Abstract
Impaired object naming is a core deficit in post-stroke aphasia, which can manifest as errors of commission – producing an incorrect word or a nonword – or as errors of omission – failing to attempt to name the object. Detailed behavioral, computational, and neurological investigations of errors of commission have played a key role in development of neurocognitive models of word production. In contrast, the neurocognitive basis of omission errors is radically underspecified despite being a prevalent phenomenon in aphasia and other populations. The prevalence of omission errors makes their neurocognitive basis important for characterizing an individual’s deficits and, ideally, for personalizing treatment and evaluating treatment outcomes. The present study leveraged established relationships between lesion location and errors of commission to investigate omission errors in picture naming. Omission error rates from the Philadelphia Naming Test for 123 individuals with post-stroke aphasia were analyzed using support vector regression lesion-symptom mapping. Omission errors were most strongly associated with left frontal and mid-anterior temporal lobe lesions. Computational model analysis further showed that omission errors were positively associated with impaired semantically-driven lexical retrieval rather than phonological retrieval. These results suggest that errors of omission in aphasia predominantly arise from lexical-semantic deficits in word retrieval and selection from a competitor set.
Keywords: aphasia, lesion-symptom mapping, picture naming, word production, omission errors
Introduction
Difficulty naming familiar objects is the quintessential symptom of post-stroke aphasia. Such difficulties often manifest as errors of commission, which include production of a word that is semantically or phonologically related to the correct response, or production of a nonword. Detailed analysis of object naming errors has provided important insights into the cognitive and neural organization of the processes that underpin word production (e.g., Schwartz, 2014).
In the literature on word production, theoretical frameworks differ in their characterization of the timing, number and nature of the stages of processing, the role of extra-linguistic factors on processing, and the flow of activation through the system. However, theories generally agree that producing a word from meaning (e.g., such as when naming a familiar object) begins with visual-semantic processes that activate the semantic features of the object (e.g., cat) and semantically-related concepts (e.g., dog). Activation from these semantic features is propagated forward to activate a set of candidate words, from which a word is selected. Word selection is followed by retrieval of that word’s phonological form (cf. Caramazza, 1997), ending with articulatory planning and execution. Lexical-semantic deficits (prior to the phonological stages of this process) cause production of a semantically-related word instead of the target – semantic errors such as pig → “cow” (e.g., Dell, Schwartz, Nozari, Faseyitan, & Coslett, 2013; Levelt, Roelofs, & Meyer, 1999; Ueno, Saito, Rogers, & Lambon Ralph, 2011). Deficits at the post-word selection stages of this process, which correspond to phonological production deficits, cause misselection or misordering of sounds that results in production of phonologically related words or nonwords – phonological errors such as shell → “sell” or “chell” (e.g., Dell et al., 2013; Roelofs, 2014).
In addition to behavioral and computational investigations that have revealed the cognitive basis of these common commission error types, lesion-symptom mapping studies have revealed their neural correlates. Semantic errors in picture naming are strongly associated with damage to the anterior temporal lobe (Butler, Brambati, Miller, & Gorno-Tempini, 2009; Campo et al., 2016; Damasio, Tranel, Grabowski, Adolphs, & Damasio, 2004; Lambon Ralph, McClelland, Patterson, Galton, & Hodges, 2001; Mesulam et al., 2009, 2013; Mirman, Zhang, Wang, Coslett, & Schwartz, 2015; Schwartz et al., 2009; Walker et al., 2011), presumably because this region is an important hub for semantic cognition (Lambon Ralph, Jefferies, Patterson, & Rogers, 2017). In contrast, phonological errors in word production are associated with damage to posterior superior temporal and inferior parieto-frontal regions (Buchsbaum et al., 2011; Fridriksson et al., 2016; Mirman, Chen, et al., 2015; Mirman, Zhang, et al., 2015; Schwartz, Faseyitan, Kim, & Coslett, 2012).
Errors of omission — failure to attempt to name the object — comprise a significant proportion of naming failures across the various clinical subtypes of aphasia, and in dementia as well. Unfortunately, the lack of an overt naming attempt makes it challenging to draw conclusions about their underlying origins. As a result, compared to the classic errors of commission, errors of omission are much less well studied and less well understood (though see Halai, Woollams, & Lambon Ralph, 2018). In addition to shedding new light on the dynamics of the word production system, a better understanding of omission errors in picture naming may have clinical implications. The prevalence of omissions in aphasia and dementia make their neurocognitive basis important for characterizing an individual’s deficits, which can inform the personalization of treatment and evaluation of treatment outcomes.
As described above, research from multiple independent laboratories has converged to a very clear picture of the distinct neural bases of semantic versus phonological-based naming errors, aligning with the prevailing view of separable processing origins for the two types. Leveraging these established facts about commission errors and examining the neuroanatomical basis of omission errors in aphasia may provide new insights into naming impairment. Models of naming identify four possible functional causes of omission errors (Dell, Lawler, Harris, & Gordon, 2004), each of which makes a different prediction regarding the lesion site(s) that should be most strongly associated with omission errors.
1. A deficit in core semantic knowledge or in the connections between semantic knowledge and lexical representations undermines activation or retrieval of a lexical item.
Core semantic deficits are associated with anterior temporal lobe (ATL) damage (Bozeat, Lambon Ralph, Patterson, Garrard, & Hodges, 2000; Lambon Ralph et al., 2017; Mummery et al., 2000; Nestor, Fryer, & Hodges, 2006) and unilateral left ATL damage is specifically associated with semantic deficits in picture naming (Damasio et al., 2004; Lambon Ralph et al., 2001; Mesulam et al., 2009; Schwartz et al., 2009; Walker et al., 2011). Omission errors could arise because no lexical representation receives sufficient semantic input to become activated. This could occur either because core semantic knowledge has been degraded or because the connections between (relatively intact) semantic knowledge and lexical representations have been degraded. Although these are somewhat distinct mechanisms, both core semantic deficits and degraded semantic-lexical connections are associated with ATL damage; therefore, in either of these two cases, omission errors should be associated with ATL damage.
2. An executive function deficit undermines selection among competing lexical alternatives.
Competitive selection deficits are associated with inferior frontal gyrus (IFG) damage (Mirman & Graziano, 2013; Piai, Riès, & Swick, 2016; Riès, Dronkers, & Knight, 2016; Schnur et al., 2009; Schnur, Schwartz, Brecher, & Hodgson, 2006). The critical element across different computational mechanisms of competitive selection is that the selected word must be substantially more activated than other, competing, words (for a discussion of selection deficits in aphasia see Mirman & Britt, 2014). Failure to resolve competition would occur when multiple words are approximately equally active, regardless of whether that activation level is low or high. If omission errors result from failure to resolve competition for lexical selection, they should be primarily associated with IFG damage.
3. A lexical-phonological, phonological, or motor speech deficit either prevents any production or produces a phonological or articulatory error that is suppressed by a speech monitoring process.
These three sources of errors are combined here because they are all associated with damage to a system known as the dorsal speech stream, which is comprised of posterior superior temporal and inferior parieto-frontal regions (Buchsbaum et al., 2011; Fridriksson et al., 2016; Hickok, 2012; Mirman, Chen, et al., 2015; Mirman, Zhang, et al., 2015; Schwartz et al., 2012). One classical explanation of omission errors is that they constitute instances of failed retrieval of a holistic word form representation (i.e., lexeme in Levelt et al., 1999). In this case, omission errors should localize to the posterior aspect of this dorsal stream: left posterior superior temporal cortex (Graves, Grabowski, Mehta, & Gordon, 2007; Graves, Grabowski, Mehta, & Gupta, 2008; Indefrey, 2011; Indefrey & Levelt, 2004; Wilson, Isenberg, & Hickok, 2009). In contrast, omissions may be covert phonological errors arising from problems in the retrieval, assembly, or articulation of constituent phonemes after target word form retrieval, which are suppressed by a speech monitoring process. If so, omissions will localize to inferior parieto-frontal regions of the dorsal stream (Mirman, Chen, et al., 2015; Mirman, Zhang, et al., 2015; Schwartz et al., 2012). An association with damage to the precentral gyrus and insula would implicate an articulatory basis for such covert phonological errors (Baldo, Wilkins, Ogar, Willock, & Dronkers, 2011; Basilakos, Rorden, Bonilha, Moser, & Fridriksson, 2015; Itabashi et al., 2016). Importantly, although this dorsal pathway reaches the frontal lobe, damage associated with phonological and articulatory errors is posterior to IFG damage that is associated with lexical selection deficits, making it possible to distinguish these two hypotheses. If omission errors are (suppressed) motor speech or phonological errors, then they should also be associated with damage to the dorsal speech production system.
4. Omission errors are generated by deficits outside the systems that produce other naming errors.
For example, omission errors could arise from impaired visual object recognition (i.e., agnosia). To be distinct from the semantic deficit hypothesis (see #1 above), these would have to be apperceptive agnosic deficits generally associated with occipital or posterior inferior temporal damage. Such deficits are rare in post-stroke aphasia, perhaps because those regions are rarely affected by middle cerebral artery strokes, which are the primary cause of post-stroke aphasia. More generally, the “independence model” (Ruml, Caramazza, Shelton, & Chialant, 2000) hypothesis that omission errors are caused by other deficits highlights the importance of a broader analysis to avoid missing contributions outside of the a priori regions enumerated above.
Because errors of commission have been the subject of such extensive study using behavioral, computational, and lesion-symptom methods, the space of possible hypotheses regarding errors of omission in post-stroke aphasia is strongly constrained. Each of the hypotheses in this limited set is based on a specific cognitive deficit that has a well-established lesion location correlate. In the present study, we used multivariate lesion-symptom mapping to adjudicate among these conflicting hypotheses in order to provide new insights into the cognitive basis of omission errors in post-stroke aphasia. To facilitate direct evaluation of these hypotheses, we also conducted lesion-symptom mapping analyses of semantic and phonological commission errors in object naming, and of performance on the Camel and Cactus Test, a non-verbal test of semantic cognition. Overlap between the lesion correlates of omission errors and these three landmarks will localize omissions within the neural system that supports picture naming. Finally, we used a computational model of picture naming (the interactive two-step model) to evaluate whether degradation of lexical-semantic or lexical-phonological connections contributes to omissions error rates.
Method
The data were drawn from a large-scale study of language processing following left hemisphere stroke. Analyses of other language deficits in earlier subsets of the participants have been reported in several previous articles (Mirman, Chen, et al., 2015; Mirman & Graziano, 2013; Mirman, Zhang, et al., 2015; Schwartz et al., 2009, 2011, 2012; Thothathiri, Kimberg, & Schwartz, 2012; Walker et al., 2011), which also provide more detailed descriptions of the participants and imaging methods. The study was carried out in accordance with protocols approved by the Institutional Review Boards at the Einstein Healthcare Network and University of Pennsylvania School of Medicine.
The present study examined omission errors produced on the Philadelphia Naming Test (PNT) (Roach, Schwartz, Martin, Grewal, & Brecher, 1996), which is comprised of 175 black and white line drawings of familiar objects from a broad range of semantic categories. Omission errors were defined as failure to produce a complete naming attempt, including silence or only a word fragment, personally-relevant circumlocutions (e.g., “I know what that is”), and vague descriptions (e.g., “It’s a thing”). Semantically-relevant descriptions (i.e., multiword responses that characterize the object or explain its function or purpose) are similar to semantic errors, both cognitively and neuroanatomically (Schwartz et al., 2011). Including semantic descriptions as omission errors would have risked re-discovering that semantic errors are associated with ATL damage, so these were excluded to avoid biasing the results toward a semantic account.
The participants were 123 individuals with aphasia secondary to left hemisphere stroke (not bilateral or solely subcortical). All had English as their first language, were right-handed prior to stroke, and were able to produce at least one correct response on the PNT. Participants were tested outside the acute phase, at least one month post onset, with almost all tested in the chronic phase (120/123 were at least 3 months post onset). The participant sample consisted of 52 females and 71 males, with mean age 57.6 (range = 26-79) and mean 14.3 years of education (range = 6-21). The sample included a wide range of aphasia sub-types (51 anomic, 34 Broca’s, 23 conduction, 11 Wernicke’s, 2 transcortical motor, 1 transcortical sensory, and 1 global) and aphasia severity based on the Western Aphasia Battery (Kertesz, 1982) aphasia quotient (Mean = 72.1, SD = 19.0, range = 25.2-97.9). Average PNT picture naming accuracy was 61.6% correct with scores from the full range of performance (1.1% - 97.7% correct).
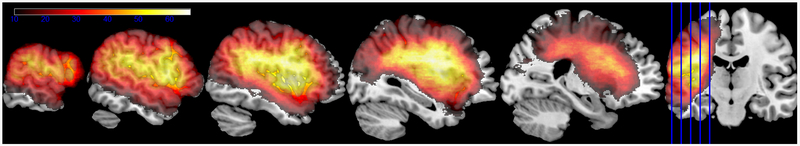
Lesion location was assessed based on MRI (n=68) or CT (n=55) brain scans, following the same procedures as previous studies of this data set (or sub-sets of these data). For the MRI scans, lesions were manually segmented on each participant’s T1-weighted structural image, then the structural scans and lesion maps were registered to the Montreal Neurological Institute (MNI) space Colin27 template by an automated process (Avants, Schoenemann, & Gee, 2006). For the CT scans, the lesion was drawn directly onto the Colin27 template after rotating it (pitch only) to match the approximate slice plane of the participant’s scan. Lesion coverage included the lateral portion of the left hemisphere exclusive of the occipital lobe and the medial and posterior inferior temporal lobe (Figure 1). Only voxels where at least 10 participants had lesions were included in the analysis (a total of 389,502 voxels) to provide a stable comparison of lesioned vs. non-lesioned performance. Both the coding of the naming data and the lesion drawing were done by individuals who were blind to the hypotheses tested here.
Figure 1.
Lesion overlap for the 123 participants (left hemisphere stroke) on the MNI space Colin27 template. The color scale ranges from 10 lesions (minimum for inclusion in analyses) to 66 (maximum observed overlap).
Lesion-symptom mapping analyses were performed using support vector regression (SVR-LSM; Zhang, Kimberg, Coslett, Schwartz, & Wang, 2014). SVR-LSM leverages a multivariate machine learning algorithm to discover lesion-behavior relationships. Compared to standard mass-univariate voxel-based lesion-symptom mapping methods (e.g., Wilson, 2017), SVR-LSM is better able to capture independent contributions of multiple brain regions to performance and is less sensitive to differences in statistical power that arise from differences in proportion of participants with lesions in each voxel. These advantages are particularly important for the present study because omissions could arise from multiple independent causes and at least one of the hypothesized regions of interest (the ATL) lies near the outer edge of the lesion coverage. As a standard pre-processing step for SVR-LSM, each participant’s lesion vector was normalized by dividing each voxel’s binary lesion status value by the square root of the total lesion volume. This also serves as an effective control for the impact of lesion volume, referred to as “direct total lesion volume control” (Zhang et al., 2014). SVR parameters (cost =1.0, gamma = 3.5) for the primary analysis were selected based on a grid search with 5-fold cross-validation to maximize prediction accuracy (as suggested by Zhang et al.). Follow-up analyses used the same parameters for consistency. SVR-LSM produces a voxel-wise map of raw regression β values. Statistical significance for the β values was calculated using a permutation test (2000 permutations) and corrected at false discovery rate (FDR) (Genovese, Lazar, & Nichols, 2002; Zhang et al., 2014) q < 0.05. The final results include only voxels that passed the FDR-correct threshold, were in the top 5% of raw β values, and comprised clusters larger than 50 voxels.
To facilitate direct comparisons between different error types, we conducted additional SVR-LSM analyses of two key types of errors of commission in picture naming that have been analyzed in previous lesion-symptom studies: (1) semantic errors, which include semantically-related single-word errors and semantically appropriate multi-word descriptions (Dell et al., 2013; Schwartz et al., 2009; Walker et al., 2011), and reflect semantic or semantic-to-lexical mapping deficits (i.e., Hypothesis #1 above); (2) phonological errors, which include nonword errors (neologisms) and phonologically-related word errors (Halai et al., 2018; Schwartz et al., 2012), which reflect phonological retrieval or planning deficits (i.e., Hypothesis #3 above). We also analyzed performance on the Camel and Cactus Test (CCT; Bozeat et al., 2000), a nonverbal test of semantic cognition in which participants are required to choose which one of four pictured objects goes best with a probe object (e.g., for the camel probe, the choices are cactus, tree, sunflower, and roses). The CCT provides a measure of semantic knowledge without requiring word production and, because many of the trials require focusing on specific semantic properties in order to identify the relationship between the probe and the correct response, it is also thought to be a measure of semantic control (Lambon Ralph et al., 2017). These three analyses serve as landmarks to help localize omission errors within the neural system that supports picture naming.
Results
Across all participants, the mean omission error rate was 10.3% (range = 0% - 84%). SVR-LSM of omission errors identified a total of 19,219 significant voxels (Figure 2A). These voxels comprised two main clusters: a frontal cluster that included middle frontal gyrus and inferior frontal gyrus (especially pars triangularis), with some extension into precentral gyrus, and a temporal cluster that included the mid-anterior portion of the middle temporal gyrus and temporal pole. These results are consistent with both an executive and a semantic basis for omission errors. The remaining supra-threshold voxels were scattered throughout the middle cerebral artery territory without substantial clusters in the dorsal language route. At FDR q < 0.05, up to 5% of supra-threshold voxels can be expected to be false positives (Bennett, Wolford, & Miller, 2009), so these small clusters are within the margin of false positives that can be expected. This pattern of results provided no support for a phonological basis for omission errors.
Figure 2.

A. Results of SVR-LSM analysis of omission error proportions. Voxels shown in red had permutation-based p < 0.003 (FDR q < 0.05).
B. Results of SVR-LSM analysis of omission error proportions (red), Camel and Cactus Test score (blue), semantic error proportion (including semantic descriptions; green), and phonological error proportion (nonword and formal errors; violet). Only voxels that survived FDR correction (q < 0.05) are shown. The omission error results overlap with the semantic error results in the temporal lobe and with the CCT results in the frontal lobe, but not with the phonological error results.
Figure 2B shows SVR-LSM results along with results from SVR-LSM of semantic errors, phonological errors, and performance on the Camel and Cactus Test. Table 1 quantitatively describes the overlap (and lack thereof) by listing the number of supra-threshold voxels for each of these analyses by anatomical region (defined by AAL atlas). These comparisons confirmed that the lesion correlates of omissions overlap with lesion correlates of performance on the Camel and Cactus Test (in the frontal lobe) and with the lesion correlates of semantic errors (in the mid-anterior temporal lobe), but not with the lesion correlates of phonological errors. In sum, these results indicate that frontal and mid-anterior temporal lesions are the primary neural correlates of omission errors in picture naming in post-stroke aphasia and suggest that they have two primary causes: impaired selection among competing lexical alternatives and impaired semantically-driven word retrieval. In the next section, we report computational model analyses that were conducted in order to further evaluate the claim that omission errors are caused by deficits in semantically-driven lexical retrieval rather than deficits in phonological retrieval and planning.
Table 1.
Voxels surviving FDR correction for each SVR-LSM analysis in regions of interest (ROI) defined by the AAL atlas.
| ROI | Omission errors | CCT | Semantic Errors | Phonological Errors |
|---|---|---|---|---|
| FDR threshold | 0.003 | 0.001 | 0.0005 | 0.001 |
| Frontal_Mid_L | 6249 | 1324 | - | - |
| Frontal_Inf_Tri_L | 3248 | 1702 | 224 | - |
| Temporal_Mid_L | 5871 | - | 754 | - |
| Temporal_Pole_Sup_L | 949 | - | - | - |
| Temporal_Inf_L | 827 | - | 242 | - |
| Frontal_Mid_Orb_L | 489 | 40 | - | - |
| Frontal_Inf_Oper_L | 367 | 442 | - | - |
| Postcentral_L | 292 | - | - | 3692 |
| Frontal_Inf_Orb_L | 291 | 1 | - | - |
| Insula_L | 242 | 542 | 203 | - |
| Temporal_Pole_Mid_L | 238 | - | - | - |
| Temporal_Sup_L | 69 | - | - | 267 |
| Putamen_L | 38 | 146 | - | - |
| Caudate_L | 24 | - | - | - |
| Thalamus_L | 16 | 3 | - | - |
| Frontal_Sup_L | 8 | - | - | - |
| Hippocampus_L | 1 | - | - | - |
| Angular_L | - | 74 | - | - |
| Parietal_Inf_L | - | 151 | - | 175 |
| Parietal_Sup_L | - | 295 | - | - |
| Occipital_Mid_L | - | 46 | - | - |
| Rolandic_Oper_L | - | - | - | 24 |
| SupraMarginal_L | - | - | - | 1233 |
Model-Based Analysis
The broad conceptual model of naming described in the Introduction has been implemented in an explicit computational model known as the “interactive two-step model” (Dell, Martin, & Schwartz, 2007; Dell et al., 2013; Schwartz, Dell, Martin, Gahl, & Sobel, 2006). This model makes a clear distinction between semantic and phonological aspects of word production and accounts for variability in patterns of naming errors across a large, unselected sample of people with aphasia in terms of just two parameters: an “s-weight” that determines the ability of semantic knowledge of the picture to drive lexical retrieval, and a “p-weight” that determines the ability of lexical activation to drive phonological retrieval for production. Given a participant’s distribution of correct responses, semantic errors, phonological errors, mixed errors, nonword errors, and unrelated responses, the model provides a description of that participant’s naming system in terms of an s-weight and a p-weight1. Note that omission errors are not included in the estimation of s-weights and p-weights, so these model parameters provide a quantitative way to test whether omission errors are related to deficits at semantically-driven lexical retrieval (quantified by the s-weight) or phonological planning and execution (quantified by the p-weight). In addition, because s-weight and p-weight parameter estimates are based on the distribution across multiple error types, they provide a more comprehensive estimate of deficits in different aspects of word production than a direct correlation between, for example, semantic and omission errors.
Since this analysis is not constrained by availability of structural lesion data, a larger set of PNT data was downloaded (on 20 June 2017) from the Moss Aphasia Psycholinguistics Project Database (Mirman et al., 2010) (www.mappd.org). This data set consisted of PNT data from 273 participants with aphasia. Some of the participants had s-weight and p-weight parameters that were higher than the default value for neurologically healthy adults (0.6). Because these hypernormal weight estimates may reflect poor model fit to an individual participant’s data, the analysis was restricted to participants with s-weight and p-weight estimates below the control level (N = 241). For participants who completed the PNT multiple times, only the first administration was used. Logistic regression was used to analyze rate of omission errors as a function of s-weights, p-weights, and their interaction. The results revealed that both kinds of weights were significant predictors of omission error rate, but in opposite directions: higher p-weights were associated with more omission errors (Estimate = 45.9, SE = 4.74, p < 0.001), whereas higher s-weights were associated with fewer omission errors (Estimate = −57.4, SE = 5.52, p < 0.0001). The interaction was also statistically significant (Estimate = −1410, SE = 247.5, p < 0.0001). Figure 3 depicts this relationship between omission error proportions and s- and p-weights. The highest rates of omission errors were observed for individuals with low s-weights (impaired semantic-to-lexical mapping) and high p-weights (relatively spared lexical-to-phonological mapping).
Figure 3.
Relationship between omission error proportions and s-weights and p-weights. Hotter colors indicate higher proportion of omission errors estimated by logistic regression of data for 241 participants from the Moss Aphasia Psycholinguistics Project Database.
The association between omission errors and lower s-weights converges with the SVR-LSM result in suggesting that failure of semantic knowledge to drive lexical access is a key contributor to omission errors. The association between omission errors and higher p-weights may reflect that phonological feedback to the lexical level can exacerbate the lexical selection challenge, especially when low s-weights have created a cohort of weakly active lexical candidates. For example, if the target is CAT and low s-weights have weakly activated CAT and DOG, phonological feedback may additionally activate COG, thus adding yet another lexical competitor and making lexical selection more difficult. A related phenomenon was observed in a recent study of homophone picture naming (e.g., deer), which found that homophone counterparts compete and exert a detrimental effect at the lexical-semantic level but cooperate and exert a facilitative effect at the phonological level (Middleton, Chen, & Verkuilen, 2015). These strikingly opposite effects were reflected in semantic and omission errors at the lexical-semantic level, and in phonological errors at the phonological level.
Discussion
Although omission errors in picture naming are common in aphasia, the cognitive and neural bases of these errors are poorly understood. Models of word production define three distinct possible causes of omission errors and their neural correlates: (1) if omission errors are due to lexical-semantic deficits, then they should have the same neural correlates as semantic errors – ATL damage; (2) if omission errors are due to deficits in lexical selection, then they should have the same neural correlates as other lexical selection deficits – inferior frontal damage; and (3) if omission errors are due to phonological deficits, then they should have the same neural correlates as phonological (word and nonword) errors – posterior superior temporal and inferior parieto-frontal damage. The present SVR-LSM analyses localized the neuroanatomical correlates of omission errors to the left frontal and anterior temporal regions, consistent with the lexical-semantic and lexical selection deficit hypotheses, and not consistent with the phonological deficit hypothesis. The computational model analysis further suggested that omission errors are associated with degraded connections between semantic and lexical representations, not degraded connections between lexical and phonological representations. Together, these results provide computational and neural evidence that omission errors in picture naming in aphasia are primarily due to lexical-semantic deficits in word retrieval and selection.
Computational models of picture naming generally agree that word retrieval begins with visual-semantic processes that activate the semantic features of the picture (e.g., cat) and semantically-related concepts (e.g., dog). Activation from these semantic features is propagated forward through intermediate levels to phonological and articulatory planning and execution. Along the way, the target word will be selected from among the semantically-related cohort.
Converging evidence from functional neuroimaging and neuropsychological studies have identified bilateral ATL as a critical “hub” for semantic cognition (Lambon Ralph et al., 2017; Mummery et al., 2000; Nestor et al., 2006; Patterson, Nestor, & Rogers, 2007), with the left ATL being particularly important for verbal semantic processing, including picture naming (Butler et al., 2009; Campo et al., 2016; Lambon Ralph et al., 2001; Mesulam et al., 2013; Mirman, Zhang, et al., 2015). Damage to the left ATL may weaken either the activation of the semantic features (Lambon Ralph et al., 2001; Ueno et al., 2011) or the mapping (connections) from semantic features to words (Dell et al., 2004, 2013; Schwartz et al., 2006). The present SVR-LSM results suggest that omission errors in picture naming arise, in part, when activation of semantic knowledge is insufficient to guide retrieval of the correct lexical representation. Whether resulting from degraded semantic activation or degraded connections, weak semantic input to lexical representations, combined with normal activation noise, can produce two different behavioral outcomes: (1) a semantically related word is selected, resulting in a semantic error, or (2) no word becomes activated strongly enough (e.g., relative to some absolute or relative threshold) to be selected, resulting in no naming attempt being produced – an omission error (a similar finding is reported by Halai et al., 2018). Which of these two outcomes is observed depends on the absolute activation of the different candidates: if a non-target word reaches sufficiently high activation, then the response will be a semantic error; if no candidate word reaches sufficiently high activation, then no response will be produced.
The present results strongly associate omission errors with lexical-semantic deficits rather than phonological deficits, but they do not distinguish between core semantic (“storage”) deficits and deficits in semantically-driven lexical retrieval (“access”). It has been proposed that semantic deficits in semantic dementia (also known as the semantic variant of primary progressive aphasia) reflect degradation of the semantic store whereas semantic deficits in post-stroke aphasia reflect impaired access to relatively intact semantic knowledge, although there is currently no adequate cognitive or computational account for this difference (for a review see Mirman & Britt, 2014). Despite these behavioral differences and differences in the etiology of brain damage, the neural correlates of semantic errors in picture naming are remarkably consistent – in both the semantic dementia and the post-stroke aphasia groups, semantic errors are associated with ATL damage. As a result, the present finding that omission errors are also associated with ATL damage suggests a lexical-semantic deficit cause, though it does not distinguish between impaired semantic representations (core semantic deficit) and impaired connections between intact semantic representations and lexical representations (lexical access deficit).
Neuroimaging and neuropsychological studies suggest that inferior frontal regions are critical to efficient lexical selection (Mirman & Graziano, 2013; Piai et al., 2016; Riès et al., 2016; Schnur et al., 2009, 2006). The present SVR-LSM results suggest that difficulty resolving lexical competition contributes to failure to produce a picture naming response. Lexical selection requires that one lexical representation become substantially more active than the others, so it can be selected. If no single lexical item is able to achieve activation sufficiently greater than the others – possibly because high noise or impaired inhibition (competition) leaves multiple candidates approximately equally activated – then no lexical item will be selected, resulting in no response being produced.
The present study specifically examined omission errors in picture naming in aphasia secondary to left hemisphere stroke. Phenomenologically similar kinds of omission errors, such as “tip-of-the-tongue” states in neurologically intact speakers, may have other causes. This study only considered structural lesion information from individuals with left hemisphere stroke, so it does not address the role of the right hemisphere or of spared perilesional left hemisphere regions. The present study included a relatively large and diverse sample of participants with left-hemisphere stroke. It also used a multivariate lesion-symptom mapping method (SVR-LSM) that is particularly well-suited to detecting contributions of distinct brain regions, as in the present results. Thus, the results provide strong evidence that omission errors in picture naming are primarily associated with damage to left frontal and mid-anterior temporal regions, and suggest that they arise from combined deficits of lexical selection and semantic cognition. Omission errors are inherently difficult to study due to the lack of an overt response. The present results shed new light on this aspect of the quintessential deficit in post-stroke aphasia.
Acknowledgements:
We thank Myrna F. Schwartz for making these data available for analysis and for helpful comments on an early draft of this article, and Kristin Graziano, Casey Ferrara, and Adelyn Brecher for their help preparing the data for analysis.
Disclosure of interest:
Qi Chen – Was funded by grants by the Natural Science Foundation of China (Grant 31671135 and 31300834) and the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2016).
Erica Middleton – Was funded by NIH grants R03DC012426 and R01DC015516.
Daniel Mirman – Was funded by NIH grant R01DC010805 and received funding for a trip from the American Speech-Language-Hearing Association.
Footnotes
A detailed description of the model and parameter-fitting procedure is provided elsewhere (Schwartz et al., 2006) and an online model-fitting tool is available at http://langprod.cogsci.illinois.edu/cgi-bin/webfit.cgi.
References
- Avants BB, Schoenemann PT, & Gee JC (2006). Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Medical Image Analysis, 10, 397–412. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Wilkins DP, Ogar J, Willock S, & Dronkers NF (2011). Role of the precentral gyrus of the insula in complex articulation. Cortex, 47(7), 800–807. 10.1016/j.cortex.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Basilakos A, Rorden C, Bonilha L, Moser D, & Fridriksson J (2015). Patterns of Poststroke Brain Damage That Predict Speech Production Errors in Apraxia of Speech and Aphasia Dissociate. Stroke, 46(6), 1561–1566. 10.1161/STROKEAHA.115.009211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CM, Wolford GL, & Miller MB (2009). The principled control of false positives in neuroimaging. Social Cognitive and Affective Neuroscience, 4(4), 417–422. 10.1093/scan/nsp053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson KE, Garrard P, & Hodges JR (2000). Non-verbal semantic impairment in semantic dementia. Neuropsychologia, 38(9), 1207–1215. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Baldo JV, Okada K, Berman KF, Dronkers NF, D’Esposito M, & Hickok GS (2011). Conduction aphasia, sensory-motor integration, and phonological short-term memory - an aggregate analysis of lesion and fMRI data. Brain and Language, 119(3), 119–128. 10.1016/j.bandl.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CR, Brambati SM, Miller BL, & Gorno-Tempini ML (2009). The neural correlates of verbal and nonverbal semantic processing deficits in neurodegenerative disease. Cognitive and Behavioral Neurology, 22(2), 73–80. 10.1097/WNN.0b013e318197925d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo P, Poch C, Toledano R, Igoa JM, Belinchón M, García-Morales I, & Gil-Nagel A (2016). Visual object naming in patients with small lesions centered at the left temporopolar region. Brain Structure and Function, 221(1), 473–485. 10.1007/s00429-014-0919-1 [DOI] [PubMed] [Google Scholar]
- Caramazza A (1997). How Many Levels of Processing Are There in Lexical Access? Cognitive Neuropsychology, 14(1), 177–208. 10.1080/026432997381664 [DOI] [Google Scholar]
- Damasio H, Tranel D, Grabowski TJ, Adolphs R, & Damasio AR (2004). Neural systems behind word and concept retrieval. Cognition, 92(1–2), 179–229. 10.1016/j.cognition.2002.07.001 [DOI] [PubMed] [Google Scholar]
- Dell GS, Lawler EN, Harris HD, & Gordon JK (2004). Models of errors of omission in aphasic naming. Cognitive Neuropsychology, 21(2), 125–45. 10.1080/02643290342000320 [DOI] [PubMed] [Google Scholar]
- Dell GS, Martin N, & Schwartz MF (2007). A case-series test of the interactive two-step model of lexical access: Predicting word repetition from picture naming. Journal of Memory and Language, 56(4), 490–520. 10.1016/j.jml.2006.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell GS, Schwartz MF, Nozari N, Faseyitan OK, & Coslett HB (2013). Voxel-based lesion-parameter mapping: Identifying the neural correlates of a computational model of word production. Cognition, 128(3), 380–396. 10.1016/j.cognition.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Yourganov G, Bonilha L, Basilakos A, Den Ouden D-B, & Rorden C (2016). Revealing the dual streams of speech processing. Proceedings of the National Academy of Sciences, 113(52), 15108–15113. 10.1073/pnas.1614038114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, & Nichols TE (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage, 15(4), 870–878. 10.1006/nimg.2001.1037 [DOI] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, & Gordon JK (2007). A neural signature of phonological access: distinguishing the effects of word frequency from familiarity and length in overt picture naming. Journal of Cognitive Neuroscience, 19(4), 617–31. 10.1162/jocn.2007.19.4.617 [DOI] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, & Gupta P (2008). The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. Journal of Cognitive Neuroscience, 20(9), 1698–1710. 10.1162/jocn.2008.20113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halai AD, Woollams AM, & Lambon Ralph MA (2018). Triangulation of language-cognitive impairments, naming errors and their neural bases post-stroke. NeuroImage: Clinical, 17, 465–473. 10.1016/j.nicl.2017.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok GS (2012). Computational neuroanatomy of speech production. Nature Reviews Neuroscience, 13(2), 135–145. 10.1038/nrn3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P (2011). The spatial and temporal signatures of word production components: a critical update. Frontiers in Psychology, 2(October), 255 10.3389/fpsyg.2011.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, & Levelt WJM (2004). The spatial and temporal signatures of word production components. Cognition, 92, 101–144. [DOI] [PubMed] [Google Scholar]
- Itabashi R, Nishio Y, Kataoka Y, Yazawa Y, Furui E, Matsuda M, & Mori E (2016). Damage to the left precentral gyrus is associated with apraxia of speech in acute stroke. Stroke, 47(1), 31–36. 10.1161/STROKEAHA.115.010402 [DOI] [PubMed] [Google Scholar]
- Kertesz A (1982). Western Aphasia Battery. New York: Grune & Stratton. [Google Scholar]
- Lambon Ralph MA, Jefferies E, Patterson K, & Rogers TT (2017). The neural and computational bases of semantic cognition. Nature Reviews Neuroscience, 18(1), 42–55. 10.1038/nrn.2016.150 [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson KE, Galton CJ, & Hodges JR (2001). No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience, 13(3), 341–356. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, & Meyer AS (1999). A theory of lexical access in speech production. Behavioral and Brain Sciences, 22(1), 1–75. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Rogalski EJ, Wieneke C. a, Cobia D, Rademaker A, Thompson CK, & Weintraub S (2009). Neurology of anomia in the semantic variant of primary progressive aphasia. Brain, 132(9), 2553–2565. 10.1093/brain/awp138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Wieneke CA, Hurley RS, Rademaker A, Thompson CK, Weintraub S, & Rogalski EJ (2013). Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain, 136(Pt 2), 601–618. 10.1093/brain/aws336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton EL, Chen Q, & Verkuilen J (2015). Friends and foes in the lexicon: homophone naming in aphasia. Journal of Experimental Psychology. Learning, Memory, and Cognition, 41(1), 77–94. 10.1037/a0037778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, & Britt AE (2014). What we talk about when we talk about access deficits. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1634), 1–14. 10.1098/rstb.2012.0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Chen Q, Zhang Y, Wang Z, Faseyitan OK, Coslett HBB, & Schwartz MF (2015). Neural Organization of Spoken Language Revealed by Lesion-Symptom Mapping. Nature Communications, 6(6762), 1–9. 10.1038/ncomms7762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, & Graziano KM (2013). The Neural Basis of Inhibitory Effects of Semantic and Phonological Neighbors in Spoken Word Production. Journal of Cognitive Neuroscience, 25(9), 1504–1516. 10.1162/jocn [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Strauss TJ, Brecher AR, Walker GM, Sobel P, Dell GS, & Schwartz MF (2010). A large, searchable, web-based database of aphasic performance on picture naming and other tests of cognitive function. Cognitive Neuropsychology, 27(6), 495–504. 10.1080/02643294.2011.574112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Zhang Y, Wang Z, Coslett HBB, & Schwartz MF (2015). The ins and outs of meaning: Behavioral and neuroanatomical dissociation of semantically-driven word retrieval and multimodal semantic recognition in aphasia. Neuropsychologia, 76, 208–219. 10.1016/j.neuropsychologia.2015.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson KE, Price CJ, Ashburner J, Frackowiak RS, & Hodges JR (2000). A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Annals of Neurology, 47(1), 36–45. [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, & Hodges JR (2006). Declarative memory impairments in Alzheimer’s disease and semantic dementia. NeuroImage, 30(3), 1010–20. 10.1016/j.neuroimage.2005.10.008 [DOI] [PubMed] [Google Scholar]
- Patterson KE, Nestor PJ, & Rogers TT (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience, 8, 976–987. 10.1038/nrn2277 [DOI] [PubMed] [Google Scholar]
- Piai V, Riès SK, & Swick D (2016). Lesions to Lateral Prefrontal Cortex Impair Lexical Interference Control in Word Production. Frontiers in Human Neuroscience, 9(January), 1–13. 10.3389/fnhum.2015.00721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riès SK, Dronkers NF, & Knight RT (2016). Choosing words: Left hemisphere, right hemisphere, or both? Perspective on the lateralization of word retrieval. Annals of the New York Academy of Sciences, 1369(1), 111–131. 10.1111/nyas.12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach A, Schwartz MF, Martin N, Grewal RS, & Brecher AR (1996). The Philadelphia Naming Test: Scoring and rationale. Clinical Aphasiology, 24, 121–133. [Google Scholar]
- Roelofs A (2014). A dorsal-pathway account of aphasic language production: The WEAVER++/ARC model. Cortex, 59(1874), 33–48. 10.1016/j.cortex.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Ruml W, Caramazza A, Shelton JR, & Chialant D (2000). Testing Assumptions in Computational Theories of Aphasia. Journal of Memory and Language, 43(2), 217–248. 10.1006/jmla.2000.2730 [DOI] [Google Scholar]
- Schnur TT, Schwartz MF, Brecher AR, & Hodgson C (2006). Semantic interference during blocked-cyclic naming: Evidence from aphasia. Journal of Memory and Language, 54(2), 199–227. 10.1016/j.jml.2005.10.002 [DOI] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, & Thompson-Schill SL (2009). Localizing interference during naming: Convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proceedings of the National Academy of Sciences, 106(1), 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF (2014). Theoretical analysis of word production deficits in adult aphasia. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 369(1634), 20120390 10.1098/rstb.2012.0390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Dell GS, Martin N, Gahl S, & Sobel P (2006). A case-series test of the interactive two-step model of lexical access: Evidence from picture naming. Journal of Memory and Language, 54(2), 228–264. 10.1016/j.jml.2005.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Faseyitan OK, Kim J, & Coslett HB (2012). The dorsal stream contribution to phonological retrieval in object naming. Brain, 135(12), 3799–3814. 10.1093/brain/aws300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Brecher AR, Faseyitan OK, Dell GS, … Coslett HB (2011). A neuroanatomical dissociation for taxonomic and thematic knowledge in the human brain. Proceedings of the National Academy of Sciences, 108(20), 8520–8524. 10.1073/pnas.1014935108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan OK, Brecher AR, Dell GS, & Coslett HB (2009). Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain, 132(12), 3411–3427. 10.1093/brain/awp284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thothathiri M, Kimberg DY, & Schwartz MF (2012). The Neural Basis of Reversible Sentence Comprehension: Evidence from Voxel-based Lesion Symptom Mapping in Aphasia. Journal of Cognitive Neuroscience, 24(1), 212–222. 10.1162/jocn_a_00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Saito S, Rogers TT, & Lambon Ralph MA (2011). Lichtheim 2: Synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron, 72(2), 385–396. 10.1016/j.neuron.2011.09.013 [DOI] [PubMed] [Google Scholar]
- Walker GM, Schwartz MF, Kimberg DY, Faseyitan OK, Brecher AR, Dell GS, & Coslett HB (2011). Support for anterior temporal involvement in semantic error production in aphasia: New evidence from VLSM. Brain and Language, 117(3), 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM (2017). Lesion-symptom mapping in the study of spoken language understanding. Language, Cognition and Neuroscience, 32(7), 891–899. 10.1080/23273798.2016.1248984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Isenberg AL, & Hickok GS (2009). Neural correlates of word production stages delineated by parametric modulation of psycholinguistic variables. Human Brain Mapping, 30(11), 3596–3608. 10.1002/hbm.20782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kimberg DY, Coslett HB, Schwartz MF, & Wang Z (2014). Multivariate lesion-symptom mapping using support vector regression. Human Brain Mapping, 35(12), 5861–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]