Abstract
Objective:
Anxiety is common in pediatric chronic pain and is related to a higher risk for poor outcomes; thus, there is a need for effective clinical screening methods to identify youth with chronic pain and co-occurring anxiety. The Screen for Child Anxiety Related Disorders (SCARED) is a validated measure that defines clinically significant anxiety using the traditional clinical cut-off, but in pain populations, may fail to screen in youth with subclinical anxiety that may also be at increased risk. Two studies aimed to devise a clinically meaningful approach to capture anxiety severity in pediatric chronic pain.
Methods:
Study 1 (n=959) and Study 2 (n=207) were completed at two separate pediatric pain clinics, where the SCARED was administered along with measures of disability, activity limitations, pain intensity, quality of life, and pain catastrophizing. Groups with different levels of anxiety were compared on clinical outcomes via multivariate analyses of variance (MANOVAs) or independent samples T-tests.
Results:
A tertile solution suggested the following anxiety groupings based on the SCARED: minimal (0–12), subclinical (13–24), and clinical (≥25). Across both studies, the tertile solution was generally superior in classifying different levels of pain-related outcomes.
Discussion:
Future directions include testing the utility of this anxiety classification system to identify youth with subclinical levels of anxiety for early intervention focused on both pain and anxiety management.
Keywords: Anxiety, Pediatric, Chronic Pain, Functional Disability, SCARED
Anxiety [1], affects up to two thirds of youth with chronic pain [2–4], can be persistent [5], and is associated with increased functional disability [6–9] over the long term [5]. High anxiety may also correspond to higher levels of pain [6]. In general, youth with chronic pain and comorbid anxiety may function poorly regardless of pain intensity [7, 10], experience poorer health-related quality of life [7, 8, 11, 12] and higher pain catastrophizing [8, 11]. Anxiety also attenuates responsiveness to pain coping interventions [13]. Although it is important to identify anxiety in youth with chronic pain, a comprehensive assessment is impractical in most medical settings. Therefore, an efficient anxiety screening method is needed.
The Screen for Child Anxiety Related Disorders (SCARED), is a brief, psychometrically validated measure of child anxiety [14, 15] used in pediatric pain populations [11]. Higher SCARED scores are related to greater functional disability [6], pain intensity [6], pain catastrophizing [11], and poorer quality of life [11] in pediatric chronic pain samples. Although the SCARED has a traditional cut-off (score ≥ 25) to indicate clinical anxiety [14, 15], which has been shown to be related to greater pain intensity and functional disability in pediatric functional abdominal pain [6], this cut-off has not been tested for youth with a variety of chronic pain conditions.
It is important to identify a clinically meaningful classification of anxiety in the context of pediatric chronic pain, as co-occurring anxiety may increase the risk for adverse pain-related outcomes, even if anxiety levels fall within the subclinical range in the current classification system. Prior efforts have been made to develop clinically relevant indicators of symptom severity in pediatric chronic pain [16, 17], specifically for functional disability [18] and child pain catastrophizing [19], suggesting that three symptom levels (e.g., mild, moderate, severe), determined by calculating the percent of individuals within different score distributions, appropriately categorize patients when compared with four symptom level categories. However, it is unknown if there is a clinically meaningful way to categorize anxiety symptoms beyond presence or absence of clinical anxiety to understand risk for pain-related impairment in pediatric chronic pain.
The goal was to develop a clinically meaningful anxiety classification system for pediatric chronic pain using data from 2 large pediatric pain treatment centers. Consistent with clinical cut-offs developed for other measures [16, 17], we predicted that three levels of anxiety would correspond to progressively poorer outcomes and tested this against the traditional two-level cut-off and a four level classification.
Materials and Methods
Participants
Participants were youth with chronic pain who completed an evaluation at one of two multidisciplinary pediatric pain management centers located within Midwestern children’s hospitals in the United States. Study 1 consisted of 959 patients presenting to one pediatric chronic pain clinic. Study 2, consisted of 207 patients at the second pain clinic. A psychometric study of the SCARED in pediatric chronic pain was previously published based on a subsample of youth in Study 1 [11]. However, this prior investigation did not examine clinical cut-offs of anxiety. Participants were included in either study if they reported pain for at least two months during a pain clinic evaluation between December 2009 and October 2014, and were between 8 and 18 years of age. Patients were excluded if they had a significant developmental delay that affected their ability to complete measures or if they were unable to comprehend English. Patients were referred from a variety of medical settings (e.g., rheumatology, gastroenterology, primary care), usually if pain symptoms and associated disability failed to resolve following treatment in these settings.
Procedure
As part of the standard multi-disciplinary pain clinic evaluation at both sites, patients completed clinical assessment measures including demographic information, average pain intensity ratings, and clinical impairment measures (e.g., functional disability, quality of life). In Study 1, all measures were obtained as part of usual care. In Study 2, select measures were administered if patients agreed to participate in a larger IRB-approved research project. The majority of patients were willing to complete the measures, and >55% ultimately completed study materials (e.g., non-completion was namely due to limited time available during the medical visit). There were no significant gender or age differences between those who completed the measures and those who did not complete the measures. IRB approval was obtained at both sites to analyze the data.
Measures
Unless otherwise indicated, the following measures were collected in both studies.
Demographic information.
Demographic information was collected from the electronic medical record (EMR) including sex, age, race, ethnicity as well as information regarding the duration and location of pain.
Anxiety.
The Screen for Child Anxiety Related Disorders, Child Report (SCARED) is a widely used instrument to assess for the presence of clinically significant anxiety symptoms in youth based on the DSM-IV-TR [21] anxiety disorder diagnoses. Of note, the recent revisions in The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-V) included minimal changes with regard to the criteria for the anxiety disorders assessed with the SCARED [1]; thus, we believe the measure remains an appropriate indicator of clinically significant anxiety. The SCARED is validated for use in children between the ages of 8 and 18 years [14, 15], and in pediatric pain samples [11]. Patients report the frequency of their anxiety symptoms over the past three months on a 41-item measure with responses including “Not True,” “Sometimes True,” and “Often True”. Total scores range from 0–82, with higher scores reflecting greater levels of anxiety. The presence of clinically significant anxiety is traditionally defined as a total score ≥25, which is the traditional cut-off point based on psychometric research indicating optimal sensitivity and specificity [14, 15] for clinically anxious youth. The internal consistency for the total score of the SCARED for both studies (i.e., 1 and 2) was 0.94
Average pain intensity.
Average pain intensity in the past week was collected via patient self-report using a 0–10 numeric rating scale [22].
Pain Catastrophizing.
The Pain Catastrophizing Scale, Child Version (PCS), contains 13 items related to the child’s thoughts and feelings about pain when the child is in pain [19]. Response items range from not at all (0) to mildly (1), moderately (2), severely (3), and extremely (4). Total scores range from 0 to 52, with higher scores reflecting greater pain catastrophizing. The PCS includes three clinical reference points: low (0–14), moderate (15– 25), and high (26 and greater) [17]. The PCS internal consistencies were .94 and .92 for Studies 1 and 2, respectively.
Quality of Life.
The Pediatric Quality of Life Inventory (PedsQL) is a youth self-report measure of health-related quality of life (HRQOL) [23]. The PedsQL 4.0 is a 23-item measure and has been validated for use with children and adolescents aged 8–18 years in both community and pediatric settings [23]. Age appropriate versions were used for children (8–12) and adolescents (13–18). The total score ranges from 0 to 100, with higher scores indicating better HRQOL. For this investigation, the total score was used. This measure was collected in Study 1 only, with internal consistency at α = 0.90.
Pain-related Disability.
Study 1.
The Child Activity Limitations Questionnaire (CALQ) is a 21-item youth self-report measure of functional disability [24]. The respondent reports how difficult 21 activities are due to pain on a 6-point Likert scale ranging from 0 (Not at all difficult) to 5 (Extremely difficult). Total score range from 0 to 105. The CALQ is a questionnaire based on of the Child Activity Limitations Interview [25] and has demonstrated good internal consistency (α = .91), and construct and discriminant validity as a self-report questionnaire [24]. The scores of participants who omitted four or fewer items were adjusted upward based on the total score of completed items and number of omitted items (Adjusted Total Score = [Total Score]*[21/(21-Number of Items Omitted)]) [26]. Data with four or more missing items were considered invalid. The internal consistency for this measure was α = 0.95.
Study 2.
The Functional Disability Inventory (FDI) [18] is a 15-item, self-report instrument assessing a child’s perception of his or her difficulty completing common daily activities because of pain. It has good evidence of psychometric validity and reliability [27, 28], has been used in multiple pediatric pain populations, and has limited clinician burden in terms of length, administration, scoring, and interpretation [28]. Items are scored on a 5-point Likert scale, ranging from 0 to 4 (“No Trouble” to “Impossible”). Total scores range from 0 to 60. Higher scores indicate greater functional disability. Clinical cut-off scores were developed to represent no/minimal (≤12), moderate (13–29), and severe (≥30) levels of disability [16]. The internal consistency for the FDI was α = 0.88. Prior research has shown the FDI and CALQ are significantly correlated [26].
Data Analytic Approach
Statistical analyses were accomplished using IBM SPSS Version 23[29] and Mplus 7.4. This investigation was conducted on pediatric pain patients who completed the SCARED; missing data on individual SCARED items was found to be less than 0.007% of items. Analyses to test proposed classification models for the SCARED (i.e., tertile, quartile and traditional cut point groupings) were based on data from the sample in Study 1. Descriptive statistics on patient demographics (age, race, sex; see Table 1), pain characteristics (duration and location; see Table 2), and clinical measures (anxiety, disability, pain intensity, pain catastrophizing, quality of life; see Table 3) were computed. Tertile and quartile groups were compared using Multivariate Analyses of Variance (MANOVAs) using MLR estimation in Mplus 7.4 in order to determine the clinical utility of the reference points. These analyses was conducted separately for each study. Missing data were handled using maximum likelihood parameter estimation [30]. Specifically, demographic (age, gender, race) and theoretical (pain location, duration, anxiety severity indices) variables were included in all analyses as auxiliary correlates of missing data. All possible post-hoc follow-up pairwise comparisons were conducted for these analyses, and Type-1 error inflation was controlled using the False Discovery Rate (FDR) technique [31], which has been shown to have superior Type-1 error rates and statistical power balance when compared to Bonferroni. Independent samples T-tests were used to examine the traditional cut-off developed for the SCARED in relation to the outcome variables.
Table 1.
Demographic characteristics
| Study 1 (n=959) | Study 2 (n=207) | |||
|---|---|---|---|---|
| m | sd | m | sd | |
| Age (8–18 years) | 14.03 | 2.43 | 14.20 | 2.63 |
| Sex | N | % | N | % |
| Male | 281 | 29.3 | 48 | 23.2 |
| Female | 678 | 70.7 | 159 | 76.8 |
| Race | ||||
| White | 762 | 79.5 | 181 | 87.4 |
| African-American | 84 | 8.8 | 9 | 4.3 |
| Hispanic | 49 | 5.1 | 2 | 1.0 |
| Asian | 2 | 0.2 | 3 | 1.4 |
| Native American | 3 | 0.1 | 0 | 0 |
| More than one race | 41 | 4.3 | 5 | 2.4 |
| Other | 13 | 1.4 | 1 | 0.5 |
| Not reported | 5 | 0.5 | 6 | 2.9 |
Note. There was a significantly greater proportion of White participants in Study 2 as compared with Study 1 (Chi-square = 6.87, p < 0.01).
Table 2.
Pain characteristics by study
| Study 1 (n=959) | Study 2 (n=207) | ||||
|---|---|---|---|---|---|
| Pain Location | n | % | n | % | |
| Head | 392 | 40.9 | 7 | 3.4 | |
| Extremities | 168 | 17.5 | 20 | 9.7 | |
| Back | 126 | 13.1 | 61 | 29.5 | |
| Abdominal | 126 | 13.1 | 47 | 22.7 | |
| Joint | 14 | 1.5 | 36 | 17.4 | |
| Generalized | 26 | 2.7 | 22 | 10.6 | |
| Other | 54 | 5.7 | 7 | 3.5 | |
| Pain Duration | |||||
| 2–3 months | 102 | 10.6 | 23 | 11.1 | |
| 3–6 months | 180 | 18.8 | 27 | 13.0 | |
| 7–11 months | 102 | 10.6 | 29 | 14 | |
| 1–3 years | 346 | 36.1 | 65 | 31.4 | |
| More than 3 years | 203 | 21.2 | 42 | 20.3 | |
Note. For Study 2, generalized pain was diagnosed as fibromyalgia; For Study 2, other pain included chest wall (n=4), pelvic (n=2), shoulder only (n=1); For Study 1, other pain locations included chest (26), jaw (n=2), and missing (n=38); In Study 1, pain duration was not reported for 26 individuals; In Study 2, pain duration was not reported for 21 individuals. There was significantly greater head pain (chi-squared = 106.24, p<0.0001), extremity pain (chi-squared = 7.66, p < 0.01), and back pain (chi-squared =34.02, p<0.0001) in Study 1. There was significantly greater abdominal pain (chi-squared = 12.43, p<0.001), joint pain (chi-squared = 103.99, p < 0.0001), and generalized pain (chi-squared = 26.99, p< 0.0001) in Study 2. There were more participants with a 3–6 month pain duration in Study 1 (chi-square = 3.92, p < 0.05).
Table 3.
Means and correlations of study measures
| M(SD) | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Study 1 (N=959) | |||||||
| 1. Average Pain (0–10) | 6.5(2.0) | 1.00 | |||||
| 2. Pain Catastrophizing (0–52) | 24.9(12.8) | .37* | 1.00 | ||||
| 3. Activity Limitations (0–105) | 40.2(22.7) | .41* | .45* | 1.00 | |||
| 4. Quality of Life (0–92) | 58.2(17.7) | −.35* | −.48* | −.76* | 1.00 | ||
| 5. Anxiety (0–82) | 20.4(14.5) | .18* | .56* | −.50* | .30* | 1.00 | |
| Study 2 (N=207) | |||||||
| 1. Average Pain (0–10) | 5.7(1.9) | 1.00 | |||||
| 2. Pain Catastrophizing (0–52) | 26.7(11.1) | .41* | 1.00 | ||||
| 3. Functional Disability (0–60) | 24.5(11.7) | .48* | .42* | 1.00 | |||
| 4. Anxiety (0–82) | 23.8(14.8) | .05 | .47* | .22* | 1.00 | ||
Note.
p<0.01; (x-y) = range. There was a statistically significant difference between Studies 1 and 2 in anxiety levels (t(1164) = −3.01, p<0.001) and pain intensity (t(1142) = 4.84, p<.001).
Selection of SCARED cut-offs
There are two main methods which can be used to determine clinical cut-offs for a patient reported outcome measures [32]. These include anchor-based approaches (e.g., sensitivity and specificity analyses such as the receiver operating characteristic (ROC) curves) and distribution-based approaches, the latter of which categorizes the approach used in the current investigation. Distribution approaches compare the change in measure scores to some measure of variability, such as the standard deviation (SD). This approach is considered more statistically rigorous than using anchor-based approaches such as ROC curves (which utilizes arbitrary cut-offs, and requires a dichotomous response variable).
A seminal paper [33] detailing a widely used distribution-based method found that optimal cut points to classify pain intensity are those that best predict different levels of functional interference. This paper tested different boundaries of mild, moderate, and severe against each other using ANOVAs, and the largest F ratio for the between category effect was considered optimal [34]. Such an approach has been used by other researchers [35, 36]. Thus, a similar approach was employed in the current investigation. Our approach differs slightly as we have compared 3 distinct groups akin to “mild” “moderate” and “severe” to 2 and 4 groups respectively, to determine if the three group solution was statistically superior in predicting differences between the outcome measures.
Quartile and tertile groups of the SCARED scores were developed based on the distributions of scores from the mean from Study 1 (See Figure 1), a large sample and thus well-powered for determining cut-off scores. This method is considered optimal for use in a larger sample size that is less prone to variability [32], and is consistent with previously used methods to determine clinical reference points [16, 17] of functional disability [18] and child pain catastrophizing [19]. Clinically meaningful reference points for the SCARED were evaluated by examining differences in clinical outcomes (i.e., functional disability, activity limitations, pain, quality of life, and pain catastrophizing) using quartile and tertile groups, in addition to examining differences in clinical outcomes based on the traditional clinical cut-off developed for the SCARED. Clinical utility was defined as significant differences in clinical outcome measures across the different anxiety severity groups.
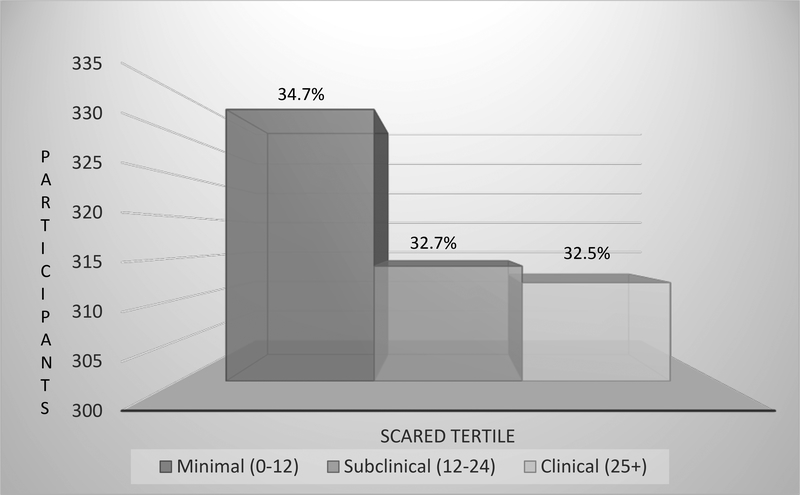
Figure 1.
Distribution of SCARED scores based of minimal, subclinical, and clinical anxiety categories.
Post Hoc Sensitivity Analyses [14, 15]. In line with prior research [33], MANOVAs were used to compare the sensitivity of different cut-offs for the optimal solution developed in the current investigation. Specifically, differences in cut-off scores that reflected at least a 5% score difference were statistically compared. This approach has been used to examine SCARED score differences in prior literature [37].
Results
Study 1
Clinical Characteristics
The sample was predominantly female (70%) and Caucasian (80%) with a mean age of 14 years (See Table 1). The majority of the sample experienced head pain, followed by pain of the extremities, back, and abdomen (Table 2). For more than half the sample, pain duration was one year or longer. The sample was generally categorized by moderate levels of pain, activity limitations, anxiety, and pain catastrophizing, and lower than normative quality of life (see Table 3) [23]. Higher levels of anxiety were significantly correlated with higher levels of pain intensity, activity limitations, pain catastrophizing, and a poorer quality of life. Age was significantly and negatively correlated with anxiety levels with a younger age being associated with a higher level of anxiety (r = −.07, p < 0.05), although the size of the effect and the amount of shared variance (r2 = .0049) was small. Females had higher levels of anxiety (M = 21.81, SD = 14.68) compared with males (M = 17.15, SD = 13.34; t (957) = −4.78, p < 0.001).
Clinical reference points
Mean differences in outcome measures for the tertile solution are reported in Table 4. Across tertiles indicating minimal (0–12), subclinical (13–24), and clinical (25+) anxiety based on the SCARED, the distribution was as follows: minimal, n = 333 (34.7%); subclinical, n = 314 (32.7%); and clinical, n = 312 (32.5%). There were significant differences between all anxiety groups on pain catastrophizing, health-related quality of life, and activity limitations (Table 5). Significant differences in pain intensity were found between the clinical anxiety group and the minimal and subclinical anxiety groups.
Table 4.
Pain outcomes by minimal, subclinical, and clincial anxiety
| Clinical Outcome M(SD) | Minimal (0–12) | Subclinical (13–24) | Clinical (25+) | |
|---|---|---|---|---|
| Study 1 (N=959) | ||||
| Pain Intensity | 6.2(1.97) | 6.3(2.07) | 6.9(1.85) | |
| Pain Catastrophizing | 17.6(11.4) | 25.5(11.1) | 33.0(10.2) | |
| Quality of Life | 66.3(16.2) | 58.3(16.0) | 48.1(15.8) | |
| Activity Limitations | 36.4(24.4) | 43.8(23.4) | 51.3(22.7) | |
| Study 2 (N=207) | ||||
| Pain Intensity | 5.3(1.94) | 6.2(1.87) | 5.7(1.8) | |
| Pain Catastrophizing | 19.4(10.8) | 26.6(9.7) | 31.4(9.8) | |
| Functional Disability | 21.1(12.2) | 25.9(11.3 ) | 25.8(11.2 ) | |
Note. SCARED = Screen for Child Anxiety and Related Disorders.
Table 5:
Differences between tertile groups on clinical outcomes
| Clinical Outcome | Group Comparisons | Estimate | SE | Wald Z | P | Cohen’s d |
|---|---|---|---|---|---|---|
| Study 1 (N=959) | ||||||
| Pain Intensity | Clinical v. Subclinical | 0.71 | 0.16 | 4.47 | <.001 | 0.36 |
| Subclinical v. Minimal | 0.11 | 0.16 | 0.70 | 0.49 | - | |
| Clinical v. Minimal | 0.82 | 0.15 | 5.40 | <.001 | 0.43 | |
| Pain Catastrophizing | Clinical v. Subclinical | 8.22 | 0.85 | 9.65 | <.001 | 0.77 |
| Subclinical v. Minimal | 8.07 | 0.88 | 9.19 | <.001 | 0.73 | |
| Clinical v. Minimal | 16.29 | 0.86 | 18.98 | <.001 | 1.50 | |
| Quality of Life | Clinical v. Subclinical | −11.74 | 1.25 | −9.38 | <.001 | 0.75 |
| Subclinical v. Minimal | −7.07 | 1.25 | −5.65 | <.001 | 0.44 | |
| Clinical v. Minimal | −18.82 | 1.28 | −14.75 | <.001 | 1.16 | |
| Activity Limitations | Clinical v. Subclinical | 8.42 | 1.8 | 4.68 | <.001 | 0.38 |
| Subclinical v. Minimal | 6.87 | 1.84 | 3.74 | <.001 | 0.29 | |
| Clinical v. Minimal | 15.29 | 1.84 | 8.29 | <.001 | 0.66 | |
| Study 2 (N=207) | ||||||
| Pain Intensity | Clinical v. Subclinical | 0.53 | 0.31 | 1.69 | 0.09 | - |
| Subclinical v. Minimal | 1.12 | 0.38 | 2.98 | <.0.01 | 0.58 | |
| Clinical v. Minimal | 0.59 | 0.33 | 1.78 | 0.07 | - | |
| Pain Catastrophizing | Clinical v. Subclinical | 5.75 | 1.85 | 3.11 | <0.01 | 0.57 |
| Subclinical v. Minimal | 6.34 | 2.18 | 2.91 | <0.01 | 0.59 | |
| Clinical v. Minimal | 12.09 | 1.81 | 6.67 | <.001 | 1.20 | |
| Functional Disability | Clinical v. Subclinical | 0.25 | 2.00 | 0.13 | 0.90 | - |
| Subclinical v. Minimal | 6.07 | 2.36 | 2.57 | <0.05 | 0.51 | |
| Clinical v. Minimal | 5.82 | 2.00 | 2.90 | <0.01 | 0.52 | |
False Discovery Rate (FDR) Type-1 error control was used for all pairwise comparisons.
For quartiles, the anxiety groups were defined as follows, with higher numbers indicating increasing levels of anxiety: I (score range 0–9), n = 247 (25.8%), II (10–17), n = 236 (24.6%); III (18–29), n = 253 (26.4%), and IV (30+), n = 223 (23.3%). The results showed fewer differences between the quartile groups as compared to the tertile groups (See Table 6). For pain intensity, analyses failed to differentiate group I from groups II and III. For activity limitations, groups II and III failed to significantly differ. Pain catastrophizing and quality of life continued to significantly differ across all four anxiety groups.
Table 6:
Difference between quartile groups on clinical outcomes
| Clinical Outcome | Group Comparisons | Estimate | SE | Wald Z | p | Cohen’s d |
|---|---|---|---|---|---|---|
| Study 1 (N=959) | ||||||
| Pain Intensity |
IV v. III IV v. II IV v. I III v. II III v. I II v. I |
0.49 0.89 0.82 0.40 0.33 0.07 |
0.18 0.19 0.18 0.19 0.18 0.19 |
2.76 4.74 4.64 2.13 1.87 0.39 |
<0.01 <.001 <.001 0.03 0.06 0.69 |
0.25 0.45 0.43 0.20 - - |
| Pain Catastrophizing |
IV v. III IV v. II IV v. I III v. II III v. I II v. I |
7.50 11.90 19.39 4.40 11.88 7.48 |
0.95 1.01 0.94 1.02 0.95 1.02 |
7.93 11.75 20.53 4.33 12.52 7.37 |
<.001 <.001 <.001 <.001 <.001 <.001 |
0.73 1.10 1.91 0.39 1.13 0.68 |
| Quality of Life |
IV v. III IV v. II IV v. I III v. II III v. I II v. I |
−12.06 −15.36 −23.29 −3.29 −11.22 −7.93 |
1.42 1.46 1.48 1.39 1.41 1.45 |
−8.50 −10.54 −15.79 −2.36 −7.95 −5.47 |
<.001 <.001 <.001 0.02 <.001 <.001 |
0.78 0.99 1.46 0.21 0.71 0.50 |
| Activity Limitations |
IV v. III IV v. II IV v. I III v. II III v. I II v. I |
10.12 10.65 19.37 0.53 9.26 8.73 |
2.05 2.14 2.18 2.03 2.07 2.15 |
4.93 4.98 8.91 0.26 4.48 4.05 |
<.001 <.001 <.001 0.79 <.001 <.001 |
0.46 0.47 0.83 - 0.40 0.37 |
| Study 2 (N=207) | ||||||
| Pain Intensity |
IV v. III IV v. II IV v. I III v. II III v. I II v. I |
0.54 0.02 0.31 0.52 0.85 0.33 |
0.33 0.38 0.39 0.39 0.40 0.45 |
1.64 0.06 0.79 1.33 2.12 0.75 |
0.10 0.95 0.42 0.18 0.03* 0.46 |
− - - - - |
| Pain Catastrophizing |
IV v. III IV v. II IV v. I III v. II III v. I II v. I |
3.91 9.56 13.51 5.65 9.61 3.95 |
1.70 1.89 2.33 1.91 2.35 2.48 |
2.30 5.07 5.80 2.97 4.10 1.59 |
0.02* <0.001 <0.001 <0.01 <0.001 0.11 |
− 0.98 1.31 0.60 0.95 - |
| Functional Disability |
IV v. III IV v. II IV v. I III v. II III v. I II v. I |
2.93 4.64 7.57 1.71 4.64 2.92 |
1.95 2.43 2.35 2.41 2.33 2.74 |
1.50 1.91 3.23 0.71 1.99 1.07 |
0.13 0.05 <0.01 0.48 0.05 0.29 |
− - 0.66 - - - |
False Discovery Rate (FDR) Type-1 error control was used for all pairwise comparisons;
revealed non-significant after FDR correction.
When using the traditional cut-off score for the SCARED (which corresponded exactly to the “clinical” anxiety cut-off based on the tertile classification above), n = 312 (32.5%) of the participants were defined as clinically anxious. Clinically significant anxiety was found to be significantly associated with higher levels of pain catastrophizing (t (945) = −16.48, p < .001), pain intensity (t (939)= −5.72, p < .001), poorer quality of life (t (953) = 12.69, p < .001), and more activity limitations (t (848)=−7.30, p < .001).
Study 2
Clinical Characteristics
The sample demographics were similar to Study 1, with the majority of the sample identified as female (>75%) and Caucasian (>85%; See Table 1). Participants were 14.2 years on average. The most common pain subtypes were back, abdominal, and joint (See Table 2). Half the sample reported a pain duration of one year or more. Similar to Study 1, the sample was categorized by moderate levels of pain, disability, anxiety, and pain catastrophizing (see Table 3). Higher levels of anxiety were significantly correlated with higher levels of functional disability and pain catastrophizing, but not with pain intensity (Table 3). Anxiety levels were not significantly related to age (r = −.08, p = .29). Males (M = 20.17, SD = 14.27) and females (M = 24.88, SD = 14.79) had comparable levels of anxiety, (t (205) = −1.95, p = 0.052).
Clinical reference points
Using the same tertile SCARED reference points as calculated in Study 1, the anxiety groups in Study 2 were distributed as follows: minimal, n = 53 (25.6%); subclinical, n = 56 (27.1%); and clinical, n = 98 (47.3%). As in Study 1, MANOVAs revealed significant differences across anxiety groups (see Table 5). Specifically, there were significant differences between all anxiety groups on pain catastrophizing, and between minimal and subclinical groups on pain intensity, with results in the expected direction (i.e., groups categorized by higher levels of anxiety reported higher levels of catastrophizing and pain intensity). Significant differences were also found between the minimal group as compared with the subclinical and clinical groups on functional disability.
The following is the distribution for the anxiety quartile groups: I (score range 0–9), n = 36 (17.4%), II (10–17), n = 43 (20.8%), III (18–29), n = 59 (28.5%), and IV (30+), n = 69 (33.3%). Significant differences were found in pain catastrophizing and functional disability across some of the anxiety categories, but pain intensity was not significantly different between groups. Specifically, group comparisons revealed fewer significant differences between the quartile groups as compared to the tertile groups (See Table 6). For pain intensity, analyses revealed that all anxiety groups failed to differentiate. Functional disability only differed in terms of group I versus IV. For pain catastrophizing, groups I and II and groups III and IV failed to differentiate.
Using the traditional clinical cut-off for the SCARED (≥25), n = 98 (47.3%) of the sample met criteria for clinically significant anxiety. Clinically significant anxiety was found to be significantly associated with higher levels of pain catastrophizing (t (197) = −5.66, p < .001). However, the associations between clinically significant anxiety and pain intensity and pain-related disability were not significant.
Results Summary
Based on the results, the tertile solution may be a superior method for detecting clinically meaningful changes across pain-related outcome measures as compared to the quartile solution and the traditional cut-off of the SCARED.
Post Hoc Sensitivity Analysis Results
MANOVAs were used to compare the sensitivity of different minimal versus subclinical cut-offs for the tertile solution developed in the current investigation (See Table 7). To compare SCARED cut-offs, a 3-point range above (scores of 15 versus 16) and below (scores of 9 versus 10), the established minimal and subclinical tertile cut-off determined in the current investigation (scores of 12 versus 13) was used. This 3-point difference corresponds to at least a 5% score difference, which has been used for comparison in prior literature [37]. The minimal versus subclinical tertile cut-off developed using the distribution-based method detailed in the Methods section above performed superiorly to the cut-offs >5% above and below these values.
Table 7.
Sensitivity Analysis of Minimal versus Subclinical Tertile Cut-off Scores
| Minimal and subclinical cut-off SCARED scores | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Original (12 versus 13) | >5% (15 versus 16) | <5% (9 versus 10) | |||||||
| Z | mean diff | p | Z | mean diff | p | Z | mean diff | p | |
| Study 1 | |||||||||
| Pain | −0.66 | −0.11 | 0.511 | −0.60 | −0.10 | 0.548 | −0.08 | −0.01 | 0.937 |
| PCS | −9.16 | −8.05 | 0.000 | −8.04 | −7.47 | 0.000 | −10.07 | −8.84 | 0.000 |
| QOL | 5.64 | 7.05 | 0.000 | 4.58 | 6.06 | 0.000 | 6.63 | 8.56 | 0.000 |
| CALQ | −3.74 | −6.88 | 0.000 | −2.83 | −5.45 | 0.005 | −4.40 | −8.45 | 0.000 |
| Study 2 | |||||||||
| Pain | −2.94 | −1.10 | 0.003 | −2.42 | −1.02 | 0.009 | −1.48 | −0.60 | 0.138 |
| PCS | −3.52 | −7.04 | 0.000 | −3.80 | −8.05 | 0.000 | −2.69 | −6.10 | 0.007 |
| FDI | −2.66 | −6.20 | 0.008 | −2.26 | −6.16 | 0.009 | −2.06 | −4.99 | 0.040 |
Note. Bold values indicate the greatest Z score across the three groups.
The original minimal/subclinical cut-off developed in this study had the largest Wald Z score values in 3 out of 7 analyses conducted for both pain (Study 1 and 2) and FDI, suggesting utility across both studies. While the cut-off that was 5% lower also had the largest Wald Z scores in 3 out of 7 analyses (for pain catastrophizing [Study 1], quality of life, and the CALQ), significant results were only found in Study 1. Lastly, the 5% higher cut-off had a greater Wald Z score value in only 1 of out of 7 instances, for pain catastrophizing (Study 2). Of note, sensitivity analyses were not conducted to compare different subclinical versus severe cut-offs because this traditional cut-off has already been established as a significant indicator of clinical anxiety
Discussion
Findings from two investigations of youth with a variety of pain conditions demonstrate the utility of identifying a clinically meaningful classification of anxiety in pediatric chronic pain patients. Support for use of a three group classification emerged as the most clinically meaningful approach to categorize anxiety using the SCARED. The conventional cut-off (presence or absence of clinically significant anxiety) captures a significant proportion (32.5% to 47.3%) of youth that may be at risk for adverse outcomes. Thus, the inclusion of the “subclinical” category allows for identification of additional youth with moderate levels of anxiety who may nevertheless be adversely affected by their condition. Individuals falling into the “subclinical” category (i.e., SCARED score of 13–24) would not otherwise have been identified as being at increased risk using the traditional clinical cut-off (SCARED score of ≥25). Identification of a moderately anxious group allows for clinicians to take a more nuanced and individualized approach to treatment planning. Additionally, this investigation suggests that clinicians may need to take more notice of the “worrier” or modestly anxious child as even modest increases in anxiety symptoms appear to correspond to notable impairments in several domains of pain-related functioning.
Across both studies, three cut-points on the SCARED significantly distinguished youth with chronic pain in terms of increasing severity of activity limitations/functional disability, pain catastrophizing, quality of life, and to a lesser extent, pain intensity. This is important because in using the traditional cut-off, groups failed to differ in terms of both pain intensity and functional disability (Study 2). These findings extend prior psychometric research on the utility of the SCARED in a pediatric pain sample [11] and allow clinicians the flexibility to move beyond the single clinical cut-off originally developed for the SCARED [14, 15] when evaluating youth with chronic pain.
Strengths of this investigation include use of two large pediatric samples and empirically validated clinical measures, which allows for greater generalizability to other pediatric chronic pain clinics. Generalizability of results is also strengthened by the variety of pain conditions represented across the two investigations. Another strength of these studies is the focus on child- reported anxiety symptoms (versus parent proxy measures of child anxiety or other parent-reported outcome measures) which may underrepresent children’s symptoms. The current investigation intentionally focused on child-reported anxiety because prior research suggests that children in general outpatient and pediatric chronic pain samples are generally better informants of their own anxiety in comparison to parent report [38] [6, 39]. Finally, given that pediatric chronic pain is common and youth with chronic pain present to multiple care settings (e.g., primary care, specialty care), the utility of capturing anxiety using a “minimal,” “subclinical,” and “clinical” distinction may be broadly useful across medical settings, though further testing is needed.
Limitations include some differences in clinical profiles and measures used across the investigations. Pain subtype composition differed across studies. For example, there was a higher proportion of youth with headache in Study 1, which is important to note because youth with headaches may experience less disability than youth with other chronic pain conditions [40]. The differences in representation of pain subtypes across studies may be because different institutions have different referral patterns which may result in these differences. Although measures of anxiety, pain, and pain-related catastrophizing were consistent across the two studies, functional impairment due to pain was collected using different measures, and data on pain-related quality of life was only collected in Study 1. Although the measures used to assess disability at both sites have each been validated in pediatric pain samples, we foresee that similar issues may arise at other pain clinic sites, where the SCARED and other clinic-specific outcome measures are not uniformly administered, potentially impacting future generalizability of these classifications. The SCARED holds promise as a useful instrument in clinical settings given that it is clinically meaningful, corresponds to poorer pain-related outcomes, and is also significantly associated with response to behavioral treatment for pediatric chronic pain [13]. It may also assist medical providers less familiar with the nuances of anxiety to differentiate the level of symptoms necessary for referral for behavioral interventions.
Additionally, while also an important measure shown to be related to functional impairment in pediatric chronic pain [12], it should be noted that pain catastrophizing assesses a specific form of anxiety (catastrophic cognitions regarding pain) and is correlated with anxiety but is not necessary nor sufficient to capture the more global nature of anxiety disorders [12]. So, clinics collecting one but not the other may not be fully capturing the full spectrum of anxiety symptoms.
In recent years, there has been increasing interest in creating national registries of pain conditions to enhance large multi-site investigations with a common core of brief patient-reported outcomes. Although the SCARED is relatively brief and feasible to use in clinical settings, it may still be somewhat burdensome in the context of a larger registry. One direction for future research would be to create a briefer version of the SCARED using only the most informative and discriminating items (e.g., via item response theory) and examine whether the instrument performs better in a clinical setting than the newer and more unidimensional assessments of anxiety (e.g., the PROMIS pediatric anxiety scale [41]) in identification of risk for poor outcomes.
Future directions may also include using anxiety cut-offs based on the SCARED to inform more tailored or stratified behavioral intervention approaches to better treat pediatric chronic pain. For example, a child with minimal anxiety levels may benefit from pain-focused psychoeducation and a brief preventative intervention that can potentially be administered during a medical clinic visit. On the other hand, those with subclinical levels of anxiety may require intervention with a behavioral specialist that jointly addresses anxiety and pain. Those with clinical levels of anxiety may require longer and a more intensive cognitive-behavioral interventions that specifically target reduction in anxiety symptoms along with other pain management efforts. Development of such nuanced algorithms for treatment referral can provide the best dose of treatment for the magnitude and severity of clinical symptoms. In addition, it will be important to evaluate the predictive validity of the tertile solution of the SCARED in future longitudinal studies.
In summary, it is important to recognize and address anxiety in youth with chronic pain, as even subclinical levels of anxiety can have significant negative consequences with regard to pain and functioning in youth. The utility of the three group categorization of the SCARED to capture anxiety may be clinically useful for identification of individuals at risk for poorer outcomes, and may ultimately inform the development of tailored behavioral interventions for youth with chronic pain.
Acknowledgments
Preparation of this paper was supported in part by NIH grants # K23 AT009458–01A1, a training grant awarded to the first author (Cunningham), and # K24 AR056687, a midcareer mentorship award to the last author (Kashikar-Zuck). There are no conflicts of interest.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed). Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
- 2.Dufton LM, Dunn MJ, Compas BE. Anxiety and somatic complaints in children with recurrent abdominal pain and anxiety disorders. J Pediatr Psychol 2009;34:176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knook LM, Konijnenberg AY, van der Hoeven J, et al. Psychiatric disorders in children and adolescents presenting with unexplained chronic pain: What is the prevalence and clinical relevancy? Euro Child Adoles Psychiatry 2011;20:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashikar-Zuck S, Parkins IS, Graham TB, et al. Anxiety, mood, and behavioral disorders among pediatric patients with juvenile fibromyalgia syndrome. Clin J Pain 2008;24:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulvaney S, Lambert EW, Garber J, et al. Trajectories of symptoms and impairment for pediatric patients with functional abdominal pain: A 5-year longitudinal study. J Am Acad Child Adolesc Psychiatry 2006;45:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham NR, Cohen MB, Farrell MK, et al. Concordant parent-child reports of anxiety predict impairment in youth with functional abdominal pain. Journal of Pediatr Gastroenterol Nutr 2014;60:312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen LL, Vowles KE, Eccleston C. The impact of adolescent chronic pain on functioning: Disentangling the complex role of anxiety. J Pain 2010;11:1039–1046. [DOI] [PubMed] [Google Scholar]
- 8.Benore E, D’Auria A, Banez GA, et al. The influence of anxiety reduction on clinical response to pediatric chronic pain rehabilitation. Clin J Pain 2015;31:375–83. [DOI] [PubMed] [Google Scholar]
- 9.Wendland M, Jackson Y, Stokes LD. Functional disability in paediatric patients with recurrent abdominal pain. Child Care Health 2010;36:516–23. [DOI] [PubMed] [Google Scholar]
- 10.Simons LE, Sieberg CB, Claar RL. Anxiety and functional disability in a large sample of children and adolescents with chronic pain. Pain Res Manag 2012;17:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jastrowski Mano KE, Evans JR, Tran ST, et al. The Psychometric Properties of the Screen for Child Anxiety Related Emotional Disorders in Pediatric Chronic Pain. J Pediatr Psychol 2012;37:999–1011. [DOI] [PubMed] [Google Scholar]
- 12.Tran ST, Jastrowski Mano K, Hainsworth KR, et al. Distinct influences of anxiety and pain catastrophizing on functional outcomes in children and adolescents with chronic pain. Journal of Pediatr Psychol 2015:1–10. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham NR, Jagpal A, Tran ST, et al. Anxiety Adversely Impacts Response to Cognitive Behavioral Therapy in Children with Chronic Pain. J Pediatr 2016;171:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birmaher B, Brent D, Chiappetta L, et al. Psychometric properties of the Screen for Anxiety Related Emotional Disorders (SCARED): A replication study. J Am Acad Child Adolesc Psychiatr 1999;38:1230–1236. [DOI] [PubMed] [Google Scholar]
- 15.Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatr 1997;36:545–553. [DOI] [PubMed] [Google Scholar]
- 16.Kashikar-Zuck S, Flowers S, Claar R, et al. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain 2011;152:1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pielech M, Ryan M, Logan D, et al. Pain catastrophizing in children with chronic pain and their parents: proposed clinical reference points and reexamination of the Pain Catastrophizing Scale measure. Pain 2014;155:2360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol 1991;16:39–58. [DOI] [PubMed] [Google Scholar]
- 19.Crombez G, Bijttebier P, Eccleston C, et al. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain 2003;104:639–646. [DOI] [PubMed] [Google Scholar]
- 20.Hyams JS, Di Lorenzo C, Saps M, et al. Childhood Functional Gastrointestinal Disorders: Child/Adolescent. Gastroenterol 2016;150:1456–1468.e2. [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed). Washington, DC: American Psychiatric Publishing, 2000. [Google Scholar]
- 22.von Baeyer CL, Spagrud LJ, McCormick JC, et al. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. PAIN 2009;143:223–227. [DOI] [PubMed] [Google Scholar]
- 23.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 1999;37:126–39. [DOI] [PubMed] [Google Scholar]
- 24.Hainsworth KR, Davies WH, Khan KA, et al. Development and preliminary validation of the child activity limitations questionnaire: flexible and efficient assessment of pain-related functional disability. J Pain 2007;8:746–52. [DOI] [PubMed] [Google Scholar]
- 25.Palermo TM, Witherspoon D, Valenzuela D, et al. Development and validation of the Child Activity Limitations Interview: a measure of pain-related functional impairment in school-age children and adolescents. Pain 2004;109:461–70. [DOI] [PubMed] [Google Scholar]
- 26.Palermo TM, Lewandowski AS, Long AC, et al. Validation of a self-report questionnaire version of the Child Activity Limitations Interview (CALI): The CALI-21. Pain 2008;139:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain 2006;121:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palermo TM, Long AC, Lewandowski AS, et al. Evidence-based assessment of health-related quality of life and functional impairment in pediatric psychology. J Pediatr Psychol 2008;33:983–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Released IC IBM SPSS Statistics for Windows, Version 23.0. IBM Corp; Armonk, NY, 2015. [Google Scholar]
- 30.Enders CK. Applied missing data analysis. New York: Guilford, 2010. [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Rl Stat Soc Series B Methodol 1995:289–300. [Google Scholar]
- 32.Copay AG, Subach BR, Glassman SD, et al. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J 2007;7:541–6. [DOI] [PubMed] [Google Scholar]
- 33.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 1995;61:277–84. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava MS, Carter EM. An introduction to applied multivariate statistics. North-Holland, 1983. [Google Scholar]
- 35.Hirschfeld G, Zernikow B. Variability of “optimal” cut points for mild, moderate, and severe pain: Neglected problems when comparing groups. Pain 2013;154:154–159. [DOI] [PubMed] [Google Scholar]
- 36.Hirschfeld G, Zernikow B. Cut points for mild, moderate, and severe pain on the VAS for children and adolescents: what can be learned from 10 million ANOVAs? Pain 2013;154:2626–2632. [DOI] [PubMed] [Google Scholar]
- 37.Caporino NE, Sakolsky D, Brodman DM, et al. Establishing Clinical Cutoffs for Response and Remission on the Screen for Child Anxiety Related Emotional Disorders (SCARED). J Am Acad Child Adolesc Psychiatry 2017;56:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosi S, Canals J, Hernandez-Martinez C, et al. Parent-child agreement in SCARED adn its relationsip to anxiety symptoms. J Anxiety Disord 2010;24:129–133. [DOI] [PubMed] [Google Scholar]
- 39.Tran ST, Jastrowski Mano KE, Anderson Khan K, et al. Patterns of anxiety symptoms in pediatric chronic pain as reported by youth, mothers, and fathers. Clin Pract in Pediatr Psychol 2016;4:51. [Google Scholar]
- 40.Kashikar-Zuck S, Goldschneider KR, Powers SW, et al. Depression and Functional Disability in Chronic Pediatric Pain. Clinical J Pain 2001;17:341–349. [DOI] [PubMed] [Google Scholar]
- 41.DeWalt DA, Gross HE, Gipson DS, et al. PROMIS® pediatric self-report scales distinguish subgroups of children within and across six common pediatric chronic health conditions. Qual Life Res 2015;24:2195–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]