Abstract
Leveraging the multifunctional nature of citrate in chemistry and inspired by its important role in biological tissues, a class of highly versatile and functional citrate-based materials (CBBs) has been developed via facile and cost-effective polycondensation. CBBs exhibiting tunable mechanical properties and degradation rates, together with excellent biocompatibility and processability to be successfully applied in vitro and in vivo for applications ranging from soft to hard tissue regeneration, as well as for nanomedicine designs. We summarize in the review, chemistry considerations for CBBs design to tune polymer properties and to introduce functionality with a focus on the most recent advances, biological functions of citrate in native tissues with the new notion of degradation products as cell modulator highlighted, and the applications of CBBs in wound healing, nanomedicine, orthopedic, cardiovascular, nerve and bladder tissue engineering. Given the expansive evidence for citrate’s potential in biology and biomaterial science outlined in this review, it is expected that citrate based materials will continue to play an important role in regenerative engineering.
Keywords: citric acid, elastomer, antimicrobial, osteogenic differentiation, metabolic regulation, regenerative engineering
1. Introduction
The expanding field of regenerative engineering, a convergence of advanced materials design, stem cell biology, and developmental biology for the restoration or regeneration of complex tissues [1], necessitates an ever increasing array of biomaterials enabling tailored properties and functionalities, together with a comprehensive yet in-depth understanding of tissue responses to those biomaterials in specific biological settings. While it is expected that naturally derived autografts and allografts as well as native materials such as collagen or hyaluronic acid will provide the optimal replication of the native tissue, the severe limitations of these materials in terms of supply and cost, as well as the inherent heterogeneity and limited tunability of their properties led to the development of synthetic alternatives. Thermoplastic materials such as poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA) and poly(caprolactone) (PCL) have thus far been the materials of choice for biomedical applications due to their high degree of processability and approval for the use in the Food and Drug Administration (FDA) approved devices; however, these materials are still limited by their plastic mechanics, slow degradation rates, limited cell interactions, and limited potential for functionalization.
Poly(glycerol sebacate) (PGS), the first generation of elastomeric thermoset materials, have emerged in recent years as an effective alternative to thermoplastics with mechanics that more closely resemble the native cross-linked extracellular matrix (ECM), leading to improved cell interactions and gradual transfer of mechanical loading from scaffolds to regenerating tissue concomitant with material degradation [2, 3]. While PGS has gained wide acceptance in the biomaterials field, its limited mechanical strength and harsh cross-linking conditions have called for improved elastomeric materials. To address these limitations, Yang et al. synthesized a family of poly(diol citrates) utilizing multifunctional citric acid as the key and cornerstone monomer [4, 5] and a facile and simple polycondensation reaction, inaugurating a new family of citrate based biomaterials (CBBs) possessing a wide range of tunable mechanical properties and degradation rate [6]. The large number of functional pendent carboxyl and hydroxyl groups present in CBBs from citrate has allowed multiple functionalities, including incorporation of bioactive moieties, introduction of additional cross-link networks, as well as imparting intrinsic fluorescence, serving as a potent example of utilizing simple chemistry to endow functional complexity.
Additionally, the widespread presence and biological function of citrate, notably represented by its role of metabolic regulation, antimicrobial potential, mineralization regulation, anticoagulant effect, neuronal excitability regulation, and renal stone prevention, have suggested CBBs’ suitability in vitro and in vivo, where they have demonstrated excellent biocompatibility, adequate immune response, antimicrobial properties and hemocompatibility. The versatility brought by citrate chemistry and citrate biology have led to the application of CBBs to many areas of regenerative engineering, including wound healing, nanomedicine, orthopedic, cardiovascular, nerve and bladder tissue engineering. Further, the emerging interest in degradation product as cell regulator bridges citrate chemistry and citrate biology in biomaterials design, presaging future directions of CBBs design not only functionalized through chemistry, but with intrinsic cell regulatory capabilities. Based on our previous review that summarizes the fundamental rationale and functionalization strategies of CBBs [6], this review intends to offer an overview of 1) fundamental polymer syntheses with a detailed discussion of tunable polymer properties; 2) the chemistry considerations for versatile and functionalized CBBs design with a focus on the most recent advances in materials design, device fabrication, as well as comprehensive understanding of fluorescence mechanism, 3) the biological function of citrate in different tissues based on the rediscoveries of materials degradation products as cell modulators, as well as 4) the current and updated applications of CBBs in regenerative engineering based on the citrate biology considerations, aiming at inspiring the next generation of biomaterials innovation to match the diverse biomedical needs.
2. Fundamental Citrate-based Polymer Syntheses and Properties
2.1. Polymer Syntheses
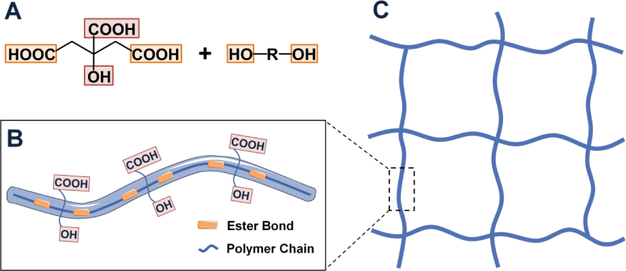
The synthesis of citrate-based biomaterials (CBBs) was first reported by Yang et al. in 2004 [4] with poly(octamethylene citrate) (POC), composed of the starting materials citric acid and 1,8octanediol and synthesized via a simple, catalyst-free, one pot polycondensation reaction. Citric acid, containing three carboxyl groups and one hydroxyl group (Fig. 1A), is a biocompatible, reactive, and multifunctional monomer which contributes to the formation of hydrolysable ester bonds in combination with diol monomers to constitute pre-polymer chains (Fig. 1B). The tetra functional nature of the citrate monomer concurrently provides pendent carboxyl and hydroxyl groups that can be preserved during pre-polymer synthesis, not only contributing to further polymer network cross-linking post-polymerization, but also providing active sites for introducing functionalities. 1, 8-Octanediol is the largest aliphatic diol that remains water soluble with no reported toxicity, a property that ensures no insoluble complexes remain following degradation [4]. The pre-polymer can be purified by precipitation in water followed by freeze-drying, and dissolved in multiple solvents including ethanol and 1,4-dioxane. Dissolved pre-polymer is stable and can be further cross-linked, conferring elasticity through the formation of 3D polymer networks similar to those naturally occurring in polymers such as collagen, proteoglycan, and elastin. In pre-polymer form it is readily processable into multiple sample with geometries and microarchitectures, such as films, cylindrical blocks, tubular or sponge-like scaffolds, biphasic or triphasic scaffolds, and nano- or micro-particles.
Fig. 1. Design rationale of citrate-based materials.
(A) Multifunctional citric acid contributes to ester bond formation in combination with diols (yellow) to prepare pre-polymer chain, while preserving valuable pendant chemistry (Red) (B) for further cross-linking into polymeric network (C), and for additional functionalization.
2.2. Polymer Properties
Generally, ideal materials for biological applications should be biocompatible and biodegradable, allowing for adequate cell loading, while possessing mechanical and physio-chemical properties that are suitable for the target application. Understanding the following materials properties of biomaterials is the first step towards elucidating its potential for different applications.
2.2.1. Physio-chemical Properties
POC is a transparent and almost colorless polyester. Fourier transform infrared (FTIR) analysis of its pre-polymer, whose average molecular weight (Mw) is 1088 (Mn=1085), showed intensive signature carbonyl bands within 1690–1750 cm−1 confirming the formation of ester bonds [4] as well as an intense -OH stretch at 3464 cm−1 suggesting that the hydroxyl groups are hydrogen bonded.1H nuclear Magnetic Resonance (NMR) spectroscopy further confirms that the composition of the pre-polymer is approximately 1:1 citric acid:1,8-octanediol, which agrees with the initial reaction feeding ratio [4]. Differential Scanning Calorimetry (DSC) thermograms showed no crystallization or melting temperature and an apparent glass transition temperature (Tg) between −10 to ~0°C, confirming that POC is amorphous at 37 °C, the physiological temperature. The pedant carboxyl and hydroxyl groups present in citric acid are involved in the formation of the ester linkages during the post-crosslinking step. The crosslinking density increases with cross-linking time and temperature (60, 80, 100, or 120 °C), and is altered by polymer synthesis conditions (initial monomer molar ratio) and the cross-linking conditions (no vacuum or under vacuum). A simple and nondestructive method has been developed [7] to determine the cross-linking density based on equilibrium swelling and dynamic mechanical analysis (DMA). Notably, controlling cross-linking density allows for the tuning of mechanical properties, degradation rate, and chemical functionalities to meet specific requirements.
2.2.2. Mechanical Properties
POC films display a stress-strain curve typical for soft elastomeric materials [4, 5], with a tensile strength as high as 6.1±1.4 MPa and a Young’s modulus ranging from 0.92± 0.02 to 16.4±3.4 MPa, comparable to that of elastin derived from bovine ligament (tensile strength and Young’s modulus of 2 MPa and 1.1 MPa) [8]. The elongation at break reaches as high as 265±10%, similar to that of human arteries (260%) [9] and bovine elastin (150%) [8]. It is theorized that both the covalently cross-linking framework of ester bonds and the hydrogen bonding interactions between hydroxyl groups contribute to the elastic nature of POC [4, 5]. The mechanical properties of the elastomer are directly proportional to the crosslinking density, and are easily tailored by changing synthesis and cross-linking conditions. As many tissues in our body such as the heart, blood vessels, lung, bladder, nerves, and skin, have elastomeric properties, soft elastomeric POC is uniquely suited for the development of compliant scaffolds for tissue engineering [4].
2.2.3. Biodegradability
Biodegradation is a highly desired property for biomaterials. The degradation pattern and degradation rates of materials together with the possible toxicity of degradation products collectively affect the materials’ application potential. POC degrades by cleavage of the ester bonds through surface erosion [6], which offers great controllability and advantages over bulk degradation in terms of presenting a more linear loss of mechanical properties during degradation while avoiding an abrupt accumulation of degradation products that cause undesired tissue response. In addition, POC is fully degradable and its degradation rate is inversely proportional to the cross-linking density. Thus, by changing the synthesis and crosslinking conditions, the degradation of POC can be tuned from a few days to over a year in order to meet the requirement of a specific tissue.
2.2.4. Biocompatibility
The biocompatibility of POC originates from the intrinsic biocompatibility of citric acid and 1,8octanediol. Citric acid is a well-known non-toxic metabolic intermediate of living organism, and is included in a wide range of FDA approved applications, while 1,8-octanediol has been used in cosmetics as plasticizer [10], and a comprehensive biocompatibility evaluation of these two monomers to different cells has been performed to confirm their intrinsic biocompatibility [11]. On the other hand, the biocompatibility of any additional building block built into the POC network for further modification or functionalization should be considered at the onset of materials design. In vitro and in vivo biocompatibility test results have indicated that POC is a suitable basal material for several tissue engineering applications. For example, the growth and viability of human aortic smooth muscle cells (HASMC) and human aortic endothelial cells (HAEC) on POC films are at least as good as or better than that on Poly (L-lactic acid) (PLLA) [4, 5]. In addition, degradation products that readily come into contact with host tissues could exert great impact on the long-term tissue response and should be included for a comprehensive biocompatibility evaluation. Cytotoxicity evaluation of the fully dissolved POC degradation products to 3T3 mouse fibroblast suggests a similar cytotoxicity of POC degradation products to that of PLGA [12]. In vivo biocompatibility evaluation with Sprague-Dawley rats via subcutaneous implantation displays a slightly acute inflammatory response at 1 week after implantation which fades away after 1 month and there is no or minimal sign of chronic inflammatory response. POC also induces a fibrous capsule with a thickness similar to that for poly(glycerol sebacate) (PGS) and thinner than FDA approved poly(L-Lactide-co-glycolide) PLGA [4, 5]. Long-term in vivo evaluation of POC plugs in rabbit femoral condyle models further confirms no evident inflammation at the implant bone interface, better than the PLLA group [13].
2.3. Diol Selection
The material properties of CBBs are manifestations of the underlying monomer building blocks, and thus selection of diols reacted with citrate is a powerful tool for imparting design flexibility and altering the mechanical properties, degradation rates, and other physical properties such as swelling beyond the effects of altered cross-linking conditions and cross-link density. Based on POC synthesis, materials with increasing aliphatic diol length (C4–C12) display decreased material stiffness, increased elasticity, and slower degradation [5]. Further, by increasing diol length to C12-C16 and diol feeding ratio, CBBs capable of shape memory have been synthesized. Such materials display hydrophobic micro-domains consisting of thermodynamically favorable associations of the hydrophobic diol chains and increased Tg versus POC, allowing them to be molded into complex geometries when heated above their Tg which are then fixed when the material is cooled [14]. Thermal cycling through the transition point can be used to change the material geometry many times. Partial or complete replacement of hydrophobic aliphatic diols with hydrophilic diols such as poly(ethylene glycol) (PEG), poly(vinyl alcohol), and xylitol [15] leads to increased hydrophilic character or even water solubility, particularly advantageous in applications that involve organic solvent sensitive proteins or cells. Increased feeding ratio of hydrophilic and small diols leads to stiffer materials with faster degradation rates. Further, modifying CBBs with non-aliphatic diols can impart unique functional properties. Notably, through the incorporation of N-methyldiethanolamine (MDEA), a nitrogen containing diol, the mechanical properties of POC can be increased due to the enhanced hydrogen bonding of the tertiary amine and the introduction of positive charges within the network. Concomitantly, the generation of local alkaline pH due to the amine also increases degradation rate.
3. Chemistry Considerations for Citrate-based Biomaterials Design
Citrate chemistry not only contributes to the formation of the ester bond framework, but also allows for introduction of other cross-linked networks or advanced functionalities, serving as a potent example of utilizing simple chemistry to endow functional complex to meet the increasing demands of biomedical applications.
3.1. Introduction of Radical Cross-linked Network
One critical limitation of thermoset CBBs is their inability to be rapidly set into three dimensional shapes, negatively affecting their application to complex geometries or systems. Radical polymerization, as opposed to thermal polycondensation, is capable of rapid curing and fixation of the elastomer. Through incorporation of unsaturated double-bond moieties, such as acrylate, fumarate [16], and vinyl containing (maleic acid or maleic anhydride) monomers, rapid crosslinking under UV irradiation or redox initiation can be achieved [17]. For example, the photocrosslinkable biodegradable elastomer, poly(octamethylene maleate citrate) (POMC) with vinyl-groups incorporated into the POC polymer backbone may be crosslinked using free radical polymerization and/or thermal polycondensation [18, 19]. This dual cross-linking mechanism extends the range of polymer mechanical properties and endows the polymer with enhanced and versatile processability, leading to its combination with micro-electro-mechanical systems (MEMS) and fabrication of complex 3D microchanneled scaffolds [19]. For example, by advanced new 3D stamping technique, POMC has recently been fabricated into a AngioChip scaffold with built-in branching microchannel networks to generate perusable vasculature enabling direct surgical anastomosis [20]. Meanwhile, POMC enables the microfabricated lattice design into scaffold with shape memory, using a combination of soft-lithography and injection molding, for functional tissue delivery via injection [21]. Notably, incorporation of maleic anhydride in POMC also enabled the ultra-finely tuning of optical properties to possess a higher refractive index than POC with an index difference of ~0.003, comparable to that between the cladding and the core of conventional silica optical fibers. Both POC and POMC also show low absorption which enables organ scale light delivery and collection. Therefore, by leveraging the processibility of the two polymers, a biodegradable, flexible, step-index and low-loss optical fibers consisting of a POC cladding and a POMC core has been fabricated for potential deeptissue light delivery, sensing and imaging applications [22]. Addition of vinyl moieties into water soluble CBBs has endowed the polymer, poly(ethylene glycol) maleate citrate (PEGMC), with injectablity and in situ cross-linking capability when combined with crosslinker and initiator, thus providing advantages including minimally invasive injection, conformation to the wound shape and localized drug or cell delivery [17]. For example, by selecting acrylic acid as the crosslinker and 2,2’-azobis(2-methylpropionamidine) dihydrochloride (AAPH) as the initiator, an injectable and visible light-curing system based on PEGMC has been developed with gelation time in the range of the preferred light-curing times for composite resin materials in current dental practices, indicating its potential application as an endodontic drug delivery vehicle [23]. Further, through this strategy, carboxyl and hydroxyl groups can be preserved for the bioconjugation of bioactive factors such as antimicrobial peptides to minimize the risk of bacterial infection [24].
As a further modification, water soluble CBBs functionalized with acrylate containing groups were utilized for free radical coupling with N-isopropylacrylamide (NIPAAm) initiated by 2,2’azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (AIBN), generating the PNIPAAm analogue, thermoresponsive gel poly(polyethylene glycol citrate-co-N-isopropylacrylamide) (PPCN) [25]. PPCN displays gelation at physiological temperature; however, unlike traditional PNIPAAm, gelation is not accompanied by significant shrinkage, making PPCN a suitable candidate for void filling as well as a vehicle for cell and drug delivery [26, 27]. Initial studies demonstrated the efficient cell encapsulation of both human umbilical vein endothelial cells (HUVECs) and human aortic smooth muscle cells (HASMCs) with both cell lines displaying continued viability and proliferation up to 5 and 7 days, respectively. Additionally, HASMCs remained viable in vitro up to 72 days and PPCN was successfully injected subcutaneously in rats with minimal immune response [25]. Finally, poly(dodecamethylene citrate) was successfully methacrylated and combined with diethyl fumarate (solvent), Irgacure (photoinitiator), and Sudan 1 (UV absorbing agent) to create a resin bath 3D printing system (microCLIP) successfully used to print customized bioresorbable vascular stents [28]. The above systems amply demonstrate the power of free radical chemistry to improve CBB processability and clinical applicability.
3.2. Introduction of Urethane Bonds
The development of a soft, strong yet elastic biodegradable polyester to meet the mechanical requirements for a wide range of applications remains a challenge in the biomaterials field, since traditional crosslinked polyesters are inherently weak and the strategy of increasing crosslinking density sacrifices elasticity while improving strength. By doping urethane bonds into the polyester chains between ester crosslinks, a new class of polymer named crosslinked urethane-doped polyesters (CUPEs) has been developed by reacting with hexamethylene diisocyanate (HDI) with pendant hydroxyl groups as a chain extender to possess enhanced hydrogen bonding within polymer network, thus significantly improving mechanical strength to be 41.07± 6.85 MPa with a corresponding elongation at break of 222.66 ±27.84%. Importantly, when fabricated into CUPE scaffolds [29, 30], the tensile strength of biophasic scaffolds remained 5.02±0.70MPa, which was greater than native blood vessels (1.43±0.60 MPa) [30], while fabricated CUPE nerve guides with biomimetic multichannel structure still display an ultimate peak stress of 1.38±0.22 MPa with a corresponding elongation at break of 122.76±42.17%, comparable to that of native nerve guides [31]. Even when fabricated into porous triphasic vascular scaffolds, a suture retention strength of 1.79±0.11N [32] was obtained meeting the standard (1.7 N) for surgical suturing.
3.3. Introduction of Click Chemistry
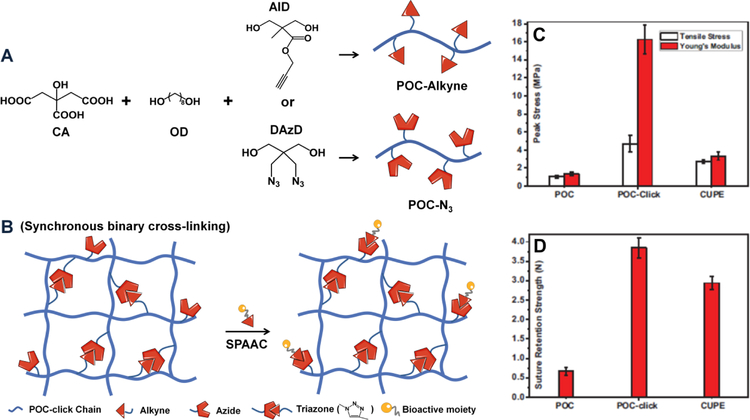
Another strategy to improve the mechanical properties of CBBs is to introduce click chemistry as a secondary cross-linking mechanism by introducing azide and alkyne diols into pre-POC polymer chains to generate azide (pre-POC-N3) and alkyne (pre-POC-Al) functionalized POC prepolymer, the mixture of which can be post-polymerized to generate POC-click, with a dual crosslinking mechanism: the thermal triazole ring-forming click reaction between azide and alkyne groups and esterification between carboxyl and hydroxyl groups (Fig. 2) [33]. Due to the dual-crosslinking mechanism as well as the rigid property of the triazole rings, improved bulk mechanical strength (as high as 41.32MPa) has been attained. More importantly, extra functional azide groups can be designed on POC-click polymers for further modification or conjugation of heat-liable bioactive molecules. For example, collagen mimetic peptide P15 that promotes endothelial cell adhesion and proliferation has been successfully conjugated to POC-click through strain-promoted azide-alkyne cycloaddition (SPAAC), another click reaction performed in aqueous solution at room or body temperature. MDEA can be further incorporated in the POC-click backbone [34] which is beneficial through acceleration of the degradation rate of POC-M-click, counteracting the delayed polymer degradation caused by the rigid triazole rings of POC-click.
Fig. 2. Incorporation of click chemistry into citrate-based materials.
(A) Synthesis of clickable POC pre-polymers POC-N3 and POC-Alkyne. (B) POC-click polymer network generated by mixing POC-N3 and POC-Alkyne pre-polymers via synchronous binary cross-linking mechanism (thermal click reaction and esterification) and further bioactive moieties incorporation via strainpromoted azide-alkyne cycloaddition (SPAAC). The mechanical properties, tensile strength, Young’s modulus (C), and suture retention strengths (D) of POC, POC-click and CUPE tubular biphasic scaffolds (Panel C and D reproduced with permission from figure 2 of Reference 33).
3.4. Introduction of Biomimetic Network with Adhesive Properties
Inspired by the strong wet adhesive properties of mussels, catechol-containing amino acid 3,4-dihydroxyl-phenylalanine (DOPA) has been incorporated into water soluble degradable citrate-based elastomer pre-polymer to develop a novel family of biodegradable and strong wet-tissue adhesives [35] termed iCMBA (Fig. 3A). iCMBA pre-polymer can be dissolved in Phosphate Buffered Saline (PBS) and then mixed with the oxidizing agent sodium (meta) periodate (PI) as an initiator for catechol-mediated crosslinking to form adhesive hydrogels within 18±2s. Oxidation of the catechol hydroxyl side chains of DOPA not only promotes the hydrogel crosslinking by turning into ortho-quinone, which subsequently triggers intermolecular cross-linking, but also contributes to its strong adhesion ability towards biological surfaces through covalent bonding with nucleophilic groups on wet-tissues [36]. The cross-linked hydrogel displays a wet adhesion strength that is 2.5–8.0 times stronger than that of clinically utilized fibrin glue. In addition, the gelling time, mechanical strength and degradation rate of iCMBA can be tuned by the molecular weight of PEG, the feeding ratio of dopamine and the oxidant (PI)-to-pre-polymer ratio. Click chemistry (copper-catalyzed azide-alkyne cycloaddition) and alkyne modified gelatin can also be introduced to iCMBA to improve the wet adhesion strength (highest adhesion strength: 223.11±15.94 KPa; 13 times higher than clinically used fibrin) and slow down its initial degradation rate (Fig. 3B) [37]. In addition, by incorporating clinically applied and inexpensive anti-fungal agent, 10-undecylenic acid (UA), iCMBAs has been engineered to possess longterm anti-bacteria and anti-fungal capability (Fig. 3C), serving as an adequate bioadhesive candidate for wound closure and bone regeneration [38], especially when infections are a major concern.
Fig. 3. Incorporation of dopamine into citrate-based materials.
(A) Schematic representation of iCMBA synthesis and mussel-inspired iCMBA network via catechol-mediated cross-linking after mixing with oxidizing agent sodium (meta) periodate (PI). (B) Adhesion strength and (C) degradation profile of normal iCMBA, clickable iCMBA cross-linked by PI, CuSO4-NaLAc-HEPES, or both (Panel B and C adapted with permission from figure 5 and figure 4 of Reference 37, respectively). Anti-fungal performance of AbAf iCBMA against C. albicans in terms of (D) fungal survival ratios and (E) inhibition halos after direct exposure of cross-linked hydrogels to C. albicans after 24 h of incubation (Panel D and E adapted with permission from figure 7 of Reference 38).
3.5. Introduction of Photoluminescence
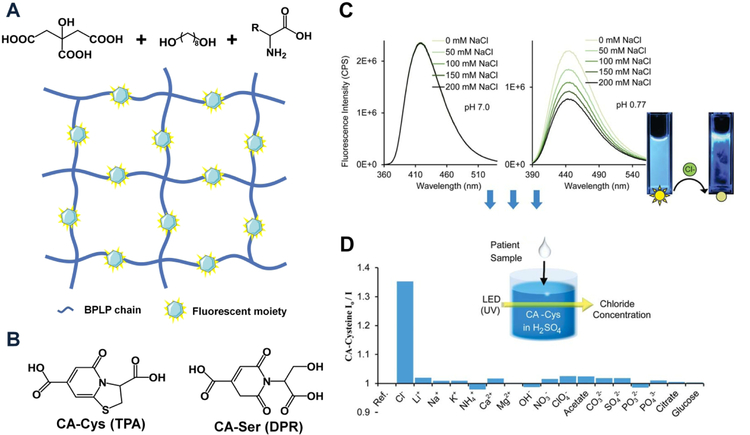
Intrinsic photoluminescent properties can be introduced to CBBs by reacting essential α-amino acids with citric acid and aliphatic diols via a one-pot polycondensation to develop a novel series of biodegradable photoluminescent polymers (BPLPs) (Fig. 4A) [12]. In particular, BPLPcysteine (BPLP-Cys) and BPLP-serine (BPLP-Ser) present excellent photoluminescent properties including high quantum yields of up to 62.33%, tunable fluorescent emission from 303 to 725 nm, and impressive photostability compared to commercial Rhodamine B and Fluorescein. Thermally cross-linked BPLPs offer advantages over previously developed aromatic fluorescent polymers or organic dyes due to their demonstrated biocompatibility and controlled degradation. The great processability of BPLPs enables their micro/nanofabrication into films or tissue engineering scaffolds as well as different nanocarriers which could greatly impact tissue engineering, drug delivery and bioimaging. The 6-membered ring structure formed by citric acid and α-amino acids has been identified to be the fluorescent moiety in BPLP. These fluorescent moieties can also be released from BPLP during degradation, as CA-Cys has been found in the alkaline hydrolysis productions of BPLP-Cys [39]. Meanwhile, a family of citratebased fluorescent small molecules have been synthesized via a one-pot reaction of citric acid with a primary amine or amino acid. A deeper and comprehensive understanding of two distinct types of fluorescent moiety have been identified (Fig. 4B) [39]: a thiozolopyridine (TPA) family such as CA-Cys, with high quantum yield, exceptional photostability and long lifetime, and a dioxopyridine (DPR) family such as CA-Ser with relatively lower quantum yield, multiple lifetimes, and solvent-dependent band shifting [39]. The fluorescence mechanism for the two types of molecule were also proposed: the fluorescence of TPAs comes from π-π* electronic excitation which leads to emission from the lowest energy band, similar to that of most organic dyes, while DRPs likely represent a distinct fluorescence mechanism, probably derived from the aliphatic tertiary nitrogen. Theses mechanistic discoveries have laid a solid foundation for further development of new citrate-based fluorophores, which have shown great promise in a diversity of applications, such as molecular labeling, live cell imaging and fluorescent sensing [39–41]. For example, CA-Cys reacted by citric acid and cysteine (Cys) is an excellent candidate for fluorescent chloride sensors through a dynamic quenching mechanism, which possesses great potential for the diagnosis of cystic fibrosis (Fig. 4C&D) [40]. Based on it, a smart phone-based chloridometer has been further developed for point-of-care diagnostics of cystic fibrosis with its analytical performance for sweat chloride detection optimized for clinical applications [41]. Effort has also been made to introduce urethane bonds into BPLP pre-polymer to improve its mechanical properties [32]. Moreover, BPLP pre-polymer possessing multiple pendant hydroxyl groups can initiate the ring-opening polymerization of lactones, thus leading to the development of a new generation of BPLP-polylactide copolymers such as BPLP-co-Poly(L-lactide) (BPLPPLLA) [42] and BPLP-Poly(lactide-co-glycolide) (BPLP-PLGA) [43]. These copolymers inherit the intrinsic photoluminescence from BPLP enabling non-invasive tracking of polymer degradation, while retaining the thermoplastic and mechanical properties from polylactides, improving processability by taking advantage of the sophisticated techniques developed for thermoplastic materials. Various modalities of BPLP-PLLA/BPLP-PLGA including films, scaffolds, particles, and fibers have been fabricated, expanding their application in biomedical engineering.
Fig. 4. Photoluminescent citrate-based materials.
(A) Schematic representation of BPLP synthesis and photoluminescent polymeric framework after thermal cross-linking. (B) Two distinct types of fluorescent moiety: a thiozolopryidine (TPA) family such as CA-Cys and a dioxopyridine (DPR) family such as CA-Ser. (C) Chloride quenches the fluorescence of CA-Cys strictly under acidic conditions. (D) Comparison of quenching efficiency of common ions to the fluorescence of CA-Cys at pH 1.3 (Panel C and D adapted from figure 2 of Reference 40 with permission from the Royal Society of Chemistry).
4. Biomaterial Degradation Products as Cell Regulators
Beyond biocompatibility, biomaterials in medical applications are closely in contact with biological systems, and their innate physical and chemical properties have been viewed as important regulators for cell function and tissue regeneration [44–46]. The interaction between cells and biomaterials is complex and dynamic, and has been reviewed elsewhere [44]: in brief, cells interacting with biomaterials are able to sense the materials’ inherent properties, such as substrate stiffness, surface topography, surface chemical functionality, as well as biodegradability [44, 47], and translate those cues into intracellular signaling activities that governing specific cell functions. Meanwhile, cells are capable of remodeling their own microenvironment by secreting extracellular matrix [48] or producing specific enzymes to degrade the materials [49] releasing more degradation products. Thus, understanding of how inherent material properties regulate cell functions represents a tremendous opportunity to develop the next generation of dynamic biomaterials. Among all the signals that materials give to cells, degradation by-products released from biomaterials during degradation, largely ignored beyond biocompatibility evaluation, has surfaced as a cell regulator provided by materials to direct cell behaviors [44]. In fact, many of the degradation fragments of naturally occurring proteins, such as the 20 KD fragment of natural collagen matrix (endostatin), [50–52], the hyaluronan degradation fragment (3–10 disaccharides) [53], the 29 KD fragment of fibronectin [54], and the 38-KD fragment of plasminogen (agiostatin) [55], all have been found to have an impact on endothelial cell behavior. Specifically, endostatin, representing the C-terminal fragment of collagen α1 (XVIII), was found to present in the conditioned medium of a hemangioendothelioma cell line and in the basement membrane zone around blood vessels [50–52], and numerous studies have pointed out its anti-angiogenesis effect either through inhibiting endothelial cell proliferation or by regulating endothelial cell apoptosis. In addition, fibrin degradation products (fragments D and E) are also capable of modulating the proliferation of smooth muscle cells (SMCs) [56]. More recently, different ions, especially calcium [57–59], magnesium [60–62], zinc [63–65] and strontium [66–68], as well as inorganic phosphate [69] released from inorganic minerals or metal implants have gained a lot of attention to influence the stem cell osteo-phenotype and specific osteoblast and osteoclast functions. In the case of synthetic polymeric materials, a few specific degradation by-products have also been emerging as cell regulators. For example, the lactic acid by-products [70, 71] that liberated from poly (lactic acid)-b-PEG-b-poly (lactic acid) hydrogels during gel degradation was found to facilitate the proliferation and differentiation of encapsulated neuron precursor cells. Interestingly, it has been revealed that citrate released from CBB degradation could also serve as a signal that materials provided to favor osteo-phenotype progression of human mesenchymal stem cells (hMSCs) in terms of elevated ALP expression [72] and increased calcium nodule formation [73].
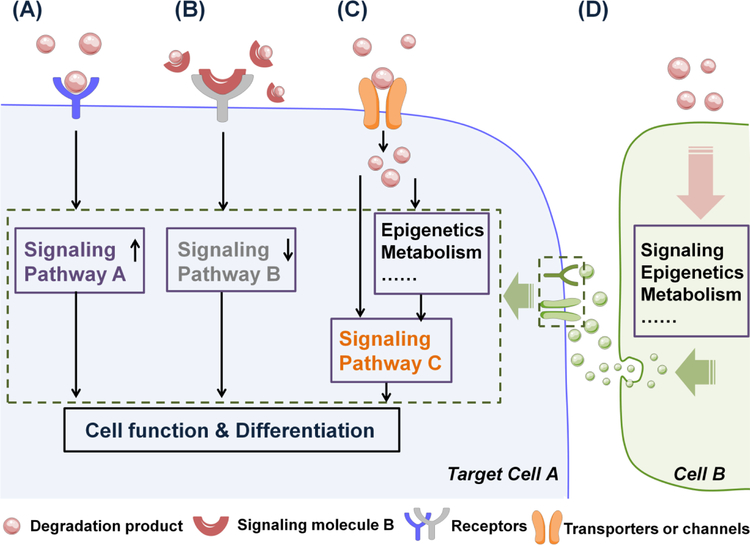
Increasing evidence has placed a spotlight on these degradation products, the neglected form of signals that materials could provide to cells. A unique opportunity for further exploration of the effects of a wide range of materials degradation products has thus arisen. Based on existing studies, degradation by-products could be related to cell function in four different ways (Fig. 5). First, degradation by-products themselves may function as signaling molecules, upon interacting with specific cell membrane receptors they could activate a series of signaling cascades to alter cell function. For example, endostatin inhibits endothelial angiogenesis mediated by plasma membrane αvβ3/α5β1-integrins [50], or by regulating FGF2 signaling [52], while hyaluronan degradation products stimulate endothelial cell proliferation, migration and tube formation mostly through activating CD44, which could lead to the activation of protein kinase C (PKC) and subsequently mitogen-activated protein (MAP) kinases. Alternative signaling pathways involving hyaluronan-mediated angiogenesis have also been proposed and reviewed elsewhere [53]. Second, degradation products may also interact with certain alreadyknown signaling molecules in the extracellular space, thereby affecting the availability of those signaling molecules which eventually influences cell behavior. The effect of lactic acid that released from biomaterials on neuron cells may belong to this category, as it can scavenge extracellular free radicals [70], which reduces the free radical-induced damages to cells and subsequently modulates intracellular redox state, leading to alleviated cell death and increased ATP production. Another example is that citrate molecule, despite not derived from materials, when supplemented exogenously, has been speculated to regulate neuronal excitability through chelating with Ca2+ and Mg2+, greatly altering the availability of these ions to modulate the ionotropic N-methyl-D-aspartate (NMDA) receptors [74, 75]. Third, plasma membrane transporters or channels may mediate the entry of certain degradation products into cells, which directly or indirectly regulates cell function. A good example is inorganic phosphate from calcium phosphate-bearing matrices uptaken by hMSCs through their cell membrane phosphate transporter, solute carrier family 20, member 1 (SLC20a1) for ATP synthesis as key substrates in cell energy metabolism. ATP then could be exported out of cells to generate an ATP metabolite, adenosine, which acts as an autocrine/paracrine signaling molecule through adenosine A2b receptor to support osteogenic differentiation [69]. Similarly, it has been revealed that type L voltage-gated calcium channels mediates the transport of Ca2+ across cell membrane to increase bone morphogenetic protein-2 (BMP-2) expression through protein kinase C (PKC)-MAPK or ERK kinases 1/2 (MEK1/2) signaling pathway, providing valuable understanding of the effect of ceramic materials on cells in situ [57]. Lastly, degradation products may affect the target cell function through paracrine signaling. Magnesium-induced bone formation is a good example of this: magnesium derived from biodegradable magnesium implants could induce the periosteum neuron cells to produce and secret more calcitonin generelated polypeptide-α (CGRP), which subsequently enhances the osteogenic differentiation of periosteum-derived stem cells by activating their cAMP-responsive element binding protein 1 (CREB1) and osterix for robust bone formation [60]. A combination of mechanism is also pervasive. For example, the effect of magnesium on periosteum neuron cells is mediated by the cell membrane magnesium transporter 1 (MAGT1) and transient receptor potential cation channel, subfamily M, member 7 (TRPM7), and the magnesium uptake leads to accumulation of terminal synaptic vesicles releasing more CGRP for stem cells [60], representing a combined mechanism of transporter-mediated signaling and paracrine signaling induced by implantderived magnesium.
Fig. 5. The ways degradation products may link to cell function and differentiation.
(A) Degradation products may function as signaling molecules to promote signaling pathway A by interacting with cell membrane receptors, leading to altered cell function and differentiation. (B) Degradation products may interact with already known signaling molecule B to down-regulate signaling pathway B, which affecting cell function and differentiation. (C) Degradation products may enter cells via cell membrane transporters or channels to regulate cell functions directly or indirectly through signaling pathway C. (D) Degradation products may indirectly regulate target cell function and differentiation via modulating the paracrine signaling between target cell A and neighboring cell B.
In addition, one has to note that all the existing studies above only focus on single fragment or one specific compound (ion or monomer) in the entire pool of exact degradation products, which is much more complicated with various fragments or compounds mixed together in most of cases. Given one of the key degradation products can serve as cell regulator, one can speculate that other unidentified degradation products could possibly also participate in cell modulation to some extent. Therefore, a full and comprehensive picture of the collective regulatory effect of all degradation products to different cells is far from complete, requiring a deeper understanding of the degradation mechanism, the advanced analytical technologies to identify each component in degradation products, and the ability to screen for the effects of materials degradation products in a diverse and extensive range. Taken together, the new concept of degradation products as cell regulator is emerging, representing huge opportunities to inspire new ways of thinking about material degradation products as bioactive moieties. In particular, citrate-based materials, with their regulatory role in hMSCs osteo-differentiation preliminarily shown, warrant further in depth understanding to elucidate how citrate links the chemistry of materials building blocks with the chemical reactions inside cells, which could support the notion of viewing degradation products as cell regulator on one hand, and on the other hand motivate dynamic citrate-based materials development.
5. Biology Considerations for Biomaterial Design and Application
Currently, the regulatory role of citrate as degradation products on cell functions remains underexplored, despite citrate’s ubiquitously presence in living organism: when viewed systemically, citrate is not evenly distributed in human native tissues and has tissue-specific multifunctional biological roles. On the cellular level, citrate is located at the crossroads of cell metabolism where its critical regulatory role in cell energy metabolism has been increasingly appreciated in recent years. Understanding of citrate’s multifunctional roles in biological tissues (summarized in Table 1) could provide inspiration towards further exploration of the regulatory mechanism of citrate in different organs, meanwhile fueling the improvement and application of CBBs to the next generation in specific medical situations.
Table 1.
Summery of citrate biology in different biological tissues
| Location | Specific Target | Function | Mechanism Type | Ref |
|---|---|---|---|---|
| Microbial | Bacteria | Citric acid inhibits or kills bacteria by reducing intracellular pH | 1) | [101–105] |
| Bacteria cell wall | Citrate serves as a potent permeabilizer to display antimicrobial effects that synergistic with antibiotics by chelating with Ca2+ and Mg2+ | 2) | [101, 105, 108,109] | |
| Musculoskeletal system | Bone matrix | Citrate functions as a biomineralization regulator to stabilize bone apatite and control mineralization | 2) | [113–119] |
| Osteoblast-like cells (MG 63) | Citrate elevates alkaline phosphatase production | Possibly 3) | [72] | |
| Mesenchymal stem cells (MSCs) | Citrate increases the calcium nodule formation | Possibly 2) and 3) | [135] | |
| Blood and Cardiovascular system | Clotting cascade | Citrate serves as potent anticoagulant agent by chelating with Ca2+ | 2) | [153, 154] |
| Endothelial cells | Citrate reduces endothelial dysfunction, and promotes endothelial sprouting as well as the production of angiogenic growth factors | Possibly 3) | [155, 136] | |
| Red blood cells | Citrate uptake maintains cell homeostasis by metabolizing into di-carboxylates or transamination intermediates | 3) | [156] | |
| Immune cells (monocytes or lymphocytes) | Citrate serves as biosynthetic substrates for important mediators of inflammation | 3) | [97, 98] | |
| Central nerve system | Neuron cells | Citrate regulates neuronal excitability by chelating with cations and/or as energy substrates | 2) and/or 3) | [74, 75,174] |
| Renal system | Renal calcium salts and proteins | Citrate reduces calcium supersaturation and increase the activity of Tamm-Horsfall proteins to prevent renal stone formation | 2) | [82] |
| Cancer | Prostate cancer cells | Citrate enters cells and is consumed to increase their metastasis | 3) | [178, 180, 181] |
| Liver cancer cells | Citrate uptake serves as energy and biosynthetic substrates to support cell proliferation | 3) | [182] | |
| Gastric, lung, breast and pancreatic cancer | Citrate inhibits cancer cell proliferation by suppressing glycolysis | 3) | [183, 184] |
Note: Reduced pH value;
Chelation of cations such as Ca2+ and Mg2+;
Metabolic regulation
5.1. Citrate’s Role in Native Tissue
Citric acid is one of the most important tricarboxylic acids with pKa values of 2.9, 4.3 and 5.6 [76], meaning that in most human tissues, citric acid predominantly presents as citrate, a trivalent anion (~95%) [77]. Citrate, well-known as a key metabolic intermediate in the mitochondrial tricarboxylic acid (TCA) cycle of living organisms, displays a tissue-specific distribution pattern and may have a impact on various tissues as well as the microbes interacting with tissues, such as metabolic regulation, antimicrobial potential, mineralization regulation, anticoagulant effect, neuronal excitability regulation, and renal stone prevention. As an anion, citrate serves as a key chelating agent of physiologically important divalent cations, especially Ca2+, Fe2+ and Mg2+. It is well-known that more than 90% of total citrate is accumulated in human bone and citrate also concentrates in other healthy or pathological biomineralized tissues, such as teeth dentin [78], kidney stones and atheroscleroic plaques [79]. Besides, citrate is found in blood [80] and other soft tissues, such as prostate [81], kidney [82], and brain. Currently, ionic chelation potential of citrate is still considered as the major mechanism underlying citrate’s biological function. With the advent of rediscoveries and regained interest in cell metabolism, it has pointed out a new connection between the understanding of citrate as a metabolic regulator and citrate’s biological function.
5.2. Citrate in Cell Metabolism as Metabolic Regulator
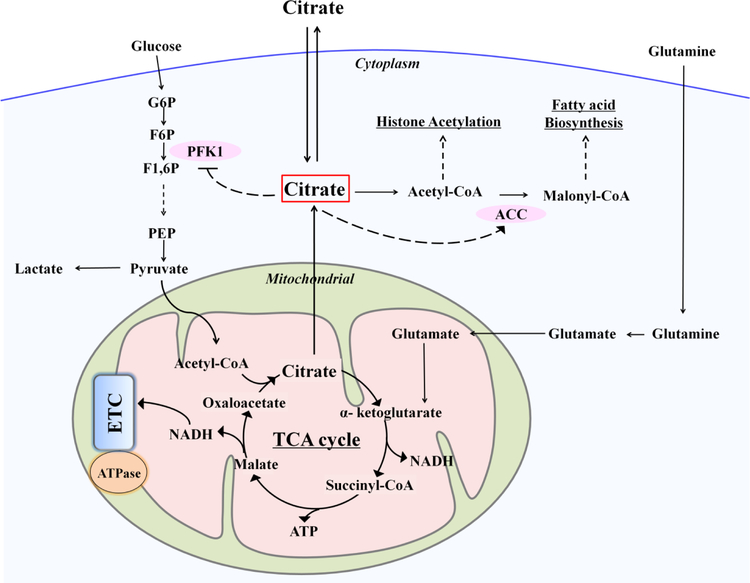
Cell metabolism refers to all chemical reactions that occur in living organisms, in which multi-enzyme systems are highly coordinated to support life. It involves catabolism that breaks down complex energy-rich molecules such as glucose to yield energy, as well as anabolism which consumes energy to build up complex molecules and structures such as lipids or proteins. It is now becoming increasingly evident that metabolism, discovered hundreds of years ago and traditionally regarded as a bystander, acts as an essential regulator that crosstalks with multiple signaling pathways [83], developmental signals [84] and epigenetic networks [85] to steer cell proliferation, regulate cell differentiation, and modulate physiological cell responses. Citrate, a well-known intermediate metabolite, plays a key regulatory role in maintaining energy homeostasis [76, 86, 87], since it is located at the crossroads of metabolism (Fig. 6): Citrate is metabolized not only as an energy substrate in the TCA cycle to produce the majority of cellular ATP but also serves as a source of cytosolic precursor for fatty acid biosynthesis [76, 86–88]. Changes in citrate concentration could influence the activity of key enzymes in both ATP producing catabolic and ATP consuming anabolic pathways [86, 87, 89, 90]. For example, citrate is a negative regulator of the rate-limiting glycolytic enzyme phosphofructokinase-1 (PFK1), and an allosteric activator of acetyl-CoA carboxylase (ACC), the enzyme that catalyses the formation of malonyl-CoA for fatty acid biosynthesis. Given the robust regulatory role of citrate in metabolism, the transportation of citrate is strictly controlled [76]. Specifically, citrate is synthesized in mitochondria catalyzed by citrate synthase (CS) and can be exported to the cytosol through mitochondrial citrate carrier (CIC) when the cellular ATP level is abundant and the energy demand of cells is low [87]. Meanwhile, Glutamine replenishes the TCA cycle through glutaminolysis, which results in the generation of α-ketoglutarate. In cytosol, the enzyme ATP citrate lyase (ACL) is responsible for converting citrate into Acetyl-CoA, which is the only and direct substrate for lipid biosynthesis, and also is required for histone acetylation induced by either growth factor or differentiation [91]. For instance, increased citrate was required by lymphocyte cells upon stimulation by extracellular growth factors, for fatty acid synthesis during the initiation of lymphocyte cell growth [92], by up-regulation of CS. In some cases cytosol citrate is further exported to extracellular spaces for specialized purposes. For example, both prostate epithelial cells [93] and cerebella astrocytes have been found to produce and release large amounts of citrate from mitochondria to their microenvironment. On the other hand, extracellular citrate can also be taken up via plasma membrane transporter SLC13a5 [94] by specialized cells such as hepatic cells [95], metastatic cancer cells [96] to meet their high energy or high biosynthetic demands. Exogenous addition of citrate can be also consumed by activated lymphocyte cells for fatty acid synthesis to meet the demand of cell growth [92].
Fig. 6. Metabolic regulatory role of citrate in maintaining energy homeostasis.
Citrate locates at the crossroads of metabolism: is metabolized not only as an energy substrate in the TCA cycle to produce the majority of cellular ATP but also serves as a source of cytosolic precursor for fatty acid biosynthesis. Changes in citrate concentration could influence the activity of key enzymes: such as the PFK1 in glycolysis and the ACC in fatty acid synthesis. (PFK1, phosphofructokinase 1; ACC, acetyl-CoA carboxylase; G6P, glucose-6-phosphate; F6P, fructose-6-phosphate; F1,6P, fructose-1,6-phosphate; PEP, phosphoenolpyruvate; ETC, electron transfer chain)
The impact of metabolic regulation has been increasingly appreciated, especially on stem cells and immune cells, which undergo dramatic metabolism reprogramming in response to their altered microenvironment. The essential role of altered citrate metabolism for immune cells upon stimulation serves as a good example. Citrate transportation is also involved in inflammatory response of monocytes or lymphocytes, as following lipopolysaccharide (LPS) stimulation, more cytosol citrate, which could be obtained by exporting from mitochondrial [97] or by uptake of exogenous citrate [98], is required for the generation of acetyl-CoA and oxaloacetate, precursors for important mediators of inflammation, such as NO, reactive oxygen species (ROS) and arachidonic acid (which is used to generate prostaglandins). Although more study is required to identify the exact role of citrate at different concentrations in regulating inflammation, transient acute but no chronic inflammatory response induced by POC in vivo [3] as introduced in Section 2.2.4 implicates the potential favorable role of citrate to regulate host defense and inflammation, which is worthy of further exploration. More recently, intermediates of metabolism such as lactate and succinate, have been rediscovered as active metabolic signals mediated by binding to specific cytoplasmic membrane receptors [99, 100]. For example, binding of succinate to its receptor GPR91 [99], a G protein-coupled receptor, could trigger intracellular calcium release and inhibit cAMP production, eventually enhancing the immunity of dendritic cells [100]. These break-through findings point out a brand new scenario, in which change in specific metabolite concentration does not represent just a metabolic shift or altering in energy demand; rather, it is also possible to manifest as a form of signaling-mediated regulation of biological processes. Although whether citrate present similar extrametabolic function remains unexplored, this new concept of metabolite may provide a new potential connection or a brand new perspective to understand the citrate biological role in different tissues that will be discussed below.
5.3. Citrate as Antimicrobial Agent and in Wound Healing
Wound healing is a complex and multi-phase process comprised of hemostasis, inflammation, proliferation, and remodeling, which is affected by multiple factors. Microbial infection is a major medical factor that impairs wound healing and hinders the application of biomaterials for medical applications. Citric acid has been widely used in dental rinses and as a root canal irrigant for antimicrobial cleansing [101] and even for germicidal purposes when its concentration reached as high as 25% to 50% [102, 103]. As an organic acid, the potential antimicrobial effect of citric acid is probably due to its pH effect, lowering the intracellular pH of bacteria and leading to decreased enzyme activity, DNA damage [104] or suppressed nicotinamide adenine dinucleotide (NADH) oxidation [105]. The relatively acidic environment created by citric acid is also beneficial for wound healing in other aspects [106]: for example, by promoting oxygen release to the wound bed. Studies have revealed lowering pH by 0.9 units resulted in a 5-fold increase in oxygen release from hemoglobin, greatly affecting the available supply of oxygen to tissues [107]. Apart from the pH effect, more importantly, sodium citrate has also been found to possess superior antimicrobial effects against a wide range of oral or blood derived [101, 108], Gram-positive or Gram-negative, bacterial or fungus microbials. In addition to its anti-coagulant effect at 0.5%, 2% Citrate presents substantial inhibitory effect on bacterial growth in platelet and red blood cell suspension [101] without causing any damage to the cells, and the antimicrobial property of citrate also makes it a superior catheter locking solution over heparin [108]. Of note, citrate at concentrations as little as 1% greatly enhances the antimicrobial effect of antibiotics including vancomycin [101] and gentamicin [108], which would be of great importance in fighting antibiotic-resistant bacteria. The mechanism underlying the citrate antimicrobial effect is also proposed [101, 105, 108, 109] to result from its potent chelating with cations Ca2+ and Mg2+ that are critical in stabilizing bacteria cell walls, especially for Gram-negative bacteria [110], since the chelating agent disodium-ethylenediaminetetraacetate (EDTA) exhibits a similar but less effective inhibitory effect on bacteria growth [108] and the citrate-derived antimicrobial effect is abolished by addition of Mg2+. Therefore, citrate is considered as a potent permeabilizer of bacteria, whose incorporation of into biodegradable polymer chains is a rationale approach to endow the polymer with inherent antimicrobial properties preventing microbial growth.
In fact, almost all citrate-based materials (POC, BPLP, CUPE, POMC [105] and iCMBA [38]) effectively inhibit the growth of E. coli and S. aureus without additional antibiotics, silver nanoparticles, or other chemicals. Among all the tested polymers, POC exhibits the highest antimicrobial efficacy, most likely due to its high citrate feeding ratio and faster degradation rate, supporting our notion that it is the citrate that provides potent antimicrobial effect, which could further be tuned by altering citrate ratio and degradation rate. Given hydrogels, among all the wound dressing modalities, have shown a wide range of advantages, such a high degree of swelling to avoid the formation of fluid filled pockets, in situ cross-linking capability ensuring complete wound closure, and the cross-linked network offering a further platform for controlled drug delivery, an in situ cross-linkable biodegradable hydrogel system (iFBH) based on PEGMC in combination with PEGDA and initiator has been developed as a wound dressing for skin wounds [24]. A two-component injection of PEGMC/PEGDA solution as component A and initiator solution as component B simultaneously can lead to the formation of hydrogel via redox-crosslinking covering the wound area. Conjugation of different antimicrobial peptides including CHRG01, ABU, TEMP-A, or ALA5 to PEGMC was performed by EDC/NHS to further boost its anti-infection capacity. A similar two component injectable and in situ crosslinking hydrogel system named iDEEP (injectable Drug Eluting Elastomeric Polymer) [111] was also developed to assist surgery procedures, particularly endoscopic mucosal resection (EMR), which is a minimally invasive endoscopic procedure developed to remove dysplastic and cancer lesions limited to the mucosa or submucosal top layer of the gastrointestinal (GI) tract. Specifically, after injection of PEGMC/PEGDA based component A and the redox initiator based component B in a 2:1 ratio into the submucosa top layer to form a soft and biodegradable hydrogel in situ, the diseased submucosa layer was effectively separated from the underlying muscle layer with a much higher elevation height than that of clinically used saline solution and sodium hyaluronate. Meanwhile, the hydrogel system enables the delivery of therapeutic doses of rebamipide, which is a mucosal protective and ulcer-healing drug that aids the healing of EMR-induced damage. In addition, efforts have been made to develop an injectable PEGMC-based light-cured hydrogel system in combination with acrylic acid and initiator, with great potential for covering the exposed vital pulp in direct pulp capping treatment [23], as citric acid has been commonly included in dental rinses [101] for antimicrobial purposes previously.
The clinical application of natural or synthetic bioadhesives as wound closure agents have advanced surgeries by facilitating surgical operations, improving patient compliance and reducing healthcare costs [36, 112]. With this in mind, a family of injectable citrate-based mussel-inspired bioadhesives (iCMBA) has been developed [35, 36] for sutureless wound closure. iCMBAs possessed stronger wet tissue adhesion strength versus clinically used fibrin glue, controllable degradability, tissue-like elastomeric mechanical properties and excellent cyto/tissue compatibility. Meanwhile, iCMBAs crosslinked by sodium metaperiodate (PI) showed excellent intrinsic anti-bacterial capability since both citric acid and PI exhibit inhibition against S. aureus and E. coli growth [38]. Moreover, the capability of iCMBAs to close wounds was tested with full-thickness wounds (2 cm long ×0.5 cm deep) on the dorsum of Sprague-Dawley rats. iCMBA solution was shown to stop bleeding instantly and suturelessly close wounds within 2 min. As compared to conventional sutures, iCMBAs at day 7 elicited the same level of inflammation, accompanied with significantly more collagen production at day 28, consistent with improved tensile strength in the iCMBA treated group. Also, iCMBAs were fully degraded day 28, supporting iCMBAs’ potential for biological applications. In addition, click chemistry has been introduced into the mussel-inspired cross-linking network of iCMBA [37], which greatly improved the cohesive strength under wet conditions to 223.11±15.94 KPa, almost 13 times higher than that of conventional fibrin glue, while decreasing the initial degradation, critical for sustaining mechanical integrity in the early tissue regeneration stage. Further modification of iCMBA with anti-fungal activity can be achieved by a two-step synthesis: first, 10-undecenoyl chloride as the anti-fungal agent is synthesized with citric acid to generate anti-fungal undecylenate citric acid (U-CA) [38], which was subsequently incorporated into iCMBAs prepolymer to generate a new family of anti-bacterial and anti-fungal iCBMA termed AbAf iCs. With the strong wet tissue adhesion strength retained, the new AbAf iCs possessed not only antibacterial but also excellent anti-fungal activity for wound closure, especially when bacterial and fungal infections are a major concern for wound healing.
5.4. Citrate in Bone and Bone Tissue Engineering
In the early 1960s, it was found that citrate molecules make up about 5 wt% of the total organic component in bone and that over 90% of the body’s total citrate content is located in the skeletal system [113–115], revealing citrate as an indispensible component in bone. Recently, the important structural role of citrate in regulating bone hydroxylapatite-like (HA) minerals has been uncovered: citrate molecules are not only studded on the surface of apatite nanocrystals [116] and bridged between the apatite mineral platelets, stabilizing bone apatite [117, 118], but also control the size and crystallinity of HA particles [119]. In addition to citrate-HA interactions, citrate-collagen (the primary bone organic matrix) interactions promote collagen biomineralization by improving the wetting effect at the collagen-mineral interface to directly regulate the mineralization process [120]. All the above studies demonstrate that citrate is a strongly bound and integral part of native bone, serving as a key mineralization regulator. Intriguingly, the citrate involved in bone mineralization has been found to be derived from intracellular citrate metabolism of differentiating osteoblasts, as uncovered by a recent study [121]. Specifically, in response to osteogenic stimulation, hMSCs undergo metabolic reprogramming with a metabolic shift from glycolysis to oxidative respiration [122]. Meanwhile, gene expression of citrate synthase (CS) and its activity [121, 123] elevate along with increased uptake of zinc, which inhibits aconitase-mediated citrate oxidation [121, 124–127]. Collectively, it leads to citrate accumulation in mitochondria, which subsequently exported via CIC to extracellular spaces incorporated in newly formed bone.
Although numerous studies have revealed a favorable effect of alkaline citrate (e.g. K citrate) therapy to bone mineral density in healthy adults [128] or patients with nephrolithiasis [129, 130], up to now it was believed such a favorable effect is derived from neutralization of the endogenous acid load leading to positive calcium and phosphate balance. However, SLC13a5 gene expression is found to be upregulated significantly in newly formed rat bone [131] and also at the early stage of osteointegration [132], which is in agreement with the sharply increased accumulation of intraperitoneally injected14C labeled citrate in the soft callus after bone fracture [133], pointing out a potential link between citrate biology and bone formation. Moreover, SLC13a5 deficiency could lead to impaired bone formation and defective tooth enamel development [134]. Excitingly, soluble citrate released from citrate-based materials during degradation, after supplemented in osteogenic medium, has been found to elevate the alkaline phosphatase (ALP) of osteoblast-like MG63 cells [72] and to increase the calcium nodule formation of human mesenchymal stem cells (hMSCs) [135]. In addition, solute citrate has shown angiogenic effect on endothelial cells in terms of promoting endothelial sprouting in rat aortic rings, and increasing the production of angiogenic growth factors, VEGF and FGF [136]. Given that bone formation involves the differentiation of hMSCs to bone-forming osteoblasts producing a large amount of bone matrix, and involves the formation of new blood vessels transporting growth factors and nutrients, all the results support the notion that citrate may also have an indispensable yet unexplored impact on bone formation, meanwhile support the use of citrate in bone biomaterial design.
In light of the structural and potential biological role in native bone, a series of citrate-based materials have been developed to composite with HA particles better mimicking the composition of native bone for orthopedic applications. POC/HA is the first generation of citrate-based composites [137] and was processed into tissue fixation screws by compression molding and machining. Interestingly, POC/HA composites demonstrated minimal chronic inflammatory response and impressive osteointegration with the surrounding tissue after 6 weeks of implantation in rabbit femoral condyles [137]. Long-term in vivo response of POC/HA up to 26 weeks has been evaluated with its long-term biocompatibility and increased ingrowth of new bone demonstrated [13]. By collecting tissue samples one year post implantation, significantly increased bone formation was found in POC/HA implants compared with PLLA. It is believed that the incorporation of osteoconductive HA not only enhances the bioactivity, but also serves as a buffer to the acidic functional groups and products generated from POC degradation. On the other hand, POC in turn could improve the processability and mechanical properties of bioceramic implants due to its biocompatibility, adjustable mechanical properties, and controllable degradation rates from a few months to almost 1 year. The –COOH pendant chemistry in POC is believed to prompt calcium chelation to facilitate polymer/HA interaction [138–141], resulting in its ability to incorporate up to 65 wt% HA in POC/HA composites, which is not possible with previous biodegradable PLLA based polymers, as only 25–30% HA can be incorporated into PLLA before it becomes too brittle to process. Our rheological study further confirmed the presence of chelating interactions of –COOH of another citrate-based polymer, poly(ethylene glycol) maleate citrate (PEGMC) with HA particles [141]. The ex vivo injectability of this composite has been demonstrated with a porcine femoral head, confirming the feasibility of injecting PEGMC/HA using minimally invasive techniques into collapsed femoral heads for reinforcement. Further in situ cross-linking of poly(ethylene glycol) diacrylate (PEGDA) with PEGMC/HA not only stabilizes the composite but also allows additional control over the degradation and mechanical properties of the resulting cross-linked PEGMC/HA [135]. Moreover, the strongest polymer/HA composites based on citrate polymers possess a compressive strength of ~250 MPa, which is very impressive as very few polymer/HA composites are able to show compressive strength comparable to that of native human cortical bone (100–230 MPa) [142, 143].
The mechanical properties of citrate-based composites could be further tuned by changing the HA component and cross-linking conditions as well as the choice of different CBBs. A series of new citrate-based biodegradable elastomers have recently been developed to composite with HA. Such composites have subsequently been fabricated into different modalities, such as porous cylindrical or disk-like [13, 72, 144] scaffolds [34], and biphasic scaffolds [145]. For example, the mechanically strong CUPE and POC-Click have been composited with 65 wt% HA to fabricate disk-shaped scaffolds with 70% porosity [144], and their efficacy as bone grafts has been evaluated in a critical-sized cranial defect model. These scaffolds lead to enhanced bone formation as well as promoted early-stage angiogenesis with minimal signs of inflammation after 1, 3 and 6 months of implantation. Moreover, their excellent processability and mechanical properties further enable more complicated scaffold design for simulating the architecture of native bone. For example, POC-Click/HA has been fabricated into biphasic scaffolds with 70% internal phase porosity and various external phase with porosities between 5% and 50% in order to mimic the bimodal distribution of porous cancellous and compact cortical bone, respectively [145], while maintaining compressive strengths up to 37.45±3.83 MPa. The resulting biphasic scaffolds were evaluated in a rabbit model with 10 mm long segmental radial defects, with significantly increased BMD comparable to autologous bone grafts after 5 weeks of implantation, prominent periosteal remodeling around the scaffold, and an almost completely repaired and bridged medullary cavity after 15 weeks. On the other hand, a new generation of polymer blends (CBPB/HA) based on POC, CUPE and HA [72] has also been developed using soft yet strong CUPE as the major fraction of the polymer blends while maintaining valuable free pendant carboxyl groups for HA chelation. Given that the synthesis of CPUE and POC can be controlled to tune their degradation from a few weeks to more than a year, a wide range of control on the degradation of CPBP/HA could be provided by controlling each polymer during the synthesis as well as the ratio between the two polymers [146–148]. More importantly, release of citrate from CPBP/HA was confirmed by HPLC and increased expression of osteogenic gene encoding alkaline phosphatase (ALP) and osterix in C2C12 cells cultured on CPBP/HA composites. Together with the impressive in vivo data showing almost full osteointegration, substantial new bone formation, and minimal fibrous tissue encapsulation after implantation into rabbit lateral femoral condyle for 6 weeks, the study not only confirmed the favorable role of CPBP/HA in bridging cavity defects and promoting bone healing, but also bridged the gap in orthopedic biomaterial design and osteoblast cell culture.
The promising in vivo bone responses of citrate-based composite in bridging defects and promoting bone formation motivated the development of CBB/HA composites for other specific regeneration applications, such as spinal fusion [34], comminuted bone fracture healing [73] and bone-tunnel healing during ligament reconstruction [149]. For example, spinal fusion is a surgical procedure, responsible for 50% of bone transplantations, that fuses two or more vertebrae into one single structure for the treatment of cervical vertebra instability, intervertebral disc injury, spinal degeneration and deformity [150]. Selection of materials for the intervertebral filler is a critical factor to affect fusion outcome. To achieve fast bone fusion, Nmethyldiethanolamine (MDEA) has been introduced into POC-Click to develop a new type of fast degradable, mechanically strong clickable material to composite with HA, named POC-MClick/HA. The resulting material was fabricated into matchstick-like porous scaffolds which can be easily implanted in the spinal interbody spaces and their efficacy in supporting quick spinal fusion was evaluated in a rabbit model of intervertebral fusion. Impressively, POC-M-Click/HA compared with PLLA/HA exhibited s substantially increased early bone formation, a significantly higher fusion rate than that of PLLA/HA scaffolds at 8 weeks after surgery, and a higher mechanical strength of fused spinal samples at 12 weeks. Comminuted bone fractures (CBF), which are recognized as one of the most difficult orthopedic conditions to treat, were treated with citrate-based bioadhesive iCMBA with strong adhesive strength composited with HA to be minimally invasively injected into the fracture site, avoiding open surgery and severe trauma, and to be favorable for bone fixation and bone healing simultaneously [73]. After mixing with PI solution as cross-linker, iCMBA/HA that could set within 2–4 min and fully degraded in 30 days was injected in a rabbit comminuted radial facture model. The enhanced bone formation at all time points, the greater quality of bone healing revealed by biomechanical tests, the complete degradability of iCMBA and the complete incorporation of HA particles into new bone sufficiently confirmed the efficacy of such injectable iCMBA/HA as an ideal candidate for CBF treatment. Anterior cruciate ligament (ACL) reconstruction by bone-patellar tendon-bone (BPTB) grafts is another specific scenario in which CBB/HA composite has been applied to take advantage of its excellent osteointegration and osteopromotive capability [149], because the fixation within bone tunnels is a determinant factor affecting the eventual repair outcome. Specifically, a tricomponent BPTB graft with porous POC/HA as its two bone-bonding ends and with braided PLLA fiber as the synthetic intra-articular section was fabricated and its efficacy in ACL reconstruction was evaluated in rabbit models presenting numerous tissue infiltration and functional interlocking within the bone tunnels.
Successful delivery of osteogenic progenitor cells to the wound environment is critical for accelerating bone regeneration; however, direct injection of cells typically results in limited cell retention and tissue regeneration. To address this limitation, thermoresponsive PPCN was mixed with gelatin to improve adhesion and survival versus the polymer alone [26, 151]. PPCN/gelatin was able to successfully encapsulate BMP9 stimulated MSCs. In vivo, ectopic bone formation was observed, with woven and mineralized, trabecular bone-like structures evident in the BMP9 stimulated cell group. Further study in critical sized cranial defects using BMP9-transduced calverial cells encapsulated in PPCN/gelatin also demonstrated its effectiveness [26]. Groups treated with encapsulated BMP9-transduced cells displayed significant reductions in defect size after 12 weeks and more complete osteointegration and mature bone formation versus non transduced cell/hydrogel and hydrogel only groups. Taken together, these results represent a significant advance over other CBBs such as POC and CUPE, which cannot encapsulate cells, as well as hydrogels such as PEGMC that display limited adhesion and proliferation of encapsulated cells.
5.5. Citrate in Blood and Cardiovascular Tissue Engineering & Nanomedicine
In blood, the physiological citrate concentration has been estimated to be 100–150 μM [77] and any marked change in plasma citrate concentration may be related to pathological conditions serving as a biomarker for diagnosis [77, 152]. Citrate, similar to EDTA, heparin, and oxalic acid, is commonly and widely used in hospitals as an anticoagulant, taking advantage of its calciumchelating properties to inhibit the clotting cascade. Citrate is also contained in clinically used interdialytic locking solution of haemodialysis catheters [153] for local anticoagulation. It also serves as a better anticoagulant versus heparin in the extracorporeal circuit during continuous renal replacement therapy, improving clinical parameters in terms of less bleeding and better circuit survival [154]. In addition, one in vitro study demonstrated that citrate treatment reduces hyperglycaemic-induced endothelial dysfunction (endothelial apoptosis and PKC activation), which is an early manifestation of vascular atherosclerosis [155], and also decreases the secretion of pro-inflammatory factors from endothelial cells, indicating the therapeutic potential of citrate to abolish endothelial dysfunction. When exposed to hypoxia, red blood cells (RBC) in vivo or ex vivo has also shown to reprogram its metabolism by uptaking citrate and metabolizing it into di-carboxylates or transamination intermediates (e.g. α-ketoglutarate and glutamate), contributing to the homeostasis of RBC [156]. The indispensable role of citrate for immune cells to respond to infections [97, 98] as introduced in Section 5.2 serves as another example that emphasizing that citrate is essential for maintaining the homeostasis and supporting the function of white blood cells. The above studies greatly implicated the potential hemocompatibility of CBBs, which in fact has been assessed using POC films previously [157] and presented as reduced platelet adhesion, platelet activation, and reduced thrombogenicity as compared to PLGA and expanded polytetrafluoroethylene (ePTFE), which have been approved by the FDA for blood contact devices, supporting the notion that CBBs may serve as adequate candidate basal materials to design vascular grafts/coatings for tissue engineering, therapeutic carriers for nanomedicine, or any other blood contact applications.
There is substantial clinical need for readily available synthetic blood-vessel grafts, given that cardiovascular diseases remain the leading cause of mortality in United States [158]. Currently, expanded polytetrafluoroethylene (ePTFE) is one of the mostly used synthetic grafts for the replacement of medium-to-large diameter blood vessels (ID>6 mm) with high blood flow and low resistance conditions. However, in small diameter blood vessels, their application is still limited by early graft thrombosis [159]. Modification of the graft surface or development of alternative synthetic grafts has been regarded as a promising strategy to address the problem by presenting a more antithrombogenic surface and promoting endothelialization of the graft lumen. Therefore, given the antithrombogenic potential of POC versus ePTFE, a homogeneous layer of elastomeric POC has been coated to the luminal surface of ePTFE vascular grafts via spinshearing of pre-polymer followed by post-polymerization. Such a polymeric coating has reduced thrombogenicity in vitro presented as substantially inhibited platelet adhesion, reduced platelet aggregation, as well as delayed clotting of plasma [160]. Importantly, POC modification can improve the surface compatibility, resulting in supportive endothelialization in vitro and in vivo without affecting the inflammatory response, which was evaluated in a porcine artery bypass model for 1 [160] and 4 weeks [161]. Moreover, the available carboxyl and hydroxyl groups on coated POC provide researchers more flexibility to introduce functionalities. For example, another well-known antithrombogenic and anticoagulant agent, heparin, has been immobilized by EDC/NHS on POC coatings for enhanced antithrombogenic effect and improved endothelialization [162]. Such a polymer coating has also demonstrated its potential as an adequate substrate to embed drug loading PLGA microparticles for enhanced and quicker reendothelialization [163]. In addition, biodegradable, hemocompatible and elastomeric POC itself has been fabricated into a tubular biphasic scaffold comprised of a nonporous phase providing a continuous surface for endothelialization and maintaining the bulk mechanical strength, together with a connected porous phase expected to facilitate the expansion and maturation of smooth muscle cells (SMCs) [164]. In an effort to replace ePTFE as small-diameter blood vessel grafts, POC biphasic scaffolds demonstrated mechanical properties similar to those of native vessel. By fabricating such a biphasic scaffold with the elastic yet strong CUPE, its mechanical properties were further enhanced, with a tensile strength (5.02±0.70 MPa) greater than native tissue (1.43±0.60 MPa), tunable burst pressure ranging from 1500 mmHg to 2600 mmHg, and an adequate suture retention value of 2.45±0.23 N [30]. The excellent mechanical property of CUPE enabled further biomimetic design of triphasic scaffolds replicating the stratified architecture of native vessels [32] without sacrificing the mechanical compliance. POC-Click vessels were also fabricated to click peptide P15 to the surface, enhancing cell interactions and promoting angiogenesis [33]. In addition, the AngioChip scaffolds designed with branched microchannel network based on POMC have shown to support a confluent and functional endothelialization on the luminal network surface after perfuse the microchannels with endothelial cell suspension. More importantly, together with either human embronic stem cells (hESC) derived or neonatal rate cardiomyocytes, AngioChip scaffolds confer the engineering of fully endothelialized cardiac tissues with the thickness of 1.75–2 mm, with high cell viability under perfusion, and with the capability of surgical anastomosis to host vasculature [20]. In another study, POMC, when fabricated into injectable cardiac patches with microfabricated lattice design and loaded with cardiomyocytes, have shown to significantly improve cardiac function following myocardial infarction in a rat, serving as a flexible scaffold for invasive delivery of functional cardiac tissues [21].
Emerging evidence has emphasized the hemocompatibility of nanomedicine as hemoincompatibility could lead to mostly mild but occasionally severe or fatal allergic reactions [165]. In this respect, CBBs with demonstrated hemocompatibility could act as an ideal biomaterial for nanomedicine design, which has been verified in numerous studies during the last decade. Specifically, CUPE has been made into nanoparticles (Nps) by precipitation and the CUPE Nps were endowed with multi-functionality by conjugation with glycoprotein lb (GPlb) to target the injured arterial wall after injection, as well as with anti-CD34 antibodies to recruit endothelial progenitor cells (EPC) to the damaged areas of arteries [166]. In this way, the smart Nps with their hemocompatibility and excellent biocompatibility to human aortic endothelial cells (HAECs) could mimic native platelets, binding to and even covering the vWF rich injured blood vessels and substantially inhibiting the native platelet binding. On the other hand, the attached Nps form a nanoscaffold, attracting circulating EPCs as evaluated in a rat balloon angioplasty injury model, which subsequently lead to a significant increase in endothelial cell number as well as a ~60% increase in the degree of re-endothelialization compared to the untreated group after 21 days. In addition, the development of BPLP with intrinsic fluorescent properties has fueled a new generation of CBBs-based nanomedicine with much wider applications [12]. Various modality of BPLP theranostic nanomedicines such as photostable nanoparticles prepared via precipitation of BPLP pre-polymer [12], intrinsically photoluminescent polylactide BPLP-PLA or BPLP-PLGA Nps [42, 43], amphiphilic self-assembled ABPLP micelles [167] via conjugation of hydrophilic methoxy poly(ethylene glycol) (MPEG) blocks to hydrophobic BPLP blocks, and photocrosslinkable PBPLP nanogels [168, 169] by free-radical crosslinking of a vinyl-containing water-soluble BPLP pre-polymer have been designed for controlled drug delivery with real-time fluorescence-based imaging capacity and with the potential of adding further advanced functionalities for specific purposes. For example, in an immune cell-mediated drug delivery strategy, muramyl tripeptide can be conjugated onto BPLP-PLA Nps to enhance the Nps uptake by macrophages so that more anti-cancer drug PLX4032 can be loaded on macrophages to be delivered to melanoma cells via native immune cell-cell binding with potent anti-melanoma efficacy [170]. In another targeted molecular imaging strategy, a selective ligand of neuropeptide YY1 receptor has been linked to BPLP-PLA to target Y1 receptor over-expressed breast cancer cells, while by encapsulating liquid tetradecafluorohexane (C6F14) within BPLPPLA through an emulsion-evaporation process, a type of new dual-imaging enabled nanobubble was fabricated for safe, efficient and active targeted breast cancer imaging [171]. In addition, due to the excellent processability and the valuable reactive pendant groups of BPLPs, fluorescent imaging could be combined with other diagnostic modalities commonly applied in clinical settings to overcome the limitations associated with the stand-alone systems, increasing imaging precision. For example, by chemically conjugating water-soluble BPLP or BPLP prepolymer to magnetic nanoparticles (MNPs), the resulting Nps provide dual-imaging capability [172] with BPLP enabling fluorescent imaging with high sensitivity while MNPs act as negative contrast agents for magnetic resonance imaging (MRI) with exceptional tissue contrast, penetration depth and high spatial resolution.
5.6. Citrate in Central Nervous System and Nerve Regeneration
In the cerebrospinal fluid (CSF) of the central nervous system (CNS), filling and surrounding the brain and spinal cord, high concentrations of citrate are detected (~400 μM), which are believed to be maintained by cerebella astrocytes [173]. Studies using13C glucose incubated with astrocytes [74, 173, 174] provides direct evidence to support that astrocytes in fact export endogenous synthesized citrate in the TCA cycle to extracellular spaces, maintaining the citraterich microenvironment in cerebrospinal fluid, which is thought to indispensably support neuron function in the CNS [75]. Interestingly, neurons co-cultured with astrocytes could further stimulate an increased release of citrate from astrocytes [174], indicating the crosstalk between astrocytes and neurons. Although the functional importance of citrate in the CSF still remains elusive, its chelating capability of Ca2+ and Mg2+, greatly altering the availability of these ions has been emphasized, since both ions play a pivotal role in regulating neuronal excitability through modulating the ionotropic N-methyl-D-aspartate (NMDA) receptors [74, 75]. Although the viewpoint of whether neurons could import exogenous citrate is still controversial, given relatively low citrate uptake by both neuron and astrocytes [74], the expression of SLC13a5, the plasma membrane transporter responsible for citrate import has been identified on MAP2positive primary cerebrocortical neurons. The direct evidence for neurons using extracellular citrate for either glutamate synthesis or energy production is still lacking; however, such a citrate-rich microenvironment in the CSF strongly suggests a potential functional application of CBBs in spinal cord procedures, such as repair of the spinal cord, which possesses little spontaneous regeneration. Meanwhile, given that SLC13a5 expression has been identified on cerebrocortical neurons and the interest in SLC13a5 expression has been rising in recent years, it would be of great interest to detect whether it expresses on peripheral neuron cells and to explore the citrate effect to peripheral nerve engineering. In fact, in order to assist peripheral nerve repair by bridging transected nerves, the soft yet strong CUPE has been fabricated into biomimetic nerve guides with multiple longitudinally oriented channels simulating the native endoneurial microtubular structure and an external non-porous sheath mimicking the native epineurium structure [31]. The resulting multichanneled CUPE guides display a tensile peak stress as high as 1.38±0.22 MPa with a corresponding elongation at break of 122.76±42.17%, which are comparable to that of native nerve tissue, together with suture retention strengths as high as 2.36±0.11N. More importantly, CUPE nerve conduits elicited evenly distributed myelinated axons at the central conduit with new regenerated fiber population and density comparable to nerve autograft controls at 8 weeks after implantation into a 1 cm rat sciatic nerve defect.
5.7. Citrate in Renal System and Bladder Regeneration
In renal system, citrate chelation of Ca2+ is helpful for preventing its precipitation and reducing urinary supersaturation of calcium salts. Together with its effect on increasing the activity of proteins in urine such as Tamm-Horsfall proteins that could inhibit calcium oxalate aggregation, the protective effect of citrate on renal stone formation [82] justifies the application of citrate-based materials in renal applications. In fact, POC thin films have demonstrated their efficacy by serving as an suitable scaffold for cell transplantation [175] in partial bladder regeneration. Specifically, POC films in the study displayed a Young’s modulus of 138 KPa with a corresponding elongation of 137%, close to native bladder tissue. After co-cultured mesenchymal stem cells (MSCs) with urothelial cells (UCs) on POC films, the cell-polymer composite was transplanted into nude rat with supratrigonal cystectomy for bladder augmentation, which exhibited typical bladder architecture with numerous muscle bundle formation, retention of smooth muscle contractile proteins as well as a high muscle/collagen ratio after 10 weeks. Besides, POC films also supported the cotransplatation of spina bifida bone marrow stem/progenitor cells to enhance urinary bladder regeneration in terms of increasing tissue vascularization, inducing peripheral nerve growth and supporting urothelium regeneration in grafted areas of animal model [176]. All these results indicates that POC provides a suitable microenvironment for the transplanted cells to participated in the regeneration of bladder.
5.8. Other Implications from Citrate Biology
The prostate is a unique organ that produces, accumulates and releases large amounts of citrate into prostatic fluid, with concentrations reaching up to 180 mM [93]. It is believed that prostate epithelial cells are responsible for the accumulated citrate in prostatic fluid through a unique mechanism capable of producing and exporting large amounts of metabolic citrate from mitochondria to extracellular spaces. Although there is basically no clinical need for replacement of diseased prostatic tissue [177], the highly enriched citrate in native prostate strongly implicates that CBBs could serve as an ideal base material in combination with stem cells to prepare in vitro prostate models for a wide range of prostate physiology or pathology studies, or to fabricate a prostate-on-chip for drug screening applications. Interestingly, a substantial decrease in citrate production has been found in prostate cancer, as most citrate is consumed by prostate cancer cells [178]. Therefore, citrate has the potential to serve as a biomarker for prostate cancer diagnosis [179]. Exogenous citrate (at 1 mM) can also be used by human prostate cancer cells to enhance their in vitro metastatic behaviors [180, 181]. Given all cancer cells undergo metabolic transformation to rely mostly on glycolysis while with high demand for cellular energy and biosynthetic substrates, exogenous citrate is expected to be critical for maintaining energy homeostasis of cancer cells. In fact, silencing of SLC13a5 transporter in hepatocarcinoma cells (suppressing of citrate uptake) results in inhibited cell proliferation by reducing cell energy and decreasing phospholipid content, which deactivate oncogenic mechanistic target of rapamycin (mTOR) signaling, modulated by AMPK (AMP-activated protein kinase), the central cell energy sensor molecule [182]. Interestingly, when its concentration increases to 5 mM or 10 mM, exogenous citrate starts to inhibit the proliferation of multiple cancer cells, such as gastric cancer [183], lung cancer, breast cancer, and pancreatic cancer [184], mainly by inhibiting glycolysis. All the studies implicate the therapeutic potential of citrate for cancer treatment and further comprehensive studies are needed to determine the toxic concentration range to specific cancer types. In addition, it is worthy to note that although citrate has been found to reduce radical surge [185] or alleviate certain cell dysfunction affected by reactive oxygen species (ROS) [155], the existing results are still preliminary and additional research is needed to confirm the antioxidant effect of citrate, since citrate chelating with ferrous ions was also found to elevate intracellular oxidative stress leading to increased ROS production [186, 187]. Therefore, despite its great importance, this aspect of citrate biology is not discussed in the present review.
6. Concluding Remarks
Leveraging the multifunctional nature of citric acid, CBBs represent a series of simple platform materials possessing a high degree of tunability and functionalization potential while maintaining favorable in vitro and in vivo compatibility, which are highly desired for either clinical translation or regenerative engineering application. Beyond polymer chemistry, a discussion of the multifunctional role of citrate in biological systems provides a brand new perspective to understand the significance of materials-derived citrate in different applications, particularly when increasing evidence has pointed out that degradation products of biomaterials are a neglected form of signal that materials may provide to cells. Future research is expected to follow up elucidating the relation between citrate present in CBBs and cell or tissue fate. Such understanding will inspire and enable the design of materials with tuned degradation rates and citrate release profiles to directly influence tissue regeneration. Additionally, improved processing through the adaptation of CBBs to 3D printing and MEMS technologies will greatly enhance the complexity attainable with these materials, while the continued expansion of functionalities enabled by CBB modification promises to expand their application to new areas of biomedicine.
Acknowledgement
This work was supported in part by National Institutes of Health awards (CA182670, EB024829, AR071316) and National Science Foundation award (CMMI 1537008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
Dr. Yang and The Pennsylvania State University have a financial interest in Aleo BME, Inc. and Acuitive Technologies, Inc. These interests have been reviewed by the University’s Institutional and Individual Conflict of Interest Committees and are currently being managed by the University.
References:
- [1].Laurencin CT, Khan Y, Regenerative engineering, Science Translational Medicine, 4 (2012) 160ed9. [DOI] [PubMed] [Google Scholar]
- [2].Rai R, Tallawi M, Grigore A, Boccaccini AR, Synthesis, properties and biomedical applications of poly (glycerol sebacate)(PGS): a review, Progress in Polymer Science 37(8) (2012) 1051–1078. [Google Scholar]
- [3].Wang Y, Ameer GA, Sheppard BJ, Langer R, A tough biodegradable elastomer, Nature Biotechnology 20(6) (2002) 602–606. [DOI] [PubMed] [Google Scholar]
- [4].Yang J, Webb AR, Ameer GA, Novel citric acid-based biodegradable elastomers for tissue engineering, Advanced Materials 16(6) (2004) 511–516. [Google Scholar]
- [5].Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA, Synthesis and evaluation of poly(diol citrate) biodegradable elastomers, Biomaterials 27(9) (2006) 1889–98. [DOI] [PubMed] [Google Scholar]
- [6].Tran RT, Yang J, Ameer GA, Citrate-Based Biomaterials and Their Applications in Regenerative Engineering, Annual Review of Materials Research 45 (2015) 277–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Webb AR, Yang J, Ameer GA, A new strategy to characterize the extent of reaction of thermoset elastomers, Journal of Polymer Science Part A: Polymer Chemistry 46(4) (2008) 1318–1328. [Google Scholar]
- [8].Gosline J, Lillie M, Carrington E, Guerette P, Ortlepp C, Savage K, Elastic proteins: biological roles and mechanical properties, Philosophical Transactions of the Royal Society of London B: Biological Sciences 357(1418) (2002) 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee MC, Haut RC, Strain rate effects on tensile failure properties of the common carotid artery and jugular veins of ferrets, Journal of Biomechanics 25(8) (1992) 925–927. [DOI] [PubMed] [Google Scholar]
- [10].Pommet M, Redl A, Guilbert S, Morel M-H, Intrinsic influence of various plasticizers on functional properties and reactivity of wheat gluten thermoplastic materials, Journal of Cereal Science 42(1) (2005) 81–91. [Google Scholar]
- [11].Ma C, Gerhard E, Lin Q, Xia S, Armstrong AD, Yang J, In vitro cytocompatibility evaluation of poly(octamethylene citrate) monomers toward their use in orthopedic regenerative engineering, Bioactive Materials 3(1) (2018) 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang J, Zhang Y, Gautam S, Liu L, Dey J, Chen W, Mason RP, Serrano CA, Schug KA, Tang L, Development of aliphatic biodegradable photoluminescent polymers, Proceedings of the National Academy of Sciences 106(25) (2009) 10086–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chung EJ, Kodali P, Laskin W, Koh JL, Ameer GA, Long-term in vivo response to citric acid-based nanocomposites for orthopaedic tissue engineering, Journal of Materials Science. Materials in Medicine 22(9) (2011) 2131–8. [DOI] [PubMed] [Google Scholar]
- [14].Serrano MC, Carbajal L, Ameer GA, Novel biodegradable shape‐memory elastomers with drug‐releasing capabilities, Advanced Materials 23(19) (2011) 2211–2215. [DOI] [PubMed] [Google Scholar]
- [15].Bruggeman JP, Bettinger CJ, Nijst CL, Kohane DS, Langer R, Biodegradable Xylitol‐Based Polymers, Advanced Materials 20(10) (2008) 1922–1927. [Google Scholar]
- [16].Zhao H, Ameer GA, Modulating the mechanical properties of poly (diol citrates) via the incorporation of a second type of crosslink network, Journal of Applied Polymer Science 114(3) (2009) 1464–1470. [Google Scholar]
- [17].Gyawali D, Nair P, Zhang Y, Tran RT, Zhang C, Samchukov M, Makarov M, Kim HK, Yang J, Citric acid-derived in situ crosslinkable biodegradable polymers for cell delivery, Biomaterials 31(34) (2010) 9092–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gyawali D, Tran RT, Guleserian KJ, Tang L, Yang J, Citric-acid-derived photo-crosslinked biodegradable elastomers, Journal of biomaterials science. Polymer edition 21(13) (2010) 1761–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tran RT, Thevenot P, Gyawali D, Chiao JC, Tang L, Yang J, Synthesis and characterization of a biodegradable elastomer featuring a dual crosslinking mechanism, Soft Matter 6(11) (2010) 2449–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Keller G, Sefton MV, Radisic M, Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis, Nature Materials 15(6) (2016) 669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Montgomery M, Ahadian S, Huyer LD, Rito ML, Civitarese RA, Vanderlaan RD, Wu J, Reis LA, Momen A, Akbari S, Flexible shape-memory scaffold for minimally invasive delivery of functional tissues, Nature Materials 16(10) (2017) 1038. [DOI] [PubMed] [Google Scholar]
- [22].Shan D, Zhang C, Kalaba S, Mehta N, Kim GB, Liu Z, Yang J, Flexible biodegradable citrate-based polymeric step-index optical fiber, Biomaterials 143 (2017) 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Komabayashi T, Wadajkar A, Santimano S, Ahn C, Zhu Q, Opperman LA, Bellinger LL, Yang J, Nguyen KT, Preliminary study of light-cured hydrogel for endodontic drug delivery vehicle, Journal of Investigative and Clinical Dentistry 7(1) (2016) 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xie Z, Aphale NV, Kadapure TD, Wadajkar AS, Orr S, Gyawali D, Qian G, Nguyen KT, Yang J, Design of antimicrobial peptides conjugated biodegradable citric acid derived hydrogels for wound healing, Journal of Biomedical Materials Research Part A 103(12) (2015) 3907–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yang J, van Lith R, Baler K, Hoshi RA, Ameer GA, A thermoresponsive biodegradable polymer with intrinsic antioxidant properties, Biomacromolecules 15(11) (2014) 3942–52. [DOI] [PubMed] [Google Scholar]
- [26].Dumanian ZP, Tollemar V, Ye J, Lu M, Zhu Y, Liao J, Ameer GA, He T-C, Reid RR, Repair of critical sized cranial defects with BMP9-transduced calvarial cells delivered in a thermoresponsive scaffold, PloS One 12(3) (2017) e0172327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhu Y, Hoshi R, Chen S, Yi J, Duan C, Galiano RD, Zhang HF, Ameer GA, Sustained release of stromal cell derived factor-1 from an antioxidant thermoresponsive hydrogel enhances dermal wound healing in diabetes, Journal of Controlled Release 238 (2016) 114–122. [DOI] [PubMed] [Google Scholar]
- [28].Ware HOT, Farsheed AC, Baker E, Ameer G, Sun C, Fabrication speed optimization for high-resolution 3D-printing of bioresorbable vascular scaffolds, Procedia CIRP 65 (2017) 131–138. [Google Scholar]
- [29].Tran RT, Thevenot P, Zhang Y, Gyawali D, Tang L, Yang J, Scaffold Sheet Design Strategy for Soft Tissue Engineering, Materials 3(2) (2010) 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dey J, Xu H, Nguyen KT, Yang J, Crosslinked urethane doped polyester biphasic scaffolds: Potential for in vivo vascular tissue engineering, Journal of Biomedical Materials Research. Part A 95(2) (2010) 361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tran RT, Choy WM, Cao H, Qattan I, Chiao JC, Ip WY, Yeung KW, Yang J, Fabrication and characterization of biomimetic multichanneled crosslinked-urethane-doped polyester tissue engineered nerve guides, Journal of Biomedical Materials Research. Part A 102(8) (2014) 2793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang Y, Tran RT, Qattan IS, Tsai YT, Tang L, Liu C, Yang J, Fluorescence imaging enabled urethane-doped citrate-based biodegradable elastomers, Biomaterials 34(16) (2013) 4048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guo J, Xie Z, Tran RT, Xie D, Jin D, Bai X, Yang J, Click chemistry plays a dual role in biodegradable polymer design, Advanced Materials 26(12) (2014) 1906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tang J, Guo J, Li Z, Yang C, Xie D, Chen J, Li S, Li S, Kim GB, Bai X, Zhang Z, Yang J, Fast degradable citrate-based bone scaffold promotes spinal fusion, Journal of materials chemistry. B, Materials for Biology and Medicine 3(27) (2015) 5569–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mehdizadeh M, Weng H, Gyawali D, Tang L, Yang J, Injectable citrate-based musselinspired tissue bioadhesives with high wet strength for sutureless wound closure, Biomaterials 33(32) (2012) 7972–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mehdizadeh M, Yang J, Design strategies and applications of tissue bioadhesives, Macromolecular Bioscience 13(3) (2013) 271–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Guo J, Kim GB, Shan D, Kim JP, Hu J, Wang W, Hamad FG, Qian G, Rizk EB, Yang J, Click chemistry improved wet adhesion strength of mussel-inspired citrate-based antimicrobial bioadhesives, Biomaterials 112 (2017) 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Guo J, Wang W, Hu J, Xie D, Gerhard E, Nisic M, Shan D, Qian G, Zheng S, Yang J, Synthesis and characterization of anti-bacterial and anti-fungal citrate-based mussel-inspired bioadhesives, Biomaterials 85 (2016) 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xie Z, Kim JP, Cai Q, Zhang Y, Guo J, Dhami RS, Li L, Kong B, Su Y, Schug KA, Yang J, Synthesis and characterization of citrate-based fluorescent small molecules and biodegradable polymers, Acta Biomaterialia 50 (2017) 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kim JP, Xie Z, Creer M, Liu Z, Yang J, Citrate-based fluorescent materials for low-cost chloride sensing in the diagnosis of Cystic Fibrosis, Chemical Science 8(1) (2017) 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang C, Kim JP, Creer M, Yang J, Liu Z, A smartphone-based chloridometer for pointof-care diagnostics of cystic fibrosis, Biosensors and Bioelectronics 97 (2017) 164–168. [DOI] [PubMed] [Google Scholar]
- [42].Xie Z, Zhang Y, Liu L, Weng H, Mason RP, Tang L, Nguyen KT, Hsieh JT, Yang J, Development of intrinsically photoluminescent and photostable polylactones, Advanced Materials 26(26) (2014) 4491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hu J, Guo J, Xie Z, Shan D, Gerhard E, Qian G, Yang J, Fluorescence imaging enabled poly(lactide-co-glycolide), Acta Biomaterialia 29 (2016) 307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Murphy WL, McDevitt TC, Engler AJ, Materials as stem cell regulators, Nature Materials 13(6) (2014) 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Crowder SW, Leonardo V, Whittaker T, Papathanasiou P, Stevens MM, Material cues as potent regulators of epigenetics and stem cell function, Cell Stem Cell 18(1) (2016) 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Patel AK, Tibbitt MW, Celiz AD, Davies MC, Langer R, Denning C, Alexander MR, Anderson DG, High throughput screening for discovery of materials that control stem cell fate, Current Opinion in Solid State and Materials Science 20(4) (2016) 202–211. [Google Scholar]
- [47].Lane SW, Williams DA, Watt FM, Modulating the stem cell niche for tissue regeneration, Nature Biotechnology 32(8) (2014) 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Stuart MAC, Boehm H, Li B, Vogel V, Extracellular-matrix tethering regulates stem-cell fate, Nature Materials 11(7) (2012) 642–649. [DOI] [PubMed] [Google Scholar]
- [49].Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA, Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked threedimensional hydrogels, Nature Materials 12(5) (2013) 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R, Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins, Proceedings of the National Academy of Sciences 100(8) (2003) 4766–4771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [51].O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J, Endostatin: an endogenous inhibitor of angiogenesis and tumor growth, Cell 88(2) (1997) 277–285. [DOI] [PubMed] [Google Scholar]
- [52].Dixelius J, Larsson H, Sasaki T, Holmqvist K, Lu L, Engström Å, Timpl R, Welsh M, Claesson-Welsh L, Endostatin-induced tyrosine kinase signaling through the Shb adaptor protein regulates endothelial cell apoptosis, Blood 95(11) (2000) 3403–3411. [PubMed] [Google Scholar]
- [53].Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S, Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways, Matrix Biology 26(1) (2007) 58–68. [DOI] [PubMed] [Google Scholar]
- [54].Homandberg G, Williams J, Grant D, Schumacher B, Eisenstein R, Heparin-binding fragments of fibronectin are potent inhibitors of endothelial cell growth, The American Journal of Pathology 120(3) (1985) 327. [PMC free article] [PubMed] [Google Scholar]
- [55].O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J, Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma, Cell 79(2) (1994) 315–328. [DOI] [PubMed] [Google Scholar]
- [56].Ahmann KA, Weinbaum JS, Johnson SL, Tranquillo RT, Fibrin degradation enhances vascular smooth muscle cell proliferation and matrix deposition in fibrin-based tissue constructs fabricated in vitro, Tissue Engineering Part A 16(10) (2010) 3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Barradas AM, Fernandes HA, Groen N, Chai YC, Schrooten J, van de Peppel J, van Leeuwen JP, van Blitterswijk CA, de Boer J, A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells, Biomaterials 33(11) (2012) 3205–15. [DOI] [PubMed] [Google Scholar]
- [58].Aquino-Martínez R, Artigas N, Gámez B, Rosa JL, Ventura F, Extracellular calcium promotes bone formation from bone marrow mesenchymal stem cells by amplifying the effects of BMP-2 on SMAD signalling, PloS One 12(5) (2017) e0178158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Honda Y, Anada T, Kamakura S, Nakamura M, Sugawara S, Suzuki O, Elevated extracellular calcium stimulates secretion of bone morphogenetic protein 2 by a macrophage cell line, Biochemical and Biophysical Research Communications 345(3) (2006) 1155–1160. [DOI] [PubMed] [Google Scholar]
- [60].Zhang Y, Xu J, Ruan YC, Yu MK, O’Laughlin M, Wise H, Chen D, Tian L, Shi D, Wang J, Chen S, Feng JQ, Chow DH, Xie X, Zheng L, Huang L, Huang S, Leung K, Lu N, Zhao L, Li H, Zhao D, Guo X, Chan K, Witte F, Chan HC, Zheng Y, Qin L, Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats, Nature Medicine 22(10) (2016) 1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wu L, Luthringer BJ, Feyerabend F, Schilling AF, Willumeit R, Effects of extracellular magnesium on the differentiation and function of human osteoclasts, Acta Biomaterialia 10(6) (2014) 2843–2854. [DOI] [PubMed] [Google Scholar]
- [62].Wu L, Feyerabend F, Schilling AF, Willumeit-Römer R, Luthringer BJ, Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture, Acta Biomaterialia 27 (2015) 294–304. [DOI] [PubMed] [Google Scholar]
- [63].Saino E, Grandi S, Quartarone E, Maliardi V, Galli D, Bloise N, Fassina L, De Angelis M, Mustarelli P, Imbriani M, In vitro calcified matrix deposition by human osteoblasts onto a zinc-containing bioactive glass, European Cells & Materials 21(2) (2011) 59–72. [DOI] [PubMed] [Google Scholar]
- [64].Jin G, Qin H, Cao H, Qiao Y, Zhao Y, Peng X, Zhang X, Liu X, Chu PK, Zn/Ag microgalvanic couples formed on titanium and osseointegration effects in the presence of S. aureus, Biomaterials 65 (2015) 22–31. [DOI] [PubMed] [Google Scholar]
- [65].Qiao Y, Zhang W, Tian P, Meng F, Zhu H, Jiang X, Liu X, Chu PK, Stimulation of bone growth following zinc incorporation into biomaterials, Biomaterials 35(25) (2014) 68826897. [DOI] [PubMed] [Google Scholar]
- [66].Zhang W, Wang G, Liu Y, Zhao X, Zou D, Zhu C, Jin Y, Huang Q, Sun J, Liu X, The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration, Biomaterials 34(13) (2013) 3184–3195. [DOI] [PubMed] [Google Scholar]
- [67].Qiu K, Zhao XJ, Wan CX, Zhao CS, Chen YW, Effect of strontium ions on the growth of ROS17/2.8 cells on porous calcium polyphosphate scaffolds, Biomaterials 27(8) (2006) 12771286. [DOI] [PubMed] [Google Scholar]
- [68].Kokesch-Himmelreich J, Schumacher M, Rohnke M, Gelinsky M, Janek J, ToF-SIMS analysis of osteoblast-like cells and their mineralized extracellular matrix on strontium enriched bone cements, Biointerphases 8(1) (2013) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yu-Ru YH Shih V, Phadkea Ameya, Kang Heemin, Hwange Nathaniel S., Carof Eduardo J., Nguyenf Steven, Siua Michael, Theodorakisf Emmanuel A., Gianneschif Nathan C., Vecchiog Kenneth S., Chien Shu, Leeh,i,1 Oscar K., and Varghese Shyni, Calcium phosphatebearing matrices induce osteogenic differentiation of stem cells through adenosine signaling, Proceedings of the Natuional Academy of Sciences 111(3) (2013) 990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lampe KJ, Bjugstad KB, Mahoney MJ, Impact of degradable macromer content in a poly (ethylene glycol) hydrogel on neural cell metabolic activity, redox state, proliferation, and differentiation, Tissue Engineering Part A 16(6) (2010) 1857–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lampe KJ, Namba RM, Silverman TR, Bjugstad KB, Mahoney MJ, Impact of lactic acid on cell proliferation and free radical-induced cell death in monolayer cultures of neural precursor cells, Biotechnology and Bioengineering 103(6) (2009) 1214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tran RT, Wang L, Zhang C, Huang M, Tang W, Zhang C, Zhang Z, Jin D, Banik B, Brown JL, Xie Z, Bai X, Yang J, Synthesis and characterization of biomimetic citrate-based biodegradable composites, Journal of biomedical materials research. Part A 102(8) (2014) 2521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Xie D, Guo J, Mehdizadeh M, Tran RT, Chen R, Sun D, Qian G, Jin D, Bai X, Yang J, Development of Injectable Citrate-Based Bioadhesive Bone Implants, Journal of materials chemistry. B, Materials for Biology and Medicine 3 (2015) 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Westergaard N, Sonnewald U, Unsgård G, Peng L, Hertz L, Schousboe A, Uptake, release, and metabolism of citrate in neurons and astrocytes in primary cultures, Journal of Neurochemistry 62(5) (1994) 1727–1733. [DOI] [PubMed] [Google Scholar]
- [75].Westergaard N, Waagepetersen HS, Belhage B, Schousboe A, Citrate, a ubiquitous key metabolite with regulatory function in the CNS, Neurochemical Research 42(6) (2017) 1–6. [DOI] [PubMed] [Google Scholar]
- [76].Mycielska ME, Patel A, Rizaner N, Mazurek MP, Keun H, Patel A, Ganapathy V, Djamgoz MB, Citrate transport and metabolism in mammalian cells: prostate epithelial cells and prostate cancer, BioEssays : news and reviews in molecular, Cellular and Developmental Biology 31(1) (2009) 10–20. [DOI] [PubMed] [Google Scholar]
- [77].Costello LC, Franklin RB, Plasma Citrate Homeostasis: How It Is Regulated; And Its Physiological and Clinical Implications. An Important, But Neglected, Relationship in Medicine, HSOA Journal of Human Endocrinology 1(1) (2016) 005. [PMC free article] [PubMed] [Google Scholar]
- [78].Hartles R, Leaver A, Citrate in mineralized tissues—I: Citrate in human dentine, Archives of Oral Biology 1(4) (1960) 297–303. [DOI] [PubMed] [Google Scholar]
- [79].Reid DG, Duer MJ, Jackson GE, Murray RC, Rodgers AL, Shanahan CM, Citrate occurs widely in healthy and pathological apatitic biomineral: mineralized articular cartilage, and intimal atherosclerotic plaque and apatitic kidney stones, Calcified Tissue International 93(3) (2013) 253–60. [DOI] [PubMed] [Google Scholar]
- [80].Ganapathy V, Fei Y, Biological role and therapeutic potential of NaCT, a sodium-coupled transporter for citrate in mammals, Proceedings-Indian National Science Academy Part B 71(2) (2005) 83. [Google Scholar]
- [81].Costello LC, Franklin RB, Zou J, Feng P, Bok R, Swanson MG, Kurhanewicz J, Human prostate cancer ZIP1/zinc/citrate genetic/metabolic relationship in the TRAMP prostate cancer animal model, Cancer Biololy Therapy 12(12) (2011) 1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Caudarella R, Vescini F, Urinary citrate and renal stone disease: the preventive role of alkali citrate treatment, Arch Ital Urol Androl 81(3) (2009) 182–187. [PubMed] [Google Scholar]
- [83].Metallo CM, Vander Heiden MG, Metabolism strikes back: metabolic flux regulates cell signaling, Genes & Development 24(24) (2010) 2717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Agathocleous M, Harris WA, Metabolism in physiological cell proliferation and differentiation, Trends in cell biology 23(10) (2013) 484–92. [DOI] [PubMed] [Google Scholar]
- [85].Lu C, Thompson CB, Metabolic regulation of epigenetics, Cell Metabolism 16(1) (2012) 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mycielska ME, Milenkovic VM, Wetzel CH, Rümmele P, Geissler EK, Extracellular Citrate in Health and Disease, Current Molecular Medicine 15(10) (2015) 884–891. [DOI] [PubMed] [Google Scholar]
- [87].Iacobazzi V, Infantino V, Citrate--new functions for an old metabolite, Journal of Biological Chemistry 395(4) (2014) 387–99. [DOI] [PubMed] [Google Scholar]
- [88].Rives ML, Shaw M, Zhu B, Hinke SA, Wickenden AD, State-Dependent Allosteric Inhibition of the Human SLC13A5 Citrate Transporter by Hydroxysuccinic Acids, PF-06649298 and PF-06761281, Molecular Pharmacology 90(6) (2016) 766–774. [DOI] [PubMed] [Google Scholar]
- [89].Willmes DM, Birkenfeld AL, The Role of INDY in Metabolic Regulation, Computational and Structural Biotechnology Journal 6 (2013) e201303020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wegner A, Meiser J, Weindl D, Hiller K, How metabolites modulate metabolic flux, Current Opinion in Biotechnology 34 (2015) 16–22. [DOI] [PubMed] [Google Scholar]
- [91].Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB, ATPcitrate lyase links cellular metabolism to histone acetylation, Science 324(5930) (2009) 10761080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].MacPherson S, Horkoff M, Gravel C, Hoffmann T, Zuber J, Lum JJ, STAT3 Regulation of Citrate Synthase Is Essential during the Initiation of Lymphocyte Cell Growth, Cell Reports 19(5) (2017) 910–918. [DOI] [PubMed] [Google Scholar]
- [93].Kavanagh J, Isocitric and citric acid in human prostatic and seminal fluid: implications for prostatic metabolism and secretion, The Prostate 24(3) (1994) 139–142. [DOI] [PubMed] [Google Scholar]
- [94].Bergeron MJ, Clemencon B, Hediger MA, Markovich D, SLC13 family of Na(+)-coupled di- and tri-carboxylate/sulfate transporters, Molecular Aspects of Medicine 34(2–3) (2013) 299312. [DOI] [PubMed] [Google Scholar]
- [95].Huard K, Brown J, Jones JC, Cabral S, Futatsugi K, Gorgoglione M, Lanba A, Vera NB, Zhu Y, Yan Q, Zhou Y, Vernochet C, Riccardi K, Wolford A, Pirman D, Niosi M, Aspnes G, Herr M, Genung NE, Magee TV, Uccello DP, Loria P, Di L, Gosset JR, Hepworth D, Rolph T, Pfefferkorn JA, Erion DM, Discovery and characterization of novel inhibitors of the sodium-coupled citrate transporter (NaCT or SLC13A5), Scientific Reports 5 (2015) 17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Mycielska ME, Dettmer-Wilde K, Rümmele P, Schmidt KM, Prehn C, Milenkovic V, Jagla W, Madej MG, Lantow M, Schladt MT, Extracellular citrate affects critical elements of cancer cell metabolism and supports cancer development in vivo, Cancer Research (2018) canres. 2959.2017. [DOI] [PubMed] [Google Scholar]
- [97].Infantino V, Convertini P, Cucci L, Panaro MA, Di Noia MA, Calvello R, Palmieri F, Iacobazzi V, The mitochondrial citrate carrier: a new player in inflammation, The Biochemical Journal 438(3) (2011) 433–6. [DOI] [PubMed] [Google Scholar]
- [98].Ashbrook M, McDonough K, Pituch J, Christopherson P, Cornell T, Selewski D, Shanley T, Blatt N, Citrate modulates lipopolysaccharide‐induced monocyte inflammatory responses, Clinical & Experimental Immunology 180(3) (2015) 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].He W, Miao FJ-P, Lin DC-H, Schwandner RT, Wang Z, Gao J, Chen J-L, Tian H, Ling L, Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors, Nature 429(6988) (2004) 188–193. [DOI] [PubMed] [Google Scholar]
- [100].Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido-Perrig N, Schwärzler C, Junt T, Voshol H, Meingassner JG, Triggering the succinate receptor GPR91 on dendritic cells enhances immunity, Nature Immunology 9(11) (2008) 1261–1269. [DOI] [PubMed] [Google Scholar]
- [101].Shanbrom E, Use of citric acid as antimicrobial agent or enhancer or as anticancer agent, Google Patents, 2002. [Google Scholar]
- [102].Smith JJ, Wayman BE, An evaluation of the antimicrobial effectiveness of citric acid as a root canal irrigant, Journal of Endodontics 12(2) (1986) 54–58. [DOI] [PubMed] [Google Scholar]
- [103].Scelza M.r.F.Z., Pierro V, Scelza P, Pereira M, Effect of three different time periods of irrigation with EDTA-T, EDTA, and citric acid on smear layer removal, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 98(4) (2004) 499–503. [DOI] [PubMed] [Google Scholar]
- [104].Mani-Lopez E, García HS, López-Malo A, Organic acids as antimicrobials to control Salmonella in meat and poultry products, Food Research International 45(2) (2012) 713–721. [Google Scholar]
- [105].Su L-C, Xie Z, Zhang Y, Nguyen KT, Yang J, Study on the antimicrobial properties of citrate-based biodegradable polymers, Frontiers in Bioengineering and Biotechnology 2 (2014) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Gethin G, The significance of surface pH in chronic wounds, Wounds UK 3(3) (2007) 52. [Google Scholar]
- [107].Leveen HH, Falk G, Borek B, Diaz C, Lynfield Y, Wynkoop BJ, Mabunda GA, Rubricius JL, Christoudias GC, Chemical acidification of wounds. An adjuvant to healing and the unfavorable action of alkalinity and ammonia, Annals of Surgery 178(6) (1973) 745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Weijmer MC, Debets‐Ossenkopp YJ, van de Vondervoort FJ, ter Wee PM, Superior antimicrobial activity of trisodium citrate over heparin for catheter locking, Nephrology Dialysis Transplantation 17(12) (2002) 2189–2195. [DOI] [PubMed] [Google Scholar]
- [109].Nagaoka S, Murata S, Kimura K, Mori T, Hojo K, Antimicrobial activity of sodium citrate against Streptococcus pneumoniae and several oral bacteria, Letters in Applied Microbiology 51(5) (2010) 546–551. [DOI] [PubMed] [Google Scholar]
- [110].Helander I, Mattila‐Sandholm T, Fluorometric assessment of Gram‐negative bacterial permeabilization, Journal of Applied Microbiology 88(2) (2000) 213–219. [DOI] [PubMed] [Google Scholar]
- [111].Tran RT, Palmer M, Tang SJ, Abell TL, Yang J, Injectable drug-eluting elastomeric polymer: a novel submucosal injection material, Gastrointestinal Endoscopy 75(5) (2012) 10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Manolakis I, Noordover BA, Vendamme R, Eevers W, Novel L‐DOPA‐Derived Poly (ester amide) s: Monomers, Polymers, and the First L‐DOPA‐Functionalized Biobased Adhesive Tape, Macromolecular Rapid Communications 35(1) (2014) 71–76. [DOI] [PubMed] [Google Scholar]
- [113].Dickens F, The citric acid content of animal tissues, with reference to its occurrence in bone and tumour, The Biochemical Journal 35(8–9) (1941) 1011–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Eastoe JE, Eastoe B, The organic constituents of mammalian compact bone, The Biochemical Journal 57(3) (1954) 453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Dixon TF, Perkins HR, Citric acid and bone metabolism, The Biochemical Journal 52(2) (1952) 260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hu YY, Rawal A, Schmidt-Rohr K, Strongly bound citrate stabilizes the apatite nanocrystals in bone, Proceedings of the National Academy of Sciences 107(52) (2010) 224259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hartles RL, Citrate in mineralized tissues, Advanced Oral Biology 1 (1964) 225–253. [DOI] [PubMed] [Google Scholar]
- [118].K.H.M Erika Daviesa, Wonga Wai Ching, Pickardc Chris J., Reida David G., Skepperb Jeremy N., Duera a.M.J. Citrate bridges between mineral platelets in bone, Proceedings of the National Academy of Sciences 111(14) (2014) E1354–E1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Hu YY, Liu XP, Ma X, Rawal A, Prozorov T, Akinc M, Mallapragada SK, SchmidtRohr K, Biomimetic Self-Assembling Copolymer−Hydroxyapatite Nanocomposites with the Nanocrystal Size Controlled by Citrate, Chemistry of Materials 23(9) (2011) 2481–2490. [Google Scholar]
- [120].Shao C, Zhao R, Jiang S, Yao S, Wu Z, Jin B, Yang Y, Pan H, Tang R, Citrate Improves Collagen Mineralization via Interface Wetting: A Physicochemical Understanding of Biomineralization Control, Advanced Materials 30(8) (2018) 1704876–83. [DOI] [PubMed] [Google Scholar]
- [121].Fu X, Li Y, Huang T, Yu Z, Ma K, Yang M, Liu Q, Pan H, Wang H, Wang J, Guan M, Runx2/Osterix and Zinc Uptake Synergize to Orchestrate Osteogenic Differentiation and Citrate Containing Bone Apatite Formation, Advanced Science (2018) e1700755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Pattappa G, Heywood HK, de Bruijn JD, Lee DA, The metabolism of human mesenchymal stem cells during proliferation and differentiation, Journal of Cellular Physiology 226(10) (2011) 2562–70. [DOI] [PubMed] [Google Scholar]
- [123].Forni MF, Peloggia J, Trudeau K, Shirihai O, Kowaltowski AJ, Murine mesenchymal stem cell commitment to differentiation is regulated by mitochondrial dynamics, Stem Cells 34(3) (2016) 743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Karner CM, Esen E, Okunade AL, Patterson BW, Long F, Increased glutamine catabolism mediates bone anabolism in response to WNT signaling, The Journal of Clinical Investigation 125(2) (2015) 551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].C. M Franklin1 Renty B., Zou1 Jing, Reynolds2 Mark A. and Costello Leslie C., Evidence that osteoblasts are specialized citrate-producing cells that provide the citrate for incorporation into the structure of bone, The Open Bone Journal 6 (2014) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Franklin RB, Chellaiah M, Zou J, Reynolds MA, Costello LC, Evidence that Osteoblasts are Specialized Citrate-producing Cells that Provide the Citrate for Incorporation into the Structure of Bone, The Open Bone Journal 6 (2014) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Costello LC, Chellaiah MA, Zou J, Reynolds MA, Franklin RB, In vitro BMP2 stimulation of osteoblast citrate production in concert with mineralized bone nodule formation, Journal of Regenerative Medicine & Tissue Engineering 4 (2015) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Jehle S, Hulter HN, Krapf R, Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial, The Journal of Clinical Endocrinology and Metabolism 98(1) (2013) 207–17. [DOI] [PubMed] [Google Scholar]
- [129].Pak CY, Peterson RD, Poindexter J, Prevention of spinal bone loss by potassium citrate in cases of calcium urolithiasis, The Journal of Urology 168(1) (2002) 31–34. [PubMed] [Google Scholar]
- [130].Vescini F, Buffa A, La Manna G, Ciavatti A, Rizzoli E, Bottura A, Stefoni S, Caudarella R, Long-term potassium citrate therapy and bone mineral density in idiopathic calcium stone formers, Journal of Endocrinological Investigation 28(5) (2005) 218–222. [DOI] [PubMed] [Google Scholar]
- [131].Mantila Roosa SM, Liu Y, Turner CH, Gene expression patterns in bone following mechanical loading, Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 26(1) (2011) 100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Thalji G, Gretzer C, Cooper LF, Comparative molecular assessment of early osseointegration in implant-adherent cells, Bone 52(1) (2013) 444–53. [DOI] [PubMed] [Google Scholar]
- [133].Petrovich YA, Podorozhnaya R, Kichenko S, Dmitriev I, Labeled Citrate Metabolism in Bone Fractures and Impaired Innervation, Bulletin of Experimental Biology and Medicine 136(1) (2003) 84–87. [DOI] [PubMed] [Google Scholar]
- [134].Irizarry AR, Yan G, Zeng Q, Lucchesi J, Hamang MJ, Ma YL, Rong JX, Defective enamel and bone development in sodium-dependent citrate transporter (NaCT) Slc13a5 deficient mice, PloS One 12(4) (2017) e0175465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Gyawali D, Nair P, Kim HK, Yang J, Citrate-based Biodegradable Injectable hydrogel Composites for Orthopedic Applications, Biomaterials Science 1(1) (2013) 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Binu S, Soumya S, Sudhakaran P, Metabolite control of angiogenesis: angiogenic effect of citrate, Journal of Physiology and Biochemistry 69(3) (2013) 383–395. [DOI] [PubMed] [Google Scholar]
- [137].Qiu H, Yang J, Kodali P, Koh J, Ameer GA, A citric acid-based hydroxyapatite composite for orthopedic implants, Biomaterials 27(34) (2006) 5845–54. [DOI] [PubMed] [Google Scholar]
- [138].Rhee SH, Tanaka J, Effect of citric acid on the nucleation of hydroxyapatite in a simulated body fluid, Biomaterials 20(22) (1999) 2155–60. [DOI] [PubMed] [Google Scholar]
- [139].Rhee SH, Tanaka J, Hydroxyapatite formation on cellulose cloth induced by citric acid, Journal of materials science. Materials in medicine 11(7) (2000) 449–52. [DOI] [PubMed] [Google Scholar]
- [140].Yokoyama A, Yamamoto S, Kawasaki T, Kohgo T, Nakasu M, Development of calcium phosphate cement using chitosan and citric acid for bone substitute materials, Biomaterials 23(4) (2002) 1091–101. [DOI] [PubMed] [Google Scholar]
- [141].Jiao Y, Gyawali D, Stark JM, Akcora P, Nair P, Tran RT, Yang J, A rheological study of biodegradable injectable PEGMC/HA composite scaffolds, Soft Matter 8(5) (2012) 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Liu HN, Webster TJ, Mechanical properties of dispersed ceramic nanoparticles in polymer composites for orthopedic applications, International Journal of Nanomedicine 5 (2010) 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Johnson AJW, Herschler BA, A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair, Acta Biomaterialia 7(1) (2011) 16–30. [DOI] [PubMed] [Google Scholar]
- [144].Sun D, Chen Y, Tran RT, Xu S, Xie D, Jia C, Wang Y, Guo Y, Zhang Z, Guo J, Yang J, Jin D, Bai X, Citric acid-based hydroxyapatite composite scaffolds enhance calvarial regeneration, Scientific Reports 4 (2014) 6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Guo Y, Tran RT, Xie D, Wang Y, Nguyen DY, Gerhard E, Guo J, Tang J, Zhang Z, Bai X, Yang J, Citrate-based biphasic scaffolds for the repair of large segmental bone defects, Journal of Biomedical Materials Research. Part A 103(2) (2015) 772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Dey J, Xu H, Nguyen KT, Yang J, Crosslinked urethane doped polyester biphasic scaffolds: Potential for in vivo vascular tissue engineering, Journal of Biomedical Materials Research A 95A(2) (2010) 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Dey J, Xu H, Shen J, Thevenot P, Gondi SR, Nguyen KT, Sumerlin BS, Tang L, Yang J, Development of biodegradable crosslinked urethane-doped polyester elastomers, Biomaterials 29(35) (2008) 4637–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Dey J, Tran RT, Shen JH, Tang LP, Yang J, Development and Long-Term In Vivo Evaluation of a Biodegradable Urethane-Doped Polyester Elastomer, Macromolecular Materials and Engineering 296(12) (2011) 1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Chung EJ, Sugimoto MJ, Koh JL, Ameer GA, A biodegradable tri‐component graft for anterior cruciate ligament reconstruction, Journal of Tissue Engineering and Regenerative Medicine 11(3) (2017) 704–712. [DOI] [PubMed] [Google Scholar]
- [150].Kaiser MG, Eck JC, Groff MW, Watters III WC, Dailey AT, Resnick DK, Choudhri TF, Sharan A, Wang JC, Mummaneni PV, Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 1: introduction and methodology, Journal of Neurosurgery: Spine 21(1) (2014) 2–6. [DOI] [PubMed] [Google Scholar]
- [151].Ye J, Wang J, Zhu Y, Wei Q, Wang X, Yang J, Tang S, Liu H, Fan J, Zhang F, A thermoresponsive polydiolcitrate-gelatin scaffold and delivery system mediates effective bone formation from BMP9-transduced mesenchymal stem cells, Biomedical Materials 11(2) (2016) 025021. [DOI] [PubMed] [Google Scholar]
- [152].Fraenkl SA, Muser J, Groell R, Reinhard G, Orgul S, Flammer J, Goldblum D, Plasma citrate levels as a potential biomarker for glaucoma, Journal of Ocular Pharmacology and Therapeutics 27(6) (2011) 577–580. [DOI] [PubMed] [Google Scholar]
- [153].Stas KJ, Vanwalleghem J, Moor BD, Keuleers H, Trisodium citrate 30% vs heparin 5% as catheter lock in the interdialytic period in twin‐or double‐lumen dialysis catheters for intermittent haemodialysis, Nephrology Dialysis Transplantation 16(7) (2001) 1521–1522. [DOI] [PubMed] [Google Scholar]
- [154].Oudemans-van Straaten HM, Ostermann M, Bench-to-bedside review: citrate for continuous renal replacement therapy, from science to practice, Critical Care 16(6) (2012) 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Bryland A, Wieslander A, Carlsson O, Hellmark T, Godaly G, Citrate treatment reduces endothelial death and inflammation under hyperglycaemic conditions, Diabetes & Vascular Disease Research 9(1) (2012) 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Nemkov T, Sun K, Reisz JA, Yoshida T, Dunham A, Wen EY, Wen AQ, Roach RC, Hansen KC, Xia Y, Metabolism of citrate and Other carboxylic acids in erythrocytes as a Function of Oxygen saturation and refrigerated storage, Frontiers in Medicine 4 (2017) 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Motlagh D, Allen J, Hoshi R, Yang J, Lui K, Ameer G, Hemocompatibility evaluation of poly(diol citrate) in vitro for vascular tissue engineering, Journal of Biomedical Materials Research Part A 82A(4) (2007) 907–916. [DOI] [PubMed] [Google Scholar]
- [158].Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Heart disease and stroke statistics—2017 update: a report from the American Heart Association, Circulation 135(10) (2017) e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Pashneh-Tala S, MacNeil S, Claeyssens F, The tissue-engineered vascular graft—past, present, and future, Tissue Engineering Part B: Reviews 22(1) (2015) 68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Yang J, Motlagh D, Allen JB, Webb AR, Kibbe MR, Aalami O, Kapadia M, Carroll TJ, Ameer GA, Modulating expanded polytetrafluoroethylene vascular graft host response via citric acid-based biodegradable elastomers, Advanced Materials 18(12) (2006) 1493–98. [Google Scholar]
- [161].Kibbe MR, Martinez J, Popowich DA, Kapadia MR, Ahanchi SS, Aalami OO, Jiang Q, Webb AR, Yang J, Carroll T, Ameer GA, Citric acid-based elastomers provide a biocompatible interface for vascular grafts, Journal of Biomedical Materials Research. Part A 93(1) (2010) 314–24. [DOI] [PubMed] [Google Scholar]
- [162].Hoshi RA, Van Lith R, Jen MC, Allen JB, Lapidos KA, Ameer G, The blood and vascular cell compatibility of heparin-modified ePTFE vascular grafts, Biomaterials 34(1) (2013) 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Xu H, Nguyen KT, Brilakis ES, Yang J, Fuh E, Banerjee S, Enhanced endothelialization of a new stent polymer through surface enhancement and incorporation of growth factor-delivering microparticles, Journal of Cardiovascular Translational Research 5(4) (2012) 519–27. [DOI] [PubMed] [Google Scholar]
- [164].Yang J, Motlagh D, Webb AR, Ameer GA, Novel biphasic elastomeric scaffold for small-diameter blood vessel tissue engineering, Tissue Engineering 11(11–12) (2005) 18761886. [DOI] [PubMed] [Google Scholar]
- [165].Szebeni J, Hemocompatibility testing for nanomedicines and biologicals: predictive assays for complement mediated infusion reactions, European Journal of Nanomedicine 4(1) (2012) 33–53. [Google Scholar]
- [166].Su L-C, Xu H, Tran RT, Tsai Y-T, Tang L, Banerjee S, Yang J, Nguyen KT, In situ re-endothelialization via multifunctional nanoscaffolds, ACS Nano 8(10) (2014) 10826–10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Gyawali D, Zhou S, Tran RT, Zhang Y, Liu C, Bai X, Yang J, Fluorescence imaging enabled biodegradable photostable polymeric micelles, Advanced Healthcare Materials 3(2) (2014) 182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Manry D, Gyawali D, Yang J, Size optimization of biodegradable fluorescent nanogels for cell imaging, Journal of High School Research 2(1) (2009) 1–7. [Google Scholar]
- [169].Gyawali D, Kim JP, Yang J, Highly photostable nanogels for fluorescence-based theranostics, Bioactive Materials 3(1) (2017) 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Xie Z, Su Y, Kim GB, Selvi E, Ma C, Aragon‐Sanabria V, Hsieh JT, Dong C, Yang J, Immune Cell‐Mediated Biodegradable Theranostic Nanoparticles for Melanoma Targeting and Drug Delivery, Small 13(10) (2017) 1603121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Li J, Tian Y, Shan D, Gong A, Zeng L, Ren W, Xiang L, Gerhard E, Zhao J, Yang J, Neuropeptide YY 1 receptor-mediated biodegradable photoluminescent nanobubbles as ultrasound contrast agents for targeted breast cancer imaging, Biomaterials 116 (2017) 106–117. [DOI] [PubMed] [Google Scholar]
- [172].Wadajkar AS, Kadapure T, Zhang Y, Cui W, Nguyen KT, Yang J, Dual-imaging enabled cancer-targeting nanoparticles, Advanced Healthcare Materials 1(4) (2012) 450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Sonnewald U, Westergaard N, Krane J, Unsgård G, Petersen S, Schousboe A, First direct demonstration of preferential release of citrate from astrocytes using [13 C] NMR spectroscopy of cultured neurons and astrocytes, Neuroscience Letters 128(2) (1991) 235–239. [DOI] [PubMed] [Google Scholar]
- [174].Meshitsuka S, Aremu DA, 13C heteronuclear NMR studies of the interaction of cultured neurons and astrocytes and aluminum blockade of the preferential release of citrate from astrocytes, JBIC Journal of Biological Inorganic Chemistry 13(2) (2008) 241–247. [DOI] [PubMed] [Google Scholar]
- [175].Sharma AK, Hota PV, Matoka DJ, Fuller NJ, Jandali D, Thaker H, Ameer GA, Cheng EY, Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly (1, 8-octanediol-co-citrate) based thin films, Biomaterials 31(24) (2010) 6207–6217. [DOI] [PubMed] [Google Scholar]
- [176].Sharma AK, Bury MI, Fuller NJ, Marks AJ, Kollhoff DM, Rao MV, Hota PV, Matoka DJ, Edassery SL, Thaker H, Cotransplantation with specific populations of spina bifida bone marrow stem/progenitor cells enhances urinary bladder regeneration, Proceedings of the National Academy of Sciences 110(10) (2013) 4003–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Yamzon JL, Kokorowski P, Koh CJ, Stem cells and tissue engineering applications of the genitourinary tract, Pediatric Research 63(5) (2008) 472–477. [DOI] [PubMed] [Google Scholar]
- [178].Costello LC, Franklin RB, The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots, Molecular Cancer 5 (2006) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Costello L, Franklin R, and P Narayan, Citrate in the diagnosis of prostate cancer, The Prostate 38(3) (1999) 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Mycielska ME, Broke-Smith TP, Palmer CP, Beckerman R, Nastos T, Erguler K, Djamgoz MB, Citrate enhances in vitro metastatic behaviours of PC-3M human prostate cancer cells: status of endogenous citrate and dependence on aconitase and fatty acid synthase, The international Journal of Biochemistry & Cell Biology 38(10) (2006) 1766–77. [DOI] [PubMed] [Google Scholar]
- [181].Mycielska ME, Palmer CP, Brackenbury WJ, Djamgoz MB, Expression of Na+dependent citrate transport in a strongly metastatic human prostate cancer PC-3M cell line: regulation by voltage-gated Na+ channel activity, The Journal of Physiology 563(Pt 2) (2005) 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Li Z, Li D, Choi EY, Lapidus R, Zhang L, Huang S-M, Shapiro P, Wang H, Silencing of solute carrier family 13 member 5 disrupts energy homeostasis and inhibits proliferation of human hepatocarcinoma cells, Journal of Biological Chemistry 292(33) (2017) 13890–13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Lu Y, Zhang X, Zhang H, Lan J, Huang G, Varin E, Lincet H, Poulain L, Icard P, Citrate induces apoptotic cell death: a promising way to treat gastric carcinoma?, Anticancer Research 31(3) (2011) 797–805. [PubMed] [Google Scholar]
- [184].Ren J-G, Seth P, Ye H, Guo K, Hanai J.-i., Husain Z, Sukhatme VP, Citrate Suppresses Tumor Growth in Multiple Models through Inhibition of Glycolysis, the Tricarboxylic Acid Cycle and the IGF-1R Pathway, Scientific Reports 7(1) (2017) 4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Delvecchio FC, Brizuela RM, Khan SR, Byer K, Li Z, Zhong P, Preminger GM, Citrate and vitamin E blunt the shock wave-induced free radical surge in an in vitro cell culture model, Urological Research 33(6) (2005) 448–452. [DOI] [PubMed] [Google Scholar]
- [186].Chen L-C, Hsu C, Chiueh CC, Lee W-S, Ferrous citrate up-regulates the NOS2 through nuclear translocation of NFκB induced by free radicals generation in mouse cerebral endothelial cells, PloS One 7(9) (2012) e46239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Mohanakumar K, Bartolomeis AD, WU RM, Yeh K, Sternberger L, PENG SY, Murphy D, Chiueh C, Ferrous‐Citrate Complex and Nigral Degeneration: Evidence for Free‐radical Formation and Lipid Peroxidation, Annals of the New York Academy of Sciences 738(1) (1994) 392–399. [DOI] [PubMed] [Google Scholar]