Abstract
A 3,5-bis((2-iodophenyl)ethynyl)pyridinium scaffold was synthesized which introduces the use of methanesulfonyl withdrawing groups to polarize iodine halogen bonding units for anion binding. We investigate the capability of this receptor to bind halides in polar media, while further probing the structure-property relationship of this well-polarized yet under-explored halogen bonding system.
Graphical Abstract
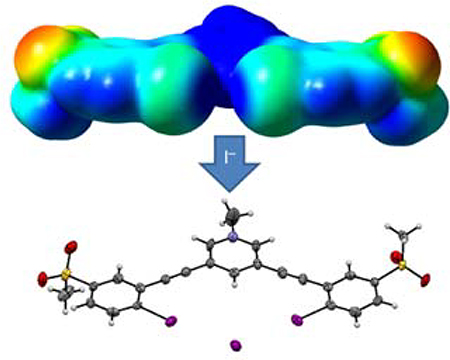
Easy-to-synthesize methanesulfonyl substituents are introduced as a way to polarize C-I halogen bond donors for reversible halide binding in competitive media.
Halogen bonding (XB) forces have emerged in the field of supramolecular chemistry as an attractive alternative to more common reversible interactions (e.g., hydrogen bonding).1,2 Polarized halogen atoms generate an electron deficient σ–hole capable of forming a highly directional non-covalent bond with nucleophilic species at a near 180° bond angle (R–X…Y).3 Recently, scaffolds that utilize σ–hole interactions including halogen,4–11 chalcogen,12–14 and pnictogen15 bonding have brought exciting new developments to the field of anion recognition. Initially, halogen bonding had primarily been explored in crystal engineering16–17 and in the gas phase;18–19 however, further investigation of these interactions have focused on their efficacy in solution phase as well.20–23 Halogen bonding interactions are comparable in strength to those of hydrogen bonding, and furthermore, they demonstrate a surprising resilience to interference from competitive, polar solvents.24–26 These attributes have generated a growing interest in halogen bonding from the supramolecular community over the past 20 years.
The water-resiliece of XB interactions is an attractive feature for reversible anion receptors with potential applications in biomedical,27–28 environmental,29–30 and agricultural areas,31–32 where conventional anion sensors meet competition from polar protic media. Previously examined XB-containing scaffolds have utilized perfluoroaryl/alkyl,33 pyridinium,34–35 imidazolium,36–38 and triazolium39–41 functional groups as electron withdrawing sources to generate the electron deficient σ–hole binding site. While perfluorinated compounds have previously demonstrated significant XB polarization capabilities, they can limit host functionalization. Para-positioned withdrawing groups allow for facile functionalization of the receptor’s binding moiety while still generating suitable XB donor sites. The size of the σ–hole has been shown to directly correlate to the strength of the polarizing group.43 To the best of our knowledge, our new scaffold (1+) is the first to introduce neutral methanesulfonyl polarizing groups for XB donors in anion receptors, which we found surprising given the Hammett sigma parameter for this functional group (σp = 0.72)44 exceeds those of other neutral withdrawing groups such as trifluoromethyl (σp = 0.54),44 suggesting sulfones should be very good at polarizing halogen bond donors as well.
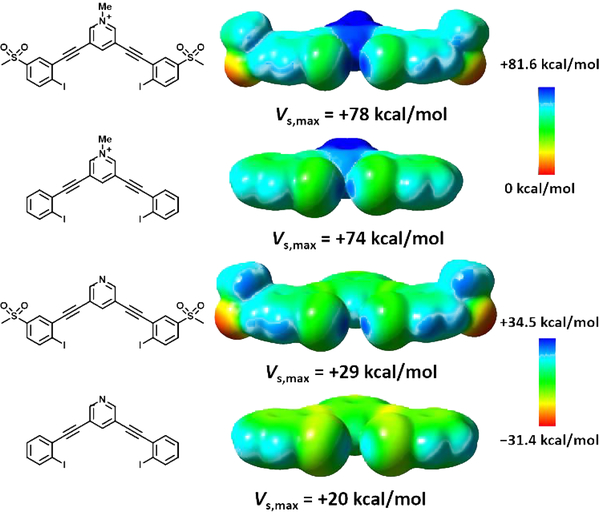
Herein, we report a new bidentate anion receptor (1+, Scheme 1) with a 3,5-diethynylpyridinium central core coupled to two 2-iodo-5-(methanesulfonyl)phenyl units to yield a binding pocket with two XB donor recognition sites designed to bind and sense anionic guests reversibly. Host 1+ integrates the methanesulfonyl-polarized XB recognition units within a highly conjugated arylethynyl backbone that can generate a spectroscopic response upon binding in solution.45–47 In addition, the cationic N-methylpyridinium core generates a positive potential to the receptor for electrostatic binding and promotes solubility in more polar solvent systems. The computed electrostatic potential surface of 1+ (Fig. 1) illustrates the partial positive charge enhancement from the methanesulfonyl groups on both iodines along the R-X axis and highlights the convergence of the two σ-holes within a single binding pocket with a determined Vs,max of +78 kcal/mol. A computed electrostatic potential surface for the analogus pyridinium receptor without methanesulfonyl groups (instead substituted with H atoms in the para-position to the XB donors) demonstrated a Vs,max of +74 kcal/mol. The addition of the sulfone groups in the para-position thus leads to a +4 kcal/mol increase. To assess methanesulfonyl’s withdrawing potential in the absensce of a charged pyridinium group, ectrostatic potential surfaces were also determined for a non-methylated neutral varient of the receptor scaffold (Fig. 1). The addition of the sulfone groups leads to a +9 kcal/mol increase in Vs,max at the site of the σ–hole, suggesting it is a suitable source of polarization for neutral XB donors as well.
Scheme 1.
Synthesis of XB anion recognition scaffold 1·PF6.
Fig 1.
Molecular electrostatic potential surfaces (isovalue= 0.001 a.u.) calculated at the B97-D3/Def2-TZVP level of theory for compounds 1+ and 5 along with congeners lacking methanesulfonyl substituents indicate areas of rich electron density (red) and depleted electron density (blue). Vs,max values shown for the σ-holes.
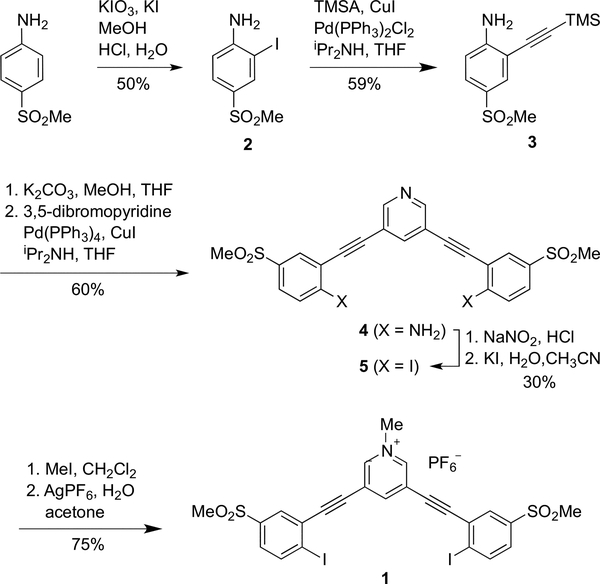
The receptor scaffold was synthesized as shown in Scheme 1. 4-Methanesulfonylaniline48 was iodinated with KI/KIO3 to give 2, which was then cross-coupled with (trimethylsilyl)acetylene (TMSA) to afford 3.49 Desilation in mildly basic MeOH and a second Sonogashira cross-coupling with 3,5-dibromopyridine yielded bis-aniline intermediate 4. Diazotization followed by addition of KI furnished neutral 5, which was alkylated with MeI to produce the charged pyridinium product 1+ with iodide (I−) as the counterion (1·I). To assess binding affinities more accurately, the iodide was exchanged for the more labile hexafluorophsophate7 (PF6−) to give 1·PF6.
Crystals of 1+ with the counteranions PF6−, I−, Br−, and Cl− suitable for X-ray diffraction were grown via slow evaporation methods.50 Interestingly, each anion afforded a structure that displayed profound conformational differences in the host with respect to how and where guest binding occurs. As previously seen with the arylethynyl-bisurea hydrogen bonding receptors, the halogen bonding 3,5-bis(2-iodophenyl)ethynylpyridinium scaffold can adopt each of the three possible binding conformations we have observed in this receptor class (W-, S-, and U-shaped) in the presence of each of the aforementioned anions.45,51
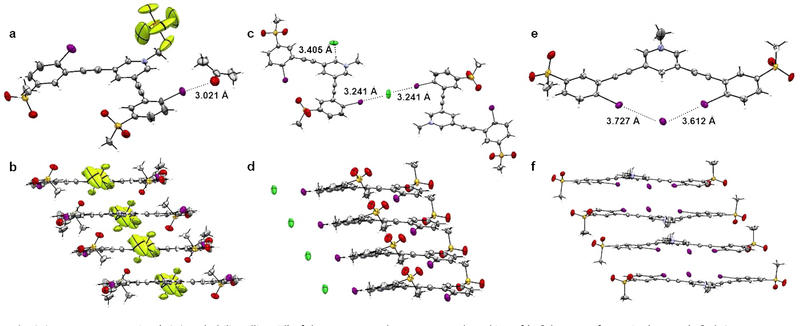
Crystals of 1·PF6 were grown by slow evaporation of acetone (Fig. 2a). Crystal packing shows the receptor units stacked head-to-tail in off-set pairs in which PF6− sits in the pocket of one receptor and over the pyridinium core of a second receptor in a complementary position (Fig. 2b). Notably, the structure reveals the PF6− counterion does not halogen bond with the polarized iodine atoms, which are instead twisted out of the central binding pocket toward the external pyridinium, in a W-like conformation. Instead, an acetone molecule forms an XB interaction with one of the externally facing iodine atoms with a R–I···O bond length of 3.021(7) Å, resulting in a 14% reduction of the sum of van der Waals radii and a bond angle of 173.5°, which is near the ideal 180° angle for halogen bonds.52 The second XB donor interacts with an adjacent receptor’s sulfonyl oxygen to a weaker extent at a R–I···O distance of 3.106(6) Å at a 158.0° angle, showing a slight deviation from the idealized 180°.
Fig. 2.
ORTEP representation (50% probability ellipsoid) of the X-ray crystal structures and stacking of (a & b, W-conformation) 1-PF6, (c & d, S-conformation) 1-Cl. and (e & f, U-conformation) 1·I.
The molecular structure of 1·Cl, from crystals grown by evaporation of MeOH, demonstrates a change from the W-shaped structure seen in 1·PF6 to an S-type conformation where one XB donor is pointing inward towards the binding pocket and the other pointed outwards towards the pyridinium. The structure reveals a 1:1 stoichiometric ratio of Cl− to 1+ in the solid state with two different Cl− binding modes. Two independent receptors are seen bridging a Cl− between them through halogen bonds, resulting in a bis-monodentate binding external from the idealized binding pocket near the pyridinium at an R–I···Cl− distance of 3.241(2) Å and a binding angle of 168.9° (Fig. 2c). A second Cl− ion is found in the molecular plane forming a hydrogen bond with a pyridinium C(H) on the side opposite to the halogen bond at a C(H)···Cl− distance of 3.405 Å, thus balancing the complex charge between the two receptors and two anions. The bond distance is seen to exhibit a 15% shortening between the van der Waals and ionic radii.53 The second XB donor is twisted inward toward the binding pocket and below the molecular plane, forming the S-shape conformation which allows for a XB interaction with the sulfonyl oxygen of a second host 1·Cl molecule (Fig. 2d) at a distance and angle of 3.146(1) Å and 176.5°, respectively. This interaction between the polarizing methanesulfonyl oxygen and the XB donor shows the susceptibility for individual host molecules to interact with one another to form dimerized complexes. An almost identical binding mode was observed in the crystal structure of 1·Br. While the adopted conformormation remains the same, the Brˉ is held at a distance and angle of 3.407(2) Å and 167.2°, respectively, resulting in a 14% reduction of the sum of the ionic and van der Waals radii (Fig. S4).53
Complex 1·I demonstrates another notable change in the structural conformation to the U-shape, producing a bidentate binding pocket (Fig. 2e) within a 1:1 host:iodide complex. The complex again packs in a head-to-tail paired pattern, with receptors slightly offset from one another (Fig. 2f). Both XB donor units are pointing inward toward the central binding pocket, coordinating a lone I− with a high degree of planarity. The I− anion is held in the central pocket at I···I− distances of 3.727(6) and 3.612(8) Å, a shortening in the sum of the ionic and van der Waals radii of 11% and 14%, respectively.53 While the lengthening of the interaction distance typically denotes weaker binding, the second XB interaction provides an additional stabilizing force in binding I− over the monodentate Cl− bound system. Additionally, the R–I···I− bond angles are 172.2° and 174.5°, further confirming the highly directional XB interaction elicited from the σ-hole created by the p-methanesulfonyl substituents.
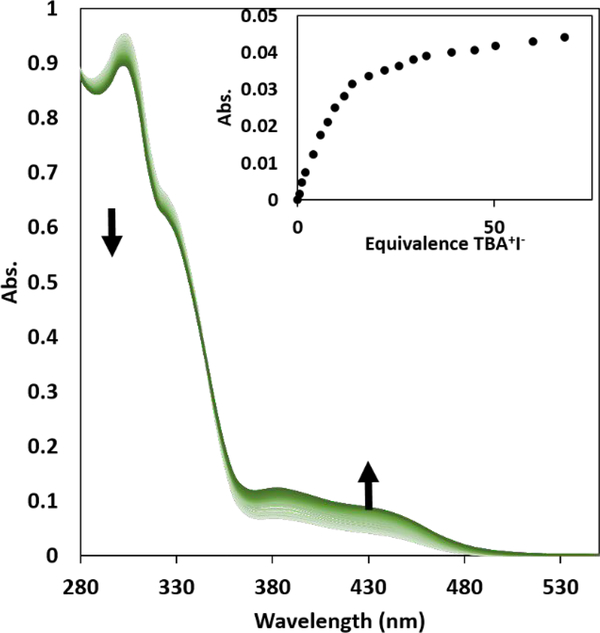
The strength of the anion binding interactions of 1·PF6 with a range of halides was determined by UV-Vis spectroscopy. UV-Vis titrations were performed with 30 μΜ solutions of 1·PF6 in DMSO-1.4 wt% water (see SI), with the anions introduced as tetrabutylammonium salts. DMSO was chossen in order to assess binding in a competitive solvent and as a stepping stone for future binding studies in water mixtures. Association constants (Ka) were determined by fitting the change in the absorbance to a 1:1 binding model using Bindfit.54 As chloride, bromide, or iodide were titrated into the solution, the absorption band at 300 nm decreased and the bands at 380 and 430 nm increased (Fig. 3), generating an isosbestic point at 345 nm. Despite the competitive nature of the polar solvent, and possible competitive halogen bonding between the C-I donor and sulfonyl oxygen, receptor 1·PF6 binds Cl−, Br−, and I− with Ka values of 940, 690, and 3900 M−1, respectively (Table 1).
Fig. 3.
UV-Vis spectroscopic titration of 1·PF6 with TBA+I− in wet DMSO (1.4% water w/w) at a concentration of 30 μΜ. Inset shows the absorption binding isotherm upon addition of TBA+I− at 430 nm.
Table 1.
Calculated association constants (Ka) for 1-PF6 in DMSO (1.4% water w/w) obtained by fitting to a 1:1 host-guest model in Bindfit.54
| Guest | Ka (M−1)a |
|---|---|
| Cl− | 940 |
| Br− | 690 |
| I− | 3900 |
Values represent an average of three UV-Vis titrations at a host concentration of 30 μM. Anions added as tetrabutylammonium (TBA+) salts in wet DMSO. Error is less than ±15%.
As expected from the bidentate binding shown in the solid-state, 1·PF6 exhibited the greatest affinity for I− over the other halide anions, with a Ka three times larger than that of the more basic Cl− guest. This preference is attributed to the size of the binding pocket coupled with the constraints of the near-180° XB bonding angle. Crystal structures for 1·Cl and 1·Br demonstrated monodentate binding of the smaller Cl− and Br− anions outside of the pocket. To effectively bind inside the pocket would reqiure a greater deviation from the 180° bonding angle thus generating repulsion from the electron belt surounding the σ-hole. The larger sized ionic radius of I− allows the host to better coordinate it using both XB donors while still maintaining nearly 180° angles from both of the R–I···X− positions, making I− clearly the best fit for the binding pocket.54 Overall, given the pocket dimensions and required bite angles for the target anion to XB donors, I− is the most effectively coordinated between the two XB donors in the scaffold. These structural features as well as possible solvation effects result in the observed binding preferences.
In conclusion, we have synthesized a new ditopic 3,5-bis((2-iodophenyl)ethynyl)pyridinium receptor 1+ with XB anion recognition sites that showcase the utility of the electron-withdrawing methanesulfonyl group to polarize XB donors. Crystal structures revealed both an external monodentate and internal bidentate binding model for Cl− and I−, respectively. These differences in binding modes can be attributed to the receptor’s rigid architecture and large pocket size coupled with the highly directional 180° XB interactions that make the binding pocket more suitable for the larger I− anion. This is supported by binding data that revealed an inherent selectivity for I− over the more basic, yet smaller Cl− and Br− anions. These attributes suggest the XB-based anion-binding receptor 1·PF6 will open new avenues for anion detection in highly competitive media.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01-GM087398. This work was also supported by the Bradshaw and Holzapfel Research Professorship in Transformational Science and Mathematics to DWJ. We thank the US National Science Foundation (CHE-1625529) for support in the form of an instrumentation grant. The authors thank Joshua A. Barker for collecting a mass spectrum reported in the SI of this paper.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic Supplementary Information (ESI) available: synthetic methods and characterization, UV titration data and method, computational data, and x-ray crystallography. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Cavallo G, Metrangolo P, Milani R, Pilati T, Priimagi A, Resnati G and Terraneo G, Chem. Rev 2016, 116, 2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown A and Beer PD, Chem. Commun 2016, 52, 8645. [DOI] [PubMed] [Google Scholar]
- 3.Lim JYC and Beer PD, Chem 2018, 4, 731. [Google Scholar]
- 4.Alessandra DS, Alessandra F, Rosalba L, Pierangelo M, Tullio P and Giuseppe R, Chem. Eur. J 2003, 9, 3974.12916124 [Google Scholar]
- 5.Metrangolo P, Pilati T, Terraneo G, Biella S and Resnati G, CrystEngComm 2009, 11, 1187. [Google Scholar]
- 6.Mele A, Metrangolo P, Neukrich H, Pilati T, and Resnati G, J. Am. Chem Soc 2005, 127, 14972. [DOI] [PubMed] [Google Scholar]
- 7.Caballero A, White NG and Beer PD, Angew. Chem. Int. Ed 2011, 50, 1845. [DOI] [PubMed] [Google Scholar]
- 8.Sarwar MG, Dragisic B, Sagoo S and Taylor MS, Angew. Chem. Int. Ed 2010, 49, 1674. [DOI] [PubMed] [Google Scholar]
- 9.Sarwar MG, Dragisic B, Salsberg LJ, Gouliaras C, Taylor MS, J. Am. Chem. Soc, 2010, 132, 1646. [DOI] [PubMed] [Google Scholar]
- 10.Zapata F, Caballero A, Molina P, Alkorta I and Elguero J, J. Org. Chem, 2014, 79, 6959. [DOI] [PubMed] [Google Scholar]
- 11.Riel AMS, Decato DA, Sun J, Massena CJ, Jessop MJ, and Berryman OB, Chem. Sci, 2018, 9, 5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benz S, Macchione M, Verolet Q, Mareda J, Sakai N and Matile S, J. Am. Chem. Soc, 2016, 138, 9093. [DOI] [PubMed] [Google Scholar]
- 13.Lim JYC, Marques I, Thompson AL, Christensen KE, Félix V, and Beer PD, J. Am. Chem. Soc, 2017, 139, 3122. [DOI] [PubMed] [Google Scholar]
- 14.Garrett GE, Carrera EI, Seferos DS and Taylor MS, Chem. Commun, 2016, 52, 9881. [DOI] [PubMed] [Google Scholar]
- 15.Qiu J, Unruh DK and Cozzolino AF, J. Phys. Chem. A, 2016, 120, 9257. [DOI] [PubMed] [Google Scholar]
- 16.Metrangolo P and Resnati G, Chem. Eur. J, 2001, 7, 2511. [DOI] [PubMed] [Google Scholar]
- 17.Metrangolo P, Neukirch H, Pilati T and Resnati G, Acc. Chem. Rev, 2005, 38, 386. [DOI] [PubMed] [Google Scholar]
- 18.Legon AC, Chem. Eur. J, 1998, 4, 1890. [Google Scholar]
- 19.Legon AC, Angew. Chem. Int. Ed 1999, 38, 2686. [DOI] [PubMed] [Google Scholar]
- 20.Beale TM, Chudzinski MG, Sarwar MG and Taylor MS, Chem. Soc. Rev, 2013, 42, 1667. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Riel AMS and Berryman OB, New J. Chem, 2018, 42, 10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarwar MG, Dragisić B, Dimitrijević E, and Taylor MS, Chem. Eur. J, 2013, 19, 2050. [DOI] [PubMed] [Google Scholar]
- 23.Pang X and Jin WJ, New J. Chem, 2015, 39, 5477. [Google Scholar]
- 24.Robertson CC, Perutz RN, Brammer L and Hunter CA, Chem. Sci, 2014, 5, 4179. [Google Scholar]
- 25.Langton MJ, Serpell CJ and Beer PD, Angew. Chem. Int. Ed, 2016, 55, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langston MJ, Robinson SW, Marques I, Félix V and Beer PD, Nat. Chem, 2014, 6, 10393. [DOI] [PubMed] [Google Scholar]
- 27.Jentzsch AV, Pure Appl. Chem, 2015, 87, 15. [Google Scholar]
- 28.Wilcken R, Zimmermann MO, Lange A, Joerger AC and Boeckler FM, J. Med. Chem, 2013, 56, 1363. [DOI] [PubMed] [Google Scholar]
- 29.Massena CJ, Riel AMS, Neuhaus GF, Decato DA and Berryman OB, Chem. Commun, 2015, 51, 1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark JF, Clark DL, Whitener GD, C Schroeder N and Strauss SH, Environ. Sci. Technol, 1996, 30, 3124. [Google Scholar]
- 31.Barendt TA, Docker A, Marques I, Félix V and Beer PD, Angew. Chem. Int. Ed, 2016, 55, 11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith VH and Schindler DW, Trends Ecol. Evol, 2009, 24, 201. [DOI] [PubMed] [Google Scholar]
- 33.Dimitrijevic E, Kvak O and Taylor MS, Chem. Commun, 2010, 46, 9025. [DOI] [PubMed] [Google Scholar]
- 34.Massena CJ, Wageling NB, Decato DA, Rodriguez EM, Rose AM and Berryman OB, Angew. Chem. Int. Ed, 2016, 55, 12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amendola V, Bergamaschi G, Boiocchi M, Fusco N, La Rocca MV, Linati L, Lo Presti E, Mella M, Metrangolo P and Miljkovic A, RSC Adv, 2016, 6, 67540. [Google Scholar]
- 36.Caballero A, White NG and Beer PD, Angew. Chem. Int. Ed, 2011, 50, 1845. [DOI] [PubMed] [Google Scholar]
- 37.Cametti M, Raatikainen K, Metrangolo P, Pilati T, Terraneo G and Resnati G, Org. Biomol. Chem, 2012, 10, 1329. [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty S, Dutta R and Ghosh P, Chem. Commun, 2015, 51, 14793. [DOI] [PubMed] [Google Scholar]
- 39.40Tepper R, Schulze B, Jäger M, Friebe C, Scharf DH, Görls H and Schubert US, J. Org. Chem, 2015, 80, 3139. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz-Botella S, Vidossich P, Ujaque G, Peris E and Beer PD, RSC Adv, 2017, 7, 11253. [Google Scholar]
- 41.47Lim JYC and Beer PD, New J. Chem, 2018, 42, 10489. [Google Scholar]
- 42.Dumele O, Wu D, Trapp N, Goroff N and Diederich F, Org. Lett, 2014, 16, 4722. [DOI] [PubMed] [Google Scholar]
- 43.Bauzá A, Quinonero D, Frontera A and, Deyà PM, Phys. Chem. Chem. Phys, 2011, 13, 20371. [DOI] [PubMed] [Google Scholar]
- 44.Hansch C, Leo A and Taft RW, Chem. Rev, 1991, 91, 165. [Google Scholar]
- 45.Carroll CN, Naleway JJ, Haley MM and Johnson DW, Chem. Soc. Rev, 2010, 39, 3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engle JM, Carroll CN, Johnson DW and Haley MM, Chem. Sci 2012, 3, 1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vonnegut CL, Tresca BW, Johnson DW and Haley MM, Chem. Asian J 2015, 10, 522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng J-B, Gong J-L and Wu X-F, RSC Adv, 2014, 4, 29273. [Google Scholar]
- 49.Sonogashira K, Tohda Y and Hagihara N, Tetrahedron Lett, 1975, 16, 4467. [Google Scholar]
- 50.CCDC 1868183–1868185 and 1875924 contain the supplementary crystallographic data for these compounds. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre. For additional x-ray crystallography experimental details see the SI.
- 51.Carroll CN, Berryman OB, Johnson CA, Zakharov LN, Haley MM and Johnson DW, Chem. Commun, 2009, 2520. [DOI] [PubMed] [Google Scholar]
- 52.Metrangolo P, Pilati T, Terraneo G, Biella S and Resnati G, CrystEngComm, 2009, 1187. [Google Scholar]
- 53.Shannnon R, Acta Crystallogr., Sect. A, 1976, A32, 751. [Google Scholar]
- 54. http://app.supramolecular.org/bindfit/
- 55.Kilah NL, Wise MD and Beer PD, Cryst. Growth Des, 2011, 11, 4565. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.