Abstract
Although sensitive detection of pathological cognitive aging requires accurate information about the trajectory of normal cognitive aging, prior research has revealed inconsistent patterns of age-cognition relations with cross-sectional and longitudinal comparisons. Age trends in four cognitive domains were compared in over 5,000 adults with cross-sectional data, and in almost 1,600 adults with three-occasion longitudinal data. Quasi-longitudinal comparisons, which are similar to cross-sectional comparisons in that there is no prior test experience, and are similar to longitudinal comparisons in that the participants are from the same birth cohorts, were also reported. The age trends in quasi-longitudinal comparisons more closely resembled those in cross-sectional comparisons than those in longitudinal comparisons, which suggests that, at least up until about age 65, age-cognition relations in longitudinal comparisons are distorted by prior test experience. Results from cross-sectional and quasi-longitudinal comparisons, which can be assumed to have minimal test experience effects, imply that normal cognitive aging is characterized by nearly linear declines from early adulthood in speed, and accelerating declines in memory and reasoning. However, vocabulary knowledge increased until the decade of the 60’s in all three types of comparisons.
Keywords: cross-sectional, longitudinal, cohort differences, retest effects
It is widely recognized that memory and other cognitive abilities deteriorate in pathological conditions such as dementia, but less is known about the relations between age and cognition in the absence of disease. This is unfortunate because accurate description of normal aging is essential to provide a baseline against which abnormal aging can be contrasted. That is, pathological functioning cannot be accurately defined in the absence of information about normal functioning.
Characterization of non-pathological cognitive aging is important for at least three additional reasons. First, precise specification of the trajectory of normal aging is valuable to evaluate the plausibility of potential causes of the phenomenon. For example, interpretations emphasizing the importance of a discrete event, such as menopause or retirement, on the relations between age and cognitive functioning would be called into question if cognitive performance was found to decline continuously from early adulthood. Second, identification of the earliest age of cognitive decline is important in establishing the optimal period for interventions intended to minimize or prevent decline. To illustrate, interventions targeted at older adults may not be very effective if a considerable amount of cognitive decline has already occurred. And third, even if they are small relative to differences associated with pathology, gradual changes that accumulate over a period of decades could have negative effects on quality of life, and accurate description of those changes is an important first step in their ultimate prevention.
Relations between age and cognitive functioning are commonly assessed with either cross-sectional (between-person) or longitudinal (within-person) comparisons. Cross-sectional age trends in measures of cognitive functioning are quite robust as nearly linear patterns of decline have been reported at different periods in historical time (e.g., Foster & Taylor, 1920; Jones & Conrad, 1933; Kaufman et al., 2016; Figure 2.6 in Salthouse, 2010a; Figure 1 in Salthouse, 2016b), in samples of adults who presumably had high levels of motivation because the tests were used for vocational selection (e.g., Fozard & Nuttall, 1971; Trembly & O’Connor, 1966), and with different modes of data collection such as stimuli presented, and/or responses recorded, via television (e.g., Broadbent & Gregory, 1965), internet (e.g., Hampshire et al., 2012; Hartshorne & Germine, 2015; Johnson et al., 2010; Logie & Maylor, 2009; Murre et al., 2013; Sternberg et al., 2013), telephone (e.g., Lachman et al., 2014), or in the context of video games (e.g., Lee et al., 2012; Thompson et al., 2014).
However, because the comparisons are based on different people at each age, individuals of varying ages may not be equivalent in all important respects. Moreover, even if the samples of participants at different ages did not differ in any relevant characteristics other than age, cross-sectional comparisons based on people of different ages only provide indirect information about change. Direct measurement of change requires longitudinal comparisons in which the same individuals are assessed at each age.
In contrast to the approximately linear age-cognition relations apparent in cross-sectional comparisons, longitudinal comparisons often reveal increasing or stable relations between age and cognition in young and middle-aged adults, followed by negative changes at older ages (e.g., Bielak et al., 2012; Caselli et al., 2009; Ferrer et al., 2004; Finkel et al. 1998; Giambra et al., 1995; Huppert & Whittington, 1993; Lamar et al., 2003; McArdle et al., 2002; Mitchell et al., 2012; Parisi et al., 2011; Ronnlund & Nilsson, 2006; Ronnlund et al., 2005; Schaie, 2013; Schaie & Hertzog, 1983; Singh-Manoux et al., 2012; van der Elst et al., 2008; van Dijk et al., 2008; Zelinski & Burnight, 1997).
Two major factors, cohort differences and practice effects, have been postulated to contribute to the discrepancy between cross-sectional and longitudinal age trends. The cohort interpretation is based on the idea that people of different ages in cross-sectional comparisons belong to different birth cohorts, and thus might have had different educational and cultural experiences throughout their lives that could have influenced their level of cognitive performance. Because longitudinal comparisons involve the same people (who are thus from the same birth cohort) at different ages, they are not subject to this type of age-cohort confound.
The practice interpretation focuses on the fact that successive assessments in longitudinal comparisons not only occur when the individual is older, but also when he or she has had prior experience with the cognitive tests. This raises the possibility that at least some of the longitudinal change in performance could be attributable to effects of test experience rather than to effects related to aging or maturation. Because participants in cross-sectional studies are only tested once, cross-sectional comparisons are not affected by this type of test experience, or practice, effect.
Resolution of the discrepancy between the age-cognition relations in the two types of comparisons therefore largely depends on whether cross-sectional comparisons are misleading because of cohort differences, or whether longitudinal comparisons are misleading because of practice effects. A research design originally introduced by Schaie and colleagues (e.g., Schaie et al., 1973; Schaie & Strother, 1968) is particularly valuable in this respect because it provides estimates of age-cognition relations among people of the same birth cohort without a contamination of prior test experience. That is, in the quasi-longitudinal method (which Schaie termed the independent-samples same-cohort method), the differences in performance of different people from the same birth cohort who are tested in different years, and hence at different ages, are used as an estimate of within-cohort change without a confound of prior test experience. For example, one-half of a sample of people born in 1960 could be tested in 2010 when they were 50 years old, and the other half could be tested in 2020 when they were 60 years old. Because both groups are from the same birth cohort (i.e., the 1960 birth year), and are only tested once, the difference in performance between 50-year-olds in 2010 and 60-year-olds in 2020 can be postulated to reflect effects of age without confounds associated with different birth cohorts or prior test experience. The difference in these two groups can be compared with the cross-sectional difference between 50-year-olds and 60-year-olds in either 2010 or 2020 in which the difference reflects cohort differences in addition to age, and with the observed longitudinal change in which the difference between the 2010 and 2020 assessments reflects test experience effects in addition to effects of age. A finding that quasi-longitudinal age trends resembled cross-sectional age trends would therefore imply that test experience effects were more important determinants of age-cognition relations than cohort effects, whereas a finding of similar age trends in quasi-longitudinal and longitudinal comparisons would imply that cohort effects were more important than test experience effects.
Very few comparisons of cross-sectional, longitudinal, and quasi-longitudinal age-cognition relations have been reported because of the need to collect data from relatively large samples of adults of different ages at different periods of time. Some comparisons of this type were reported by Schaie and colleagues (e.g., Schaie et al., 1973; Schaie & Strother, 1968), but their interpretations of the results were challenged by later researchers (e.g., Horn & Donaldson, 1976; Salthouse, 1991). Quasi-longitudinal comparisons were reported across two occasions in subsets of the current sample of participants (Salthouse, 2013, 2014a), and in analyses of multiple-occasion data from two other projects (Salthouse, 2016a).
The goal of the current project was to further investigate the trajectory of normal cognitive aging by comparing age trends with quasi-longitudinal, as well as cross-sectional and longitudinal, methods in the same moderately large sample of participants and with the same combination of cognitive tests. The analyses in this report extend the earlier studies by examining age relations across three longitudinal occasions spanning an average interval of nearly six years, and providing numerical estimates of the age relations with cross-sectional, longitudinal, and quasi-longitudinal data.
Method
Participants
Community-residing adults were recruited by advertisements, flyers, and referrals from other participants. Informed consent was obtained from all participants, and the project was approved by the local Institutional Review Board. Data collection started in 2001, with new participants recruited in subsequent years continuing through 2017. Longitudinal assessments began in 2004, and continued with average intervals between occasions of about 3 years. Approximately 79% of the participants identified themselves as white, and 12% as black, with the remainder classifying themselves as American Indian, Asian, or a mixture of several ethnicities. Characteristics of the 5,098 participants with cross-sectional data, and of the subset of 1,598 participants with three-occasion longitudinal data, are summarized in Table 1. On average the participants reported themselves to be in very good to excellent health, had completed over 15 years of education, and had above-average estimated IQs. The average interval between the first and third occasion for the longitudinal participants was 5.9 years.
Table 1.
Participant characteristics by age decade in cross-sectional and 3-occasion longitudinal data
| Decade | N | Age | Sex | Health | Educ | MMSE | Est. lQ | T1–T3 lnt. |
|---|---|---|---|---|---|---|---|---|
| Cross Sectional | ||||||||
| 20’s | 915 | 23.1 (3.2) | .58 (.49) | 2.0 (0.9) | 14.6 (2.1) | 28.8 (1.7) | 106.4 (12.5) | NA |
| 30’s | 510 | 34.3 (2.8) | .69 (.46) | 2.1 (0.8) | 15.7 (2.9) | 28.5 (1.8) | 107.3 (15.2) | NA |
| 40’s | 792 | 45.0 (2.9) | .71 (.45) | 2.2 (0.9) | 15.2 (2.7) | 28.4 (1.9) | 107.4 (15.6) | NA |
| 50’s | 1166 | 54.5 (2.8) | .71 (.46) | 2.2 (0.9) | 15.7 (2.6) | 28.3 (2.0) | 109.3 (15.2) | NA |
| 60’s | 907 | 64.1 (2.8) | .66 (.48) | 2.1 (0.9) | 16.3 (2.8) | 28.4 (1.9) | 111.7 (13.5) | NA |
| 70’s | 567 | 74.2 (2.8) | .58 (.49) | 2.4 (0.9) | 15.9 (2.9) | 28.1 (1.9) | 109.3 (13.5) | NA |
| 80’s | 241 | 83.0 (2.5) | .51 (.50) | 2.6 (0.8) | 16.1 (3.1) | 27.2 (2.5) | 105.9 (13.8) | NA |
| All | 5098 | 50.6 (18.0) | .65 (.48) | 2.2 (0.9) | 15.6 (2.7) | 28.4 (1.9) | 109.2 (14.3) | NA |
| Longitudinal | ||||||||
| 20’s | 124 | 23.0 (3.5) | .62 (.49) | 2.0 (0.9) | 14.1 (2.0) | 28.5 (1.8) | 106.6 (13.1) | 6.0 (2.2) |
| 30’s | 121 | 34.9 (3.0) | .75 (.43) | 2.3 (0.8) | 15.5 (2.4) | 28.2 (1.9) | 107.3 (17.4) | 6.3 (2.2) |
| 40’s | 298 | 45.2 (2.9) | .70 (.46) | 2.1 (0.9) | 15.4 (2.4) | 28.5 (1.7) | 109.2 (15.2) | 6.4 (2.2) |
| 50’s | 454 | 54.3 (2.9) | .72 (.45) | 2.1 (0.9) | 16.0 (2.7) | 28.6 (1.7) | 112.5 (14.8) | 5.9 (1.9) |
| 60’s | 382 | 64.1 (2.8) | .67 (.47) | 2.1 (0.9) | 16.5 (2.6) | 28.7 (1.6) | 113.8 (13.3) | 5.6 (1.7) |
| 70’s | 183 | 74.0 (2.9) | .59 (.49) | 2.3 (0.9) | 16.3 (2.9) | 28.6 (1.6) | 113.3 (13.0) | 5.6 (1.7) |
| 80’s | 36 | 82.8 (2.6) | .50 (.51) | 2.5 (0.8) | 16.6 (4.0) | 27.8 (2.0) | 111.1 (11.7) | 5.3 (1.8) |
| All | 1598 | 54.0 (14.6) | .68 (.47) | 2.2 (0.9) | 15.9 (2.7) | 28.6 (1.7) | 111.5 (14.5) | 5.9 (2.0) |
Note: Values in parentheses are standard deviations. Sex refers to proportion of females, health is a self-rating on a scale from 1 for excellent to 5 for poor, Educ is years of education, MMSE is score on the Mini Mental State Exam (Folstein et al., 1975), Est. IQ is an estimate of IQ based on age-adjusted scores on three tests found to be highly related to Wechsler IV full scale IQ (Salthouse, 2014b), and T1–T3 Int. is the number of years between the first and third longitudinal occasion. NA indicates that the estimate is not applicable
Selectivity of attrition in this project has been examined in several earlier articles (e.g., Salthouse, 2010b; 2014b), where it was reported that among older adults the returning participants had higher levels of cognitive performance on the initial occasion than non-returning participants, but if anything, this pattern was reversed among young adults.
Tests
Cognitive functioning was evaluated with scores on 13 tests, representing four cognitive domains. (Tests of spatial visualization were also administered but results with those measures are not reported here because they were very similar to the results with measures of reasoning.) Episodic memory was assessed with a Paired Associates test (Salthouse et al., 1996) and with two subtests of the Wechsler Memory Scale (Wechsler, 1997b), Logical Memory and Word Recall. Reasoning was assessed with the Raven’s Progressive Matrices (Raven, 1962), Letter Sets (Ekstrom et al., 1976), and Shipley Abstraction (Zachary, 1986) tests. Perceptual speed was assessed with the Digit Symbol subtest (Wechsler, 1997a), and the Letter Comparison and Pattern Comparison (Salthouse & Babcock, 1991) tests. Vocabulary was assessed with four tests (i.e., the WAIS–III Vocabulary subtest [Wechsler, 1997a], the Woodcock–Johnson Picture Vocabulary subtest [Woodcock & Johnson, 1990], and Synonym and Antonym Vocabulary tests [Salthouse, 1993]).
Analyses
In order to maximize reliability and generalizability, and minimize idiosyncratic aspects of single measures, the analyses were conducted on composite scores created by averaging the z-scores from the three, or four for vocabulary, tests representing a given cognitive domain. All of the z-scores were based on the means and standard deviations of the scores in the first occasion completed by all participants. Coefficient alphas for the composite scores based on the scores in relevant tests as items were .80 for memory, .85 for reasoning, .85 for speed, and .91 for vocabulary.
Cross-sectional age relations were estimated by the coefficient for age in linear regression analyses predicting the composite cognitive score. Longitudinal age relations were estimated by the average of the linear slopes, computed separately for each participant, relating the composite cognitive score to the intervals, in years, between the first and second, and between the second and third, occasions. Quasi-longitudinal age relations were estimated by the coefficient for test year in linear regression analyses predicting the composite cognitive score, with birth year and estimated IQ as covariates. Including birth year as a covariate had the effect of conducting the analyses at the average birth year (or cohort), and controlling estimated IQ had the effect of minimizing possible selection differences associated with participant recruitment in different test years (cf. Salthouse, 2013). Separate estimates were derived with each method in participants grouped in successive 10-year age ranges. In order to provide more stable estimates and increase statistical power, values were also reported for two extreme age groups, age 25 to 45 and age 65 to 85.
Results
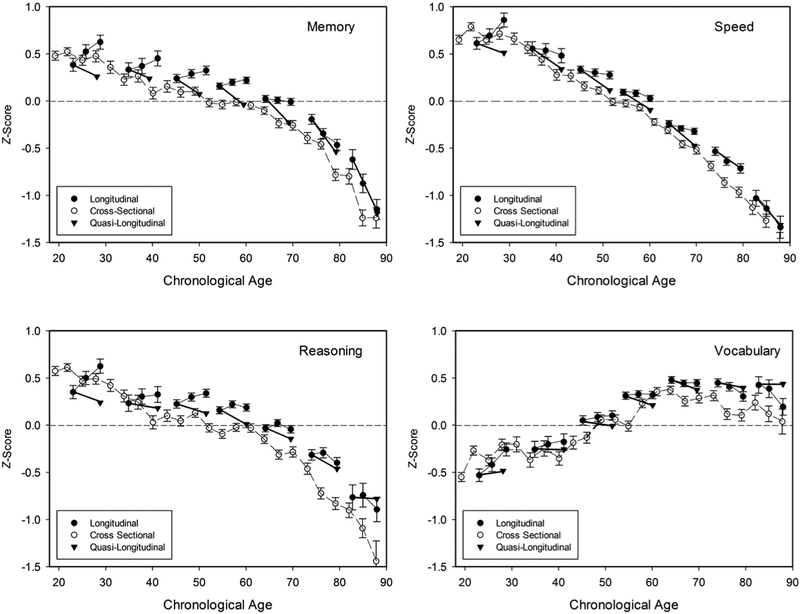
The four panels of Figure 1 portray means of the cross-sectional data, the three-occasion longitudinal data, and the quasi-longitudinal estimates over a time period equal to the interval between the first and third longitudinal occasions. That is, the average longitudinal interval between the first and third occasions was 5.9 years, and thus the quasi-longitudinal estimates represent performance differences in adults from the same average birth year who were tested for the first time an average of 5.9 years apart (see Salthouse, 2014a; 2016a, for more details).
Figure 1 –
Means and standard errors of the cross-sectional and three-occasion longitudinal data, and estimates of quasi-longitudinal relations in four cognitive domains. The quasi-longitudinal trajectories are portrayed as originating at the first longitudinal occasion, and extending over an interval equal to the average longitudinal interval. Quasi-longitudinal values are only reported for the Time 1 and Time 3 occasions to minimize clutter in the figures.
Inspection of the figure reveals that there was a similar pattern with the memory and reasoning measures of positive longitudinal and negative cross-sectional and quasi-longitudinal age relations before about age 65, followed by negative values with each method above that age. Decline was evident with the speed measures with each type of comparison, and at all ages except the youngest. The age relations with vocabulary measures were generally similar in each type of comparison, with a shift from increases at young ages to stability or declines after about age 60. Nevertheless, it is noteworthy that the vocabulary measures were higher among adults in their 70’s and 80’s than among those in their 20’s, 30’s, and 40’s.
Estimates of the age relations per year with the three methods are reported in Table 2. It can be seen that the results were quite consistent with the patterns in the figure. Specifically, for the memory and reasoning measures the longitudinal age relations were positive until the decade of the 60’s after which they were negative, but both the cross-sectional and quasi-longitudinal age relations were negative at all ages. The age relations were negative with each method in all but the youngest ages for the speed measures, and were small and inconsistent for the vocabulary measures.
Table 2.
Age relations, in standard deviation units per year, for longitudinal, cross-sectional, and quasi-longitudinal comparisons by cognitive ability domain and age decade
| Age Group | Longitudinal | Cross-Sectional | Quasi-Longitudinal |
|---|---|---|---|
| Memory | |||
| 20’s | .046* | −.004 | −.021 |
| 30’s | .018 | −.023 | −.016 |
| 40’s | .021* | −.001 | −.028 |
| 50’s | .016* | −.002 | −.033* |
| 60’s | −.003 | −.027* | −.042* |
| 70’s | −.045* | −.035* | −.058* |
| 80’s | −.080* | −.062* | −.097* |
| 25–45 | .026* | −.018* | −.027* |
| 65–85 | −.034* | −.045* | −.073* |
| Speed | |||
| 20’s | .046* | .002 | −.017 |
| 30’s | −.011 | −.046* | −.037* |
| 40’s | −.005 | −.021 | −.037* |
| 50’s | −.008 | −.017 | −.032* |
| 60’s | −.010 | −.035* | −.040* |
| 70’s | −.030* | −.039* | −.030 |
| 80’s | −.053* | −.047* | −.048 |
| 25–45 | −.000 | −.028* | −.042* |
| 65–85 | −.024* | −.047* | −.047* |
| Reasoning | |||
| 20’s | .054* | −.009 | −.019* |
| 30’s | .021 | −.046* | −.009 |
| 40’s | .019* | .008 | −.017 |
| 50’s | .008 | −.007 | −.025* |
| 60’s | .002 | −.038* | −.019* |
| 70’s | −.015 | −.062* | −.025* |
| 80’s | −.041 | −.056* | −.003 |
| 25–45 | .028* | −.025* | −.012* |
| 65–85 | −.014 | −.049* | −.025* |
| Vocabulary | |||
| 20’s | .048* | .030* | .008 |
| 30’s | .014* | −.024 | .000 |
| 40’s | .011* | .038* | −.010 |
| 50’s | .002 | .024 | −.017 |
| 60’s | −.006 | −.010 | −.018 |
| 70’s | −.026* | −.014 | −.009 |
| 80’s | −.039 | −.012 | .002 |
| 25–45 | .020* | .002 | −.003 |
| 65–85 | −.020* | −.011* | −.012 |
p<.01
The patterns were more salient in the 25-to-45 and 65-to-85 groups. That is, in the 25-to-45 age group there were similar negative age relations in the cross-sectional and quasi-longitudinal comparisons, but either positive or stable age relations in the longitudinal comparisons. In contrast, the age relations were negative with each type of comparison and each cognitive domain in the 65–85 age group.
Discussion
Dramatically different age relations with cross-sectional and longitudinal comparisons have contributed to uncertainty about the nature of normal cognitive aging, particularly in adults under about 65 years of age. That is, increased age is associated with lower levels of cognitive performance in cross-sectional comparisons, but is frequently associated with higher levels of cognitive performance in longitudinal comparisons. The two data collection methods differ with respect to the existence of one (longitudinal) or multiple (cross-sectional) birth cohorts, and in the potential presence (longitudinal) or absence (cross-sectional) of practice, or test experience, effects. The current study was motivated by the assumption that quasi-longitudinal comparisons may be informative in identifying the most important determinants of age-cognition relations. That is, quasi-longitudinal comparisons differ from cross-sectional comparisons in that they involve participants from a single birth cohort, and thus different age trends would be expected in quasi-longitudinal and cross-sectional comparisons if cohort differences are important determinants of age-cognition relations. However, unlike longitudinal comparisons, quasi-longitudinal comparisons do not involve prior experience with the tests, and thus quasi-longitudinal and longitudinal comparisons would be expected to have different age trends if test experience effects are important determinants of age-cognition relations.
A major finding in the study was that quasi-longitudinal age trends in each cognitive domain were much more similar to cross-sectional, than to longitudinal, age trends. These results imply that the divergent patterns in the cross-sectional and longitudinal comparisons among young and middle-aged adults in this study are primarily attributable to positive effects of prior test experience in longitudinal comparisons, rather than to the existence of different birth cohorts in cross-sectional comparisons.
The discrepancy between cross-sectional and longitudinal age-cognition relations was most pronounced with memory and reasoning measures, with a smaller discrepancy evident with speed and vocabulary measures. This variation across ability domains may be attributable to the greater likelihood of the development of strategies acquired after experience with memory and reasoning tests, compared to speed and vocabulary tests which may be relatively unaffected by strategies.
The differences between cross-sectional and longitudinal age trends also varied with age, as they were largest in adults under about 65 years of age, with similar age-cognition relations with all three types of age comparisons among older adults. Other studies involving participants across a wide age range have also reported a convergence of cross-sectional and longitudinal trajectories in adults 65 years of age and older (e.g., Alder et al., 1990; Huppert & Whittington, 1993; Ronnlund et al., 2005; Schaie, 2005; Zelinski & Burnight, 1997; also see figures in Salthouse, 2009; 2010b,c; 2011).
It is instructive to consider alternative approaches that could be used to identify age-cognition trajectories. For example, one method of portraying age relations with longitudinal data is with synthetic gradients in which the values from a second longitudinal occasion in one age group are aligned with the values from an initial longitudinal occasion in the next older age group (e.g., Ronnlund et al., 2005; Schaie, 2013). Although connecting values from successive age groups has the advantage of portraying longitudinal data in a format similar to cross-sectional data, this form of representation does not alter the longitudinal age relations. In particular, this method does not distinguish determinants of within-person change associated with age from those associated with prior test experience.
Various types of statistical models have been used to attempt to separate longitudinal change into a component associated with age, and a component associated with prior test experience (e.g., Ferrer et al., 2004; McArdle et al., 2002; Rabbitt et al., 2001; 2004). The models have varied in the analytical methods, and in the form of the growth functions for different types of influences. However, nearly all of the studies using these methods have reported positive values for the estimates of experience effects, which implies that the age-cognition relations were underestimated by the observed longitudinal changes. Apparently only one study involving adults under about 65 years of age has compared the experience-partialled age estimates derived from these models with the age estimates based on cross-sectional and longitudinal comparisons. In that report, McArdle et al. (2002) found nearly identical values of the age-cognition relations for the model-based experience-independent age estimate and for the age estimate from the cross-sectional comparison, both of which were more negative than the estimate from the longitudinal comparison.
Another approach that has been used to estimate test experience effects in longitudinal data is based on the difference in performance between participants tested for the first time with those of the same age tested for the second time. This twice-minus-once-tested difference can then be subtracted from the observed longitudinal change to obtain an estimate of the experience-independent component of longitudinal change. This method has been used in several studies with participants across a wide age range, and in each cased the adjusted age estimates were less positive than the age relations based on traditional longitudinal comparisons (e.g., Ronnlund et al., 2005; Schaie, 2013). Salthouse (2010b) compared these experience-controlled age-cognition estimates with age-cognition estimates from cross-sectional and longitudinal comparisons in data from a subset of the participants from the current study. Of particular interest were the results for adults between 19 and 53 years of age, in which the experience-controlled estimates were much closer to the cross-sectional estimates than to the longitudinal estimates.
This brief review indicates that a similar pattern of less positive longitudinal age relations has been found with different methods of estimating, and controlling, test experience influences. At least among adults younger than about 65 years of age, unadjusted longitudinal comparisons often underestimate the negative relations between age and measures of cognitive functioning.
Several limitations of the study should be noted. For example, assessment of health status was based on crude self reports, and it is possible that some of the participants were experiencing various types of pathologies. Second, the longitudinal interval was relatively short, and greater cohort influences, or smaller test experience influences, might have been apparent with longer intervals. It is nevertheless important to note that a large discrepancy between cross-sectional and longitudinal age trends was evident for young and middle-aged adults in these data. Third, all of the analyses were conducted on composite scores from group data, and it remains to be seen whether similar patterns would be evident in analyses at the level of separate cognitive tests, or on data from individual participants. Fourth, relatively little is known about possible distortions in the age-cognition relations derived from cross-sectional, longitudinal, and quasi-longitudinal comparisons, and the results could be misleading if systematic biases exist in one or more methods. And finally, only two possible determinants of age differences were considered in this study, and other influences, such as those associated with period effects or selective attrition, could also be contributing to the discrepancy between cross-sectional and longitudinal age comparisons.
Despite these limitations, the study has a number of important strengths, such as moderately large samples of participants with each type of data, and examination of a variety of different cognitive measures. Furthermore, the quasi-longitudinal results reported here are consistent with results from prior analyses on subsets of these data across two occasions (Salthouse, 2013; 2014b; 2016a), and with results of analyses of independent data from the Betula Project and from the Seattle Longitudinal Study (Salthouse, 2016a).
To summarize, longitudinal comparisons are essential for assessing within-person change, but results of analyses reported here and elsewhere indicate that they may be misleading as a reflection of the trajectory of normal aging, particularly in adults under about 65 years of age. Specifically, age-cognition relations with longitudinal comparisons can be distorted because of positive effects associated with prior experience with the tests. Estimates from cross-sectional and quasi-longitudinal comparisons are not confounded by across-occasion test experience effects, and thus they may provide the best estimates of the trajectory of normal cognitive aging. Both cross-sectional and quasi-longitudinal comparisons indicate modest declines for memory and reasoning abilities until about age 65 when the decline accelerates, and nearly linear declines in speed from the decade of the 30’s, with an increase followed by modest decline after the 60’s for vocabulary. These patterns, and particularly the early declines in cognitive functioning in presumably healthy adults, should be recognized when attempting to distinguish abnormal or pathological cognitive declines from normal cognitive aging.
Acknowledgments
This research was supported by National Institute on Aging Grant RO1AG024270. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
References
- Alder AG, Adam J, & Arenberg D (1990). Individual-differences assessment of the relationship between change in and initial level of adult cognitive functioning. Psychology and Aging, 5, 560–568. [DOI] [PubMed] [Google Scholar]
- Bielak AAM Anstey KJ, Christensen H, & Windsor TD (2012). Activity engagement is related to level, but not change in cognitive ability across adulthood. Psychology and Aging, 27, 219–228. [DOI] [PubMed] [Google Scholar]
- Broadbent DE & Gregory M (1965). Some confirmatory results on age differences in memory for simultaneous stimulation. British Journal of Psychology, 56, 77–80. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborn D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, Locke DEC, Snyder CH, Alexander GE, Rademakers R, & Reiman EM (2009). Longitudinal modeling of age-related memory decline and the APOE e4 effect. New England Journal of Medicine, 361, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, & Dermen D (1976). Manual for Kit of Factor-Referenced Cognitive Tests. Princeton, NJ: Educational Testing Service. [Google Scholar]
- Ferrer E, Salthouse TA, Stewart W, & Schwartz B (2004). Modeling age and retest processes in longitudinal studies of cognitive abilities. Psychology and Aging, 19, 243–259. [DOI] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL, Plomin R, & McClearn GE (1998). Longitudinal and cross-sectional twin data on cognitive abilities in adulthood: The Swedish Adoption/Twin Study of Aging. Developmental Psychology, 34, 1400–1413. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Foster JC & Taylor GA (1920). The applicability of mental tests to persons over fifty years ago. Journal of Applied Psychology, 4, 39–58. [Google Scholar]
- Fozard JL & Nuttall RL (1971). General aptitude test battery scores for men differing in age and socioeconomic status. Journal of Applied Psychology, 55, 372–379. [Google Scholar]
- Giambra LM, Arenberg D, Zonderman AB, Kawas C, & Costa PT (1995). Adult life span changes in immediate visual memory and verbal intelligence. Psychology and Aging, 10, 123–139. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Highfield RR, Parkin BL, & Owen AM (2012). Fractionating human intelligence. Neuron, 76, 1225–1237. [DOI] [PubMed] [Google Scholar]
- Hartshorne JK & Germine LT (2015). When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychological Science, 26, 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JL & Donaldson G (1976). On the myth of intellectual decline in adulthood. American Psychologist, 31, 701–719. [DOI] [PubMed] [Google Scholar]
- Huppert FA & Whittington JE (1993). Changes in cognitive function in a population sample In Cox BD, Huppert FA, & Whichelow MJ (Eds.). The Health and Lifestyle Survey: Seven Years On. Aldershot, England: Dartmouth Publishing Co. [Google Scholar]
- Johnson W, Logie RH, & Brockmole JR (2010). Working memory tasks differ in factor structure across age cohorts: Implications for dedifferentiation. Intelligence, 38, 513–528. [Google Scholar]
- Jones HE, & Conrad HS (1933). The growth and decline of intelligence: A study of a homogeneous group between the ages of ten and sixty. Genetic Psychology Monographs, 13, No. 3. [Google Scholar]
- Kaufman AS, Salthouse TA, Schieber C & Chen H (2016). Age differences and educational attainment across the lifespan on three generations of Wechsler Adult Scales. Journal of Psychoeducational Assessment, 34, 421–441. [Google Scholar]
- Lachman ME, Agrigoroaei S, Tun PA, & Weaver SL (2014). Monitoring cognitive functioning: Psychometric properties of the Brief Test of Adult Cognition by Telephone. Assessment, 21, 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Resnick SM, & Zonderman AB (2003). Longitudinal changes in verbal memory in older adults: Distinguishing the effects of age from repeat testing. Neurology, 60, 82–86. [DOI] [PubMed] [Google Scholar]
- Lee H, Baniqued PL, Cosman J, Mullen S, McAuley E, Severeson J, & Kramer AF (2012). Examining cognitive function across the lifespan using a mobile application. Computers in Human Behavior, 28, 1934–1946 [Google Scholar]
- Logie RH, & Maylor EA (2009). An internet study of prospective memory across adulthood. Psychology and Aging, 24, 767–774. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, & Woodcock RW (2002). Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Developmental Psychology, 38, 115–142. [PubMed] [Google Scholar]
- Mitchell MB, Cimino CR, Benitez A, Brown CL, Gibbons LE, et al. (2012). Cognitively stimulating activities: Effects of cognition across four studies with up to 21 years of longitudinal data. Journal of Aging Research, Article ID 461592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre JMJ, Janssen SMJ, Rouw R & Meeter M (2013). The rise and fall of immediate and delayed memory for verbal and visuospatial information from late childhood to late adulthood. Acta Psychologica, 142, 96–107. [DOI] [PubMed] [Google Scholar]
- Parisi JM, Gross AL, Rebok GW, & Saczynski JS (2011). Modeling change in memory performance and memory perceptions: Findings from the ACTIVE study. Psychology and Aging, 26, 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P, Diggle P, Holland F, & McInnes L (2004). Practice and drop-out effects during a 17-year longitudinal study of cognitive aging. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences, 59, 84–97. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Diggle P, Smith D, Holland F, & McInnes L (2001). Identifying and separating the effects of practice and of cognitive ageing during a large longitudinal study of elderly community residents. Neuropsychologia, 39, 532–543. [DOI] [PubMed] [Google Scholar]
- Raven J (1962). Advanced progressive matrices, Set II. London, United Kingdom: Lewis. [Google Scholar]
- Ronnlund M & Nilsson L-G (2006). Adult life-span patterns in WAIS-R Block Design performance: Cross-sectional versus longitudinal age gradients and relations to demographic factors. Intelligence, 34, 63–78. [Google Scholar]
- Ronnlund M, Nyberg L, Backman L & Nilsson L-G, (2005). Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and Aging, 20, 3–18. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1991). Theoretical Perspectives on Cognitive Aging. Hillsdale, N.J.: Lawrence Erlbaum Associates. [Google Scholar]
- Salthouse TA (1993). Speed and knowledge as determinants of adult age differences in verbal tasks. Journal of Gerontology: Psychological Sciences, 48, P29–P36. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2009). When does age-related cognitive decline begin? Neurobiology of Aging, 30, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2010a). Major Issues in Cognitive Aging. New York: Oxford University Press. [Google Scholar]
- Salthouse TA (2010b). Influence of age on practice effects in longitudinal neurocognitive change. Neuropsychology, 24, 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2010c). Selective review of cognitive aging. Journal of International Neuropsychological Society, 16, 754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2011). Neuroanatomical substrates of age-related cognitive decline. Psychological Bulletin, 137, 753–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2013). Within-cohort age differences in cognitive functioning. Psychological Science, 24, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2014a). Why are there different age relations in cross-sectional and longitudinal comparisons of cognitive functioning? Current Directions in Psychological Science, 23, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2014b). Selectivity of attrition in longitudinal studies of cognitive functioning. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences, 69, 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2016a). Aging cognition unconfounded by prior test experience. Journal of Gerontology: Psychological Sciences, 71, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2016b). Continuity of cognitive change. Psychonomic Bulletin and Review, 23, 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, & Babcock RL (1991). Decomposing adult age differences in working memory. Developmental Psychology, 27,763–776. [Google Scholar]
- Salthouse TA, Fristoe N, & Rhee SH (1996). How localized are age-related effects on neuropsychological measures? Neuropsychology, 10, 272–285. [Google Scholar]
- Schaie KW (2005). Developmental influences on adult intelligence: The Seattle Longitudinal Study. New York, NY: Oxford University Press. [Google Scholar]
- Schaie KW (2013). Developmental influences on adult intelligence: The Seattle Longitudinal Study (2nd ed.). New York, NY: Oxford University Press. [Google Scholar]
- Schaie KW, & Hertzog C (1983). Fourteen-year cohort-sequential analyses of adult intellectual development. Developmental Psychology, 19, 531–543. [Google Scholar]
- Schaie KW, Labouvie GV, & Buech BU (1973). Generational and cohort-specific differences in adult cognitive functioning: A fourteen-year study of independent samples. Developmental Psychology, 9, 151–166. [Google Scholar]
- Schaie KW, & Strother CR (1968). A cross-sequential study of age changes in cognitive behavior. Psychological Bulletin, 70, 671–680. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Kivimaki M, Glymour AM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE & Dugravot A (2012). Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. British Medical Journal. 344: d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg DA, Ballard K, Hardly JL, Katz B, Doraiswamy PM, & Scanlon M (2013). The largest human cognitive performance dataset reveals insights into the effects of lifestyle factors and aging. Frontiers in Human Neuroscience, 7, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JJ, Blair MR, & Henrey AJ (2014). Over the hill at 24: Persistent age-related cognitive motor decline in reaction times in an ecologically valid video game task begins in early adulthood. PLoS ONE, 9, e94215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembly D & O’Connor J (1966). Growth and decline of natural and acquired intellectual characteristics. Journal of Gerontology, 21, 9–12. [DOI] [PubMed] [Google Scholar]
- Van der Elst W, van Boxtel MPJ, van Breukelen GJP, & Jolles J (2008). Detecting the significance of changes in performance on the Stroop Color-Word Test, Rey’s Verbal Learning Test, and the Letter Digit Substitution Test: The regression-based change approach. Journal of the International Neuropsychological Society, 14, 71–80. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, van Gerven PWM, van Boxtel MPJ, van der Elst W, & Jolles J (2008). No protective effects of education during normal cognitive aging: Results from the 6-year follow-up of the Maastricht Aging Study. Psychology and Aging, 23, 119–130. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997a). Wechsler Adult Intelligence Scale (3rd ed.). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (1997b). Wechsler Memory Scale (3rd ed.). San Antonio, TX: Psychological Corporation. [Google Scholar]
- Woodcock RW, & Johnson MB (1990). Woodcock–Johnson Psycho-Educational Battery-Revised. Allen, TX: DLM. [Google Scholar]
- Zachary RA (1986). Shipley Institute of Living Scale—Revised. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Zelinski EM & Burnight KP (1997). Sixteen-year longitudinal and time lag changes in memory and cognition in older adults. Psychology and Aging, 12, 503–513. [DOI] [PubMed] [Google Scholar]