Abstract
Purpose
We studied BCR-ABL1 transcript levels in patients with chronic myeloid leukemia in chronic phase (CML-CP) at 3, 6, and 12 months after starting imatinib to identify molecular milestones that would predict for overall survival (OS) and other outcomes more reliably than serial marrow cytogenetics.
Patients and Methods
We analyzed 282 patients with CML-CP who received imatinib 400 mg/d as first-line therapy followed by dasatinib or nilotinib if treatment with imatinib failed. We used a receiver operating characteristic curve to identify the cutoffs in transcript levels at 3, 6, and 12 months that would best predict patient outcome. We validated our findings in an independent cohort of 95 patients treated elsewhere.
Results
Patients with transcript levels of more than 9.84% (n = 68) at 3 months had significantly lower 8-year probabilities of OS (56.9% v 93.3%; P < .001), progression-free survival, cumulative incidence of complete cytogenetic response, and complete molecular response than those with higher transcript levels. Similarly, transcript levels of more than 1.67% (n = 87) at 6 months and more than 0.53% (n = 93) at 12 months identified high-risk patients. However, transcript levels at 3 months were the most strongly predictive for the various outcomes. When we compared OS for the groups defined molecularly at 6 and 12 months with the usual cytogenetic milestones, categorization by transcript numbers was the only independent predictor for OS (relative risk, 0.207; P < .001 and relative risk, 0.158; P < .001, respectively).
Conclusion
A single measurement of BCR-ABL1 transcripts performed at 3 months is the best way to identify patients destined to fare poorly, thereby allowing early clinical intervention.
INTRODUCTION
The introduction of tyrosine kinase inhibitors (TKIs) has proved to be a major advance in the management of patients with chronic myeloid leukemia in chronic phase (CML-CP).1 In the first year of treatment, patients are most commonly monitored by regular examination of the bone marrow and are classified as responders or nonresponders on the basis of the achievement of major cytogenetic response (MCyR) or complete cytogenetic response (CCyR) at given time points.1 For patients who achieve CCyR, BCR-ABL1 fusion transcripts are often monitored thereafter by real-time quantitative polymerase chain reaction (RQ-PCR), because increasing transcript levels identify patients most likely to relapse.2 Recently, efforts have been made to define molecular milestones that predict patient outcome more reliably than the cytogenetics,3–5 but to date, such findings are not fully reproducible. The problem is due in part to the lack of standardization of the RQ-PCR technology (an issue now being addressed6,7) and in part to the fact that the most common molecular milestone—major molecular response (MMR; equivalent to a 3 log reduction in BCR-ABL1 transcripts)—does not predict the most relevant outcomes, such as overall survival (OS) or survival without progression to advanced phase (ie, progression-free survival [PFS]).1,4,8
In this study, we identified molecular milestones at 3, 6, and 12 months after starting imatinib that strongly predict for OS and other outcomes. These milestones can be applied in other centers by using a conversion factor that allows laboratory staff to express transcript results on the widely accepted international scale6; thus, they can be used in clinical practice to guide therapeutic decisions.
PATIENTS AND METHODS
Patients and Therapy
Between June 2000 and December 2010, 282 consecutive adult patients with CML-CP seen at our institution received imatinib 400 mg daily as first-line therapy as described elsewhere.9 Patients gave written informed consent for their data to be used in this analysis. Table 1 lists patient characteristics. The median follow-up was 69 months (range, 17 to 131 months). During follow-up, 118 patients discontinued imatinib and received nilotinib (n = 37), dasatinib (n = 72), or an allogeneic stem-cell transplantation (n = 9). In addition, 22 patients underwent transplantation after second-line therapy failed. Dasatinib and nilotinib were administered as described elsewhere.10 CP, complete hematologic response, CCyR, and MCyR were defined by using conventional criteria.9,11
Table 1.
Patient Characteristics (N = 282)
| Characteristics | No. | % |
|---|---|---|
| Age, years | ||
| Median | 46.3 | |
| Range | 13-86.4 | |
| Sex | ||
| Male | 157 | 55.7 |
| Female | 125 | 44.3 |
| Sokal risk group* | ||
| Low | 88 | 31.8 |
| Intermediate | 111 | 40.1 |
| High | 78 | 28.1 |
| Interval since diagnosis, months | ||
| Median | 1.5 | |
| Range | 0-6 | |
| Chromosomal abnormalities in addition to the Philadelphia chromosome† | 16 | 6.0 |
| Splenomegaly‡ | 186 | 66.4 |
| Spleen size ≥ 10 cm below the costal margin | 75 | 26.8 |
| White cell count × 109/L | ||
| Median | 140.5 | |
| Range | 5.8-645 | |
| Platelet count × 109/L | ||
| Median | 396 | |
| Range | 108-2,267 | |
| Hemoglobin, g/L | ||
| Median | 116.0 | |
| Range | 33.0-172.0 | |
| Peripheral blood blasts (%) | ||
| Median | 1 | |
| Range | 0-14 | |
| Peripheral blood basophils (%) | ||
| Median | 2.6 | |
| Range | 0-18 | |
We could not calculate the Sokal score in five patients because of missing data.
The most common additional abnormalities were a second Philadelphia chromosome and trisomy of chromosome 8. Data were missing in 17 patients.
Two patients had missing data; splenomegaly was defined as a palpable spleen in the physical examination and measured in centimeters below the costal margin.
Detection of BCR-ABL1 Transcripts
BCR-ABL1 transcripts were measured in the blood at 6- to 12-week intervals by using RQ-PCR as described previously.5,6,12,13 Results were expressed as percent ratios relative to an ABL1 internal control, with original laboratory values converted to the international scale.6 Complete molecular response (CMR) was defined by the finding of two consecutive samples with no detectable transcripts having an ABL1 control with more than 40,000 copies. The median ABL1 control in the CMR samples was 84,000 copies.
Statistical Methods
Probabilities of OS, PFS, and event-free survival (EFS) were calculated by using the Kaplan-Meier method. An event was defined as loss of a CCyR or complete hematologic response, progression to advanced phase, death, or imatinib discontinuation. The probabilities of cytogenetic and molecular responses were calculated by using the cumulative incidence (CI) procedure. Current CCyR survival (c-CCyRS) was defined as the probability of being alive and in CCyR at a given time point. The c-CCyRS is the analog of “current leukemia free-survival,” a term we developed to describe behavior of patients after allogeneic stem-cell transplantation14; it recognizes the fact that patients may relapse and regain remission with alternative therapy. OS, PFS, and EFS were compared by using the log-rank test or a Cox regression model. CI and c-CCyRS were examined by using Fine-Gray regression or a modified Cox regression model for recurrent events, respectively.15–18 Univariate and multivariate analyses were performed in accordance with standard methods; variables found to be significant at the P < .10 level were entered in the multivariate analysis. Unless stated in the text, all the analyses were performed on an intention-to-treat basis.
RESULTS
Outcome and Responses
The 8-year CIs of CCyR, MMR, and CMR with imatinib therapy were 76.8%, 51.8%, and 13.5%, respectively. The 8-year probabilities of OS and PFS on a intention-to-treat basis were 84.3% and 83.7%, respectively. During follow-up, 118 patients (41.8%) discontinued imatinib because of lack of efficacy (85 patients) or intolerance (33 patients), resulting in an 8-year probability of imatinib failure-free survival of 50.7%. Appendix Table A1 (online only) depicts the prognostic influence of patient characteristics on the various outcomes.
BCR-ABL1 Transcript Levels at 3, 6, and 12 Months Strongly Predict for Most Relevant Clinical Outcomes
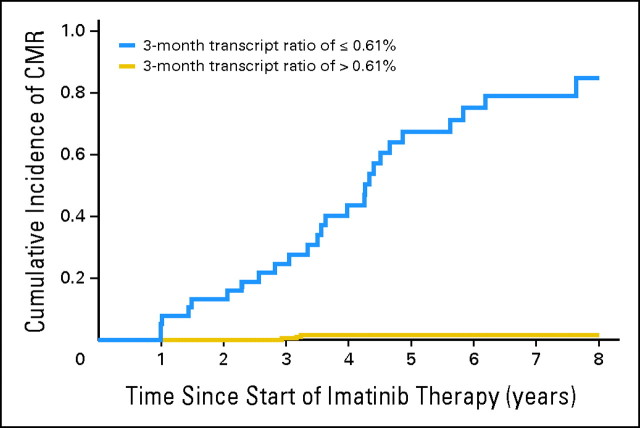
The BCR-ABL1 transcript levels at 3, 6, and 12 months significantly predicted for OS, PFS, and EFS and for the probabilities of achieving CCyR, MMR, and CMR (Table 2). We used a receiver operating characteristic curve to identify the optimal cutoff in transcript level that would allow us to classify the patients as high risk or low risk with maximal sensitivity and specificity for each individual outcome and time point (Table 2). For example, at 3 months, patients with transcript levels below 9.84% had significantly better 8-year OS (93.3% v 56.9%; P < .001; Fig 1), although the cutoff with maximal sensitivity and specificity for CMR was 0.61% (8-year CI of CMR, 84.7% v 1.5%; P < .001; Fig 2). Interestingly, we found that at each time point, the optimal cutoffs identified for OS, PFS, EFS, and CI of CCyR were similar; for example, at 3 months, the cutoffs identified for OS, PFS, EFS, and CI of CCyR were 9.84%, 9.54%, 9.84%, and 8.58%, respectively, but the optimally predictive cutoffs for MMR and, in particular, for CMR were significantly lower (ie, 2.81% and 0.61%, respectively), indicating that a subset of patients destined to achieve CMR can be identified at early time points on the basis of the speed of their response. The cutoffs identified for OS—9.84% at 3 months, 1.67% at 6 months, and 0.53% at 12 months—also predict strongly for all the other outcomes (Table 2). This, together with the fact that OS is the most relevant outcome, made us choose the cutoffs identified for OS to define the transcript-based prognostic system.
Table 2.
RR for OS, PFS, and EFS at 8 Years and Cumulative Incidences of CCyR, MMR, and CMR According to the Transcript Level at 3, 6, and 12 Months
| Outcome | RR for Transcript Level (Log) |
Cutoff (%) | No. of Patients at Risk | 8-Year Probability of the Outcome |
8-Year Probability of the Outcome According to Risk Group |
||||
|---|---|---|---|---|---|---|---|---|---|
| RR | P | % | P | No. of Patients | % | P | |||
| BCR-ABL1 transcript level at 3 months | |||||||||
| OS | 0.161 | < .001 | < .001 | ||||||
| Low risk | ≤ 9.84 | 211 | 93.3 | N/A | N/A | ||||
| High risk | > 9.84 | 68 | 56.9 | N/A | N/A | ||||
| PFS | 0.162 | < .001 | < .001 | < .001 | |||||
| Low risk | ≤ 9.54 | 208 | 92.8 | 211 | 92.8 | ||||
| High risk | > 9.54 | 71 | 57.0 | 68 | 55.5 | ||||
| EFS | 0.102 | < .001 | < .001 | < .001 | |||||
| Low risk | ≤ 9.84 | 211 | 65.1 | 211 | 65.1 | ||||
| High risk | > 9.84 | 66 | 6.9 | 66 | 6.9 | ||||
| CCyR | 5.17 | < .001 | < .001 | < .001 | |||||
| Low risk | ≤ 8.58 | 169 | 99.4 | 180 | 97.9 | ||||
| High risk | > 8.58 | 79 | 21.7 | 68 | 14.8 | ||||
| MMR | 12.98 | < .001 | < .001 | < .001 | |||||
| Low risk | ≤ 2.81 | 141 | 82.5 | 210 | 70.1 | ||||
| High risk | > 2.81 | 137 | 21.1 | 68 | 0 | ||||
| CMR | 10.95 | < .001 | < .001 | < .001 | |||||
| Low risk | ≤ 0.61 | 57 | 84.7 | 211 | 19.3 | ||||
| High risk | > 0.61 | 222 | 1.5 | 68 | 0 | ||||
| BCR-ABL1 transcript level at 6 months | |||||||||
| OS | 0.342 | < .001 | < .001 | N/A | N/A | ||||
| Low risk | ≤ 1.67 | 187 | 93.7 | N/A | N/A | ||||
| High risk | > 1.67 | 87 | 74.7 | ||||||
| PFS | 0.328 | < .001 | < .001 | < .001 | |||||
| Low risk | ≤ 1.73 | 188 | 92.8 | 187 | 92.8 | ||||
| High risk | > 1.73 | 86 | 68.9 | 87 | 69.4 | ||||
| EFS | 0.195 | < .001 | < .001 | < .001 | |||||
| Low risk | ≤ 1.67 | 186 | 70.7 | 186 | 70.7 | ||||
| High risk | > 1.67 | 87 | 18.3 | 87 | 18.3 | ||||
| CCyR | 5.033 | < .001 | < .001 | < .001 | |||||
| Low risk | ≤ 2.70 | 98 | 92.0 | 82 | 93.9 | ||||
| High risk | > 2.70 | 66 | 24.7 | 82 | 35.8 | ||||
| MMR | < .001 | < .001 | |||||||
| Low risk | 6.854 | < .001 | ≤ 0.73 | 136 | 81.6 | 176 | 72.5 | ||
| High risk | > 0.73 | 123 | 20.4 | 83 | 13.9 | ||||
| CMR | 4.405 | < .001 | < .001 | .006 | |||||
| Low risk | ≤ 0.21 | 73 | 42.7 | 187 | 21.0 | ||||
| High risk | > 0.21 | 197 | 6.1 | 83 | 4.0 | ||||
| BCR-ABL1 transcript level at 12 months | |||||||||
| OS | 0.398 | < .001 | < .001 | N/A | N/A | ||||
| Low risk | ≤ 0.53 | 164 | 95.4 | N/A | N/A | ||||
| High risk | > 0.53 | 93 | 74.7 | ||||||
| PFS | 0.333 | < .001 | < .001 | < .001 | |||||
| Low risk | ≤ 0.53 | 164 | 94.9 | 164 | 94.9 | ||||
| High risk | > 0.53 | 92 | 73.1 | 92 | 73.1 | ||||
| EFS | 0.280 | < .001 | < .001 | < .001 | |||||
| Low risk | ≤ 0.57 | 168 | 82.1 | 164 | 75.0 | ||||
| High risk | > 0.57 | 78 | 41.4 | 82 | 27.6 | ||||
| MMR | 5.639 | < .001 | < .001 | < .001 | |||||
| Low risk | ≤ 0.22 | 90 | 81.6 | 121 | 78.4 | ||||
| High risk | > 0.22 | 114 | 20.4 | 83 | 12.3 | ||||
| CMR | 4.139 | < .001 | < .001 | .005 | |||||
| Low risk | ≤ 0.036 | 59 | 52.1 | 158 | 18.8 | ||||
| High risk | > 0.036 | 182 | 4.1 | 83 | 4.1 | ||||
NOTE. Table shows the cutoff in transcript levels that distinguishes low-risk and high-risk groups with maximal sensitivity and specificity for each outcome, the 8-year probability of outcome in each of the two groups created by applying the cutoffs identified for each outcome, and the 8-year probabilities for the various outcomes according to the risk group defined by OS.
Abbreviations: CCyR, complete cytogenetic response; CMR, complete molecular response; EFS, event-free survival; MMR, major molecular response; OS, overall survival; PFS, progression-free survival; RR, relative risk.
Fig 1.
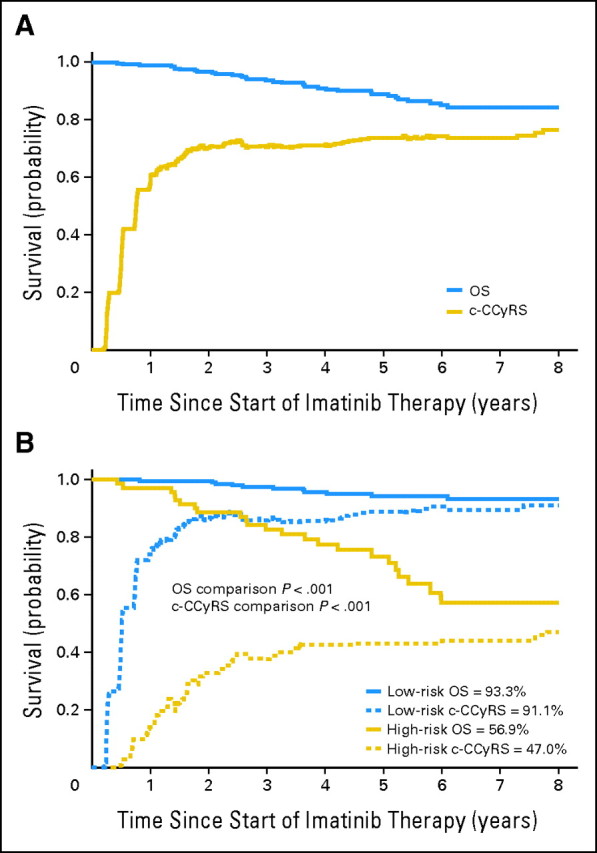
Eight-year probability of overall survival (OS) and current complete cytogenetic response survival (c-CCyRS) in the whole population and with patients stratified by risk group defined by BCR-ABL1 transcript level at 3 months. (A) The probability of 8-year survival in the whole population was 84.3%. During follow-up, 34 patients died, 12 of causes unrelated to leukemia. When only the leukemia-related deaths were taken into account, the 8-year probability of OS was 89.2%. The 8-year probability of c-CCyRS was 76.6%, which represents the probability of being alive and in remission (complete cytogenetic response [CCyR]) at a given time point; thus, the curve fluctuates as patients gain or lose CCyR (or die). It is interesting to observe that after the third year, the c-CCyRS curve remains practically unchanged. (B) Eight-year probability of OS and c-CCyRS for patients according to the risk group defined by transcript levels at 3 months (high-risk BCR-ABL1/ABL1 ratio > 9.84% [n = 68]; low-risk BCR-ABL1/ABL1 ≤ 9.84% [n = 211]). The high-risk group (red lines) had a significantly lower OS (56.9% v 93.3%; P < .001) and c-CCyRS (47.0% v 91.1%; P < .001) than the low-risk group (blue). The 6- and 12-month landmark analyses show similar results: OS was 74.7% versus 93.7% (P < .001) and 74.7% versus 95.4% (P < .001) and c-CCyRS was 53.1% versus 91.7% (P < .001) and 53.3% versus 91.3% (P = .001), respectively.
Fig 2.
Eight-year cumulative incidence of complete molecular response (CMR) for patients receiving imatinib therapy according to the BCR-ABL1 transcript level at 3 months. The transcript level at 3 months identifies those patients with higher probability of achieving CMR on imatinib therapy. The 57 patients who had a 3-month transcript ratio ≤ 0.61% (blue line) had an 8-year cumulative incidence (CI) of CMR of 84.7%, and the 222 patients with a ratio of more than 0.61% (gold line) had a CI of CMR of only 1.5% (P < .001). Similar thresholds with high predictive power could be identified for 6 and 12 months (Table 2). Patients in the low-risk group defined at 3, 6, and 12 months also had significantly higher CI of CMR (Table 2).
These Transcript-Based Prognostic Categories Can Be Applied to Other Patient Populations When the Transcript Ratio Is Expressed on the International Scale
We similarly classified 95 patients treated with first-line imatinib therapy at the Royal Liverpool University Hospital according to their transcript levels at 3, 6, and 12 months, after converting their local RQ-PCR results to the international scale. At 3, 6, and 12 months, 32 of 95, 40 of 94, and 37 of 83 patients still receiving imatinib therapy belonged to the high-risk group as defined by RQ-PCR. At each time point, the high-risk patients had significantly poorer OS—69.1% versus 98.3% (P = .003), 71.2% versus 98.1% (P = .009), and 74.4% versus 98.0% (P = .01)—than the low-risk patients. Similarly, patients in the high-risk group had significantly lower PFS, EFS, c-CCyRS, CI of CCyR, and CI of CMR (data not shown).
Use of Second-Line or Subsequent Therapies Has Little Impact on the Prognostic Value of Early Measurement of BCR-ABL1 Transcript Levels
During follow-up, 118 patients experienced failure of imatinib therapy and required alternative therapy. To further explore the impact of early measurement of transcript levels on outcome, irrespective of the use of rescue treatment, we calculated the c-CCyRS (ie, the probability of being alive and in CCyR at a given time point as defined above). The 8-year c-CCyRS for the whole population was 76.6% (Fig 1A). High-risk patients had a significantly worse 8-year probability of c-CCyRS than low-risk patients: 47.0% versus 91.1% at 3 months (P < .001) and 53.1% versus 91.3% at 6 months (P = .01; Fig 1B). These findings indicate that the prognostic value of early measurement of the residual leukemia burden retains its value even for patients requiring treatment with second-generation TKIs.
Patient Outcome Predicted at 3 Months
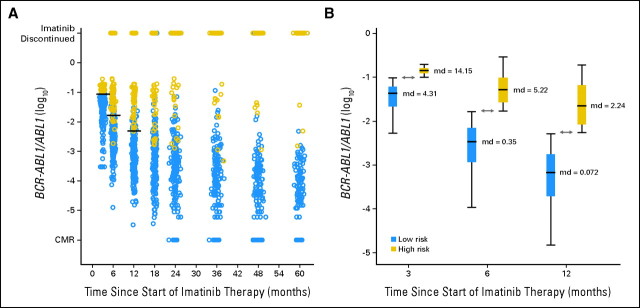
We classified the patients as high risk or low risk according to their transcript levels at 3, 6, and 12 months and entered the resulting variables in a Cox regression model together with the appropriate pretherapy variables shown in Appendix Table A1 (see Results). The 3-month transcript level (higher or lower than 9.84%) was the only independent predictor for OS (relative risk [RR], 7.33; P < .001), PFS (RR, 7.16; P < .001), EFS (RR, 9.71; P < .001), and c-CCyRS (RR, 0.431; P < .001), which indicates that this measurement at 3 months is the most informative. Indeed, 18 of the 23 patients who eventually died of CML-related causes and 28 of the 37 who are still alive but were not in CCyR at their latest follow-up belonged to the high-risk group defined at 3 months, and use of transcript cutoffs at 6 and 12 months did not identify any additional deaths or cytogenetic failures. Conversely, the high-risk group defined at 3 months contained only 68 patients in total; the 6-and 12-month groups contained 87 and 93 patients, respectively; thus, the 3-month time point is the one with the best sensitivity and specificity. Figure 3 shows the evolution of the transcript level according to the prognostic risk group defined at 3 months.
Fig 3.
Evolution of the transcript level according to the 3-, 6-, and 12-month risk group. Transcript levels are expressed on a log10 scale. (A) Evolution of the transcript level over time. Patients are classified as high risk (gold circles) and low risk (blue circles) according to their transcript level at 3 months (higher or lower than 9.84%). The horizontal black lines represent the transcript level that defines the 3-month (9.84%), 6-month (1.67%), and 12-month (0.53%) risk groups. The majority of patients (all but six) who are classified as high risk at 3 months are also classified as high risk at 6 months, and all are classified as high risk at 12 months. The transcript level declines over time in the low-risk patients, although it remains comparatively high in the high-risk population. Many of the high-risk patients eventually abandon imatinib therapy because of unsatisfactory response, loss of response, or progression. (B) Comparison of transcript levels in the two risk groups defined at 3, 6, and 12 months. Although the optimal cutoffs to define the groups at 3, 6, and 12 months are 9.84%, 1.67%, and 0.53%, the majority of the patients had a transcript level much higher or much lower than the cutoff selected for a given time. The median value (md) of the transcript level is also specified in the figure as a BCR-ABL1/ABL1 ratio (%). The double arrowed lines represent the value of the cutoffs used to define the groups on a logarithmic scale (9.84%, 1.67%, and 0.53%). CMR, complete molecular response.
Prognostic Value of the 3-Month Transcript Level Is Independent of the Imatinib Dose Intensity Received by the Patient
Ninety-three patients (33.0%) temporarily discontinued imatinib therapy or had the dose reduced because of adverse effects. These patients had significantly higher 3-month transcript levels than the remaining 186 patients (8.49% v 5.83%; P = .001). This raised the question of whether the transcript level in these patients can be interpreted in the same way as it is in the rest of the population. To investigate this, we subclassified these patients into two groups: (1) those with nonhematologic toxicity and (2) those with hematologic toxicity. The first group (23 patients) had transcript levels similar to those of the 186 imatinib-tolerant patients (7.15% v 5.83%; P = .82);the 70 patients with hematologic toxicity had a significantly higher level (8.91% v 5.83%; P < .001). Patients with nonhematologic toxicity had prognoses identical with those of the imatinib-tolerant patients (data not shown), but the 70 patients with hematologic intolerance to imatinib had a worse 8-year CI of CCyR (RR, 0.44; P < .001), c-CCyRS (RR, 0.531; P < .001), and OS (RR, 2.33; P = .02). Furthermore, dose intensity of imatinib lost its prognostic significance when the transcript level was introduced into the multivariate model (data not shown), reinforcing the notion that the transcript level at early time points affects prognosis independently of the intensity of the dose of imatinib the patient has received.
Transcript Levels at 6 and 12 Months Identify Those Patients in CCyR Who Are Destined to Fare Poorly
Of the 166 patients who had achieved CCyR at 12 months, 41 had also achieved MMR. Patients who had achieved MMR had OS (96.3% v 92.4%; P = .5) and EFS (93.7% v 80.4%; P = .08) similar to those of patients who were unable to achieve MMR. The achievement of MMR at 18 months also lacked prognostic value (data not shown). We tried to identify levels of transcripts at 6 and 12 months above which patients in CCyR were destined to fare worse than those with lower levels. At 6 months and 12 months, 23 of the 109 and 20 of the 166 patients who were in CCyR had a transcript level higher than 0.53% (the value we had previously identified as defining the 12-month high-risk group); these patients had significantly worse OS (65.8% v 95.9%; P < .001 and 81.5% v 94.9%; P = .01) and EFS (37.3% v 72.0%; P < .001 and 29.5% v 74.3%; P < .001) than the 86 and 146 patients with lower transcript levels.
Measurement of Transcript Levels at 6 and 12 Months Is More Informative Than Bone Marrow Cytogenetic Data for Identifying Patients With a Worse Prognosis
Assessing bone marrow cytogenetic responses at 6 and 12 months is probably the most common method of determining a patient's response to therapy and is used to guide clinical decisions. Table 3 shows the RR for OS and complete response according to the cytogenetic responses. Patients who achieved an MCyR at 6 months and patients who achieved an MCyR or CCyR at 12 months had better outcomes. By using OS and c-CCyRS as end points, we compared the prognostic value of cytogenetic assessment with the RQ-PCR results by performing multivariate analysis for each time point, including the appropriate variables listed in Table 3 and Appendix Table A1. The transcript levels at 6 months (higher or lower than 1.67%) and 12 months (higher or lower than 0.53%) were the only independent predictors for OS (RR, 4.83; P < .001 and RR, 6.33; P < .001) and c-CCyRS (RR, 0.486; P < .001 and RR, 0.545; P < .001).
Table 3.
RRs for 8-Year OS and c-CCyRS According to the Cytogenetic and Molecular Responses Achieved at 6 or 12 Months
| Variable | No. of Patients | 8-Year OS |
8-Year c-CCyRS |
||
|---|---|---|---|---|---|
| RR | P | RR | P | ||
| 6 months | |||||
| CCyR* | .29 | .12 | |||
| Yes | 106 | 1 | 1 | ||
| No | 157 | 3.44 | 0.815 | ||
| MCyR* | .033 | .01 | |||
| Yes | 170 | 1 | 1 | ||
| No | 93 | 2.30 | 0.715 | ||
| MiCyR* | .29 | .23 | |||
| Yes | 223 | 1 | 1 | ||
| No | 40 | 2.15 | 0.727 | ||
| BCR-ABL1/ABL1 | < .001 | < .001 | |||
| Good response (≤ 1.67%) | 87 | 1 | 1 | ||
| Poor response (≥ 1.67%) | 187 | 4.83 | 0.486 | ||
| 12 months | |||||
| CCyR† | .016 | .003 | |||
| Yes | 164 | 1 | 1 | ||
| No | 89 | 2.88 | 0.674 | ||
| MCyR† | .08 | .007 | |||
| Yes | 199 | 1 | 1 | ||
| No | 54 | 2.40 | 0.714 | ||
| BCR-ABL1/ABL1 | < .001 | < .001 | |||
| Good response (≤0.53%) | 164 | 1 | 1 | ||
| Poor response (≥0.53%) | 93 | 6.33 | 0.545 | ||
Abbreviations: c-CCyRS, current complete cytogenetic response survival; CCyR, complete cytogenetic response; MCyR, major cytogenetic response; MiCyR, minor cytogenetic response; OS, overall survival; RR, relative risk.
Data missing in 11 patients
Data missing in four patients.
DISCUSSION
The observation that BCR-ABL1 transcript levels measured early in the course of CML-CP treatment with imatinib may have prognostic value is not new; we and others have previously shown that patients with relatively low transcript levels at early time points had outcome superior to those with higher levels.9,19–21 We show here that BCR-ABL1 transcript measurements performed at 3, 6, or 12 months can identify those patients with relatively poor survival, poor PFS, and poor c-CCyRS, but the data suggest that the 3-month assessment is the most important one and may preclude the need for measurements at later intervals. Thus for the three time points studied, we established transcript cutoff values that were based on outcomes observed for the patient population, which may be preferable to using somewhat arbitrarily defined levels of log reduction from a starting baseline. This led to a new set of definitions for molecular responses: transcript levels ≤ 9.84% at 3 months, ≤ 1.67% at 6 months, and ≤ 0.53% at 12 months. It is also possible to refine the proposed definitions of molecular response to better identify those patients who are more likely to achieve CMR on imatinib (Fig 2). We have validated our results by applying these definitions of response to an independent cohort of patients who were treated and had their transcript levels analyzed in a different specialist center.
We found that the 3-month milestone is indeed the most informative and the 6- and 12-month assessments contributed little (if anything) more to identifying patients with a high risk of progression. This suggests that therapeutic strategies for which patients are supposed to achieve successive milestones at specific time points, for example MCyR at 6 months and then CCyR at 12 months and so on, could be abandoned in favor of a single assessment at an early time point that would direct high-risk patients to alternative therapy.
The prognostic value of the 3-month assessment was independent of whether or not patients had had their dose of imatinib temporarily reduced or discontinued because of adverse effects. Thus, finding high transcript levels in a given patient at 3 months is a major cause for concern for clinicians; this also applies in patients in whom the high levels can be attributed to hematologic toxicity, because this cohort of patients has lower OS, PFS, and CI of CCyR than the rest of the population.
The 8-year c-CCyRS, which reflects the probability of being alive and in CCyR, was 76.6%. Interestingly, the c-CCyRS remained relatively constant after the third year, reflecting the low probability of later events in patients with CML treated with TKIs. Patients with low transcript levels at 3, 6, or 12 months had an excellent c-CCyRS (Fig 1). For example, the probability of being alive and in CCyR at 8 years for patients with a good 3-month molecular response was 91.2% (95.4% if the non–leukemia-related deaths were excluded), whereas high-risk patients had only a 47.0% probability of being alive and in CCyR at 8 years. This is consistent with the conclusion that second-line TKIs have only partial success in rescuing the patients for whom treatment with imatinib has failed. Thus the poor prognosis reflected by the high transcript level at early time points was maintained despite the fact that an individual patient may experience treatment failure with imatinib and subsequently receive a variety of apparently more efficacious agents. Clearly, it is important to develop new therapeutic strategies for this cohort of patients.
As we have previously shown,8,22 we found that the patients who were in MMR at 12 or 18 months had better EFS when we analyzed the whole patient population and when we limited the analysis to patients who were in CCyR, but the achievement of MMR at 12 or 18 months did not have any predictive value for OS (P = .21 and P = .42), PFS (P = .26 and P = .35), or c-CCyRS (P = .32 and P = .44) in the whole population or indeed when the analysis was limited to patients who were in CCyR. This reflects the limitations of basing the definition of therapeutic targets on arbitrarily predefined cutoffs (ie, 3 log reduction in transcripts or 0.1% on the international scale) rather than trying to find the appropriate cutoffs that discriminate between patients with different clinical outcomes.
Finally, we compared our risk groups on the basis of molecular response at 6 and 12 months with the cytogenetic milestones normally used in clinical settings. Patients who achieved MCyR at 6 months and patients who achieved MCyR or CCyR at 12 months had significantly better OS and complete response (Table 3), but cytogenetic responses were not independent predictors for these outcomes when the molecular responses were taken into account.
The superior value of early molecular assessment over serial cytogenetic studies is not readily explained. It could reflect the statistical unreliability of extrapolating cytogenetic results from a small number of analyzable metaphases. It could also reflect the fact that the population of dividing Philadephia-positive cells that is identified in the bone marrow is poorly representative of a specialized, more primitive population of leukemia cells less susceptible to eradication by TKI from the outset or more readily able to acquire resistance in the first few years of therapy. This hypothetical population might be better characterized by their capacity to express more BCR-ABL1 transcripts.
In conclusion, in experienced hands, a single measurement of BCR-ABL1 transcripts performed at 3 months is the most accurate way to identify those patients with CML-CP treated with imatinib who are destined to fare poorly, thus allowing early clinical intervention.
Appendix
Table A1.
RR for OS, PFS, EFS and Cumulative Incidence of CCyR, MMR, and CMR, and c-CCyRS at 8 Years According to the Pretherapy Characteristics of the Patients
| Variable | OS |
PFS |
EFS |
CCyR |
MMR |
CMR |
c-CCyRS |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | RR | P | |
| Age (years) | 1.021 | .14 | 1.017 | .14 | 1.006 | .36 | 0.990 | .74 | 1.002 | .75 | 1.013 | .23 | 0.998 | .64 |
| Sex (female) | 0.983 | .96 | 0.963 | .91 | 1.000 | .96 | 1.142 | .31 | 1.719 | .04 | 6.491 | < .001 | 1.007 | .953 |
| Sokal risk group | .002 | .003 | .27 | < .001 | .02 | .09 | < .001 | |||||||
| Low | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Intermediate | 2.279 | 1.646 | 1.260 | 0.800 | 0.814 | 0.570 | 0.786 | |||||||
| High | 5.532 | 3.944 | 1.388 | 0.506 | 0.622 | 0.492 | 0.498 | |||||||
| CE | 1.655 | .5 | 2.180 | .21 | 0.761 | .52 | 1.171 | .51 | 1.411 | .29 | 1.101 | .47 | 1.096 | .75 |
| Splenomegaly (cm) | 1.056 | .03 | 1.045 | .06 | 1.029 | .007 | 0.958 | < .001 | 0.938 | < .001 | 0.847 | < .001 | 0.935 | < .001 |
| White cell count (×109/L) | 1.003 | .01 | 1.002 | .05 | 1.002 | < .001 | 0.997 | < .001 | 0.996 | < .001 | 0.989 | < .001 | 0.998 | .01 |
| Platelet count (×109/L) | 1.000 | .91 | 1.000 | .57 | 1.001 | .006 | 1.001 | .005 | 1.001 | .03 | 1.001 | .001 | 1.000 | .64 |
| Hemoglobin (g/dL) | 0.733 | .001 | 0.790 | .005 | 0.878 | .01 | 1.207 | < .001 | 1.221 | < .001 | 1.258 | .02 | 1.116 | .001 |
| Peripheral blood blasts (%) | 1.082 | .07 | 1.094 | .06 | 1.121 | .001 | 0.928 | .005 | 0.953 | .12 | 0.648 | .01 | 0.964 | .11 |
| Peripheral blood basophils (%) | 1.058 | .25 | 1.033 | .51 | 0.974 | .31 | 1.023 | .18 | 1.030 | .13 | 1.027 | .19 | 1.017 | .34 |
Abbreviations: c-CCyRS, current complete cytogenetic response survival; CCyR, complete cytogenetic response; CMR, complete molecular response; CE, chromosomal abnormalities in addition to the Philadelphia chromosome; EFS, event-free survival; MMR, major molecular response; OS, overall survival; PFS, progression-free survival; RR, relative risk.
Footnotes
Processed as a Rapid Communication manuscript. See accompanying editorial on page 223; listen to the podcast by Dr Stone at www.jco.org/podcasts
Supported by the National Institute for Health Research Biomedical Research Centre Funding Scheme.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Dragana Milojkovic, Novartis (C), Bristol-Myers Squibb (C) Stock Ownership: None Honoraria: Richard E. Clark, Novartis, Bristol-Myers Squibb, Pfizer; Jane F. Apperley, Novartis, Bristol-Myers Squibb, ChemGenex Research Funding: David Marin, Novartis, Bristol-Myers Squibb; Richard E. Clark, Novartis, Bristol-Myers Squibb; Jane F. Apperley, Novartis, Bristol-Myers Squibb; John M. Goldman, Novartis, Bristol-Myers Squibb, Ariad Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: David Marin
Administrative support: Jane F. Apperley
Provision of study materials or patients: David Marin, Jane F. Apperley
Collection and assembly of data: David Marin, Amr R. Ibrahim, Claire Lucas, Lihui Wang, Jane F. Apperley, Christos Paliompeis
Data analysis and interpretation: David Marin, Amr R. Ibrahim, Claire Lucas, Gareth Gerrard, Richard M. Szydlo, Richard E. Clark, Jane F. Apperley, Dragana Milojkovic, Marco Bua, Jiri Pavlu, Alistair Reid, Katayoun Rezvani, John M. Goldman, Letizia Foroni
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1. Marin D: Current status of imatinib as frontline therapy for chronic myeloid leukemia Semin Hematol 47: 312– 318,2010 [DOI] [PubMed] [Google Scholar]
- 2. Marin D Khorashad JS Foroni L , etal: Does a rise in the BCR-ABL1 transcript level identify chronic phase CML patients responding to imatinib who have a high risk of cytogenetic relapse? Br J Haematol 145: 373– 375,2009 [DOI] [PubMed] [Google Scholar]
- 3. Hughes TP Kaeda J Branford S , etal: Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia N Engl J Med 349: 1423– 1432,2003 [DOI] [PubMed] [Google Scholar]
- 4. Druker BJ Guilhot F O'Brien SG , etal: Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia N Engl J Med 355: 2408– 2417,2006 [DOI] [PubMed] [Google Scholar]
- 5. Marin D Kaeda J Szydlo R , etal: Monitoring patients in complete cytogenetic remission after treatment of CML in chronic phase with imatinib: Patterns of residual leukaemia and prognostic factors for cytogenetic relapse Leukemia 19: 507– 512,2005 [DOI] [PubMed] [Google Scholar]
- 6. Hughes T Deininger M Hochhaus A , etal: Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: Review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results Blood 108: 28– 37,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foroni L Wilson G Gerrard G , etal: Guidelines for the measurement of BCR-ABL1 transcripts in chronic myeloid leukaemia Br J Haematol 153: 179– 190,2011 [DOI] [PubMed] [Google Scholar]
- 8. Marin D Milojkovic D Olavarria E , etal: European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor Blood 112: 4437– 4444,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Lavallade H Apperley JF Khorashad JS , etal: Imatinib for newly diagnosed patients with chronic myeloid leukaemia: Incidence of sustained responses in an intention-to-treat analysis J Clin Oncol 26: 3358– 3363,2008 [DOI] [PubMed] [Google Scholar]
- 10. Milojkovic D Nicholson E Apperley JF , etal: Early prediction of success or failure of treatment with second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia Haematologica 95: 224– 231,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kantarjian HM Dixon D Keating MJ , etal: Characteristics of accelerated disease in chronic myelogenous leukemia Cancer 61: 1441– 1446,1988 [DOI] [PubMed] [Google Scholar]
- 12. Kaeda J, Chase A, Goldman JM: Cytogenetic and molecular monitoring of residual disease in chronic myeloid leukaemia Acta Haematol 107: 64– 75,2002 [DOI] [PubMed] [Google Scholar]
- 13. Kaeda J O'Shea D Szydlo RM , etal: Serial measurement of BCR-ABL transcripts in the peripheral blood after allogeneic stem cell transplantation for chronic myeloid leukemia: An attempt to define patients who may not require further therapy Blood 107: 4171– 4176,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Craddock C Szydlo RM Klein JP , etal: Estimating leukemia-free survival after allografting for chronic myeloid leukemia: A new method that takes into account patients who relapse and are restored to complete remission Blood 96: 86– 90,2000 [PubMed] [Google Scholar]
- 15. Kleinbaum DG: Kleinbaum DG, Klein M. Recurrent event survival analysis Survival Analysis: A Self-Learning Text 2005. New York, NY: Springer-Verlag [Google Scholar]
- 16. Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk J Am Stat Assoc 94: 496– 509,1999 [Google Scholar]
- 17. Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk Ann Stat 16: 1140– 1154,1988 [Google Scholar]
- 18. Domenech JM, Navarro JB: Analisis de eventos recurrentes y de riesgos competitivos: Analisis de la supervivencia y modelo de riesgos proporcionales de Cox [in Spanish] 2011. Barcelona, Spain: Signo [Google Scholar]
- 19. Hanfstein B Müller MC Erben P , etal: Molecular response after 3 months of 1st line imatinib therapy is predictive for treatment failure and disease progression in patients with chronic phase chronic myeloid leukemia: A follow-up analysis of the German CML Study IV Presented at the 52nd ASH Annual Meeting and Exposition December 4-7, 2010 Orlando, FL abstr 360 [Google Scholar]
- 20. Hughes TP Hochhaus A Branford S , etal: Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: An analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood 116: 3758– 3765,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang L Pearson K Ferguson JE , etal: The early molecular response to imatinib predicts cytogenetic and clinical outcome in chronic myeloid leukaemia Br J Haematol 120: 990– 999,2003 [DOI] [PubMed] [Google Scholar]
- 22. Ibrahim AR Eliasson L Apperley JF , etal: Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy Blood 117: 3733– 3736,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]