Abstract
We here investigate how APRIL impacts immune regulatory T cells and directly contributes to the immunosuppressive multiple myeloma (MM) bone marrow (BM) microenvironment. First, APRIL receptor TACI expression is significantly higher in regulatory T cells (Tregs) than conventional T cells (Tcons) from the same patient, confirmed by upregulated Treg markers, i.e., Foxp3, CTLA-4. APRIL significantly stimulates proliferation and survival of Tregs, whereas neutralizing anti-APRIL monoclonal antibodies (mAbs) inhibit theses effects. Besides TACI-dependent induction of cell cycle progression and anti-apoptosis genes, APRIL specifically augments Foxp3, IL-10, TGFβ1, and PD-L1 in Tregs to further enhance Treg-inhibited Tcon proliferation. APRIL further increases MM cell-driven Treg (iTreg) via TACI-dependent proliferation associated with upregulated IL-10, TGFβ1, and CD15s in iTreg, which further inhibits Tcons. Osteoclasts producing APRIL and PD-L1 significantly block Tcon expansion by iTreg generation, which is overcome by combined treatment with anti-APRIL and -PD1/PD-L1 mAbs. Finally, APRIL increases IL-10-producing B regulatory cells (Bregs) via TACI on BM Bregs of MM patients. Taken together, these results define novel APRIL actions via TACI on Tregs and Bregs to promote MM cell survival, providing the rationale for targeting APRIL/TACI system to alleviate the immunosuppressive BM milieu and improve patient outcome in MM.
Keywords: A proliferation inducing ligand (APRIL), Transmembrane Activator and CAML Interactor (TACI), regulatory T (Treg), conventional T (Tcon), multiple myeloma (MM), tumor-induced Treg (iTregs), Osteoclast (OC), interleukin-10, IL-10, tumor growth factor beta, TGFβ
INTRODUCTION
Multiple myeloma (MM) development and progression is associated with evolving genetic aberrations and alterations in the bone marrow (BM) microenvironment which promote malignant plasma cell (PC) growth while suppressing host immunity. Indeed, MM is characterized by recurrent infections due to immune deficiency, as well as bone lesions due to hyperactive osteoclasts (OCs). Moreover, the suppressive immune microenvironment underlies drug resistance and disease relapse. To date, however, the regulatory mechanisms of MM-related immune cell dysfunction have not been fully characterized.
Regulatory T cells (Tregs), traditionally defined as CD4+CD25+Foxp3+, are essential components of immune surveillance to maintain immune homeostasis and self-tolerance.1 Tregs are broadly divided by lineage into thymic-derived naturally occurring Tregs (nTregs) from CD4+CD8+ T-cells, and peripheral Tregs induced from naïve CD4+ T cells (iTregs).2 The latter are generated via cell-cell contact and/or cytokine-dependent mechanisms, i.e., TGF-β, IL-10, to prevent cellular and humoral immune responses.3 The function of nTregs and iTregs are quite similar, and it is difficult to distinguish them. Recently, Tregs have been associated with long-lived PCs in the BM, further suggesting their role in controlling homeostasis of PC populations.4
Increasing evidence indicates that the expansion of Tregs contributes to impaired anti-tumor immune responses resulting in immune escape and progression of solid and blood cancers, including MM.5–12 Tumor cells can positively interact with Tregs to inhibit tumor-specific CD8+ and CD4+ T effector cell function and exhaust effector cells in the tumor microenvironment.13–16 In MM patients, the proportion of circulating functional Tregs in T cells were increased, which correlated with disease burden and higher risk of progression.9–12, 17, 18 Elevated Treg numbers in MM patients can be derived from naïve CD4 T cells by stimulation with tumor cells and tumor bystander cells.12, 19–21 As shown in ex vivo cocultures, MM cells significantly induce generation of iTreg from Tcons.12, 21, 22 CD38-expressing Tregs (both nTregs and iTregs) have been identified and characterized as immune modulators in MM patients.12, 23, 24 Importantly, therapeutic CD38 targeting monoclonal antibodies (mAbs) deplete CD38-expressing Tregs and stimulate T and NK effector cell function.12,25,23 Overexpressed Foxp3 and CTLA4 in BM samples further supports a local accumulation of immunosuppressive Tregs in the MM microenvironment.26 Finally, MM cells directly drive Tregs via a positive feedback loop in a transplantation mouse model to promote disease progression and inferior outcome.27
A proliferation-inducing ligand (APRIL), a critical PC growth and survival factor, binds with high affinity to B cell maturation antigen (BCMA), the most specific MM antigen expressed at high levels in malignant PCs of all MM patients.28, 29 Most recently, targeting BCMA by novel immunotherapies has achieved impressive clinical responses in relapsed and refractory MM.28–32 Importantly, constitutive in vivo activation of APRIL/BCMA signaling promotes MM cell progression and induction of immune inhibitory factors in MM cells.33 In addition, MM cell growth is significantly reduced in APRIL-deficient SCID mice, indicating that APRIL by itself can induce in vivo MM progression.34 Myeloma-supporting OCs produce APRIL35–38 and PD-L139 in the BM, and OCs further block autologous T cell proliferation via immune checkpoint molecules including PD-L1.39 However, it is not yet known whether Tregs mediate OC-induced immunosuppression and whether APRIL regulates these processes.
APRIL also binds to transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI),40 which is expressed at lower levels and reduced frequency in patient MM cells when compared with BCMA.35, 41 Unlike BCMA that is only important in long-lived and malignant PCs but not normal B cells, TACI can negatively or positively regulate B cell responses.42–46 Results from TACI and APRIL knockout mice indicate their roles in serum IgA production42, 47–49, and TACI requires heparan sulfate proteoglycans (i.e., CD138) for APRIL-induced IgA production.44,50 At present, it is unclear whether APRIL directly acts on immune regulatory T- and B-linage cells through TACI to downregulate effector T cells in MM.
In this study we examined whether APRIL promotes immunosuppression in MM. We demonstrated that APRIL signaling via TACI significantly upregulates proliferation, survival, and immune inhibitory function of both Tregs and Bregs. Furthermore, targeting APRIL, alone and together with PD1/PD-L1 blockade, decreases OC-induced immune suppression in the MM BM microenvironment. These studies provide the framework for targeting APRIL to overcome immunosuppression, enhance MM cell cytotoxicity, and improve patient outcome.
MATERIALS AND METHODS
Flow cytometric analysis and cell sorting
Immunofluorescence analysis was performed using BD FACSCanto™ II and BD LSRFortessa™ flow cytometer. Data were analyzed using FlowJo Version 8.6.6 (TreeStar Inc) and FACSDiva Version 5.0 acquisition/analysis software (BD Biosciences). Anti-CD3 (APC/Cy7, SK7), anti-CD8 (FITC, SK1), anti-CD8 (APC/Cy7, SK1), anti-FOXP3 (Alexa Fluor 647, 259D/C7), anti-CD15s (FITC, CSLEX1), and anti-CD4 (FITC, RPA-T4) were obtained from BD Biosciences. Anti-CD4 (Brilliant Violet 421, RPA-T4), anti-CD25 (PE, M-A251), anti-TACI (PE,1A1), anti-TACI (PE/Cy7, 1A1), anti-CD38 (PE/Cy7, HB-7), anti-IL-10 (FITC, JES3–9D7) and anti-IL-10 (PE/Cy7, JES3–9D7), and anti-TGFβ1 (PE, TW4–6H10) were obtained from BioLegend (San Diego, CA). The LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen) was used to identify viable cells.
Tcon suppression assay
Tcons were stained by CellTrace CFSE or Violet Cell Proliferation Kit (Invitrogen), and Tregs were stained by CellTrace Violet (CTV) Cell Proliferation Kit (Invitrogen). Tcons (50,000 cells/well) were cultured alone or with autologous Tregs in 96-well plates at various ratios in the presence of APRIL-containing media or clones of antagonistic anti-APRIL mAbs. Tcons were then stimulated with anti-CD3/CD28 beads (Miltenyi Biotec) according to the manufacturer’s recommendation. Proliferation (CFSE- or CTV-diluted fractions) of indicated cells was measured by FACS analysis.
The generation of iTregs in ex vivo cocultures
MM cells, pretreated with mitomycin C (Sigma) to prevent their proliferation, were washed twice and then cocultured with CD3 T cells or Tcons (CD4+CD25-) in 96-well culture plates.12 T cells or Tcons alone were used as controls. Recombinant human APRIL (200 ng/ml, unless specified) and/or antagonistic anti-APRIL mAbs (A1, clone 01A33, 50; A2, clone Aprily-1, Invitrogen) were added into cocultures for 4 or 7d. Culture media was replenished on day 4. The cells were collected for FACS analysis to determine the frequency and phenotype of iTregs.
CFSE-dilution-based proliferation assay
Tcons or Tregs were pre-stained by CellTrace CFSE or Violet (CTV) Cell Proliferation Kit (Invitrogen), and then plated in the presence or absence of anti-CD3/CD28 beads (Miltenyi Biotec) with or without APRIL and/or anti-APRIL mAbs. After 4 or 7d, cells were collected and analyzed by FACS analysis.
Statistical analysis
Experiments were done in triplicate and repeated > 2 times. A representative experiment (mean ± SD) was selected for figures, except when otherwise indicated. Comparisons between 2 groups were performed with Student’s t-test. All statistical analyses were performed with GraphPad software (Prism Version 7.03, San Diego, CA, USA). A p value ± 0.05 was considered statistically significant.
RESULTS
Regulatory T cells (Tregs) express significantly higher TACI than paired conventional T (Tcon)
To define a potential immune regulation of APRIL on T cells which lack BCMA expression, TACI protein levels, as mean fluorescence intensities (MFIs), was first assessed using flow cytometry analysis, on the cell membrane of T cell subsets harvested from MM patients (n=47). Patient samples included 1 MGUS, 2 SMM, and 8 newly diagnosed MM Pts who were untreated. There were 24 Pts who received Lenalidomide bortezomib dex induction, 2 patients in response posttransplant, and 10 Pts with relapsed/refractory MM. Among T cells freshly isolated from peripheral blood (PB) or bone marrow (BM) aspirates of MM patients (n=47), CD4+ (and CD8+) CD25high T cells have >3–5-fold higher TACI expression than CD4+ (and CD8+) CD25low T cells (Supplemental Fig. S1A, S1B left panel). Significantly higher TACI were also observed on CD4+ (and CD8+) CD25low T cells than CD4+ (and CD8+) CD25- conventional T (Tcon). TACI is hardly detected on Tcons since MFIs for TACI and isotype control are almost superimposed. In contrast to Tcons (CD4+CD25-), regulatory T cells (Treg, CD4+CD25+Foxp3+) express the highest TACI levels (Figure 1a). CD8 Tregs, CD8+CD25+Foxp3+ cells, which are functionally suppressive51 and increased in MM patients,22 also express higher levels of TACI than CD8+CD25- Tcons (Supplemental Fig. S1B right panel). Next, suppressive cytokine IL-10 was simultaneously measured with TACI and Foxp3 within CD4+CD25+Foxp3+ Tregs. Highest IL-10 levels were found in CD4+CD25+Foxp3high subsets which express highest TACI (Supplemental Fig. S1C). Furthermore, TACI levels are highest on IL-10+Foxp3+ T cell subsets, despite their low frequencies (<2%) within CD4+ T cells (Supplemental Fig. S1D upper panel). In contrast to IL-10-Foxp3- cells which occupy ~95% CD4 T cells and lack TACI expression, IL-10-Foxp3+ and IL-10+Foxp3+ subsets, which account for <2–4% CD4+T cells, have 6–8-fold higher TACI expression (Supplemental Fig. S1D lower panel).
Figure 1. Regulatory T cells (Tregs) express significantly higher TACI than conventional T cells (Tcons) freshly isolated from the same patient.
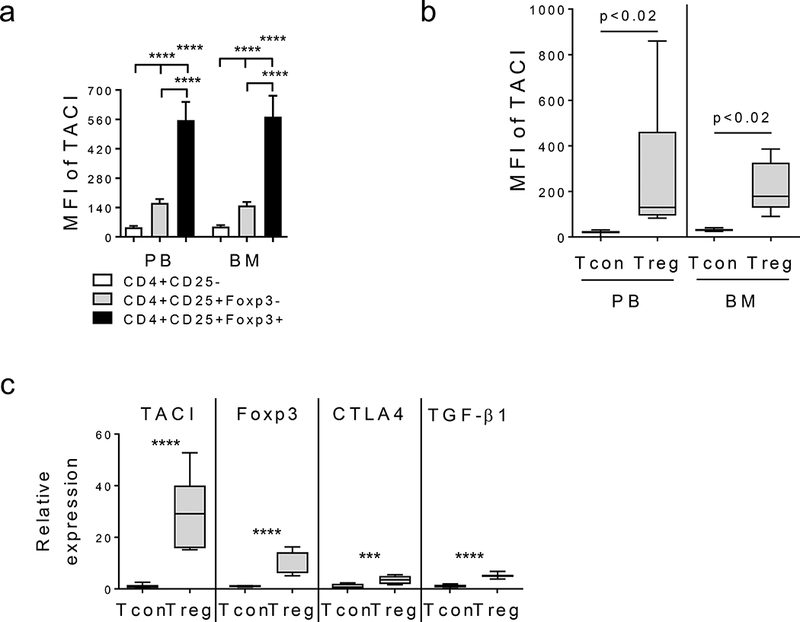
(a) Using flow cytometry analysis, median fluorescence intensity (MFI) of TACI was determined in indicated subsets of CD4+ T cells of PB and BM compartments from MM patients (n=47). TACI protein levels are highest on regulatory T subset (Treg, CD4+CD25+Foxp3+, black) followed by CD4+CD25+Foxp3-subset (gray). TACI MFIs in conventional T cells (Tcon, CD4+CD25-, white) are as similar as isotype control Ab. (b) TACI MFIs are shown for Treg vs paired Tcon from additional 9 MM patients. (c) Tregs were separated from Tcons from 9 MM patients followed by RNA extraction to quantitate TACI transcripts by qRT-PCR. Foxp3, CTLA4, and TGFβ serve as control genes to identify Tregs. Expression levels were normalized by internal control GAPDH then shown are relative expression levels in Tregs vs Tcons* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
TACI protein levels are significantly elevated on Tregs when compared with autologous Tcons in both PB and BM compartments from the same MM patient (n=9, p<0.02) (Figure 1b). More than 4–40-fold and 3–15-fold increase in TACI MFIs were seen in Tregs vs paired Tcons. Significantly, TACI transcripts are higher in Tregs vs matched Tcons from normal donors (n=2. p< 0.01, Supplemental Fig. S1E) and MM patients (n=9, Figure 1c, Supplemental Fig. S1F p<0.0001). More than 4–12-fold and > 17–52-fold higher levels of TACI transcripts were detected in Tregs than Tcons from normal donors and MM patients, respectively. Elevated levels of Foxp3 (>7–16 fold) and CTLA4 (>3–9-fold) were confirmed in Tregs vs paired Tcons. TACI levels are significantly correlated with CTLA-4 (r=0.9715, p<0.0001). Additional negative immune regulators including TGFβ (p<0.0001) and IL-10 (p<0.0003) are significantly increased in Treg vs paired Tcon of MM patients (Figure 1c, Supplemental Fig. S1F). Thus, protein and transcript of TACI are expressed at significantly increased levels in Tregs vs Tcons from the same individual.
APRIL significantly supports viability and blocks apoptosis of Tregs, dependent on TACI-mediated induction of key growth and survival genes
To determine whether TACI expression is functional on Tregs, APRIL was added to freshly purified Tregs vs autologous Tcons, cultured in media containing low IL-2 (5 ng/ml) without CD3/CD28 beads. APRIL, in a time dependent manner, promoted viability of Tregs vs Tcons from the same individual (MM patient in Figure 2a, normal donors in Supplemental Fig. S2). Furthermore, APRIL significantly inhibited caspase 3/7 and caspase 8 activity in Tregs vs Tcons, indicating that APRIL blocks apoptosis in Tregs (Figure 2b). Conversely, antagonistic anti-APRIL monoclonal antibodies (mAbs) abrogated APRIL-induced proliferation and survival of Tregs. An anti-TACI blocking mAb only significantly neutralized APRIL-induced effects on Tregs but not Tcons (Figure 2c and Supplemental Fig. S2B).
Figure 2. APRIL signals via TACI to significantly increase viability and inhibit apoptosis in Treg vs paired Tcon.
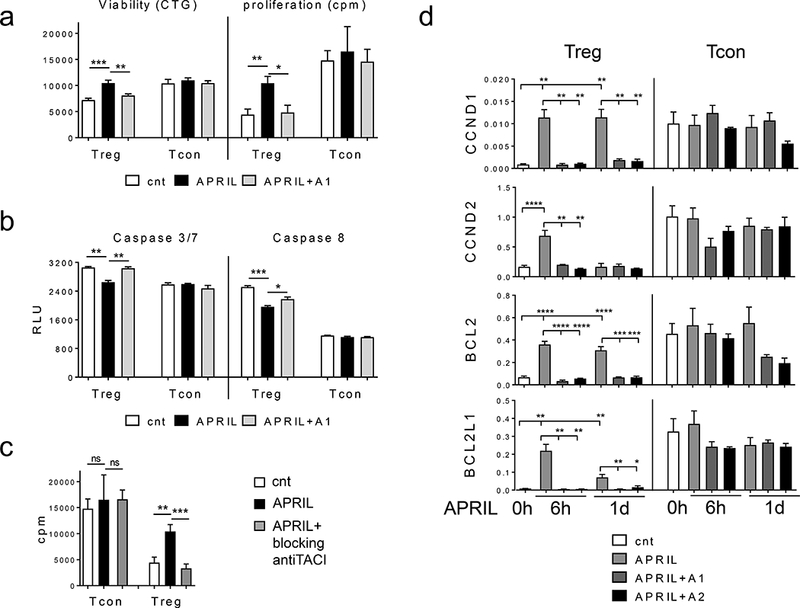
Purified Treg and Tcon cells from the same patient were incubated with recombinant human APRIL in media containing low dose IL-2 (5 ng/ml) with or without neutralizing anti-APRIL mAb (A1, clone 01A) followed by luminescence CellTiter-Glo (CTG) (left) and [3H] thymidine incorporation (right) assays (a), as well as CTG-based caspase activity assay (b). Similar results were obtained from additional 3 samples. (c) Purified Tregs and paired Tcons (n=2) were incubated with APRIL for 6h and 1d in the presence or absence of blocking anti-APRIL mAbs (A1, A2). Expression levels of indicated genes were then determined using qRT-PCR normalized by internal controls GAPDH. cnt, control media * p < 0.02, ** p < 0.005, *** p < 0.001, **** p < 0.0001.
Using quantitative qRT-PCR, key growth and survival genes were next assayed in Tregs compared with Tcons purified from the same individual (n>3) and cultured in low dose IL-2 culture media, with or without APRIL. Following 6h incubation, APRIL significantly induced expression of cell cycle progression genes CCND1 and CCND2, as well as anti-apoptotic genes BCL2 and BCL2L1/BCLxL, in Tregs but not Tcons (Supplemental Fig. S2D). Addition of APRIL every other day further sustained upregulation of these target genes in Tregs vs Tcons (data not shown). Neutralizing anti-APRIL mAbs completely blocked APRIL-induced expression of these target genes (Figure 2d), confirming specific TACI dependency in Tregs vs autologous Tcons in response to APRIL stimulation. Furthermore, these results confirmed that freshly isolated Tcons (CD4+CD25-) barely express TACI (Figure 1).
APRIL signaling through TACI significantly induces immune suppressive genes in Tregs, thereby enhancing inhibitory effects of Tregs on autologous Tcons
We next asked whether APRIL modulates immunoregulatory function of Tregs by examining changes in the expression of key suppressive molecules in Tregs following APRIL stimulation. More than 11-, 4-, and 5-fold higher mRNA expression of Foxp3, IL-10, and TGFβ were seen in Treg vs Tcon, respectively (Figure 3, Supplemental Fig. S3). Importantly, APRIL further enhanced Foxp3 and IL-10 at 6h, followed by a time-dependent induction of PD-L1 and TGFβ1, only in Tregs. PD-L1 and TGFβ1 were further upregulated until d3. In contrast, APRIL did not induce expression of these immune inhibitory cytokines and the checkpoint genes in paired Tcons. In the presence of antagonistic anti-APRIL mAbs, APRIL-triggered increased expression of Foxp3, IL-10, TGFβ1, and PD-L1 are completely blocked at 6h and sustained to 1d after treatments (Figure 3). Thus, APRIL selectively augments critical immune suppressive cytokine and checkpoint genes in Tregs, but not Tcon. These data further indicate that TACI expression specifically mediates APRIL-induced immune suppressive action of Tregs.
Figure 3. APRIL selectively induces immune regulatory and suppressive genes in Treg but not Tcon.
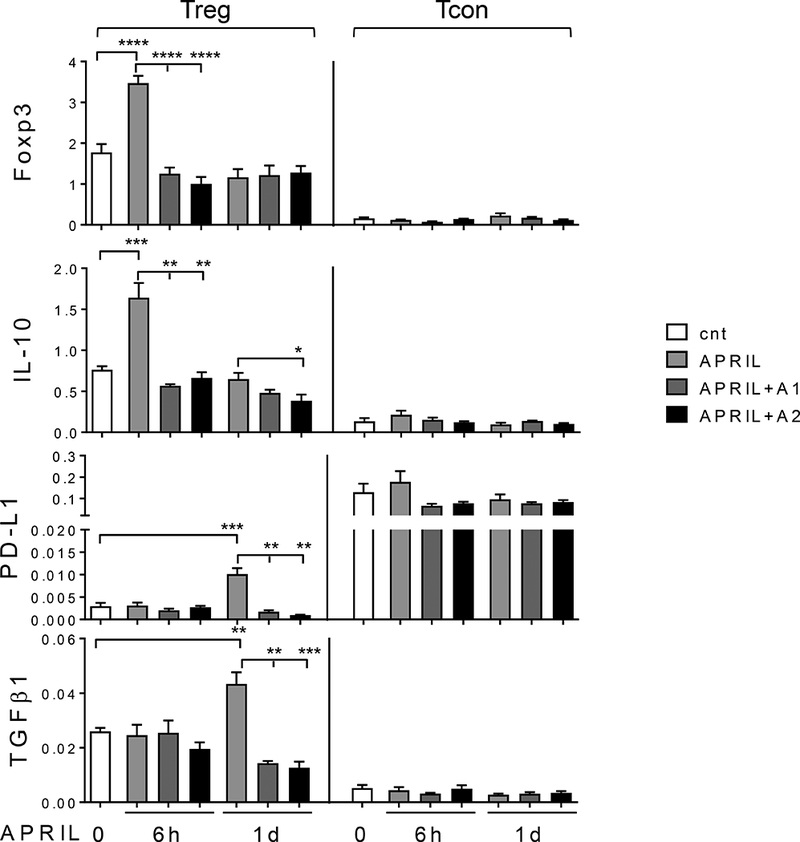
Treg (left) and Tcon (right) cells freshly purified from the same individual (n=5) were incubated with APRIL, in the presence of antagonistic anti-APRIL mAbs (A1, A2). cnt, control media. Expression levels of indicated genes by qRT-PCR were normalized by internal controls GAPDH and 18S. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
APRIL enhances Treg-mediated inhibition of Tcon proliferation
We next tested the effect of APRIL on Treg-mediated inhibition of Tcon proliferation. APRIL was added to cocultures of purified Tcons pre-labeled with CFSE and stimulated with CD3/CD28 microbeads at various ratios of autologous Tregs to Tcons. Using flow cytometric analysis to determine % CFSE-diluted Tcon representing fractions of the proliferative Tcons, the addition of Treg to Tcon (1:1) completely blocked proliferation of Tcons (Figure 4a). With lower ratios of Tregs to Tcons, the inhibition by Treg of Tcon proliferation was proportionally reduced. In the cocultures of Treg to Tcon (1:16), Tregs did not inhibit proliferative Tcons (Figure 4, Supplemental Fig. S4). Importantly, APRIL potentiated Treg inhibition of Tcon growth, in a dose- (Figure 4b, Supplemental Fig. S4A) and time (Figure 4c, Supplemental Fig. S4B)-dependent manner. Conversely, antagonistic anti-APRIL mAbs overcame APRIL-enhanced Treg suppression of Tcon proliferation. These results further confirm that APRIL action on Tregs further enhances their suppression of paired Tcons.
Figure 4. APRIL potentiates Treg-inhibited proliferation of autologous Tcon cells.
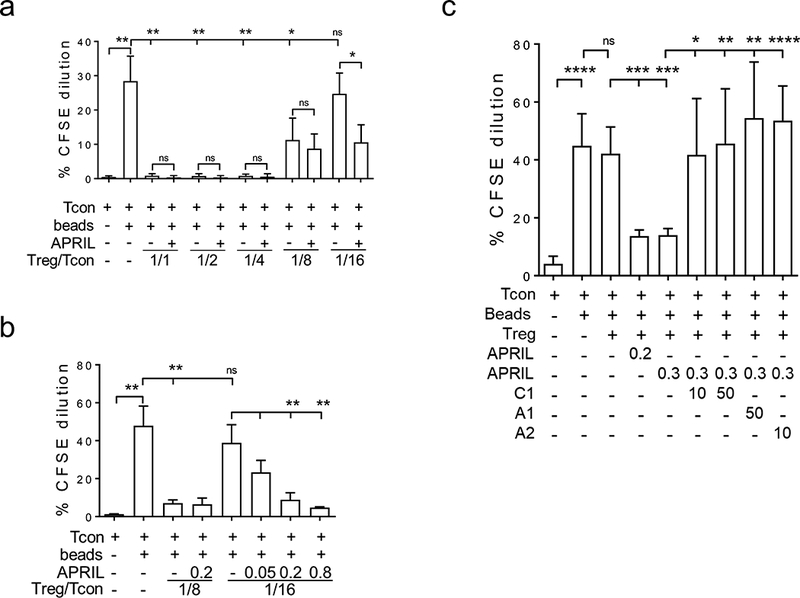
a, Purified Tcons were stained with 5μM CFSE and then stimulated with CD3/CD28 beads (beads) in the presence or absence of autologous Tregs at indicated ratios of Treg/Tcon, with or without APRIL (200 ng/ml). b, Beads-stimulated Tcons were cocultured with autologous Tregs for 7d at lower ratios of Treg/Tcon in serial dilutions of APRIL (μg/ml). c, Tcons were cocultured with Tregs at a low Treg/Tcon ratio with APRIL (μg/ml) in the presence or absence of neutralizing anti-APRIL mAb (μg/ml) for 7d. C1, chimeric homolog of A1 (01A). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Generation of functional Tregs (iTregs) induced by MM cells is further augmented by APRIL dependent on increased iTreg proliferation
We next addressed whether APRIL further increased MM-induced iTreg from CD3 T cells in ex vivo co-cultures, mimicking increased Tregs during disease progression. Following 3d cocultures, MM cells (i.e., U266, RPMI8226, JJN3) significantly induced the % iTreg (CD25+Foxp3+) to >10-fold within CD4+ T subset (Supplemental Fig. S5A). The percentages of iTregs continued to rise at d7 (Figure 5a). Fractions of CD8 iTreg (CD8+CD25+Foxp3+) were also significantly increased (Supplemental Fig. S5B-D). APRIL further augmented generation of iTreg within both CD4+ and CD8 T cells at d3 and continued to d7 in ex vivo cocultures of MM cells with T cells (Figure 5a, Supplemental Fig. S5). Conversely, anti-APRIL mAbs specifically blocked APRIL-enhanced iTreg induced by MM cells.
Figure 5. APRIL further enhances proliferative and suppressive MM-induced iTregs in ex vivo cocultures.
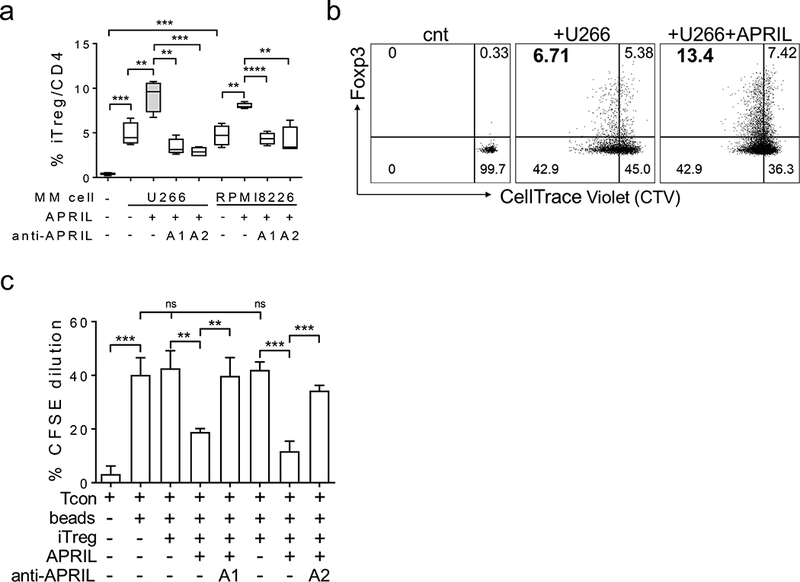
a, Mitomycin C-pre-treated U266 or RPMI8226 MM cells were washed and cocultured with T cells for 3d in the presence or absence of APRIL. Neutralizing anti-APRIL mAbs (A1 or A2) were also added as indicated. Percentages of CD4+CD25+Foxp3+ iTreg gated in CD4 T cells were determined by flow cytometry analysis. b, Tcons were pre-stained with Cell Trace Violet (CTV) and cocultured with U266 MM cells in APRIL-containing media. Shown are the dot plots of a representative experiment. c, MM cell-induced iTreg were purified from the cocultures and subjected to CFSE-dilution assays to determine fractions of autologous Tcon proliferation under indicated conditions. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
To further define the mechanism of APRIL-enhanced MM-induced iTreg, Tcon cells (CD4+CD25-) were pre-labeled with CellTrace Violet (CTV) prior to cocultures with U266 MM cells, with or without APRIL. By quantifying the % CTV-T cells, MM cells were demonstrated to significantly stimulate the proliferative iTreg cell fraction (Figure 5b, Supplemental Fig. S6A). The % CTV-Foxp3+CD4+CD25+ was increased from 0% to 7.24 ± 0.27% (n=3, p < 0.0001) following 7d cocultures. A representative dot plot (Figure 5b) showed an increase from 0 to 6.71% and from 0.33 to 5.38% in percentages of proliferative iTreg and resting iTreg (CTV+Foxp3+CD4+CD25+), respectively. Importantly, APRIL further upregulated % proliferative iTreg from 6.71 to 13.4% (Figure 5b). Three repeated experiments show that APRIL further increased proliferative iTreg from 7.24 ± 0.27% to 11.28 ± 1.1 (n=3, p<0.02) (Supplemental Fig. S6A). A slight increase in the resting iTreg fraction following APRIL treatment did not reach statistical significance when compared with untreated groups. In contrast, the proliferative Tcon (CTV-Foxp3-CD4+) fraction remained unchanged or slightly decreased (Supplemental Fig. S6B). Furthermore, TACI MFIs remain highest on iTreg, and APRIL did not further increase TACI on iTreg in ex vivo cocultures (data not shown).
APRIL triggers immune suppressive effects in MM cell-induced iTreg in IL-10-dependent and -independent mechanisms
To confirm that APRIL enhanced iTreg function, we next purified iTreg from ex vivo cocultures and assessed iTreg inhibition on the proliferation of Tcons. At high ratio of iTreg to Tcon, iTregs, significantly blocked the growth of autologous Tcons (data not shown), consistent with previous reports.12, 21 While cultures at lower iTreg to Tcon ratios (1:16) did not change growth of Tcon, APRIL still promoted blockade of iTreg on Tcon proliferation (p<0.005, Figure 5c). Conversely, neutralizing anti-APRIL mAbs overcome APRIL-enhanced suppressive effects of iTreg on Tcon.
We further showed that percentages of IL10+ and TGFβ+ iTreg within CD4 T cells were significantly increased when compared with control T cells in the absence of MM cells (p<0.0001, Supplemental Fig. S6C). APRIL further augmented the % IL10+ TGFβ+ iTreg (p<0.05). CD15s (sialyl Lewis x), another highly specific marker of activated and most suppressive effector Treg,52 was also significantly increased in iTregs. Fractions of IL10+ and CD15s+ CD8+ iTreg were similarly increased by APRIL (Supplemental Fig S6D). TGFβ secretion was significantly increased by APRIL in ex vivo cocultures (Supplemental Fig. S6E). These data strongly suggest that IL-10, TGFβ, and CD15s regulate APRIL-enhanced immune suppressive capabilities of MM cell-induced iTreg.
Tregs contribute to Osteoclast (OC)-induced immune suppression on Tcons
We next asked whether OCs induce iTreg to block Tcons. We further confirmed whether APRIL and PD-L1, which are produced by OCs,33, 39 regulate OC suppression on Tcons. OCs significantly induced generation of CD4 and CD8 iTreg from T cells following 7d cocultures (Figure 6a, Supplemental Fig. S7A). Antagonistic anti-APRIL mAb partially reduced OC-induced iTregs. OC culture supernatants further upregulated MM cell-induced CD4+ and CD8+ iTreg cells, which was significantly blocked in the presence of anti-APRIL mAbs (Figure 6b, Supplemental Fig. S7B). OCs inhibited expansion of Tcons whereas anti-APRIL, or -PD1, or -PD-L1 mAbs partially reverted OC-inhibited Tcon proliferation (Figure 6c, Supplemental Fig. S7C). Furthermore, combined treatments of anti-APRIL with either -PD1 or -PD-L1 further overcame OC suppression on Tcons. These results indicate that OC-downregulated Tcon number is mediated by increased Tregs and soluble factors including APRIL and PD-L1.
Figure 6. Osteoclasts induce generation of iTreg dependent on APRIL secretion.
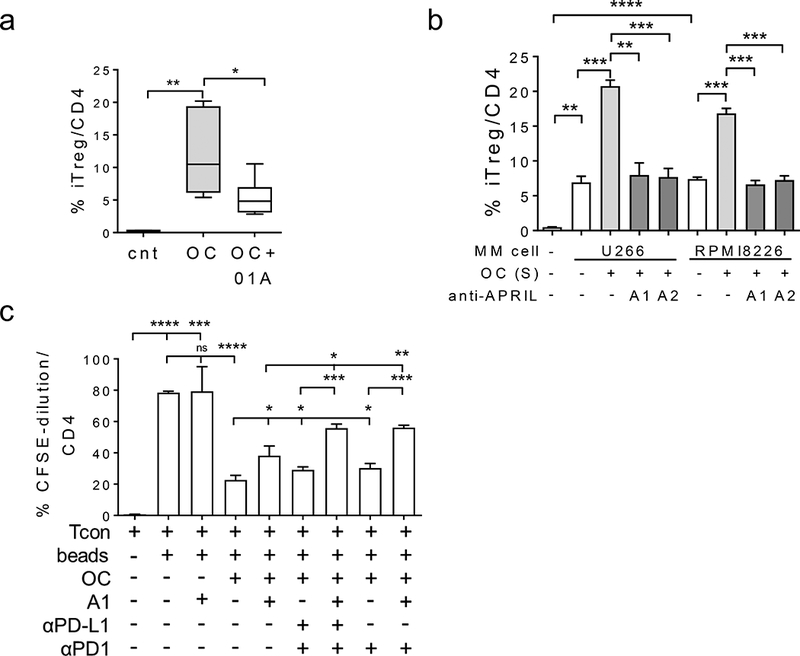
(a) Osteoclasts (OC) were differentiated from CD14+ cells following 3-week stimulation with M-CSF and RANKL and then co-cultured with autologous T cells for 7d in the presence or absence of anti-APRIL mAbs (A1, 10 μg/ml). Generation of iTreg was determined by gating CD25+Foxp3+ in CD4+ T cells. (b), CD3 T cells were cultured with supernatants (S) from 3-week OC cultures from the same donors for 7d under indicated conditions. Percentages of CD25+Foxp3+ iTreg in CD4+ T were determined. (c) CD3 T cells, pre-stained with CFSE, were co-cultured with OCs from the same donor under indicated conditions for 7d followed by flow cytometric analysis to determine fractions of proliferative Tcons. When noted, antagonistic anti-APRIL mAbs A1 or A2 (50 μg/ml) or anti(α)-PD-L1 or -PD1 mAbs (10 μg/ml) were added. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Shown here are data from 1 representative experiment with triplicates for each condition. Similar results were obtained using 2 additional samples.
APRIL affects function of BM-derived MM Bregs via TACI
Since Bregs can regulate Treg immunobiology and that BM-derived Bregs (CD19+CD24highCD38high) closely interact with MM cells in the BM microenvironment to mitigate responses to monoclonal antibody treatment (i.e., elotuzumab) treatment,53 we finally examined TACI expression in Bregs from MM patients. Bregs, when compared with naïve B cells (CD19+CD24low/-CD38low), showed a significantly elevated TACI levels (p< 0.02, Supplemental Fig. S8A). BCMA is undetectable in Breg, naïve B, and memory B (CD19+CD24highCD38low/-) cells (data not shown). Following treatment with lipopolysaccharides (LPS) which significantly induces IL-10 production from Breg,53 TACI levels are significantly increased in Bregs (p< 0.02) but not naïve and or memory B cells.
BM mononuclear cells from MM patients were further incubated with APRIL in the presence or absence of inhibiting anti-APRIL mAb, followed by flow cytometry analysis to quantitate % Breg in B cells and % IL-10 production in Bregs. APRIL significantly upregulated % Breg in B cells (Figure 7a) from 14.59 ± 1.36 % to 25.2 ± 0.69 % (p = 0.0004, n=4, Supplemental Fig. S8B). Importantly, APRIL further increased functional Bregs as IL-10 production in Bregs was significantly enhanced from 15.02 ± 0.88 % to 29.22 ± 3.33% (p < 0.007, Figure 7b). Conversely, an anti-APRIL mAb abolished APRIL-induced increases in Breg number and IL-10 production.
Figure 7. MM BM-derived regulatory B cells express TACI to specifically mediate APRIL-induced IL-10 production.
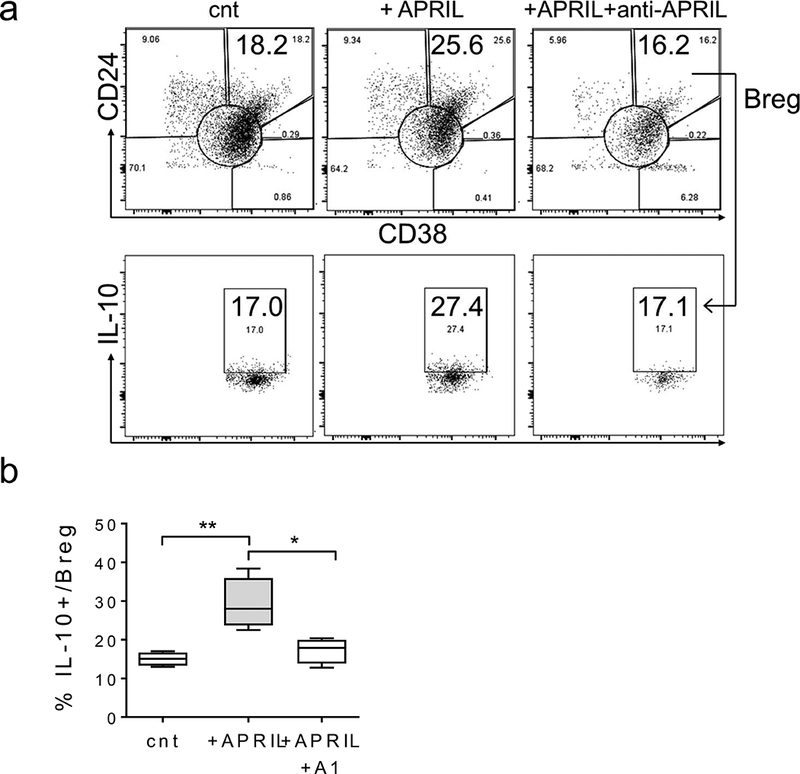
BM mononuclear cells from MM patients were incubated with APRIL in the presence of anti-APRIL mAb for 7d. Shown in (a) are dot plots of a representative experiment. (b) Percentages of IL-10+ Bregs (CD19+CD24highCD38high) was determined using flow cytometry analysis from 4 samples. * p < 0.02, ** p < 0.01, *** p < 0.0005, **** p < 0.0001.
DISCUSSION
We here identify novel function of APRIL signaling via TACI in Tregs and Bregs of MM patients to inhibit effector T cells, thereby promoting an immunosuppressive BM microenvironment. APRIL, abundantly secreted from MM-promoting OCs, significantly upregulates pro-survival and proliferative, as well as suppressive, capabilities of Tregs dependent on TACI. APRIL selectively enhances MM cell- and OC-driven iTregs to potentiate their inhibitory effects on Tcons by upregulating immune suppressive molecules including Foxp3, IL-10, TGFβ, PD-L1, CD15s. Conversely, blocking the APRIL-TACI axis using antagonistic anti-APRIL mAbs, alone and with PD1/PD-L1 checkpoint inhibitors, downregulates these immune regulatory cells, thereby alleviating the suppressive BM microenvironment.
First, Tregs (CD4+/CD8+ CD25+FOXP3+) were shown to have significantly elevated TACI when compared with matched Tcons (CD4+/CD8+ CD25-) freshly harvested from the same individuals. Increased TACI protein and mRNA in Tregs vs paired Tcons is further confirmed by significantly increased expression of genes critical for Treg identify and function such as Foxp3, CTLA-4, TGFβ, IL-10. Importantly, TACI levels are highly correlated with CTLA-4 (r=0.9715, p<0.0001), indicating that TACI may directly regulate the immune suppressive function of Tregs. We found that TACI expression is also significantly higher on IL-10+Foxp3-CD4+T cells when compared with IL-10-Foxp3-CD4+ T cells (Supplemental Fig. S1C). This small sub-population of T cells (IL-10+Foxp3-) can inhibit the proliferative Tcons (CD4+CD25-) in an IL-10-independent manner and with similar efficiency as CD4+CD25+Foxp3+ Tregs.54 Although TACI is also induced in activated Tcon cells, TACI levels are significantly higher on immunosuppressive Tregs than activated Tcons. Importantly, we here delineate an APRIL-dependent mechanism of Treg immunobiology, which will provide the framework for novel cancer immunotherapies.
APRIL significantly stimulates proliferation and survival of Tregs via TACI-dependent induction of genes including CCND1/2, BCL2, BCL2L1/BCLxL. Importantly, APRIL-increased growth and survival in Tregs vs Tcons were inhibited by neutralizing anti-APRIL and -TACI mAbs. APRIL further protects Tregs by inhibiting caspase 3/7 and 8 activities, as well as inducing anti-apoptotic molecules. Most importantly, APRIL augments the production of immune inhibitory factors in Tregs including Foxp3, IL-10, TGFβ, and PD-L1. In contrast, these essential Treg-related genes are expressed only at low levels in Tcons purified from the same individual, and their expression is unaffected by APRIL. As expected, Tregs abrogate the proliferation of autologous Tcons stimulated with CD3/CD28 beads in a Treg to Tcon ratio-dependent manner. APRIL, in a dose- and time-dependent fashion, promotes suppression of Tcons by Tregs even at low Treg to Tcon ratios, but does not significantly affect Tcon growth. Conversely, antagonistic anti-APRIL mAbs block APRIL-enhanced immune suppression induced by Tregs.
Since iTregs resulting from MM cell-induced conversion from Tcons in ex vivo cocultures are as highly suppressive as nTreg,12, 21, 22, 27 we demonstrated that APRIL selectively enhances iTreg-mediated inhibition of Tcon proliferation. TACI levels are significantly higher in iTregs than Tcons in cocultures with MM cells. Significantly, in the presence of MM cells, APRIL preferentially upregulates proliferation of iTreg (CD4+CD25+Foxp3+) subsets, but not remaining Tcon (CD25-Foxp3-) (Figure 5b, Supplemental Fig. S6A-B). It is likely that elevated TACI protein on iTregs permits APRIL-induced downstream targets to further promote expansion of immunosuppressive iTregs. Importantly, IL-10-dependent and -independent (i.e., TGFβ1, CD15s) mechanisms occur in purified iTregs which block proliferation of Tcon from the same individual, an effect which is further potentiated by APRIL. These results confirm the importance of APRIL signaling via TACI in enhancing the immune suppressive capabilities of Tregs (both iTregs and nTregs) on matched Tcons.
We here for the first time demonstrate that APRIL induces Foxp3 in Tregs via TACI. Foxp3, a master transcriptional factor critical for the development, function, and lineage commitment of Tregs, has been widely used as a Treg specific marker. Our results strongly indicate that APRIL-mediated active immune suppression is dependent on TACI expression. Neutralizing anti-TACI reagents inhibited these APRIL-induced targets (data not shown). APRIL further increases TGFβ and PD-L1 at later time points, following IL-10 and Foxp3 upregulation in Tregs. Thus, APRIL, via TACI, preferentially induces multiple immune inhibitors and checkpoint molecules in Tregs to further sustain a local suppressive tumor milieu. APRIL also upregulates IL-10+Bregs derived from MM BM via TACI, not BCMA. Since Bregs can facilitate the conversion of T cells to Tregs and inhibit effector T cells via both IL-10-dependent and -independent mechanisms,55, 56 our results indicate that Bregs further upregulate APRIL-induced Tregs in the MM BM milieu, at least in part, mediated by IL-10. Importantly, neutralizing anti-APRIL mAbs abrogate APRIL-induced increased Breg numbers and IL-10 production.
Our studies show that OCs, a key source of APRIL and PD-L1 in the MM BM, stimulate iTregs to suppress Tcon proliferation, establishing Treg as a crucial cellular factor mediating OC-inhibited immune suppression, as has been shown recently.39 These results, coupled with immune suppressive molecules induced in MM cells by APRIL,33 identify positive feedback loops between malignant PCs, Tregs, and Bregs to further exacerbate immune evasion and MM progression. Our results further confirm an immunosuppressive role of APRIL in tumor progression and drug resistance in multiple human cancers and related animal models.33, 34, 49, 57, 58
In summary, our current results provide new insights into Treg immunobiology which may be highly relevant for improved treatment strategies. Importantly, our current results support targeting APRIL/TACI axis using anti-APRIL, alone and in combination with PD1/PD-L1 blockade, to further modulate Tregs and Bregs, ameliorate immunosuppression, restore immune surveillance, and improve patient outcome in MM. They also support strategies to further build on clinical trials to improve the clinical efficacy of BCMA-based CAR T cells in MM29, 30, 32, 59 including utilizing APRIL-based CAR T cells for dual antigen targeting of both BCMA and TACI in MM.60
Supplementary Material
Key points:
APRIL signaling via TACI on Tregs and Breg contributes to the immunosuppressive MM BM milieu.
Besides MM cells, therapeutic anti-APRIL mAbs may further affect Treg and Breg, thereby attenuating myeloma- and OC-induced immunosuppression.
ACKNOWLEDGEMENTS
We thank Drs. G An, L Zhang, XY Feng, Y Xu, and L Qiu during the early phase of this study. The authors also thank clinical research coordinators of the LeBow Institute for Myeloma Therapeutics and the Jerome Lipper Multiple Myeloma Center of the Dana-Farber Cancer Institute for support and help.
Financial support: This work was supported in part by grants from the National Institutes of Health Grants RO1–124929 to Dr. Nikhil C. Munshi; P50–100007, and PO1–155258 to Drs. Kenneth C. Anderson and Nikhil C. Munshi, and RO1–50947 to Dr. Kenneth C. Anderson. Dr. Kenneth C. Anderson is an American Cancer Society Clinical Research Professor
Conception and design: Y.-T. Tai, K.C. Anderson;
Development of methodology: L. Lin, LJ Xing, T Yu, C. Acharya, Y.-T. Tai
Acquisition of data (provided reagents, facilities, etc.): L. Lin, LJ Xing, S.-F. Cho, T Yu, K. Wen, P. Hsieh, C. Acharya
Reagents and Materials: J. Dulos, A.v. Elsas.
Analysis and interpretation of data (statistical analysis, biostatistics analysis): L. Lin, LJ Xing, S.-F. Cho, T Yu, Y.-T. Tai
Provided acquired and managed patients: Munshi, P. Richardson, K.C. Anderson
Writing, review, and/or revision of the manuscript: Y.-T. Tai, J. Dulos, A.v. Elsas., K.C. Anderson
Study supervision: Y.-T. Tai, K.C. Anderson
Footnotes
CONFLICT OF INTEREST
N.C.M. serves on advisory boards to Millennium, Celgene, and Novartis. K.C.A. serves on advisory boards Celgene, Millennium and Gilead Sciences and is a Scientific founder of OncoPep and C4 Therapeutics. P. Richardson is on advisory board of Celgene, Millennium and Johnson & Johnson. J.D. and A.v.E. are employees of Aduro Biotech Europe. All other authors declare no competing financial interests.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133: 775–787. [DOI] [PubMed] [Google Scholar]
- 2.Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother 2007; 56: 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell JD, Cook G, Robertson SE, Fraser A, Boyd KS, Gracie JA, et al. Suppression of IL-2-induced T cell proliferation and phosphorylation of STAT3 and STAT5 by tumor-derived TGF beta is reversed by IL-15. J Immunol 2001; 167: 553–561. [DOI] [PubMed] [Google Scholar]
- 4.Glatman Zaretsky A, Konradt C, Depis F, Wing JB, Goenka R, Atria DG, et al. T regulatory cells support plasma cell populations in the bone marrow. Cell Rep 2017; 18: 1906–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12: 298–306. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res 2017; 27: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 2014; 27: 1–7. [DOI] [PubMed] [Google Scholar]
- 8.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res 2007; 13: 6947–6958. [DOI] [PubMed] [Google Scholar]
- 9.Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood 2006; 107: 3940–3949. [DOI] [PubMed] [Google Scholar]
- 10.Feyler S, von Lilienfeld-Toal M, Jarmin S, Marles L, Rawstron A, Ashcroft AJ, et al. CD4(+)CD25(+)FoxP3(+) regulatory T cells are increased whilst CD3(+)CD4(−)CD8(−)alphabetaTCR(+) double negative T cells are decreased in the peripheral blood of patients with multiple myeloma which correlates with disease burden. Br J Haematol 2009; 144: 686–695. [DOI] [PubMed] [Google Scholar]
- 11.Muthu Raja KR, Rihova L, Zahradova L, Klincova M, Penka M, Hajek R. Increased T regulatory cells are associated with adverse clinical features and predict progression in multiple myeloma. PloS one 2012; 7: e47077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng X, Zhang L, Acharya C, An G, Wen K, Qiu L, et al. Targeting CD38 suppresses induction and function of T regulatory cells to mitigate immunosuppression in multiple myeloma. Clin Cancer Res 2017; 23: 4290–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest 2013; 123: 2447–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcgammaRs, leading to antitumor efficacy. Immunol Cell Biol 2014; 92: 475–480. [DOI] [PubMed] [Google Scholar]
- 15.Paiva B, Mateos MV, Sanchez-Abarca LI, Puig N, Vidriales MB, Lopez-Corral L, et al. Immune status of high-risk smoldering multiple myeloma patients and its therapeutic modulation under LenDex: a longitudinal analysis. Blood 2016; 127: 1151–1162. [DOI] [PubMed] [Google Scholar]
- 16.Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity 2017; 46: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannopoulos K, Kaminska W, Hus I, Dmoszynska A. The frequency of T regulatory cells modulates the survival of multiple myeloma patients: detailed characterisation of immune status in multiple myeloma. Br J Cancer 2012; 106: 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muthu Raja KR, Kubiczkova L, Rihova L, Piskacek M, Vsianska P, Hezova R, et al. Functionally suppressive CD8 T regulatory cells are increased in patients with multiple myeloma: a cause for immune impairment. PloS one 2012; 7: e49446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiteside TL, Schuler P, Schilling B. Induced and natural regulatory T cells in human cancer. Expert Opin Biol Ther 2012; 12: 1383–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Front Immunol 2013; 4: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frassanito MA, Ruggieri S, Desantis V, Di Marzo L, Leone P, Racanelli V, et al. Myeloma cells act as tolerogenic antigen-presenting cells and induce regulatory T cells in vitro. European journal of haematology 2015; 95: 65–74. [DOI] [PubMed] [Google Scholar]
- 22.Feyler S, Scott GB, Parrish C, Jarmin S, Evans P, Short M, et al. Tumour cell generation of inducible regulatory T-cells in multiple myeloma is contact-dependent and antigen-presenting cell-independent. PloS one 2012; 7: e35981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016; 128: 384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai YT, Anderson KC. A new era of immune therapy in multiple myeloma. Blood 2016; 128: 318–319. [DOI] [PubMed] [Google Scholar]
- 25.Tai YT, Anderson KC. Targeting CD38 alleviates tumor-induced immunosuppression. Oncotarget 2017; 8: 112166–112167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braga WM, da Silva BR, de Carvalho AC, Maekawa YH, Bortoluzzo AB, Rizzatti EG, et al. FOXP3 and CTLA4 overexpression in multiple myeloma bone marrow as a sign of accumulation of CD4(+) T regulatory cells. Cancer Immunol Immunother 2014; 63: 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawano Y, Zavidij O, Park J, Moschetta M, Kokubun K, Mouhieddine TH, et al. Blocking IFNRA1 inhibits multiple myeloma-driven Treg expansion and immunosuppression. J Clin Invest 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res 2013; 19: 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tai YT, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014; 123: 3128–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tai YT, Anderson KC. Targeting B-cell maturation antigen in multiple myeloma. Immunotherapy 2015; 7: 1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016; 128: 1688–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood 2017; 130: 2594–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai YT, Acharya C, An G, Moschetta M, Zhong MY, Feng X, et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood 2016; 127: 3225–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthes T, McKee T, Dunand-Sauthier I, Manfroi B, Park S, Passweg J, et al. Myelopoiesis dysregulation associated to sustained APRIL production in multiple myeloma-infiltrated bone marrow. Leukemia 2015; 29: 1901–1908. [DOI] [PubMed] [Google Scholar]
- 35.Moreaux J, Cremer FW, Reme T, Raab M, Mahtouk K, Kaukel P, et al. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood 2005; 106: 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tucci M, Ciavarella S, Strippoli S, Brunetti O, Dammacco F, Silvestris F. Immature dendritic cells from patients with multiple myeloma are prone to osteoclast differentiation in vitro. Experimental hematology 2011; 39: 773–783 e771. [DOI] [PubMed] [Google Scholar]
- 37.Yaccoby S, Pennisi A, Li X, Dillon SR, Zhan F, Barlogie B, et al. Atacicept (TACI-Ig) inhibits growth of TACI(high) primary myeloma cells in SCID-hu mice and in coculture with osteoclasts. Leukemia 2008; 22: 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abe M, Kido S, Hiasa M, Nakano A, Oda A, Amou H, et al. BAFF and APRIL as osteoclast-derived survival factors for myeloma cells: a rationale for TACI-Fc treatment in patients with multiple myeloma. Leukemia 2006; 20: 1313–1315. [DOI] [PubMed] [Google Scholar]
- 39.An G, Acharya C, Feng X, Wen K, Zhong M, Zhang L, et al. Osteoclasts promote immune suppressive microenvironment in multiple myeloma: therapeutic implication. Blood 2016; 128: 1590–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marsters SA, Yan M, Pitti RM, Haas PE, Dixit VM, Ashkenazi A. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Current biology : CB 2000; 10: 785–788. [DOI] [PubMed] [Google Scholar]
- 41.Tai YT, Li XF, Breitkreutz I, Song W, Neri P, Catley L, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res 2006; 66: 6675–6682. [DOI] [PubMed] [Google Scholar]
- 42.Yan M, Wang H, Chan B, Roose-Girma M, Erickson S, Baker T, et al. Activation and accumulation of B cells in TACI-deficient mice. Nat Immunol 2001; 2: 638–643. [DOI] [PubMed] [Google Scholar]
- 43.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med 2005; 201: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakurai D, Hase H, Kanno Y, Kojima H, Okumura K, Kobata T. TACI regulates IgA production by APRIL in collaboration with HSPG. Blood 2007; 109: 2961–2967. [DOI] [PubMed] [Google Scholar]
- 45.Tsuji S, Cortesao C, Bram RJ, Platt JL, Cascalho M. TACI deficiency impairs sustained Blimp-1 expression in B cells decreasing long-lived plasma cells in the bone marrow. Blood 2011; 118: 5832–5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Carmona Y, Cols M, Ting AT, Radigan L, Yuk FJ, Zhang L, et al. Differential induction of plasma cells by isoforms of human TACI. Blood 2015; 125: 1749–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity 2001; 14: 573–582. [DOI] [PubMed] [Google Scholar]
- 48.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A 2004; 101: 3903–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Planelles L, Carvalho-Pinto CE, Hardenberg G, Smaniotto S, Savino W, Gomez-Caro R, et al. APRIL promotes B-1 cell-associated neoplasm. Cancer cell 2004; 6: 399–408. [DOI] [PubMed] [Google Scholar]
- 50.Guadagnoli M, Kimberley FC, Phan U, Cameron K, Vink PM, Rodermond H, et al. Development and characterization of APRIL antagonistic monoclonal antibodies for treatment of B-cell lymphomas. Blood 2011; 117: 6856–6865. [DOI] [PubMed] [Google Scholar]
- 51.Correale J, Villa A. Role of CD8+ CD25+ Foxp3+ regulatory T cells in multiple sclerosis. Ann Neurol 2010; 67: 625–638. [DOI] [PubMed] [Google Scholar]
- 52.Miyara M, Chader D, Sage E, Sugiyama D, Nishikawa H, Bouvry D, et al. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc Natl Acad Sci U S A 2015; 112: 7225–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Tai YT, Ho M, Xing L, Chauhan D, Gang A, et al. Regulatory B cell-myeloma cell interaction confers immunosuppression and promotes their survival in the bone marrow milieu. Blood Cancer J 2017; 7: e547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol 2004; 172: 5986–5993. [DOI] [PubMed] [Google Scholar]
- 55.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010; 32: 129–140. [DOI] [PubMed] [Google Scholar]
- 56.Mauri C, Menon M. Human regulatory B cells in health and disease: therapeutic potential. J Clin Invest 2017; 127: 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreaux J, Legouffe E, Jourdan E, Quittet P, Reme T, Lugagne C, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004; 103: 3148–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang G, Wang F, Ding W, Wang J, Jing R, Li H, et al. APRIL induces tumorigenesis and metastasis of colorectal cancer cells via activation of the PI3K/Akt pathway. PloS one 2013; 8: e55298. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Hipp S, Tai YT, Blanset D, Deegen P, Wahl J, Thomas O, et al. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 2017; 31: 1743–1751. [DOI] [PubMed] [Google Scholar]
- 60.Lee L, Draper B, Chaplin N, Philip B, Chin M, Galas-Filipowicz D, et al. An APRIL-based chimeric antigen receptor for dual targeting of BCMA and TACI in multiple myeloma. Blood 2018; 131: 746–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


