Intensive chemotherapy will induce a complete morphologic remission (CR) in many persons with acute myeloid leukemia (AML). Even with continued treatment, however, relapse is common.1,2 Many pre-treatment factors, most notably cytogenetic and molecular profiles, are associated with disease recurrence and shorter survival and allow outcome prediction, albeit with limited accuracy, at the individual patient level.3–5 It is increasingly recognized that post-treatment information provides valuable insight into which remissions are of higher quality. One important characteristic of more durable remissions is the lack of measurable residual disease (MRD).6 On the other hand, whether it matters that a remission is obtained early, i.e. with the first cycle of chemotherapy, has remained controversial. Data from historic and contemporary trials with double induction chemotherapies showed patients who achieved a CR with the first induction cycle were less likely to relapse than those requiring 2 courses of therapy to enter CR.7,8 Contrasting these findings, an analysis of 6 ECOG (now ECOG-ACRIN) trials conducted between 1983 and 1993 indicated patients who achieved a CR after 1 or 2 cycles of induction chemotherapy had similar prognoses.9 The relationship between timing of remission achievement and outcome has not been examined in contemporary cohorts of adults treated with 7+3. Here, we used data from participants of 5 SWOG trials between 1983 and 2015 and studied the association between prognosis and need for 7+3 reinduction therapy and how it has changed over time.
We analyzed 1,247 patients randomized to 7+3 in treatment arms of five National Cancer Institute (NCI)/National Clinical Trials Network (NCTN) trials conducted by SWOG. We analyzed patients age 65 and younger: S860010 (n=530), S903111 (n=98), S933312 (n=57), S010613 (n=301), and S120314 (n=261). S8600 enrolled patients between 11/1986 and 12/1991, S9031 between 11/1991 and 1/1995, S9333 between 1/1995 and 12/1998, S0106 between 5/2004 and 8/2009, and S1203 between 12/2012 and 11/2015. While all studies were limited to patients with newly-diagnosed AML, the exact eligibility criteria differed between these trials. For example, trials S9031 and S9333 were restricted to patients age 55 and older; the other studies included patients age 18 and older. Patients with secondary AML were not eligible for S0106, which tested the addition of the CD33 antibody-drug conjugate gemtuzumab ozogamicin to induction chemotherapy. There were also differences in the chemotherapy regimens administered since, in all five trials,7+3 was given as per standard at that time. Specifically, in S8600, S9031, and S9333, the cytarabine and daunorubicin doses were 200 mg/m2 and 45 mg/m2, respectively, In S0106, doses used were 100 mg/m2 and 60 mg/m2, respectively. Finally, in S1203, doses of 100 mg/m2 for cytarabine and 90mg/m2 for daunorubicin were used. Institutional review boards of participating institutions approved all protocols, and patients were treated according to the Declaration of Helsinki.
CR was defined conventionally (<5% marrow blasts by morphology, absolute neutrophil count [ANC] ≥1,000/µL, platelets ≥100,000/µL).2 Overall survival (OS) was measured from the date of study registration/randomization to date of death due to any cause; patients last known to be alive were censored at the date of last contact. Relapse-free survival (RFS) was measured from the date of CR to the first of relapse from CR or death due to any cause; patients last known to be alive in CR were censored at the date of last contact. OS and RFS were estimated using the Kaplan-Meier method. Multivariable Cox regression models included covariates (modeled quantitatively unless otherwise specified): age at study registration, gender, cytogenetic risk, pre-study white blood cell counts (WBC), pre-study platelets, pre-study marrow blasts, type of AML (secondary vs. de novo), indicator of receiving reinduction and study/protocol. We analyzed study/protocol separately and also grouped the studies by twenty-year period (S8600/S9031/S9333 representing 1980s and 1990s vs. S0106/S1203 representing 2000s and 2010s). The earlier of these groups (S8600/S9031/S9333) corresponds to the period of time covered in the analyses previously reported by ECOG-ACRIN.9 NPM1 and FLT3 mutation data were available for participants of S0106 and S1203 and separate regression models in restricted to these two trials were fit that included these molecular data. To control for the fact that patients needed to live long enough to receive reinduction, we performed landmark analyses among the patients who were alive at day 45. However, to allow more direct comparison with the data previously reported by ECOG-ACRIN which were not based on landmark analyses,9 we also performed analyses among all patients. All analyses were performed with R (http://www.rproject.org) .
Table 1 summarizes the characteristics of the 1,247 patients included in our analysis, group by decade of treatment (S8600 vs. S9031/S9333 vs. S0106 vs. S1203). Consistent with the differences in eligibility criteria noted above, patients enrolled in S9031/S9333 were older than those on the other studies. The proportion of patients with performance status 2 and higher decreased over time. In contrast, CR rates were higher in the studies conducted more recently compared to the earlier ones. In parallel with improved CR rates, OS and RFS were higher in S0106 and S1203 compared to S8600 and S9031/S9333 (Figure 1A,B).
TABLE 1.
Characteristics of study cohort
| S8600 (n=530) |
S9031/S9333 (n=156) |
S0106 (n=301) |
S1203 (n=261) |
P-value | |
|---|---|---|---|---|---|
| Median age, years (range) | 45 (15–64) | 61 (56–65) | 48 (18–60) | 48 (19–60) | <0.001 |
| Gender, n (%) Female Male |
247 (47) 283 (53) |
70 (45) 86 (55) |
147 (49) 154 (51) |
131 (50) 130 (50) |
0.68 |
| Performance status, n (%) 0–1 2–4 |
374 (73) 140 (27) |
114 (75) 38 (25) |
255 (85) 44 (15) |
221 (85) 40 (15) |
<0.001 |
| Cytogenetic risk, n (%) Favorable Intermediate Unfavorable Missing |
9 (9) 56 (58) 32 (33) 97 |
5 (5) 68 (62) 36 (33) 109 |
42 (19) 126 (57) 55 (25) 223 |
28 (11) 168 (66) 60 (23) 256 |
0.0035 |
|
NPM1, FLT3/ITD status, n (%) NPM1- and/or FLT3/ITD+ NPM1+ and FLT3- NPM1, FLT3/ITD unknown |
N/A |
N/A |
153 (84) 30 (16) 183 |
144 (85) 25 (15) 169 |
0.76 |
| Type of AML, n (%) De novo Secondary |
506 (95) 24 (5) |
122 (78) 34 (22) |
301 (100) 0 (0) |
236 (90) 25 (10) |
<0.001 |
| Laboratory studies at baseline WBC (x103/µL), median (range) Platelets (x103/µL) Marrow blasts (%) |
18 (0–416) 54 (2–700) 74 (0–99) |
13 (1–274) 49 (6–1,200) 61 (10–99) |
12 (0–244) 55 (7–9,300) 66 (3–100) |
13 (1–800) 50 (4–8,500) 60 (0–100) |
0.029 0.045 <0.001 |
| Number of induction courses, n (%) 1 2 |
381 (72) 149 (28) |
126 (82) 27 (18) |
234 (78) 66 (22) |
198 (76) 63 (24) |
0.036 |
| Induction response, n (%) CR on first induction CR on reinduction No CR |
219 (41) 68 (13) 243 (46) |
74 (48) 7 (5) 72 (47) |
178 (59) 32 (11) 91 (30) |
137 (52) 27 (10) 97 (37) |
<0.001 |
Figure 1. Treatment outcomes in study cohort.
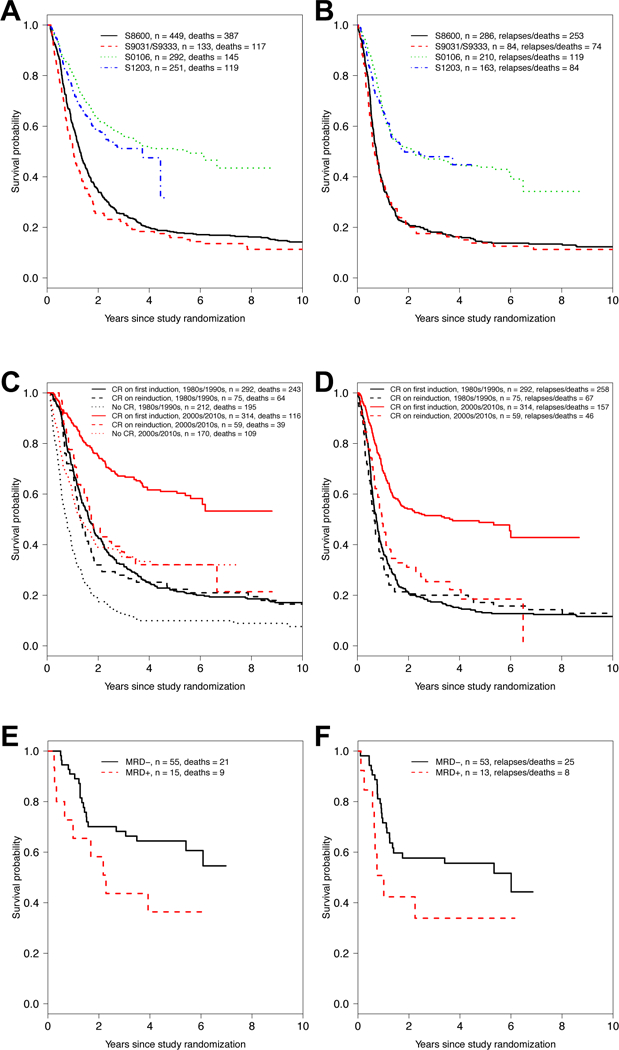
(A) Overall survival and (B) relapse-free survival by trial/decade. (C) Overall survival and (D) relapse-free survival by CR status and decade of therapy (1980s/1990s versus 2000s/2010s). (E) Overall survival and (F) relapse-free survival in patients treated on S0106 who achieved morphologic CR with the first cycle of 7+3, stratified by results from MRD test, assessed centrally by multiparameter flow cytometry.
We then built multivariable models to examine the relationship between CR achievement with the second cycle of 7+3 and OS as well as RFS in the older cohort of studies (S8600, S9031, and S9333) as well as the newer cohort of studies (S0106 and S1203) separately. After adjustment for age, gender, performance status, cytogenetic risk, WBC, platelet count, percentage of blasts in the bone marrow, and type of AML, CR achievement only upon reinduction chemotherapy was not statistically significantly associated with OS (hazard ratio (HR)=1.19 [95% confidence interval: 0.89–1.59], P=0.25; Supplementary Table 1) or RFS (HR=1.15 [0.86–1.54], P=0.34; Supplementary Table 2) in the older studies. These findings are similar to those reported by Rowe and colleagues on 1,980 adults with newly-diagnosed AML treated on 6 consecutive ECOG trials conducted in the 1980s and early 1990s.9 In the ECOG trial analysis, 5- and 10-year DFS and OS was not significantly affected by the need for 1 cycle or 2 cycles of induction chemotherapy in any of the 6 trials, even after multivariable adjustment. Using the same methodology as the prior ECOG analysis (analyzing all patients instead of performing a landmark analysis), we found similar results to the landmark analyses above for both OS (HR=1.21 [0.91–1.63], P=0.19) and RFS (HR=1.14 [0.85–1.53], P=0.37).
In contrast to the data obtained from the older studies, we found receiving 2 cycles of induction chemotherapy before CR is documented was associated with worse outcome in the S0106 and S1203 trials. Specifically, in these two trials, CR achievement only upon reinduction chemotherapy was statistically significantly associated with worse OS (HR=1.82 [1.24–2.66], P=0.002; Supplementary Table 3) or RFS (HR=1.90 [1.34–2.70], P<0.001; Supplementary Table 4). Fitting a model with an interaction between time-period and reinduction, that interaction was significant (for OS: P=0.046; for RFS: P=0.016). Kaplan-Meier estimates of OS and RFS, stratified by older vs. newer studies summarize these results graphically (Figure 1C,D).
These findings indicate adults with newly-diagnosed AML treated on more recent cooperative group trials who achieve remissions early, i.e. with the first cycle of 7+3 chemotherapy, have better survival outlooks than those who need 2 cycles of chemotherapy to enter a CR, even after adjustment for other risk factors. These data are reminiscent of those obtained from trials of double-induction chemotherapy regimens.7,8 Why the relationship between time of remission achievement and outcome with 7+3 therapy is not seen in older studies is unclear. Possibilities may include the change in anthracycline dose over time (i.e. chemotherapy resistance may be more apparent after the first cycle of chemotherapy with more intense regimens), selection of patients who receive on-study reinduction therapy, and changes in frequency/routine of transplantation, among others.
Finally, having found evidence that early achievement of morphologic CR was associated with survival with 7+3 chemotherapy, we examined whether data from MRD tests provide additional prognostic information in such patients. For S0106, we previously reported patients in remission without flow cytometric evidence of MRD after completion of induction chemotherapy (either 1 or 2 courses of 7+3) had better outcomes than patients who achieved CR with up to 2 courses of chemotherapy but had a positive MRD test.15 Here, we limited these analyses to patients who achieved a morphologic CR with the first cycle of 7+3 and had data from MRD testing available (n=70). Acknowledging limited power in these analyses because of a relatively small sample size, we found having a negative MRD test (n=55) after cycle 1 was associated with statistically significantly better OS (P=0.049) and a trend toward better RFS (P=0.098) compared to having a positive MRD test after cycle 1 (n=15; Figure 1E,F). Patients who tested negative for MRD after the first cycle of 7+3 also had significantly better RFS (p=0.02) and a statistically non-significant trend (p=0.11) toward better OS than those who achieved a MRD test-negative CR only after 2 therapy cycles (Supplementary Figure 1). Together, our findings not only highlight the prognostic significance of achieving a remission early but also support the value of “MRDneg CR” as recently proposed by the European LeukemiaNet2 as new response entity.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to gratefully acknowledge the important contributions of the late Dr. Stephen H. Petersdorf to SWOG and to study S0106. Research reported in this publication was supported by a grant from the National Cancer Institute/National Institutes of Health grants CA180888 and CA180819. R.B.W. is a Leukemia & Lymphoma Society Scholar in Clinical Research.
Footnotes
Supplementary information is available at Leukemia’s website.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- 1.Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015; 373(12): 1136–1152. [DOI] [PubMed] [Google Scholar]
- 2.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129(4): 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter RB, Othus M, Burnett AK, Löwenberg B, Kantarjian HM, Ossenkoppele GJ, et al. Resistance prediction in AML: analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia 2015; 29(2): 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walter RB, Othus M, Paietta EM, Racevskis J, Fernandez HF, Lee JW, et al. Effect of genetic profiling on prediction of therapeutic resistance and survival in adult acute myeloid leukemia. Leukemia 2015; 29(10): 2104–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 2016; 374(23): 2209–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hourigan CS, Gale RP, Gormley NJ, Ossenkoppele GJ, Walter RB. Measurable residual disease testing in acute myeloid leukaemia. Leukemia 2017; 31(7): 1482–1490. [DOI] [PubMed] [Google Scholar]
- 7.Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council’s Adult and Childhood Leukaemia Working Parties. Br J Haematol 1999; 107(1): 69–79. [DOI] [PubMed] [Google Scholar]
- 8.Terwijn M, van Putten WL, Kelder A, van der Velden VH, Brooimans RA, Pabst T, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol 2013; 31(31): 3889–3897. [DOI] [PubMed] [Google Scholar]
- 9.Rowe JM, Kim HT, Cassileth PA, Lazarus HM, Litzow MR, Wiernik PH, et al. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer 2010; 116(21): 5012–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weick JK, Kopecky KJ, Appelbaum FR, Head DR, Kingsbury LL, Balcerzak SP, et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study. Blood 1996; 88(8): 2841–2851. [PubMed] [Google Scholar]
- 11.Godwin JE, Kopecky KJ, Head DR, Willman CL, Leith CP, Hynes HE, et al. A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest oncology group study (9031). Blood 1998; 91(10): 3607–3615. [PubMed] [Google Scholar]
- 12.Anderson JE, Kopecky KJ, Willman CL, Head D, O’Donnell MR, Luthardt FW, et al. Outcome after induction chemotherapy for older patients with acute myeloid leukemia is not improved with mitoxantrone and etoposide compared to cytarabine and daunorubicin: a Southwest Oncology Group study. Blood 2002; 100(12): 3869–3876. [DOI] [PubMed] [Google Scholar]
- 13.Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013; 121(24): 4854–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Manero G, Othus M, Pagel JM, Radich JP, Fang M, Rizzieri DA, et al. SWOG S1203: a randomized phase III study of standard cytarabine plus daunorubicin (7+3) therapy versus idarubicin with high dose cytarabine (IA) with or without vorinostat (IA+V) in younger patients with previously untreated acute myeloid leukemia (AML) [abstract]. Blood 2016; 128(22): 901. [Google Scholar]
- 15.Othus M, Wood BL, Stirewalt DL, Estey EH, Petersdorf SH, Appelbaum FR, et al. Effect of measurable (‘minimal’) residual disease (MRD) information on prediction of relapse and survival in adult acute myeloid leukemia. Leukemia 2016; 30(10): 2080–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


