Abstract
Nanomedicine is a promising, noninvasive approach to reduce atherosclerotic plaque burden. However, drug delivery is limited without the ability of nanocarriers to sense and respond to the diseased microenvironment. In this study, we developed nanomaterials from peptide amphiphiles (PAs) that respond to the increased levels of matrix metalloproteinases 2 and 9 (MMP2/9) or reactive oxygen species (ROS) found within the atherosclerotic niche. We tethered a pro-resolving therapeutic, Ac2–26, derived from annexin-A1 protein, to PAs using peptide linkages that cleave in response to MMP2/9 or ROS. We found that by adjusting the molar ratios and processing conditions, we promoted co-assembly with a PA containing an apolipoprotein A1-mimetic peptide to create a targeted, therapeutic nanofiber (ApoA1-Ac2–26 PA). The ApoA1-Ac2–26 PAs demonstrated release of Ac2–26 within 24 hours after treatment with MMP2 or ROS. The niche-responsive ApoA1-Ac2–26 PAs were cytocompatible and reduced macrophage activation from interferon gamma and lipopolysaccharide treatment, evidenced by decreased nitric oxide production. Interestingly, we found that the linkage chemistry of ApoA1-Ac2–26 PAs significantly affected macrophage uptake and retention. Taken together, these findings demonstrate the potential of PAs to serve as an atheroma niche-responsive nanocarrier system to modulate the inflammatory microenvironment, with implications for atherosclerosis treatment.
Keywords: atherosclerosis, nanomedicine, peptide amphiphile, drug delivery, Ac2–26, immunotherapy
1. Introduction
Cardiovascular disease is the leading cause of death and morbidity in the world.[1] Atherosclerosis, the narrowing of blood vessels due to plaque accumulation, is a major contributor to the pathophysiology of cardiovascular disease.[2] Atherosclerosis is characterized by an impaired lipid metabolism and nonresolving inflammatory response.[3][4] [5][6] This disease progresses asymptomatically, precluding patients from seeking treatment until the extent of plaque burden has reached an advanced stage that impairs blood flow. To restore blood flow, invasive surgical interventions are required and include balloon angioplasty with or without stenting, bypass grafting, or endarterectomy.[7] However, these procedures cause injury to the intervention site and carry the risk of restenosis, a narrowing of the vessel lumen due to cell ingrowth caused by an exaggerated wound healing response.[8] Thus, the development of noninvasive therapeutic strategies to alleviate plaque burden is of critical importance. Nanomedicine is a promising approach to manage atherosclerosis as nanoparticles can be delivered intravenously and provide targeted delivery of therapeutics that would otherwise cause toxicity when administered systemically.[9]
An emerging therapeutic strategy to reduce plaque burden is to address the cell-mediated inflammation involved in atherosclerosis.[10][11] In particular, Ac2–26, a peptide derived from the glucocorticoid annexin A1 protein, can resolve inflammation within atherosclerotic lesions through its pleiotropic interactions with formyl peptide receptor 2 (FPR2), which is expressed on vascular endothelial cells, leukocytes, and macrophages. Binding between Ac2–26 and FPR2 may reduce plaque burden by limiting leukocyte infiltration, enhancing phagocytosis of apoptotic cells, and reducing pro-inflammatory macrophage activation.[11][12][13] Still, without targeted delivery of Ac2–26 and other pro-resolving therapeutics to atheroma, broad immunosuppression remains a major risk.[14] Additionally, the majority of nanocarriers for atherosclerosis therapeutic delivery rely upon passive diffusion or hydrolysis for drug release, risking premature drug loss during intravenous transport, as well as lack of drug release at the plaque site.[10] The development of nanomaterials that can target and respond to the atheroma niche are vital to improving the safety, efficacy, and clinical translation of atherosclerosis nanomedicine.
Recently, Dou et al. reported the first nanocarriers that could deliver a therapeutic in response to the atheroma niche in vivo.[15] Rapamycin encapsulated within β-cyclodextrin nanoparticles and modified by either acetylation or 4-(hydroxymethyl)phenylboronic acid pinacolester, generated pH- and reactive oxygen species (ROS)-responsive nanocarriers, respectively. This nanocarrier system significantly enhanced the therapeutic effects of rapamycin in ApoE−/− mice after intraperitoneal injection in comparison to non-responsive poly(lactide-co-glycolide) nanoparticle controls, delaying atherosclerosis progression and promoting plaque stability.[15] While a major advancement, the β-cyclodextrin system did not contain an atheroma-targeting moiety, excluding it from being used to deliver therapeutics that cause systemic toxicity.
Peptide amphiphiles (PAs) are an encouraging nanocarrier platform to treat atherosclerosis due to their modularity, biocompatibility, and lack of immunogenicity.[16] We recently developed PAs incorporating a bioactive epitope, the apolipoprotein A1-mimetic peptide 4F (ApoA1 PAs), that binds to oxidized low density lipoproteins.[7] The ApoA1 PAs could self-assemble to form nanofibers under physiological conditions and, upon intravenous injection, targeted early- and late-stage atherosclerotic lesions within low density lipoprotein receptor knockout mice.[7] Co-assembling ApoA1 PAs with PAs that contain therapeutics covalently attached through redox- and protease-sensitive linkages could provide a targeted, atheroma niche-responsive nanocarrier with controlled drug release. ROS- and matrix metalloproteinase 2 and 9 (MMP2/9)-cleavable oligopeptide moieties would be strong candidates as they can be incorporated into PAs during peptide synthesis and are present at elevated levels within atherosclerotic lesions in comparison to healthy tissues.[4][17] For example, oligoprolines are oxidized by macrophage-secreted ROS.[18] As well, several peptide sequences are cleavable by MMP2/9.[19] In this study, we evaluate the hypothesis that Ac2–26 tethered to PAs using ROS- and MMP2/9-cleavable peptide sequences can be co-assembled with plaque-targeting ApoA1 PAs to generate nanofibers that have the potential to release immunotherapeutics in response to the atheroma niche. We test this hypothesis by determining parameters that promote ApoA1-Ac2–26 PA nanofiber formation and characterize the ability of the PAs to reduce macrophage activation towards a pro-inflammatory phenotype in vitro.
2. Results and Discussion
2.1. Design and characterization of atheroma niche-responsive PAs
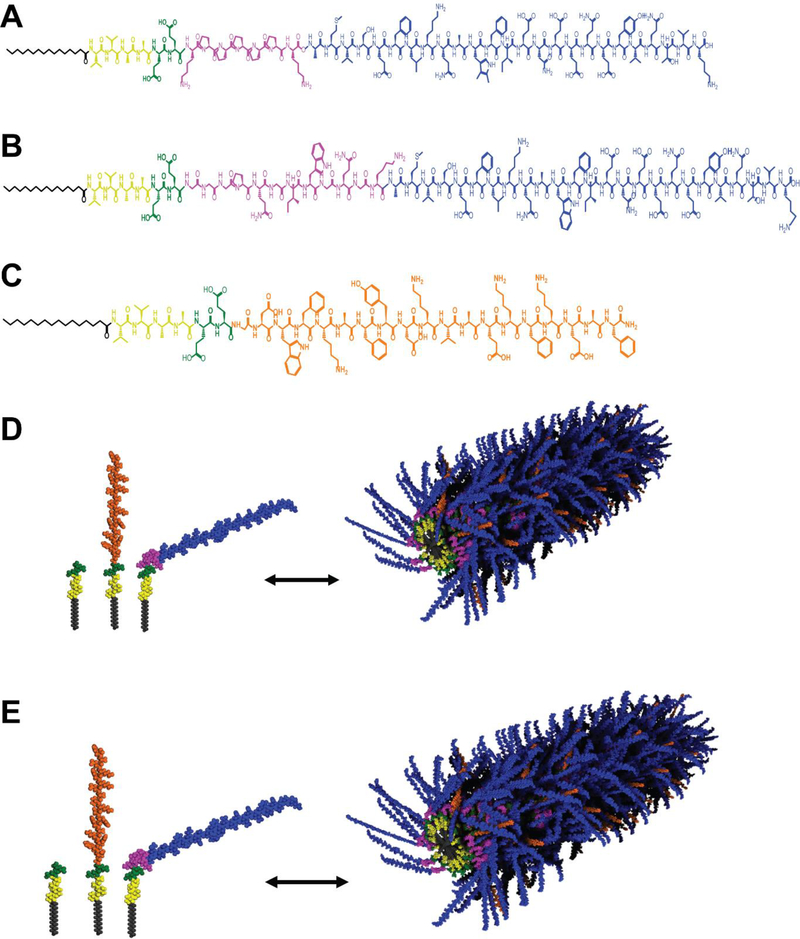
We developed atheroma niche-responsive linkages using ROS- or MMP2/9-sensitive peptides due to their ease of PA incorporation during peptide synthesis, and their bioresorbable properties. Arginine, histidine, lysine, and proline are readily oxidized by metal-catalyzed oxidative systems.[20] Of these residues, only proline is capable of forming tertiary amide bonds, which are more susceptible to oxidation than secondary amide bonds.[21] In addition, the mechanism of proline oxidation to glutamic semialdehyde does not involve the formation of reactive carbonyl groups that may aggravate existing inflammation within the atherosclerotic lesion.[22] Supporting their potential for cleavage by pro-inflammatory cells within the atherosclerotic niche, prolines incorporated into poly(ethylene glycol) (PEG) and poly(ε-caprolactone) scaffolds are degradable by M1 polarized murine macrophages within nine days in vitro.[18] Hence, for this study, we designed ROS-responsive Ac2–26 PAs (ROS-Ac2–26 PA) by incorporating five prolines, flanked by lysine residues to enhance solubility (Schematic 1A).
Schematic 1.
Chemical structures of (A) ROS-Ac2–26 PA, (B) MMP-Ac2–26 PA, and (C) ApoA1 PA. Molecular graphics of (D) ROS-Ac2–26-ApoA1 PA or (E) MMP-Ac2–26-ApoA1 PA nanofibers formed by self-assembly of three PAs: the PA backbone (E2 filler PA) containing an alkyl tail (gray), β-sheet forming peptide sequence (yellow), and charged region to enhance solubility (green); ApoA1 PA with 4F peptide (orange), and PAs with pro-resolving Ac2–26 (blue) attached with a ROS- or MMP2/9-cleavable linkage (pink)
The MMP2/9-sensitive peptide sequence GGGPQG↓IWGQGK (abbreviated as PQ, with ↓ denoting cleavage site) is cleavable by human aortic smooth muscle and blood-derived endothelial cell co-culture within a PEG hydrogel system, enabling three-dimensional microvessel formation in vitro.[23] Given the increased production of MMP2/9 by vascular and immune cells in the atherosclerotic niche, we designed an MMP2/9-sensitive PA containing the PQ sequence to link Ac2–26 (MMP-Ac2–26 PA) into the PA backbone (Schematic 1B). An atheroma-targeting, therapeutic nanocarrier may be created through co-assembly with ApoA1 PA and either ROS- or MMP-Ac2–26 PAs to generate supramolecular nanofibers (Schematic 1C-E). All PAs were synthesized using solid phase peptide synthesis and validated for expected molecular weight through liquid chromatography coupled with mass spectrometry (Supplemental Figure 1).
2.2. ApoA1-Ac2–26 PA co-assembly and characterization
We co-assembled ROS- or MMP-cleavable Ac2–26 PAs with ApoA1 PA to generate an atheroma-targeted immunotherapeutic. However, direct co-assembly of ROS- or MMP-Ac2–26 PAs with ApoA1 PAs did not result in nanofiber formation as each PA formed aggregates in aqueous solution, shown through transmission electron microscopy (TEM, Supplemental Figure 2). Co-assembly with a diluent PA can enhance nanofiber formation.[7] For example, we have previously shown that co-assembly of the ApoA1 PA with E2 filler PA enabled nanofiber formation without compromising PA bioactivity.[7] Accordingly, we sought to determine the PA co-assembly parameters that would support nanofiber formation between the E2 filler PA, ROS- or MMP-Ac2–26 PA, and ApoA1 PA.
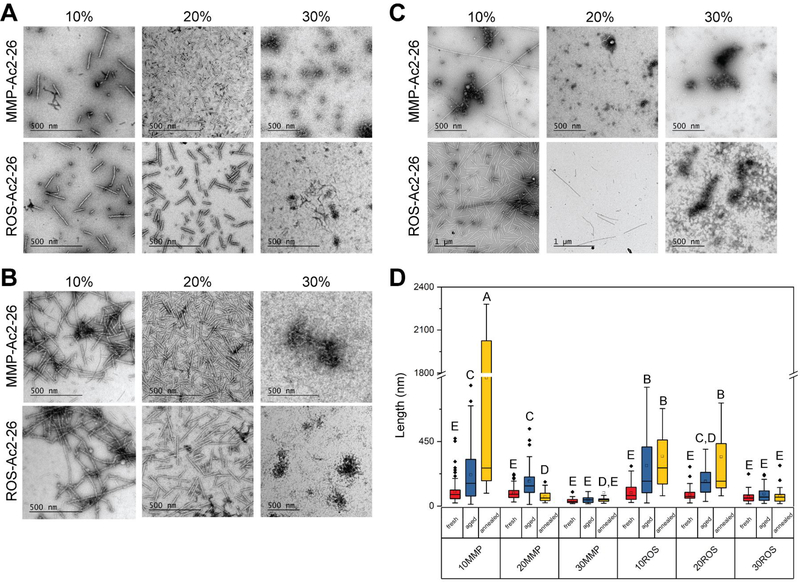
An essential condition for the co-assembled ApoA1-Ac2–26 PA is that it contains a minimum of 40 mol% ApoA1 PA to target atheroma.[7] The processing conditions for PA co-assembly can significantly affect nanofiber structure as self-assemblies exist at distinct energy landscapes, ranging from a metastable thermodynamic state with short fibers to the thermodynamically favored state of long nanofibers.[24][25] Annealing PAs by temporarily increasing the temperature to 80°C provides thermal energy to assist nanofiber elongation.[25] Aging the PAs also enhanced nanofiber formation by allowing the fibers more time to self-assemble towards a more energetically favorable state. For these reasons, we investigated the effect of aging and annealing PAs, as well as varying molar ratios of ROS- or MMP-Ac2–26 PA and E2 filler PA, on ApoA1-Ac2–26 PA nanofiber formation. Based on the average fiber length and lack of aggregates, we found that PAs co-assembled from 10 mol% MMP- or ROS-Ac2–26 PA, 50 mol% E2 filler PA, and 40% ApoA1 PA (hereafter referred to as 10% MMP- or ROS-Ac2–26 PAs), aged at least 24 hours at 4°C were the most conducive for nanofiber formation (Figure 1). The median lengths of 10% MMP-Ac2–26 and 10% ROS-Ac2–26 PA nanofibers were 159 nm and 174 nm, respectively.
Figure 1:
Determining parameters for ApoA1-Ac2–26 PA nanofiber formation. PAs were co-assembled based upon molar ratio of the MMP- or ROS-Ac2–26 PA therapeutic with 40 mol% ApoA1 PA and the remainder as E2 filler PA. The PAs were imaged using TEM after being (A) freshly dissolved, (B) aged for 24 hours, or (C) annealed for 30 minutes at 80°C. (D) PA nanostructure length was quantified with conditions not containing the same letter significantly different, p<0.05, n ≥ 32 nanostructures analyzed per condition
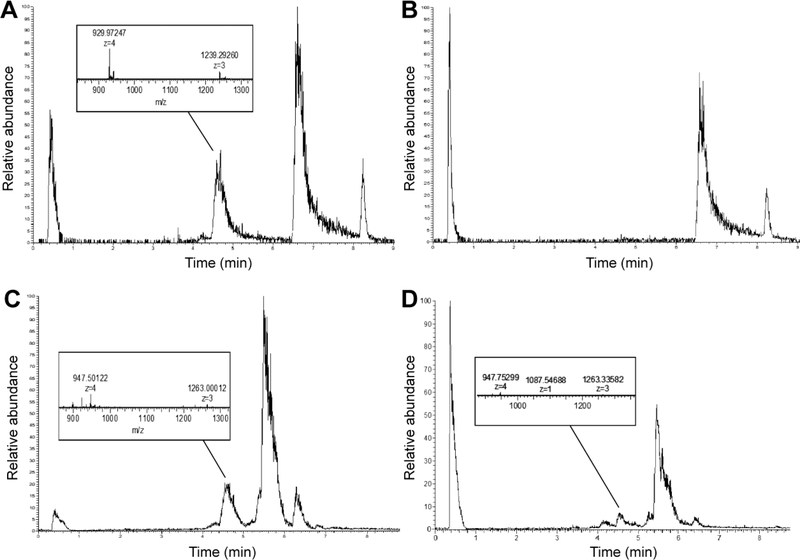
To confirm that Ac2–26 is released from the PA nanofibers upon exposure to biochemical cues overexpressed in atheroma microenvironment, we treated the PAs with MMP2 or SIN-1. SIN-1 spontaneously decomposes to yield superoxide and nitric oxide in aqueous solutions.[26] The MMP-Ac2–26 PA nanofibers released Ac2–26 after 24 hours of treatment with 40 nM MMP2, evidenced by product elution times and molecular weights matching that of the Ac2–26 peptide and the cleaved MMP2-responsive peptide sequence (4.4–4.8 minutes, 3716 g/mol, Figure 2A-B, Supplemental Figure 3). Similarly, the ROS-Ac2–26 PAs released Ac2–26 after 24 hours of treatment with 100 μM SIN-1 as shown by the presence of products near the expected elution time and molecular weight of Ac2–26 (4.4–4.8 minutes, 3295–3791 g/mol, Figure 2C-D). The range of molecular weights correspond to the Ac2–26 peptide attached to residual oligoprolines. Surprisingly, the untreated ROS-Ac2–26 PA also showed the presence of cleaved Ac2–26 peptide. One possible explanation for this result is that ambient atmospheric oxygen caused some cleavage to occur during the assay period.
Figure 2:
Representative chromatographs and mass spectra indicating Ac2–26 release from PA nanofibers. (A) 10% MMP-Ac2–26 PA treated with 40 nM MMP2 for 24 hours and analyzed for a product scan of m/z=940–948. (B) 10% MMP-Ac2–26 PA without MMP2 treatment. The peak at 6.5–7 minutes contains uncleaved MMP-Ac2–26 PA and ApoA1 PA, the peak near 8.2 minutes indicates E2 filler PA (C) ROS-Ac2–26 PA treated with 100 μM SIN-1 for 24 hours and analyzed for a product scan of m/z=947–1030. (D) ROS-Ac2–26 PA without SIN-1 treatment. Uncleaved ROS-Ac2–26 PA is present in the peaks at both 5.4 and 6.5 minutes.
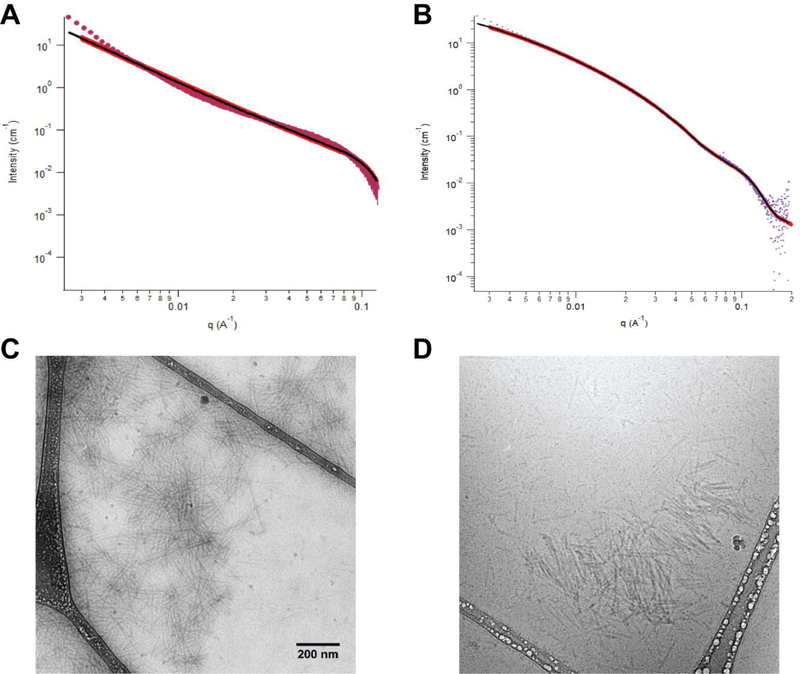
We further characterized the 10% MMP- and ROS-Ac2–26 PAs for their physical structure, charge, and stability. Using small angle x-ray scattering (SAXS), we examined the intensity versus the scattering vector of the Guinier regime in the low q region and determined the slope as −1.6 for 10% ROS-Ac2–26 PAs and −1.8 for 10% MMP-Ac2–26 PAs (Supplemental Figure 4).[27] A slope of −1 is observed for E2 filler PA and indicates a cylindrical shape while a slope of −2 is associated with lamellar structures.[27] As a result, the ApoA1-Ac2–26 PAs could be interpreted as flattened, elongated structures; a mixture of flattened and cylindrical structures, or two cylindrical shapes stacked atop each other.[28] Fitting the SAXS data to a polydisperse core-shell cylinder model, we found similar core radius (1.8 vs. 2.1 nm) and radial shell thickness (2.2 vs. 2.0 nm) for the 10% ROS-Ac2–26 PA and 10% MMP-Ac2–26 PAs, respectively (Figure 3A-B). In addition, we found that both the 10% MMP-Ac2–26 and 10% ROS-Ac2–26 PA nanofiber structures were not disrupted by serum proteins, as shown by cryogenic TEM images after reconstitution in solution containing 10% fetal bovine serum, providing support for their stability upon intravenous injection (Figure 3C-D).
Figure 3:
Characterization of PA structure using SAXS for (A) 10% MMP-Ac2–26 and (B) 10% ROS-Ac2–26 PAs. Plot indicates scattering intensity vs. wave vector. The solid red line represents the best fit of polydisperse core shell cylinder model form factor. The black line indicates the region where the data was fit to the model. PA stability in serum-containing solution as assessed through cryoEM for (C) 10% MMP-Ac2–26 and (D) 10% ROS-Ac2–26 PAs. Scale bar equals 200 nm, images in panel C and D are taken at the same magnification.
Given the importance of the α-helical character of ApoA1 for lipid binding, we examined the secondary structure of MMP- and ROS-ApoA1-Ac2–26 PA nanofibers using circular dichroism spectroscopy. The MMP- and ROS-ApoA1-Ac2–26 PAs retained the α-helical character of the ApoA1 PA at both room temperature (25°C) and physiological temperature (37°C, Figure 4A).[29] In contrast, the E2 filler PA had dominant β-sheet character, as expected, while the Ac2–26 peptide and MMP- or ROS-Ac2–26 PAs exhibited random coil secondary structure. The 10% ROS-Ac2–26 PAs had a negative overall charge of −13.9 ± 0.6 mV at 25°C that was maintained at 37°C, and the 10% MMP-Ac2–26 PAs also had a negative overall charge of −18.1 ± 0.5 mV at 25°C that was maintained at 37°C (Figure 4B). The 10% MMP-Ac2–26 PAs are expected to be more negatively charged than the 10% ROS-Ac2–26 PAs due to the greater number of negatively charged residues in the MMP2/9-cleavable linkage than the oligoproline linkage. To predict whether the PAs would maintain nanofiber structure upon dilution in the bloodstream, we performed a Nile Red Assay to determine the critical aggregation concentration (CAC). We found the CAC to be near 32 μM and 38 μM for the 10% MMP-Ac2–26 and 10% ROS-ApoA1-Ac2–26 PAs, respectively (Figure 4C). This concentration is 25-fold lower than the injection concentration of PAs, and beyond the 20-fold dilution expected for PA dilution in the bloodstream.[7]
Figure 4:
Characterization of ApoA1-Ac2–26 PA nanofibers for secondary structure through (A) circular dichroism spectroscopy and (B) zeta potential. Nile Red was used to determine the critical aggregation concentration (CAC) for (C) 10% MMP-Ac2–26 and (D) 10% ROS-Ac2–26 PAs based upon the blue shift at decreasing PA concentrations (inset).
2.3. ApoA1-Ac2–26 PA cytocompatibility and therapeutic potential
Based on the critical role of macrophages in driving inflammation-mediated progression of atherosclerosis, we utilized a murine macrophage cell line, J774.2, to assess the cytocompatibility and therapeutic effects of ApoA1-Ac2–26 PAs.[5] The macrophages expressed >90% of CD11b, a pan-macrophage marker, and 14.5% of FPR2, the target ligand for Ac2–26 (Supplemental Figure 5A-B).[30] The potential for the macrophages to produce ROS was confirmed by >90% iNOS expression after overnight treatment with 100 ng/mL IFN-γ followed by 24 hours of treatment with 10 μg/mL lipopolysaccharide (LPS, Supplemental Figure 5C-D).[31] MMP2/9 production from J774.2 macrophages was confirmed using zymography (Supplemental Figure 6).
We assessed ApoA1-Ac2–26 PAs effects upon cell viability through a MUSE® Count and Viability Assay Kit, which utilizes fluorescence to indicate cell membrane barrier function. Neither the 10% MMP-Ac2–26 PAs nor 10% ROS-Ac2–26 PAs showed significant cytotoxicity (p>0.6) in comparison to the untreated or Ac2–26 peptide controls after 24 hours of treatment (Figure 5A). Interestingly, we found that the rate and extent of PA uptake varied with linker sequence, with the ROS-cleavable PAs demonstrating a near 18-fold increase in cellular uptake in comparison to MMP-cleavable PAs after one hour of treatment (Figure 5B,Supplemental Figure 7). While PA uptake was significantly increased for both 10% MMP- and ROS-Ac2–26 PAs after 24 hours, the ROS-cleavable PAs maintained a significant 2-fold increase in comparison to the MMP-cleavable PAs. Further, the ROS-cleavable PAs were retained within the cell whereas the majority of the MMP-cleavable PAs were found in the extracellular space. Both MMP- and ROS-cleavable ApoA1-Ac2–26 PAs were processed in the endosomal or lysosomal compartments based on colocalization to lysosomal-associated membrane protein 1 (LAMP1, Figure 5C-D). The average Manders coefficient values for PA to LAMP1 vs. LAMP1 to PA colocalization were 0.55 and 0.89 for 10% ROS-Ac2–26 PAs and 0.48 and 0.79 for 10% MMP-Ac2–26 PAs. These results indicate that while half of the PAs are being processed within lysosomal or endosomal compartments, the other half may be localizing to other intracellular compartments or were recycled from the endosome/lysosome to the cellular membrane or extracellular space.[32][33] Taken together, these results indicate that the ROS- and MMP2/9-cleavable linker chemistry affects macrophage uptake and retention of ApoA1-Ac2–26 PAs and may also cause differences in lysosomal or endosomal processing.
Figure 5:
Cytocompatibility and cellular uptake characterization of ApoA1-Ac2–26 PAs. (A) The effect of 24 hours of treatment with 10% MMP-Ac2–26 PA, 10% ROS-Ac2–26 PA, or Ac2–26 peptide on J774.2 macrophage viability. No significant differences (p<0.05) were observed between groups. n ≥ 3 independent experiments per condition. (B) Cellular uptake of PAs assessed by the integrated pixel density of Alexa Fluor 546-tagged PAs found within the cell. *p<0.05, #p<0.05 vs. 24 hours 10% ROS-Ac2–26 PA, ^p<0.05 vs. 24 hours 10% MMP-Ac2–26 PA. n ≥ 32 cells analyzed per condition. (C) Manders colocalization coefficient values after 24 hours of treatment with 10% ROS- or MMP-Ac2–26 PAs. M1 indicates colocalization of AF546 PA pixels vs. LAMP1 pixels, M2 indicates the reverse. *p<0.05, n ≥ 180 cells analyzed per condition. (D) Representative images of ApoA1-Ac2–26 PA colocalization to macrophages after 24 hours of treatment. Scale bar equals 20 μm.
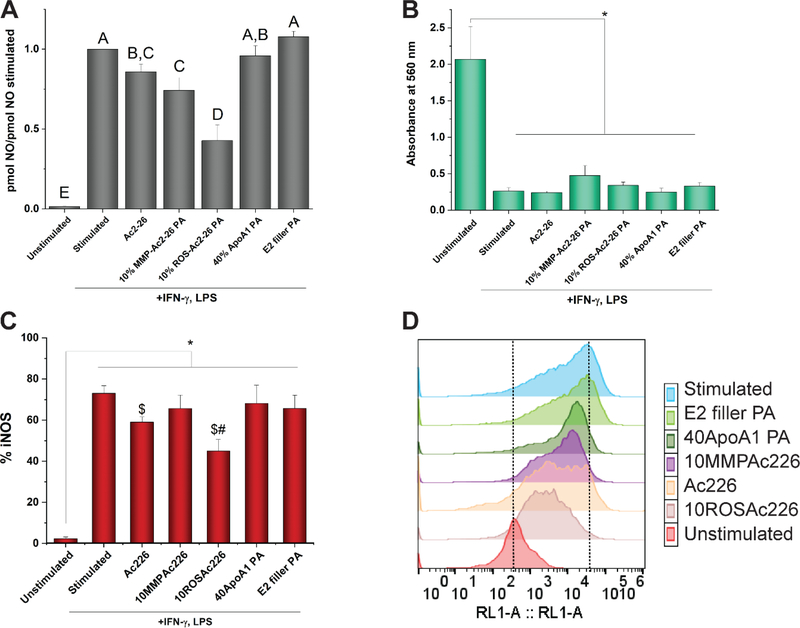
To determine whether the Ac2–26 peptide maintained its pro-resolving potential upon cleavage from PAs, we utilized an in vitro assay to simulate the pro-inflammatory macrophages characteristic of the atherosclerotic niche.[4] We found that after 24 hours of treatment with Ac2–26, 10% MMP-Ac2–26, or 10% ROS-Ac2–26, macrophage activation by LPS and IFN-γ was significantly reduced (p=0.0207, p=0.0005, p<0.0001, respectively) based upon decreased nitric oxide production relative to the stimulated control (Figure 6A). Further, the 10% ROS-Ac2–26 PA caused significantly greater effects on decreased nitric oxide production in comparison to 10% MMP-Ac2–26 PA (p=0.0002) and Ac2–26 (p<0.0001) treatments. The additional PAs in the 10% MMP- and ROS-Ac2–26 PA co-assemblies—ApoA1 PA and E2 filler PA— did not cause significant decreases in nitric oxide production in comparison to the stimulated control (p=0.5534, p=0.2606, respectively), indicating that the MMP- or ROS-cleavable Ac2–26 PAs were responsible for the therapeutic effects. While stimulating the macrophages with LPS and IFN-γ significantly reduced cell metabolic activity (p<0.0001), as measured by NAD(P)H-dependent oxidoreductase enzyme activity through an MTT assay, the Ac2–26 peptide and PA treatments did not significantly affect cellular metabolism in comparison to the stimulated control (p>0.37, Figure 6B).
Figure 6:
Analysis of ApoA1-Ac2–26 PA effects upon macrophage activation and metabolic activity. (A) Ratio of nitric oxide (NO) production in comparison to the stimulated control. Letters not connected by the same letter are significantly different (p<0.05). n ≥ 7 independent experiments for each condition. Error bars indicate S.E.M. (B) Cellular metabolic activity measured by an MTT assay using absorbance at 560 nm. n=3 per condition. *p<0.05. (C) Percentage of cells expressing iNOS assayed by flow cytometry. *p<0.05, $p<0.05 vs. Stimulated, #p<0.05 vs. 40ApoA1 PA, n ≥ 4 per condition. (D) Representative histogram for effect of PA and peptide treatments upon iNOS expression. RL1-A indicates the APC channel used to measure fluorescence. The dotted lines indicate reference peak values for unstimulated and stimulated conditions.
In addition, treatment with 10% ROS-Ac2–26 PAs decreased intracellular iNOS expression of stimulated macrophages (p=0.0022) to a similar extent as the Ac2–26 peptide (p=0.3075, Figure 6C-D). In contrast, the 10% MMP-Ac2–26 PA had no effect upon iNOS expression in comparison to the stimulated control (p=0.2970). A potential explanation for the enhanced therapeutic effect of the 10% ROS-Ac2–26 PAs in comparison to the 10% MMP-Ac2–26 PAs is faster cleavage and corresponding release of Ac2–26 due to the increased production of ROS from macrophages under stimulating conditions. In addition, the enhanced cellular uptake and retention of the 10% ROS-Ac2–26 PAs versus the 10% MMP-Ac2–26 PAs may provide additional pathways for Ac2–26 to decrease pro-inflammatory macrophage activation. For example, Liu et al. recently demonstrated that Ac2–26 induces anti-inflammatory effects within the cytosol of microglia cells by affecting the small molecular chaperone heat shock factor-binding protein 1 (HSPB1) binding to IKKβ, ultimately causing its degradation to reduce TNF-α expression, which positively regulates iNOS expression.[34][35]
3. Conclusion
In conclusion, we developed a bioresorbable nanocarrier with the potential to target atheroma and provide controlled immunotherapeutic delivery to the atherosclerotic niche. We found that the pro-resolving peptide Ac2–26 could be conjugated to a PA and multiplexed with additional PAs to create a nanocarrier with the potential to target atherosclerotic plaque. The release of Ac2–26 is controlled by peptide linkages that cleave in response to endogenous signals overexpressed in the plaque microenvironment—MMP2 and ROS. The released Ac2–26 maintained its bioactivity to reduce macrophage activation towards a pro-inflammatory phenotype within 24 hours of treatment.
Future work will examine the ability of ApoA1-Ac2–26 PAs to reduce plaque burden in vivo and examine the mechanistic pathways responsible for its anti-inflammatory effects. In addition, further investigations will examine the kinetics of PA cleavage within the atherosclerotic niche.
4. Experimental Section
PA synthesis:
All PAs were synthesized using standard 9-fluorenyl methoxycarbonyl chemistry with Rink Amide 4-methylbenzhydrylamine resin and contained a C-terminal amide to improve stability, as previously described.[7] E2 filler PA, or the PA backbone, consists of palmitoyl (C16) attached to V2A2E2. ROS-Ac2–26 PAs incorporated Ac2–26 to the PA backbone via five prolines, and additional lysine residues to enhance solubility (C16V2A2E2-KP5K-AMVSEFLKQAWFIENEEQEYVQTVK-NH2). The MMP-Ac2–26 PAs contained Ac2–26 attached to backbone PA via the MMP2/9-sensitive PQ peptide (C16V2A2E2-GGGPQGIWGQGK-AMVSEFLKQAWFIENEEQEYVQTVK-NH2). ApoA1 PAs contained the 4F peptide attached to backbone PA through a glycine linkage (C16V2A2E2-G-DWFKAFYDKVAEKFKEAF-NH2). To visualize PA cellular uptake and localization, E2 filler PAs were synthesized to contain a fluorescent molecule, Alexa Fluor 546-C5-maleimide, attached by reaction with a cysteine thiol residue (C16V2A2E2-C) and reacted with 2- to 3-fold molar excess PA.[7] All PAs were purified by high performance liquid chromatography (HPLC), and characterized by LC-MS.
ApoA1-Ac2–26 PA co-assembly:
ApoA1-Ac2–26 PAs were co-assembled from ApoA1 PA, E2 filler PA, and ROS- or MMP-Ac2–26 PAs at varying molar ratios, and dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP, Sigma-Aldrich) at 2 mg/mL before probe sonication with a Q700 Sonicator (Qsonica, 10% amplitude, 110 V, 20 kHz) in a water bath for 15 minutes. ApoA1 PA controls were co-assembled as previously described, using 40 mol% ApoA1 PA and 60 mol% E2 filler PA dissolved in HFIP and mixed with a probe sonicator for five minutes.[7] The resulting PA co-assemblies were frozen in liquid nitrogen and evaporated under high vacuum for 2 hours or until dry. PAs were reconstituted in deionized water with pH adjusted to 7.5–8.0 using 200 mM NaOH, and probe sonicated for a total of 1–2 minutes with 10-second on/off pulse cycles before freezing in liquid nitrogen. All PAs were lyophilized (Labconco Freezone 1L Freeze Dryer System) and stored at −20°C until use.
ROS-Ac2–26 and MMP-Ac2–26 PA cleavage assays:
To examine ROS-mediated cleavage, we utilized 3-morpholinosydnonimine hydrochloride (SIN-1, Sigma-Aldrich or abcam). ROS-Ac2–26 PAs were reconstituted to 0.5 mM in phosphate buffer and treated with 100 μM SIN-1 for 24 hours at 37°C. For MMP2/9-mediated cleavage, we employed human recombinant MMP2 (R&D systems, carrier-free), activated with freshly prepared 1 mM p-aminophenylmercuric acetate (APMA, Sigma-Aldrich) for one hour at 37°C. The APMA disrupts the cysteine-zinc bond in MMP2 responsible for enzyme latency by liberation of the sulfhydryl group.[36] 10% MMP-Ac2–26 PA co-assemblies were prepared at 0.5 mM in a cleavage buffer containing 50 mM tricine, 50 mM NaCl, 50 μM ZnCl2, and 10 mM CaCl2 at pH 7.4, and treated with 40 nM human recombinant MMP2 for 24 hours at 37°C. At the end of each time point, the samples were stored at −20°C, effectively stopping the cleavage reactions. Samples were analyzed at room temperature, 0.04–0.3 mg/mL with 0.1% NH4OH using a ThermoFisher Q Exactive HF-X (ThermoFisher, Bremen, Germany) mass spectrometer coupled with a Waters Acquity H-class liquid chromatograph system. Samples were introduced via a heated electrospray source at a flow rate of 0.6 mL/minute. Electrospray source conditions were set as: spray voltage 4.7 kV, sheath gas (nitrogen) 45 arb, auxiliary gas (nitrogen) 30 arb, sweep gas (nitrogen) 0 arb, capillary temperature 350°C, capillary voltage 40 V, and tube lens voltage 100 V. The mass range was set to 150–2000 m/z. All measurements were recorded at a resolution setting of 120,000. Separations were conducted on a Waters Acquity UPLC BEH C18 column (2.1 × 50 mm, 1.7 μm particle size). LC conditions were set at 100 % water with 0.1% formic acid (A) ramped linearly over 9.8 minutes to 95% acetonitrile with 0.1% formic acid (B) and held until 10.2 minutes. At 10.21 minutes the gradient was switched back to 100% A and allowed to re-equilibrate until 11.25 minutes. Injection volume for all samples was 3 μL. Data was analyzed on Xcalibur (ThermoFisher, Breman, Germany).
TEM:
Conventional TEM images were taken on a FEI Tecnai T-12 TEM at 80 kV with a Gatan Orius® 2k x 2k CCD camera. PAs at 1 mM in phosphate buffered saline (PBS) were prepared for TEM by pipetting 8 μL atop copper supports that were covered with thin carbon foil 400-mesh and treated with glow discharge. After two minutes, samples were rinsed with deionized water and stained with 2% uranyl acetate for two minutes before imaging. PA nanofiber length was quantified manually using Fiji open source image processing software. Cryogenic TEM was performed on a JEOL 1230 using 100 kV accelerating voltage and Gatan 831 CCD camera as previously described.[37] 10% ROS- or MMP-Ac2–26 PAs were aged for four days at 1 mM, 4°C in PBS containing 10% v/v fetal bovine serum (FBS, heat inactivated, Gibco). PAs were pipetted at 7.0 μL volumes onto 300-mesh copper grids with lacey carbon support (Electron Microscopy Sciences) that were treated with glow discharge for one minute. Samples were blotted twice at one second per blot before plunging plunged into liquid ethane using a Vitrobot Mark IV (FEI) vitrification robot operating at 95% humidity. After vitrification, the samples were transferred under liquid nitrogen to a Gatan 626 cryo-holder.
Small angle x-ray scattering (SAXS):
SAXS characterization of the PA nanofibers was performed at the Advanced Photon Source in the Argonne National Laboratory on beamline 5-ID-D, DuPont-Northwestern-Dow Collaborative Access Team Synchrotron Research Center. Samples were prepared at 5 mM in PBS solution, aged at least 24 hours at 4°C, and analyzed in 1.5 mm quartz capillaries (Charles Supper) at 17 keV with a charged couple device photon detector located 245 cm behind the sample. Scattering intensities were collected using wave vector q range of 0.0024 to 0.40 Å−1. q represents the scattering vector and is calculated as q = 4 π sin (θ)/λ, with θ as the scattering angle between the incident beam and detector and λ the X-ray wavelength. The plots of scattering intensity versus q were obtained using NIST software Igor Pro v8. The scattering intensities of PBS were subtracted from the PA samples using the Irena SAS macro, and the resulting plots were fitted using NCNR Analysis macro to a polydisperse core shell cylinder model as previously described.[38]
Circular dichroism spectroscopy:
PA samples were analyzed at 0.5 mM in 0.1 M phosphate buffer at pH 7.4 with a 0.1 mm pathlength Suprasil® quartz cuvette (Sigma-Aldrich) using a Chirascan™-plus Circular Dichroism Spectrometer (Applied Photophysics). Samples were analyzed at 25°C or 37°C from 185 to 260 nm with 0.3 nm step size and analysis time of 1.25 seconds per data point. Spectrum data was averaged from two scans with the phosphate buffer values subtracted.
Zeta potential:
The PA charge was characterized using a Zetasizer Nano ZS (Malvern). Samples were prepared at 0.5 mM and passed through a 0.2 μm filter (Acrodisc® Syringe Filters with Supor® Membrane, Pall). At least 10 scans were taken per measurement, three measurements per sample at 25°C and 37°C.
Nile Red assay:
The critical aggregation concentration (CAC) for PA nanofibers was determined by diluting the PAs in Nile Red solution as previously described.[39] Nile Red undergoes a blue shift in fluorescence as the hydrophobic character of PAs in the solvent increases, with a corresponding increase in fluorescence intensity due to reduced twisted intramolecular charge transfer.[40] 10% MMP- and ROS-Ac2–26 PAs were diluted from 1 mM to 100 nM in PBS containing of 2.5 μM Nile Red and aged for 24 hours at 4°C. The PAs were aliquotted in triplicate onto a 96-well plate, excited at 550 nm, and the fluorescence read from 580–450 nm. The CAC was determined by plotting the log of the concentration with the corresponding maximum fluorescence intensity, and calculating the concentration at the intersection of the baseline and tangent line to the rising curve.[41]
Macrophage culture and characterization:
J774.2 murine macrophages (Sigma-Aldrich) were expanded at 4–9 × 105 cells/mL in growth media consisting of Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich) containing 4.5 g/L glucose, 2 mM L-glutamine, 10% v/v FBS, 100 U/m penicillin, and 100 μg/mL streptomycin. Cells were passaged using Accutase™ (StemCell Technologies) and used at passage 6–9 for all experiments. The cells were detached with Accutase™ and gentle scraping, counted, and re-suspended in flow cytometry buffer containing 2% w/v bovine serum albumin (BSA, heat shock fraction free of proteases, globulin, and fatty acids, Sigma-Aldrich) and 0.1% w/v sodium azide (Sigma-Aldrich). To minimize non-specific binding of immunoglobin to Fc receptors, macrophages were incubated with anti-mouse CD16/32 antibody (TrueStain FcX™, Biolegend) at 1 μg/1×105 cells for 10 minutes at 4°C. APC anti-mouse CD11b antibody or APC Armenian hamster IgG isotype control antibody (Biolegend) were added at 0.4 μg/1×105 cells, FPR2 antibody (Novus Biologics) was added at 2 μg/1×105 cells, and all samples were incubated for 30 minutes at 4°C. The cells were washed once with flow cytometry buffer by centrifugation at 300 x g. FPR2 samples were further treated with goat anti-rabbit Alexa Fluor 488 secondary antibody (Invitrogen) at 2 μg/1×105 cells for 20 minutes at 4°C, followed by a wash step. Unstained macrophages served as controls and contained 7AAD viability staining solution (Biolegend) at 0.1 μg/1×105. All samples were suspended in 4% paraformaldehyde (Electron Microscopy Sciences) in PBS until analysis.
Macrophage activation towards a pro-inflammatory phenotype was confirmed by increased levels of inducible nitric oxide synthase (iNOS) using flow cytometry (Supplemental Figure 5A-B). Macrophages were activated to simulate a pro-inflammatory, M1 phenotype using 100 ng/mL of interferon gamma (IFN-γ, mouse carrier-free protein, Biolegend), added to 4 × 105 cells for 12–16 hours, rinsed once, and treated with 10 μg/mL lipopolysaccharide (LPS, derived from Escherichia coli 0111:B4, Sigma-Aldrich) for 24 hours. Flow cytometry was performed by permeabilizing detached cells with 0.0625% Triton-X for 10 minutes at 4°C. Macrophages were rinsed with flow cytometry buffer by centrifugation before adding APC-iNOS monoclonal antibody (eBioscience™) at 0.12 μg/1×105 cells for 30 minutes at 4°C. The cells were rinsed once more before re-suspension in 4% paraformaldehyde. All samples were analyzed using Attune NxT Acoustic Focusing Cytometer (Thermo Fisher) with at least 2000 events counted per condition.
To examine MMP2/9 production from macrophages, conditioned media was collected from tissue culture plates. Protein concentration was measured using a bicinchoninic acid assay (BCA assay, PierceTM). After measuring protein concentration, a 4–16% gradient sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE, Sigma-Aldrich and ThermoFisher Scientific) containing 1mg/mL gelatin (Sigma-Aldrich) was cast. 20 μg of total protein from each media sample was mixed with 2x non-reducing sample buffer (1.6% SDS, 8% glycerol, 0.01% bromophenol blue, 217 mM Tris-HCl) and loaded into the gelatin SDS-PAGE. Samples were passed through the gel using a constant voltage (150 V) until the bromophenol blue in the loading buffer ran out of the gel. Gels containing protein samples were then washed twice for 30 minutes with washing buffer (2.5% Triton X-100, and 50 mM Tris-HCl) to renature loaded proteins, and rinsed with incubation buffer (1% Triton X-100, 50 mM Tris-HCl, 5 mM CaCl2, and 1 μM ZnCl2). Following washes, the gelatin SDS-PAGE was incubated for 24 hours at 37°C with incubation buffer to activate gelatinases present in the samples. Gels were then stained with Coomassie Brilliant Blue (Sigma-Aldrich) for one hour and developed with de-staining solution until bands were observed. Images of the gels were taken using a desktop scanner.
Cytocompatibility:
Macrophages were seeded at 2.1 × 105 cells/cm2 on 24 well plates and treated with 32 μM of Ac2–26 or 320 μM PA co-assemblies (final Ac2–26 concentration of 32 μM) for 24 hours. Prior to addition, all PAs were mixed by vortex or water bath sonication until dissolved and passed through a 0.2-μm filter. Cells were detached by Accutase treatment for 20 minutes and counted using a MUSE® Count and Viability Assay Kit and cytometer (Emdmillipore).
Cellular uptake and localization of ApoA1-Ac2–26 PA:
PAs containing 40 mol% ApoA1 PA, 10 mol% MMP-Ac2–26 or ROS-Ac2–26 PA, 45.5 mol% E2 filler PA, and 4.5 mol% E2 filler PA with AF546 were co-assembled as described above and aged at least 1 week in PBS at 4°C to promote nanofiber formation. Macrophages were seeded onto 6-channel μ-Slide VI 0.4 (ibidi) at 2.1 × 105 cells/cm2 and allowed to adhere for 24 hours before adding 0.33 μM 10% MMP- or ROS-Ac2–26 PA and incubated at 37°C for 1 or 24 hours. At each time point, the samples were fixed with 4% paraformaldehyde for 10 minutes, followed by permeabilization with 0.125% Triton-X for 10 minutes. The samples were rinsed twice with PBS and incubated with blocking buffer consisting of 2% w/v BSA for one hour at room temperature before incubating with 20 μg/mL LAMP1 antibody (rabbit polyclonal, abcam) for 12–18 hours at 4°C. The samples were rinsed twice with PBS containing 0.01% Tween-20 at one hour per rinse followed by an hour rinse with PBS before adding 2 μg/mL donkey anti-rabbit Alexa Fluor Plus 647 (Highly cross-adsorbed secondary antibody, Invitrogen) for 12–18 hours at 4°C. The samples were rinsed twice with PBS before adding 5 μg/mL 4’,6-Diamidino-2-Phenylindole (DAPI, ThermoFisher Scientific) and phalloidin (1:1000 dilution, CruzFluor™ 488 conjugate, Santa Cruz Biotechnology) for one hour at room temperature. Samples were rinsed and stored in PBS until image analysis. Microscopy was performed using Zeiss 880 confocal microscopy at 63x magnification with numerical aperture of 1.4. Lasers at 405, 488, 561, and 633 nm were used to collect images through Multi Channel acquisition, which uses a separate scan for each channel to avoid spectral overlap. 3D images were created using z-stacks with 9–14 μm thick sections compiled from 0.22 μm slices using a line step of 1, speed of 8, and image size of 1024 × 1024 pixels at 16 bit depth. To examine colocalization of PAs to endosomes/lysosomes, we utilized Manders coefficient calculations using the Coloc 2 plugin from Fiji.[42][43]
Therapeutic effects of ApoA1-Ac2–26 PAs:
10% ROS- and MMP-Ac2–26 PAs, as well as Ac2–26 peptide were added together with 10 μg/mL LPS at 32 μM Ac2–26 to macrophages. 40% ApoA1 PA and E2 filler PA were added with LPS at epitope equivalent masses to the ApoA1-Ac2–26 PA co-assemblies. 10% ROS- and MMP-Ac2–26 PA co-assemblies were aged at least 24 hours at 4°C prior to use. All PAs and peptides were sonicated in a water bath sonicator for 15 minutes and passed through a 0.22-μm filter prior to use. The supernatant from each treatment was collected, centrifuged at 300 x g to avoid cell contamination, and diluted 1:10 with PBS prior to analysis with a Sievers Nitric Oxide Analyzer (NOA 280i, GE Water & Process Technologies). In the absence of hemoglobin, the nitric oxide produced in the cell cultures is assumed to react with dissolved oxygen to form nitrite. To convert the nitrite to nitric oxide (NO), a 1% w/v solution of potassium iodide (KI) was used as a reducing agent. Expanded details on NO quantification can be found in the Supplemental Materials.
To measure cell metabolic activity, thiazolyl blue tetrazolium bromide (MTT, Sigma-Aldrich) was added to cells at 0.4 mg/mL in complete medium for 4 hours at 37°C. The plates were then aspirated and 100 μL of DMSO added for 10–15 minutes at room temperature on a plate shaker at low speed setting. The plates were run on an Epoch plate reader using absorbance at 560 nm corrected for background at 670 nm. Flow cytometry analysis for iNOS expression followed the methods described above.
Statistical analysis:
Data are presented as mean ± SEM. Sample sizes are included in figure legends or methods for each experiment. Data were analyzed for statistical significance using one- or two-factor analysis of variance (ANOVA) followed by a post-hoc Student’s t-test. JMP® software was used for all analysis.
Supplementary Material
Acknowledgments
This study was supported in part by funding from the National Institutes of Health (1R01HL116577-01) and the University of North Carolina’s School of Medicine. E.B.P. was supported by the American Heart Association Postdoctoral Fellowship Award #18POST33960499. E.M.B. is a KL2 scholar partially supported by the UNC Clinical and Translational Science Award-K12 Scholars Program funded by the National Center for Advancing Translational Sciences, US. (KL2TR002490, 2018). B.M. was partially supported by the Uruguayan Commission for Scientific Research (CSIC) and Program for the Development of Basic Science (PEDECIBA). S.I.S acknowledges funding from the Louis A. Simpson and Kimberly K. Querrey Center for Regenerative Nanomedicine at Northwestern University through a CRN Catalyst Award. The HPLC-MS analysis utilized equipment supported by the National Science Foundation under Grant No. CHE-1726291. We gratefully acknowledge Dr. Jack Griffith and Smaranda Wilcox for assistance with conventional TEM. The UNC Electron Microscopy Facility is supported in part by the Lineberger Comprehensive Cancer Center UCRF. Peptide amphiphile synthesis was performed in the Peptide Synthesis Core Facility of the Simpson Querrey Institute at Northwestern University. The U.S. Army Research Office, the U.S. Army Medical Research and Materiel Command, and Northwestern University provided funding to develop this facility and ongoing support is being received from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205). Portions of this work were performed at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) located at Sector 5 of the Advanced Photon Source (APS). DND-CAT is supported by Northwestern University, E.I. DuPont de Nemours & Co., and The Dow Chemical Company. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Data was collected using an instrument funded by the National Science Foundation under Award Number 0960140. This material is based upon work supported by the National Science Foundation under Grant No. (CHE-1726291).
Contributor Information
Dr. Erica B. Peters, Department of Surgery, Division of Vascular Surgery and Center for Nanotechnology in Drug Delivery, University of North Carolina at Chapel Hill Chapel Hill, NC 27599, USA
Prof. Nick D. Tsihlis, Department of Surgery, Division of Vascular Surgery and Center for Nanotechnology in Drug Delivery, University of North Carolina at Chapel Hill Chapel Hill, NC 27599, USA
Dr. Mark R. Karver, Simpson Querrey Institute, Northwestern University, Chicago, IL 60611, USA
Stacey M. Chin, Department of Chemistry, Northwestern University, Evanston, IL 60208, USA
Bruno Musetti, Institute of Biological Chemistry, Universidad de la República, Montevideo, 11400, Uruguay.
Dr. Benjamin T. Ledford, Department of Surgery, Division of Vascular Surgery and Center for Nanotechnology in Drug Delivery, University of North Carolina at Chapel Hill Chapel Hill, NC 27599, USA
Prof. Edward M. Bahnson, Department of Surgery, Division of Vascular Surgery and Center for Nanotechnology in Drug Delivery, University of North Carolina at Chapel Hill Chapel Hill, NC 27599, USA Department of Cell Biology & Physiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
Prof. Samuel I. Stupp, Simpson Querrey Institute, Northwestern University, Chicago, IL 60611, USA Department of Chemistry, Northwestern University, Evanston, IL 60208, USA; Department of Materials Science & Engineering and Department of Biomedical Engineering, Northwestern University, Evanston, IL 60208, USA; Department of Medicine, Northwestern University, Chicago, IL 60611, USA.
Prof. Melina R. Kibbe, Department of Surgery, Division of Vascular Surgery and Center for Nanotechnology in Drug Delivery, University of North Carolina at Chapel Hill Chapel Hill, NC 27599, USA; Department of Biomedical Engineering, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
References
- [1].Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, De Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jim’nez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, MacKey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfghi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JHY, Alger HM, Wong SS, Muntner P, Circulation 2017, 135, e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Frostegård J, BMC Med. 2013, 11, DOI 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ross R, N. Engl. J. Med 1999, 340, 115. [DOI] [PubMed] [Google Scholar]
- [4].Tabas I, García-Cardeña G, Owens GK, J. Cell Biol 2015, 209, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tabas I, Nat. Rev. Immunol 2010, 10, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lusis AJ, Nature 2000, 407, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].So MM, Mansukhani NA, Peters EB, Albaghdadi MS, Zheng W, Rubert Pérez CM, Kibbe MR, Stupp SI, Adv. Biosyst 2018, 2, 1700123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ahanchi SS, Tsihlis ND, Kibbe MR, J. Vasc. Surg. Off. Publ. Soc. Vasc. Surg. [and] Int. Soc. Cardiovasc. Surgery, North Am. Chapter 2007, 45 Suppl A, A64. [DOI] [PubMed] [Google Scholar]
- [9].Morgan CE, Wasserman MA, Kibbe MR, Ann. Surg 2016, 263, 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokhzad O, Tabas I, Sci. Transl. Med 2015, 7, 275ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].de Jong R, Leoni G, Drechsler M, Soehnlein O, Cell Adhes. Migr 2017, 11, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maderna P, Cottell DC, Toivonen T, Dufton N, Dalli J, Perretti M, Godson C, FASEB J. 2010, 24, 4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Moraes LA, Kar S, Foo SL, Gu T, Toh YQ, Ampomah PB, Sachaphibulkij K, Yap G, Zharkova O, Lukman HM, Fairhurst AM, Kumar AP, Lim LHK, Sci. Rep 2017, 7, DOI 10.1038/s41598-017-17622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Al-Lawati H, Aliabadi HM, Makhmalzadeh BS, Lavasanifar A, Expert Opin. Drug Deliv 2018, 15, 397. [DOI] [PubMed] [Google Scholar]
- [15].Dou Y, Chen Y, Zhang X, Xu X, Chen Y, Guo J, Zhang D, Wang R, Li X, Zhang J, Biomaterials 2017, 143, 93. [DOI] [PubMed] [Google Scholar]
- [16].Webber MJ, Berns EJ, Stupp SI, Isr. J. Chem 2013, 53, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sluijter JPG, Pulskens WPC, Schoneveld AH, Velema E, Strijder CF, Moll F, De Vries JP, Verheijen J, Hanemaaijer R, De Kleijn DPV, Pasterkamp G, Stroke 2006, 37, 235. [DOI] [PubMed] [Google Scholar]
- [18].Yu SS, Koblin RL, Zachman AL, Perrien DS, Hofmeister LH, Giorgio TD, Sung HJ, Biomacromolecules 2011, 12, 4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Patterson J, Hubbell JA, Biomaterials 2010, 31, 7836. [DOI] [PubMed] [Google Scholar]
- [20].Amici A, Levine RL, Tsai L, Stadtman ER, J. Biol. Chem 1989, 264, 3341. [PubMed] [Google Scholar]
- [21].Schuessler H, Schilling K, Int. J. Radiat. Biol. Relat. Stud. physics, Chem. Med 1984, 45, 267. [DOI] [PubMed] [Google Scholar]
- [22].Stadtman ER, Levine RL, Amino Acids 2003, 25, 207. [DOI] [PubMed] [Google Scholar]
- [23].Peters EB, Christoforou N, Leong KW, Truskey GA, West JL, Cell. Mol. Bioeng 2016, 9, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Korevaar PA, Newcomb CJ, Meijer EW, Stupp SI, J. Am. Chem. Soc 2014, 136, 8540. [DOI] [PubMed] [Google Scholar]
- [25].Tantakitti F, Boekhoven J, Wang X, Kazantsev RV, Yu T, Li J, Zhuang E, Zandi R, Ortony JH, Newcomb CJ, Palmer LC, Shekhawat GS, de la Cruz MO, Schatz GC, Stupp SI, Nat. Mater 2016, 15, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Singh RJ, Hogg N, Joseph J, Konorev E, Kalyanaraman B, Arch. Biochem. Biophys 1999, 361, 331. [DOI] [PubMed] [Google Scholar]
- [27].Ochbaum G, Bitton R, Using Small-Angle X-Ray Scattering (SAXS) to Study the Structure of Self-Assembling Biomaterials, Elsevier Ltd., 2018. [Google Scholar]
- [28].Moyer TJ, Cui H, Stupp SI, J. Phys. Chem. B 2013, 117, 4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Oram JF, Heinecke JW, Future Lipidol. 2007, 2, 185. [Google Scholar]
- [30].Ho MK, Springer TA, J. Immunol 1982, 128, 2281 LP. [PubMed] [Google Scholar]
- [31].Mosser DM, Zhang X, Curr. Protoc. Immunol 2008, Chapter 14, Unit 14.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Grant BD, Donaldson JG, Nat. Rev. Mol. Cell Biol 2009, 10, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Eitan E, Suire C, Zhang S, Mattson MP, Ageing Res. Rev 2016, 32, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu L, An D, Xu J, Shao B, Li X, Shi J, Front. Mol. Neurosci 2018, 11, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fonseca SG, Romão PRT, Figueiredo F, Morais RH, Lima HC, Ferreira SH, Cunha FQ, Eur. J. Immunol 2003, 33, 2297. [DOI] [PubMed] [Google Scholar]
- [36].Galazka G, Windsor LJ, Birkedal-Hansen H, Engler JA, Biochemistry 1996, 35, 11221. [DOI] [PubMed] [Google Scholar]
- [37].Chin SM, Synatschke CV, Liu S, Nap RJ, Sather NA, Wang Q, Álvarez Z, Edelbrock AN, Fyrner T, Palmer LC, Szleifer I, de la Cruz MO, Stupp SI, Nat. Commun. 2018 91 2018, 9, 2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Matson JB, Newcomb CJ, Bitton R, Stupp SI, Soft Matter 2012, 8, 3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Moyer TJ, Kassam HA, Bahnson ESM, Morgan CE, Tantakitti F, Chew TL, Kibbe MR, Stupp SI, Small 2015, 11, 2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hawe A, Sutter M, Jiskoot W, Pharm. Res 2008, 25, 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen C, Liu G, Liu X, Pang S, Zhu C, Lv L, Ji J, Polym. Chem 2011, 2, 1389. [Google Scholar]
- [42].MANDERS EMM, VERBEEK FJ, ATEN JA, J. Microsc 1993, 169, 375. [DOI] [PubMed] [Google Scholar]
- [43].Dunn KW, Kamocka MM, McDonald JH, AJP Cell Physiol 2011, 300, C723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.