Abstract
Psychopathologic traits that arise in adolescence may increase proneness to substance use uptake as well as channel the familial transmission of substance use. Poly use is a common pattern of substance use in youth. To identify a parsimonious model of familial transmission of substance use, the current study tested whether anhedonia—a psychopathologic endophenotype manifested as the inability to experience pleasure—mediates the association of family history of substance use (FHS) with polysubstance use patterns across mid-adolescence. High school students (N=3,392) in Los Angeles, CA, completed four semiannual surveys of mental health and substance use from ages 14 to 16 years old. Use and co-use of cigarettes, alcohol, and marijuana across the four waves were reduced to 4 homogenous classes using parallel process growth mixture modeling: (1) Abstainers (N=1,629, 48.0%), (2) Experimenters (N=1,293, 38.1%), (3) Polysubstance using marijuana escalators (N=210, 6.2%), and (4) Heavy polysubstance using cigarette escalators (N=126, 3.7%). FHS was positively associated with membership in each of the three substance using trajectory groups (vs. the Abstainers group). After adjusting for depressive symptoms and other covariates, associations of FHS with membership in the Polysubstance using marijuana escalators group and with the Heavy polysubstance using cigarette escalators group (in comparison to the Abstainers or Experimenters groups) were each significantly mediated by anhedonia in youth age 14 (the proportion mediated by anhedonia: 0.33−0.42). Etiology research and intervention addressing anhedonia may have value for understanding and preventing the familial transmission of adolescent polysubstance use patterns.
Keywords: anhedonia, family substance use history, polysubstance use trajectories, adolescence
Introduction
Individuals with (vs. without) a family history of substance use problems (FHS) are more likely to exhibit early onset of substance use, rapid escalation of use during adolescence, polysubstance use, comorbid psychopathology, as well as more chronic and severe courses of substance use during adulthood (Elliot, Carey, & Bonafide, 2012). As a distal risk factor for substance use, FHS may confer risk for adolescent substance use through a mix of genetic, epigenetic, and environmental factors that mediate the familial transmission of substance use (Elliot et al., 2012). Psychopathological comorbidity often precedes substance use uptake (Colder et al., 2013) and may be a marker of the various processes that underlie the familial transmission of substance use across generations (Leventhal, Witt, & Zimmerman, 2008).
Anhedonia—diminished pleasure in response to, and interest in, pursuing rewarding stimuli—is an endophenotype of depression and other forms of psychopathology that evinces robust empirical associations with substance use (Hatzigiakoumis, Martinotti, Di Giannantonio, & Janiri, 2011; Leventhal & Zvolensky, 2015). Dimensional perspectives propose that anhedonia is normally distributed in the population, with individuals who experience low levels of anhedonia responding quickly and strongly to a variety of common rewards, and those with higher levels of anhedonia having greater difficulty experiencing pleasure in response to rewards. For some, anhedonia is a state that is acutely elevated in the context of an active psychiatric episode or in response to stress (Bogdan & Pizzagalli, 2009), becomes ‘dormant’ in between episodes, and regularly re-manifests during distress states. For others, anhedonia is a trait-like dimension with modest fluctuation across time (Lyons et al., 1995; Meehl, 2001). While anhedonic individuals have difficulty processing and responding to perceptual rewards, drugs of abuse bypass stimulus input reward processing mechanisms by directly (i.e., pharmacologically) stimulating the brain’s reward system (Leventhal et al., 2014). Consequently, it is plausible that when anhedonic (vs. non-anhedonic) youth initiate drug use, they may be more likely to escalate their frequency of use because pharmacological rewards may have unique motivational priority relative to alternative rewards due to their superior comparative efficacy in eliciting a pleasure response (Leventhal et al., 2014; Wise, 2008).
In addition to potentially increasing risk for adolescent substance use, anhedonia is a plausible mediator of familial transmission of substance use. Anhedonia is genetically transmitted (Bogdan & Pizzagalli, 2009) and gene variants linked with substance use risk may also alter neural phenotypes that underlie anhedonia (Ray et al., 2009; Wise, 2008), raising the possibility that FHS may predict offspring anhedonia. Furthermore, children raised in homes of substance users may be more likely to be subject to familial neglect and chronic stress (Yule, Wilens, Martelon, Simon, & Biederman, 2013), which are implicated in anhedonia development during adolescence (Bolton et al., 2018b; Hynes et al., 2017). Despite its heritability, anhedonia is malleable in response to intervention (Craske, Meuret, Ritz, Treanor, & Dour, 2016), including behavioral interventions that aim to increase access to and ability to savor rewards (Sussman & Leventhal, 2014), and therefore could be a prime psychopathological target for substance use prevention.
Evidence of prospective positive associations between adolescent anhedonia and subsequent substance use is limited to a few studies that each focused on single-drug use outcomes—two showing prediction of smoking (Audrain-McGovern et al., 2012; Stone, Audrain-McGovern, & Leventhal, 2017) and marijuana (Leventhal et al., 2017). While polysubstance use is a common manifestation of drug use amongst U.S. youth (Conway et al., 2013) and is associated with FHS (Jones, Calkins, Scott, Bach, & Gur, 2017; Merikangas et al., 1998), it is unknown whether polysubstance use patterns arise from anhedonia and whether anhedonia is a phenotypic marker of the familial transmission of substance use.
The current study sought to determine whether anhedonia mediates the association of FHS and adolescent substance use in a school-based longitudinal cohort of high school students. To optimally operationalize polysubstance use trajectories in adolescence, we first modeled the heterogeneity in developmental patterns of use and co-use of cigarettes, alcohol, and marijuana from age 14 to 16 years using parallel process latent growth mixture modeling. Given prior results and extant knowledge regarding longitudinal patterns of polysubstance use trajectories (Brook et al., 2014; Nelson et al., 2015), we hypothesized that variation in use and co-use patterns over time could be accurately and parsimoniously captured with the following qualitatively distinct patterns: (a) abstainers; (b) experimenters who use one or multiple substances at low levels on a time-limited basis; (c) one or more group polysubstance users who escalate use of multiple substances during adolescence and reach moderate to high levels of use.
We then tested whether the association of FHS with adolescents’ membership in specific substance use trajectory groups was mediated by anhedonia at age 14. We hypothesized that youth exposed to family members’ substance use in childhood would have higher levels of anhedonia, which in turn would predict the likelihood of membership in trajectories involving polysubstance versus those involving single-substance use or little/no use of any substance. Considering a high degree of symptomatic heterogeneity of depression, such that different symptoms within a particular syndrome often only loosely cluster together and have distinct etiologies (Krueger & Bezdjian, 2009), numerous measures of psychopathology were included as covariates, and the corresponding mediational pathway of a common psychiatric comorbidity (i.e., depressive symptoms) was also tested. Given that anhedonia may tap a single etiologically-relevant process over and above variance captured by multi-symptom composite syndrome measures of depression, mediating pathways involving anhedonia would be independent of manifest psychopathologic symptoms and play a unique role in the etiology of substance use risk.
Methods
Participants and Procedures
Data were drawn from the Happiness & Health Study, a longitudinal cohort survey of substance use and mental health among high school students in Los Angeles. Among 40 public high schools approached to participate in the study due to their diverse demographic characteristics and proximity, 10 schools participated (characteristics of participating schools, in reference to Los Angeles county public schools, appear in sTable 1 in the online supplemental material). Of the 4,100 eligible 9th grade students, 3,396 students and their parents provided active written or verbal assent and consent, respectively, and enrolled in the study. Data collection involved four semiannual assessments: baseline (T1; fall 9th grade, 2013; N surveyed=3,383, 99.6%) and 6-month (T2; spring 9th grade, 2014; N=3,292, 96.9%), 12-month (T3; fall 10th grade, 2014; N=3,281, 96.6%), and 18-month (T4; spring 10th grade, 2015; N=3,251, 95.7%) follow-ups. At each time-point, paper-and-pencil surveys were administered in students’ classrooms on site. Students not in class during data collections completed surveys by telephone, Internet, or mail (6-month follow-up: N=49, 12-month follow-up: N=142, 18-month follow-up: N=216). The University of Southern California IRB approved the study.
Measures
Substance use.
At each time-point, past 30-day frequency of cigarette, alcohol (i.e., ‘one full drink of alcohol and not just a few sips for religious purposes’), and marijuana use was measured using well-validated items based on the Youth Behavior Risk Surveillance and Monitoring the Future surveys (Eaton et al., 2010; Johnston, O’Malley, Miech, Bachman, & Schulenberg, 2016). The number of days each substance was used in the past 30 days was assessed with 9 forced choice options ranging 0–30 days. To produce trajectories that could be interpreted as days used in past 30, the ordinal responses were recoded into the following quantities count variables: 0 for (‘0 days’ response option), 2 (1–2 days), 4 (3–5 days), 8 (6–9 days), 12 (10–14 days), 17 (15–19 days), 22 (20–24 days), 27 (25–29 days), and 30 (All 30 days).
Family history of substance use (FHS).
At T1, participants reported whether anyone in their immediate family (parents, grandparents, brothers, sisters) had a history of smoking cigarettes, alcohol abuse problems, and drug abuse problems. Based on studies that support the basic concept of cumulative risk index (see Evans, Li, & Whipple, 2013), responses to these three questions were dichotomously coded (0 = No, 1 = Yes) and summed to create a cumulative FHS index (Range: 0 to 3).
Anhedonia.
At T1, an anhedonia composite score was created using two self-report measures of anhedonia selected to collectively provide adequate coverage of multiple facets of the anhedonia construct—the Snaith-Hamilton Pleasure Scale (SHAPS; Snaith et al., 1995) and Tripartite Pleasure Inventory – Desire subscale (TPI; Leventhal, 2010; Leventhal et al., 2015). The SHAPS includes 14 items assessing pleasure response to rewarding sensory stimuli, social activities, and hobbies based on how respondents have been feeling in the past few days (e.g., “I would be able to enjoy a beautiful landscape or view”). The response options ranged from 0 (Strongly disagree) to 3 (Strongly agree), and all items were summed (Cronbach’s α = .90). The TPI-Desire subscale is an 11-item measure in which participants rated the extent to which they typically desire to engage in commonly pleasant experiences that span interest/pastimes, social interaction, sensory, and goals/mastery (e.g., “How much do you usually want to eat tasty food?”). Responses were coded as 0 (Not at all), 1 (A little), 2 (Somewhat), 3 (Quite a bit), and 4 (Very much). Cronbach’s α was .79. In a prior psychometric study of 9th grade students, the SHAPS and TPI displayed convergent validity with each other (Leventhal et al., 2015). The two measures were significantly correlated in the current study (r=.33, p<.001). Both the SHAPS and TPI responses were reverse coded such that their summary scores both reflected worse anhedonia. The summary score from both measures were separately standardized (M=0, SD=1) to place them on the same scaling metric. The standardized SHAPS and TPI scores were summed to comprise an anhedonia composite with a higher score indicating greater levels of anhedonia.
Depressive symptoms.
At T1, a depressive symptom composite was created from two self-report measures. The Center for Epidemiologic Studies Depression (CESD; Radloff, 1977) is a 20-item measure of depressive symptoms experienced over the past week and rated on a 4-point response scale, ranging from 0 (Rarely or None of the time; 0–1 days) to 3 (Most or all of the time; 5–7 days), for which a total scale was computed by the sum of 20 items (α = .81). Participants also completed the 10-item Major Depression (MD) Scale of the Revised Children’s Anxiety and Depression Scale (RCADS; Chorpita, Moffitt, & Gray, 2005). For each item on the RCADS, respondents indicated how often they experienced each symptom on a 4-point scale, ranging from 0 (Never) to 3 (Always), and 10 items were summed (α =.93). Both measures have demonstrated strong psychometric properties in previous adolescent samples (Simmons, Wilkinson, & Dubicka, 2015), and were significantly correlated in the study (r=.71, p<.001). The standardized CESD and MD scores were summed to comprise a depressive symptom composite.
Covariates.
Covariates at T1 were selected to parse the relative contribution of anhedonia and depression to the familial transmission of substance use over and above extraneous processes that could potentially covary with FHS and lead to adolescent substance use. Sociodemographic characteristics, including age, gender, race/ethnicity, and highest parental education level, were assessed using self-report responses to investigator-defined forced-choice items (see response categories in sTable 2). Lifetime ever-use of other substances besides cigarettes, alcohol, and marijuana (e.g., other forms of tobacco, inhalants, cocaine, ecstasy) at T1 were assessed with 19 separate questions that were each coded as 0 (Non-use) and 1 (Any use) and summed, resulting in a cumulative drug use diversity index (Range: 0 to 19). Also, peer use of cigarettes, alcohol, and marijuana in the past 30 days (i.e., number of five closest friends who had used these substances) was assessed via self-report measures. Peer use of each substance was coded dichotomously (0 vs. ≥ 1) and summed (Range: 0 to 3). As a psychological covariate, impulsivity was measured with the 12-item premeditation scale from the UPPS Impulsive Behavior Scale (Miller, Flory, Lynam, & Leukefeld, 2003), which includes items assessing the tendency toward acting on instinct without conscious deliberation (α = .89). The generalized anxiety subscale (6 items; α = .89) from the RCADS (Chorpita et al., 2005) was measured and coded in the same fashion as the RCADS-MD. Finally, externalizing delinquent behavior was measured with a sum of frequency ratings for engaging in 11 behaviors (e.g., stealing, lying to parents; score range: 1 [never] to 6 [≥10 times]; α = .79) in the past 6 months (Thompson, Ho, & Kingree, 2007).
Analysis Plan
We used a specialized ‘parallel process’ application of growth mixture modeling (GMM; Muthén & Asparouhov, 2008), which generates latent class profiles based on patterns of covariation across three separate sets of intercepts (baseline level) and linear slopes (rate of change over time), one set of growth factors per each substance (i.e., alcohol, marijuana, and cigarettes). The analysis generates separate model solutions, with each model estimating a different number of class profiles. The model that best fit the data was selected based on model fit comparisons using a series of standard fit indices (i.e., Bayesian Information Criterion [BIC], sample-size-adjusted BIC [SSA-BIC], Akaike Information Criterion [AIC]). We also utilized the Lo–Mendell–Rubin likelihood ratio test (LMR-LRT), which compares the improvement in fit between neighboring class models (i.e., comparing k-1 and the k class models) and provides a p-value that can be used to determine if there is a statistically significant improvement in fit for the inclusion of one more class (Nylund, Asparouhov, & Muthén, 2007). Finally, we interpreted entropy values for each model, which refers to the average accuracy in assigning individuals to class profiles.
For the primary analysis, we first conducted path analyses to test if FHS was associated with membership in polysubstance use trajectory groups using multinomial logistic regression modeling excluding the anhedonia and depressive symptom composites (i.e., the ‘total effect’; Figure 2). We then tested the hypothesized mediational path model, which imbedded a multinomial logistic regression model for the substance use trajectory outcome (see Figure 3) and calculated the indirect ‘mediated’ effects of FHS T1 anhedonia composite substance use trajectory groups (vs. the Abstainers group) via Monte Carlo integration methods (Hayes, 2013). Effect sizes were calculated from the proportion of indirect effect to the total effect using a protocol of a regression-based model (Preacher & Kelley, 2011). A simultaneous pathway of FHS T1 depression composite substance use trajectory groups was also modeled to test whether mediating pathways involving anhedonia would be independent of multi-symptom composite syndrome measures of depression. The final path model adjusted for each study covariate listed in the measures section.
Figure 2.
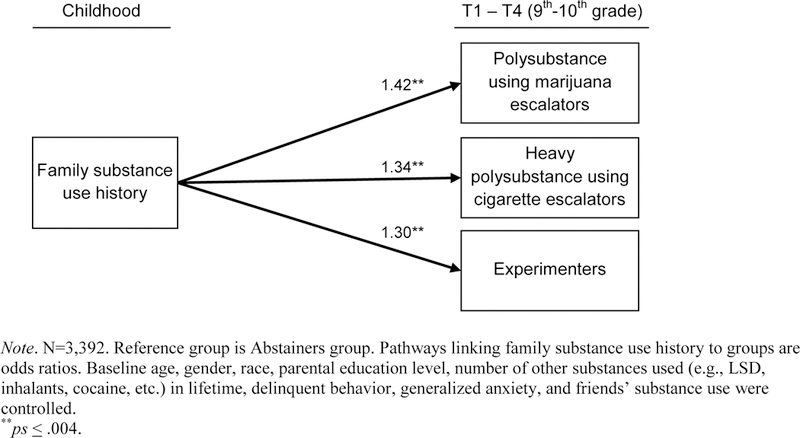
Total effect model: Association between family substance use history and membership in each substance use trajectory group (vs. Abstainers group)
Figure 3.
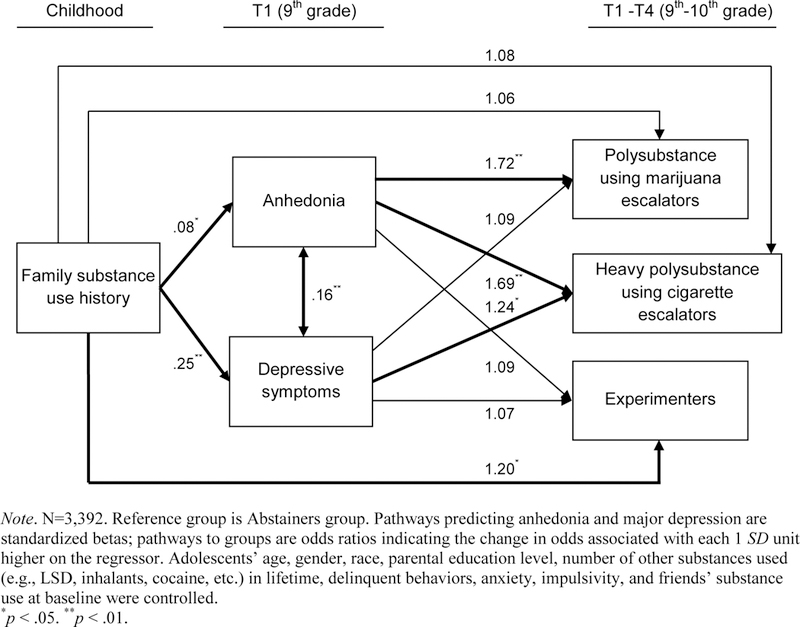
Path analysis of mediational processes of anhedonia linking family substance use history to membership in each polysubstance use trajectory group (vs. Abstainers)
All models were tested in Mplus version 7 (Muthén & Muthén, 2010) using the complex analysis function to adjust parameter standard errors for interdependence in the data due to the nesting of students by their school. All variables were standardized in the path analyses (M=0, SD=1), permitting the interpretation of each path estimate as the change in the outcome associated with each one standard deviation change in the regressor. Significance was set to .05 (two-tailed). Missing data were managed with full information maximum likelihood (FIML) estimation. Additional supplementary analyses utilizing alternative strategies of GMM analyses, missing data management, and multinomial logistic regression modeling with a different reference group were conducted (see the online supplemental material).
Results
Preliminary Analyses
Among study enrollees, 3,392 (99.9%) provided at least one data point for the substance use variables in primary GMM analyses and constituted the analytic sample (see sTable 2 for Ns of available data). As depicted in sTable 2, the sample was balanced on gender, and was sociodemographically diverse. Correlations among study variables at baseline are also presented in Table 1.
Table 1.
Correlations of study variables at baseline
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Gender | – | |||||||||||||||
| 2. Age | .08** | – | ||||||||||||||
| 3. Parental Education | .02 | −.03 | – | |||||||||||||
| 4. Cigarette use | −.01 | .02 | −.04* | – | ||||||||||||
| 5. Alcohol use | −.04* | .02 | −.09** | .40** | – | |||||||||||
| 6. Marijuana use | .01 | .04* | −.10** | .31** | .48** | – | ||||||||||
| 7. Family substance use history | −.10** | .02 | −.12** | .09** | .13** | .15** | – | |||||||||
| 8. Anhedonia (SHAPS) | .09** | .03 | −.15** | .15** | .15** | .13** | .13** | – | ||||||||
| 9. Anhedonia (TPI) | −.03 | .01 | −.12** | .07** | .04 | .06* | −.05* | .33** | – | |||||||
| 10. Depression (CESD) | −.26** | −.01 | −.07** | .11** | .10** | .07** | .24** | .28** | .03 | – | ||||||
| 11. Depression (MD) | −.21** | −.01 | −.05** | .15** | .15** | .08** | .24** | .21** | −.01 | .71** | – | |||||
| 12. Anxiety (GAD) | −.20** | −.02 | −.03 | .07** | .07** | .05* | .21** | .05* | .17** | .51** | .61** | – | ||||
| 13. Impulsivity (UPPS) | −.07** | .01 | −.04 | .14** | .15** | .11** | .24** | .14** | −.15** | .43** | .46** | .41** | – | |||
| 14. Other substance use | .02 | .09** | −.14** | .14** | .28** | .29** | .22** | .18** | −.04* | .18** | .16** | .12** | .21** | – | ||
| 15. Delinquent behavior | .05* | .06** | −.14** | .27** | .39** | .45** | .21** | .24** | .01 | .25** | .24** | .17** | .30** | .38** | – | |
| 16. Friends’ substance use | −.07** | .06** | −.16** | .18** | .35** | .38** | .25** | .18** | −.01 | .20** | .15** | .11** | .23** | .43** | .46** | – |
Note. Gender was coded: 0=female, 1=male. Parental education was coded as continuous variable (0=8th grade or less, 1=Some high school, 2=High school graduate, 3=Some college, 4=College graduate, 5=Advanced degree). Abbreviations: SHAPS = Snaith-Hamilton Pleasure Capacity Scale score. TPI = Tripartite Pleasure Inventory–Desire subscale score. CESD = Center for Epidemiologic Depression Scale score. MD = Revised Children’s Anxiety and Depression Scale (RCADS)–Major Depression Scale score. GAD = Revised Children’s Anxiety and Depression Scale (RCADS)–Generalized Anxiety Scale score. UPPS = UPPS Impulsive Behavior Scale score.
p < .05.
p < .01.
Identification and Characterizing Trajectory Groups of Cigarette, Marijuana, and Alcohol Use and Co-Use
Model selection.
Given prior evidence that the inclusion of a large group of abstainers in GMM may reduce the ability to detect meaningful variation among ever-users (Acock, 2008), we first identified the Abstainers class (N=1,763, 52.0%) who did not use any substances across the study period. We then conducted a series of GMM analyses using a subsample who reported at least one day of use of cigarettes, alcohol, or marijuana across any of the four time-points (N=1,629, 48.0%). Fit indices for a series of GMM analyses presented in sTable 3 in the online supplemental material converged to suggest a three-class solution best fit the data (AIC=45133.79, BIC=45274.08, Entropy=0.82, LMR p-value=.03). Both AIC and BIC values sharply decreased across the one-class and three-class solutions, while the difference in AIC between the three- and four-class solutions was only 413.32. The scree plot for the log-likelihood also supported the three-class solutions (Nylund et al., 2007). In the four-class solution, the LMR test comparing the improvement in fit with the three-class solution indicated that the inclusion of one more class did not significantly improve the model fit (p=.15). Additionally, compared to the three-class solution, the four-class solution included a class with a relatively small proportion of the total sample (N=26, 0.8%), which was not clearly distinguished from other polysubstance use classes.
Characterization of the trajectory group derived from the selected model.
The resulting polysubstance use trajectory profiles included four classes—the three polysubstance use groups and the Abstainers group. Descriptive statistics for all T1 study variables and observed mean past 30-day scores for each substance across T1–T4, by trajectory group, are reported in the online supplemental sTable 2 and sTable 4, respectively.
A trajectory group called “Experimenters” (N=1,293, 38.1% of the total sample including Abstainers) reported a negligible level of cigarettes, alcohol, and marijuana use in the past month (estimated mean initial number of days of use in the past 30 days at T1: marijuana=.40, cigarettes=.09, alcohol=.54). The use frequency of each substance did not significantly change during the study observation period (estimated slope mean p-values >.27; see Figure 1).
Figure 1.
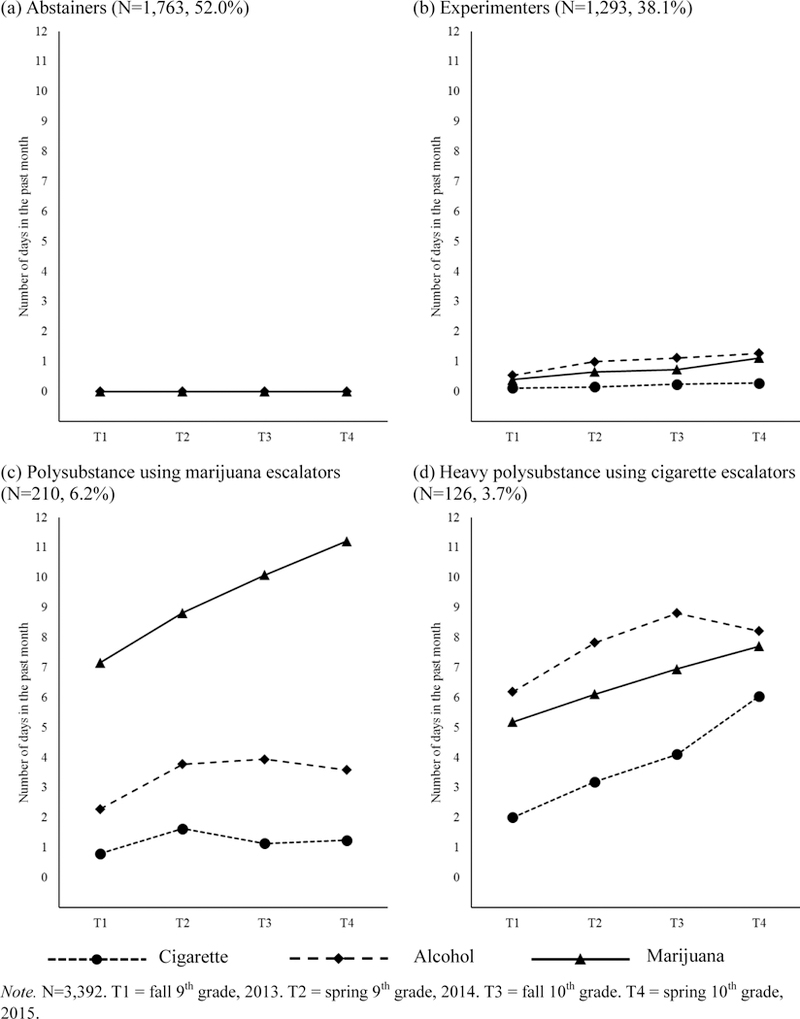
Mean cigarette, alcohol, and marijuana past-month use frequency (number of days used each substance) over time, by substance use trajectory groups
The Polysubstance using marijuana escalators group (N=210, 6.2%) reported a significant level of marijuana use (Initial level mean=7.09, p<.001) and a significantly increasing trajectory (Slope mean=1.06, p=.02) during adolescence. That is, the escalating marijuana users used 7 out of the past 30 days on average during fall of 9th grade and increased the frequency of use by about one day in the past 30 days, on average, with each successive semiannual assessment. They also showed modest levels of cigarette and alcohol use trajectories (< 4 days in the past month), which did not significantly change over time (see Figure 1).
The Heavy polysubstance using cigarette escalators group (N=126, 3.7%) a modest level of cigarette smoking that significantly increased across time (Initial level mean=2.01, p<.001; Slope mean=1.16, p=.03). They also showed higher alcohol use on average (Initial level mean=6.18, p<.001) but did not significantly change their drinking level during the follow-up period (Slope mean=1.01, p=.46). There was also a moderate frequency of marijuana use (Initial level mean=5.19, p<.001) but no significant change in marijuana use frequency over time (Slope mean=1.02, p=.68).
Anhedonia as a Mediator of the Association between Family History of Substance Use and Substance Use Trajectory Group Membership
Total effect analysis.
As illustrated in Figure 2, total effect analysis found positive associations of FHS score with each polysubstance use trajectory group (vs. Abstainers) after adjustment of all study covariates. For example, each standard deviation unit higher in FHS was associated with 42% greater odds of membership in Polysubstance using marijuana escalators (vs. Abstainers) trajectory (OR[95%CI]=1.42[1.16, 1.74], p <.001).
Mediation by anhedonia.
Illustrated in Figure 3, the mediational model including all study covariates found that the association of FHS with membership in the Polysubstance using marijuana escalators (vs. Abstainers) group was significantly mediated by the T1 anhedonia composite (bindirect effect[95%CI]=.05[0.004, 0.099], p=.02). The proportion of the total effect mediated by anhedonia was .42. Component path estimates of this mediational process indicate that that FHS was positively associated with anhedonia (β[95%CI]=.08[0.02, 0.15], p=.02), which in turn significantly predicted the membership in the Polysubstance using marijuana escalators group compared to the Abstainers group (OR[95%CI]=1.72[1.15, 2.34], p=.004).
Anhedonia also mediated the association of FHS with membership in the Heavy polysubstance using cigarette escalators (vs. Abstainers) group (bindirect effect[95%CI]=.04[0.009, 0.087], p=.02, the proportion of total effect mediated by anhedonia=.33). FHS was associated with anhedonia (β[95%CI]=.08[0.02, 0.15], p=.02), which in turn was related to membership in the Heavy polysubstance using cigarette escalators (vs. Abstainers) group (OR[95%CI]=1.69[1.07, 2.35], p=.009).
Anhedonia did not significantly mediate the association of FHS with membership in the Experimenters (vs. Abstainers) group (bindirect effect[95%CI]=.004[−0.070, 0.083], p=.76, the proportion of total effect mediated by anhedonia=.03).
Additional findings.
In the final adjusted model (Figure 3), depressive symptom composite at T1 significantly mediated the association of FHS with the Heavy polysubstance using cigarette escalators (vs. Abstainers) group (bindirect effect[95%CI]=.06[0.011, 0.122], p=.01, proportion of total effect mediated by depressive symptoms=.41), but not with the Polysubstance using marijuana escalators (vs. Abstainers) group (bindirect effect[95%CI]=.02[−0.011, 0.053], p=.35, the proportion of total effect mediated by depressive symptoms=.16) or the Experimenters (vs. Abstainers) group (bindirect effect[95%CI]=.01[−0.018, 0.062], p=.41, the proportion mediated by depressive symptoms=.08).
After adjusting for mediational processes of anhedonia and depressive symptoms, the remaining direct effect associations between FHS and membership in the Experimenters (vs. Abstainers) was significant (OR[95%CI]=1.20[1.05, 1.40], p=.03). There were not significant direct effect associations of FHS with membership in the Polysubstance using marijuana escalators (vs. Abstainers) group (OR[95%CI]=1.06[0.90, 1.24], p=.51) or the Heavy polysubstance using cigarette escalators (vs. Abstainers) group (OR[95%CI]=1.08[0.90, 1.29], p=.46).
Supplementary Analyses
Alternative method for modeling Abstainers.
To assess the adequacy of our primary GMM analysis using the subsample of 1,629 substance users (i.e., students who reported at least one day of cigarette, alcohol, or marijuana use across any of the four time-points), we conducted a series of GMM analyses using the total sample (N=3,392) including 1,763 abstainers who did not use any substance across four time-points. Considering excess zeros of past month substance use variables, we conducted the zero-inflated Poisson growth mixture model analysis (Acock, 2008). This model yielded a solution similar to the primary results and resulted in four classes (see sTable 3 and sTable 4 in the online supplemental material): Abstainers (N=2,032, 59.9%), Experimenters (N=1,023, 30.2%), Polysubstance using marijuana escalators (N=211, 6.2%), and Heavy polysubstance using cigarette escalators (N=126, 3.7%). Detailed information regarding primary and alternative GMM methods to characterize latent polysubstance use trajectory profiles are presented in the sensitivity analyses section in the online supplemental material.
Unadjusted model testing the mediation paths of anhedonia.
To address the role of study covariates, the final mediational path model with simultaneous anhedonia and depressive symptom composite mediators (presented in Figure 3) was retested without T1 covariates. The results showed a very similar pattern of findings to the final adjusted model, indicating that anhedonia significantly mediated the associations of FHS with membership in the Heavy polysubstance using cigarette escalators (vs. Abstainers) group (bindirect effect[95%CI]=.05[0.006, 0.102], p=.01, the proportion of total effect mediated by anhedonia=.22) and the Polysubstance using marijuana escalators (vs. Abstainers) group (bindirect effect[95%CI]=.04[0.008, 0.091], p=.01, the proportion of total effect mediated by anhedonia=.20). Also, an additional mediational path of anhedonia was detected this unadjusted model. The association between FHS and membership in the Experimenters (vs. Abstainers) group was significantly mediated by anhedonia (bindirect effect[95%CI]=.01[0.003, 0.021], p=.03, the proportion of total effect mediated by anhedonia=.05). Detailed parameter estimates were presented in sFigure 1 of the online supplemental material.
Post-hoc comparisons to the reference group of Experimenters.
The primary results compared the three substance-using trajectories to the Abstainers group as a referent category, leaving unclear the extent to which the risk pathways are explained by a general propensity to initiate substance use or a proneness to progress from experimentation to significant levels of use. To address this question, we re-tested the structural equation models using the Experimenters as the reference group (see sFigure 2 in the online supplemental material). Consistent with the final model results, FHS was significantly associated with adolescents’ memberships in different substance use trajectory groups via anhedonia.
Additional potential influences on the study findings.
To address the possibility that the occurrence of missing data, false reporting, and data collection method impacted our findings, we retested the final path analysis model presented in Figure 3 using: (1) a sample excluding participants who did not complete the follow-up survey at T4 (N=145, 4.3%); (2) a sample excluding participants reporting use of a fictitious drug at some point during the study period and therefore may have data of questionable validity (N=53, 1.6%); and (3) a sample without students who completed a follow-ups survey by an alternate mode of survey administration (N=255, 7.5%). No meaningful differences between primary analysis results and these sensitivity analyses results were detected (see the detailed description of these results in the sensitivity analyses section in the online supplemental material).
Discussion
This study finds the initial support of a novel model of familial transmission of adolescent substance use by showing that the prospective association between FHS in childhood and polysubstance use patterns from ages 14 to 16 was mediated by anhedonia at age 14. These pathways were empirically independent of other sociodemographic, environmental, and psychopathologic risk factors for substance use, and the pattern of results were qualitatively distinct from comparative descriptive results including depressive symptoms as a mediator linking FHS and adolescent substance use. In the context of testing this model, this study also provides some of the first evidence including: (1) polysubstance use patterns as the modal trajectory of adolescent substance use escalation; (2) an association between FHS and adolescent anhedonia, and (3) polysubstance use as a potential consequence of anhedonia in youth.
Using GMM analyses on triple conjoint trajectories of cigarette, alcohol, and marijuana use, we found four groups that parsimoniously explained heterogeneity in the developmental patterns of use of three popular substances across mid-adolescence (ages 14 to 16). Consistent with results from the National Survey on Drug Use and Health (Substance Abuse and Mental Health Services Administration, 2014), our findings showed that the majority of the total sample was classified as abstainers or experimenters who did not escalate their substance use and used cigarettes, alcohol, or marijuana only occasionally throughout mid-adolescence. Also, low prevalence of polysubstance use profiles was detected, consistent with the finding in a meta-analytic review on the trajectory overlap of cigarette, alcohol, and marijuana use across adolescence and young adulthood (Nelson et al., 2015). As Nelson et al. (2015) identified 3.9% qualified as high risk for all three trajectories and 10.5% of high school marijuana escalators, 3.7% of Heavy polysubstance users and 6.2% of polysubstance using marijuana escalators were detected in the present study.
While only a few prior studies have examined adolescents’ cigarette, alcohol, and marijuana use trajectories conjointly (Nelson et al., 2015), our findings concord with and extend a prior study that applied a parallel process GMM approach to cigarette, alcohol, and marijuana use in a sample followed across emerging and young adulthood (Brook et al., 2014). Brook et al. (2014) detected five groups with distinct trajectories of substance use: (1) nonuse; (2) use of all three substances; (3) alcohol use only; (4) marijuana and alcohol use; and (5) cigarette and alcohol use. Consistent with our findings of the identification of two poly substance use profiles with distinct developmental signatures, this study found two distinct groups of individuals who were involved in polysubstance use, including a group with high levels of both tobacco and alcohol use over time. While this study did not find evidence of escalating patterns of cigarette, alcohol or marijuana use over time (Brook et al., 2014), our study detected escalating trajectories of marijuana use and cigarette use in adolescence. It may be the case that focusing on critical developmental periods (i.e., early or mid-adolescence) can elucidate the origin of polysubstance use trajectories that may persist throughout adulthood.
After we characterized the developmental patterns of polysubstance use among adolescents, we found longitudinal associations between FHS with adolescents’ membership in specific substance use trajectory groups, which was significantly mediated by anhedonia at age 14. There are several potential explanations for the observation that anhedonia was overexpressed in youth with more exposure to FHS and, in turn, predicted substance use uptake. Anhedonia and substance use may share common genetic underpinnings, as both have been linked with gene variants in dopaminergic pathways or other neural circuits implicated in reward and motivation (Bogdan & Pizzagalli, 2009). If genetic covariance explains the link between adolescent anhedonia and subsequent polysubstance use, their association may be non-causal, and both phenotypes could merely reflect manifestations of a common genetic etiology.
Alternatively, it is possible that one of the many genetic influences on youth substance use risk is driven by a pathway in which anhedonia is a necessary and causal intermediate precursor, at least for certain cases of adolescent substance use. A similar notion has been proposed for evidence that youth novelty seeking traits mediate the association of FHS with adolescent substance use (Bidwell et al., 2015). Under this interpretation, any factor (genetic or environmental) that leads to the development of anhedonia would presumably have downstream effects on substance use risk if anhedonia is indeed a causal mechanism of risk. Being raised in a family environment in which parents or siblings have substance use problems or drug-related morbidity or mortality can be a significant source of stress (Elliott et al., 2012; Mann et al., 2005) or lead to insufficient parental nurturing (Yule et al., 2013). Stress is a known precipitant for anhedonia (Bolton et al., 2018a). Deficient reward stimulation in offspring caused by the absence of parental nurturing may promote insufficient maturation of reward circuitry during adolescence and, in turn, the behavioral expression of anhedonia (Molet et al., 2016) and anhedonia-related acquisition of drug-self administration (Bolton et al., 2018b). Thus, the familial transmission of substance use via anhedonia may not be entirely driven by genetic influences.
Following substance use initiation, youth with higher (vs. lower) anhedonia may derive greater pharmacological reward from substance exposure. Indeed, laboratory drug administration studies demonstrate that anhedonia is associated with greater sensitivity to the acute rewarding effects of d-amphetamine and nicotine administration (Cook, Spring, & McChargue, 2007; Tremblay et al., 2005). Hypersensitivity to drug reward in anhedonia may be due to an upregulation of dopamine D2 receptors caused by a chronically hypoactive dopamine release in response to natural rewards, which when challenged with a pharmacological reward, results in increased receptor occupancy and heightened psychoactive effects (Tremblay, Naranjo, Cardenas, Herrmann, & Busto, 2002). Because individuals who experience greater rewarding effects during their early substance use experiences are more likely to escalate and persist in their use (King, de Wit, McNamara, & Cao, 2011), this mechanism could explain why anhedonia was associated with an increased risk of membership in trajectories involving escalating use, in comparison to both Abstainers and Experimenters with very limited drug use exposure.
This study had limitations. In school-based studies, it is important to minimize the educational burden on teachers and students by utilizing an efficient and brief assessment strategy, which can result in measurement limitations. The measure of FHS was retrospective, self-report and based on the adolescents’ probands and therefore could be subject to inaccuracies. The FHS measure was designed to be brief and simple (i.e., yes/no responses to FHS for cigarettes, alcohol, and marijuana) and does not address the density of FHS (i.e., the number of family members with a substance use problem) or detailed information about the level of use in family members. In addition, whether polysubstance use trajectories identified in this study or other findings will generalize to other regions is unknown, although prior nationally-representative research finds that polysubstance use is common in adolescents and that anhedonia is associated with substance use in U.S. adults. Future research within and outside of the U.S. should consider social and cultural contexts in which different substances are accessible and socially-normative, possibly resulting in various patterns of polysubstance use among youth. Finally, although the possibility that anhedonia is a causal risk factor for adolescent substance use uptake is conceptually plausible, causality cannot be determined in this observational study. In addition, familial transmission of substance use may be explained by other potential mediating variables including increasing environmental exposure to drugs or facilitating drug availability and impaired parenting behaviors (Merikangas et al., 1998). These limitations notwithstanding, several implications can be drawn from this study. First, focusing on a narrow phenotype (i.e., anhedonia), with a putatively homogenous etiologic influence on substance use (i.e., desire to offset deficient reward stimulation by pursuing drug-related rewards), may be a useful conceptual premise to study substance use risk and its familial transmission in adolescence. Two, given that polysubstance use is a common pattern of adolescent substance use (Nelson et al., 2015), the application of longitudinal modeling strategies that consider covariation of use of multiple drugs, such as those used in this study, may provide a more comprehensive and parsimonious clinical picture of substance use patterns among adolescents than focusing on single-drug trajectories. Three, if anhedonia is ultimately identified to be a necessary causal intermediate phenotype that transmits familial transmission of substance use, addressing anhedonia in substance use prevention may be beneficial.
Candidate interventions that may reduce anhedonia include positive psychology approaches that teach individuals to cultivate positive affect and be mindful of positive emotions to extend their intensity and duration (Kahler et al., 2014). Behavioral activation and alternative reinforcer enhancement interventions that motivate individuals to engage in healthy rewarding activities may also be useful. Interventions incorporating such concepts have shown promise in preventing alcohol use in college students (Murphy et al., 2012; Reynolds, MacPherson, Tull, Baruch, & Lejuez, 2011). Finally, it is possible that anhedonic teens may benefit from the identification of novel and potent reinforcers which can provide strong reward stimulation to satisfy the desire for pleasure but do not pose significant health consequences. For example, prior research has found that anhedonia is associated with engagement in extreme sports (e.g., skydiving; Franken, Zijlstra, & Muris, 2006), perhaps because these activities are stimulating enough to engender a reward response in anhedonic individuals. Such activities and cultivating meaningful social connections may be powerful enough reinforcers to engender pleasure and happiness in anhedonic youth, reducing the propensity to resort to drugs as a means of obtaining pleasure (Sussman & Leventhal, 2014). Adolescent etiology and intervention research addressing such concepts may be relevant for the reduction of anhedonia-related psychopathology, substance use, and the familial transmission of risky behavioral conditions related to adverse public health consequences.
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health grant R01-DA033296. The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Part of this study was presented as a poster at the 2017 Collaborative Perspectives on Addiction Meeting. Adam M. Leventhal and Junhan Cho had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Junhan Cho and Adam M. Leventhal lead the conceptualization of the study and wrote most the manuscript text. Junhan Cho conducted the analyses. Mattew D. Stone aided in study conceptualization and provided feedback on drafts. Mattew D. Stone and Junhan Cho oversaw data management and processing.
Footnotes
Declaration of interest: The authors report no potential conflicts of interests.
REFERENCES
- Acock AC (2008). Zero-Inflated Growth and Mixture Models Using Mplus [PDF file] Retrieved from http://www.caldar.org/presentations/summer%20institute/2008/Day-2%20Aug%2014-2008/Track%201/Zero-Inflated.pdf
- Arthur MW, Hawkins JD, Pollard JA, Catalano RF, & Baglioni AJ Jr (2002). Measuring risk and protective factors for use, delinquency, and other adolescent problem behaviors: The Communities That Care Youth Survey. Evaluation Review, 26(6), 575–601. doi.org/10.1177/0193841X0202600601 [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Leventhal AM, Cuevas J, Rodgers K, & Sass J (2012). Where is the pleasure in that? Low hedonic capacity predicts smoking onset and escalation. Nicotine & Tobacco Research, 14(10), 1187–1196. 10.1093/ntr/nts017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC, Knopik VS, Audrain-McGovern J, Glynn TR, Spillane NS, Ray LA, … & Leventhal AM (2015). Novelty seeking as a phenotypic marker of adolescent substance use. Substance Abuse: Research and Treatment, 9(S1), 1–10. doi:org/10.4137/SART.S22440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, & Pizzagalli DA (2009). The heritability of hedonic capacity and perceived stress: A twin study evaluation of candidate depressive phenotypes. Psychological Medicine, 39(2), 211–218. 10.1017/S0033291708003619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Molet J, Regev L, Chen Y, Rismanchi N, Haddad E, … & Baram TZ (2018a). Anhedonia following early-life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene. Biological Psychiatry, 83(2), 137–147. doi:org/10.1016/j.biopsych.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Ruiz CM, Rismanchi N, Sanchez GA, Castillo E, Huang J, … & Mahler SV (2018b). Early-life adversity facilitates acquisition of cocaine self-administration and induces persistent anhedonia. Neurobiology of Stress, 8, 57–67. doi:org/10.1016/j.ynstr.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook JS, Lee JY, Rubenstone E, Brook DW, & Finch SJ (2014). Triple comorbid trajectories of tobacco, alcohol, and marijuana use as predictors of antisocial personality disorder and generalized anxiety disorder among urban adults. American Journal of Public Health, 104(8), 1413–1420. 10.2105/AJPH.2014.301880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpita BF, Moffitt CE, & Gray J (2005). Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behaviour Research and Therapy, 43(3), 309–322. 10.1016/j.brat.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Colder CR, Scalco M, Trucco EM, Read JP, Lengua LJ, Wieczorek WF, & Hawk LW (2013). Prospective associations of internalizing and externalizing problems and their co-occurrence with early adolescent substance use. Journal of Abnormal Child Psychology, 41(4), 667–677. doi:org/10.1007/s10802-012-9701-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Vullo GC, Nichter B, Wang J, Compton WM, Iannotti RJ, & Simons-Morton B (2013). Prevalence and patterns of polysubstance use in a nationally representative sample of 10th graders in the United States. Journal of Adolescent Health, 52(6), 716–723. doi.org/10.1016/j.jadohealth.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J, Spring B, & McChargue D (2007). Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology, 192(1), 87–95. 10.1007/s00213-006-0688-5 [DOI] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, & Dour HJ (2016). Treatment for anhedonia: A neuroscience driven approach. Depression and Anxiety, 33(10), 927–938. doi:org/10.1002/da.22490 [DOI] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Ross J, Hawkins J, … & Lim C (2010). Youth risk behavior surveillance−United States, 2009. Morbidity and Mortality Weekly Report Surveillance Summaries, 59(5), 1–142. doi:ss5905a1 [pii] [PubMed] [Google Scholar]
- Elliott JC, Carey KB, & Bonafide KE (2012). Does family history of alcohol problems influence college and university drinking or substance use? A meta-analytical review. Addiction, 107(10), 1774–1785. 10.1111/j.1360-0443.2012.03903.x [DOI] [PubMed] [Google Scholar]
- Evans GW, Li D, & Whipple SS (2013). Cumulative risk and child development. Psychological Bulletin, 139(6), 1342–1396. 10.1037/a0031808 [DOI] [PubMed] [Google Scholar]
- Franken IH, Zijlstra C, & Muris P (2006). Are nonpharmacological induced rewards related to anhedonia? A study among skydivers. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 30(2), 297–300. doi: org/10.1016/j.pnpbp.2005.10.011 [DOI] [PubMed] [Google Scholar]
- Hatzigiakoumis DS, Martinotti G, Di Giannantonio M, & Janiri L (2011). Anhedonia and substance dependence: clinical correlates and treatment options. Frontiers in Psychiatry, 2(10), 1–10. doi:org/10.3389/fpsyt.2011.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach New York, NY: Guilford Press. [Google Scholar]
- Hynes TJ, Thomas CS, Zumbusch AS, Samson A, Petriman I, Mrdja U, … & Zjadewicz M (2017). Early life adversity potentiates expression of addiction-related traits. Progress in Neuro-Psychopharmacology and Biological Psychiatry doi:org/10.1016/j.pnpbp.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, & Schulenberg JE (2016). Monitoring the future national survey results on drug use, 1975–2015: Overview, key findings on adolescent drug use Ann Arbor: Institute for Social Research, The University of Michigan. [Google Scholar]
- Jones JD, Calkins ME, Scott JC, Bach EC, & Gur RE (2017). Cannabis use, polysubstance use, and psychosis spectrum symptoms in a community-based sample of US youth. Journal of Adolescent Health, 60(6), 653–659. doi.org/10.1016/j.jadohealth.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Day A, Clerkin E, Parks A, Leventhal AM, & Brown RA (2014). Positive psychotherapy for smoking cessation: Treatment development, feasibility and preliminary results. The Journal of Positive Psychology, 9(1), 19–29. 10.1080/17439760.2013.826716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, & Cao D (2011). Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of General Psychiatry, 68(4), 389–399. 10.1001/archgenpsychiatry.2011.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, & Bezdjian S (2013). Enhancing research and treatment of mental disorders with dimensional concepts: Toward DSM-V and ICD-11. World Psychiatry, 8(1), 3–6. doi.org/10.1002/j.2051-5545.2009.tb00197.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM (2010). The tripartite pleasure inventory: A mulidimensional measure of anhedonia Los Angeles, CA: University of Southern California. [Google Scholar]
- Leventhal AM, Cho J, Stone MD, Barrington-Trimis JL, Chou CP, Sussman SY, … & Strong DR (2017). Associations between anhedonia and marijuana use escalation across mid-adolescence. Addiction, 112(12), 2182–2190. doi:org/10.1111/add.13912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Trujillo M, Ameringer KJ, Tidey JW, Sussman S, & Kahler CW (2014). Anhedonia and the relative reward value of drug and nondrug reinforcers in cigarette smokers. Journal of Abnormal Psychology, 123(2), 375–386. 10.1037/a0036384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Unger JB, Audrain-McGovern J, Sussman S, Volk HE, & Strong DR (2015). Measuring anhedonia in adolescents: A psychometric analysis. Journal of Personality Assessment, 97(5), 506–514. 10.1080/00223891.2015.1029072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Witt CF, & Zimmerman M (2008). Associations between depression subtypes and substance use disorders. Psychiatry Research, 161(1), 43–50. 10.1016/j.psychres.2007.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, & Zvolensky MJ (2015). Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion–smoking comorbidity. Psychological Bulletin, 141(1), 176–212. doi:org/10.1037/bul0000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, Toomey R, Faraone SV, Kremen WS, Yeung AS, & Tsuang MT (1995). Correlates of psychosis proneness in relatives of schizophrenic patients. Journal of Abnormal Psychology, 104(2), 390–394. doi.org/10.1037/0021-843X.104.2.390 [DOI] [PubMed] [Google Scholar]
- Mann JJ, Bortinger J, Oquendo MA, Currier D, Li S, & Brent DA (2005). Family history of suicidal behavior and mood disorders in probands with mood disorders. American Journal of Psychiatry, 162(9), 1672–1679. 10.1176/appi.ajp.162.9.1672 [DOI] [PubMed] [Google Scholar]
- Meehl PE (2001). Primary and secondary hypohedonia. Journal of Abnormal Psychology, 110(1), 188–193. doi:org/10.1037/0021-843X.110.1.188 [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, … & Rounsaville BJ (1998). Familial transmission of substance use disorders. Archives of General Psychiatry, 55(11), 973–979. 10.1001/archpsyc.55.11.973 [DOI] [PubMed] [Google Scholar]
- Miller J, Flory K, Lynam D, & Leukefeld C (2003). A test of the four-factor model of impulsivity-related traits. Personality and Individual Differences, 34(8), 1403–1418. 10.1016/S0191-8869(02)00122-8 [DOI] [Google Scholar]
- Molet J, Heins K, Zhuo X, Mei YT, Regev L, Baram TZ, & Stern H (2016). Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Translational Psychiatry, 6(1), e702 10.1038/tp.2015.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JG, Skidmore JR, Dennhardt AA, Martens MP, Borsari B, Barnett NP, & Colby SM (2012). A behavioral economic supplement to brief motivational interventions for college drinking. Addiction Research & Theory, 20(6), 456–465. doi:org/10.3109/16066359.2012.665965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B, & Asparouhov T (2008). Growth mixture modeling: Analysis with non-Gaussian random effects. In Fitzmaurice G, Davidian M, Verbeke G, & Molenberghs G (eds.), Longitudinal Data Analysis, pp.143–165. Boca Raton: Chapman & Hall/CRC Press. [Google Scholar]
- Muthén LK, & Muthén BO (2010). Mplus: Statistical analysis with latent variables: User’s guide (pp. 1998–2007). Los Angeles: Muthén & Muthén. [Google Scholar]
- Nelson SE, Van Ryzin MJ, & Dishion TJ (2015). Alcohol, marijuana, and tobacco use trajectories from age 12 to 24 years: Demographic correlates and young adult substance use problems. Development and Psychopathology, 27(1), 253–277. 10.1017/S0954579414000650 [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, & Muthén BO (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling, 14(4), 535–569. doi:org/10.1080/10705510701575396 [Google Scholar]
- Preacher KJ, & Kelley K (2011). Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychological Methods, 16(2), 93–115. 10.1037/a0022658 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Ray LA, Bryan A, MacKillop J, McGeary J, Hesterberg K, & Hutchison KE (2009). The dopamine D4 receptor (DRD4) gene exon III polymorphism, problematic alcohol use, and novelty seeking: Direct and mediated genetic effects. Addiction Biology, 14(2), 238–244. 10.1111/j.1369-1600.2008.00120.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds EK, MacPherson L, Tull MT, Baruch DE, & Lejuez CW (2011). Integration of the Brief Behavioral Activation Treatment for Depression (BATD) into a college orientation program: Depression and alcohol outcomes. Journal of Counseling Psychology, 58(4), 555–564. 10.1037/a0024634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M, Wilkinson P, & Dubicka B (2015). Measurement issues: Depression measures in children and adolescents. Child and Adolescent Mental Health, 20(4), 230–241. 10.1111/camh.12106 [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, & Trigwell P (1995). A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. British Journal of Psychiatry, 167(1), 99–103. 10.1192/bjp.167.1.99 [DOI] [PubMed] [Google Scholar]
- Stone MD, Audrain-McGovern J, & Leventhal AM (2017). Association of anhedonia with adolescent smoking susceptibility and initiation. Nicotine & Tobacco Research, 19(6), 738–742. org/10.1093/ntr/ntw177 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2014). Results from the 2013 National Survey on Drug Use and Health: Detailed tables Retrieved from Rockville, MD: http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013/NSDUH-DetTabs2013.htm [PubMed] [Google Scholar]
- Sussman S, & Leventhal AM (2014). Substance misuse prevention: Addressing anhedonia. New Directions for Youth Development, 2014(141), 45–56. 10.1002/yd.20085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MP, Ho CH, & Kingree JB (2007). Prospective associations between delinquency and suicidal behaviors in a nationally representative sample. Journal of Adolescent Health, 40(3), 232–237. 10.1016/j.jadohealth.2006.10.016 [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, & Busto UE (2002). Probing brain reward system function in major depressive disorder: Altered response to dextroamphetamine. Archives of General Psychiatry, 59(5), 409–416. 10.1001/archpsyc.59.5.409 [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, & Busto UE (2005). Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Archives of General Psychiatry, 62(11), 1228–1236. 10.1001/archpsyc.62.11.1228 [DOI] [PubMed] [Google Scholar]
- Wise RA (2008). Dopamine and reward: The anhedonia hypothesis 30 years on. Neurotoxicity Research, 14(2–3), 169–183. 10.1007/BF03033808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule AM, Wilens TE, Martelon MK, Simon A, & Biederman J (2013). Does exposure to parental substance use disorders increase substance use disorder risk in offspring? A 5‐year follow-up study. The American Journal on Addictions, 22(5), 460–465. doi:org/10.1111/j.1521-0391.2013.12048.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


