Abstract
Despite major academic and industry efforts, Alzheimer’s disease (AD) remains the only leading cause of death for which there is no disease-modifying treatment available. Disappointing clinical trials over the last several years have led to a growing consensus on the need to intervene earlier in the disease process, prior to the onset of any clinical symptoms. However, drug development at this stage is challenging given the difficulty of assessing a therapeutic benefit in subjects who are, by definition, clinically normal. The FDA recently issued new draft guidance for trials in early AD, which revised the taxonomy of AD by recognizing four stages of the disease, including an expanded view of the predementia stage. These guidelines further advance regulatory support for clinical trials in earlier stages of AD. We will discuss the basis for this change and the impact that it may have on early intervention AD trials, as well as stimulating the need for improved biomarkers and outcome measures that will be required for a disease-modifying drug to win approval.
1. INTRODUCTION
AD is the most common cause of dementia worldwide, affecting one-third of those aged over 85 years (Masters et al, 2015). It is characterized by at least a decade-long long asymptomatic phase during which there is development of AD pathology (Selkoe and Hardy, 2016). Based on the hypothetical model of disease progression proposed by Jack et al (Jack et al, 2010), which has since been supported by empirical data (Fleisher et al, 2012, Benzinger et al, 2013), it is understood that AD-related pathological changes begin approximately 15–20 years before any symptoms are manifested (Sperling et al, 2014). Current approved treatments are palliative in nature and modestly reduce symptoms in the dementia stage by targeting neurotransmitter abnormalities that occur as sequelae of AD neuropathology. For the past decade, however, drug development efforts have turned towards disease-modification and have been strongly influenced by the two key neuropathological hallmarks of AD: extracellular deposition of beta-amyloid and the subsequent formation of intraneuronal neurofibrillary tangles. The field has focused on mechanisms of action that reduce the production of beta-amyloid within the brain or accelerate its clearance. However, virtually all interventions have been tested in cohorts with clinically evident symptoms when there is already substantial pathology present. Based on the results of these studies, it is believed that, as in the other conditions such as cancer and cardiovascular disease, we will have a greater chance for success by intervening much earlier in AD before substantial neurodegeneration has occurred. One of the key challenges with drug development for AD then, is how best to design studies that will assess efficacy when there are no outward symptoms, while adhering to regulatory (i.e. FDA) requirements for efficacy claims.
2. DEFINING PREVENTION TRIAL POPULATIONS
In 2013, the Food and Drug Administration (FDA) released draft guidance on drug development for AD. That guidance built on the understanding that AD is a progressive disease with symptoms appearing long after the AD pathophysiological process has begun and proposed a disease classification that acknowledged three stages of AD: the preclinical, prodromal and dementia stages (FDA 2013). The guidance recognized the difficulty with demonstrating both cognitive and functional benefits during the pre-clinical and prodromal stages and proposed several strategies that may enable a sponsor to demonstrate a clinical benefit resulting from disease modification. For example, they endorsed the use of continuous outcome measures, such as the Clinical Dementia Rating Sum of Boxes (CDR-SB), that capture pre-dementia decline (Aisen et al,2011); yet while such measures may permit efficient trial design, they do not eliminate the need for large trials with long duration. Moreover, while the CDR-SB may represent a useful outcome measure for subjects with MCI, it lacks sensitivity in the preclinical stages of the disease (Sperling et al, 2011).
In 2018, the US Food and Drug Administration (FDA) issued new draft guidance for trials in AD, which expanded the taxonomy of AD by recognizing four stages, specifically expanding the pre-dementia continuum (FDA, 2018). The new categories include: Stage 1: ‘Preclinical’, characteristic pathophysiologic changes of AD but no evidence of clinical symptoms; Stage 2: ‘Preclinical/Prodromal’, characteristic pathophysiologic changes of AD and subtle detectable abnormalities on sensitive neuropsychological measures, but no functional impairment; Stage 3: ‘Prodromal’, characteristic pathophysiological changes, and mild but detectable functional impairment; and Stage 4: ‘dementia’.
Recognizing that we can now reliably detect biomarker changes that indicate individuals who are on the path towards Alzheimer’s disease dementia (Donohue et al, 2018), FDA would consider cognitive endpoints in clinical trials of participants in Stage 2, rather than both cognition and function. And FDA would now consider measures of biomarker change (amyloid, tau, neurodegeneration) as endpoints in clinical trials for participants in Stage 1, particularly if a pattern of effect is seen across multiple biomarkers. The guidance document acknowledges, however, that there is not good evidence that biomarker changes will predict clinical benefit and refers to a “post-approval requirement for an additional study to confirm the predicted clinical benefit.” This new classification encourages designing clinical trials for the earliest stages of AD, i.e. preclinical AD, and perhaps even earlier, but requires the identification of a) well characterized, biomarker-confirmed participant cohorts that are in the earliest stages of AD, b) outcome measures that are sensitive to subtle and relevant cognitive changes, and c) biomarkers that are predictive of disease progression and that accurately reflect target engagement and disease modification. This new FDA guidance is consistent with that proposed by the European Medicines Agency (EMA) in September 2018. The EMA points out that ‘from a regulatory perspective, the main goal of treatment in at risk population remains prevention of cognitive impairment, since no biomarker can yet be considered a valid surrogate endpoint (EMA, 2018).
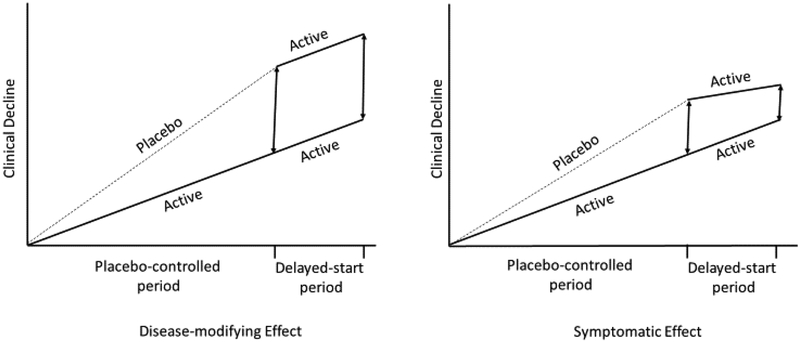
With regard to study design, the FDA guidance states ‘A randomized-start or randomized-withdrawal trial design (with clinical outcome measures) is the most convincing approach to demonstrating a persistent effect on disease course. Generally, a randomized-start design would be most appropriate for use in AD’. In the randomized-start study design, participants are randomized to drug and placebo, and at some point, placebo patients are crossed over to active treatment (Figure 1). If participants who were initially on placebo and then assigned to active treatment fail to catch up to those who received active treatment for the entire duration of the trial, a disease-modification will have been shown (Leber, 1997). This design may be a reasonable path forward, particularly as we move towards utilizing composite cognitive measures that are sensitive to subtle changes.
Figure 1. The Delayed-Start Design.
Participants are randomized to the same active treatment but starting at different times, resulting in two treatment periods: a placebo-controlled period followed by an active period. During the placebo-controlled period, participants receive either placebo or active treatment. During the delayed-start period, placebo participants are switched to active treatment and thus become delayed-start participants. Participants on active treatment during the placebo-controlled period continue to receive active treatment during the delayed-start period and are labeled as early-start participants. If the treatment difference observed at the end of the placebo-controlled period was preserved at the end of the delayed-start period (i.e. delayed-start patients do not “catch up” with the early start patients), the treatment effect is considered consistent with a disease-modifying effect.
3. BIOMARKERS FOR SAMPLE ENRICHMENT AND CONFIRMATION OF TARGET ENGAGEMENT
The role of biomarkers has become paramount in clinical drug trials for AD. Biomarkers are now widely available for the core neuropathologic features of AD, beta-amyloid plaques and tau neurofibrillary tangles. These include assays measuring cerebrospinal fluid Aβ42, total tau, and phosphorylated tau concentrations, as well as direct visualization of fibrillar amyloid and tau neurofibrillary tangle deposition using positron emission tomography. Biomarker development has been driven in part by the Alzheimer’s Disease Neuroimaging Initiative (ADNI), which set out as one of its primary objectives the validation and standardization of AD biomarkers (Weiner et al, 2015). Amyloid positivity, as measured by amyloid PET or CSF amyloid peptide measures, has become the mainstay in determining eligibility for preclinical and prodromal trials, with the resultant effect of accurately enriching the clinical trial samples for AD, but with an 80–90% screen-fail rate in preclinical studies. In addition, AD biomarkers may be useful not only for enrichment purposes but also to provide objective evidence of target engagement and disease-modifying effects (Blennow et al, 2010). Among CSF biomarkers, levels of Aβ42, total tau (ttau), and phosphorylated tau (p-tau) have been the most widely studied. The combination of low Aβ42, high t-tau, and high p-tau represents a sensitive and specific signature for prodromal AD (Blennow et al, 2015).
More recently, it has been demonstrated that progression of abnormalities on Tau PET imaging show a stronger correlation to cognitive and clinical progression of AD than do amyloid measures (Johnson et al, 2016). These findings agree with the older pathology literature, which suggests that tau tangles but not amyloid-β plaques correlate with cognition and clinical symptoms (Arriagada et al. 1992).
Finally, recent published data have identified potential blood-based biomarkers (e.g., neuronally derived exosome levels of Aβ 1–42 and phosphorylated tau,) that predict the risk for incident AD and the risk of progression (O’Bryant et al, 2017). In addition, there are now ultrasensitive measurement techniques (immuno-magnetic reduction and single-molecule array) that allow accurate analysis of blood-based biomarkers (e.g. plasma Aβ42, tau levels, neurofilament light chain (NFL)) (Mattsson et al, 2016; Mattsson et al, 2017).In one study, high-performance measurement of plasma amyloid-β biomarkers by immunoprecipitation coupled with mass spectrometry showed excellent performance in predicting brain amyloid-β burden (Nakamura et al, 2017; Ovod et al, 2017). In particular, the biomarker showed very high areas under the receiver operating characteristic curves (AUCs) with an accuracy approximately equal to 90% when compared to amyloid PET imaging and also correlated well with levels of Aβ1–42 in cerebrospinal fluid. These plasma biomarkers also have cost-benefit and scalability advantages over CSF and PET imaging techniques, potentially enabling broader clinical access and more efficient population screening.
4. IMPROVING COGNITIVE AND FUNCTIONAL OUTCOME MEASURES
Cognition may very well be the best outcome measure to predict efficacy in preclinical and prodromal AD clinical trials. The recent FDA guidance appears to endorse the view that sensitive cognitive measures may be the path forward in demonstrating efficacy in the very earliest detectable stages of AD. However, as earlier stages are selected, there will be potentially greater confounding factors including variance. To detect cognitive changes in preclinical AD, a number of sensitive composite measures have been developed (Donohue et al, 2014; Langbaum et al, 2015). The Preclinical Alzheimer’s Cognitive Composite (PACC) currently in use in the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) study, is sensitive to subtle cognitive change in preclinical AD and measures cognition across three key domains: episodic memory, executive function, and orientation. The PACC incorporates a global measure of cognition (Mini-Mental State Exam (MMSE)) with the Free and Cued Selective Reminding Test (FCSRT), Delayed Paragraph Recall, and the Digit-Symbol Substitution Test and enables the separation of subjects with and without amyloid (Donohue et al, 2014). The PACC has evolved over the past five years with improved sensitivity and specificity in detecting amyloid-related cognitive decline. The PACC was recently shown to capture decline in Aβ+ versus Aβ− clinically normal older adults within an independent study sample (Mormino et al, 2017). Most recently, a standard semantic memory measure has been integrated which adds independent information about amyloid-related cognitive decline. The PACC5, which incorporates multiple cognitive domains, appears to be effective in detecting amyloid-related cognitive decline even within the preclinical period. Although cognition can serve as a primary endpoint in trials enrolling a clinically normal population, a second measure will undoubtedly be required to demonstrate clinically meaningful results. The Cognitive Function Index (CFI) is one such measure, which assesses subjective impression of memory change (Amariglio et al, 2015).
5. ONGOING STUDIES
There are a number of ongoing secondary prevention studies that revolve around anti-amyloid mechanisms of action (Table 1).
Table 1.
Anti-Amyloid Compounds in Secondary Prevention Trials for AD
| Drug | Mechanism of Action | Phase |
|---|---|---|
| Solanezumab | Passive immunization | III |
| Crenezumab | Passive immunization | III |
| Gantenerumab | Passive immunization | III |
| Aducanumab | Passive immunization | III |
| BAN2401 | Passive immunization | II |
| CAD106 | Active immunization | II |
| Verubacestat | BACE Inhibition | Abandoned |
| Atabacestat | BACE Inhibition | Abandoned |
| Lanabacestat | BACE Inhibition | Abandoned |
| Elenbacestat | BACE Inhibition | II |
| CNP520 | BACE Inhibition | II |
BACE – Beta amyloid cleaving enzyme
5.1. Anti-amyloid Immunotherapy
The recognition of the long preclinical phase of AD has already enabled a number of secondary prevention trials. Secondary prevention in this context refers to testing interventions in individuals who have evidence that the disease process is already beginning (i.e. elevated brain amyloid) aimed at preventing the onset of symptoms and progression to the clinical stages of AD.
Solanezumab, is a humanized monoclonal IgG1 antibody directed against the mid-domain of the Aβ peptide and is being evaluated in the Dominantly Inherited Alzheimer Network Trial Unit (DIAN TU), an international, adaptive platform testing multiple drugs to slow or prevent the progression of AD in families with autosomal dominant mutations in PSEN1, PSEN2, and APP mutations (Bateman et al, 2017). The DIAN-TU trial includes 160 mutation carriers in their 30s, 40s, or 50s, who range from 15 years before to 10 years after the expected onset of symptoms. The primary outcome is the DIAN-TU Cognitive Composite, which consists of the delayed recall score from the International Shopping List Test, the Logical Memory delayed recall score from the Wechsler Memory Scale-Revised, the Digit Symbol Coding test total score from the Wechsler Adult Intelligence Scale-Revised, and the MMSE total score (Bateman et al, 2017). Solanezumab is also being tested in the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) trial (Sperling et al 2014), a multicenter, randomized, double-blind, placebo-controlled, dose-escalation, Phase III study comparing solanezumab with placebo given as infusions once every 4 weeks over 4.5 years in approximately 1,150 subjects with preclinical AD. The primary outcome of the A4 Study is rate of change on the Preclinical Alzheimer Cognitive Composite (PACC) (Donohue et al., 2014).
Crenezumab, a fully humanized immunoglobulin isotype G4 monoclonal antibody, binds to monomers and aggregated forms of Aβ with a 10-fold–higher affinity for oligomers (Adolfsson et al, 2012). Genentech has launched the CREAD Study: A Study of Crenezumab Versus Placebo to Evaluate the Efficacy and Safety in Participants With Prodromal to Mild Alzheimer’s Disease (AD), enrolling 750 individuals with prodromal AD (i.e. MCI with biomarker evidence of Aβ pathology). This trial uses change on the CDR-SB as primary outcome and a range of cognitive and functional measures as secondary outcomes using the higher dose of 60mg/kg of crenezumab. CREAD2 is a second Phase 3 clinical trial with high dose crenezumab and will recruit an additional 750 patients with prodromal or mild Alzheimer’s dementia.
Crenezumab is also being tested in the Alzheimer’s Prevention Initiative (API) trial in the large Colombian PSEN1 cohort (Tariot et al, 2018). The API APOE4 Study of CAD106 and CNP520 Versus Placebo in Participants at Risk for the Onset of Clinical Symptoms of Alzheimer’s Disease, is testing two drugs, an active immunotherapy (vaccine) CAD-106 and an oral BACE inhibitor (CNP520) and will involve more than 1,300 cognitively-healthy older adults, aged 60 to 75, at high risk of developing symptoms of Alzheimer’s as they are homozygous for apolipoprotein E4. Trial participants will receive the active immunotherapy, the oral BACE inhibitor or a placebo. The co-primary outcome measures are time to diagnosis of MCI due to Alzheimer’s Disease (AD) or dementia due to Alzheimer’s Disease and Change in the Alzheimer’s Prevention Initiative Composite Cognitive (APCC) Test Score (NCT02565511).
Gantenerumab is a fully human IgG1 antibody designed to bind with sub-nanomolar affinity to a conformational epitope on Aβ fibrils and was tested in the SCarlet RoAD, a phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group, 2-year study in prodromal AD. 797 participants were randomized to gantenerumab 105 mg or 225 mg or placebo every 4 weeks by subcutaneous injection (Ostrowitzki et al, 2017). The primary endpoint was the change from baseline to week 104 in Clinical Dementia Rating Sum of Boxes (CDR-SB) score. A futility analysis, which was performed once 50% of patients completed 2 years of treatment, led to the study being halted early due to lack of efficacy. Results from the ongoing open-label extension however, indicated that monthly injections under the skin of 900 mg and 1,200 mg gantenerumab reduced brain amyloid by up to 15 percent over six to nine months. In 2018, two phase 3 studies were launched named GRADUATE-1 (NCT03443973) and GRADUATE-2 (NCT03444870). The two multicenter, randomized, double-blind, placebo-controlled trials will enroll up to 760 participants each, to assess the efficacy and safety of high-dose gantenerumab in patients with early (prodromal to mild) Alzheimer’s disease. The primary outcome measure is change from Baseline to Week 104 in the Clinical Dementia Rating−Sum of Boxes (CDR-SOB).
Aducanumab, a high-affinity, fully human IgG1 monoclonal antibody against a conformational epitope found on Aβ, is being tested in two efficacy trials, each of which will enroll 1,350 people with MCI due to AD or mild AD as confirmed by amyloid PET. These studies follow up on a phase I study that showed robust, dose-related reduction in amyloid PET signal along with slowing of clinical progression (Sevigny et al, 2016). They will compare monthly infusions of one of two undisclosed doses of aducanumab or placebo over an 18-month treatment course; the primary outcome measures cognitive and functional decline with the CDR-SB (NCT02477800 and NCT02484547). Results from long-term follow-up of participants in this anti-amyloid treatment study showed a reduction in amyloid plaque levels in a dose- and time-dependent manner, as measured by amyloid PET while analyses of cognitive measures suggested a continued slowing of clinical decline over 48 months (CTAD 2018).
Recently, topline results were presented from the Phase II study with BAN2401, an anti-amyloid beta protofibril antibody, in 856 patients with early AD (AAIC 2018). After 18 months of treatment, participants in the highest dose arm showed slowing of progression on the Alzheimer’s Disease Composite Score (ADCOMS) along with dramatic reduction of amyloid accumulated in the brain as measured using amyloid-PET. The ADCOMS combines items from the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog), the CDR-SB scale and the MMSE (Wang et al, 2016). The results are confounded by an imbalance in ApoE4 carriers between the high dose and placebo arms but are nonetheless encouraging.
5.2. BACE Inhibition
Besides the removal of Aβ via immunotherapy, another major approach in AD therapeutic development has been to reduce production of Aβ. The Aβ peptide is cut out of APP by the sequential action of beta- and gamma-secretases (Haass et al, 2007). Recent genetic evidence shows that mutations near the betasecretase cleavage site that prevent such cleavages lead to decreased production of Aβ by approximately 40% and are protective against developing AD dementia (Jonsson et al, 2012).
The most advanced compound in clinical development was Merck’s verubecestat, a small-molecule inhibitor of beta-secretase cleaving enzymes BACE1 and BACE2. The compound was being tested in mild to moderate dementia due to AD as well as prodromal AD. Both studies ended early based on interim analyses that indicated a positive benefit/risk ratio would be unlikely. Verubecestat reduced production of Aβ42, lowering levels in cerebrospinal fluid by up to 81 percent (Egan et al 2018). Nonetheless, the compound provided no clinical benefit and plaque load was reduced by only 4 percent. This may not be surprising given that such patients have had amyloid deposition in their brains for well over a decade.
BACE inhibition was also being tested in preclinical AD as part of Janssen’s EARLY trial of atabacestat. This trial was halted when Janssen concluded that the benefit-risk ratio was no longer favorable to continue development of atabecestat for individuals with late-onset preclinical stage Alzheimer’s disease due to liver toxicity. Lanabacestat, co-developed by Eli Lilly and AstraZeneca, had a futility analysis which showed the compound would most likely miss the efficacy endpoint. This experience has raised concern that late-stage, i.e. symptomatic, AD may be too late for robust benefit from a BACE inhibitor.
Other BACE inhibitor programs continue. For example, Eisai has a Phase 3 program of elenbecestat, consisting of MISSION AD1 and AD2; each is a global trial and will compare a two-year, 50 mg once-aday course of elenbecestat to placebo in 1,330 (total 2,660 across the studies) patients age 50 to 85 who have biomarker-confirmed MCI due to AD or mild AD dementia. Change from baseline on the CDR-SB at the 2-year time point serves as the primary outcome (NCT02956486 and NCT03036280).
Most recently, concerns about an adverse effect on cognition with several BACEi drugs in development has raised concerns with this class and further studies will be required to better understand whether this is an on-target or off-target effect and to what extent it can be managed with, for example, less aggressive reductions in Aβ (CTAD, 2018).
6. PROMISING STRATEGIES
New efforts are underway to address the issue of ‘too little, too late’ in AD therapeutic trials. Additional studies are planned to test the efficacy of amyloid-reducing antibodies alone or in combination with active immunotherapeutic agents in preclinical AD. The A3 Study, (Ante-Amyloid Prevention of Alzheimer’s Disease), targets individuals at an even earlier stage: candidates will have a fibrillar amyloid load by PET that is above the mean, but not reaching the threshold for amyloid elevation used as an indication of preclinical AD in studies such as A4. These individuals may in fact be at an earlier stage of plaque development. The A3 Study design is a Phase 2b/3 double-blind, randomized, 3-arm, 4-year trial with an anti-amyloid intervention (e.g., active immunotherapy) versus placebo. The primary outcome will be rate of beta-amyloid deposition on serial Amyloid PET imaging, with additional outcomes using Tau PET imaging, CSF assays, volumetric MRI, and sensitive cognitive measures.
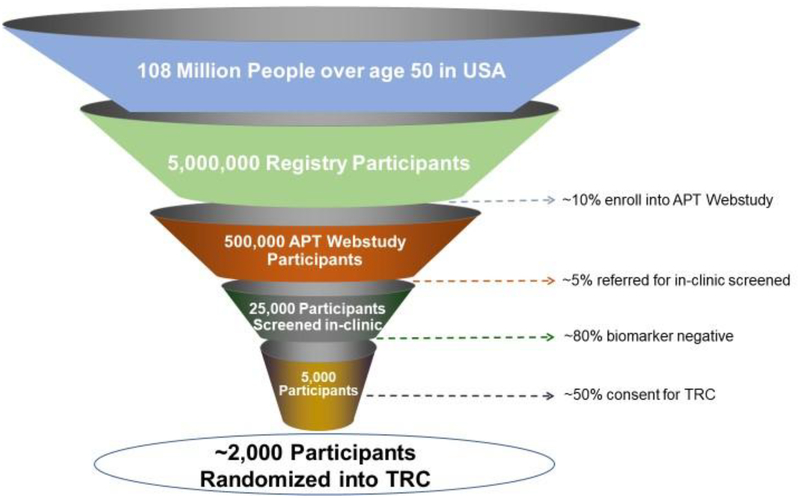
The timeframe, complexity and expense of the recruitment process and site activation for secondary prevention trials are extremely challenging, and trial enrollment represents the greatest bottleneck for drug development for AD. Large numbers of cognitively normal individuals need to be screened in order to fully enroll prevention trials. Implementation of a highly efficient approach to identify, evaluate, and enroll such participants is critical to overcoming this challenge (Figure 2). To meet the need of largescale screening of cognitively normal individuals in order to identify the approximately 20% who have elevated brain amyloid, multiple feeder registries (Brain Health Registry, Alzheimer’s Prevention Registry, HealthyBrains.org and others) filter participants based on age and cognitive status and invite older, individuals not meeting criteria for dementia to enroll in the Alzheimer Prevention Trials (APT) Webstudy, which in turn conducts unsupervised, web-based capture of demographic, medical, lifestyle and genetic factors, as well as longitudinal web-based cognitive testing and symptom questionnaires, plasma AD-biomarkers and APOE genotyping, to assess their risk of elevated brain amyloid. The initial risk algorithm is derived from analysis of ADNI data and screening data from A4 and is then iteratively updated to improve risk-prediction and confirming with in person-biomarker testing results (i.e. amyloid PET imaging). Those individuals who are biomarker confirmed for elevated brain amyloid will be enrolled into a Trial-Ready Cohort for Preclinical/Prodromal AD (TRC-PAD) which will be run across sites in the US. Participants will then be screened from the TRC-PAD and enrolled into prevention trials for which they are found to be eligible.
Figure 2. Steps in Recruiting Biomarker Eligible Participants into Preclinical/Prodromal trials.
Implementation of a web-based tool to capture demographic, genetic and longitudinal clinical and cognitive information on cognitively normal individuals interested in AD prevention trials. The data generates risk scores for AD pathology that allows selection of candidates for in-person biomarker and clinical assessment. Individuals with biomarker-confirmed evidence of brain amyloid accumulation are invited to join the Trial Ready Cohort (TRC) with semi-annual in-person follow-up visits within the network of pre-qualified clinical sites from which they can be invited to enroll in prevention trials.
7. CONCLUSIONS
One can envision a highly efficient preclinical trial design where cognitively-normal participants are identified as eligible (i.e. likely to have elevated brain amyloid) using remote cognitive testing, APOE genotyping and assessment of plasma-based biomarkers for AD, followed by biomarker confirmation (i.e. amyloid PET, cognitive testing) during in-clinic evaluation and subsequent randomization into an AD prevention trial. The recent FDA guidance appears to endorse the view that sensitive cognitive measures may be the path forward in demonstrating efficacy in the very earliest detectable stages of AD. The ongoing prevention trials should provide substantial amounts of data that will help determine whether current measures have the sensitivity and specificity required for early AD trials that truly predict clinical meaningfulness. Novel strategies such as remote cognitive testing, remote plasma biomarker analysis, the use of trial ready cohorts combined with an arsenal of anti-amyloid compounds should increase the likelihood of finding effective treatments that slow the progression of AD.
Key Points:
FDA has issued new draft guidance for trials in AD, which expanded the taxonomy of AD by recognizing four stages, specifically expanding the pre-dementia continuum.
These guidelines further advance regulatory support for clinical trials in earlier stages of AD.
Validated outcome measures sensitive to subtle yet clinically meaningful change during preclinical AD are needed.
Web-based tools are being develop in order to efficiently identify and enroll biomarker-positive individuals into a trial ready cohort of well-characterized participants for prevention trials
Anti-amyloid strategies continue to represent the most advanced mechanism of action in clinical trials.
Acknowledgments
Compliance with Ethical Standards: No sources of funding were used to assist in the preparation of this study.
Footnotes
Conflict of Interest Disclosure: MSR has nothing to disclose. PSA reports grants from NIH, FNIH and Alzheimer’s Association and consulting fees from NeuroPhage, Eli Lilly, Merck, Roche, Amgen, Abbvie, Pfizer, Novartis, Janssen, Lundbeck, Biogen, iPerian, Probiodrug, Anavex, Cohbar, Cytox, aTyr, Avanir.
REFERENCES
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Primers 2015;1:15056–15056. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 2016;8:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010. January;9(1):119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gomez MG, Langois CM, Langbaum JB, Ayutyanont N, Roontiva A, Thiyyagura P, et al. Florbetapir PET analysis of amyloid-beta deposition in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional study. Lancet Neurol. 2012;11:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger TL, Blazey T, Jack CR Jr, Koeppe RA, Su Y, Xiong C, Raichle ME, Snyder AZ, Ances BM, Bateman RJ, et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci U S A. 2013;110:E4502–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron. 2014. November 5;84(3):608–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. Draft Guidance for Industry. Alzheimer’s disease: Developing drugs for the treatment of early stage disease. 2013. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM338287.pdf.
- Aisen PS, Andrieu S, Sampaio C, Carrillo M, Khachaturian ZS, Dubois B, Feldman HH, Petersen RC, Siemers E, Doody RS, Hendrix SB, Grundman M, Schneider LS, Schindler RJ, Salmon E, Potter WZ, Thomas RG, Salmon D, Donohue M, Bednar MM, Touchon J, Vellas B. Report of the task force on designing clinical trials in early (predementia) AD. Neurology. 2011. January 18;76(3):280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. Early Alzheimer’s Disease: Developing Drugs for Treatment Guidance for Industry. 2018. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM596728.pdf
- Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS; Alzheimer’s Disease Neuroimaging Initiative. [Google Scholar]
- Leber P Slowing the progression of Alzheimer disease: methodologic issues. Alzheimer Dis Assoc Disord. 1997;11 Suppl 5:S10–21; discussion S37–9. [PubMed] [Google Scholar]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Cedarbaum J, Donohue MC, Green RC, Harvey D, Jack CR Jr, Jagust W, Morris JC, Petersen RC, Saykin AJ, Shaw L, Thompson PM, Toga AW, Trojanowski JQ; Alzheimer’s Disease Neuroimaging Initiative. Impact of the Alzheimer’s Disease Neuroimaging Initiative, 2004 to 2014. Alzheimers Dement. 2015. July;11(7):865–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010. March;6(3):131–44. [DOI] [PubMed] [Google Scholar]
- Blennow K, Dubois B, Fagan AM, Lewczuk P, de Leon MJ, Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimers Dement. 2015. January;11(1):58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79(1):110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-White ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992a;42:631–639 [DOI] [PubMed] [Google Scholar]
- O’Bryant SE, Mielke MM, Rissman RA, Lista S, Vanderstichele H, Zetterberg H, Lewczuk P, Posner H, Hall J, Johnson L, Fong YL, Luthman J, Jeromin A, Batrla-Utermann R, Villarreal A, Britton G, Snyder PJ, Henriksen K, Grammas P, Gupta V, Martins R, Hampel H, Biofluid Based Biomarker Professional Interest Area. Blood-based biomarkers in Alzheimer disease: Current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement. 2017. January; 13(1):45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, Palmqvist S, Baker D, Tan Hehir CA, Jeromin A, Hanlon D, Song L, Shaw LM, Trojanowski JQ, Weiner MW, Hansson O, Blennow K, ADNI Investigators. Plasma tau in Alzheimer disease. Neurology. 2016. October 25; 87(17):1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Andreasson U, Zetterberg H, Blennow K, Alzheimer’s Disease Neuroimaging Initiative. Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol. 2017. May 1; 74(5):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Papp KV, Rentz DM, Donohue MC, Amariglio R, Quiroz YT Early and late change on the preclinical Alzheimer’s cognitive composite in clinically normal older individuals with elevated βamyloid. Alzheimers Dement. 2017;13:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V, Fowler C, Li QX, Martins R, Rowe C, Tomita T, Matsuzaki K, Ishii K, Ishii K, Arahata Y, Iwamoto S, Ito K, Tanaka K, Masters CL, Yanagisawa K. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018. February 8;554(7691):249–254. [DOI] [PubMed] [Google Scholar]
- Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, Sullivan M, Paumier K, Holtzman DM, Morris JC, Benzinger T, Fagan AM, Patterson BW, Bateman RJ. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017. August;13(8):841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbaum JB, Hendrix SB, Ayutyanont N, Chen K, Fleisher AS, Shah RC, Barnes LL, Bennett DA, Tariot PN, Reiman EM. An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer’s disease. Alzheimers Dement. 2014. November;10(6):666–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, Weiner M, Aisen PS; Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing; Alzheimer’s Disease Neuroimaging Initiative; Alzheimer’s Disease Cooperative Study. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014. August;71(8):961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio RE, Donohue MC, Marshall GA, Rentz DM, Salmon DP, Ferris SH, et al. Tracking early decline in cognitive functionin older individuals at risk for Alzheimer disease dementia: The Alzheimer’s Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol. 2015. April;72(4):446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Benzinger TL, Berry S, Clifford DB, Duggan C, Fagan AM, Fanning K, Farlow MR, Hassenstab J, McDade EM, Mills S, Paumier K, Quintana M, Salloway SP, Santacruz A, Schneider LS, Wang G, Xiong C; DIAN-TU Pharma Consortium for the Dominantly Inherited Alzheimer Network. The DIAN-TU Next Generation Alzheimer’s prevention trial: Adaptive design and disease progression model. Alzheimers Dement. 2017. January;13(1):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, Aisen P. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014. March 19;6(228):228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolfsson O, Pihlgren M, Toni N, et al. An effector-reduced anti-β-amyloid (Aβ) antibody with unique binding properties promotes neuroprotection and glial engulfment of Aβ. J Neurosci 2012;32:9677–9689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariot PN, Lopera F, Langbaum JB, Thomas RG, Hendrix S, Schneider LS, Rios-Romenets S, Giraldo M, Acosta N, Tobon C, Ramos C, Espinosa A, Cho W, Ward M, Clayton D, Friesenhahn M, Mackey H, Honigberg L, Sanabria Bohorquez S, Chen K, Walsh T, Langlois C, Reiman EM; Alzheimer’s Prevention Initiative. The Alzheimer’s Prevention Initiative Autosomal-Dominant Alzheimer’s Disease Trial: A study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimers Dement (N Y). 2018. March 8;4:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T, Ashford E, Retout S, Hofmann C, Delmar P, Klein G, Andjelkovic M, Dubois B, Boada M, Blennow K, Santarelli L, Fontoura P; SCarlet RoAD Investigators. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther. 2017. December 8;9(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O’Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016. August 31;537(7618):50–6. [DOI] [PubMed] [Google Scholar]
- Wang J, Logovinsky V, Hendrix SB, Stanworth SH, Perdomo C, Xu L, Dhadda S, Do I, Rabe M, Luthman J, Cummings J, Satlin A. ADCOMS: a composite clinical outcome for prodromal Alzheimer’s disease trials. J Neurol Neurosurg Psychiatry. 2016. September;87(9):993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–9. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kost J, Tariot PN, Aisen PS, Cummings JL, Vellas B, Sur C, Mukai Y, Voss T, Furtek C, Mahoney E, Harper Mozley L, Vandenberghe R, Mo Y, Michelson D. Randomized Trial of Verubecestat for Mild-toModerate Alzheimer’s Disease. N Engl J Med. 2018. May 3;378(18):1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency. Guideline on the clinical investigation of medicines for the treatment of Alzheimer’s disease. https://www.ema.europa.eu/documents/scientific-guideline/guideline-clinicalinvestigation-medicines-treatment-alzheimers-disease-revision-2_en.pdf. 22February 2018.