Notation of prior abstraction presentation: An early version of this work was presented at the International Association of Gerontology and Geriatrics (IAGG) World Congress in July 2017.
Keywords: sensitivity testing, hazard of sub-distribution, multiple imputation, death, longitudinal analysis, Precipitating Events Project
Abstract
OBJECTIVES:
To address the competing risk of death in longitudinal studies of older persons, we demonstrate sensitivity analyses that evaluate robustness of associations between exposures and three outcome types: dichotomous, count, and time-to-event.
DESIGN:
A secondary analysis of data from a prospective cohort study.
SETTING:
Community-based data from the Precipitating Events Project in New Haven, CT
PARTICIPANTS:
Persons of age 70 years and older who were initially community-dwelling and without disability in the four basic activities of daily living (N = 754)
MEASUREMENTS:
Missing outcome values from decedents were multiply imputed under different scenarios. Three outcomes were examined: dichotomous fall-related hospitalization; a count (0–13) of total disability in each of the six months following discharge; days to functional recovery among those whose disability worsened in hospital. Each outcome had a different exposure: for dichotomous, indicators of being overweight or obese; for count, frailty from the Fried phenotype (0–5, where not frail=0, pre-frail=1–2, and frail=3–5); for days to recovery, vision impairment.
RESULTS:
For fall-related hospitalization, being overweight or obese lost significance when decedents were kept in the risk pool without outcome events for over 10 years. For disability count and time to recovery, with follow-up of six months, exposures only lost significance under highly implausible clinical scenarios.
CONCLUSION:
This method facilitates evaluation of potential bias from the competing risk of death in longitudinal studies for non-death outcomes that are not necessarily time-to-event. Results suggest that death introduces substantive bias when long term follow-up results in cumulatively high levels of mortality.
INTRODUCTION
In longitudinal studies of older persons, participants often die during follow-up,1 especially in studies of critical illness.2 Because many aging-related outcomes such as daily function are correlated with death, the loss of follow-up occasioned by death represents informative censoring, which can, in turn, bias the estimated associations of interest.3 Investigators must therefore find ways to either prevent or quantify that potential bias. For time-to-event analyses, the cumulative incidence approach of Fine and Gray is widely used to test the sensitivity of associations to censoring by death.4 Relative to a cause specific survival model that ignores death, a Fine and Gray analysis indicates, in a population with similarly distributed covariates and mortality during follow-up, how much the associations of interest are biased by death.5 Perhaps the most important limitation of Fine and Gray is its restriction to time-to-event outcomes, which precludes its ready application to the binary and count outcomes so prevalent in aging research.
In this educational report, we investigate a method that allows for sensitivity testing of both the significance and magnitude of associations of interest with non-death outcomes that are not necessarily time-to-event. This approach is based on application of multiple imputation to the observations longitudinally lost to death in a framework meant to provide clinical intuition regarding the competing risk of death. With data from the Precipitating Events Project,6,7, we demonstrate this for three types of outcomes: dichotomous, count, and time-to-event.
METHODS
The Precipitating Events Project
The Precipitating Events Project (PEP) is an ongoing longitudinal study of 754 community-living persons, aged 70 or older, who were initially nondisabled in four basic activities of daily living (ADLs)—bathing, dressing, walking, and transferring. The assembly of this cohort, which enrolled participants between March 1998 and October 1999, has been previously documented.6–8 For the analytical examples presented here, comprehensive home-based assessments were completed at baseline and at successive 18-month intervals through December 2013. Those comprehensive assessments updated the measurements of the Fried phenotype of frailty and of all covariates except intensive care unit (ICU) length of stay, mechanical ventilation, and shock; the latter three covariates were updated from administrative data.9 Monthly telephone interviews assessed disability from enrollment through December 2013, as described elsewhere6,8. Information on hospitalization and admission to the ICU were also obtained from the monthly interviews and supplemented by managed Medicare as needed.10
Analytical Units for the Outcomes
For the dichotomous indicator of Fall-Related Hospitalization (FRH) the analytical unit was an 18-month person-interval corresponding to the time between comprehensive interviews in the PEP study, where explanatory variables were updated at the beginning of the interval and the outcome was any FRH occurring during that interval. The PEP participants (N=754 at baseline) contributed a total of 3969 person-intervals that started from baseline through the comprehensive interview at 108 months through June 30 of 2010 (N=279 survivors). For the count and time-to-event outcomes the analytical units are the first person-admissions to hospital within each 18-month interval that included admission to the ICU; the construction of these cohorts has been described in detail previously.9,10 Because evaluations of the count and time-to-event outcomes were published in separate studies, they have differing sample sizes. The analytic sample for the disability count outcome drew from eligible PEP data through December 2013, which consisted of 266 eligible person-admissions who survived to at least one month after discharge. Analysis of the third outcome (time-to-recovery) draws from eligible PEP data through December 2012. For this time-to-recovery outcome, there were 218 eligible person-admissions surviving through the first post-discharge month with higher disability relative to admission.
Three Outcome Types with Distinct Exposures of Interest
To demonstrate what would be required to eliminate the significance of an association, we focus on the single exposure for each outcome that was the most vulnerable, i.e., closest to a p-value of 0.05,9,10 in the data truncated by death. Hereafter we refer to this as complete case data to differentiate from the datasets where post-death outcomes are imputed. For FRH the exposure of interest consisted of the indicators of being overweight or obese (relative to normal or underweight), each of which had a p-value of 0.01; for count of disability, exposure was the indicator of pre-frailty with its p-value of 0.042; for time-to-recovery, exposure was the indicator of vision impairment with its p-value of 0.031. In each outcome model the serial correlation of the repeated measures from each participant was accounted for by generalized estimating equations with an autoregressive correlation structure (dichotomous and count outcomes) or with a person-specific random intercept (time-to-recovery). All analyses were performed with SAS statistical software version 9.4.
Logistic Regression of the Dichotomous Outcome: Fall-Related Hospitalization (FRH)
FHR was modeled with multivariable logistic regression that included adjustment for the following covariates: age, sex, race, indicators of being overweight or obese, depressive symptoms, number of chronic conditions, cognitive status, slow gait, and the number of the most recent comprehensive interview (1 for baseline through 7 for 108 months). The exposure of primary interest consisted of indicators of being overweight or obese that respectively exhibited odds ratios and (95% confidence interval) of 0.5 (0.3, 0.9) and of 0.5 (0.3, 0.9), each with p-values of 0.01 in the complete case data. By adding on all the intervals that each of the 475 (63%) persons who died before June 30, 2010 were missing through the seventh comprehensive interview and subtracting out those from participants who dropped out, our datasets for the sensitivity analyses consisted of 5111 intervals (7 intervals times 754 persons less dropout). In our second scenario we imputed the missing outcomes of the post-death intervals based on an assumption of missing at random (MAR). Our third scenario kept all decedents in the dataset while assigning them no outcomes in the post-death intervals, approximating what is done in a Fine and Gray hazard of sub-distribution analysis. In our fourth scenario we assigned an outcome event to each post-death interval. Because all missing outcomes in the third and fourth scenarios were set to the same value (FRH=0 and FRH=1, respectively), these scenarios are examples of missing not at random (MNAR), in contrast with the MAR scenario that imputed the missing outcomes of decedents by drawing from the longitudinal values of all other model terms.
Negative Binomial Regression of the Count Outcome
The count outcome was functional disability from 0 – 13 in basic activities of daily living (0–4), instrumental activities of daily living (0–5), and mobility (0–4) in each of the six months following hospital discharge. Its negative binomial model adjusted for the following covariates: age, sex, race, education, body mass index, depressive symptoms, number of chronic conditions, cognitive status, pre-ICU count of disability, ICU length of stay, mechanical ventilation, shock, and number of months since the comprehensive interview. The exposure of interest was a binary indicator of pre-frailty, defined as a Fried phenotype of 1 or 2, that exhibited a rate ratio of 1.28 (1.01, 1.63) with p-value of 0.04 in complete case data. We imputed the missing monthly outcome values of the 43 persons who died during follow-up under the following scenarios: MAR, MNAR (values set to 0), MNAR (values set to 7), and MNAR (values set to 13).
Proportional Hazards Regression of the Time-To-Event Outcome
For participants who survived through the first post-discharge month with greater functional disability than at pre-admission, the outcome was number of days from hospital admission to recovery of pre-admission disability. Its proportional hazards model, which was chosen using backward selection, included person-specific random intercepts and adjustment for the following covariates: age, sex, race, increase in disability from pre-ICU, hearing impairment, body mass index, and functional self-efficacy, i.e., a measure of confidence in performing functional activities as measured with the modified self efficacy scale.11 The exposure of interest was a binary indicator of vision impairment which exhibited a hazard ratio of 0.59 (0.37, 0.95) with p-value of 0.031 in complete case data. We imputed time to recovery for the 35 persons who died during follow-up as MAR and the indicator of recovery under the following scenarios: MAR, MNAR (no decedents recover), MNAR (small positive bias for recovery), MNAR (moderate positive bias for recovery), MNAR (large positive bias for recovery), and MNAR (all decedents recover).
RESULTS
Dichotomous Outcome: Fall-Related Hospitalization (FRH)
Figure 1 depicts the odds-ratios (ORs) and (95% confidence intervals) of the indicators of being overweight and being obese (lower half) as well as that for slow gait speed (top half) in the four scenarios: complete case, MAR, MNAR where missing outcomes of decedents are set to no FHR, and MNAR where all missing outcomes of decedents are set to have FHR. Being overweight and obese are protective against FRH in the complete case scenario, for MAR imputation, and for MNAR where all missing outcomes of decedents are set to FRH. However, for MNAR where missing outcomes of decedents are set to no FRH, both indicators lose significance. The association for slow gait speed retains significance in all scenarios, but exhibits a meaningful reduction of magnitude in the MNAR scenario with no post-death FRH (reduction of 25%) and in MNAR where all post-death observations include FRH (34% reduction). The MNAR scenario with no post-death FRH approximates a Fine and Gray sub-distribution analysis, where the decedents are simultaneously kept in the risk set and prevented from experiencing the outcome. The loss of significance of being overweight and obese in that scenario indicates that death acts as an informative censoring agent that prevents heavier participants from experiencing FRH.
Figure 1.
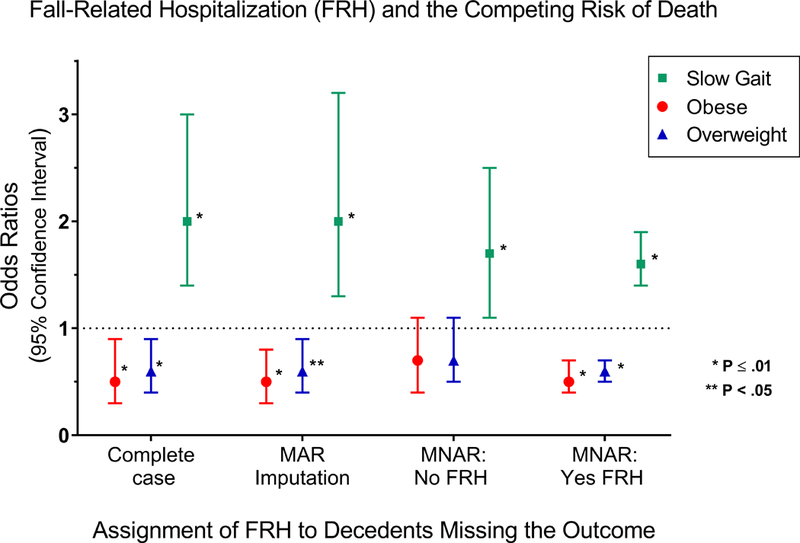
Associations (Odds-Ratios with 95% Confidence Intervals) between explanatory variables (indicators of being overweight, of being obese and of slow gait speed) and the outcome (occurrence of Fall-Related Hospitalization (FRH) in a given 18 month interval) are presented for four different data scenarios that differ in their treatment of death during follow-up over more than 10 years. The four scenarios are: complete case where follow-up is truncated by death; missing-at-random where outcomes of decedents are multiply imputed; missing not at random where all missing decedent outcomes are set to no FRH; missing not at random where all missing decedent outcome are set to FRH. The complete case data consists of 3969 person-intervals and the other scenarios consist of 5111 person-intervals. Point estimates, confidence intervals and p-values were calculated with multivariable logistic regression with generalized estimating equations and a first-order autoregressive covariance structure, and included adjustment for age, sex, race, indicators of being overweight or obese, depressive symptoms, number of chronic conditions, cognitive status, slow gait speed, and the number of the most recent comprehensive interview (1 for baseline through 7 for 108 months).
Abbreviations: MAR = missing at random; MNAR = missing not at random
Count Outcome: Count of Post-Discharge Functional Disability over Six Months
Table 1 presents the associations between the indicator of pre-frailty and count of post-discharge functional disability for complete case data and four imputed scenarios. In the MAR imputation of the outcomes of the 43 persons who die over the six months following discharge, pre-frailty exhibits a marginally significant positive association with higher functional disability. In the MNAR scenarios, the magnitude of the association diminishes as the level of disability rises in the person-months truncated by death, finally losing significance when all those person-months are set to the maximum value of 13. There are two reasons why this latter scenario does not cast reasonable doubt on pre-frailty’s association with this outcome. First, setting the missing outcomes to the maximum level limits outcome variability to such an extent that its association with any explanatory variable is greatly diminished. Second, persons needing help in all 13 functions are unlikely to survive the entire six months of follow-up.
Table 1:
Sensitivity Testing for Robustness to Death of Participants during Follow-up: Associations of Pre-Frailty with Post-Discharge Count of Disability among Older Survivors of Critical Illness
| Count Outcome: Post-Discharge Functional Disability (0 – 13)a | |||
|---|---|---|---|
| Imputation Approach for Follow-up Outcomes Missing From Decedents | Sample Size (person-months) | Indicator of Pre-Frailty (1 ≤ Fried ≤ 2) referent to Fried = 0 | |
| Rate Ratio (95% CI) | p-valueb | ||
| Complete Case | 1433 from 215 persons | 1.28 (1.01 – 1.63) | 0.042 |
| MAR | 1596 from 215 persons | 1.31 (1.04 – 1.64) | 0.020 |
| MNAR (all missing set to 0) | 1596 from 215 persons | 1.36 (1.05 – 1.76) | 0.018 |
| MNAR (all missing set to 7) | 1596 from 215 persons | 1.27 (1.01 – 1.59) | 0.041 |
| MNAR (all missing set to 13) | 1596 from 215 persons | 1.21 (0.94 – 1.56) | 0.134 |
sum of disability in four basic activities of daily living, five instrumental activities of daily living, and four mobility measures
multivariable negative binomial regression with GEE with autoregressive correlation structure and adjustment for age, sex, race, education, body mass index, depressive symptoms, number of chronic conditions, cognitive status, pre-ICU count of disability, ICU length of stay, mechanical ventilation, shock, and number of months since the comprehensive interview
Abbreviations: GEE = generalized estimating equations; MAR = missing at random; MNAR =missing not at random
Reprinted with permission of Elsevier. Copyright © 2018 Elsevier.
Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. The association of frailty with post-ICU disability, nursing home admission, and mortality. Chest. 2018 Jun;153(6):1378–1386. doi: 10.1016/j.chest.2018.03.007. Epub 2018 Mar 17. PMID: 29559308.
Time-to-event Outcome: Recovery of Pre-ICU Function within Six Months of Discharge
Table 2 presents the associations between vision impairment and time-to-recovery of pre-ICU function for complete case data and six imputed scenarios. For complete case data, for the Fine and Gray hazard of sub-distribution, and for MAR imputed outcomes, vision impairment exhibits a negative association with recovery. Relative to the 62% of non-decedents who recovered, 41% of the 35 decedents recovered under MAR imputation, a reasonable level given that decedents are typically in worse health and consequently less likely to recover. The other five scenarios are MNAR which range from none of the decedents recovering to all recovering, either through arbitrary setting of outcome values or by setting parameters during imputation that bias the decedents toward higher likelihood of recovery than from MAR imputation. The second column has been added to guide interpretation of the values chosen for the MNAR parameters. Through setting of parameters in imputation the proportion of decedents who recover is raised from 55% to 69%, at which point vision impairment loses statistical significance. Note that this level is substantively higher than the proportion of decedents who recover during MAR imputation.
Table 2.
Sensitivity Testing for Robustness to Death of Participants during Followup: Associations of Vision Impairment with Functional Recovery among Older Survivors of Critical Illness
| Datasets Used in Model of Time to Functional Recovery within Six Months of ICU Admission (N=218 in each case) |
Rate of Recovery Among 35 Decedents Censored by Deatha | Multivariable Association between Vision Impairment and Functional Recovery within Six Months | |
|---|---|---|---|
| HR (95% CI) | p-valueb | ||
| All Person-Admissions with 35 decedents not imputed (complete case results) | 0% | Cause Specific 0.59 (0.37, 0.95) | 0.031 |
| Fine and Gray 0.64 (0.42, 0.98)c | 0.038c | ||
| MAR Imputations of time to recovery and its occurrence | 41% | 0.61 (0.38, 0.96) | 0.032 |
| MNAR where all 35 decedents set to non-recovery with censoring at six months | 0% | 0.58 (0.37, 0.90) | 0.015 |
| MNAR Imputations of recovery (shift=0.8 and sigma=0.2) and MAR imputation of time to recovery | 55% | 0.61 (0.39, 0.97) | 0.038 |
| MNAR Imputations of recovery (shift=1.5 and sigma=0.5) and MAR imputation of time to recovery | 63% | 0.63 (0.39, 1.00) | 0.049 |
| MNAR Imputations of recovery (shift=2.0 and sigma=0.5) and MAR imputation of time to recovery | 69% | 0.62 (0.38, 1.01) | 0.054 |
| MNAR where all 35 decedents set to recovery at time of death | 100% | 0.72 (0.48, 1.08) | 0.114 |
among 183 not censored by death, in all cases, fixed rate of recovery (62%)
Cox regression with random intercept and adjustment for age, sex, race, increase in disability from pre-ICU, hearing impairment, body mass index, and functional self-efficacy calculated over ten imputations.
Fine and Gray hazard of sub-distribution from single imputation.
Abbreviations: HR = hazard ratio; CI = confidence interval; ICU=Intensive Care Unit;
MV = multivariable, MAR = missing at random, MNAR = missing not at random
Reprinted with permission of the American Thoracic Society. Copyright © 2018 American Thoracic Society.
Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors associated with functional recovery among older ICU survivors. American Journal of Respiratory and Critical Care Medicine 2016, Aug 1:194(3):299–307. Doi: 10.1164/rccm.201506–1256OC. PMID:26840348
The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
Sample code in SAS outlining the generation of the sensitivity datasets and their analyses for all three outcome types is available upon request from the corresponding author.
DISCUSSION
The sensitivity testing demonstrated here provides an easily interpreted means of assessing how robust an association between an explanatory variable and non-death outcome is to death during follow-up. Based on the outcome of interest, e.g., proportion of decedents assumed to recover function, it provides clinicians with intuition regarding robustness. The primary strength of this study is its demonstration of such analyses for the dichotomous, count, and time-to-event outcomes of fundamental interest to the geriatric and critical care communities. In the web-based supplements, samples of code in SAS are provided to guide those interested in pursuing this approach. A second strength is the source data, which, in amassing this large collection of hospitalizations for critical illness, draws from a cohort of initially non-disabled, community dwelling adults who have been followed monthly for over 10 years. The primary limitation for this technique is its requirement that the analyst have some knowledge with respect to both MAR and MNAR imputation. However, most practicing biostatisticians have experience with multiple imputation and can assist with this task.
In both observational studies and clinical trials, sensitivity analyses are increasingly viewed as a necessary part of epidemiological research.12 Because observational studies lack the theoretical balance of unmeasured confounders enjoyed by randomized trials, they are vulnerable to potential bias from confounders not addressed in the multivariable model. To that end VanderWeele and Ding recently proposed a sensitivity analysis based on an entity they call the E value (i.e, evidence).12 The E value represents the minimum strength of association that an unmeasured confounder would need to have with both exposure and outcome in order to eliminate the significance of a separate association of interest. Larger E values reflect higher robustness just as larger magnitudes of the index association are inherently more robust, reflecting the importance of magnitude of association in addition to its statistical significance. The sensitivity analyses proposed here differ from the E value in two important ways.
First, the sensitivity testing described here specifically evaluates how a range of hypothetical outcomes of decedents affect associations with the non-death outcome of interest. Whereas the E value indicates the magnitude of association from an unmeasured confounder needed to eliminate significance, we are evaluating the influence of death during follow-up on specific associations with a non-death outcome. Second, our approach provides direct intuition regarding what specific outcome values from decedents would be required to eliminate significance. In the example with time to functional recovery, we showed that positive bias would be required so that nearly 70% of the decedents would need to recover before significance of vision impairment was eliminated. Because that scenario is so implausible, it provides intuitive evidence that the primary association is robust to death during follow-up. It is noteworthy that while the Fine and Gray approach for this outcome also signaled robustness to death during follow-up, it did not provide any clinical intuition regarding what assumptions pertaining to death of participants would be needed to eliminate significance of a given association.
Acknowledging that the competing risk of death did not play much of a role in our examples based on count and time-to-event outcomes, it is natural to question just when it is likely to be of serious concern. Our dichotomous example (fall-related hospitalization) is quite informative in this regard. Apart from being a different outcome type, this example differs from the others in two ways that have strong bearing on the competing risk of death. First, this example draws on follow-up of more than 10 years; second, this time period coincides with the death of a decided majority (63%) of the participants. We posit that the competing risk of death introduces substantive bias when follow-up is very long (e.g., > 5 years) and results in very high cumulative mortality (e.g., > 50%). We further note that the only scenario wherein the exposure of interest (being overweight or obese) lost significance is the one that approximates the conditions of the Fine and Gray hazard of sub-distribution approach. We note that several published cases where sensitivity analyses based on the Fine and Gray hazard of sub-distribution show strong bias contributed by the competing risk of death are also characterized by long term follow-up, i.e., ≥ 5 years. For example, in Berry et al (2010) the simulated risk of a second hip fracture in the Framingham study is, by not accounting for the competing risk of death, overestimated by 37% over five years and by 75% over 10 years.13 A second example is that of Ashburner et al (2017),14 where cause specific analysis (ignoring death) estimates a 43% lower hazard of stroke over seven years among users of warfarin whereas the hazard of sub-distribution is only 13% lower over the same period.
In conclusion, sensitivity analysis is increasingly being recognized as a viable option for testing the robustness of associations to potential bias from death of participants during follow-up. While the hazard of sub-distribution approach of Fine and Gray is commonly used to address the informative censoring of death in time-to-event models, techniques for other outcome types have not been clearly delineated in the literature. This study illustrates how aging-related longitudinal research can evaluate such robustness in dichotomous, count, and time-to-event outcomes with use of multiple imputation. Its simple implementation facilitates evaluation of the potential bias from the competing risk of death in longitudinal studies for non-death outcomes that are not necessarily time-to-event. It also suggests that the competing risk of death is of greatest concern in studies that include long periods of follow-up that entail high cumulative mortality.
Impact Statement:
This work delineates sensitivity analyses to address the competing risk of death in studies of older persons, a topic that is relevant to a wide range of geriatrics studies. Using examples from the literature for three outcome types (dichotomous, count, and time-to-event) we illustrate the flexibility and utility of this approach. The references for the recent clinical research are listed immediately below:
Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors associated with functional recovery among older ICU survivors. American Journal of Respiratory and Critical Care Medicine 2016, Aug 1:194(3):299–307. Doi: 10.1164/rccm.201506–1256OC. PMID:26840348
Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. The association of frailty with post-ICU disability, nursing home admission, and mortality. Chest. 2018 Jun;153(6):1378–1386. doi: 10.1016/j.chest.2018.03.007. Epub 2018 Mar 17. PMID: 29559308.
Acknowledgments:
We thank Denise Shepard, BSN, MBA, Andrea Benjamin, BSN, Barbara Foster, and Amy Shelton, MPH, for assistance with data collection; Wanda Carr and Geraldine Hawthorne, BS, for assistance with data entry and management; Peter Charpentier, MPH, for design and development of the study database and participant tracking system; Joanne McGloin, MDiv, MBA, for leadership and advice as the Project Director.
Role of the Sponsors:
The organizations funding this study had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
This work took place at the Yale Program on Aging/Claude D. Pepper Older Americans Independence Center (P30AG021342). Dr. Ferrante is supported by a Paul B. Beeson Emerging Leaders in Aging Research Career Development Award from the National Institute on Aging (K76AG057023), the Francis Family Foundation, a GEMSSTAR award from the National Institute on Aging (R03AG050874), a Pepper Scholar award from the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342), and a T. Franklin Williams Scholar Award, with funding provided by: Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Alliance for Academic Internal Medicine-Association of Specialty Professors and the American Thoracic Society Foundation. At the start of this work, Dr. Ferrante was supported by T32AG019134. The PEP Study is supported by a grant from the National Institute on Aging (R01AG017560). Dr. Gill is the recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging.
Footnotes
Conflicts of Interest:
The authors have no conflicts of interest.
REFERENCES
- 1.Murphy TE, Han L, Allore HG, Peduzzi PN, Gill TM, Lin H. Treatment of death in the analysis of longitudinal studies of gerontological outcomes. J Gerontol A Biol Sci Med Sci. 2011;66(1):109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175(4):523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy TE, Chaudhry SI. Benefit of Warfarin in Older Persons with Atrial Fibrillation. J Am Geriatr Soc. 2017;65(1):25–26. [DOI] [PubMed] [Google Scholar]
- 4.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 5.Analysing Pintilie M. and interpreting competing risk data. Stat Med. 2007;26(6):1360–1367. [DOI] [PubMed] [Google Scholar]
- 6.Gill TM, Hardy SE, Williams CS. Underestimation of disability in community-living older persons. J Am Geriatr Soc. 2002;50(9):1492–1497. [DOI] [PubMed] [Google Scholar]
- 7.Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135(5):313–321. [DOI] [PubMed] [Google Scholar]
- 8.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291(13):1596–1602. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. The Association of Frailty With Post-ICU Disability, Nursing Home Admission, and Mortality: A Longitudinal Study. Chest. 2018;153(6):1378–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors Associated with Functional Recovery among Older Intensive Care Unit Survivors. Am J Respir Crit Care Med. 2016;194(3):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tinetti ME, Mendes de Leon CF, Doucette JT, Baker DI. Fear of falling and fall-related efficacy in relationship to functioning among community-living elders. J Gerontol. 1994;49(3):M140–147. [DOI] [PubMed] [Google Scholar]
- 12.VanderWeele TJ DP. Sensitivity analysis in observational research: introducing the E-value. Annals of Internal Medicine. 2017. [DOI] [PubMed] [Google Scholar]
- 13.Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58(4):783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashburner JM, Go AS, Chang Y, et al. Influence of Competing Risks on Estimating the Expected Benefit of Warfarin in Individuals with Atrial Fibrillation Not Currently Taking Anticoagulants: The Anticoagulation and Risk Factors in Atrial Fibrillation Study. J Am Geriatr Soc. 2017;65(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


