Abstract
Background/Objective:
In population studies, most individuals with mild cognitive impairment (MCI) do not progress to dementia in the near term, but rather remain stable MCI or revert to normal cognition. Here, we characterized MCI subgroups with different outcomes over 5 years.
Setting/Participants:
A population-based cohort (N=1603).
Measurements:
Clinical Dementia Rating (CDR); self-reported medical conditions, subjective cognitive concerns, self-rated health, depressive symptoms, blood pressure, medications, blood pressure, APOE genotype, cognitive domain composite scores.
Design:
We compared 3 MCI subgroups who progressed to dementia (n=86), stabilized at MCI (n=384), or reverted to normal (n=252), to those who remained consistently normal (n=881), defining MCI as CDR = 0.5 and dementia as CDR≥1. Using multinomial logistic regression models adjusted for demographics, we examined the associations of each group with selected baseline characteristics.
Results.
With the normal group for reference, worse subjective cognitive concerns, functional impairments, self-rated health, and depressive symptoms were associated with being in any MCI group. Taking more prescription medications was associated with being in the stable MCI and reverter groups; diabetes and low diastolic blood pressure were associated with stable MCI. The APOE4 genotype was associated with stable and progressive MCI; stroke was associated with progressive MCI. All MCI subgroups had lower mean composite scores in all cognitive domains and more operationally defined impairments in attention, language, and executive function; reverters lacked memory and visuospatial impairments.
Conclusions:
MCI subgroups with different 5-year outcomes had some distinct characteristics suggesting different underlying causes. The progressors, unlike the reverters, had a profile broadly typical of Alzheimer’s disease; the stable MCIs had other, including vascular, morbidity. These data shed light on the heterogeneity of MCI in the population.
Keywords: Aging, Epidemiology, Cognition
INTRODUCTION
Mild cognitive impairment (MCI) is an intermediate, but not necessarily transitional, cognitive state between normal cognition and dementia. 1–3 In specialty clinics, the majority of individuals meeting diagnostic criteria for MCI progress to dementia within a relatively short time. In the community setting, MCI is more heterogeneous; the majority remains stable at the MCI level, a minority progresses to dementia, while another minority reverts to normal. 4–8 Previous studies addressed the characteristics of whose MCI progressed to dementia, with features typically resembling the Alzheimer’s disease (AD) profile: older age, prominent memory loss, and the APOE4 genotype. 9–12 Those whose MCI reverts to normal cognition lack these characteristics 10, 12 although in some studies they too eventually progress to dementia.9 However, the largest group of people with MCI, those who remain stably mildly impaired, have received less attention. 13 Knowing the characteristics associated with likelihood of MCI progression can be helpful to clinicians in treatment planning.
We investigated, at the community level, the five-year outcomes of MCI: what proportions of people with MCI progressed to dementia, remained at stable MCI, and reverted to normal, and what baseline characteristics distinguished the MCI subgroups from one another and from those who remained consistently normal. Specifically, we identified their distinctive demographic, health, and cognitive characteristics, and their eventual dementia and mortality rates, compared to the normal group.
METHODS
The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) is a cohort participating in a population-based, prospective study of cognitive impairment and dementia. It comprised 1982 individuals recruited from 2006–2008 by age-stratified random sampling from the publicly available voter registration list for a group of contiguous small towns in southwestern Pennsylvania. Eligibility criteria included age 65+ years, non-institutionalized, lacking severe sensory impairment, and having decisional capacity. Details of sampling, recruitment, and assessment have been reported previously. 14–16 Participants were reassessed annually. All study procedures were approved by the University of Pittsburgh Institutional Review Board. All participants provided written informed consent.
Exposure variables.
The assessments included, but were not limited to, demographic characteristics (age, sex), education (high school graduate HSG, < HSG, >HSG), self-report of diagnoses by a health care provider, prescription and nonprescription drug intake, self-rated general health, subjective cognitive concerns,17 depression symptoms,18, 19 independence in instrumental activities of daily living (IADLs), lifestyle factors (exercise, smoking, alcohol use) and APOE genotype, as well as a brief focused physical and neurological examination. 14, 16, 20
The neuropsychological assessment comprised tests tapping the cognitive domains of attention/processing speed, memory, executive, language, and visuospatial functions. For each participant we created a standardized composite score for each domain by first standardizing each individual test score and then calculating the mean of all the standardized scores in that domain.15
Classification and Outcome variables.
We rated participants on their everyday independent cognitively-driven activity using the Clinical Dementia Rating (CDR)21 where CDR=0 is normal, CDR=0.5 is mild cognitive impairment, and CDR=1, 2, and 3 represent mild, moderate, and severe dementia. We used the CDR definition of MCI, which is independent of neuropsychological data, so that we could evaluate baseline cognitive data as predictors of different MCI outcomes. We assigned participants CDR ratings at study entry and each annual assessment. Excluding individuals with prevalent dementia (CDR >=1) at study entry, we identified four groups: consistently normal (CDR=0 at up to 10 annual visits), stable MCI (CDR=0.5 for five consecutive visits), reverting/fluctuating MCI (CDR reverts from 0.5 to 0, including any who then return to 0.5, within five visits), and progressed to dementia (CDR=0.5 progresses to CDR ≥1 within five visits).
For each MCI participant, we designated as the “index visit” the first visit at which the CDR was 0.5. We then measured outcomes over the subsequent 5-year observation period (5 annual visits). Thus, different MCI participants were followed for different 5-year calendar periods, depending on the dates of their index visits. MCI participants’ index visits serve as their “baseline;” the normal group’s baseline visits were their initial (study entry) visits. We compared the baseline characteristics of 3 MCI subgroups with those of the normal group and with one another. We also compared the reverters who remained at CDR=0 with those who returned to CDR=0.5 (fluctuators). In post-hoc analyses, we addressed attrition during the observation period, incident dementia after the observation period, and mortality.
Statistical Methods
Descriptive statistics.
To compare baseline characteristics, overall and across groups, we created indicator variables for self-rated health (poor or fair vs. good or excellent), IADL impairment (any vs. none), depressive symptoms ( >3 vs. ≤3, the 90th percentile of the MYHAT cohort), subjective cognitive concerns (any vs. none), prescription medicines (>3 vs. ≤3, a median split), APOE4 genotype (E*4 carrier vs. non-carrier); history of stroke/TIA, heart disease, and diabetes; current smoking, alcohol consumption, and physical exercise, systolic (>130 vs. ≤130 mm Hg) and diastolic (>70 vs. ≤70 mm Hg ) blood pressures. We examined cognitive domain composite scores, both as continuous variables (standardized z scores), and categorized as impaired/unimpaired at the threshold of 1.5 standard deviations (SD) below the appropriate mean.
Multivariable Models.
We fit multinomial logistic regression models treating membership in all four groups as the outcome variables, with the normal group as reference, to assess the effect of each baseline characteristic on the likelihood of belonging to each group, adjusting for demographics.
Attrition:
We examined the proportions lost to follow-up in each group during 5-year followup, and the average ages at which individuals left the study. We fit Cox proportional hazard models with time to dropout as the outcome, adjusting for demographics. As sensitivity analyses, we repeated the multinomial logistic regression models excluding those who were lost before completing 5 followup years.
Fluctuation:
We compared the reverters who remained at CDR=0 with those reverters (fluctuators) who returned to CDR= 0.5 during the 5 years.
Subsequent outcomes:
In post-hoc analyses, we examined the occurrence of incident dementia (CDR ≥ 1) and mortality beyond 5 years.
We used Stata 15.0 and R 3.4.3 for all analyses.
Additional methodological details are provided in Supplemental Text S1.
RESULTS
Of the 1982 individuals who met all eligibility criteria at study entry, 1413 (71.3%) were rated CDR=0; 546 (27.6%) were rated CDR=0.5. We excluded the following individuals: 23 with prevalent dementia; 258 with only initial assessments; 93 with no follow-up assessments after the index visit; and 5 who progressed directly from CDR=0 to CDR ≥1 without being observed at a 0.5 stage. We report here the data from the remaining 1603 individuals.
We identified four groups: Consistently normal (n=881); Reverter /fluctuator MCI (n=252; of these, 81 individuals fluctuated back to CDR=0.5 during the 5 years); Stable MCI (n=384); Progressed to dementia (n=86).
Participants were followed on average for 6.93 years; normal, reverter, stable MCI, and progressor groups were followed on average for 7.05, 8.02, 6.05, and 6.43 years. For all analyses reported in this section, except for the pairwise comparisons, all reported results compare each MCI subgroup to the consistently normal group.
Sample description (Unadjusted comparisons)
We found significant overall differences across the 4 groups in several variables in unadjusted analyses of baseline characteristics (Table 1). We followed up the significant overall differences with pairwise unadjusted comparisons (Supplemental Table S1).
Table 1.
Characteristics of entire sample and of MCI subgroups
| Characteristic at “baseline” * | Total N |
All participants N=1603 |
Consistently normal participants N=881 |
Reverters from MCI to normal N=252 |
Stable MCI N=384 |
Progressors from MCI to dementia N=86 |
Overall Kruskal-Wallis, chi-square or Fisher’s exact test: P value |
|
|---|---|---|---|---|---|---|---|---|
| Age | Mean (SD) | 1603 | 77.7 (7.6) | 75.2 (7.0) | 77.5 (7.0) | 82.3 (6.9) | 83.6 (5.7) | 0.0001 |
| Sex | % female | 1603 | 62.0 | 62.1 | 58.7 | 64.1 | 61.6 | 0.605 |
| Education | % < HS | 1603 | 13.0 | 8.3 | 12.7 | 20.3 | 29.1 | |
| % =HS | 44.8 | 44.6 | 45.2 | 47.40 | 33.7 | <0.001 | ||
| % >HS | 42.2 | 47.1 | 42.1 | 32.3 | 37.2 | |||
| Any subjective memory concerns (SMC) | % >0 | 1600 | 70.8 | 51.4 | 96.0 | 94.5 | 93.0 | <0.001 |
| Number of SMC | Median (IQR) | 1600 | 2 (4) | 1 (2) | 4 (2) | 4 (3) | 5 (4) | 0.0001 |
| Self-rated health | % Poor + fair | 1603 | 17.1 | 11.8 | 23.4 | 24.2 | 20.9 | <0.001 |
| Any IADL impairments | % >0 | 1603 | 19.6 | 8.2 | 21.0 | 38.3 | 48. | <0.001 |
| Depressive symptoms (>3) | % >3 | 1603 | 8.1 | 4.7 | 12.3 | 11.5 | 15.1 | <0.001 |
| APOE*4 genotype | % E*4 | 1478 | 20.9 | 19.2 | 21.4 | 21.9 | 34.2 | 0.1 |
| Systolic blood pressure (SBP) | % >130 | 1603 | 51.1 | 51.8 | 48.0 | 51.0 | 53.5 | 0.727 |
| Diastolic blood pressure (DBP) | % >70 | 1603 | 59.6 | 64.7 | 61.9 | 49.0 | 47.7 | <0.001 |
| H/o Stroke/TIA | % yes | 1603 | 11.8 | 8.5 | 12.3 | 18.2 | 22.1 | <0.001 |
| H/o Heart attack | % yes | 1603 | 36.6 | 35.3 | 39.3 | 37.8 | 36.1 | 0.646 |
| H/o Diabetes | % yes | 1603 | 22.2 | 20.4 | 20.2 | 27.3 | 23.3 | 0.044 |
| Current Smoking | % current | 1603 | 6.9 | 8.5 | 6.4 | 3.4 | 7.0 | 0.011 |
| Current Alcohol consumption | % current | 1603 | 80.4 | 88.3 | 79.4 | 66.7 | 62.8 | <0.001 |
| Number of prescription medications | Median (IQR) | 1603 | 4 (4) | 3 (4) | 5 (4) | 5 (5) | 4 (3) | 0.0001 |
| Exercise | % any | 1603 | 59.6 | 63.2 | 56.8 | 55.5 | 48.8 | 0.006 |
Baseline for Stable Normal group is study entry (assessment 1). Baseline for MCI groups is the “index visit” at which the participant was first classified as MCI (CDR=0.5)
SMC: Subjective Memory Concerns
IADL: Instrumental Activities of Daily Living
Mean and SD, or median and interquartile range, were compared for continuous variables, and percentages for categorical variables. To detect overall group differences, Kruskal-Wallis rank tests were performed for continuous predictors when the assumptions for one-way ANOVA were not met. Chi-square tests were performed for categorical predictors, and Fisher’s Exact Tests were conducted when sample size was small.
In unadjusted analyses of baseline cognitive functioning, all 3 MCI groups had significantly lower average composite scores in all domains than the normals and were significantly less likely to meet operational criteria for cognitive impairment in most domains (Supplemental Table S2).
Demographic, Health, and Lifestyle Comparisons Adjusted for Demographics (multivariable models)
In multinomial logistic regression models, for each independent variable (covariate), we fit a single model for all three MCI groups simultaneously, adjusting for demographics. Each relative risk ratio (RRR) indicates the effect size for a given covariate’s association with each MCI group compared to the normal group.
For example, in Table 2, all three MCI groups had significantly more subjective cognitive concerns than the normals, with RRRs of 22.58, 16.32, and 12.79 for the reverters, stable MCI, and progressors; i.e., the reverters had the strongest association with subjective concerns, followed by stable MCIs and progressors.
Table 2.
Multinomial Logistic regression comparing MCI subgroups to normal group, adjusting for demographics
| Reverters vs. Consistently Normal |
Stable MCI vs. Consistently Normal |
Progressor vs. Consistently Normal |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| RRR | SE | p-value | RRR | SE | p-value | RRR | SE | p-value | |
| >0 Subjective cognitive concerns | 22.58 | 7.46 | <0.001 | 16.33 | 4.02 | <0.001 | 12.79 | 5.64 | <0.001 |
| Number of subjective concerns | 2.52 | 0.13 | <0.001 | 2.73 | 0.14 | <0.001 | 3.02 | 0.19 | <0.001 |
| Self-rated health – good/very good/excellent (ref: poor/fair) |
0.417 | 0.77 | <0.001 | 0.364 | 0.064 | <0.001 | 0.424 | 0.128 | 0.004 |
| >0 IADL impairments | 2.629 | 0.540 | <0.001 | 4.174 | 0.732 | <0.001 | 6.150 | 1.656 | <0.001 |
| ≥ 3 depressive symptoms | 2.872 | 0.728 | <0.001 | 2.557 | 0.627 | <0.001 | 3.55 | 1.283 | <0.001 |
| APOE *4 genotype | 1.221 | 0.226 | 0.28 | 1.535 | 0.263 | 0.012 | 3.062 | 0.833 | <0.001 |
| Systolic blood pressure (SBP) | 0.844 | 0.122 | 0.242 | 0.925 | 0.123 | 0.558 | 0.976 | 0.231 | 0.919 |
| Diastolic blood pressure (DBP) | 0.993 | 0.15 | 0.96 | 0.74 | 0.1 | 0.026 | 0.757 | 0.181 | 0.245 |
| Reported Stroke/TIA | 1.518 | 0.347 | 0.068 | 2.556 | 0.502 | <0.001 | 1.967 | 0.671 | 0.047 |
| Reported Heart attack | 1.088 | 0.163 | 0.573 | 0.924 | 0.128 | 0.568 | 0.826 | 0.204 | 0.438 |
| Reported Diabetes | 1.002 | 0.18 | 0.99 | 1.605 | 0.25 | 0.002 | 1.312 | 0.369 | 0.334 |
| Smoking | 0.846 | 0.244 | 0.561 | 0.615 | 0.199 | 0.133 | 1.575 | 0.731 | 0.327 |
| Alcohol | 0.543 | 0.105 | 0.002 | 0.362 | 0.06 | <0.001 | 0.306 | 0.082 | <0.001 |
| Number of Prescription medications | 1.13 | 0.03 | <0.001 | 1.15 | 0.02 | <0.001 | 1.02 | 0.04 | 0.656 |
| Exercise (any) | 0.807 | 0.119 | 0.146 | 0.884 | 0.12 | 0.366 | 0.676 | 0.161 | 0.1 |
In contrast, IADL impairment was most strongly associated with the progressor group, followed by the stable and reverter groups, all compared to the normal group.
Fair or poor self-rated health and >3 depression symptoms were about equally associated with all MCI groups; number of prescription medications was associated only with stable MCI and reverter (but not progressor) groups.
APOE*4 carrier status was associated with stable and progressor but not reverter groups. Diabetes and diastolic blood pressure (DBP) <70 mm Hg were associated only with stable MCI.
Alcohol use was negatively associated with all 3 MCI groups; smoking and exercise were associated with none.
Cognitive Characteristics of Groups, adjusted for Demographics.
In multinomial models (Table 3), we found associations with lower composite scores in all cognitive domains with all 3 MCI subgroups, with the RRRs indicating progressively stronger effects from reverter through stable to progressor groups.
Table 3.
Multinomial Logistic regression for baseline* cognitive characteristics, adjusting for demographics
| Reverter vs. Consistently Normal | Stable MCI vs. Consistently Normal | Progressor vs. Consistently Normal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RRR | SE | p-value | RRR | SE | p-value | RRR | SE | p-value | |
| Cognitive Domain Composite Scores | |||||||||
| Attention† | 0.657† | 0.069 | <0.001 | 0.563† | 0.056 | <0.001 | 0.375† | 0.065 | <0.001 |
| Executive | 0.576 | 0.067 | <0.001 | 0.344 | 0.038 | <0.001 | 0.236 | 0.04 | <0.001 |
| Language | 0.587 | 0.071 | <0.001 | 0.274 | 0.031 | <0.001 | 0.200 | 0.031 | <0.001 |
| Memory | 0.558 | 0.065 | <0.001 | 0.236 | 0.027 | <0.001 | 0.1 | 0.019 | <0.001 |
| Visuospatial | 0.783 | 0.064 | 0.004 | 0.543 | 0.048 | <0.001 | 0.421 | 0.068 | <0.001 |
| % with Cognitive Impairment by Domain | |||||||||
| Attention | 3.309 | 1.885 | 0.036 | 3.713 | 1.874 | 0.009 | 7.954 | 4.565 | <0.001 |
| Executive | 13.243 | 10.465 | 0.001 | 19.052 | 14.339 | <0.001 | 45.407 | 35.655 | <0.001 |
| Language | 2.946 | 1.297 | 0.014 | 6.049 | 2.193 | <0.001 | 12.167 | 5.199 | <0.001 |
| Memory | 2.215 | 2.033 | 0.386 | 2.619 | 0.744 | 0.001 | 5.473 | 2.043 | <0.001 |
| Visuospatial | 1.459 | 0.518 | 0.288 | 2.074 | 0.591 | 0.001 | 4.885 | 1.774 | <0.001 |
Baseline for Stable Normal group is study entry (assessment 1). Baseline for MCI groups is the assessment at which the participant was first classified as MCI (CDR=0.5)
For example, a one-unit change in the composite attention domain score multiplied the risk of reversion by 0.657, of stable MCI by 0.563, and of progression by 0.375, compared to remaining stably normal.
Comparing proportions with operationally defined impairments in attention, language, and executive functions, we found increasingly strong effects in reverter, stable MCI, and progressor groups. In the memory and visuospatial domains, the progressors had the strongest effect followed by the stable MCIs; we found no association among reverters. The executive function domain showed the largest effect sizes in all MCI groups.
Attrition.
During the 5-year observational period, in the normal, reverter, stable MCI, and progressor groups, the proportions lost to follow-up were 29.06%, 29.76%, 70.83%, and 56.98%; the corresponding mean ages at leaving the study were 80.3, 85.4, 86.8, and 87.3 years. In Cox proportional hazard models with time to dropout as the outcome, adjusting for demographics, the stable MCI and progressor groups, but not the reverters, were significantly more likely than normals (hazard ratio 2.7, 95% confidence interval 2.3–3.3 and HR 1.5, 95% CI 1.1 – 2.0) to be lost to follow-up.
In the sensitivity analysis, (models excluding those who dropped out before 5 years of follow-up), some power was lost. Effects which were attenuated and became non-significant were: the associations of APOE4 genotype, DBP, smoking, diabetes, and memory impairment with stable MCI; alcohol consumption, language impairment and attention impairment with MCI reverters; and stroke/TIA with MCI progressors. We could not assess the effect of executive function impairment as none of the normals had executive function impairment (data not shown).
Fluctuation.
Compared with the 171 reverters who remained at CDR=0, the 81 fluctuators who returned to CDR= 0.5 during the 5 years were significantly older (p=0.022), reported more subjective cognitive concerns (p=0.039), and were more likely to report history of stroke/TIA (p=0.027). They had lower scores in executive (p=0.003), memory (p=0.014), and visuospatial (p=0.003) domains, but were more likely to have impairment only in attention (p = 0.023).
Outcomes beyond 5 years.
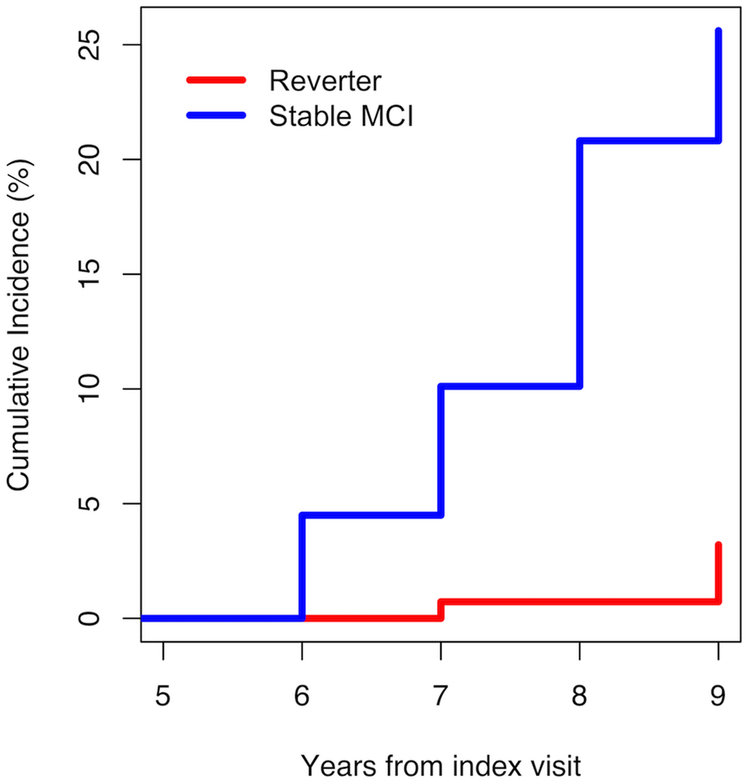
By definition, 100% of progressors developed dementia, and 0% of the normals have as yet developed dementia. Of the 171 reverters who remained at CDR=0 during the 5 years, 19 (11%) subsequently returned to CDR=0.5. Incident dementia was eventually observed in 3 (1.19%) reverters and 15 (3.91%) stable MCI cases. (Figure 1).
Figure 1.
Cumulative Incidence of Dementia In Stable MCI and Reverter Groups , Years 06–09 after Index Visit.
Mortality beyond 5 years.
Progressors have the highest proportion (44.19%) who have died thus far, with the stable, reverter, and normal groups experiencing 30.47%, 27.38%, and 21.91% mortality.
DISCUSSION
In a population-based aging cohort followed over at least 5 years, we identified a relatively small subgroup of individuals with MCI who progressed to dementia, and larger subgroups that remained stably impaired or reverted to normal. This pattern, typical of community samples in contrast to clinic samples, 4, 5, 9, 13, 22–24 has been attributed to selection factors. 6 More fundamentally, it reflects different distributions of underlying morbidities and risk factors, i.e., the heterogeneity of MCI at the population level. We identified shared as well as distinct baseline features among the three MCI groups compared with those who remained consistently normal.
As in previous studies, 9, 24 all three MCI groups were older and less well-educated than the normals, with no sex differences. The educational gradient across the four groups is consistent with lower education being an established risk factor for cognitive decline and dementia. Not unexpectedly, all three MCI groups had lower mean composite scores in all cognitive domains, and were more likely to report subjective cognitive concerns and IADL impairments. Although all MCI groups had lower mean baseline cognitive performance than normals, the reverters had the highest scores among the MCI groups, followed by the stable group and the progressors. Higher proportions of the progressor and stable MCI groups had operationally defined impairment in all domains.
The progressors’ characteristics broadly match the typical prodromal AD profile of memory impairment and APOE4 genotype, as in previous community 9, 22 and clinical studies. 10–12 They were also the only group more likely than normals to report history of stroke, consistent with evidence that combined neurodegenerative and cerebrovascular disease increases likelihood of dementia. 25, 26
In contrast, reverters resembled normals in operationally defined memory and visuospatial impairments, APOE4 genotype, and previous strokes, and had the weakest associations with cognitive and IADL impairments. However, they were the most likely to report at least one subjective cognitive concern, although the average number of concerns was fairly similar across MCI groups. Notably, the reverters who remained at CDR=0 (unlike the fluctuators who then returned to CDR=0.5) were more likely to have impaired attention, possibly reflecting transient or reversible medically or pharmaceutically derived effects. 27–31 Reverters were also more likely to take >3 prescription drugs, which might themselves contribute to reversible impairments, or reflect the presence of underlying reversible morbidities. In effect, these individuals represent potentially reversible MCI.
The stable MCI group was more likely than the normals to take prescription medications and to have diabetes and low DBP. We speculate that stable cardiovascular and metabolic abnormalities might underlie static cognitive impairment. Diabetes is a known risk factor for dementia 32 and MCI. 33 Low DBP could be attributed to factors such as medication effects, but is characteristic of heart failure which could lead to chronic hypoxemia and cerebral hypoperfusion. We found low DBP was significantly associated with history of heart failure, both overall (p=0.007) and in the stable MCI group (p=0.0001). Hypoxemia is implicated in the associations of heart failure with cognitive impairment, 34 and of chronic obstructive lung disease with MCI.35, 36 The stable MCI group was also more likely to have the APOE4 genotype which is associated not only with AD but also with hypercholesterolemia and heart disease. 37 However, unlike the progressors, those with stable MCI were not more likely than normals to report stroke, i.e., their cardiovascular disease had not led to cerebrovascular events. Notably, they experienced the highest 5-year attrition rate; in all groups, an unknown proportion of the dropouts might have evidenced dementia had they remained in the study.
With regard to established risk factors or markers of AD dementia, our results validate those of several previous community-based studies. Older age, amnestic MCI, poor memory performance, worse overall cognitive function, APOE4 genotype, and stroke are consistent predictors of MCI progression to dementia. 9, 11, 22, 24, 38, 39 In neuroimaging studies, medial temporal and global cortical atrophy, and rate of volume loss, predict progression to dementia.40 Studies of reverters show the converse. 9–12, 22, 38
However, we also investigated predictors of non-progression, and the potential roles played by factors reflecting other morbidities and lifestyle factors. The majority of individuals with MCI who remain mildly impaired, and the minority who revert to normal, may not have underlying progressive neurodegenerative or cerebrovascular processes. Yet, non-progressive impairments also have clinical and public health significance, interfering with everyday functioning, productivity, and quality of life. 41 Our data suggest that these cases of mild impairment may have causes such as diabetes and heart failure. Further, all MCI groups were significantly more likely to report depressive symptoms than normals, reflecting the well-documented relationship between depression and cognitive impairment .42–44 Here, depressive symptoms did not seem to distinguish among MCI groups, although in another study they predicted progression to dementia. 45 The cognitive impairments related to these chronic conditions may not be progressive, particularly if the underlying conditions are adequately controlled.
Our study extends the literature in comparing three distinct MCI subgroups to normal individuals over at least 5 years, and in examining variables besides neuropsychological performance and APOE genotype. Our findings regarding depression symptoms, self-rated health, prescription medications, health history, and DBP appear to be novel. While our finding of greater IADL impairment and subjective concerns in all MCI groups is as expected, the gradient of effect sizes across MCI groups is informative.
As regards strengths and limitations, our community-based assessments are less intensive than those in clinical research settings and lack neuroimaging; however, our ability to compare people with MCI to cognitively normal individuals from the same population is an advantage over research clinics that rely on highly selected volunteer or referred subjects. Variations in findings - for example, only about 1% of the reverters and 4 % of the stable MCI have as yet developed incident dementia in our study - can result from varying MCI definitions9, 22, 24 and statistical approaches. 13 The large size and population-based nature of our study cohort, and 5-year follow-up, longer than others, 46 reinforce both external and internal validity. Our study cohort, representing the older population of this study area, is largely of European descent; it should be replicated in other racial/ethnic groups.
MCI subgroups with different 5-year outcomes have some shared and some distinct characteristics. Knowing these relationships can help clinicians with early identification of prognostic subgroups so that the likely progressors and non-progressors can be targeted for appropriate interventions. All individuals with MCI need not assume that they will progress to dementia, nor should they all be subjected to the same therapeutic strategies. Despite our field’s current understandable focus on early detection of progressive dementing diseases, we should not ignore the clinical and public health significance of potentially reversible or stable MCI, which together make up a substantial proportion of mild impairment at the population level.
Supplementary Material
Unadjusted Pairwise Comparisons Between MCI Subgroups
Baseline* cognitive characteristics of MCI subgroups and normal group.
Impact Statement: We certify that this work is both novel and confirmatory of recent novel research. 9,11 The potential impact of this research on clinical care is that it describes the characteristics of individuals with mild cognitive impairment who do not progress to dementia. Clinicians need to recognize and care for these individuals understanding that their impairment may have treatable causes other than underlying neurodegenerative diseases.
ACKNOWLEDGMENTS.
The authors thank the staff and senior citizen participants in the MYHAT study, without whom the work would not exist.
Funding: The work reported here was supported in part by grants # R01AG023651, # K07AG044395, and # T32AG00181 from the National Institute on Aging, US DHHS.
Footnotes
CONFLICTS OF INTEREST. Dr. Ganguli served on the “AD Patient Journey Working Group” for Biogen, Inc., in 2016 and 2017. All other authors have no potential conflicts to disclose.
SUPPLEMENTAL TEXT FILE S1
This online file includes details of the assessment and statistical methods that are not included in the main body of the manuscript.
REFERENCES
- 1.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367:1262–1270. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. [DOI] [PubMed] [Google Scholar]
- 3.Ganguli M The unbearable lightness of MCI. Int Psychogeriatr. 2014;26:353–359. [DOI] [PubMed] [Google Scholar]
- 4.Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. [DOI] [PubMed] [Google Scholar]
- 5.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. [DOI] [PubMed] [Google Scholar]
- 7.Canevelli M, Grande G, Lacorte E, et al. Spontaneous Reversion of Mild Cognitive Impairment to Normal Cognition: A Systematic Review of Literature and Meta-Analysis. J Am Med Dir Assoc. 2016;17:943–948. [DOI] [PubMed] [Google Scholar]
- 8.Malek-Ahmadi M Reversion From Mild Cognitive Impairment to Normal Cognition: A Meta-Analysis. Alzheimer Dis Assoc Disord. 2016;30:324–330. [DOI] [PubMed] [Google Scholar]
- 9.Roberts RO, Knopman DS, Mielke MM, et al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology. 2014;82:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abner EL, Kryscio RJ, Schmitt FA, et al. Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol. 2017;81:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition: risk factors and prognosis. Neurology. 2012;79:1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park MH, Han C. Is there an MCI reversion to cognitively normal? Analysis of Alzheimer’s disease biomarkers profiles. Int Psychogeriatr. 2015;27:429–437. [DOI] [PubMed] [Google Scholar]
- 13.Aerts L, Heffernan M, Kochan NA, et al. Effects of MCI subtype and reversion on progression to dementia in a community sample. Neurology. 2017;88:2225–2232. [DOI] [PubMed] [Google Scholar]
- 14.Ganguli M, Snitz B, Vander Bilt J, Chang CC. How much do depressive symptoms affect cognition at the population level? The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatry. 2009;24:1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganguli M, Snitz BE, Lee CW, Vanderbilt J, Saxton JA, Chang CC. Age and education effects and norms on a cognitive test battery from a population-based cohort: the Monongahela-Youghiogheny Healthy Aging Team. Aging Ment Health. 2010;14:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganguli M, Chang CC, Snitz BE, Saxton JA, Vanderbilt J, Lee CW. Prevalence of mild cognitive impairment by multiple classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. Am J Geriatr Psychiatry. 2010;18:674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snitz BE, Yu L, Crane PK, Chang CC, Hughes TF, Ganguli M. Subjective cognitive complaints of older adults at the population level: an item response theory analysis. Alzheimer Dis Assoc Disord. 2012;26:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 19.Ganguli M, Gilby J, Seaberg E, Belle S. Depressive Symptoms and Associated Factors in a Rural Elderly Population: The MoVIES Project. Am J Geriatr Psychiatry. 1995;3:144–160. [DOI] [PubMed] [Google Scholar]
- 20.Ganguli M, Fu B, Snitz BE, Hughes TF, Chang CC. Mild cognitive impairment: incidence and vascular risk factors in a population-based cohort. Neurology. 2013;80:2112–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 22.Lopez OL, Becker JT, Chang YF, et al. Incidence of mild cognitive impairment in the Pittsburgh Cardiovascular Health Study-Cognition Study. Neurology. 2012;79:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unverzagt FW, Ogunniyi A, Taler V, et al. Incidence and risk factors for cognitive impairment no dementia and mild cognitive impairment in African Americans. Alzheimer Dis Assoc Disord. 2011;25:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao S, Unverzagt FW, Hall KS, et al. Mild cognitive impairment, incidence, progression, and reversion: findings from a community-based cohort of elderly African Americans. Am J Geriatr Psychiatry. 2014;22:670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nucera A, Hachinski V. Cerebrovascular and Alzheimer disease: fellow travelers or partners in crime? J Neurochem. 2018;144:513–516. [DOI] [PubMed] [Google Scholar]
- 27.Silay K, Yalcin A, Akinci S, Gursoy FG, Sener Dede D. Charlson Comorbidity Index, inappropriate medication use and cognitive impairment : Bermuda Triangle. Wien Klin Wochenschr. 2017;129:799–804. [DOI] [PubMed] [Google Scholar]
- 28.Green AR, Reifler LM, Boyd CM, Weffald LA, Bayliss EA. Medication Profiles of Patients with Cognitive Impairment and High Anticholinergic Burden. Drugs Aging. 2018;35:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell NL, Lane KA, Gao S, Boustani MA, Unverzagt F. Anticholinergics influence transition from normal cognition to mild cognitive impairment in older adults in primary care. Pharmacotherapy. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29:639–658. [DOI] [PubMed] [Google Scholar]
- 31.Richardson K, Fox C, Maidment I, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ. 2018;361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig. 2013;4:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts RO, Geda YE, Knopman DS, et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol. 2008;65:1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavery L, Vander Bilt J, Chang CC, Saxton JA, Ganguli M. The association between congestive heart failure and cognitive performance in a primary care population of elderly adults: the Steel Valley Seniors Survey. Int Psychogeriatr. 2007;19:215–225. [DOI] [PubMed] [Google Scholar]
- 35.Singh B, Mielke MM, Parsaik AK, et al. A prospective study of chronic obstructive pulmonary disease and the risk for mild cognitive impairment. JAMA Neurol. 2014;71:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Exalto LG, Biessels GJ, Karter AJ, et al. Risk score for prediction of 10 year dementia risk in individuals with type 2 diabetes: a cohort study. Lancet Diabetes Endocrinol. 2013;1:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahley RW. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl). 2016;94:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandya SY, Lacritz LH, Weiner MF, Deschner M, Woon FL. Predictors of Reversion from Mild Cognitive Impairment to Normal Cognition. Dement Geriatr Cogn Disord. 2017;43:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sachdev PS, Lipnicki DM, Crawford J, et al. Factors predicting reversion from mild cognitive impairment to normal cognitive functioning: a population-based study. PLoS One. 2013;8:e59649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macdonald KE, Bartlett JW, Leung KK, Ourselin S, Barnes J, investigators A. The value of hippocampal and temporal horn volumes and rates of change in predicting future conversion to AD. Alzheimer Dis Assoc Disord. 2013;27:168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes TF, Chang CC, Bilt JV, Snitz BE, Ganguli M. Mild cognitive deficits and everyday functioning among older adults in the community: the Monongahela-Youghiogheny Healthy Aging Team study. Am J Geriatr Psychiatry. 2012;20:836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugarman MA, Alosco ML, Tripodis Y, Steinberg EG, Stern RA. Neuropsychiatric Symptoms and the Diagnostic Stability of Mild Cognitive Impairment. J Alzheimers Dis. 2018;62:1841–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandya SY, Clem MA, Silva LM, Woon FL. Does mild cognitive impairment always lead to dementia? A review. J Neurol Sci. 2016;369:57–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Unadjusted Pairwise Comparisons Between MCI Subgroups
Baseline* cognitive characteristics of MCI subgroups and normal group.