Abstract
Aging has been regarded as a treatable condition, and delaying aging could prevent some diseases. Ovarian aging, a special type of organ senescence, is the earliest-aging organ, as ovaries exhibit an accelerated rate of aging with characteristics of gradual declines in ovarian follicle quantity and quality since birth, compared to other organs. Ovarian aging is considered as the pacemaker of female body aging, which drives the aging of multiple organs of the body. Hence, anti-ovarian aging has become a research topic broadly interesting to both biomedical scientists and pharmaceutical industry. A marked progress has been made in exploration of possible anti-ovarian agents or approaches, such as calorie restriction mimetics, antioxidants, autophagy inducers etc., over the past years. This review is attempted to discuss recent advances in the area of anti-ovarian aging pharmacology and to offer new insights into our better understanding of molecular mechanisms underlying ovarian aging, which might be informative for future prevention and treatment of ovarian aging and its related diseases.
Keywords: organ senescence, ovarian aging, pacemaker, pharmacological strategies
Introduction
As human longevity has been significantly improved, aging-related problems are markedly increasing. It is predicted that the number of people over 60 years old by 2050 will be five times than that of 1950 [1]. As the world's most populous country, China entered into the aging society 13 years ago. According to the previous population census data, the aged population in China has exceeded world average in size, growth rate and proportion. The average lifespan of Chinese people will increase to 81.9 by 2040 [2]. The primary problem of the aging population is the serious detriments caused by the aging of various organs and the decline of their functions. Organ senescence is often highly associated with a variety of diseases, such as cancer, diabetes, cardiovascular disease and obesity. The occurrence and development of these diseases lead to the decrease of the life quality and increase of the proportion of people who live with the diseases. However, fortunately, aging has been shown to be an improvable condition, and delaying aging would be a way to prevent and treat diseases [3].
One of the earlier aging organs is ovary, as it exhibits an accelerated rate of aging compared with that of other body systems. Ovarian aging is characterized by gradual declines in ovarian follicle quantity and quality, ending with menopause [4]. The ovarian aging process is complicated and affected by a number of factors, including lifestyle, medical, genetic, autoimmune, environmental, and idiopathic ones. Thus, ovarian aging can be physiologic ovarian aging, which is defined by age-specific declines of functional ovarian reserve, and also premature ovarian failure (POF) due to those aforementioned factors. For women, anti-mullerian hormone (AMH )and antral follicle counts (AFC) are currently best markers for evaluating ovarian reserve. In addition, age and menstrual cycle are also good indicators. For animals, ovarian reserve is often reflected in follicle counts at all stages. Endocrine function is mainly reflected by hormone levels and estrous cycle regularity. Reproductive ability includes pregnant rate, litter size, number of offspring per litter, and so on.
Ovarian aging is a complex process. Since birth, a large number of follicles in the ovaries have undergone atresia during development. A woman only ovulates about 500 times in her lifetime, and 99% of the follicles are wasted. Rapid deterioration in both the ovarian follicle quality and quantity is highly associated with a number of women’ disorders or diseases. The fertility of women decreases gradually with age, and after age 35, it declines more rapidly until menopause at an average age of 51 [5]. Currently, more than 15% of couples in the world face the problem of infertility in their childbearing years, which is expected to reach 7 million by 2025 [6]. What’s more, estrogen secretion decreases with the decline of ovarian function and the arrival of menopause, which then lead to multiple organ dysfunction, such as heart disease, osteoporosis, cancer, obesity, senile dementia, and so on [7]. The incidence rate of osteoporotic fractures in postmenopausal women is significantly higher than that before menopause, and the risk index is much higher than that of men of the same age. In addition, cardiovascular diseases are often called "gender difference" diseases, because of their dramatic increase in postmenopausal women [8-10]. Thus, ovarian aging is considered as the pacemaker of female body aging, which drives the aging of multiple organs of the body [11].
Hence, it becomes particularly important to study molecular events underlying this fast aging process, as doing so would help us not only better understand this process, but also develop possible strategies or approaches to slow it down for hopefully preventing ovary-aging associated diseases. The past decade has witnessed a great progress in this area. This review is aimed to discuss recent advances in the pharmacological research toward development of anti-ovarian aging agents or approaches and to offer some insights into our better understanding of the ovary aging process and its molecular events. We hope that this review with a broad collection of literature would be informative and useful to ovary researchers and others who are interested in this topic. The subtitles and classifications in this article referred to one published article regarding anti-aging pharmacological strategies [12].
The free radical theory of aging and antioxidants
The free radical theory of aging has been a classical and the most influential theory in the field of aging, since was first postulated by Denham Harman in the middle of the last century. Oxidative stress leads to changes in the ovarian microenvironment, and these changes account for ovarian senescence and the decrease of ovarian reserve [13, 14]. During the senescence process, the level of reactive oxygen species (ROS) increases, while the expression and activity of oxidative defense system related enzymes are significantly reduced. These all lead to a wide range of oxidative damage, including the cell membrane lipid peroxidation, protein oxidation and enzymes inactivation and DNA damage [15-21]. Also, ROS in follicle oocytes, corpus luteum cells and follicular fluid of elderly women who receive assisted reproductive technology (ART) increases significantly, while antioxidant enzymes decreased; this causes the low success rate of ART [22-24]. It has been shown that oxidative damage products of DNA, protein and lipid in ovarian stromal cells increase markedly with age [25]. Clinical studies have also shown that higher ROS levels exist in unfertilized eggs or low-quality embryos [23, 26]. All these studies indicate that oxidative stress plays an important role in ovarian aging. Therefore, antioxidants have been used to prevent ovarian aging
Vitamins C (ascorbic acid) and E (a-tocopherol)
Among all of the currently used antioxidants, vitamins C and E are commonly used as natural antioxidants. They are also the most studied ones. Vitamin C is the major water-soluble antioxidant, which can effectively reduce a-tocopheroxyl radicals and level of low-density lipoprotein (LDL) in cell membranes, thereby restoring a-tocopherol and inhibiting the generation of free radicals [27]. Vitamin E is the main hydrophobic antioxidant protecting cell membranes from oxidative damage by reaction with lipid radicals produced in the course of the lipid peroxidation chain reaction. The dietary vitamin E supplementation has been shown to be associated with reduced risk of atherosclerosis by reducing oxidative stress and inhibiting LDL oxidation [28]. These cellular studies are supported by an animal study, as oral administration of vitamins C and E could prevent the aging‐related negative effects on ovarian reserve (number of ovarian oocytes and number of ovulated oocytes) and oocyte quality (chromosomal aberration in metaphase II oocytes and morphological apoptotic oocytes) in mice [29]. Therefore, vitamins C and E can be useful for preventing ovary-aging.
N-acetyl-L-cysteine
There are several lines of increasing evidence for benefits of using the antioxidant N-acetyl-L-cysteine (NAC) in preventing ROS-induced damage and pathology. Previous studies have shown that NAC effectively reduces oxidative stress-induced telomere shortening, telomere fusion and chromosomal instability in oocytes in vitro, and improves oocyte and early embryo development [30–32]. Another study using NAC in drinking water for mice for two months showed that NAC can improve the quality of mouse oocytes and promote the early embryonic development. By offering 1 to 1.5 months old mice with low doses of NAC for one year, this lab also found that NAC increases the litter size and oocyte quality of 7 to 10 months old mice and also telomerase activity and telomerase length [33]. These studies indicate that appropriate treatment with the antioxidants, such as vitamins C, vitamins E or NAC, can postpone ovarian aging by reducing free radicals.
Curcumin
Curcumin is an active ingredient extracted from the dietary spice turmeric and has been utilized for medicinal purposes for thousands of years. The activity of curcumin as an antioxidant and free radical scavenger has been demonstrated by several studies [34]. Recently, more studies have demonstrated that curcumin could protect ovarian reserve and ovarian function from tissue injuries. Curcumin might be a promising treatment for cyclophosphamide-induced POF, because of its improvements in ovarian tissue histopathological damage, hormonal levels and reduced oxidative damages. It decreased vascular congestion and atretic follicles, increased healthy follicles numbers, and enhanced the levels of malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) in rat ovarian tissue [35]. In an ischemia-reperfusion rat model, curcumin could maintain and protect ovarian functions from oxidative injury [36]. Another study also indicated that curcumin can alleviate sodium arsenite-induced ovarian oxidative injury in a mouse model to a certain extent by increasing the levels of SOD and decreasing ROS and MDA expression [37]. Curcumin combined with flutamide could modulate ovarian structure and abdominal obesity in aging FSH-R haploinsufficient mice. In this model, curcumin enhanced the expression of bone morphogenetic protein-15 in the ovary and improved structural changes of in zona pellucida [38]. These studies indicate the beneficial outcomes of curcumin treatment in ovarian reserve and function protection.
Coenzyme Q10
Coenzyme Q10 (CoQ10), an oil-soluble component of nearly all cell membranes, acts as an antioxidant in cellular metabolism via inhibition of lipid peroxidation, protein, and DNA oxidation [39,40]. CoQ10 is an essential component for transporting electrons in the mitochondrial respiratory chain to produce cellular energy. Studies have shown that supplementation of CoQ10 protects cells from ROS-induced damage due to its antioxidant properties, which strengthen endogenous cellular antioxidant systems. CoQ10 also restores oocyte mitochondrial function and fertility during reproductive aging. Thus, dietary administration of CoQ10 could prevent age-related decline in oocyte quality and quantity, and oocyte-specific Pdss2-deficient induced diminished ovarian reserve [41]. Another study indicate that CoQ10 supplementation may protect ovarian reserve by counteracting ovarian aging induced by either mal-functional mitochondria or physiological programming, and reducing the expression of 8’OHdG [42]. CoQ10 may restore ovarian function and reserve of aging mice, and more researches are needed to verify its effects.
Proanthocyanidin
Proanthocyanidin is a phenolic compound exists in fruits, vegetables, nuts, seeds, wines and tea, which has been indicated to be effective in protecting tissues from oxidative damage [43]. Researchers have found that proanthocyanidin extracted from grape seed could maintain the homeostasis between cell proliferation and apoptosis, reduce oxidative damage in the D-gal-induced and natural aging ovaries, and alleviate D-gal-induced nucleus chromatin condensation, thus effectively delay the ovarian aging process in hens [44]. Currently, grape seeds have been widely produced as an antioxidant supplement and used for healthy living. More clinical trials are expected to demonstrate the role of proanthocyanidin in protecting ovarian function and delaying ovarian aging process in the human.
Quercetin and other plant polyphenols
Some plant polyphenols can also protect ovarian reserve and ovarian function by acting as antioxidants. Quercetin is a potential antioxidant and free radical scavenger that is widely found in fruits, vegetables, and leaves. Quercetin increases the antioxidant capacity of the ovary in menopausal rats and in ovarian granulosa cell culture in vitro by upregulating the expression of some oxidative stress-related genes, such as SOD-1, CAT and glutathione synthetase (GSS) [45]. Some research suggested that tea polyphenols may inhibit the transition from primordial to developing follicles, extend the entire growth phase of a follicle and reduce dominant follicle numbers per cycle. Thus, tea polyphenols increased the reserve of germ cells, inhibited oocyte apoptosis and follicle atresia during ovarian development from birth to early aged, and retard climacterium in rats [46]. Drinking even a small cup of tea per day may extent the productive life of ovary.
Hence, these studies indicate that antioxidants can prevent ovarian aging and enhance ovary function by either activating the expression of genes important for reduction of oxidative damages or directly scavenging or inactivating ROS.
Caloric restriction mimetics
Over the past years, it has been shown that improvement of cellular and physiological metabolisms could also prevent ovarian aging. One of the approaches is caloric restriction (CR), also known as dietary restriction (DR). CR by limiting the daily diet to 25% - 50% of the normal diet ensures that the body receives sufficient nutrients without malnutrition. The ability of CR to extend lifespan and delay aging have drawn noteworthy researchers’ attention since the original work was reported by McCay and colleagues eighty years ago [47]. Since then, a number of studies have confirmed that CR is the most effective means to postpone the aging of the body and extend lifespan of many organisms. Thereby it is the most inspiring discovery in the aging field [48]. CR can not only prolong the average life expectancy of rodents, but also improve the fertility and prolong the reproductive life [49], by delaying the process of ovarian aging. Although CR is so effective, its implementation is not so easy in real life practice. Thus, alternative substances have been looked for to mimic the CR effects as further detailed below. Also, the main signaling pathways that mediate the CR effects and the most promising pharmacological substances that modulate these pathways and mimic the CR effects will be discussed in the following sections.
Regulators of glycolytic metabolism
Since CR can reduce the levels of insulin, blood glucose, and increase insulin sensitivity [50], inhibitors of the enzymes involved in the process of saccharide decomposition has been searched to simulate the CR-like effect on delaying aging [51]. One of the main inhibitors of this kind is metformin that has been used for the treatment of type 2 diabetes. Metformin has been shown to effectively improve the symptoms, such as insulin resistance, hyperinsulinemia, blood lipid metabolism. It can also prevent diabetes induced large blood vessels and pathological changes of capillaries, and then reverse early diabetes. Metformin may also act as a CR mimetic since it can decrease the production of hepatic glucose, by inhibiting of the mitochondrial respiratory- chain complex-1 [52]. Recently, metformin has become a hot research topic in the field of aging. In 2015, metformin was proposed for trials in delaying human aging [3]. The current studies of metformin in ovaries are mainly limited to polycystic ovary syndrome (PCOS) and ovarian cancer. Several studies have shown that metformin can improve ovarian function in patients with PCOS [52]. It could directly affect ovarian theca cells and decreased FSH-stimulated 3β-HSD, StAR, CYP11A1 and aromatase activities in both rat granulosa cells and women with PCOS, with reduction of basal and of FSH-stimulated progesterone and estradiol levels as a consequence [53]. However, results from another study showed that metformin has no significant effect on ovarian reserve [54]. Thus, the specific effect of metformin on ovarian reserve and function in normal animals or women needs a more systematic investigation.
Inhibitors of insulin/IGF-1signal pathway
Previous studies have indicated that insulin-like growth factor-I (IGF-1) and its binding protein IGFBP-1 in follicular fluid may reflect ovarian reserve [55]. One small molecule inhibitor in metabolic enzymes, which might have a CR-like effect on ovarian aging is the synthetic glucose analog 2-deoxy-glucose (2-DG). Several studies have shown the activity and mechanisms of action of 2-DG for treating viral infection, epilepsy and cancer [56,57]. In mice, 2-DG treatment induced Foxo3 activity and inhibited primordial follicle activation, suggesting that 2-DG may be useful for fertility preservation [58].
Besides 2-DG, tamoxifen, an inhibitor of estrogen receptor (ER) often used for treatment of ER-positive breast and ovarian cancers [59,60] has also been shown to be potentially useful for improving ovarian aging. It was shown to regulate the IIS signaling involved in the occurrence of ovarian aging, and to keep the level of IGF-1 in the ovary [61]. An animal study also showed that tamoxifen can promote follicular development and AMH expression of and protect ovarian functions and the fertility of radiotherapy rats by enhancing the expression level of IGF-1 in ovaries [62].
Inhibitors of mTOR pathway
Finally, regulation of the mammalian target of rapamycin protein (mTOR) has been shown to effectively simulate CR effects, extend lifespan, and delay aging [63]. As a serine/threonine protein kinase, mTOR regulates cell growth, proliferation, differentiation and the cell cycle [64]. Recent studies have shown that mTOR-related signaling pathways play an important role in aging associated metabolic diseases, such as obesity, type 2 diabetes and cancer [65]. Inhibiting the mTOR pathway can extend the life span of several species, such as worms [66,67], fruit flies [68] and mice [69]. Analysis of gene expression profiles indicated that the mTOR pathway is closely related to human health and life span [70]. Recently, rapamycin, an inhibitor of mTOR and a new type of macrolide immunosuppressant, has also been shown to inhibit the activation of the initial follicle by regulating the mTOR and sirtuin signaling pathways, thus protecting ovarian reserve and extending the reproductive life of the ovary [71–73]. Rapamycin may provide a new strategy for patients with premature ovarian failure by protecting ovarian reserve in the near future.
Epigenetic regulators
Over the past few years, a series of epigenetic regulators have been identified, such as small molecule inhibitors of DNA methylation and histone acetyltransferases or non-coding RNAs. These regulators have the potency of treating cancer, myelodysplastic syndrome and neural degeneration [74]. Epigenetic regulations are involved in the changes of gene or protein expressions without altering DNA sequence. Epigenetic modifications are reversible. This characteristic makes small molecule epigenetic regulators attractive as aging intervention agents [75]. In the past 20 years, the role of histone and genomic epigenetic modification regulation in ovarian aging has been gradually recognized, and has become a research hotspot in this field. Abnormal regulation of the related gene expression can lead to ovarian cell apoptosis and accelerated aging, which in turn can accelerate the aging of the ovaries [76].
Sirtuin activators and resveratrol
Although little is known about the role of small molecule regulators of DNA methylases and histone acetyltransferases in ovarian senescence, there are a few studies that suggest that some of them might be useful for improvement of ovarian aging. For example, the sirtuin deacetylase family with seven members in mammalian cells plays a very important role in cell survival, apoptosis, aging, and may be one of the common life-span control family genes in eukaryotic organisms. It was shown that a SIRT1 activator can increase ovarian reserve and prolong reproductive life of obese mice induced by high-fat diet by activating SIRT1 and inhibiting mTOR signaling pathway [77]. Also, resveratrol have been reported to protect mice from aging associated infertility by activating SIRT1 gene expression, improving the number and quality of oocytes, as shown by spindle morphology and chromosome alignment [78]. Resveratrol could improve the rat ovarian reserve and prolong the reproductive life, which may be due to inhibition of the activation of primordial follicle pool and follicular atresia [79]. These studies suggest that targeting epigenetic molecules might serve as an effective approach to improve ovary functions and to delay ovarian aging, though much more need to be done.
MicroRNAs
MicroRNA profile changes in the ovaries of dwarf mice during the aging process, which suggests that these miRNAs may play vital roles in maintaining younger of ovarian phenotype [80]. Another study suggests that specific non-coding RNAs profiles are associated with age and ovarian reserve, indicating that oocyte quality might be mediated by ncRNA pathways [81]. However, there are no pharmacological strategies targeted at microRNAs to improve ovarian reserve or delaying ovarian aging process recently.
Pharmacological induction of autophagy
Autophagy is an intracellular bulk degradation system. During the process, part of the cytoplasm is enveloped in the autophagosomes, then fused and degraded by lysosomes [82]. Several clinical trials are exploring autophagy as a therapeutic target as it plays vital roles in age-associated diseases [83].
In the ovaries, germ cell death is triggered by autophagy or apoptosis during the establishment of ovarian reserve [84]. Studies also suggest that autophagy may promote the formation of the primordial follicle pool [85], which also regulates ovarian follicle atresia [86]. However, few drugs delayed ovarian aging process by targeting autophagy signal pathway. Melatonin and Resveratrol are potential autophagy inducers that may extend ovarian lifespan. Melatonin is of quite capacity to delay ovarian aging for its regulation of multiple pathways such as oxidative stress, telomeres length and SIRTs, in which autophagy plays a vital role [87–89]. Furthermore, resveratrol improved the quality of oocytes by inducing autophagy and mitochondrial synthesis in aged cows [90]. The role of autophagy in ovarian aging process needs more research, and drugs target at autophagy should be explored later.
Telomerase activators
Studies have indicated that telomere length are strongly associated with lifespan [91]. Reports on the role of the telomere and telomerase in female ovaries are still limited. Positive correlations were found between female reproductive life span, being widely accepted as ovarian reserve, and the telomere length [92]. The telomere length, serving as a biological clock, may play vital roles in limited ovarian lifespan, particularly at the cellular level [93]. Another study suggests that telomere length and telomerase activity are associated with primary ovarian insufficiency, which may indicate the progression of ovarian decline [94]. Compared to oocytes from young females, telomere length from the aged was remarkably shorter [95].
We have mentioned above that NAC could delay the oocyte aging by antioxidant activity. The telomerase activity and telomere length were also increased in the ovaries of mice treated with NAC [33]. Another study found that resveratrol protects against age-associated infertility in mice by enhancing telomerase activity and increasing telomere length in the ovaries [96]. These studies suggest that telomerase activators may extend ovarian lifespan effectively.
Hormones
Melatonin
Melatonin is a natural amine hormone secreted principally by pineal gland and released into circulation in a pulsatile fashion with the sharpest peaks in the early morning [97]. Light inhibits the secretion of melatonin and changes the time phase of melatonin rhythm. Different from the aforementioned antioxidants, melatonin possesses a variety of activities in the metabolic and physiological process. Melatonin exhibits direct free radical scavenging and indirect antioxidant effects. The main mechanism by which melatonin protects cell structure is to eliminate free radicals, inhibit the lipid peroxidation reaction and regulate activity of antioxidant and pro-oxidant enzymes. These actions of melatonin are believed to be mediated by the Keap1-Nrf2-ARE pathway and activation of sirtuin 1 (SIRT1).
The role of melatonin in preventing ovarian aging has been also reported. Previous studies have shown that shortening the time of sunshine can delay reproductive aging [98,99]. Researchers gave 10-day-old mice lifetime doses of melatonin and found that it delayed puberty arriving and the aging of the reproductive system, but had no effect on the size of the primordial follicle pool [100]. Another study showed that orally giving kunming mice at 2-3 months old age with melatonin for 12 months can significantly delay ovarian aging by increasing the total number of follicles and the number and quality of oocyte in mice, extending the telomere length, improving the fertility of old mice and reducing the ovaries the generation of ROS [101]. Melatonin treatment for 2 months can increase ovarian volume, improve estrous cycle, and maintain estrogen secretion of 13 month-old rat (middle aged) significantly, maintaining their hormone levels equivalent to that of young rats [102]. This study also showed that melatonin can extend the reproductive lifespan of the middle-aged and old rats. In a clinical study, perimenopausal and postmenopausal women of 42-62 years old were given melatonin treatment for six months. Amazingly, after the treatment, 43-49 years old women have significantly higher serum levels of LH, and their pituitary and thyroid function normally [103]. Finally, recent studies showed that melatonin can increase the primordial follicle pool size, delay ovarian aging in mice by enhancing antioxidant capacity, maintaining the telomerase activity, stimulating the SIRT1 expression and the ribosomal function [104]. These phenomena indicate that melatonin might promote the recovery of ovarian cycle and improve the elderly female fertility.
Leptin
Hormones have been shown to play a role in ovarian ageing as well. For example, leptin has been demonstrated to be essential for ovarian follicle development and fertility. The expression of the leptin receptor in ovary is regulated throughout the estrous cycle by ovarian steroids, with peak expression at ovulation, indicating a possible involvement of this hormone in follicular development and corpus luteum formation. Also, IGF-I plays an important role in leptin receptor expression during the entire estrous cycle, especially during the prepubertal period [105]. During superovulation, leptin administration with gonadotropins in aged mice increased the ovarian response, developmental competence of oocytes and ovarian VEGF expression [106]. It was also shown that leptin causes an inhibitory effect on the early follicular development in both immature and adult mice, although the underlying inhibitory mechanisms of leptin may be different [107]. Another study indicated that reduction of peripheral leptin in the circulation promotes ovarian follicle development in prepubertal female mice, suggesting that leptin acts as an inhibitor of ovarian follicle development [108]. Leptin deficiency in mice was associated with impaired folliculogenesis, which resulted in increased follicular atresia. Thereby, leptin deficiency-induced follicle impairment may be one of the causative factor of infertility [109]. The recent study showed that neonatal overfeeding induces early decline of the ovarian reserve, and acute effects of elevated circulating leptin may be responsible for the long-term reproductive outcomes, thus leading to premature ovarian ageing and changes in reproductive efficiency [110].
Dehydroepiandrosterone
Dehydroepiandrosterone (DHEA) is a hormone essential for human health, especially for women. All estrogens and nearly half of androgens are synthesized from DHEA in peripheral tissues. More studies have shown that DHEA can increase pregnancy rate, promote the steroid hormone secretion, increase the AMH expression and the number of antral follicles, which may also be due to the increased expression level of the IGF-1 [111]. Recent study indicates that DHEA supplementation prior to assisted reproductive technology (ART) improves ovarian markers such as serum AMH, inhibin B and antral follicle count (AFC) in patients with diminished ovarian reserve [112,113]. DHEA also has a pro-inflammatory immune potential and regulates the balance of CD4+/CD8+ T cells. Another study has also demonstrated that DHEA supplementation improves the ovarian reserve of women with diminished ovarian reserve which may due to its regulation of the immune response in the ovaries [114]. In a cortical autograft experimental sheep model, DHEA was applied for 10 weeks. The increased the expression of both the proliferation marker Ki-67 in granulosa cells and the follicular AMH expression at the preantral and early antral follicle stages was observed [115]. The results mentioned above suggest that DHEA may delay ovarian aging. However, long-term treatment with DHEA may affect folliculogenesis and lead to follicular atresia through interaction with AMH [116]. Thus, the concentration and duration of DHEA treatment is of great importance.
Growth hormone
Growth hormone (GH) is a peptide which can promote cell proliferation and body development in animals and humans. GH has been applied to children and adults who suffers GH deficiency clinically. Mice lack of GH receptor showed the reduction of primordial follicle pool and the decrease of their survival rate of growing follicles. Results suggest that GH may be involved in the process of primordial follicle pool recruitment via the IGF-1 pathway, and inhibition of apoptosis of follicles [117]. GH can protect ovarian function from radiation therapy damage, promote the follicular development,and improve AMH expression by increasing IGF-1expression and repressing radiation-induced oxidative damage in ovary [118].
Hormone replacement therapy
Hormone replacement therapy (HRT) is a universal treatment for premature ovarian failure (POF), mainly including estrogen therapy, estrogen and progesterone sequential therapy. Studies have shown that young POF patients treated with hormones show the development of secondary sex characters and alleviated perimenopausal syndrome [119]. Estrogen therapy can promote the endometrial proliferation in POF patients, maintain the estradiol concentration in the blood circulation and promote the pregnancy of women [120]. Estrogen and progesterone sequential therapy could enable POF patients to establish regular artificial cycles and improve pregnancy rate [121]. By comparing standard HRT treatment with percutaneous estrogen delivery combined with vaginal progesterone treatment, researchers found out that these treatments have similar effects on inhibition of FSH and LH, although the combination is more advantageous to cardiovascular protection [122]. Hence hormones-based treatments can be practically useful for improvement of ovary functions and delay of ovarian aging.
A recent study suggested that progesterone alone may protect ovarian function from ischemia-reperfusion injury through its anti-apoptotic and antioxidative properties [123]. More researches are still needed for the role of progesterone in ovarian function.
Immunomodulators
A variety of autoimmune antibodies, such as anti-nuclear antibodies and anti-ovarian tissue antibodies, have been found to be related to POF [124]. Therefore, immunotherapy is of certain significance to patients with POF. In theory, immunomodulators are effective against autoimmune POF. It has been reported that patients treated with clinical immunosuppressive agents, such as glucocorticoid can resume ovulation and pregnancy, but the effect is not so clear. Administration of the immunosuppressant diethyleneethanol and freund's adjuvant during prepubertal provided sufficient anti-GnRH for calfs to delay the onset of puberty for 112 days [125]. Treatment with corticosteroids and testosterone can significantly improve the general condition of POF mice models by reducing the levels of lymphocytes, immune globulins, and related antibodies [126]. However, the clinical application of glucocorticoid has many adverse reactions and the long-term curative effect need to be followed-up. It was shown that the effect of androgens on autoimmune POF is significant, and their side effects are relatively less significant, so the patient's compliance is better. Androgens may inhibit the immune system by regulating the hypothalamic-pituitary-gonad axis [127]. Since the optimal treatment time and duration of POF with glucocorticoid or androgen have not been determined, further studies are needed.
Traditional Chinese medicine
Traditional Chinese medicine holds that symptoms of perimenopausal women are related to the imbalance in the body of Yin and Yang, disturbance of viscera. Deficiency of the kidney energy is the fundamental cause, so it should be given priority to tonifying kidney and spleen, accompanied by protecting liver and calming nerves. Some can achieve satisfactory curative effect, such as Bushen Huoxue Recipe, Liu Wei Di Huang Jia Jian, Zuo Gui Wan Jia Jian and so on. The mechanisms of traditional Chinese medicine are complicated as the pharmacological ingredients are complex. Recent years, several studies have found that traditional Chinese medicine may regulate women nervous-endocrine system and immune system by removing free radicals, improving ovarian microcirculation and reducing cell apoptosis, and then delay ovarian aging [128,129]. The experiments of animals have revealed that, with age, the levels of nerve growth factor and norepinephrine (NE) in the ovaries, and the increase of sympathetic nervous activity were related to reproductive ability of rats. Electric acupuncture can promote the recovery of ovulation and increase fertility of older rats by reducing the level of NGF in ovary or blocking the activity of NE [130].
Intragastric administration of American ginseng gavage can protect the rat ovaries from VCD induced POF, promote the prostaglandin synthesis and ovulation, reduce the serum levels of PGE2, FSH and LH, remain the E2 to the normal level [131]. Bushen Huoxue Recipe alleviates cyclophosphamide-induced diminished ovarian reserve in mouse model by elevating the proportions of CD4+ T cells, Th1, Th17, Treg subsets and increasing serum levels of IFN-γ, TNF-α, IL-17A, IL-6 and IL-10 as well as the mRNA expressions of T-bet. These results show that Bushen Huoxue Recipe is a promising candidate to treat diminished ovarian reserve mice and this beneficial effect may be mediated through the downregulation of augmented autoimmunity [132]. Another study indicated that Bushen Huoxue Recipe might improve the ovarian reserve and enhance the ovarian function of POF mice through neo-oogenesis [133]. Bushen Tiaochong recipe might exert its beneficial role in oocyte maturation and restore diminished ovarian reserve through regulating the brain-derived neurotrophic factor pathway [134].
Yifuning, a traditional Chinese medicine recipe, has been used for many years in China, for its effects on treating climacteric syndrome. Studies suggest that it can improve the general condition of aging rats and mice, promote hormone secretion, recover estrous cycle, increase the viscera index of ovary and uterus and improve perimenopausal syndrome symptoms. It can also improve the function of natural aging reproductive organs such as ovary, uterus and vaginal, which may be related to its activities of antioxidant and promoting proliferation [135]. The Bushen compound could delay the ovarian aging of rats and significantly improve their reproductive and endocrine functions by reducing oxidative damage, promoting the production of VEGF, IGF-1 growth factor, reducing the level of TNF-α in the ovarian tissue, and effectively improving ovaries microenvironment. It can also improve the ultrastructure of ovarian tissue and enhance the immune function of the body [136]. Diosgenin improves ovarian reserve in naturally aging mice by increasing the number of primary follicles and serum levels of AMH. The mRNA expression of NOBOX, GDF9 and BMP15 was also evaluated [137]. Our recent study have also indicated that Kuntai capsule may improve damaged ovarian function, which may be related to its antioxidant and anti-apoptosis effects [138]. As the components of traditional Chinese medicine are complex, they may have several pharmacological actions and work through a variety of pathways. Traditional Chinese medicine shows a great potential in delaying ovarian aging and extending ovarian lifespan.
Conclusions
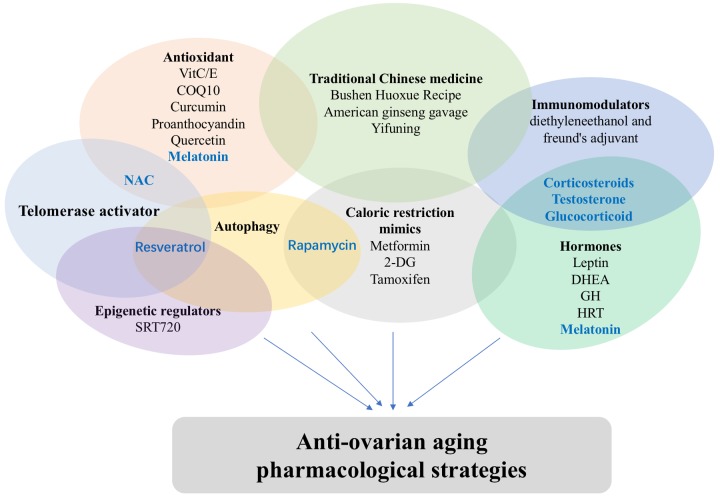
Nowadays, many young women choose to delay or avoid marriage and/or giving a birth owing to the accelerated pace of our society and various pressures from work and family. However, their ovarian reserves strictly define their best age of fertility, since a sharp decline in ovarian functions is observed after age 35, which defines a high-risk period. After 35 years-old, the rate of pregnancy markedly decreases while miscarriage and premature birth for a woman increase significantly. Consequently, the health of maternal females and child is threatened post this stage. Therefore, more systematic and careful studies of the female reproductive system, especially the mechanisms of ovarian aging and the effective delay strategies, are of great importance. Current research on ovarian aging mechanisms, such as primordial follicles activation and follicular atresia, have provided new insights into our better understanding of molecular mechanisms underlying ovarian aging delay. This rich information has been useful for development of anti-ovarian aging therapies as described above. Upon our review, some drugs may have several pharmacological actions and work through a variety of pathways (Figure 1). We believe that, upon future establishments of effective therapies, it will no longer be a fantasy to extend women's reproductive life and delay menopause with the development of medical advances in this specific area.
Figure 1.
Classification of anti-ovarian aging drugs. Some drugs may have several pharmacological actions.
ACKNOWLEDGEMENTS
The important contributions of our graduate students and colleagues in this research are gratefully acknowledged.
Footnotes
CONFLICTS OF INTEREST: The authors have no conflicts of interest.
FUNDING: This research was supported by the grant from the Key national research and development projects (2016YFC1302900) and National Natural Science Foundation of China (No.81671394, No.81370469, No.81873824).
REFERENCES
- 1.Fontana L, Kennedy BK, Longo VD, Seals D, Melov S. Medical research: treat ageing. Nature. 2014; 511:405–07. 10.1038/511405a [DOI] [PubMed] [Google Scholar]
- 2.Steel N, Ford JA, Newton JN, Davis AC, Vos T, Naghavi M, Glenn S, Hughes A, Dalton AM, Stockton D, Humphreys C, Dallat M, Schmidt J, et al. Changes in health in the countries of the UK and 150 English Local Authority areas 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018; 392:1647–61. 10.1016/S0140-6736(18)32207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Check Hayden E, and E CH. Anti-ageing pill pushed as bona fide drug. Nature. 2015; 522:265–66. 10.1038/522265a [DOI] [PubMed] [Google Scholar]
- 4.Younis JS. Ovarian aging and implications for fertility female health. Minerva Endocrinol. 2012; 37:41–57. [PubMed] [Google Scholar]
- 5.Laisk T, Tšuiko O, Jatsenko T, Hõrak P, Otala M, Lahdenperä M, Lummaa V, Tuuri T, Salumets A, Tapanainen JS. Demographic and evolutionary trends in ovarian function and aging. Hum Reprod Update. 2018. 10.1093/humupd/dmy031 [DOI] [PubMed] [Google Scholar]
- 6.Lerner-Geva L, Rabinovici J, Lunenfeld B. Ovarian stimulation: is there a long-term risk for ovarian, breast and endometrial cancer? Womens Health (Lond). 2010; 6:831–39. 10.2217/WHE.10.67 [DOI] [PubMed] [Google Scholar]
- 7.Stevenson JC. A woman’s journey through the reproductive, transitional and postmenopausal periods of life: impact on cardiovascular and musculo-skeletal risk and the role of estrogen replacement. Maturitas. 2011; 70:197–205. 10.1016/j.maturitas.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 8.Onat A, Karadeniz Y, Tusun E, Yüksel H, Kaya A. Advances in understanding gender difference in cardiometabolic disease risk. Expert Rev Cardiovasc Ther. 2016; 14:513–23. 10.1586/14779072.2016.1150782 [DOI] [PubMed] [Google Scholar]
- 9.Dorobantu M, Onciul S, Tautu OF, Cenko E. Hypertension and Ischemic Heart Disease in Women. Curr Pharm Des. 2016; 22:3885–92. 10.2174/1381612822666160414142426 [DOI] [PubMed] [Google Scholar]
- 10.Brar V, Gill S, Cardillo C, Tesauro M, Panza JA, Campia U. Sex-specific effects of cardiovascular risk factors on endothelium-dependent dilation and endothelin activity in middle-aged women and men. PLoS One. 2015; 10:e0121810. 10.1371/journal.pone.0121810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wise PM, Krajnak KM, Kashon ML. Menopause: the aging of multiple pacemakers. Science. 1996; 273:67–70. 10.1126/science.273.5271.67 [DOI] [PubMed] [Google Scholar]
- 12.Vaiserman AM, Lushchak OV, Koliada AK. Anti-aging pharmacology: promises and pitfalls. Ageing Res Rev. 2016; 31:9–35. 10.1016/j.arr.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 13.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956; 11:298–300. 10.1093/geronj/11.3.298 [DOI] [PubMed] [Google Scholar]
- 14.Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003; 5:557–61. 10.1089/152308603770310202 [DOI] [PubMed] [Google Scholar]
- 15.Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Missiroli S, Patergnani S, Poletti F, Rimessi A, Duszynski J, Wieckowski MR, Pinton P. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct. 2012; 2012:329635. 10.1155/2012/329635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brink TC, Demetrius L, Lehrach H, Adjaye J. Age-related transcriptional changes in gene expression in different organs of mice support the metabolic stability theory of aging. Biogerontology. 2009; 10:549–64. 10.1007/s10522-008-9197-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012; 2012:646354. 10.1155/2012/646354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med. 2002; 32:1102–15. 10.1016/S0891-5849(02)00826-2 [DOI] [PubMed] [Google Scholar]
- 19.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun. 2003; 305:776–83. 10.1016/S0006-291X(03)00814-3 [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B. Effect of diet on cancer development: is oxidative DNA damage a biomarker? Free Radic Biol Med. 2002; 32:968–74. 10.1016/S0891-5849(02)00808-0 [DOI] [PubMed] [Google Scholar]
- 21.Davies MJ. The oxidative environment and protein damage. Biochim Biophys Acta. 2005; 1703:93–109. 10.1016/j.bbapap.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 22.Hammadeh ME, Al Hasani S, Rosenbaum P, Schmidt W, Fischer Hammadeh C. Reactive oxygen species, total antioxidant concentration of seminal plasma and their effect on sperm parameters and outcome of IVF/ICSI patients. Arch Gynecol Obstet. 2008; 277:515–26. 10.1007/s00404-007-0507-1 [DOI] [PubMed] [Google Scholar]
- 23.Das S, Chattopadhyay R, Ghosh S, Ghosh S, Goswami SK, Chakravarty BN, Chaudhury K. Reactive oxygen species level in follicular fluid--embryo quality marker in IVF? Hum Reprod. 2006; 21:2403–07. 10.1093/humrep/del156 [DOI] [PubMed] [Google Scholar]
- 24.Oyawoye O, Abdel Gadir A, Garner A, Constantinovici N, Perrett C, Hardiman P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod. 2003; 18:2270–74. 10.1093/humrep/deg450 [DOI] [PubMed] [Google Scholar]
- 25.Lim J, Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod. 2011; 84:775–82. 10.1095/biolreprod.110.088583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility - a clinician’s perspective. Reprod Biomed Online. 2005; 11:641–50. 10.1016/S1472-6483(10)61174-1 [DOI] [PubMed] [Google Scholar]
- 27.Kavutcu M, Canbolat O, Oztürk S, Olcay E, Ulutepe S, Ekinci C, Gökhun IH, Durak I. Reduced enzymatic antioxidant defense mechanism in kidney tissues from gentamicin-treated guinea pigs: effects of vitamins E and C. Nephron. 1996; 72:269–74. 10.1159/000188853 [DOI] [PubMed] [Google Scholar]
- 28.Zaken V, Kohen R, Ornoy A. Vitamins C and E improve rat embryonic antioxidant defense mechanism in diabetic culture medium. Teratology. 2001; 64:33–44. 10.1002/tera.1045 [DOI] [PubMed] [Google Scholar]
- 29.Tarín JJ, Pérez-Albalá S, Cano A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol Reprod Dev. 2002; 61:385–97. 10.1002/mrd.10041 [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Okuka M, McLean M, Keefe DL, Liu L. Telomere susceptibility to cigarette smoke-induced oxidative damage and chromosomal instability of mouse embryos in vitro. Free Radic Biol Med. 2010; 48:1663–76. 10.1016/j.freeradbiomed.2010.03.026 [DOI] [PubMed] [Google Scholar]
- 31.Navarro PA, Liu L, Ferriani RA, Keefe DL. Arsenite induces aberrations in meiosis that can be prevented by coadministration of N-acetylcysteine in mice. Fertil Steril. 2006. (Suppl 1); 85:1187–94. 10.1016/j.fertnstert.2005.08.060 [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Trimarchi JR, Navarro P, Blasco MA, Keefe DL. Oxidative stress contributes to arsenic-induced telomere attrition, chromosome instability, and apoptosis. J Biol Chem. 2003; 278:31998–2004. 10.1074/jbc.M303553200 [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Liu M, Ye X, Liu K, Huang J, Wang L, Ji G, Liu N, Tang X, Baltz JM, Keefe DL, Liu L. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Hum Reprod. 2012; 27:1411–20. 10.1093/humrep/des019 [DOI] [PubMed] [Google Scholar]
- 34.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012; 39:283–99. 10.1111/j.1440-1681.2011.05648.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melekoglu R, Ciftci O, Eraslan S, Cetin A, Basak N. Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. J Ovarian Res. 2018; 11:33. 10.1186/s13048-018-0409-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eser A, Hizli D, Namuslu M, Haltas H, Kosus N, Kosus A, Kafali H. Protective effect of curcumin on ovarian reserve in a rat ischemia model: an experimental study. Clin Exp Obstet Gynecol. 2017; 44:453–57. [PubMed] [Google Scholar]
- 37.Wang XN, Zhang CJ, Diao HL, Zhang Y. Protective Effects of Curcumin against Sodium Arsenite-induced Ovarian Oxidative Injury in a Mouse Model. Chin Med J (Engl). 2017; 130:1026–32. 10.4103/0366-6999.204927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiwari-Pandey R, Ram Sairam M. Modulation of ovarian structure and abdominal obesity in curcumin- and flutamide-treated aging FSH-R haploinsufficient mice. Reprod Sci. 2009; 16:539–50. 10.1177/1933719109332822 [DOI] [PubMed] [Google Scholar]
- 39.Santos-Ocaña C, Do TQ, Padilla S, Navas P, Clarke CF. Uptake of exogenous coenzyme Q and transport to mitochondria is required for bc1 complex stability in yeast coq mutants. J Biol Chem. 2002; 277:10973–81. 10.1074/jbc.M112222200 [DOI] [PubMed] [Google Scholar]
- 40.Villalba JM, Navas P. Plasma membrane redox system in the control of stress-induced apoptosis. Antioxid Redox Signal. 2000; 2:213–30. 10.1089/ars.2000.2.2-213 [DOI] [PubMed] [Google Scholar]
- 41.Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, Naranian T, Chi M, Wang Y, Bentov Y, Alexis J, Meriano J, Sung HK, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015; 14:887–95. 10.1111/acel.12368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Özcan P, Fıçıcıoğlu C, Kizilkale O, Yesiladali M, Tok OE, Ozkan F, Esrefoglu M. Can Coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage? J Assist Reprod Genet. 2016; 33:1223–30. 10.1007/s10815-016-0751-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, Kuszynski CA, Joshi SS, Pruess HG. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology. 2000; 148:187–97. 10.1016/S0300-483X(00)00210-9 [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Lin X, Mi Y, Li J, Zhang C. Grape Seed Proanthocyanidin Extract Prevents Ovarian Aging by Inhibiting Oxidative Stress in the Hens. Oxid Med Cell Longev. 2018; 2018:9390810. 10.1155/2018/9390810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Qian X, Gao Q, Lv C, Xu J, Jin H, Zhu H. Quercetin increases the antioxidant capacity of the ovary in menopausal rats and in ovarian granulosa cell culture in vitro. J Ovarian Res. 2018; 11:51. 10.1186/s13048-018-0421-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo LL, Huang J, Fu YC, Xu JJ, Qian YS. Effects of tea polyphenols on ovarian development in rats. J Endocrinol Invest. 2008; 31:1110–18. 10.1007/BF03345661 [DOI] [PubMed] [Google Scholar]
- 47.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1989; 5:155–71. [PubMed] [Google Scholar]
- 48.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005; 126:913–22. 10.1016/j.mad.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 49.Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008; 7:622–29. 10.1111/j.1474-9726.2008.00409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anisimov VN. Insulin/IGF-1 signaling pathway driving aging and cancer as a target for pharmacological intervention. Exp Gerontol. 2003; 38:1041–49. 10.1016/S0531-5565(03)00169-4 [DOI] [PubMed] [Google Scholar]
- 51.Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, et al. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell. 2015; 14:497–510. 10.1111/acel.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 2012; 122:253–70. 10.1042/CS20110386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palomba S, Falbo A, Zullo F, Orio F Jr. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev. 2009; 30:1–50. 10.1210/er.2008-0030 [DOI] [PubMed] [Google Scholar]
- 54.Oner G, Ozcelik B, Ozgun MT, Ozturk F. The effects of metformin and letrozole on endometrium and ovary in a rat model. Gynecol Endocrinol. 2011; 27:1084–86. 10.3109/09513590.2011.589928 [DOI] [PubMed] [Google Scholar]
- 55.Stadtmauer L, Vidali A, Lindheim SR, Sauer MV. Follicular fluid insulin-like growth factor-I and insulin-like growth factor-binding protein-1 and -3 vary as a function of ovarian reserve and ovarian stimulation. J Assist Reprod Genet. 1998; 15:587–93. 10.1023/A:1020377209952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malm SW, Hanke NT, Gill A, Carbajal L, Baker AF. The anti-tumor efficacy of 2-deoxyglucose and D-allose are enhanced with p38 inhibition in pancreatic and ovarian cell lines. J Exp Clin Cancer Res. 2015; 34:31. 10.1186/s13046-015-0147-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xi H, Kurtoglu M, Lampidis TJ. The wonders of 2-deoxy-D-glucose. IUBMB Life. 2014; 66:110–21. 10.1002/iub.1251 [DOI] [PubMed] [Google Scholar]
- 58.Barilovits SJ, Newsom KJ, Bickford JS, Beachy DE, Rhoton-Vlasak A, Nick HS. Characterization of a mechanism to inhibit ovarian follicle activation. Fertil Steril. 2014; 101:1450–57. 10.1016/j.fertnstert.2014.01.025 [DOI] [PubMed] [Google Scholar]
- 59.Ngô C, Brugier C, Plancher C, de la Rochefordière A, Alran S, Féron JG, Malhaire C, Scholl S, Sastre X, Rouzier R, Fourchotte V, and Gynecological Cancer Study Group of Institut Curie. Clinico-pathology and prognosis of endometrial cancer in patients previously treated for breast cancer, with or without tamoxifen: a comparative study in 363 patients. Eur J Surg Oncol. 2014; 40:1237–44. 10.1016/j.ejso.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 60.Lee JY, Shin JY, Kim HS, Heo JI, Kho YJ, Kang HJ, Park SH, Lee JY. Effect of combined treatment with progesterone and tamoxifen on the growth and apoptosis of human ovarian cancer cells. Oncol Rep. 2012; 27:87–93. 10.3892/or.2011.1460 [DOI] [PubMed] [Google Scholar]
- 61.Stadtmauer L, Vidali A, Lindheim SR, Sauer MV. Follicular fluid insulin-like growth factor-I and insulin-like growth factor-binding protein-1 and -3 vary as a function of ovarian reserve and ovarian stimulation. J Assist Reprod Genet. 1998; 15:587–93. 10.1023/A:1020377209952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahran YF, El-Demerdash E, Nada AS, Ali AA, Abdel-Naim AB. Insights into the protective mechanisms of tamoxifen in radiotherapy-induced ovarian follicular loss: impact on insulin-like growth factor 1. Endocrinology. 2013; 154:3888–99. 10.1210/en.2013-1214 [DOI] [PubMed] [Google Scholar]
- 63.Ehninger D, Neff F, Xie K. Longevity, aging and rapamycin. Cell Mol Life Sci. 2014; 71:4325–46. 10.1007/s00018-014-1677-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS. Modulating mTOR in aging and health. Interdiscip Top Gerontol. 2015; 40:107–27. 10.1159/000364974 [DOI] [PubMed] [Google Scholar]
- 65.Albert V, Hall MN. mTOR signaling in cellular and organismal energetics. Curr Opin Cell Biol. 2015; 33:55–66. 10.1016/j.ceb.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 66.Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004; 131:3897–906. 10.1242/dev.01255 [DOI] [PubMed] [Google Scholar]
- 67.Tibor Vellai KT, Zhang Y, Attila L. Kovacs, László Orosz & Fritz Müller. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003; 426:620 10.1038/426620a [DOI] [PubMed] [Google Scholar]
- 68.Pankaj Kapahi BM. Tony Harper, Daniel Koslover, Viveca Sapin, and Seymour Benzer. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004; 14:885–90. 10.1016/j.cub.2004.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012; 335:1638–43. 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Passtoors WM, Beekman M, Deelen J, van der Breggen R, Maier AB, Guigas B, Derhovanessian E, van Heemst D, de Craen AJ, Gunn DA, Pawelec G, Slagboom PE. Gene expression analysis of mTOR pathway: association with human longevity. Aging Cell. 2013; 12:24–31. 10.1111/acel.12015 [DOI] [PubMed] [Google Scholar]
- 71.Zhang XM, Li L, Xu JJ, Wang N, Liu WJ, Lin XH, Fu YC, Luo LL. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene. 2013; 523:82–87. 10.1016/j.gene.2013.03.039 [DOI] [PubMed] [Google Scholar]
- 72.Tong Y, Li F, Lu Y, Cao Y, Gao J, Liu J. Rapamycin-sensitive mTORC1 signaling is involved in physiological primordial follicle activation in mouse ovary. Mol Reprod Dev. 2013; 80:1018–34. 10.1002/mrd.22267 [DOI] [PubMed] [Google Scholar]
- 73.Adhikari D, Risal S, Liu K, Shen Y. Pharmacological inhibition of mTORC1 prevents over-activation of the primordial follicle pool in response to elevated PI3K signaling. PLoS One. 2013; 8:e53810. 10.1371/journal.pone.0053810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arguelles AO, Meruvu S, Bowman JD, Choudhury M. Are epigenetic drugs for diabetes and obesity at our door step? Drug Discov Today. 2016; 21:499–509. 10.1016/j.drudis.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 75.Cacabelos R, Torrellas C. Epigenetics of Aging and Alzheimer’s Disease: Implications for Pharmacogenomics and Drug Response. Int J Mol Sci. 2015; 16:30483–543. 10.3390/ijms161226236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.von Meyenn F, Reik W. Forget the Parents: Epigenetic Reprogramming in Human Germ Cells. Cell. 2015; 161:1248–51. 10.1016/j.cell.2015.05.039 [DOI] [PubMed] [Google Scholar]
- 77.Zhou XL, Xu JJ, Ni YH, Chen XC, Zhang HX, Zhang XM, Liu WJ, Luo LL, Fu YC. SIRT1 activator (SRT1720) improves the follicle reserve and prolongs the ovarian lifespan of diet-induced obesity in female mice via activating SIRT1 and suppressing mTOR signaling. J Ovarian Res. 2014; 7:97. 10.1186/s13048-014-0097-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu M, Yin Y, Ye X, Zeng M, Zhao Q, Keefe DL, Liu L. Resveratrol protects against age-associated infertility in mice. Hum Reprod. 2013; 28:707–17. 10.1093/humrep/des437 [DOI] [PubMed] [Google Scholar]
- 79.Chen ZG, Luo LL, Xu JJ, Zhuang XL, Kong XX, Fu YC. Effects of plant polyphenols on ovarian follicular reserve in aging rats. Biochem Cell Biol. 2010; 88:737–45. 10.1139/O10-012 [DOI] [PubMed] [Google Scholar]
- 80.Schneider A, Matkovich SJ, Victoria B, Spinel L, Bartke A, Golusinski P, Masternak MM. Changes of Ovarian microRNA Profile in Long-Living Ames Dwarf Mice during Aging. PLoS One. 2017; 12:e0169213. 10.1371/journal.pone.0169213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barragán M, Pons J, Ferrer-Vaquer A, Cornet-Bartolomé D, Schweitzer A, Hubbard J, Auer H, Rodolosse A, Vassena R. The transcriptome of human oocytes is related to age and ovarian reserve. Mol Hum Reprod. 2017; 23:535–48. 10.1093/molehr/gax033 [DOI] [PubMed] [Google Scholar]
- 82.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000; 290:1717–21. 10.1126/science.290.5497.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kroemer G. Autophagy: a druggable process that is deregulated in aging and human disease. J Clin Invest. 2015; 125:1–4. 10.1172/JCI78652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Findlay JK, Hutt KJ, Hickey M, Anderson RA. How Is the Number of Primordial Follicles in the Ovarian Reserve Established? Biol Reprod. 2015; 93:111. 10.1095/biolreprod.115.133652 [DOI] [PubMed] [Google Scholar]
- 85.Li L, Fu YC, Xu JJ, Lin XH, Chen XC, Zhang XM, Luo LL. Caloric restriction promotes the reserve of follicle pool in adult female rats by inhibiting the activation of mammalian target of rapamycin signaling. Reprod Sci. 2015; 22:60–67. 10.1177/1933719114542016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hurst PR, Mora JM, Fenwick MA. Caspase-3, TUNEL and ultrastructural studies of small follicles in adult human ovarian biopsies. Hum Reprod. 2006; 21:1974–80. 10.1093/humrep/del109 [DOI] [PubMed] [Google Scholar]
- 87.Yang YC, Zhang C, Wu J, Chan WY. Melatonin as potential targets for delaying ovarian aging. Curr Drug Targets. 2018; 19. 10.2174/1389450119666180828144843 [DOI] [PubMed] [Google Scholar]
- 88.Wang YF, Sun XF, Han ZL, Li L, Ge W, Zhao Y, De Felici M, Shen W, Cheng SF. Protective effects of melatonin against nicotine-induced disorder of mouse early folliculogenesis. Aging (Albany NY). 2018; 10:463–80. 10.18632/aging.101405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tamura H, Kawamoto M, Sato S, Tamura I, Maekawa R, Taketani T, Aasada H, Takaki E, Nakai A, Reiter RJ, Sugino N. Long-term melatonin treatment delays ovarian aging. J Pineal Res. 2017; 62:e12381. 10.1111/jpi.12381 [DOI] [PubMed] [Google Scholar]
- 90.Sugiyama M, Kawahara-Miki R, Kawana H, Shirasuna K, Kuwayama T, Iwata H. Resveratrol-induced mitochondrial synthesis and autophagy in oocytes derived from early antral follicles of aged cows. J Reprod Dev. 2015; 61:251–59. 10.1262/jrd.2015-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. Telomere length in early life predicts lifespan. Proc Natl Acad Sci USA. 2012; 109:1743–48. 10.1073/pnas.1113306109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aydos SE, Elhan AH, Tükün A. Is telomere length one of the determinants of reproductive life span? Arch Gynecol Obstet. 2005; 272:113–16. 10.1007/s00404-004-0690-2 [DOI] [PubMed] [Google Scholar]
- 93.Thilagavathi J, Venkatesh S, Dada R. Telomere length in reproduction. Andrologia. 2013; 45:289–304. 10.1111/and.12008 [DOI] [PubMed] [Google Scholar]
- 94.Xu X, Chen X, Zhang X, Liu Y, Wang Z, Wang P, Du Y, Qin Y, Chen ZJ. Impaired telomere length and telomerase activity in peripheral blood leukocytes and granulosa cells in patients with biochemical primary ovarian insufficiency. Hum Reprod. 2017; 32:201–07. 10.1093/humrep/dew283 [DOI] [PubMed] [Google Scholar]
- 95.Yamada-Fukunaga T, Yamada M, Hamatani T, Chikazawa N, Ogawa S, Akutsu H, Miura T, Miyado K, Tarín JJ, Kuji N, Umezawa A, Yoshimura Y. Age-associated telomere shortening in mouse oocytes. Reprod Biol Endocrinol. 2013; 11:108. 10.1186/1477-7827-11-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu M, Yin Y, Ye X, Zeng M, Zhao Q, Keefe DL, Liu L. Resveratrol protects against age-associated infertility in mice. Hum Reprod. 2013; 28:707–17. 10.1093/humrep/des437 [DOI] [PubMed] [Google Scholar]
- 97.Claustrat B, Leston J. Melatonin: physiological effects in humans. Neurochirurgie. 2015; 61:77–84. 10.1016/j.neuchi.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 98.Place NJ, Tuthill CR, Schoomer EE, Tramontin AD, Zucker I. Short day lengths delay reproductive aging. Biol Reprod. 2004; 71:987–92. 10.1095/biolreprod.104.029900 [DOI] [PubMed] [Google Scholar]
- 99.Finley CM, Gorman MR, Tuthill CR, Zucker I. Long-term reproductive effects of a single long day in the Siberian hamster (Phodopus sungorus). J Biol Rhythms. 1995; 10:33–41. 10.1177/074873049501000103 [DOI] [PubMed] [Google Scholar]
- 100.Meredith S, Jackson K, Dudenhoeffer G, Graham L, Epple J. Long-term supplementation with melatonin delays reproductive senescence in rats, without an effect on number of primordial follicles. Exp Gerontol. 2000; 35:343–52. 10.1016/S0531-5565(00)00092-9 [DOI] [PubMed] [Google Scholar]
- 101.Song C, Peng W, Yin S, Zhao J, Fu B, Zhang J, Mao T, Wu H, Zhang Y. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci Rep. 2016; 6:35165. 10.1038/srep35165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fernández BE, Díaz E, Fernández C, Núñez P, Díaz B. Ovarian aging: melatonin regulation of the cytometric and endocrine evolutive pattern. Curr Aging Sci. 2013; 6:1–7. 10.2174/1874609811306010001 [DOI] [PubMed] [Google Scholar]
- 103.Bellipanni G, Bianchi P, Pierpaoli W, Bulian D, Ilyia E. Effects of melatonin in perimenopausal and menopausal women: a randomized and placebo controlled study. Exp Gerontol. 2001; 36:297–310. 10.1016/S0531-5565(00)00217-5 [DOI] [PubMed] [Google Scholar]
- 104.Tamura HK, Sato S, Tamura I, Maekawa R, Taketani T, Aasada H, Takaki E, Nakai A, Reiter RJ, Sugino N. Long term melatonin treatment delays ovarian aging. J Pineal Res. 2017; 62. 10.1111/jpi.12381 [DOI] [PubMed] [Google Scholar]
- 105.Gregoraszczuk EŁ, Ptak A, Wojciechowicz T, Nowak K. Action of IGF-I on expression of the long form of the leptin receptor (ObRb) in the prepubertal period and throughout the estrous cycle in the mature pig ovary. J Reprod Dev. 2007; 53:289–95. 10.1262/jrd.18071 [DOI] [PubMed] [Google Scholar]
- 106.Joo JK, Joo BS, Kim SC, Choi JR, Park SH, Lee KS. Role of leptin in improvement of oocyte quality by regulation of ovarian angiogenesis. Anim Reprod Sci. 2010; 119:329–34. 10.1016/j.anireprosci.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 107.Kikuchi N, Andoh K, Abe Y, Yamada K, Mizunuma H, Ibuki Y. Inhibitory action of leptin on early follicular growth differs in immature and adult female mice. Biol Reprod. 2001; 65:66–71. 10.1095/biolreprod65.1.66 [DOI] [PubMed] [Google Scholar]
- 108.Panwar S, Herrid M, Kauter KG, McFarlane JR. Effect of passive immunization against leptin on ovarian follicular development in prepubertal mice. J Reprod Immunol. 2012; 96:19–24. 10.1016/j.jri.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 109.Hamm ML, Bhat GK, Thompson WE, Mann DR. Folliculogenesis is impaired and granulosa cell apoptosis is increased in leptin-deficient mice. Biol Reprod. 2004; 71:66–72. 10.1095/biolreprod.104.027292 [DOI] [PubMed] [Google Scholar]
- 110.Sominsky L, Ziko I, Soch A, Smith JT, Spencer SJ. Neonatal overfeeding induces early decline of the ovarian reserve: implications for the role of leptin. Mol Cell Endocrinol. 2016; 431:24–35. 10.1016/j.mce.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 111.Fouany MR, Sharara FI. Is there a role for DHEA supplementation in women with diminished ovarian reserve? J Assist Reprod Genet. 2013; 30:1239–44. 10.1007/s10815-013-0018-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yilmaz N, Uygur D, Inal H, Gorkem U, Cicek N, Mollamahmutoglu L. Dehydroepiandrosterone supplementation improves predictive markers for diminished ovarian reserve: serum AMH, inhibin B and antral follicle count. Eur J Obstet Gynecol Reprod Biol. 2013; 169:257–60. 10.1016/j.ejogrb.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 113.Singh N, Zangmo R, Kumar S, Roy KK, Sharma JB, Malhotra N, Vanamail P. A prospective study on role of dehydroepiandrosterone (DHEA) on improving the ovarian reserve markers in infertile patients with poor ovarian reserve. Gynecol Endocrinol. 2013; 29:989–92. 10.3109/09513590.2013.824957 [DOI] [PubMed] [Google Scholar]
- 114.Zhang J, Qiu X, Gui Y, Xu Y, Li D, Wang L. Dehydroepiandrosterone improves the ovarian reserve of women with diminished ovarian reserve and is a potential regulator of the immune response in the ovaries. Biosci Trends. 2015; 9:350–59. 10.5582/bst.2015.01154 [DOI] [PubMed] [Google Scholar]
- 115.Narkwichean A, Jayaprakasan K, Maalouf WE, Hernandez-Medrano JH, Pincott-Allen C, Campbell BK. Effects of dehydroepiandrosterone on in vivo ovine follicular development. Hum Reprod. 2014; 29:146–54. 10.1093/humrep/det408 [DOI] [PubMed] [Google Scholar]
- 116.Ikeda K, Baba T, Morishita M, Honnma H, Endo T, Kiya T, Saito T. Long-term treatment with dehydroepiandrosterone may lead to follicular atresia through interaction with anti-Mullerian hormone. J Ovarian Res. 2014; 7:46. 10.1186/1757-2215-7-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Slot KA, Kastelijn J, Bachelot A, Kelly PA, Binart N, Teerds KJ. Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction. 2006; 131:525–32. 10.1530/rep.1.00946 [DOI] [PubMed] [Google Scholar]
- 118.Mahran YF, El-Demerdash E, Nada AS, El-Naga RN, Ali AA, Abdel-Naim AB. Growth Hormone Ameliorates the Radiotherapy-Induced Ovarian Follicular Loss in Rats: Impact on Oxidative Stress, Apoptosis and IGF-1/IGF-1R Axis. PLoS One. 2015; 10:e0140055. 10.1371/journal.pone.0140055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yeqing Guo SY. Treatment status and progress of premature ovarian insufficiency. Chinese journal of clinical physicians. 2015; 9(6):972-975.
- 120.Davies MC, Cartwright B. What is the best management strategy for a 20-year-old woman with premature ovarian failure? Clin Endocrinol (Oxf). 2012; 77:182–86. 10.1111/j.1365-2265.2012.04408.x [DOI] [PubMed] [Google Scholar]
- 121.Feng H. Clinical treatment of premature ovarian failure and pregnancy rate after ovulation induction. Shanxi medical journal. 2010; 39:51-53.
- 122.Langrish JP, Mills NL, Bath LE, Warner P, Webb DJ, Kelnar CJ, Critchley HO, Newby DE, Wallace WH. Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension. 2009; 53:805–11. 10.1161/HYPERTENSIONAHA.108.126516 [DOI] [PubMed] [Google Scholar]
- 123.Güleç Başer B, İslimye Taşkın M, Adalı E, Öztürk E, Hısmıoğulları AA, Yay A. Does progesterone have protective effects on ovarian ischemia-reperfusion injury? J Turk Ger Gynecol Assoc. 2018; 19:87–93. 10.4274/jtgga.2017.0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dragojević-Dikić S, Marisavljević D, Mitrović A, Dikić S, Jovanović T, Janković-Raznatović S. An immunological insight into premature ovarian failure (POF). Autoimmun Rev. 2010; 9:771–74. 10.1016/j.autrev.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 125.Prendiville DJ, Enright WJ, Crowe MA, Vaughan L, Roche JF. Immunization of prepubertal beef heifers against gonadotropin-releasing hormone: immune, estrus, ovarian, and growth responses. J Anim Sci. 1995; 73:3030–37. 10.2527/1995.73103030x [DOI] [PubMed] [Google Scholar]
- 126.Xiaobo Shi NL, Can L, Shu Q, Zhu F. Glucocorticoid or androgen therapy in mice with autoimmune premature ovarian failure. J Cent South Univ. 2009; 34:576–81. [PubMed] [Google Scholar]
- 127.Xiafei Fu YH. Advances in diagnosis and treatment of premature ovarian failure. Journal of Guangdong medical. 2010; 31(8):933-934.
- 128.Ling Xie RL. Ye Lv, Hong Ma. Mechanisms research progress of traditional Chinese medicine on delaying ovarian aging and protecting ovarian function in peri-menopausal. journal of Jiangsu traditional. Chin Med. 2005; 26:59–61. [Google Scholar]
- 129.Guoli Zhang PF, Xian Ma. Progress in experimental research on the treatment of perimenopausal syndrome by traditional Chinese medicine. Journal of Changchun university of Chinese medicine. 2015; 31(2).
- 130.Nugent BM, Tobet SA, Lara HE, Lucion AB, Wilson ME, Recabarren SE, Paredes AH. Hormonal programming across the lifespan. Horm Metab Res. 2012; 44:577–86. 10.1055/s-0032-1312593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lei Zhu JL, Xing Nannan, Han Dongwei, Kuang Haixue, and Pengling Ge American ginseng regulates gene expression to protect against premature ovarian failure in rats. BioMed Res Int. 2015; 2015:767124. 10.1155/2015/767124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang C, Song K, Ma W, Ding J, Chen Z, Zhang M. Immunomodulatory mechanism of Bushen Huoxue Recipe alleviates cyclophosphamide-induced diminished ovarian reserve in mouse model. J Ethnopharmacol. 2017; 208:44–56. 10.1016/j.jep.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 133.Song KK, Ma WW, Huang C, Ding JH, Cui DD, Tan XJ, Xiao J, Zhang MM. Effect and mechanism of Bushen Huoxue recipe on ovarian reserve in mice with premature ovarian failure. J Huazhong Univ Sci Technolog Med Sci. 2016; 36:571–75. 10.1007/s11596-016-1627-2 [DOI] [PubMed] [Google Scholar]
- 134.Xia T, Fu Y, Li S, Ma R, Zhao Z, Wang B, Chao C. Bu Shen Tiao Chong recipe restores diminished ovary reserve through the BDNF pathway. J Assist Reprod Genet. 2016; 33:795–805. 10.1007/s10815-016-0697-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liang L, Zhang XH, Ji B, Yao H, Ling XM, Guo ZJ, Deng HZ, Wu XR. Yifuning postpones ovarian aging through antioxidant mechanisms and suppression of the Rb/p53 signal transduction pathway. Mol Med Rep. 2016; 14:888–96. 10.3892/mmr.2016.5322 [DOI] [PubMed] [Google Scholar]
- 136.Yuanquan Ding SW, Nan C, Zhang L, Yuan J. Effect of Bu shen recipe on VEGF expression in ovaries of perimenopausal rats. J Tradit Chin Med. 2006; 24. [Google Scholar]
- 137.Shen M, Qi C, Kuang YP, Yang Y, Lyu QF, Long H, Yan ZG, Lu YY. Observation of the influences of diosgenin on aging ovarian reserve and function in a mouse model. Eur J Med Res. 2017; 22:42. 10.1186/s40001-017-0285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang J, Fang L, Shi L, Lai Z, Lu Z, Xiong J, Wu M, Luo A, Wang S. Protective effects and mechanisms investigation of Kuntai capsule on the ovarian function of a novel model with accelerated aging ovaries. J Ethnopharmacol. 2017; 195:173–81. 10.1016/j.jep.2016.11.014 [DOI] [PubMed] [Google Scholar]