Narasimhan B, Sustained release and stabilization of therapeutic antibodies using amphiphilic polyanhydride nanoparticles. Chem Eng Sci, 2015. 125: p. 98–107. [Google Scholar]
- 22.Adler AF, Petersen LK, Wilson JH, Torres MP, Thorstenson JB, Gardner SW, Mallapragada SK, Wannemuehler MJ, and Narasimhan B, High throughput cell-based screening of biodegradable polyanhydride libraries. Comb Chem High Through Screen, 2009. 12(7): p. 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huntimer LM, Ramer-Tait AE, Petersen LK, Ross KA, Walz KA, Wang C, Hostetter J, Narasimhan B, and Wannemuehler MJ, Evaluation of biocompatibility and administration site reactogenicity of polyanhydride-particle-based platform for vaccine delivery. Adv Healthc Mater, 2013. 2(2): p. 369–78. [DOI] [PubMed] [Google Scholar]
- 24.Vela Ramirez JE, Goodman JT, Boggiatto PM, Roychoudhury R, Pohl NLB, Hostetter JM, Wannemuehler MJ, and Narasimhan B, Safety and Biocompatibility of Carbohydrate-Functionalized Polyanhydride Nanoparticles. The AAPS Journal, 2015. 17(1): p. 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Burkersroda F, Schedl L, and Göpferich A, Why Degradable Polymers Undergo Surface Erosion or Bulk Erosion. Biomaterials, 2002. 23(21): p. 4221–4231. [DOI] [PubMed] [Google Scholar]
- 26.Brenza TM, Ghaisas S, Vela Ramirez JE, Harischandra D, Anantharam V, Kalyanaraman B, Kanthasamy AG, and Narasimhan B, Neuronal protection against oxidative insult by polyanhydride nanoparticle-based mitochondria-targeted antioxidant therapy. Nanomedicine: Nanotechnol Biol Med, 2017. 13(3): p. 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kipper MJ, Shen EE, Determan AS, and Narasimhan B, Design of an Injectable System Based on Bioerodible Polyanhydride Microspheres for Sustained Drug Delivery. Biomaterials, 2002. 23(22): p. 4405–4412. [DOI] [PubMed] [Google Scholar]
- 28.Shen EE, Kipper MJ, Dziadul B, Lim M-K, and Narasimhan B, Mechanistic Relationships between Polymer Microstructure and Drug Release Kinetics in Bioerodible Polyanhydrides. J. Controlled Release, 2002. 82(1): p. 115–125. [DOI] [PubMed] [Google Scholar]
- 29.Kelso GF, Porteous CM, Hughes G, Ledgerwood EC, Gane AM, Smith RAJ, and Murphy MP, Prevention of mitochondrial oxidative damage using targeted antioxidants. Ann New York Acad Sci, 2002. 959(1): p. 263–274. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh A, Chandran K, Kalivendi SV, Joseph J, Antholine WE, Hillard CJ, Kanthasamy A, Kanthasamy A, and Kalyanaraman B, Neuroprotection by a mitochondria-targeted drug in a Parkinson’s disease model. Free Radical Biol Med, 2010. 49: p. 1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pu C, Zhou J, Lai R, Niu Y, Nan W, and Peng X, Highly reactive, flexible yet green Se precursor for metal selenide nanocrystals: Se-octadecene suspension (Se-SUS). Nano Research, 2013. 6: p. 652–670. [Google Scholar]
- 32.Talapin DV, Rogach AL, Kornowski A, Haase M, and Weller H, Highly Luminescent Monodisperse CdSe and CdSe/ZnS Nanocrystals Synthesized in a Hexadecylamine-Trioctylphosphine Oxide-Trioctylphosphine Mixture. Nano Lett, 2001. 1: p. 207–211. [DOI] [PubMed] [Google Scholar]
- 33.Ulery BD, Phanse Y, Sinha A, Wannemuehler MJ, Narasimhan B, and Bellaire BH, Polymer Chemistry Influences Monocytic Uptake of Polyanhydride Nanospheres. Pharm. Res, 2009. 26(3): p. 683–690. [DOI] [PubMed] [Google Scholar]
- 34.Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS, and et al. , Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med, 1988. 167(4): p. 1428–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, and Persidsky Y, HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab, 2007. 27(1): p. 123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhuri A, Duan F, Morsey B, Persidsky Y, and Kanmogne GD, HIV-1 activates proinflammatory and interferon-inducible genes in human brain microvascular endothelial cells: putative mechanisms of blood-brain barrier dysfunction. J Cereb Blood Flow Metab, 2008. 28(4): p. 697–711. [DOI] [PubMed] [Google Scholar]
- 37.Bernas MJ, Cardoso FL, Daley SK, Weinand ME, Campos AR, Ferreira AJ, Hoying JB, Witte MH, Brites D, Persidsky Y, Ramirez SH, and Brito MA, Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nature Protocols, 2010. 5(7): p. 1265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaudhuri A, Yang B, Gendelman HE, Persidsky Y, and Kanmogne GD, STAT1 signaling modulates HIV-1-induced inflammatory responses and leukocyte transmigration across the blood-brain barrier. Blood, 2008. 111(4): p. 2062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanmogne GD, Singh S, Roy U, Liu X, McMillan J, Gorantla S, Balkundi S, Smith N, Alnouti Y, Gautam N, Zhou Y, Poluektova L, Kabanov A, Bronich T, and Gendelman HE, Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nanoformulated antiretroviral drugs to human brain endothelial cells. Int J Nanomed, 2012. 7: p. 2373–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bressani RF, Nowacek AS, Singh S, Balkundi S, Rabinow B, McMillan J, Gendelman HE, and Kanmogne GD, Pharmacotoxicology of monocyte-macrophage nanoformulated antiretroviral drug uptake and carriage. Nanotoxicology, 2011. 5(4): p. 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang B, Akhter S, Chaudhuri A, and Kanmogne GD, HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood-brain barrier: modulatory effects of STAT1 signaling. Microvasc Res, 2009. 77(2): p. 212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen LK, Ramer-Tait AE, Broderick SR, Kong CS, Ulery BD, Rajan K, Wannemuehler MJ, and Narasimhan B, Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants Biomaterials, 2011. 32: p. 6815–6822. [DOI] [PubMed] [Google Scholar]
- 43.Phanse Y, Lueth P, Ramer-Tait AE, Carrillo-Conde BR, Wannemuehler MJ, Narasimhan B, and Bellaire BH, Cellular Internalization Mechanisms of Polyanhydride Particles: Implications for Rational Design of Drug Delivery Vehicles. Journal of Biomedical Nanotechnology, 2016. 12(7): p. 1544–1552. [DOI] [PubMed] [Google Scholar]
- 44.Phanse Y, Carrillo-Conde BR, Ramer-Tait AE, Roychoudhury R, Pohl NLB, Narasimhan B, Wannemuehler MJ, and Bellaire BH, Functionalization of polyanhydride microparticles with di-mannose influences uptake by and intracellular fate within dendritic cells. Acta Biomater., 2013. 9: p. 8902–8909. [DOI] [PubMed] [Google Scholar]
- 45.Petersen LK, Xue L, Wannemuehler MJ, Rajan K, and Narasimhan B, The Simultaneous Effect of Polymer Chemistry and Device Geometry on the In Vitro Activation of Murine Dendritic Cells. Biomaterials, 2009. 30(28): p. 5131–5142. [DOI] [PubMed] [Google Scholar]
- 46.Ross KA, Brenza TM, Binnebose AM, Phanse Y, Kanthasamy AG, Gendelman HE, Salem AK, Bartholomay LC, Bellaire BH, and Narasimhan B, Nano-enabled delivery of diverse payloads across complex biological barriers. J Contr Rel, 2015. 219: p. 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen HB, Chang J, Wnek GE, Linhardt RJ, and Langer R, Bioerodible polyanhydrides for controlled drug delivery. Biomaterials, 1983. 4: p. 131–133. [DOI] [PubMed] [Google Scholar]
- 48.Smith JD, Morton LD, and Ulery BD, Nanoparticles as synthetic vaccines. Current Opinion in Biotechnology, 2015. 34: p. 217–224. [DOI] [PubMed] [Google Scholar]
- 49.Huntimer LM, Ross KA, Darling RJ, Winterwood NE, Boggiatto PM, Narasimhan B, Ramer-Tait AE, and Wannemuehler MJ, Polyanhydride nanovaccine platform enhances antigen-specific cytotoxic T cell responses. Technology, 2014. 02(02): p. 171–175. [Google Scholar]
- 50.Vela Ramirez JE, Tygrett LT, Hao J, Habte HH, Cho MW, Greenspan NS, Waldschmidt TJ, and Narasimhan B, Polyanhydride Nanovaccines Induce Germinal Center B Cell Formation and Sustained Serum Antibody Responses. Journal of Biomedical Nanotechnology, 2016. 12(6): p. 1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, and Brem H, Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experiance. Ann Surg Oncol, 2008. 15(10): p. 2887–2893. [DOI] [PubMed] [Google Scholar]
- 52.Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, and Alyautdin R, Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target, 2002. 10(4): p. 317–25. [DOI] [PubMed] [Google Scholar]
- 53.Goodman JT, Vela Ramirez JE, Boggiatto PM, Roychoudhury R, Pohl NLB, Wannemuehler MJ, and Narasimhan B, Nanoparticle chemistry and functionalization differentially regulates dendritic cell–nanoparticle interactions and triggers dendritic cell maturation. Part Part Syst Char, 2014. 31(12): p. 1269–1280. [Google Scholar]
- 54.Carrillo-Conde BR, Garza A, Anderegg J, and Narasimhan B, Protein adsorption on biodegradable polyanhydride microparticles. J Biomed Mater Res A, 2010. 95: p. 40–8. [DOI] [PubMed] [Google Scholar]
- 55.Vela Ramirez JE, Boggiatto PM, Wannemuehler MJ, and Narasimhan B, Polyanhydride nanoparticle interactions with host serum proteins and their effects on bone marrow derived macrophage activation. ACS Biomater Sci Eng, 2017. 3(2): p. 160–8. [DOI] [PubMed] [Google Scholar]
- 56.Bellaire BH and Narasimhan B, Anti-microbial compositions and methods, US Patent 8,449,916 2013.
- 57.Binnebose AM, Haughney SL, Martin RL, Imerman P, Narasimhan B, and Bellaire BH, Polyanhydride nanoparticle delivery platform dramatically enhances killing of filarial worms. PLoS Negl Trop Dis, 2015. 9(10): p. e0004173. doi: 10.1371/journal.pntd.0004173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullis AS, Schlichtmann BW, Narasimhan B, Cademartiri R, and Mallapragada SK, Ligand-cascading nano-delivery devices to enable multiscale targeting of anti-neurodegenerative therapeutics. Biomed Mater (in press), 2018: p. 10.1088/1748-605X/aaa778 [DOI] [PubMed] [Google Scholar]