Abstract
Antibody-mediated encephalitis defines a class of diseases wherein antibodies directed at cell-surface receptors are associated with behavioral and cognitive disturbances. One such recently described encephalitis is due to antibodies directed at alpha-amino-3-hydroxy-5-methyl-4isoxazolepropionic acid receptors (AMPAR). This entity is exceptionally rare and its clinical phenotype incompletely described. We present findings from two cases of AMPAR encephalitis that exemplify variability in the disease spectrum, and summarize findings in published cases derived from a systematic literature review. When all patients are considered together, the presence of psychiatric symptoms at presentation portended a poor outcome and was associated with the presence of a tumor. Furthermore, we provide evidence to suggest that the topography of magnetic resonance imaging abnormalities in reported cases mirrors the distribution of AMPARs in the human brain. The potential for neurological improvement following immunomodulatory therapy together with the favorable outcome reported in most cases emphasizes the importance of testing for autoantibodies against neuronal cell-surface proteins, including AMPAR, in patients with clinical and neuroimaging findings suggestive of autoimmune encephalitis. Close attention to the clinical phenotype may inform the presence of malignancy and long-term prognosis.
Keywords: Autoimmune encephalitis, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, paraneoplastic encephalitis, limbic encephalitis
Introduction
Autoimmune encephalitis is increasingly recognized as an important, eminently treatable cause of subacute neurologic deterioration, with a prevalence that rivals infectious encephalitis in industrialized countries2–4. Patients typically present with memory deficits, encephalopathy or psychiatric symptoms1. Autoimmune encephalitidies associated with autoantibodies directed against neuronal cell-surface antigens have garnered particular attention over the past decade due to their unique clinical phenotype, association with catastrophic decline, and remarkable potential for dramatic and sustained recovery following treatment with immunomodulatory agents4. Of these, encephalitis associated with autoantibodies against N-methyl-D-aspartate receptors (NMDAR) is the most common2 and best defined, with symptoms, signs and diagnostic findings elucidated through case series enrolling hundreds of patients5,6. Prompt recognition of patients with antibody-mediated encephalitis is critical as long-term outcomes are inversely related to time-to-treatment5,7–10. Therefore, it is important to clarify the phenotypes of rare syndromes to improve recognition of affected patients and minimize morbidity and mortality.
Antibodies against the GluA1 or GluA2 subunits of the alpha-amino-3-hydroxy-5methyl-4-isoxazolepropionic acid receptor (AMPAR) are recognized to associate with encephalitis11. AMPAR encephalitis is extremely rare12, with clinical experience reported through relatively small case series. As the number of reported cases has increased, it has become apparent that the clinical phenotype of AMPAR encephalitis is broad13. In support of this point, we present two exemplar cases that highlight clinical variability, and consolidate the extant case-series and case-reports, providing a comprehensive overview of the demographic, clinical presentation and malignancy patterns that define this disease. Particular attention is paid to describing the associations between clinically measurable symptoms and signs, disease-associated malignancy, reported outcomes and the neurobiology of the AMPAR. Better characterization of the clinical phenotype and malignancy risk of this entity lays the groundwork for earlier recognition and earlier initiation of definitive treatment.
Methods
Clinical Cases
Patients with AMPAR encephalitis were prospectively enrolled in existing research studies. Study protocols were approved by the Washington University School of Medicine Human Research Protections Office. Written informed consent was obtained from all patients or their delegate.
AMPAR antibodies were detected using indirect immunofluorescence (IFA) and cell based assays (CBA) performed at the Mayo Clinic (Rochester, Minnesota). Briefly, IFA was performed by applying specimen to frozen mouse composite tissue, washed and treated with fluorescein-conjugated IgG. CBA was performed by applying the specimen to a slide containing transfected and nontransfected HEK-293 cells. Fluorescein-conjugated IgG was then applied, and binding-patterns interpreted. In cases where the IFA pattern suggested an AMPAR antibody and the CBA was positive, further quantification was performed*.
Systematic Review and Data Extraction
An extensive literature review was undertaken to identify published cases of AMPAR encephalitis defined by the identification of a typical clinical phenotype and associated AMPAR antibodies in the serum or CSF (Figure 1). A medical librarian (LES) searched Ovid Medline 1946-, Embase.com 1947-, Scopus 1823-, Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), and Clinicaltrials.gov 1997- in April 2018, yielding 632 unique citations. Search strategies for each database are detailed in Appendix 1. Corresponding abstracts were reviewed for eligibility, yielding 57 manuscripts, which were reviewed in full. Twenty-six manuscripts did not identify unique cases of AMPAR encephalitis, or primarily reported on other disease processes (e.g., Rasmussen’s encephalitis), and were excluded. Data were extracted from unique cases reported in the remaining 31 manuscripts concerning demographics (e.g., age, gender), clinical phenotype, results of laboratory and imaging investigations, and outcome. Clinical phenotype at presentation was characterized by the presence or absence of five symptoms: confusion, limbic encephalitis, amnesia, convulsions, and psychiatric disturbances, consistent with prior reports14. We acknowledge the potential for overlap between terminology “limbic encephalitis” (describing altered level of consciousness, seizures and psychoses) and confusion (connoting altered level of consciousness, amnesia or other cognitive impairment). In this analysis, we use the terms used by the original authors, recognizing the nested nature of this nomenclature. The modified Rankin Score (mRS) was the most frequently reported measure of disability. When possible, mRS values were extracted at time of presentation and longest follow up. When not directly reported, the clinical description was used to estimate the mRS, consistent with validated criteria15. Outcomes were dichotomized as favorable (mRS 0–2) or unfavorable (mRS ≥3), consistent with other published approaches5,6. Additional variables regarding treatment and presence or absence of a tumor were also extracted.
Figure 1.
PRISMA Diagram. PRISMA Diagram summarizing manuscript selection from systematic literature review.
Statistical Analysis
Statistical analyses were performed in R (version 3.5.1). The relationship between demographic and clinical variables on outcomes was quantified using logistic regression (dichotomized mRS). Logistic regression was also performed to investigate the effect of demographic and clinical variables on tumor status (present or absent). Statistical significance was defined as p<0.05.
Magnetic Resonance Imaging Lesions
The areas of abnormal brain magnetic resonance imaging (MRI) findings were extracted and classified according to cortical anatomic regions defined in Freesurfer16. Involved regions corresponded to those identified in the manuscript text, or depicted in published figures as abnormal or affected. The most common lesion type was T2 hyperintensity.
Gene Expression Data
Regional GluA1 and GluA2 gene expression was extracted from the Allen Brain Atlas17 by performing a “Gene Search” for “GluA1” and “GluA2”. Expression data in the Allen Brain Atlas are derived by microarray and expressed in each region as a z-score relative to the mean expression across all brain regions from 6 human brains. Each target is assayed via several probes. These probes are highly correlated, and were thus averaged. The correlation between GluA1 and GluA2 expression was high (r=0.87) across indexed regions and were, therefore, also averaged. The Allen Brain Atlas is sampled at a spatial frequency that is denser than reported MRI lesions. Thus, the Allen Brain Atlas data was down-sampled by averaging expression from samples that fall within the anatomical boundaries of individual brain FreeSurfer regions to facilitate correlation analysis.
Results
Case 1: Encephalopathy and dystonia following thymectomy
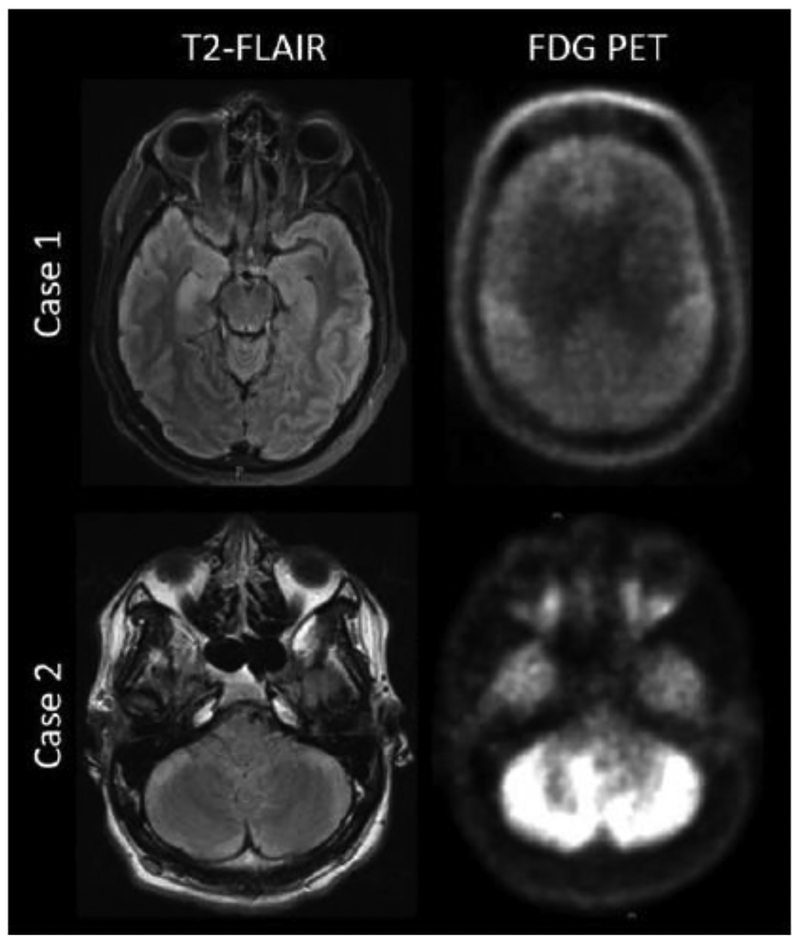
A 44 year-old man with myasthenia gravis developed disorientation, forgetfulness, labile mood, hallucinations and dystonia five weeks following thymectomy. An extensive work-up completed at an outside hospital was normal, including brain MRI and whole-body fluorodeoxyglucose positron emission tomography (FDG-PET) scan. Diagnostic lumbar puncture revealed lymphocytic pleocytosis, supportive of an inflammatory process. Serum antibody testing was performed at a reference laboratory, and confirmed autoantibodies against AMPAR (1:256) and CRMP-5 (in addition to known anti-acetylcholine receptor antibodies). He was treated sequentially with intravenous methylprednisolone (1g × 5 days), intravenous immunoglobulin (2g/kg divided over 5 days) and a single dose of rituximab (375 mg/m2), and discharged to a rehabilitation facility. After 3.5 weeks, he developed marked encephalopathy with inability to follow commands, severe ticks/bruxism, diffusely increased muscle tone, and periods of hypoventilation requiring intubation and admission to an intensive care unit. Repeat evaluation demonstrated generalized slowing on an electroencephalogram (EEG), bilateral hippocampal T2/FLAIR hyperintensities on brain MRI, and global cerebral hypometabolism measured by repeat brain FDG-PET (Figure 2). Repeat diagnostic lumbar puncture was acellular, with normal protein (47 mg/dL) and glucose (49mg/dL). IgG index was elevated. Elevated CSF AMPAR antibody titres were detected (1:256) by cell-based assay completed at a reference laboratory. Computerized tomography (CT) of the chest, abdomen and pelvis, showed no lesions concerning for recurrent thymoma or a new malignancy. Intravenous methylprednisolone (1g/day × 5 days), intravenous immunoglobulins (2g/kg divided over 5 days) and four weekly doses of rituximab (375 mg/m2 IV Q7days × 4) were provided, with gradual resolution of encephalopathy. He was discharged to a rehabilitation facility four weeks later. Two weeks following discharge, his mental status had improved to the point that he was fully oriented and could carry out his activities of daily living. Five months later (10 months from symptom onset), mRS was 2, with mild persistent short-term memory deficits. Fifteen months later (20 months from symptom onset), he had successfully returned to work as a business manager (mRS=0).
Figure 2:
Representative brain magnetic resonance and fluorodeoxyglucose positron emission tomography imaging from AMPAR encephalitis patients. Axial magnetic resonance T2 fluidattenuated inversion recovery (FLAIR) images (left) are shown alongside of fluorodeoxyglucose positron emission tomography (FDG-PET) images (right) from exemplar cases presenting with AMPAR encephalitis. In Case 1, T2-FLAIR reveals bilateral, right greater-than left hippocampal hyperintensities. FDG-PET demonstrates global hypometabolism with sparing of bilateral motor cortices. Case 2 demonstrates T2-FLAIR hyperintensities in the bilateral cerebellum, with corresponding FDG-PET hypermetabolism.
Case 2: Subacute cognitive decline
An 18 year-old previously healthy high school student presented to an outside hospital with a 5month history of declining school performance, forgetfulness, behavioral change (withdrawn affect) and poor hygiene. A thorough evaluation failed to confirm a diagnosis. Empiric intravenous methylprednisolone (1g × 5 days) was provided for possible autoimmune encephalitis, and he was transferred to our academic center for a second opinion. On arrival, his exam showed severe abulia, ocular flutter, and asymmetric appendicular and truncal ataxia. Brain MRI demonstrated T2/FLAIR hyperintensities with contrast enhancement in the bilateral cerebellar hemispheres, corresponding to areas of increased metabolism on FDG-PET (Figure 2). Repeat CSF analysis confirmed a lymphocytic pleocytosis (197 nucleated cells per high-powered field; 97% lymphocytes) with normal protein (25mg/dL) and glucose (70mg/dL). Flow cytometry and cytology did not suggest hematologic malignancy. AMPAR antibodies were detected in the CSF by cell-based assay completed at a reference laboratory (titers not reported). No malignancy was identified on CT chest/abdomen/pelvis or whole body FDG-PET. He was treated with intravenous immunoglobulin (2g/kg divided over 5 days) and rituximab (375 mg/m2 IV Q7days × 4), and discharged with a prolonged oral steroid taper. Two years later, he had enrolled in a post-secondary degree program and was asymptomatic (mRS=0).
Systematic Literature Review
Systematic literature review revealed an additional 81 patients with AMPAR encephalitis. Sufficient clinical data were reported for 53 of these cases, yielding a final cohort of 55 patients for analysis (Figure 1). Extracted patient-specific data is detailed in Appendix 2 (patients included in analyses) and Appendix 3 (patients excluded for missing data). AMPAR antibodies were identified in the serum (n=41) or CSF (n=45) of all included patients. In 40 patients, antibody testing was performed in both serum and CSF. In these cases, AMPAR antibodies were detected in the serum and CSF of 32 patients, and in the serum or CSF of 4 patients each.
Demographic features and clinically relevant symptoms and signs are presented in Table 1. Logistic regressions were performed to determine if the presence of a presenting symptom depended on age, sex, or time to diagnosis or treatment (Table 2). Psychiatric complaints at presentation were more common in younger patients (z=−2.08, p=0.038). Confusion as a presenting complaint was associated with diagnostic delay; limbic encephalitis was more common in women; amnesia and psychiatric symptoms were associated with a longer delay until diagnosis (p<0.10). Other clinical symptoms and signs were reported sporadically, including focal weakness (n=5), involuntary movements (n=6), autonomic dysfunction (n=2), upper motor neuron signs (n=6), apraxia (n=10), aphasia (n=6), sensory symptoms (n=2), ataxia or other cerebellar signs (n=10). It is unclear whether the low prevalence of these findings reflected true rarity in AMPAR encephalitis, or under-recognition/reporting—a common issue in retrospective studies.
Table 1.
Demographics
| Variable | Range | Mean | N Missing |
| Sex | 19 M/36 F | ||
| Age (years) | 14 – 92 | 53.1 | |
| mRS (presentation) | 2 – 5 | 3.94 | 24 |
| mRS (follow-up) | 0 – 6 | 1.80 | |
| Presentation to Diagnosis (wks) | 0.5 – 52 | 13.0 | 27 |
| Presentation to Treatment (wks) | 1 – 52 | 7.8 | 21 |
| Clinical Symptoms | N | ||
| Limbic Encephalitis | 18 | ||
| Confusion | 27 | ||
| Amnesia | 29 | ||
| Convulsions | 16 | ||
| Psychiatric Complaints | 26 | ||
| Clinical Studies | N | % Positive | N Missing |
| Tumor Identified | 34 | 66.7 | 4 |
| - Thymus | 15 | ||
| - Lung | 10 | ||
| - Breast | 5 | ||
| - Ovarian | 4 | ||
| Brain MRI Abnormal | 44 | 86.2% | 4 |
| EEG Abnormal | 13 | 44.8% | 26 |
| Routine CSF Abnormal | 34 | 66.7% | 4 |
Table 2.
Logistic regression of symptom at presentation against demographic data
| Confusion at Presentation | ||
| Term | z Value | p Value |
| Age | −1.2 | 0.25 |
| Sex | −0.82 | 0.41 |
| Time to Diagnosis | 1.66 | 0.096 |
| Time to Treatment | −1.30 | 0.19 |
| Limbic Encephalitis at Presentation | ||
| Term | z Value | p Value |
| Age | 1.25 | 0.21 |
| Sex | 1.68 | 0.092 |
| Time to Diagnosis | −1.58 | 0.11 |
| Time to Treatment | 1.27 | 0.20 |
| Amnesia at Presentation | ||
| Term | z Value | p Value |
| Age | −1.03 | 0.30 |
| Sex | −1.49 | 0.14 |
| Time to Diagnosis | 1.65 | 0.099 |
| Time to Treatment | −0.92 | 0.36 |
| Convulsions at Presentation | ||
| Term | z Value | p Value |
| Age | −1.12 | 0.26 |
| Sex | 0.041 | 0.96 |
| Time to Diagnosis | 1.22 | 0.22 |
| Time to Treatment | −1.04 | 0.30 |
| Psychiatric Symptoms at Presentation | ||
| Term | z Value | p Value |
| Age | −2.08 | 0.038 |
| Sex | −0.82 | 0.42 |
| Time to Diagnosis | 1.68 | 0.092 |
| Time to Treatment | −1.38 | 0.17 |
A disease-associated malignancy was reported in 34 cases (62%), most commonly lung carcinoma and thymoma. No malignancy was identified in 17 (31%) cases following variably comprehensive investigations. Data concerning malignancy was not presented for 4 (7%) cases. To determine the clinical factors that predicted the presence of malignancy, we fit a logistic regression model of malignancy presence against variables corresponding to demographics (age, sex) and clinical phenotype (presence of confusion, limbic encephalitis, amnesia, convulsions, psychiatric symptoms; Table 3). Only the presence of psychiatric symptoms predicted tumor presence (z = 2.06, p = 0.040, OR 4.9 [95% CI 1.2 – 25.3]).
Table 3.
Logistic Regression Predicting Favorable Outcome (mRS 0–2) and Presence of Disease-Associated Malignancy
| Predicting Favorable Outcome (mRS < 3) | ||
| Term | z Value | p Value |
| mRS at presentation | −1.05 | 0.29 |
| Age | −1.78 | 0.076 |
| Sex (Female) | 1.58 | 0.11 |
| Confusion at presentation | 1.85 | 0.064 |
| Limbic Encephalitis at presentation | −0.60 | 0.55 |
| Convulsions at presentation | −1.48 | 0.15 |
| Psychiatric symptoms at presentation | −2.12 | 0.034 |
| Predicting Presence of a Tumor | ||
| Term | z Value | p Value |
| Age | 1.21 | 0.23 |
| Sex (Female) | 0.14 | 0.89 |
| Confusion at presentation | −0.34 | 0.73 |
| Limbic Encephalitis at presentation | 0.26 | 0.80 |
| Convulsions at presentation | 0.63 | 0.53 |
| Psychiatric symptoms at presentation | 2.06 | 0.040 |
Beyond clinical signs and symptoms, diagnostic tests recommended in the evaluation of patients with suspected autoimmune encephalitis (i.e., MRI, LP, EEG1) were variably informative. Routine CSF studies were abnormal in approximately two-thirds (67%) of patients, where “abnormal” was defined by the reference laboratory. EEG was less sensitive with abnormalities detected in 44% (most commonly non-specific slowing). Brain MRI was frequently abnormal (86% of cases) with a stereotyped topography including a clear predilection for bilateral temporal lobes (Figure 3). Prior observation suggested that the topography of MRI abnormalities was related to the topography of GluA1 and GluA2 expression (i.e., AMPAR density)11. To test this hypothesis, we extracted the z-scored mean GluA1 and GluA2 expression from the Allen Brain Atlas17. In regions where there were brain MRI abnormalities, the mean zscored GluA1 and GluA2 expression was 0.58, indicating that the average expression in these regions was ~1/2 of a standard deviation above mean expression across the entire brain. These zscores ranged from −0.77 – 1.86 (N.B. the only negative z-score was in the cerebellum). The distribution of z-scores was significantly greater than 0† (t=4.17, p=0.001), confirming that the density of AMPAR expression was greater in these regions, compared to the rest of the brain on average. Within the regions involved from the MRI analysis, there was a significant relationship between the number of patients demonstrating an MRI abnormality in an area and the mean expression of GluA1 and GluA2 within the Allen Brain Atlas (Spearman rho=0.63, p=0.016), suggesting that regions richer in AMPAR were more likely to have MRI abnormality.
Figure 3:
Map depicting the distribution of brain magnetic resonance imaging abnormalities reported in AMPAR encephalitis patients. Frequency of imaging abnormality by anatomic region as defined by Freesurfer. Incidence of imaging abnormality is coded by color, where grey indicates no imaging abnormalities reported. Right hemisphere is shown since left and right sided data were combined. Temporal lobe was the most frequently involved cortical region.
Immunomodulatory therapies were provided to all patients; although, the agent of choice and duration of treatment varied widely within and between institutions. Forty-five patients (82%) received steroids of variable formulations and doses; 35 (64%) received intravenous immunoglobulin; 16 (29%) underwent plasma exchange. Second-line therapies were provided to fewer patients, including rituximab (n=10, 18%), cyclophosphamide (n=4, 7%), azathioprine (n=5, 9%) and mycophenolate mofetil (n=1, 2%).
In general, outcomes were favorable (Figure 4), with 46 patients (84%) surviving to follow-up. In patients where both mRS at presentation and at follow up were reported, there was a significant improvement in mRS (t(30)=6.38, p<10−6, d=1.1). Importantly, mRS at presentation did not predict mRS at follow up (r=0.026, p=0.89). The clinical factors (i.e., demographic features and clinical phenotype) that portend a particular prognosis (i.e., dichotomized mRS: favorable 0–2, unfavorable ≥3) were investigated by logistic regression (Table 3), controlling for mRS at presentation (0–5) (i.e., differences in baseline presentation). Psychiatric symptoms at presentation were associated with an unfavorable prognosis at follow up (z=−2.12, p=0.034). There was a trend towards younger age (z=−1.78, p=0.076) and the presence of confusion at presentation (z=1.85, p=0.064) associating with better prognoses.
Figure 4:
mRS at last follow-up. Histogram showing mRS at last follow up. The most common outcome was mRS 1, with apparent skew towards better outcomes.
Nine patients with AMPAR encephalitis died (16%), most commonly of complications related to underlying malignancy (mean time from presentation, 54 weeks). Of the remainder, one patient each died of status epilepticus (onset 112 weeks after presentation), urosepsis (52 weeks after presentation), myocardial infarction (105 weeks after presentation) and withdrawal of life-sustaining therapies (8 weeks after presentation).
Discussion
We summarize local experience with two patients and findings from a systematic review of reported AMPAR encephalitis cases. Our findings emphasize the high degree of variability in age-at-symptomatic onset, with AMPAR encephalitis diagnosed in patients in the 2nd through 10th decades of life. Further, symptoms, signs and outcomes observed in patients with AMPAR encephalitis were highly variable. Additionally, using logistic regression, we offer preliminary evidence suggesting an association between psychiatric symptoms, disease-associated malignancy and less favorable outcomes. Finally, we demonstrate a relationship between the topography of reported MRI abnormalities and the anatomical distribution of AMPAR reported in the Allen Brain Atlas. Together these findings may be applied to improve recognition of patients with possible AMPAR, improving coordination of diagnostic testing, and facilitating earlier intervention with the goal of improving long-term outcomes.
Of the diagnostic tests routinely performed in patients with suspected autoimmune encephalitis (neuroimaging, CSF analyses, EEG), detection of MRI T2/FLAIR hyperintensities appeared to be the most sensitive, with abnormalities reported in 86% of cases (but specificity is likely low). Together these findings reiterate that, while the results of diagnostic tests may support a diagnosis of autoimmune encephalitis, no routine test (or combination of tests) is sufficient to rule-in or -out specific causes of autoimmune encephalitis1. In patients with suspected autoimmune encephalitis, detection of AMPAR autoantibodies is assumed to be reasonably specific for AMPAR encephalitis, with low rates of seropositivity (<0.1%) reported in healthy and neurologically ill cohorts22. This finding is reassuring, in light of ongoing discussions concerning the positive and negative predictive value of testing for other cell-surface antigens in healthy controls and individuals with other neurological diseases18–21. Ultimately, however, larger methodologically sound studies are needed to determine the positive and negative predictive value of specific investigations in well-defined populations.
The clinical entity of AMPAR encephalitis was first recognized in ten patients with limbic encephalitis11, but is now known to encompass a more diverse set of clinical phenotypes14,23. In the case of AMPAR encephalitis, the physiologic mechanism appears to be related to removal of AMPAR from the synapse,11 leading to antibody-dependent changes in ion flux24,25. AMPA channels belong to a family of glutamatergic ionotropic receptors that mediate synaptic plasticity, synaptic homeostasis, learning and memory26. Functionally, AMPAR are related to NMDAR through their classic involvement in synaptic plasticity27; however the clinical entities associated with autoantibodies directed against these cell-surface receptors have some differences. These may reflect differences in the electrophysiology of the specific channels or differences in the topographic expression of receptors throughout the central nervous system. AMPARs are broadly implicated in neurologic function and broadly distributed in the cortex. Patient derived antibodies target hippocampus, cerebellum and basal ganglia in experimental models,23 which is where AMPARs are most heavily expressed. This may account in large part for the prevalence of limbic encephalitis at disease presentation, while autoantibody engagement of widely-distributed (but lower density) AMPARs throughout the brain26 may explain the wide variations in the phenotype. This more general involvement may also explain the global atrophy and hypometabolism reported in a single case of AMPAR encephalitis28,29.
AMPAR encephalitis is a rare condition12. As a result, there exists no prospective or meticulously controlled outcome data pertaining to patient demographics, clinical phenotype, associated malignancy or treatment efficacy. In lieu of higher quality data, we suggest that comprehensive analyses of existing cases provides a reasonable means of summarizing the clinical phenotype. Additionally, statistical models in this sample suggest that variations in the clinical phenotype (i.e., clinical symptoms and signs), may account for a reasonable proportion of variability in clinically relevant findings, including association with malignancy and outcome measures. Although it would be imprudent to overstate the clinical significance of relative risk or odds ratios based on such limited retrospective information, these early findings suggest that, as more patients are identified, it may be possible to use clinically-measurable variables to predict tumor presence and mRS at follow up, allowing diagnostic and therapeutic approaches to be tailored to the individual patient. However, further studies are needed to decipher the relationship between time-to-treatment and clinical outcomes, and the comparative efficacy of standard immunotherapies.
Conclusions
AMPAR encephalitis is associated with a broad clinical phenotype, high treatment-responsiveness and generally favorable outcomes. Careful databasing of new cases will facilitate more definitive study in the future, with the potential that readily-measurable clinical details may be used to inform the likelihood of disease-associated malignancy and long-term prognoses.
Supplementary Material
Acknowledgements
On behalf of all authors, the corresponding author states that there are no conflicts of interest. The authors have no relevant disclosures to report.
Appendix 1.
Search Strategies
Ovid Medline
Date Searched: 4/3/18
Applied Database Supplied Limits: n/a
Number of Results: 228
Full Search Strategy:
exp Receptors, AMPA/ OR exp alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid/ OR (alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid).mp. OR (AMPAR OR AMPAR).mp. OR ((AMPA) ADJ10 (antibod*).mp.) OR ((AMPA OR quisqualate) ADJ2 (receptor*).mp.) AND (exp Encephalitis/ OR (Encephalitis* OR encephalopath* OR encephalomyelitis OR cerebritis OR enkephalitis OR leucoencephalitis OR myeloencephaliti*).mp. OR ((brain) ADJ1 (inflammation).mp.) OR ((allergic) ADJ1 (leucoencephalopath*).mp.) OR ((cerebral) ADJ1 (ventriculitis).mp.))
Embase
Date Searched: 4/3/18
Applied Database Supplied Limits: n/a
Number of Results: 550
Full Search Strategy:
‘AMPA receptor’/exp OR ‘alpha amino 3 hydroxy 5 methyl 4 isoxazolepropionic acid’/exp OR ‘a-amino3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor’ OR (AMPA NEAR/10 antibod*) OR AMPAR OR AMPAR OR ((AMPA OR quisqualate) NEAR/2 (receptor*)) AND (‘encephalitis’/exp OR Encephalitis* OR encephalopathy* OR encephalomyelitis OR cerebritis OR enkephalitis OR leucoencephalitis OR myeloencephaliti* OR ((brain) NEAR/1 (inflammation)) OR ((allergic) NEAR/1 (leucoencephalopath*)) OR ((cerebral) NEAR/1 (ventriculitis)))
Cochrane
Date Searched: 4/3/18
Applied Database Supplied Limits: n/a
Number of Results from each database in Cochrane
CDSR: 1
CENTRAL: 4
DARE:
Full Search Strategy:
([mh “Receptors, AMPA”] OR [mh “alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid”] OR “alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid” OR AMPAR OR AMPAR OR ((AMPA) NEAR/10 (antibody*)) OR ((AMPA OR quisqualate) NEAR/2 (receptor*))) AND ([mh encephalitis] OR Encephalitis* OR encephalopath* OR encephalomyelitis OR cerebritis OR enkephalitis OR leucoencephalitis OR myeloencephaliti* OR ((brain) NEAR/1 (inflammation)) OR ((allergic) NEAR/1 (leucoencephalopath*)) OR ((cerebral) NEAR/1 (ventriculitis)))
Scopus
Date Searched: 4/3/18
Applied Database Supplied Limits: n/a
Number of Results: 455
Full Search Strategy:
TITLE-ABS-KEY(AMPAR OR AMPAR OR “alpha-Amino-3-hydroxy-5-methyl-4isoxazolepropionic Acid” OR ((AMPA) W/10 (antibody*)) OR ((AMPA OR quisqualate) W/2 (receptor*))) AND TITLE-ABS-KEY(Encephalitis* OR encephalopath* OR encephalomyelitis OR cerebritis OR enkephalitis OR leucoencephalitis OR myeloencephaliti* OR ((brain) W/1 (inflammation)) OR ((allergic) W/1 (leucoencephalopath*)) OR ((cerebral) W/1 (ventriculitis)))
ClinicalTrials.gov
Date Searched: 4/3/18
Number of Results: 0
Report, as accurately as possible, what you did. Searches in Clinicaltrials.gov must be much for simple then those used for other databases.
In expert search: (AMPA receptor OR quisqualate receptor) AND (encephalitis)
Appendix 2.
Please see attached xlsx file for supplemental data table of subjects included in these analyses
Appendix 3.
Please see attached xlsx file for supplemental data table of subjects excluded from these analyses for incomplete data
Footnotes
References
- 1.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. doi: 10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166–177. doi: 10.1002/ana.25131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The Frequency of Autoimmune N-Methyl-D-Aspartate Receptor Encephalitis Surpasses That of Individual Viral Etiologies in Young Individuals Enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54(7):899–904. doi: 10.1093/cid/cir1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalmau J, Graus F. Antibody-Mediated Encephalitis. N Engl J Med. 2018;378(9):840851. doi: 10.1056/NEJMra1708712 [DOI] [PubMed] [Google Scholar]
- 5.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for longterm outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–165. doi: 10.1016/S1474-4422(12)70310-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098. doi: 10.1016/S1474-4422(08)70224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanagan EP, McKeon A, Lennon VA, et al. Autoimmune Dementia: Clinical Course and Predictors of Immunotherapy Response. Mayo Clin Proc. 2010;85(10):881–897. doi: 10.4065/mcp.2010.0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKeon GL, Robinson GA, Ryan AE, et al. Cognitive outcomes following anti-Nmethyl-D-aspartate receptor encephalitis: A systematic review. J Clin Exp Neuropsychol. 2018;40(3):234–252. doi: 10.1080/13803395.2017.1329408 [DOI] [PubMed] [Google Scholar]
- 9.Finke C, Kopp UA, Prüss H, Dalmau J, Wandinger K-P, Ploner CJ. Cognitive deficits following anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2012;83(2):195–198. doi: 10.1136/jnnp-2011-300411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finke C, Prüss H, Heine J, et al. Evaluation of Cognitive Deficits and Structural Hippocampal Damage in Encephalitis With Leucine-Rich, Glioma-Inactivated 1 Antibodies. JAMA Neurol. 2017;74(1):50–59. doi: 10.1001/jamaneurol.2016.4226 [DOI] [PubMed] [Google Scholar]
- 11.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2010;65(4):424–434. doi: 10.1002/ana.21589.AMPA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byun JI, Lee ST, Jung KH, et al. Prevalence of antineuronal antibodies in patients with encephalopathy of unknown etiology: Data from a nationwide registry in Korea. J Neuroimmunol. 2016;293:34–38. doi: 10.1016/j.jneuroim.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 13.Graus F, Boronat A, Xifro X, et al. The expanding clinical profile of anti-AMPA receptor encephalitis. Neurology. 2010;74:857–859. doi: 10.1212/WNL.0b013e3181d3e404 [DOI] [PubMed] [Google Scholar]
- 14.Hoftberger R, van Sonderen A, Houghton D, et al. Encephalitis and AMPA receptor antibodies: Novel findings in a case series of 22 patients. Neurology. 2015;84:2403–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJA, Van Gijn J. Interobserver Agreement for the Assessment of Handicap in Stroke Patients. Stroke. 1988;19(5):604607. [DOI] [PubMed] [Google Scholar]
- 16.Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/J.NEUROIMAGE.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489(7416):391–399. doi: 10.1038/nature11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo-Gómez E, Oliveira B, Tapken D, et al. All naturally occurring autoantibodies against the NMDA receptor subunit NR1 have pathogenic potential irrespective of epitope and immunoglobulin class. Mol Psychiatry. 2017;22(12):1776–1784. doi: 10.1038/mp.2016.125 [DOI] [PubMed] [Google Scholar]
- 19.Hammer C, Stepniak B, Schneider A, et al. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry. 2014;19(10):1143–1149. doi: 10.1038/mp.2013.110 [DOI] [PubMed] [Google Scholar]
- 20.Hara M, Martinez-Hernandez E, Ariño H, et al. Clinical and pathogenic significance of IgG, IgA, and IgM antibodies against the NMDA receptor. Neurology. 2018: 10.1212/WNL.0000000000005329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steiner J. Prevalence ofN-Methyl-D-AspartateReceptor Autoantibodies in thePeripheral Blood: Healthy Control SamplesRevisited. JAMA psychiatry. 2014;71(7):838–839. doi: 10.1038/mp.2013.110.3 [DOI] [PubMed] [Google Scholar]
- 22.Dahm L, Ott C, Steiner J, et al. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann Neurol. 2014;76(1):82–94. doi: 10.1002/ana.24189 [DOI] [PubMed] [Google Scholar]
- 23.Joubert B, Kerschen P, Zekeridou A, et al. Clinical spectrum of encephalitis associated with antibodies against the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor: Case series and review of the literature. JAMA Neurol. 2015;72(10):1163–1169. doi: 10.1001/jamaneurol.2015.1715 [DOI] [PubMed] [Google Scholar]
- 24.Haselmann H, Mannara F, Werner C, et al. Human Autoantibodies against the AMPA Receptor Subunit GluA2 Induce Receptor Reorganization and Memory Dysfunction. Neuron. 2018:1–15. doi: 10.1016/j.neuron.2018.07.048 [DOI] [PubMed] [Google Scholar]
- 25.Gleichman AJ, Panzer JA, Baumann BH, Dalmau J, Lynch DR. Antigenic and mechanistic characterization of anti-AMPA receptor encephalitis. Ann Clin Transl Neurol. 2014;1(3):180–189. doi: 10.1002/acn3.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprengel R. Role of AMPA receptors in synaptic plasticity. Cell Tissue Res. 2006;326(2):447–455. doi: 10.1007/s00441-006-0275-4 [DOI] [PubMed] [Google Scholar]
- 27.Genoux D, Montgomery JM. Glutamate receptor plasticity at excitatory synapses in the brain. Clin Exp Pharmacol Physiol. 2007;34(10):1058–1063. doi: 10.1111/j.14401681.2007.04722.x [DOI] [PubMed] [Google Scholar]
- 28.Wei YC, Liu CH, Lin JJ, et al. Rapid progression and brain atrophy in anti-AMPA receptor encephalitis. J Neuroimmunol. 2013;261(1–2):129–133. doi: 10.1016/j.jneuroim.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 29.Spatola M, Stojanova V, Prior JO, Dalmau J, Rossetti AO. Serial brain 18FDG-PET in anti-AMPA receptor limbic encephalitis. J Neuroimmunol. 2014;271(1–2):53–55. doi: 10.1016/j.jneuroim.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 30.Bataller L, Galiano R, Garcia-Escrig M, et al. Reversible paraneoplastic limbic encephalitis associated with antibodies to the AMPA receptor. Neurology. 2010;74(3):265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omi T, Kinoshita M, Nishikawa A, et al. Clinical Relapse of Anti-AMPA Receptor Encephalitis Associated with Recurrence of Thymoma. Intern Med. 2018:8–10. doi: 10.2169/internalmedicine.9682-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang S, Qin J, Li J, et al. Rapidly progressive neurological deterioration in anti-AMPA receptor encephalitis with additional CRMP5 antibodies. Neurol Sci. 2016;37(11):18531855. doi: 10.1007/s10072-016-2680-0 [DOI] [PubMed] [Google Scholar]
- 33.Poster Session-Monday. Eur J Neurol. 2016;23:601–879. doi: 10.1111/ene.13094 [DOI] [Google Scholar]
- 34.Takemoto K, Iwanari H, Tada H, et al. Optical inactivation of synaptic AMPA receptors erases fear memory. Nat Biotechnol. 2016;35(1):38–47. doi: 10.1038/nbt.3710 [DOI] [PubMed] [Google Scholar]
- 35.Lahiri S, Duberstein Coad S. Alpha-Amino-3-Hydroxy-5-Methyl-4-Isoxazoleproprionic Acid (AMPA) Receptor Mediated Limbic Encephalitis in a Fourteen-Year-Old (P05.114). Neurology. 2013;80(7 Supplement). [Google Scholar]
- 36.Liu HS, Ren HT, Zhou LX, et al. Clinical analysis of paraneoplastic neurological syndrome associated with thymoma. J Chinese Med Assoc. 2017;97(35):2770–2774. doi: 10.3760/CMA.J.ISSN.0376-2491.2017.35.013 [DOI] [PubMed] [Google Scholar]
- 37.van Den Tooren H, Maskery M, Kobylecki C, Rathod N, Siripurapu R, Mckee D. Presumed tuberculous meningitis relapsing after steroid reduction: a case of AMPA receptor antibody-associated encephalitis. Eur J Neurol. 2016;23:854.26806538 [Google Scholar]
- 38.Mittal MK, Rabinstein AA, Hocker SE, Pittock SJ, M Wijdicks EF, McKeon A. Autoimmune Encephalitis in the ICU: Analysis of Phenotypes, Serologic Findings, and Outcomes. Neurocrit Care. 2016;24(2):240–250. doi: 10.1007/s12028-015-0196-8 [DOI] [PubMed] [Google Scholar]
- 39.Saraya A, Mahavihakanont A, Shuangshoti S, et al. Autoimmune causes of encephalitis syndrome in Thailand: Prospective study of 103 patients. BMC Neurol. 2013;13. doi: 10.1186/1471-2377-13-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elamin M, Lonergan R, Killeen RP, O’Riordan S, Tubridy N, McGuigan C. Posterior cortical and white matter changes on MRI in anti-ampa receptor antibody encephalitis. Neurol Neuroimmunol NeuroInflammation. 2015;2(4):1–2. doi: 10.1212/NXI.0000000000000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dishner E, Majid-Moosa A, Shamim S, Mora A. When Two Antibodies Cause One Disease. Chest. 2015;148(4):272A. doi: 10.1378/chest.2274489 [DOI] [Google Scholar]
- 42.Schou M, Sæther SG, Borowski K, et al. Prevalence of serum anti-neuronal autoantibodies in patients admitted to acute psychiatric care. Psychol Med. 2016;46(16):3303–3313. doi: 10.1017/S0033291716002038 [DOI] [PubMed] [Google Scholar]
- 43.Zekeridou A, McKeon A, Lennon VA. Frequency of synaptic autoantibody accompaniments and neurological manifestations of thymoma. JAMA Neurol. 2016;73(7):853–859. doi: 10.1001/jamaneurol.2016.0603 [DOI] [PubMed] [Google Scholar]
- 44.Boangher S, Mespouille P, Filip C-M, Goffette S. Small-Cell Lung Cancer with Positive Anti-NMDAR and Anti-AMPAR Antibodies Paraneoplastic Limbic Encephalitis. Case Rep Neurol Med. 2016;2016:1–3. doi: 10.1155/2016/3263718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quaranta G, Maremmani AGI, Perugi G. Anti-AMPA-Receptor Encephalitis Presenting as a Rapid-Cycling Bipolar Disorder in a Young Woman with Turner Syndrome. Case Rep Psychiatry. 2015;2015:1–5. doi: 10.1155/2015/273192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linnoila JJ, Binnicker MJ, Majed M, Klein CJ, McKeon A. CSF herpes virus and autoantibody profiles in the evaluation of encephalitis. Neurol - Neuroimmunol Neuroinflammation. 2016;3(4):e245. doi: 10.1212/NXI.0000000000000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaeem Z, Luk C, Anderson D, Blevins G, Siddiqi Z. AMPA-R limbic encephalitis associated with systemic lupus erythematosus. Neurology. 2018;90(15 Supplement):P5.386. [DOI] [PubMed] [Google Scholar]
- 48.De Mesa C, Crump M. Anti-AMPA Receptor Limbic Encephalitis Presenting with Ovarian Teratoma, Encephalopathy and Autonomic Instability. Pm&R. 2013;5(9):S273S274. doi: 10.1016/j.pmrj.2013.08.473 [DOI] [Google Scholar]
- 49.Pinto L, Simabukuro M, Spera R, et al. AMPA receptor antibody encephalitis in a young man associated with atypical findings. Case Report. Neurology. 2016;86(16):Supplement. [Google Scholar]
- 50.Takahashi C, Holmes S, Wong M. A rare case of AMPA-R antibody positive paraneoplastic limbic encephalitis. Neurology. 2017;88(16):Supplement. [Google Scholar]
- 51.Li X, Mao YT, Wu JJ, Li LX, Chen XJ. Anti-AMPA receptor encephalitis associated with thymomatous myasthenia gravis. J Neuroimmunol. 2015;281:35–37. doi: 10.1016/j.jneuroim.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 52.Dogan Onugoren M, Deuretzbacher D, Haensch CA, et al. Limbic encephalitis due to GABAB and AMPA receptor antibodies: a case series. J Neurol Neurosurg Psychiatry. 2015;86(9):965–972. doi: 10.1136/jnnp-2014-308814 [DOI] [PubMed] [Google Scholar]
- 53.Zhu M, Yu X, Liu C, et al. Hashimoto’s encephalitis associated with AMPAR2 antibodies: A case report. BMC Neurol. 2017;17(1):1–5. doi: 10.1186/s12883-017-0823-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei Y-C, Huang C-C, Liu C-H, Kuo H-C, Lin J-J. Peripheral neuropathy in limbic encephalitis with anti-glutamate receptor antibodies: Case report and systematic literature review. Brain Behav. 2017;7(9):e00779. doi: 10.1002/brb3.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graus F, Saiz A, Lai M, et al. Neuronal surface antigen antibodies in limbic encephalitis: Clinical-immunologic associations. Neurology. 2008;71(12):930–936. doi: 10.1212/01.wnl.0000325917.48466.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.