Summary
Plant immunity consists of two arms: pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI), induced by surface-localized receptors, and effector-triggered immunity (ETI), induced by intracellular receptors. Despite the little structural similarity, both receptor types activate similar responses with different dynamics.
To better understand phosphorylation events during ETI, we employed a phosphoproteomic screen using an inducible expression system of the bacterial effector avrRpt2 in Arabidopsis thaliana and identified 109 differentially phosphorylated residues of membrane-associated proteins upon activation of the intracellular RPS2 receptor.
Interestingly, several RPS2-regulated phosphosites overlap with sites that are regulated during PTI, suggesting that these phosphosites may be convergent points of both signaling arms. Moreover, some of these sites are residues of important defense components including the NADPH oxidase RBOHD, ABC-transporter PEN3, calcium-ATPase ACA8, non-canonical Gα protein XLG2, and H+-ATPases. In particular, we found that S343 and S347 of RBOHD are common phosphorylation targets during PTI and ETI. Our mutational analyses showed that these sites are required for the production of reactive oxygen species during both PTI and ETI, and immunity against avirulent bacteria and virulent necrotrophic fungus.
We provide, for the first time, large-scale phosphoproteome data of ETI thereby suggesting crucial roles of common phosphosites in plant immunity.
Keywords: Plant immunity, Reactive oxygen species (ROS), Protein phosphorylation, Arabidopsis, effectors, pathogen-associated molecular patterns (PAMPs), bacteria, fungi
Introduction
Recognition of pathogenic microorganisms is the first crucial step in the immune response of plants aimed at inhibiting pathogen ingress. Typically, the plant immune system is represented by two distinct arms (Jones & Dangl, 2006; Dodds & Rathjen, 2010). The first arm is initiated upon the perception of pathogen-associated molecular patterns (PAMPs) by cell surface-localized pattern recognition receptors (PRRs), leading to PAMP-triggered immunity (PTI). Plant PRRs are either receptor-like kinases (RLKs) or receptor-like proteins (Boutrot & Zipfel, 2017). Successful pathogens cause disease using effector molecules that interfere with PTI. Bacterial effectors are often proteins secreted into plant cells via the type III secretion system. For example, the pathogen effector AvrRpm1 from Pseudomonas syringae pv maculicola, as well as AvrB and AvrRpt2 from Pseudomonas syringae pv tomato (Pto), target the Arabidopsis membrane-localized protein RPM1-INTERACTING PROTEIN-4 (RIN4) that is a regulator of PTI (Chung et al., 2014; Lee et al., 2015). Phosphorylation of RIN4 by AvrB through RPM1-INDUCED PROTEIN KINASE (RIPK) and degradation of RIN4 by AvrRpt2 are recognized by the intracellular nucleotide-binding domain leucine-rich repeat (NLR)-type immune receptors RESISTANCE TO P. SYRINGAE PV MACULICOLA-1 (RPM1) and RESISTANT TO P. SYRINGAE-2 (RPS2), respectively (Spoel & Dong, 2012; Khan et al., 2016). Activation of NLRs results in effector-triggered immunity (ETI).
ETI is considered to be a stronger and faster response than PTI (Jones and Dangl, 2006). PTI and ETI share some signaling components, such as Ca2+ and MAP kinase (MAPK) cascades (Tsuda et al., 2013; Yu et al., 2017). Also, they share immune responses such as transcriptional reprogramming, the generation of apoplastic reactive oxygen species (ROS), and the production and secretion of antimicrobial compounds. However, the activated immune responses during ETI are generally more prolonged and robust than those during PTI (Dodds & Rathjen, 2010; Tsuda & Katagiri, 2010; Thomma et al., 2011). These observations suggest that the same signaling components or enzymes are similarly regulated during PTI and ETI, while the dynamics and strength of the activation are different. However, it is currently unclear how NLRs transduce the signal downstream and how PRRs and NLRs, localized to different cellular compartments, activate similar components to induce related defense outputs.
ROS have various roles in immune signaling and are produced by RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD) during both PTI and ETI, but with different kinetics: ROS accumulation is only detectable hours after activation of RPM1 and RPS2 by cognate effectors (Torres et al., 2002). Recent research clarified the molecular mechanisms underlying signaling from PAMP recognition to ROS burst. Upon recognition of the flagellin 22 (flg22) peptide, derived from bacterial flagellin, the PRR FLAGELLIN SENSING-2 (FLS2) associates with its co-receptor RLK BRI1-ASSOCIATED RECEPTOR KINASE-1 (BAK1). The FLS2-BAK1 interaction induces phosphorylation and activation of both proteins, resulting in the phosphorylation of the receptor-like cytoplasmic kinase (RLCK) BOTRYTIS-INDUCED KINASE-1 (BIK1). Subsequently, BIK1 directly phosphorylates RBOHD, responsible for the apoplastic ROS burst observed within minutes after PRR activation (Kadota et al., 2014; Li et al., 2014; Kadota et al., 2015). Although phosphorylation-mediated activation of RBOHD is well studied during PTI, it is currently unknown whether similar RBOHD phosphorylation patterns are induced during ETI.
To gain insights into ETI signaling, we previously analyzed changes in abundance of plasma membrane-localized proteins during ETI mediated by RPS2 using transgenic Arabidopsis lines expressing the bacterial effector avrRpt2 under a dexamethasone (Dex)-inducible promoter (McNellis et al., 1998; Elmore et al., 2012). Among the significantly upregulated proteins after activation of RPS2, many proteins belong to the protein kinase superfamily, suggesting that protein phosphorylation has a crucial role during RPS2-mediated ETI. However, phosphorylation-based regulation of immune regulatory networks during ETI is still largely unknown. Jones et al. examined quantitative changes in the phosphoproteome of Arabidopsis infiltrated with Pto DC3000 (avrRpm1) and found that only one protein (the large subunit of Rubisco) was highly phosphorylated after activation of RPM1 (Jones et al., 2006), while the exact phosphorylation site was not elucidated. The low success rate of phosphosite identification when avirulent pathogens are used may be due to the difficulty to synchronously induce only ETI in sufficient cells without activating PTI.
Here, we employed an unbiased phosphoproteome screen using an avrRpt2-inducible expression system, and successfully identified differentially phosphorylated membrane-associated proteins during the early stages of ETI. Interestingly, several RPS2-regulated phosphorylation sites overlap with sites differentially phosphorylated during PTI as previously published. Our results suggest that these common phosphorylation sites may be convergent points of both immune signaling arms. For example, PAMP responsive RBOHD phosphorylation sites, S343 and S347, are also highly phosphorylated during ETI. Functional analyses revealed that these RBOHD sites are required for ROS production during both PTI and ETI, as well as for full resistance against avirulent Pto strains and a virulent necrotrophic fungus. Our study provides important phosphoproteome data contributing to our understanding of the ETI signaling network and showing commonalities and differences with the PTI signaling network.
Materials and Methods
Plant materials and growth conditions
Arabidopsis plants were grown in a controlled growth chamber under 70% relative humidity at 23°C and a light intensity of 85 μmol m−2 s−1. A 10 h-light and 14 h-dark photoperiod was applied. Plant genotypes are described in Methods S1.
Phosphopeptide sample preparation
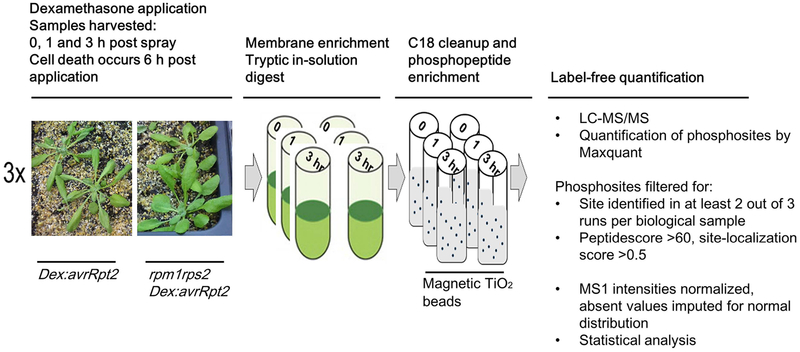
In each of three biological replicates, flats of Dex:avrRpt2 and control rpm1rps2/Dex:avrRpt2 Arabidopsis plants were grown for 5 weeks. Subsequently, plants were sprayed with an aqueous solution containing 30 μM dexamethasone (Dex) and 0.025 % Silwett L-77. In total 10 g of leaf tissue was harvested per sample at 0, 1 and 3 h after spraying Dex and flash-frozen in liquid nitrogen. Details of the phosphopeptide enrichment method are described in Methods S1.
LC-MS/MS and data analysis
Phosphopeptide samples were separately analyzed by a QExactive Plus Orbitrap mass spectrometer (Thermo Fisher Scientific) LC-MS/MS for a total of 18 runs at the UC Davis Proteomics Core. LC-MS/MS settings and specifics are described in Methods S1. LC-MS/MS raw files were analyzed using the Maxquant (version 1.5.1.0) and Perseus (version 1.5.0.15) software packages (Tyanova et al., 2016a; Tyanova et al., 2016b). Raw data files were searched against a FASTA database containing the Arabidopsis thaliana proteome. Statistical analysis is described in Methods S1. Raw data and search engine results are available at the PRIDE ProteomeXchange website under accession number PXD010440 (Vizcaino et al., 2013). Details of quantified phosphosites are shown in Tables S1–S4. Search parameters for Maxquant can be found in Table S5. Highest intensities and spectra per phosphosite are reported in Table S6.
Selected Reaction Monitoring (SRM) and data analysis
FLAG-RBOHD proteins extracted from rbohD/35S:FLAG-RbohD were immunoprecipitated with anti-FLAG antibody and separated by SDS-PAGE (NuPAGe®, Invitrogen). After staining with Coomassie Brilliant Blue G-250 CBB (SimplyBlue™ stain, Invitrogen), the proteins were cut out and digested with trypsin as described previously (Kadota et al., 2014). Intensities of phosphopeptides were normalized by the intensity of control non-phosphorylated RBOHD peptides. For details see Methods S1, transitions used for SRM are presented in Table S7.
Disease assays
Bacterial disease assays were performed as described previously (Kim et al., 2005). Plectosphaerella cucumerina BMM (PcBMM) disease assays and fungal biomass determination were performed as described previously (Torres et al., 2013). A detailed description is described in Methods S1. Primers used for quantitative PCR analysis are shown in Table S8.
ROS staining and measurements
The ROS staining protocol was adapted from an earlier described 3,3’-diaminobenzidine (DAB, Sigma) staining protocol (Thordal-Christensen et al., 1997) and described in detail in Methods S1.
Results
An unbiased phosphoproteome screen reveals ETI-dependent differential phosphorylation sites
Previous phosphoproteomic studies identified a large number of differentially phosphorylated proteins during PTI (Benschop et al., 2007; Nühse et al., 2007; Rayapuram et al., 2014; Mattei et al., 2016). To identify differential phosphorylation sites during ETI, we used Dex:avrRpt2 Arabidopsis lines to activate RPS2 synchronously and effectively eliminate the effect of any bacterial PAMPs which would activate PTI. It has been reported that AvrRpt2 can also weakly activate RPM1 (Kim et al., 2009). Therefore, we generated an rpm1rps2/Dex:avrRpt2 control line which does not induce ETI (Fig. S1a). We confirmed that both lines show comparable Dex-inducible avrRpt2 transcript expression and RIN4 protein degradation (Fig. S1b, c). By comparing the two lines, we aimed to identify differentially phosphorylated sites that are dependent on the activation of RPS2.
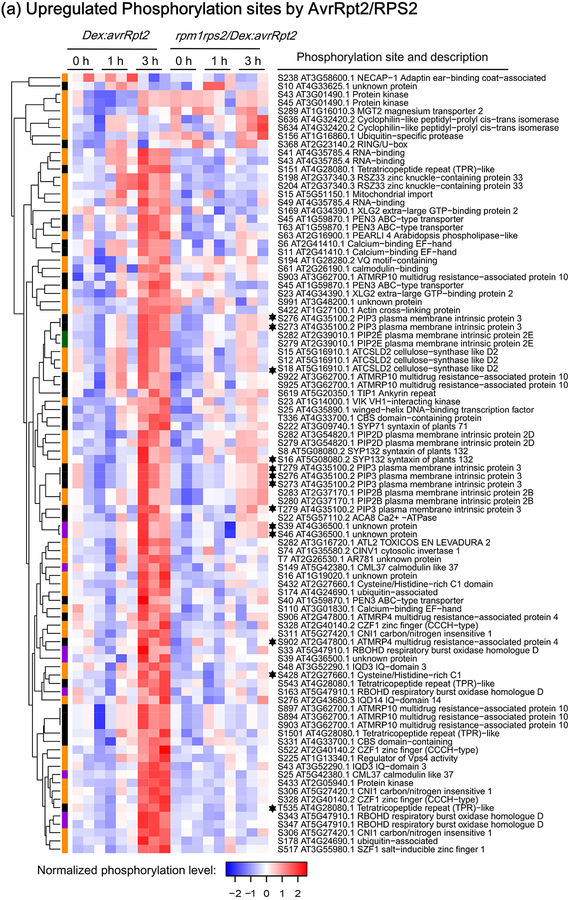
We decided to analyze membrane fractions because RPS2 associates to the plasma membrane and we expected that early phosphorylation events occur at the plasma membrane (Qi & Katagiri, 2009). In addition, membrane fractions are less complex in protein composition compared to total protein fractions, thus enabling us to perform label-free quantification using single MS runs. Before or after treatment with Dex for 1 h or 3 h, total membrane fractions of Dex:avrRpt2 and rpm1rps2/Dex:avrRpt2 were digested with trypsin followed by phosphopeptide enrichment using magnetic TiO2 beads and identification by mass spectrometry (Fig. 1). Label-free quantitative analysis followed by ANOVA (p≤0.05) revealed 264 unique phosphorylation sites to be statistically significantly changed in abundance in at least one condition (Table S1). These sites include (1) those regulated by activated RPS2, (2) those regulated by AvrRpt2 independent of RPS2 activation, (3) those regulated by the Dex treatment independently of AvrRpt2 and/or RPS2, and (4) those differentially phosphorylated solely depending on the plant genotype. To extract differentially phosphorylated sites after avrRpt2 expression and remove the sites dependent only on plant genotypes, we performed t-test between 0 h and 1 h, and 0 h and 3 h time points after Dex treatment in each genotype (p≤0.05). We found that 111 unique sites were significantly differentially phosphorylated after Dex treatment at 1 h or 3 h in Dex:avrRpt2. A total of 12 sites were highly phosphorylated after Dex treatment in both lines, but only two of these phosphorylation sites (S280 and S283 of PIP2B) were phosphorylated to a similar degree in both lines, suggesting that for these sites the increased phosphorylation at these two sites is caused by avrRpt2 expression or Dex treatment independently on RPS2 and AvrRpt2. The other 10 sites were phosphorylated in rpm1rps2/Dex:avrRpt2, but the ratio increase was at least twice more in Dex:avrRpt2. These data suggest that, although phosphorylation at these 10 sites can potentially also be induced by AvrRpt2 or Dex application alone, phosphorylation of these sites is significantly enhanced upon RPS2 activation (Table S1). In summary, we identified 109 sites differentially phosphorylated upon activation of RPS2. Out of these 109 sites, 84 sites were increased in phosphorylation (Fig. 2a and Table S2), and 25 sites were decreased in phosphorylation (Fig. 2b, Table S3). Hereafter, we call these sites “RPS2-regulated phosphorylation sites”. The majority of the upregulated phosphorylation sites are statistically significantly phosphorylated at 3 h after Dex spray, while the majority of downregulated phosphorylation sites are dephosphorylated already at 1 h after Dex spray. We also investigated the identified phosphosites for MAPK and CALCIUM-DEPENDENT PROTEIN KINASE (CPK) phosphorylation motifs. We found that 32 phosphosites and 10 phosphosites out of 84 RPS2-upregulated phosphosites contain MAPK- and CPK- phosphorylation motif, respectively (Table S2).
Figure 1.
Schematic representation of the large-scale phosphoscreen. Individual steps are highlighted and annotated. For further details see the experimental procedures in the Materials and Methods section.
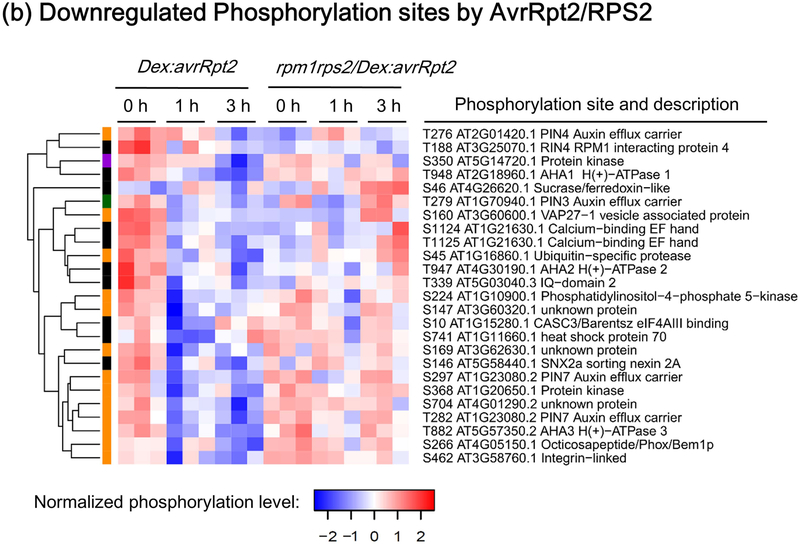
Figure 2.
Heat-maps showing RESISTANT TO P. SYRINGAE-2 (RPS2)-regulated phosphorylation sites. The phosphorylation sites shown are differentially phosphorylated upon RPS2 activation (one-way ANOVA p≤0.05 + t-test p≤0.05). (a) Phosphorylation sites significantly upregulated by RPS2 in Dex:avrRpt2 plants. (b) Phosphorylation sites significantly downregulated by RPS2 in Dex:avrRpt2 plants. Dendrograms were obtained by hierarchical clustering to represent Euclidian distances of normalized expression profiles. The colored sidebar indicates the regulation at the protein level in total protein membrane fractions (Supporting Information Table S1): black, sites for which the protein levels were not significantly altered amongst treatments; magenta, sites for which protein levels were significantly increased in Dex:avrRpt2 plants upon application of dexamethasone (Dex); green, sites for which protein levels were significantly decreased in rpm1rps2/Dex:avrRpt2 plants upon Dex application; orange, sites for which no peptides were detected in total protein samples; black stars, the phosphorylation sites upregulated in both lines after Dex treatment.
Changes in the phosphorylation intensities on proteins could potentially also be explained by alterations in protein abundance or a change in protein localization. Therefore we also analyzed total protein abundance in non-phosphopeptide enriched fractions of the samples (Table S1–3). These data revealed that the changes in abundance of some phosphorylation sites could be attributed to the changes in protein abundance at the membrane. We could not detect all proteins for which phosphorylated residues were quantified, potentially due to the low abundance of these proteins. Consequently, changes in phosphorylation intensities of such low abundant proteins could be the result of changes in protein abundance at the membrane with a similar level of phosphorylation after AvrRpt2 expression, changes in phosphorylation status after AvrRpt2 expression or both. Of note, even for the abundant proteins whose protein levels were detectable in the non-phosphopeptide enriched samples, we cannot fully exclude the possibility that a decrease in phosphorylation would be caused by selective degradation and/or altered localization of the phosphorylated protein.
Overlap in RPS2- and FLS2-induced phosphorylation sites
We found that 14 out of 109 RPS2-regulated phosphorylation sites overlap with previously identified flg22/FLS2-regulated phosphorylation sites (the phosphorylation at 11 sites are increased, and 3 sites are decreased during both ETI and PTI) (Table 1). Since our phosphoproteome strategy is not fully identical in approach compared to previous studies aimed to identify PAMP-regulated phosphorylation sites (Benschop et al., 2007; Nühse et al., 2007), there might, in fact, be more overlap in phosphorylation sites regulated during both ETI and PTI. Examples of such overlapping sites are the phosphorylation sites in RBOHD, the ABC-transporter PENETRATION-3 (PEN3), the plasma membrane-localized calcium-ATPase AUTOINHIBITED Ca2+ ATPASE-8 (ACA8) and non-canonical Gα protein EXTRA-LARGE GTP-BINDING PROTEIN-2 (XLG2) (Benschop et al., 2007; Nühse et al., 2007, Table 1, Table S4). For most of these sites, total protein levels did not change significantly during RPS2 activation (Table 1 and S4). Below we discuss these proteins in more detail.
Table 1.
Common residues differentially phosphorylated upon activation of RPS2 and flg22 treatment.
| ETI (AvrRpt2 expression by Dex treatment) | PTI (flg22 treatment) | CPKs or MAPKs phosphorylation motif | |||||
|---|---|---|---|---|---|---|---|
| Ratio Dex 3 h / 0 h in Dex:avrRpt2 | Ratio Dex 3 h / 0 h in rpm1rps2/Dex:avrRpt2 | Ratio flg22/mock | |||||
| Phosphorylation | Protein level | Phosphorylation | Protein level | Phosphorylation | |||
| RBOHD (AT5G47910) | S163 | 10.97 | 3.32 | 1.39 | 1.28 | 3.72*1 | |
| S343 | 13.08 | 0.83 | 4.7*1, 20.69*2, 4 | ||||
| S347 | 13.83 | 0.94 | 3.5*1, 20.69*2, 4 | φ-X-X-X-X-S/T-X-B*7 | |||
| PEN3 (AT1G59870) | S40 | 6.87 | 1.44 | 0.86 | 0.98 | 8.21*2 | φ-X-X-X-X-S/T-X-B*7 |
| S45 | 27.23*5, 12.85*6 | 0.18*5, 1.34*6 | 4.66*2 | ||||
| ACA8 (AT5G57110) | S22 | 20.80 | 1.50 | 2.48 | 0.68 | 3.74*2 | φ-X-X-X-X-S/T-X-B*7 |
| XLG2 (AT4G34390) | S23 | 13.23 | nd | 0.32 | nd | Not quantified*3 | |
| S169 | 3.02 | 0.20 | Not quantified*3 | ||||
| CBS domain-containing protein (AT4G33700) | S331 | 5.65 | 1.11 | 1.07 | 0.66 | 1.8*1 | |
| T336 | 5.96 | 0.70 | 2.9*1 | S/T-P*8 | |||
| PEARLI 4 (AT2G16900) | S63 | 9.95 | nd | 1.33 | nd | 4.2*1 | S/T-P*8 |
| AHA1 (AT2G18960) | T948 | 0.10 | 1.06 | 0.78 | 0.90 | 0.33*2 | |
| AHA2 (AT4G30190) | T947 | 0.22 | 1.07 | 0.97 | 0.92 | 0.42*2 | |
| AHA3 (AT5G57350) | T882 | 0.22 | nd | 0.60 | nd | 0.38*2 | |
ETI, effector-triggered immunity; PTI, PAMP-triggered immunity.
Phosphorylation data after flg22 treatment is extracted from the papers indicated. The data are shown as fold change. For the data of RPS2, the fold change of the phosphorylated sites are calculated based on raw intensities. The fold change of the protein levels is calculated based on the Maxquant label-free quantitation intensities transformed to raw intensities (see Methods S1). Significantly differentially phosphorylated sites with a ratio higher than 1.5 or lower than 0.5 were kept, see also Tables S1–S3.
The phosphorylation ratio at 10 min after flg22 treatment (Benschop et al., 2007)
The best phosphorylation ratio at 7 min after flg22 treatment out of 5 reps is shown (Nühse et al., 2007).
Phosphorylated but not quantified (Liang et al., 2016)
Double phosphorylated peptide
Calculated based on singly phosphorylated phosphopeptide intensities (Table S1 Multiplicity = __1)
Calculated based on doubly phosphorylated phosphopeptide intensities (Table S1 Multiplicity = __2)
nd, not detected
CPK phosphorylation motif: φ-X-X-X-X-S/T-X-B (S/T is the phosphorylated residue, B is a basic residue, φ is a hydrophobic residue, X is any residue) (Huang et al., 2001; Huang & Huber, 2001; Hernandez Sebastia et al., 2004)
MAPK phosphorylation motif S/T-P (S/T is the phosphorylated residue)
RPS2 signaling strongly induced RBOHD phosphorylation at residues at S163, S343, and S347 (Fig. 2, Table 1). We also found induced phosphorylation at S33. However, care must be taken for this site, because RBOHD protein amounts were also increased to a similar extent as S33 phosphorylation upon RPS2 activation (Table S4). Treatment with flg22, elf18 or chitin induces RBOHD phosphorylation at residues S163, S343, and S347 and these phosphorylated residues are required for full ROS production upon PTI (Dubiella et al., 2013; Kadota et al., 2014; Li et al., 2014). In particular, BIK1 phosphorylates RBOHD S343 and S347, and CPKs phosphorylate RBOHD residues S163 and possibly S347 during PTI. Recently, it was found that the MAP4K SIK1 is also involved in the full phosphorylation on S339 and S347 during PTI (Zhang et al., 2018).
PEN3 is involved in non-host resistance against the powdery mildew fungus Blumeria graminis fsp. hordei (Bgh), but also has broader roles in resistance, including ETI signaling (Johansson et al., 2014). PEN3 delivers indole glucosinolates to the apoplast at the plasma membrane (Kwon et al., 2008). In addition, the pen3 mutant shows defects in cell death induction by avirulent bacteria such as Pto DC3000 (avrRpm1) and Pto DC3000 (avrRps4), and an avirulent isolate of the oomycete Hyaloperonospora arabidopsis (Hpa) (Piasecka et al., 2015). We found that RPS2 signaling induced phosphorylation of PEN3 at S40 and S45, which are also reported as flg22/FLS2-inducible phosphorylation sites (Table 1, and Table S4). Moreover, these sites were recently shown to be required for the function of PEN3 in immunity against Bgh (Underwood & Somerville, 2017). These results suggest that PEN3 is activated during both PTI and ETI by phosphorylation at common residues, which may be required for transport of indole glucosinolates or other antimicrobials to restrict pathogen colonization.
ACA8 forms a protein complex with FLS2 and is involved in flg22-inducible Ca2+ signaling (Frei dit Frey et al., 2012). Flg22 treatment/perception induces the phosphorylation at ACA8 S22, and this N-terminal phosphorylation is important for the interaction of ACA8 with CALMODULIN (CaM), leading to ACA8 activation (Giacometti et al., 2012). We found that RPS2 activation similarly induced S22 phosphorylation (Table 1, Table S4), suggesting CaM-mediated activation of ACA8 during both PTI and ETI. Interestingly, CPK16 can phosphorylate S22 of ACA8 in vitro (Giacometti et al., 2012), suggesting a role of CPK16 in the PTI and ETI signaling pathways. CBL-INTERACTING PROTEIN KINASE-9 (CIPK9) in complex with the plasma membrane Ca2+ sensor CALCINEURIN B-LIKE PROTEIN-1 (CBL1) phosphorylates ACA8, thereby regulating its activity (Costa et al., 2017). However, the role of ACA8, as well as CPK16, CIPK9 and CBL1 during ETI remains to be elucidated.
XLG2 is a member of the heterotrimeric G proteins and participates in signaling with the Gβ protein GTP-BINDING PROTEIN BETA-1 (AGB1), and the Gγ proteins G PROTEIN GAMMA-SUBUNIT-1 (AGG1) and AGG2 (Zhu et al., 2009; Chakravorty et al., 2015). We found an increase in S23 and S169 phosphorylation of XLG2 after activation of RPS2, while we could not detect XLG2 total protein in the non-phosphopeptide enriched fractions (Table 1). XLG2 interacts with FLS2 and BIK1 and functions to attenuate proteasome-mediated degradation of BIK1 (Liang et al., 2016; Wang et al., 2018). After flg22 recognition, XLG2 is phosphorylated by BIK1, dissociates from AGB1, and the phosphorylated XLG2 enhances ROS production, possibly through modulation of RBOHD (Liang et al., 2016; Wang et al., 2018). Multiple sites of XLG2, including S23 and S169, are phosphorylated in flg22-treated protoplast cells (Liang et al., 2016), suggesting these are common phosphorylation sites during both PTI and ETI. Although the roles of these phosphorylation sites need to be further elucidated, our results suggest that XLG2 phosphorylation may also contribute to RBOHD-mediated ROS production during ETI.
RPS2 and FLS2 activation results in dephosphorylation of H+-ATPases
Plasma membrane H+-ATPases (AHAs) generate the proton motive force across the plasma membrane necessary to activate ion and metabolite transport (Morsomme & Boutry, 2000). AHAs also play a pivotal role in the opening of stomata, which are important entry sites for bacteria during natural infection (Yamauchi et al., 2016). We found that RPS2 activation resulted in C-terminal dephosphorylation of AHA1 at T948 and at homologous sites of AHA2 (T947) and AHA4 (T955) (Fig. 2, Table 1, Fig. S2a and Table S4). Interestingly, FLS2 activation similarly induces dephosphorylation of AHA1 at T948 and AHA2 at T947 (Nühse et al., 2007).
RPS2 regulated changes in phosphorylation status of defense-associated proteins
In addition to the overlapping PTI and ETI phosphorylation sites, we discovered a set of novel sites involved in RPS2 signaling on a variety of proteins, including defense-associated proteins. These sites are on proteins such as the kinase RIPK and PLASMA MEMBRANE INTRINSIC PROTEIN (PIP) aquaporins (Fig. 2, Table S2).
Currently, the involvement of RIPK in the RPS2 signaling pathway is unknown (Liu et al., 2011). However, we found that RPS2 activation induced dramatic phosphorylation of RIPK at the residue S433 (Fig. 2 and Table S2). The importance of RIPK phosphorylation during RPS2-mediated ETI requires future investigation since we did not detect RIPK total protein in the non-phosphopeptide enriched fractions (Table S1).
PIP aquaporins can transport water and H2O2 between the apoplast and cytoplasm. C-terminal PIP phosphosites are strongly conserved among PIPs and required for their activation (Maurel et al., 2015). Arabidopsis PIP1E transports apoplastic H2O2 produced by RBOH after PAMP treatment to the cytoplasm (Tian et al., 2016). This transport is required for the full activation of immune responses including pathogenesis-related gene expression and callose deposition. PIP2A is also required for intracellular accumulation of H2O2 as well as stomatal closure induced by PAMPs (Rodrigues et al., 2017). We found that several conserved residues at the C-terminal region of PIPs such as S279 and S282 of PIP2D and PIP2E, and S273, S276, and S279 of PIP3 were highly phosphorylated upon avrRpt2 expression in the presence of RPS2 (Fig. 2, S2b, and Table S4). PIPs may be activated during ETI and transport both water and H2O2 to the cytoplasm in a similar manner as during PTI. Recently, the importance of an aqueous apoplast for successful bacterial proliferation was shown (Xin et al., 2016). In line with our results, AvrRpt2-induced ETI failed at inducing water soaking (Xin et al., 2016). It is tempting to speculate that activation of PIPs contributes to reducing aqueous space in the apoplast thereby suppressing bacterial growth.
In addition to the upregulated phosphorylations, the activation of RPS2 resulted in dephosphorylation of the auxin transporter PIN-FORMED-3 (PIN3) at T279 and possibly PIN4 at T276 and PIN7 at T282, reflecting a possible crosstalk of ETI signaling and auxin signaling (Fig. 2, S2c and Table S3). Interestingly, AvrRpt2 promotes virulence of Pto DC3000 by stimulating turnover of key auxin transcription repressors Aux/IAA, thereby activating auxin responses (Cui et al., 2013). Although the role of the phosphorylation of these PIN residues needs to be further elucidated, RPS2-mediated dephosphorylation of PINs may change the localization of these auxin transporters thereby altering auxin transport to suppress AvrRpt2-induced auxin signaling (Lofke et al., 2013).
Interestingly, we also found AvrRpt2 itself to be phosphorylated at S97. However, we only observed S97 phosphorylation in the absence of the RPM1 and RPS2 receptors (Fig. 2 and Table S1). This suggests a possible involvement of AvrRpt2 phosphorylation for its virulent function and suppression of AvrRpt2 phosphorylation during ETI signaling. However, we could not correlate this phosphorylation to AvrRpt2 protein levels, as we did not detect AvrRpt2 protein in unenriched protein samples by mass spectrometry (Table S1). We instead detected a similar avrRpt2 gene expression pattern by semi-quantitative PCR (Fig. S1b).
Phosphorylation of common RBOHD residues is required for ROS production during ETI
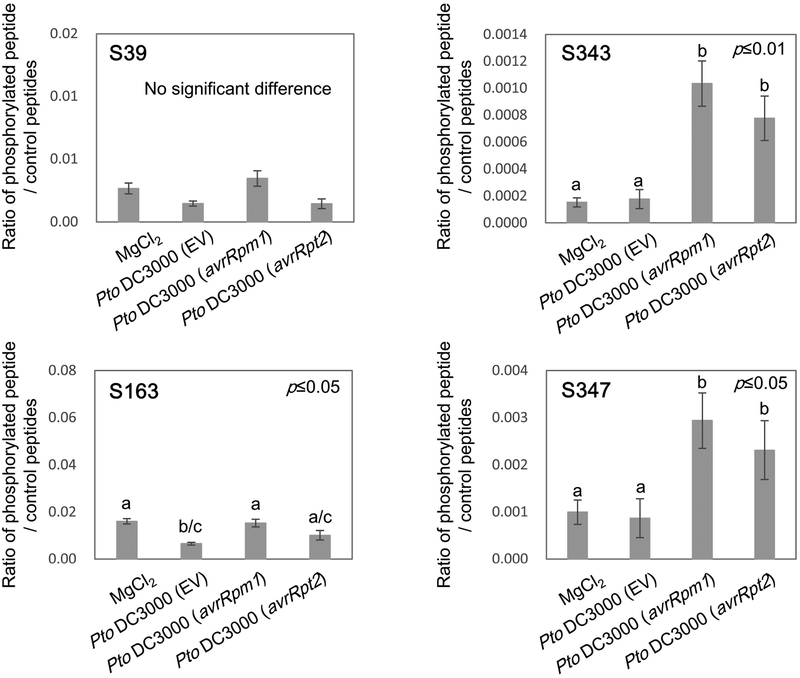
We hypothesize that the common PTI and ETI phosphorylation targets are potential convergent points of immune signaling and should therefore tightly regulate the activity of such proteins. Since RBOHD contains several such common phosphosites, we sought to clarify the importance of these common RBOHD sites during PTI and ETI. To confirm that RBOD phosphorylation during ETI identified in our large-scale screen also occurs upon bacterial infection, we syringe-inoculated leaves of rbohD plants complemented with 35S:FLAG-RBOHD, with Pto DC3000 EV (empty vector), Pto DC3000 (avrRpm1), Pto DC3000 (avrRpt2) or 10 mM MgCl2 solution. We collected the leaves at 6 h after inoculation because avirulent effector-mediated transcriptional changes are the most highly induced at 6 h after infiltration with Pto DC3000 (avrRpm1) and Pto DC3000 (avrRpt2) (Mine et al., 2018) and ROS are also actively produced at this time point (Torres et al., 2002). After collecting the infiltrated leaves, FLAG-RBOHD protein was immunopurified and individual RBOHD phosphorylation sites were quantified by selected reaction monitoring (SRM) mass spectrometry on a triple quadrupole mass spectrometer. Both avirulent strains, Pto DC3000 (avrRpm1) and Pto DC3000 (avrRpt2), but not virulent Pto DC3000 EV or 10 mM MgCl2 solution, induced phosphorylation at residues S343 and S347 (Fig. 3). In contrast to as in our large-scale phosphoscreen, residue S163 was not highly phosphorylated 6 h after bacterial infection, suggesting that S163 phosphorylation may be transient upon bacterial infection. In addition, the phosphorylation at the residue S39, which is a BIK1-dependent flg22-induced RBOHD phosphorylation site (Kadota et al., 2014; Li et al., 2014), was not detectable after infection with any of the bacteria under the conditions tested.
Figure 3.
Avirulent bacteria induce phosphorylation of RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD) at specific residues. Selected reaction monitoring (SRM) analysis of the phosphorylation sites 6 h after infiltration with 10 mM MgCl2 solution, Pseudomonas syringae pv tomato (Pto) DC3000 EV (empty vector), Pto DC3000 (avrRpm1) or Pto DC3000 (avrRpt2) using a triple quadruple mass spectrometer. Values are means ±SE of three biological replicates. Different letters indicate significantly different values at p≤0.05 for S163 and S347, or at p≤0.01 for S343 (one-way ANOVA, Tukey post hoc test).
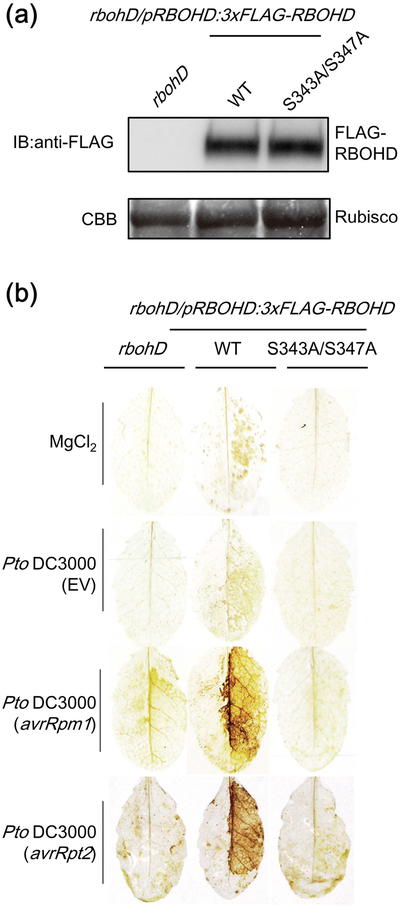
To investigate the role of RBOHD phosphorylation sites S343 and S347 on the ROS production during ETI, we generated transgenic Arabidopsis rbohd mutant lines expressing either the wild-type (WT) or the S343A/S347A variant of FLAG-tagged RBOHD driven by its endogenous promoter. Both lines expressed comparable amounts of RBOHD protein (Fig. 4a) and did not exhibit any obvious morphological growth phenotypes (Fig. S3a). Interestingly, S343A/S347A plants lost the ability to induce ROS production after infiltration with avirulent Pto DC3000 (avrRpm1) or Pto DC3000 (avrRpt2), while WT plants induced a strong H2O2 accumulation (Fig. 4b). Moreover, the S343A/S347A plants did not show ROS production upon flg22 treatment (Fig. S3b) (Nühse et al., 2007). These results demonstrate that S343 and S347 are required for ROS production during both PTI and ETI. Importantly, S343 and S347 are highly conserved in RBOHD homologs amongst different plants (Fig. S4), as well as in most Arabidopsis RBOHs (Kadota et al., 2014). Furthermore, S318 and S322 of RBOHC (corresponding to S343 and S347 in RBOHD) are required for ROS production and root hair formation (Takeda et al., 2008). Together, these results support the idea that these phosphorylation sites play crucial roles in the regulation of RBOHs in various signaling pathways.
Figure 4.
RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD) phosphorylation sites S343 and S347 are required for reactive oxygen species (ROS) production during effector-triggered immunity (ETI). (a) Immunoblot showing similar protein levels of FLAG-tagged RBOHD protein in rbohD mutants expressing 3xFLAG-RBOHD WT and the S343A/S347A variant. Coomassie stain (CBB) shows rubisco protein to demonstrate equal loading. (b) 3,3’-diaminobenzidine (DAB)-mediated H2O2 staining in rbohD and rbohD mutants expressing 3xFLAG-RBOHD WT and the S343A/S347A variant 8 h after infiltration with the bacteria. The right half of the leaves were infiltrated with 10 mM MgCl2 solution, Pseudomonas syringae pv tomato (Pto) DC3000 EV, Pto DC3000 (avrRpm1) or Pto DC3000 (avrRpt2). We repeated three times with similar results (4–5 leaves per genotype were stained each time).
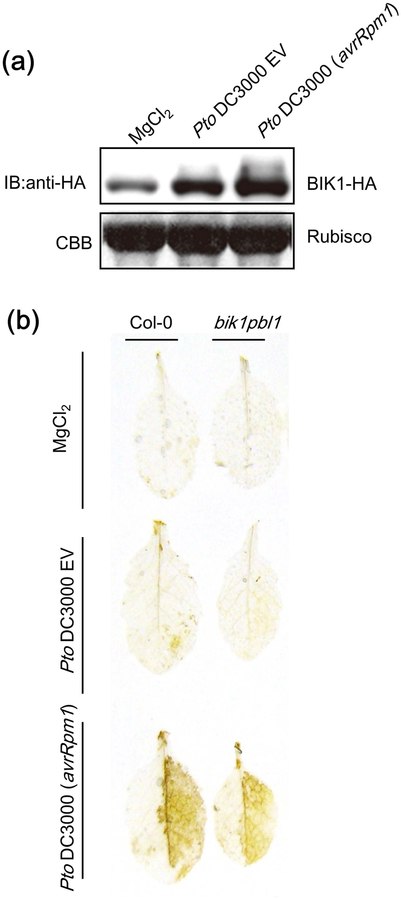
BIK1 phosphorylates both S343 and S347 of RBOHD during PTI, and the bik1 knockout mutant exhibits reduced S343/S347 phosphorylation and PTI-mediated ROS burst (Kadota et al., 2014; Li et al., 2014). Therefore, we investigated the involvement of BIK1 in ROS production during ETI. We found that BIK1 protein accumulated upon infection with Pto DC3000 (avrRpm1) and to a lesser extent Pto DC3000 EV (Fig. 5a). PBS1-LIKE-1 (PBL1) is a kinase functionally redundant with BIK1, and a bik1pbl1 double mutant shows less PAMP-inducible ROS production and RBOHD phosphorylation (Zhang et al., 2010; Kadota et al., 2014; Li et al., 2014). However, bik1pbl1 double mutants induced H2O2 accumulation similar to Col-0 upon infection with Pto DC3000 (avrRpm1) (Fig. 5b), suggesting that BIK1 may not play a major role in RBOHD phosphorylation during ETI. This is consistent with the fact that RPS2 activation did not induce significant phosphorylation at other BIK1-mediated phosphorylation sites such as S39 and S339, suggesting that other kinases may phosphorylate RBOHD at S343 and S347 during RPS2-mediated ETI.
Figure 5.
The bik1pbl1 double mutant does not exhibit a defect in reactive oxygen species (ROS) accumulation during effector-triggered immunity (ETI). (a) BOTRYTIS-INDUCED KINASE-1 (BIK1) protein accumulates after infection with Pseudomonas syringae pv tomato (Pto) DC3000 (avrRpm1). pBIK1:BIK1-HA plants were inoculated with 5 mM MgCl2, Pto DC3000 EV or Pto DC3000 (avrRpm1) (2.5 × 107 cfu (colony-forming units) ml−1) and BIK1-HA protein amount was determined by immunoblot analyses using anti HA antibody. (b) H2O2 accumulation in Col-0 and bik1pbl1 mutant after inoculation with 10 mM MgCl2, Pto DC3000 EV or Pto DC3000 (avrRpm1) (2.5 × 107 cfu ml−1). H2O2 accumulation was detected by 3,3’-diaminobenzidine (DAB). The experiments were performed three times with similar results.
The autoimmunity phenotype of rbohD and semi-dwarf phenotype of rbohDrhohF can be complemented with the RBOHD S343A/S347A variant
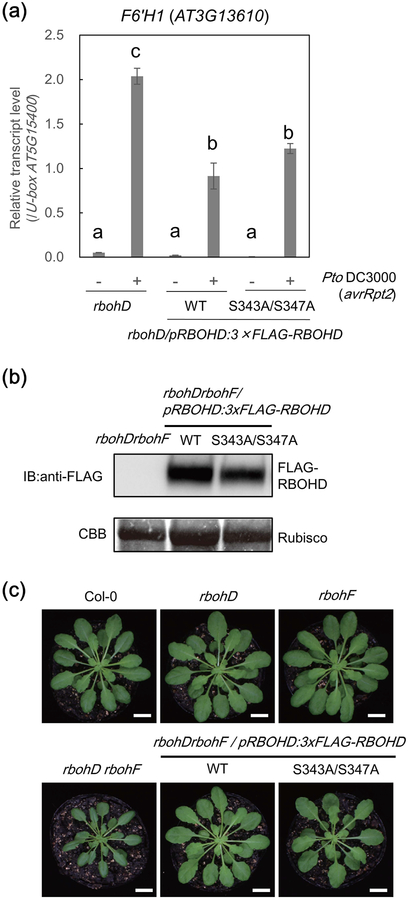
In addition to a direct toxic effect of ROS to microbial pathogens, the RBOHD-induced ROS burst has critical roles in immunity including: stomatal closure which hampers entrance of pathogens through these pores, callose deposition which prevents pathogen invasion and long distance immune signaling which induces resistance against secondary infections (Suzuki et al., 2011; Kadota et al., 2015). However, the role of RBOHD-mediated ROS in resistance against avirulent pathogens is still unclear, mostly due to a lack of genetic evidence. rbohD mutants do not produce ROS upon infection with avirulent Pto strains, and an avirulent Hpa isolate Emco5 (Torres et al., 2002; Gao et al., 2013). However, the rbohD and rbohDrbohF double mutants (RBOHF is functionally redundant with RBOHD in many immune responses) are resistant to virulent and avirulent pathogens due to constitutive or inducible activation of immune responses (Torres et al., 2002; Marino et al., 2012). Notably, rbohD plants hyper-accumulate salicylic acid, ethylene, the antimicrobial compound scopoletin, and PR-1 gene transcripts upon pathogen challenge (Pogany et al., 2009; Chaouch et al., 2012; Kadota et al., 2014). Indeed, we observed that rbohD mutants expressed the higher amount of transcripts of FERULOYL COA ORTHO-HYDROXYLASE 1 (F6’H1), a key enzyme for scopoletin biosynthesis, after infection with Pto DC3000 (avrRpt2) (Fig. 6a). Interestingly, however, this phenotype was suppressed in the rbohD plants expressing the WT or S343A/S347A RBOHD variant, suggesting that overactivation of immunity in the null rbohD mutant may compensate for the loss of ROS-based immunity. Notably, similar constitutive or overactivation of immunity was seen in null mutants of other positive regulators of immune signaling like BAK1, BIK1, POWDERY MILDEW RESISTANT-4 (PMR4) and MITOGEN-ACTIVATED PROTEIN KINASE-4 (MPK4) (Petersen et al., 2000; Nishimura et al., 2003; He et al., 2007; Kemmerling et al., 2007; Zhang et al., 2010; Zhang et al., 2012; Zhang et al., 2017). A possible explanation is that these components are guarded by NLR proteins, as seen for MPK4 (Zhang et al., 2012), whose kinase activity on CALMODULIN-BINDING RECEPTOR-LIKE CYTOPLASMIC KINASE 3 (CRCK3) is monitored by NLR protein SUMM2 (Lolle et al., 2017; Zhang et al., 2017).
Figure 6.
RBOHD-S343A/S347A variant can complement overactivation of immune-related gene expression in rbohD and the semi-dwarf autoimmune phenotype of rbohDrbohF. (a) Loss of RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD) leads to increased expression of FERULOYL COA ORTHO-HYDROXYLASE 1 (F6’H1) upon bacterial perception. Gene expression of F6’H1 in the leaves infiltrated with Pseudomonas syringae pv tomato DC3000 (avrRpt2) (2.5 × 107 cfu (colony-forming units) ml−1) or 10 mM MgCl2 solution for 24 h was measured by qPCR analysis. The relative transcript levels were calculated by normalization to the U-box housekeeping gene transcript (At5g15400). Data are mean ±SE of three technical replicates. Different letters indicate significantly different values at p ≤ 0.05 (one-way ANOVA, Tukey post hoc test). The experiments were performed three times with similar results. (b) Immunoblot showing equal FLAG-RBOHD protein level in leaves of rbohDrbohF/pRBOHD:3xFLAG-RBOHD (WT) and rbohDrbohF/pRBOHD:3xFLAG-RBOHD (S343A/S347A) lines. Leaf tissue of the rbohDrbohF double mutant was used as negative control. Coomassie stain (CBB) shows rubisco protein to demonstrate equal loading. (c) Growth phenotypes of six-week-old plants. Bars, 1 cm.
Although the rbohD mutant does not show obvious phenotypes under standard growth conditions, the rbohDrbohF double mutant is semi-dwarf and shows a strong autoimmune phenotype including the development of necrotic lesions and callose deposition in mature leaves (Torres et al., 2002). To determine if S343 or S347 phosphorylation plays a role in this phenotype, we generated rbohDrbohF mutant lines expressing the WT or the S343A/S347A variant of RBOHD at similar levels (Fig. 6b). Remarkably, the semi-dwarf phenotype of the rbohDrbohF double mutant was recovered by expression of the WT as well as expression of the S343A/S347A RBOHD variant (Fig. 6c), showing that the presence of RBOHD protein but not the phosphorylation at S343 or S347 is required for the normal growth and suppression of the autoimmunity in rbohD and rbohDrbohF mutants (Fig. 6c). These results suggest that the activity of RBOHD is not required for the suppression of the autoimmunity and supports a hypothesis in which RBOHD and RBOHF are guarded by one or more NLRs.
RBOHD phosphorylation of S343 or S347 is required for immunity
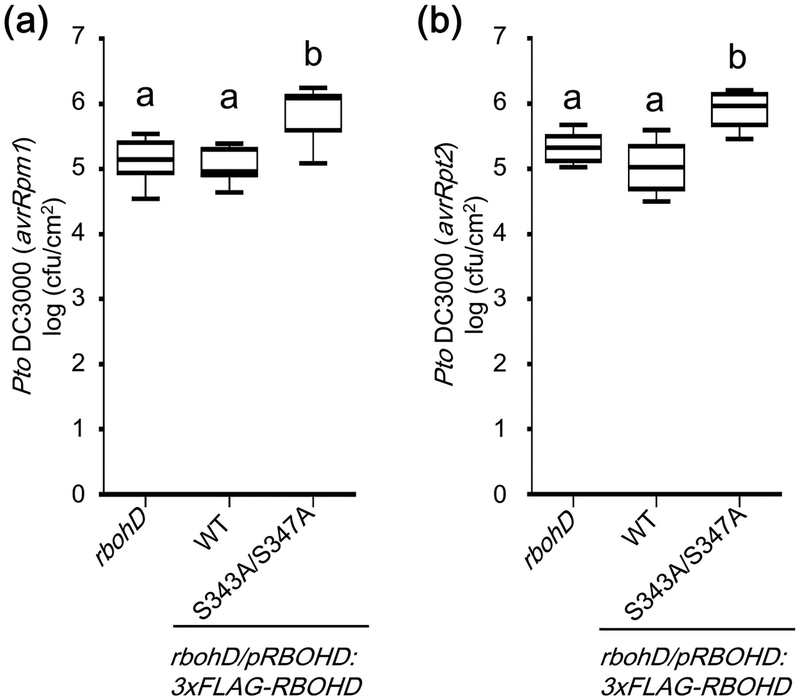
Although ROS are involved in various immune responses, there is no clear genetic evidence showing the crucial roles of ROS in resistance against avirulent pathogens. Since rbohD lines expressing the RBOHD S343A/S347A variant do not show any obvious auto-immune-related phenotypes, we used these lines to study the relevance of S343 and S347 phosphorylation in ETI. Pto DC3000 (avrRpm1) and Pto DC3000 (avrRpt2) grew significantly more in the rbohD mutant expressing the RBOHD S343A/S347A variant than in the mutant expressing WT RBOHD (Fig. 7), showing that phosphorylation at S343 and S347 is important for resistance against these avirulent bacterial strains.
Figure 7.
RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD) phosphorylation sites S343 and S347 are required for effector-triggered immunity (ETI). Bacterial growth of Pseudomonas syringae pv tomato (Pto) DC3000 (avrRpm1) (a) and Pto DC3000 (avrRpt2) (b) in rbohD and rbohD expressing 3xFLAG-RBOHD WT or the S343A/S347A variant. Bacteria were syringe infiltrated in three leaves per one plant at a concentration of 1 × 105 cfu (colony-forming units) ml−1. Three days post infiltration, leaves were harvested to determine bacterial growth. Data are means ±SD of 8 replicates. Solid horizontal lines within the boxes show median. Different letters indicate significantly different values at p ≤ 0.01 (one-way ANOVA, Tukey post hoc test). These experiments were repeated three times with similar results.
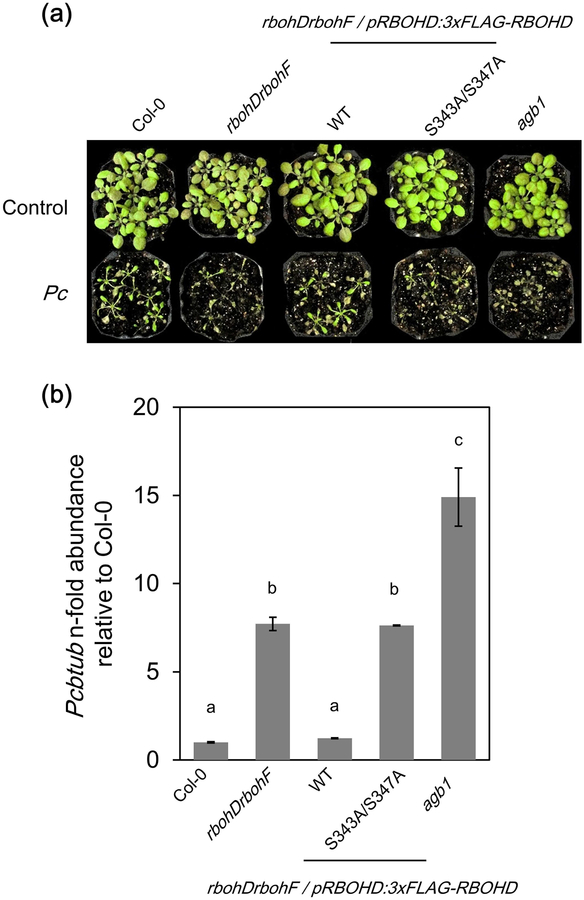
In addition, we used the rbohDrbohF lines with the RBOHD S343A/S347A variant to test the resistance against the necrotrophic fungus PcBMM. Both RBOHD and RBOHF are required for the resistance against PcBMM, as rbohDrbohF mutant was shown to display enhanced susceptibility to PcBMM (Torres et al., 2013; Morales et al., 2016). As reported, Col-0 and WT RBOHD complementation lines were resistant to PcBMM (Fig. 8). In contrast, the rbohDrbohF and RBOHD S343A/S347A plants were significantly more susceptible like the agb1 mutant (impaired in Gβ subunit of heterotrimeric G protein), which is a susceptible control (Torres et al., 2013) (Fig. 8). These results suggest that the RBOHD residues S343 and S347 contribute to the resistance against PcBMM. The rbohD single mutants and the corresponding complementation lines were all resistant to PcBMM (Fig. S5), indicating that RBOHF has a functional redundancy with RBOHD in immunity against PcBMM. The importance of S343 and S347 in the resistance suggests that plants actively phosphorylate RBOHD and produce ROS in response to PcBMM possibly through the recognition of unidentified PAMPs or DAMPs. The result also suggests that the susceptibility of rbohDrbohF is likely not due to the overactivation of salicylic acid-dependent pathway which antagonizes jasmonic acid-based resistance against necrotrophic pathogens (Thaler et al., 2012), but due to the loss of RBOHD activation and ROS production.
Figure 8.
The double mutant rbohDrbohF shows weakened resistance to virulent Plectosphaerella cucumerina (PcBMM isolate). (a) Symptoms of 18-d-old plants after spray inoculation with PcBMM (4 × 106 spores ml−1). (b) Quantification of PcBMM biomass. Fungal DNA was quantified by qPCR at 6 d post-inoculation (dpi) using specific primers for PcBMM β-TUBULIN and normalized to Arabidopsis thaliana UBIQUITIN 10 gene. Bars represent averages (±SE) of fungal DNA levels relative to Col-0 plants from two replicates. Statistical analysis was performed by ANOVA, corrected with Bonferroni post hoc test. Different letters indicate significant differences (p ≤ 0.05). The experiment was repeated twice with similar results.
Discussion
RPS2 and FLS2 trigger similar phosphorylation patterns at immune components
Previous studies showed that similar responses are induced during PTI and ETI, although dynamics and amplitude between the two signaling arms differ (Dodds & Rathjen, 2010; Tsuda & Katagiri, 2010; Thomma et al., 2011). Our study reveals a common set of differentially phosphorylated sites during both PTI and ETI. Such common sites may be convergent points of both signaling pathways, and phosphorylation or dephosphorylation of such residues likely plays a crucial role in regulating the activity of defense components. In support of this, we found that phosphorylation at specific conserved residues in RBOHD is required for ROS production in both PTI and ETI. In addition, we found commonly phosphorylated sites during PTI and ETI in PEN3 and ACA8. Importantly, previous mutational analyses showed that these phosphorylation sites of PEN3 and ACA8 are required for their activation (Giacometti et al., 2012; Underwood & Somerville, 2017).
We also found a commonly dephosphorylated site, T948 on AHA1 during PTI and ETI. Phosphorylation at T948 of AHA1 creates a binding site for a 14-3-3 protein, and 14-3-3 interaction alters the orientation of the autoinhibitory C-terminal domain, resulting in AHA1 activation (Svennelid et al., 1999). Thus, dephosphorylation of AHA1 at T948 would cause dissociation of 14-3-3 leading to inactivation of AHA1. The AHA1 inactivation may lead to the inhibition of stomatal opening thereby preventing bacterial entry (Yamauchi et al., 2016).
Which kinases phosphorylate such common sites? Candidates include CPKs that play critical roles during PTI and ETI (Boudsocq et al., 2010; Gao et al., 2013). Indeed, some of our identified common sites contain a CPK phosphorylation motif (S347 of RBOHD, S40 of PEN3 and S22 of ACA8, Table S1–S3). In particular, CPK10 was shown to phosphorylate synthetic peptides carrying S45 of PEN3 in vitro (Curran et al., 2011), and CPK16 phosphorylates S22 of ACA8 in vitro (Giacometti et al., 2012). However, the involvement of CPK10 and CPK16 in PTI and ETI is unknown. CPK4/5/6/11 were shown to be required for the PAMP-inducible ROS burst (Boudsocq et al., 2010; Dubiella et al., 2013), and CPK1/2 were shown to be required for full activation of the RPM1- and RPS2-mediated ROS burst (Gao et al., 2013), suggesting the involvement of these CPKs in the phosphorylation of RBOHD during both PTI and ETI. CPK4/5/6/11 phosphorylate RBOHD at S163 and S347 in vitro, and these sites also encompass a CPK phosphorylation motif (Kadota et al., 2014). CPK 2/4/11 also phosphorylate RBOHD at S148 in vitro (Gao et al., 2013), while it is unknown whether CPK1/2 phosphorylate S163 and S347 in vitro or during ETI. The accumulating evidence suggests that multiple CPKs are involved in phosphorylation of RBOHD and other defense components during PTI and ETI. However, since CPKs are not able to phosphorylate S343 (which does not possess a CPK phosphorylation motif) in vitro (Kadota et al., 2014), it is likely that there are other kinase(s) that phosphorylate RBOHD during ETI (Fig. S6).
During PTI, the RLCK BIK1 phosphorylates RBOHD at residues S343 and S347 (Kadota et al., 2014; Li et al., 2014). This suggests that BIK1 or similar RLCKs might phosphorylate RBOHD during ETI. However, there is no current experimental evidence showing the involvement of BIK1 in ETI. Our data show that the bik1pbl1 mutant does not affect RBOHD-mediated ROS production during ETI (Fig. 5). It is possible that other RLCKs act redundantly with BIK1 and PBL1 in the phosphorylation of RBOHD S343 and S347 during ETI. Notably, RPS2 activation by AvrRpt2 induces accumulation of many RLCKs including BIK1, PBL2, PBL32 and RIPK at 6 h after Dex treatment in Dex:avrRpt2 plants (Elmore et al., 2012). RPS2 activation by AvrRpt2 also induces the phosphorylation of RIPK at 3 h post-Dex treatment (Fig. 2), suggesting the possible involvement of RIPK in RPS2 signaling. It would be interesting to investigate the involvement of these RLCKs in RBOHD phosphorylation by using multiple knockout mutants. Interestingly, the MAP4 Kinase SIK1 was recently found to also able to phosphorylate RBOHD during PTI (Zhang et al., 2018). However, it remains to be elucidated if SIK1 contributes to RBOHD phosphorylation during ETI.
MPK3 and MPK6 play critical roles in PTI and ETI signaling, and it is likely that these MPKs phosphorylate similar downstream proteins during PTI and ETI (Tsuda et al., 2013; Su et al., 2018). Examples of such proteins are WRKY transcription factors and 1-AMINO-CYCLOPROPANE-1-CARBOXYLIC ACID SYNTHASES (ACSs), which are the key enzymes of the ethylene biosynthesis pathway (Liu & Zhang, 2004; Adachi et al., 2015; Sheikh et al., 2016). In addition, in our dataset, for example, residues S63 of PEARLI4 and T336 of the CBS domain-containing protein contain an S/T-P MPK phosphorylation motif (Table 1 and S4). Hence, it is likely that MPKs such as MPK3 and MPK6 phosphorylate these sites (Sorensson et al., 2012).
MVQ1 is a negative regulator of WRKY-mediated defense gene expression during PTI and is phosphorylated upon PAMP treatment (Pecher et al., 2014). We found increased phosphorylation of S/T-P site S194 after activation of RPS2 (Table S1). Although direct evidence is not present, it is tempting to speculate that MVQ1 is phosphorylated during PTI and ETI by MPK3 and MPK6 to regulate WRKY-mediated gene expression.
The kinases phosphorylating common PTI and ETI phosphorylation sites in vivo are still largely unknown. The identification of these kinases is important in our understanding of how different receptors, localized to different cellular compartments, regulate the same defense components. Moreover, clarification of their activation mechanisms may further explain commonalities and differences in PTI and ETI signaling. For example, differences in the dynamics and strength of kinase activation during PTI and ETI may cause differential phosphorylation dynamics of the defense components and subsequent immune signaling outputs (Tsuda et al., 2013).
RPS2-regulated phosphorylation sites
In addition to common PTI and ETI phosphorylation sites, we identified undiscovered and novel phosphosites involved in RPS2-signaling. Some of these sites are located on important immune components including PIP aquaporins. However, the kinases and phosphatases directly phosphorylating and dephosphorylating RPS2-regulated phosphosites remain largely unknown. CPKs and MPKs are strong candidate kinases for phosphorylating these sites because many RPS2-regulated phosphorylation sites contain CPK phosphorylation motifs and/or MAPK phosphorylation motifs (Table S1). Some CPKs, including CPK1, phosphorylate synthetic peptides carrying S282 of PIP2D and T279 of PIP3 in vitro, both of which are RPS2-regulated phosphosites (Curran et al., 2011). Further evidence comes from the RPS2-upregulated phosphosites, S11 of CALCIUM-BINDING EF-HAND FAMILY PROTEIN (AT2G41410) and S238 of ADAPTIN EAR-BINDING COAT-ASSOCIATED PROTEIN-1 (NECAP-1), which contain an S/T-P motif (Table S1). In addition, their phosphorylations were identified in planta after activation of MPK3/MPK6 by the expression of a constitutively active MEKDD mutant protein (Hoehenwarter et al., 2013). This suggests the possible involvement of MPK3/MPK6 in the phosphorylation of these residues. Identification and characterization of kinases and phosphatases regulating RPS2 signaling components, in addition to mutational analyses of the corresponding phosphorylation sites, would aid in resolving the RPS2 signaling network at the molecular level.
RBOHD-mediated ROS production is crucial for ETI
Our study revealed that RBOHD-mediated ROS production is required for the resistance against avirulent bacteria and thus for successful ETI. We also found RBOHD-mediated ROS contributes to the resistance against a virulent fungus. A remaining question is how ROS contributes to the resistance against these pathogens. Although ROS are believed to be directly toxic to pathogens (Lambeth, 2004), ROS are also likely to function as a signaling molecule to change the redox status and affect enzymatic activities and gene expression. In this context, it is interesting that PIPs, which transport H2O2 to the cytoplasm (Maurel et al., 2015), was phosphorylated at activation sites during ETI (Fig. 2, S3b, and Table S4). This suggests that plants regulate the import of H2O2 produced via RBOHD, thereby actively altering the cellular redox status upon ETI. In addition, import of H2O2 through PIPs during ETI might, for example, induce stomatal closure as these components play critical roles in stomatal closure (Kwak et al., 2003; Mersmann et al., 2010; Rodrigues et al., 2017). Taken together, our data suggest that plants activate ROS production and the ROS-mediated signaling occurs in large part by protein phosphorylation.
Final remarks
In summary, our study provides the first large-scale phosphoproteome data of NLR-mediated ETI adding important pieces to our understanding of the ETI signaling network. Among the identified differential phosphorylation sites, we found phosphorylation sites common to PTI and ETI signaling on a number of important defense-related proteins. Some of these phosphorylation sites have critical roles in the activation or inactivation of the corresponding proteins and thereby mediate immune signaling. The observations made in this study enhance our understanding of immune signaling regulation during ETI and PTI. The future identification of proteins regulating the common phosphorylation sites, such as kinases, will further clarify the commonalities and differences of ETI and PTI at the molecular level.
Supplementary Material
Table S1 Summary of significantly differentially phosphorylated sites and corresponding protein levels.
Methods S1 Supplemental methods describing materials, phosphoproteomic analysis, reactive oxygen species (ROS) assay, qRT-PCR, western blotting and disease assays.
Table S2 Summary of RESISTANT TO P. SYRINGAE-2 (RPS2)-upregulated phosphorylation sites.
Table S3 Summary of RESISTANT TO P. SYRINGAE-2 (RPS2)-downregulated phosphorylation sites.
Table S4 Summary of the phosphorylation sites discussed in the manuscript.
Table S5 Maxquant search parameters for LC-MS/MS data analysis.
Table S6 MS1 intensities and MS/MS counts for selected phosphorylation sites.
Table S7 Specific transitions used for selected reaction monitoring (SRM) on RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD).
Table S8 Primers used in this study.
Figure S1 Dexamethasone (Dex)-inducible avrRpt2 expression in Arabidopsis.
Figure S2 Alignments of differentially phosphorylated sites in PIP, AHA and PIN proteins.
Figure S3 RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD) phosphorylation sites S343 and S347 are required for flagellin 22 (flg22)-inducible reactive oxygen species (ROS) burst.
Figure S4 Conservation of identified RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD) phosphorylation sites in the phosphoproteomic screen.
Figure S5 The rbohD/pRBOHD:3xFLAG-RBOHD (S343A/S347A) complementation line is resistant to Plectosphaerella cucumerina (PcBMM isolate).
Figure S6 Schematic representation of RESISTANT TO P. SYRINGAE-2 (RPS2)domains and the phosphorylation sites during PAMP-triggered immunity (PTI) and effector-triggered immunity (ETI).
Acknowledgments
We thank all the members of the Shirasu, Zipfel, and Coaker lab for fruitful discussions, and the UC Davis proteomics core, Dr. Mitch Elmore, Mrs. Naomi Watanabe, Mrs. Noriko Maki, and Mrs. Mamiko Kouzai for technical support. We also thank Dr. Jian-Min Zhou for providing materials. This work was supported by the European Research Council (to C.Z.), The Gatsby Charitable Foundation (to C.Z.), JSPS KAKENHI Grant Numbers JP16H06186 and JP16KT0037 (to Y.K), JP15H05959 and JP17H06172 (to K.S), JP16J0071 (to Y.G), the National Institute of Health RO1GM092772 (to G.C.), the US Department of Agriculture USDA-NIFA 2015-67013-23082 (to G.C) and by the Ministerio de Economía y Competitividad of Spain (BIO2015–64077-R to A. M. and M.A.T). T.W.H.L. was supported by a Rubicon grant of the Netherlands Organisation for Scientific Research (NWO).
References
- Adachi H, Nakano T, Miyagawa N, Ishihama N, Yoshioka M, Katou Y, Yaeno T, Shirasu K, Yoshioka H. 2015. WRKY Transcription factors fhosphorylated by MAPK regulate a plant immune NADPH oxidase in Nicotiana benthamiana. Plant Cell 27(9): 2645–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop JJ, Mohammed S, O’Flaherty M, Heck AJ, Slijper M, Menke FL. 2007. Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol Cell Proteomics 6(7): 1198–1214. [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J. 2010. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 464(7287): 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrot F, Zipfel C. 2017. Function, discovery, and exploitation of plant pattern recognition receptors for broad-spectrum disease resistance. Annu Rev Phytopathol 55: 257–286. [DOI] [PubMed] [Google Scholar]
- Chakravorty D, Gookin TE, Milner MJ, Yu Y, Assmann SM. 2015. Extra-large G rroteins expand the repertoire of subunits in Arabidopsis heterotrimeric G protein signaling. Plant Physiol 169(1): 512–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouch S, Queval G, Noctor G. 2012. AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J 69(4): 613–627. [DOI] [PubMed] [Google Scholar]
- Chung EH, El-Kasmi F, He Y, Loehr A, Dangl JL. 2014. A plant phosphoswitch platform repeatedly targeted by type III effector proteins regulates the output of both tiers of plant immune receptors. Cell Host Microbe 16(4): 484–494. [DOI] [PubMed] [Google Scholar]
- Costa A, Luoni L, Marrano CA, Hashimoto K, Koster P, Giacometti S, De Michelis MI, Kudla J, Bonza MC. 2017. Ca2+-dependent phosphoregulation of the plasma membrane Ca2+-ATPase ACA8 modulates stimulus-induced calcium signatures. J Exp Bot 68(12): 3215–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui FH, Wu SJ, Sun WX, Coaker G, Kunkel B, He P, Shan LB. 2013. The Pseudomonas syringae Type III rffector AvrRpt2 promotes pathogen virulence via stimulating Arabidopsis auxin/indole acetic acid protein turnover. Plant Physiology 162(2): 1018–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran A, Chang IF, Chang CL, Garg S, Miguel RM, Barron YD, Li Y, Romanowsky S, Cushman JC, Gribskov M, et al. 2011. Calcium-dependent protein kinases from Arabidopsis show substrate specificity differences in an analysis of 103 substrates. Front Plant Sci 2: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN, Rathjen JP. 2010. Plant immunity: towards an integrated view of plant–pathogen interactions. Nature Reviews Genetics 11(8): 539–548. [DOI] [PubMed] [Google Scholar]
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte CP, Schulze WX, Romeis T. 2013. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci U S A 110(21): 8744–8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore JM, Liu J, Smith B, Phinney B, Coaker G. 2012. Quantitative proteomics reveals dynamic changes in the plasma membrane during Arabidopsis immune signaling. Mol Cell Proteomics 11(4): M111 014555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschen-Lippold L, Jiang X, Elmore JM, Mackey D, Shan L, Coaker G, Scheel D, Lee J. 2016. Bacterial AvrRpt2-like cysteine proteases block activation of the Arabidopsis mitogen-activated protein kinases, MPK4 and MPK11. Plant Physiol 171(3): 2223–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei dit Frey N, Mbengue M, Kwaaitaal M, Nitsch L, Altenbach D, Haweker H, Lozano-Duran R, Njo MF, Beeckman T, Huettel B, et al. 2012. Plasma membrane calcium ATPases are important components of receptor-mediated signaling in plant immune responses and development. Plant Physiol 159(2): 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen X, Lin W, Chen S, Lu D, Niu Y, Li L, Cheng C, McCormack M, Sheen J, et al. 2013. Bifurcation of Arabidopsis NLR immune signaling via Ca2+-dependent protein kinases. PLoS Pathog 9(1): e1003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti S, Marrano CA, Bonza MC, Luoni L, Limonta M, De Michelis MI. 2012. Phosphorylation of serine residues in the N-terminus modulates the activity of ACA8, a plasma membrane Ca2+-ATPase of Arabidopsis thaliana. J Exp Bot 63(3): 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J. 2007. BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17(13): 1109–1115. [DOI] [PubMed] [Google Scholar]
- Hernandez Sebastia C, Hardin SC, Clouse SD, Kieber JJ, Huber SC. 2004. Identification of a new motif for CDPK phosphorylation in vitro that suggests ACC synthase may be a CDPK substrate. Arch Biochem Biophys 428(1): 81–91. [DOI] [PubMed] [Google Scholar]
- Hoehenwarter W, Thomas M, Nukarinen E, Egelhofer V, Rohrig H, Weckwerth W, Conrath U, Beckers GJ. 2013. Identification of novel in vivo MAP kinase substrates in Arabidopsis thaliana through use of tandem metal oxide affinity chromatography. Mol Cell Proteomics 12(2): 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JZ, Hardin SC, Huber SC. 2001. Identification of a novel phosphorylation motif for CDPKs: phosphorylation of synthetic peptides lacking basic residues at P-3/P-4. Arch Biochem Biophys 393(1): 61–66. [DOI] [PubMed] [Google Scholar]
- Huang JZ, Huber SC. 2001. Phosphorylation of synthetic peptides by a CDPK and plant SNF1-related protein kinase. Influence of proline and basic amino acid residues at selected positions. Plant Cell Physiol 42(10): 1079–1087. [DOI] [PubMed] [Google Scholar]
- Johansson ON, Fantozzi E, Fahlberg P, Nilsson AK, Buhot N, Tor M, Andersson MX. 2014. Role of the penetration-resistance genes PEN1, PEN2 and PEN3 in the hypersensitive response and race-specific resistance in Arabidopsis thaliana. Plant J 79(3): 466–476. [DOI] [PubMed] [Google Scholar]
- Jones AM, Bennett MH, Mansfield JW, Grant M. 2006. Analysis of the defence phosphoproteome of Arabidopsis thaliana using differential mass tagging. Proteomics 6(14): 4155–4165. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. 2006. The plant immune system. Nature 444(7117): 323–329. [DOI] [PubMed] [Google Scholar]
- Kadota Y, Shirasu K, Zipfel C. 2015. Regulation of the NADPH Oxidase RBOHD during plant immunity. Plant Cell Physiol 56(8): 1472–1480. [DOI] [PubMed] [Google Scholar]
- Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JD, Shirasu K, Menke F, Jones A, et al. 2014. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell 54(1): 43–55. [DOI] [PubMed] [Google Scholar]
- Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Qamar SA, Mengiste T, Betsuyaku S, Parker JE, Mussig C, et al. 2007. The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol 17(13): 1116–1122. [DOI] [PubMed] [Google Scholar]
- Khan M, Subramaniam R, Desveaux D. 2016. Of guards, decoys, baits and traps: pathogen perception in plants by type III effector sensors. Curr Opin Microbiol 29: 49–55. [DOI] [PubMed] [Google Scholar]
- Kim MG, da Cunha L, McFall AJ, Belkhadir Y, DebRoy S, Dangl JL, Mackey D. 2005. Two Pseudomonas syringae type III effectors inhibit RIN4-regulated basal defense in Arabidopsis. Cell 121(5): 749–759. [DOI] [PubMed] [Google Scholar]
- Kim MG, Geng X, Lee SY, Mackey D. 2009. The Pseudomonas syringae type III effector AvrRpm1 induces significant defenses by activating the Arabidopsis nucleotide-binding leucine-rich repeat protein RPS2. Plant J 57(4): 645–653. [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. 2003. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22(11): 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Bednarek P, Schulze-Lefert P. 2008. Secretory pathways in plant immune responses. Plant Physiol 147(4): 1575–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD. 2004. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4(3): 181–189. [DOI] [PubMed] [Google Scholar]
- Lee D, Bourdais G, Yu G, Robatzek S, Coaker G. 2015. Phosphorylation of the plant immune regulator RPM1-INTERACTING PROTEIN4 enhances plant plasma membrane H+-ATPase activity and inhibits flagellin-triggered immune responses in Arabidopsis. Plant Cell 27(7): 2042–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y, et al. 2014. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15(3): 329–338. [DOI] [PubMed] [Google Scholar]
- Liang X, Ding P, Lian K, Wang J, Ma M, Li L, Li L, Li M, Zhang X, Chen S, et al. 2016. Arabidopsis heterotrimeric G proteins regulate immunity by directly coupling to the FLS2 receptor. Elife 5: e13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Elmore JM, Lin ZJ, Coaker G. 2011. A receptor-like cytoplasmic kinase phosphorylates the host target RIN4, leading to the activation of a plant innate immune receptor. Cell Host Microbe 9(2): 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang S. 2004. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16(12): 3386–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofke C, Luschnig C, Kleine-Vehn J. 2013. Posttranslational modification and trafficking of PIN auxin efflux carriers. Mech Dev 130(1): 82–94. [DOI] [PubMed] [Google Scholar]
- Lolle S, Greeff C, Petersen K, Roux M, Jensen MK, Bressendorff S, Rodriguez E, Somark K, Mundy J, Petersen M. 2017. Matching NLR immune receptors to autoimmunity in camta3 mutants using antimorphic NLR alleles. Cell Host Microbe 21(4): 518–529e514. [DOI] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A, Pauly N. 2012. A burst of plant NADPH oxidases. Trends Plant Sci 17(1): 9–15. [DOI] [PubMed] [Google Scholar]
- Mattei B, Spinelli F, Pontiggia D, De Lorenzo G. 2016. Comprehensive analysis of the membrane phosphoproteome regulated by oligogalacturonides in Arabidopsis thaliana. Front Plant Sci 7: 1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Boursiac Y, Luu DT, Santoni V, Shahzad Z, Verdoucq L. 2015. Aquaporins in plants. Physiol Rev 95(4): 1321–1358. [DOI] [PubMed] [Google Scholar]
- McNellis TW, Mudgett MB, Li K, Aoyama T, Horvath D, Chua NH, Staskawicz BJ. 1998. Glucocorticoid-inducible expression of a bacterial avirulence gene in transgenic Arabidopsis induces hypersensitive cell death. Plant J 14(2): 247–257. [DOI] [PubMed] [Google Scholar]
- Mersmann S, Bourdais G, Rietz S, Robatzek S. 2010. Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol 154(1): 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine A, Seyfferth C, Kracher B, Berens ML, Becker D, Tsuda K. 2018. The defense phytohormone signaling network enables rapid, high-amplitude transcriptional reprogramming during effector-triggered immunity. Plant Cell 30(6): 1199–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Kadota Y, Zipfel C, Molina A, Torres MA. 2016. The Arabidopsis NADPH oxidases RbohD and RbohF display differential expression patterns and contributions during plant immunity. J Exp Bot 67(6): 1663–1676. [DOI] [PubMed] [Google Scholar]
- Morsomme P, Boutry M. 2000. The plant plasma membrane H+-ATPase: structure, function and regulation. Biochim Biophys Acta 1465(1–2): 1–16. [DOI] [PubMed] [Google Scholar]
- Nühse TS, Bottrill AR, Jones AME, Peck SC. 2007. Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. The Plant Journal 51(5): 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC. 2003. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301(5635): 969–972. [DOI] [PubMed] [Google Scholar]
- Pecher P, Eschen-Lippold L, Herklotz S, Kuhle K, Naumann K, Bethke G, Uhrig J, Weyhe M, Scheel D, Lee J. 2014. The Arabidopsis thaliana mitogen-activated protein kinases MPK3 and MPK6 target a subclass of ‘VQ-motif’-containing proteins to regulate immune responses. New Phytol 203(2): 592–606. [DOI] [PubMed] [Google Scholar]
- Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE, et al. 2000. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 103(7): 1111–1120. [DOI] [PubMed] [Google Scholar]
- Piasecka A, Jedrzejczak-Rey N, Bednarek P. 2015. Secondary metabolites in plant innate immunity: conserved function of divergent chemicals. New Phytol 206(3): 948–964. [DOI] [PubMed] [Google Scholar]
- Pogany M, von Rad U, Grun S, Dongo A, Pintye A, Simoneau P, Bahnweg G, Kiss L, Barna B, Durner J. 2009. Dual roles of reactive oxygen species and NADPH oxidase RBOHD in an Arabidopsis–Alternaria pathosystem. Plant Physiol 151(3): 1459–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Katagiri F. 2009. Purification of low-abundance Arabidopsis plasma-membrane protein complexes and identification of candidate components. Plant J 57(5): 932–944. [DOI] [PubMed] [Google Scholar]
- Rayapuram N, Bonhomme L, Bigeard J, Haddadou K, Przybylski C, Hirt H, Pflieger D. 2014. Identification of novel PAMP-triggered phosphorylation and dephosphorylation events in Arabidopsis thaliana by quantitative phosphoproteomic analysis. J Proteome Res 13(4): 2137–2151. [DOI] [PubMed] [Google Scholar]
- Rodrigues O, Reshetnyak G, Grondin A, Saijo Y, Leonhardt N, Maurel C, Verdoucq L. 2017. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc Natl Acad Sci U S A 114(34): 9200–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh AH, Eschen-Lippold L, Pecher P, Hoehenwarter W, Sinha AK, Scheel D, Lee J. 2016. Regulation of WRKY46 transcription factor function by mitogen-activated protein kinases in Arabidopsis thaliana. Front Plant Sci 7: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensson C, Lenman M, Veide-Vilg J, Schopper S, Ljungdahl T, Grotli M, Tamas MJ, Peck SC, Andreasson E. 2012. Determination of primary sequence specificity of Arabidopsis MAPKs MPK3 and MPK6 leads to identification of new substrates. Biochem J 446(2): 271–278. [DOI] [PubMed] [Google Scholar]
- Spoel SH, Dong X. 2012. How do plants achieve immunity? Defence without specialized immune cells. Nat Rev Immunol 12(2): 89–100. [DOI] [PubMed] [Google Scholar]
- Su J, Yang L, Zhu Q, Wu H, He Y, Liu Y, Xu J, Jiang D, Zhang S. 2018. Active photosynthetic inhibition mediated by MPK3/MPK6 is critical to effector-triggered immunity. PLoS Biol 16(5): e2004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. 2011. Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14(6): 691–699. [DOI] [PubMed] [Google Scholar]
- Svennelid F, Olsson A, Piotrowski M, Rosenquist M, Ottman C, Larsson C, Oecking C, Sommarin M. 1999. Phosphorylation of Thr-948 at the C terminus of the plasma membrane H+-ATPase creates a binding site for the regulatory 14-3-3 protein. Plant Cell 11(12): 2379–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. 2008. Local positive feedback regulation determines cell shape in root hair cells. Science 319(5867): 1241–1244. [DOI] [PubMed] [Google Scholar]
- Thaler JS, Humphrey PT, Whiteman NK. 2012. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17(5): 260–270. [DOI] [PubMed] [Google Scholar]
- Thomma BP, Nurnberger T, Joosten MH. 2011. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 23(1): 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. 1997. Subcellular localization of H2O2 in plants. H2O2 accumulation in papilae and hypersensitive response during the barley-powdery mildew interaction. The Plant Journal 11(6): 1187–1194. [Google Scholar]
- Tian S, Wang X, Li P, Wang H, Ji H, Xie J, Qiu Q, Shen D, Dong H. 2016. Plant aquaporin AtPIP1;4 links Apoplastic H2O2 induction to disease immunity pathways. Plant Physiol 171(3): 1635–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD. 2002. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci U S A 99(1): 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Morales J, Sanchez-Rodriguez C, Molina A, Dangl JL. 2013. Functional interplay between Arabidopsis NADPH oxidases and heterotrimeric G protein. Mol Plant Microbe Interact 26(6): 686–694. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Katagiri F. 2010. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol 13(4): 459–465. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Mine A, Bethke G, Igarashi D, Botanga CJ, Tsuda Y, Glazebrook J, Sato M, Katagiri F. 2013. Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana. PLoS Genet 9(12): e1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyanova S, Temu T, Cox J. 2016a. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc 11(12): 2301–2319. [DOI] [PubMed] [Google Scholar]
- Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, Mann M, Cox J. 2016b. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods 13(9): 731–740. [DOI] [PubMed] [Google Scholar]
- Underwood W, Somerville SC. 2017. Phosphorylation is required for the pathogen defense function of the Arabidopsis PEN3 ABC transporter. Plant Signal Behav 12(10): e1379644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaino JA, Cote RG, Csordas A, Dianes JA, Fabregat A, Foster JM, Griss J, Alpi E, Birim M, Contell J, et al. 2013. The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res 41(Database issue): D1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Grubb LE, Wang J, Liang X, Li L, Gao C, Ma M, Feng F, Li M, Li L, et al. 2018. A regulatory module controlling homeostasis of a plant immune kinase. Mol Cell 69(3): 493–504e496. [DOI] [PubMed] [Google Scholar]
- Xin XF, Nomura K, Aung K, Velasquez AC, Yao J, Boutrot F, Chang JH, Zipfel C, He SY. 2016. Bacteria establish an aqueous living space in plants crucial for virulence. Nature 539(7630): 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi S, Takemiya A, Sakamoto T, Kurata T, Tsutsumi T, Kinoshita T, Shimazaki K. 2016. The plasma membrane H+-ATPase AHA1 plays a major role in stomatal opening in response to blue light. Plant Physiol 171(4): 2731–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Feng B, He P, Shan L. 2017. From chaos to harmony: responses and signaling upon microbial pattern recognition. Annu Rev Phytopathol 55: 109–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, Zou Y, Gao M, Zhang X, Chen S, et al. 2010. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7(4): 290–301. [DOI] [PubMed] [Google Scholar]
- Zhang M, Chiang Y-H, Toruño TY, Lee D, Ma M, Liang X, Lal NK, Lemos M, Lu Y-J, Ma S, et al. 2018. The MAP4 Kinase SIK1 ensures robust extracellular ROS burst and antibacterial immunity in plants. Cell Host & Microbe 24(3): 379–391.e375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Liu Y, Huang H, Gao M, Wu D, Kong Q, Zhang Y. 2017. The NLR protein SUMM2 senses the disruption of an immune signaling MAP kinase cascade via CRCK3. EMBO Rep 18(2): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wu Y, Gao M, Zhang J, Kong Q, Liu Y, Ba H, Zhou J, Zhang Y. 2012. Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe 11(3): 253–263. [DOI] [PubMed] [Google Scholar]
- Zhu H, Li GJ, Ding L, Cui X, Berg H, Assmann SM, Xia Y. 2009. Arabidopsis extra large G-protein 2 (XLG2) interacts with the Gbeta subunit of heterotrimeric G protein and functions in disease resistance. Mol Plant 2(3): 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Summary of significantly differentially phosphorylated sites and corresponding protein levels.
Methods S1 Supplemental methods describing materials, phosphoproteomic analysis, reactive oxygen species (ROS) assay, qRT-PCR, western blotting and disease assays.
Table S2 Summary of RESISTANT TO P. SYRINGAE-2 (RPS2)-upregulated phosphorylation sites.
Table S3 Summary of RESISTANT TO P. SYRINGAE-2 (RPS2)-downregulated phosphorylation sites.
Table S4 Summary of the phosphorylation sites discussed in the manuscript.
Table S5 Maxquant search parameters for LC-MS/MS data analysis.
Table S6 MS1 intensities and MS/MS counts for selected phosphorylation sites.
Table S7 Specific transitions used for selected reaction monitoring (SRM) on RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD).
Table S8 Primers used in this study.
Figure S1 Dexamethasone (Dex)-inducible avrRpt2 expression in Arabidopsis.
Figure S2 Alignments of differentially phosphorylated sites in PIP, AHA and PIN proteins.
Figure S3 RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD) phosphorylation sites S343 and S347 are required for flagellin 22 (flg22)-inducible reactive oxygen species (ROS) burst.
Figure S4 Conservation of identified RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD) phosphorylation sites in the phosphoproteomic screen.
Figure S5 The rbohD/pRBOHD:3xFLAG-RBOHD (S343A/S347A) complementation line is resistant to Plectosphaerella cucumerina (PcBMM isolate).
Figure S6 Schematic representation of RESISTANT TO P. SYRINGAE-2 (RPS2)domains and the phosphorylation sites during PAMP-triggered immunity (PTI) and effector-triggered immunity (ETI).