Abstract
RATIONALE:
The most frequently occurring phthalate, di-(2-ethylhexyl)phthalate (DEHP) causes adverse effects on glucose homeostasis and insulin sensitivity in several cell models and epidemiological studies. However, there is no information on the molecular interaction of phthalates and one of the key regulators of the metabolism, the peroxisome proliferator-activated receptor gamma (PPARγ) available thus far. Since the endogenous ligand of PPARγ 15-deoxy-delta-12,14-prostaglandin J2 (15Δ-PGJ2) features structural similarity to DEHP and its main metabolites produced in human hepatic metabolism mono(2-ethylhexyl)phthalate (MEHP) and mono(2-ethyl-5-oxohexyl)phthalate (MEOHP), we tested the hypothesis of direct interactions between PPARγ and DEHP or its transformation products.
METHODS:
Hydrogen/deuterium exchange mass spectrometry (HDX-MS) and docking were conducted to obtain structural insights into the interactions and surface plasmon resonance (SPR) analysis to reveal information about binding levels. To confirm the activation of PPARγ upon ligand binding on the cellular level, the GeneBLAzer® bioassay was performed.
RESULTS:
HDX-MS and SPR analyses demonstrated that the metabolites MEHP and MEOHP, but not DEHP itself bind to the ligand binding pocket of PPARγ. This binding lead to typical activation-associated conformational changes, as observed with its endogenous ligand 15Δ-PGJ2. Furthermore, the reporter gene assay confirmed productive interaction. DEHP was inactive up to a concentration of 14 μM, while the metabolites MEHP and MEOHP were active at low micromolar concentrations.
CONCLUSIONS:
In summary, this study gives structural insights into the direct interaction of PPARγ with MEHP and MEOHP and shows that the DEHP transformation products may modulate the lipid metabolism through PPARγ pathways.
Keywords: PPARγ, phthalates, DEHP, MEHP, MEOHP, hydrogen/deuterium exchange
1. Introduction
The metabolic syndrome is accompanied by obesity, insulin resistance, type 2 diabetes mellitus as well as cardiovascular diseases. One of the key regulators of the pathways associated with glucose uptake and lipid metabolism is the peroxisome proliferator-activated receptor gamma (PPARγ).1 This receptor belongs to the group of PPARs which are nuclear receptors encoded by different genes and expressed in various tissues. PPARγ is mainly expressed in adipose tissue and modulates the differentiation of sprouting metabolically active adipocytes that are insulin-sensitive.2 Furthermore, it supports the enrichment of lipids and the hypertrophy of mature fat cells by decreasing their insulin sensitivity.3 PPARγ, like all nuclear receptors, is a transcription factor that contains a DNA-binding domain and a ligand-binding domain and is activated following binding of a specific ligand what in turn induces the translocation of the PPARγ from cytosol to nucleus.4 Corepressors dissociate, coactivators associate and a heterodimer with the retinoid-X-receptor (RXR) is formed. This complex can subsequently bind to PPARγ responsive elements within promoter regions of specific target genes, hence regulating the expression of these genes.5 The highly affine (EC50 = 2 μM) endogenous ligand of PPARγ is 15-deoxy-delta-12,14-prostaglandin J2 (15Δ-PGJ2). Produced from fatty acids, 15Δ-PGJ2 is a suitable representative PPARγ ligand for lipid metabolism.6 Nevertheless, the size of the ligand binding domain (LBD) of PPARγ (1200 Å3) allows the interaction with several structurally diverse ligands and thus, diverse biological effects can be triggered.7, 8
Phthalates have been used as plasticizers for more than 50 years to increase the flexibility of polyvinylchloride (PVC) products. They can be found in floor coverings, food and drink packaging materials and medical devices as blood bags and tubing9, 10 and thus have become ubiquitously distributed contaminants. The most frequently used phthalate is bis-(2-ethylhexyl)phthalate (DEHP). DEHP and its main metabolites mono-(2-ethylhexyl)phthalate (MEHP) and mono-(2-ethyl-5-oxohexyl)phthalate (MEOHP) have been detected in blood, urine and adipose tissue.11 In vivo studies showed that female mice being exposed to DEHP gained weight and body fat and had decreased insulin sensitivity.12, 13 These observations were specific for female mice and independent from food intake. This is in line with results that phthalates might influence hormone signalling by interacting with the estrogen receptor.14 Furthermore, a positive correlation between phthalate metabolites in urine and serum with abdominal obesity as well as with insulin resistance in adult male patients was shown.15 This association with obesity raises the question of a mechanistic link between phthalates and the altered functionality of adipose tissue, for which PPARγ is a key regulator.
Therefore, the objective of this study was to evaluate the ability of DEHP and its main metabolites to bind to PPARγ. Since it is known that PPARγ undergoes conformational changes upon effective binding5, hydrogen/deuterium exchange (HDX) mass spectrometry (MS) was chosen to examine PPARγ ligand binding. HDX-MS is a widely used method for protein structure investigation16–20 that allows studying protein/ligand interactions due to its ability to monitor non-covalent properties in solution.21, 22 All investigated ligands share structural similarity with 15Δ-PGJ2 (Figure 1). With HDX-MS, binding data of PPARγ and its potential ligands DEHP, MEHP, and MEOHP were revealed. Using protein/ligand docking, the respective binding sites of the ligands within the PPARγ binding pocket could be further characterized. To compare the binding strength of the tested ligands surface plasmon resonance (SPR) spectroscopy was applied. Furthermore, the molecular interaction was studied on cellular level using a reporter gene assay based on GeneBLAzer® PPAR gamma-UAS-bla 293H cells.23
Figure 1: Structures of the investigated ligands.
Presented are the chemical structures of the investigated ligands 15Δ-PGJ2 and Rosiglitazone (positive controls), as well as DEHP, MEHP and MEOHP.
2. Materials and Methods
2.1. Hydrogen/deuterium exchange mass spectrometry
Solution-phase HDX-MS experiments were carried out using a fully automated system (Waters Corporation). Human His-tagged PPARγ (59.2 kDa, 0.32 mg/ml, 0.025 M Tris-HCl, pH 8.0, 50 % (v/v) Glycerol, 0.01 mM EDTA, 75 mM NaCl) was obtained from Thermo Fisher Scientific. The investigated ligands 15Δ-PGJ2 (Santa Cruz Biotechnology), DEHP (Accu Standard), MEHP (Accu Standard) and MEOHP (Santa Cruz Biotechnology) were solved in 0.025 M Tris-HCl pH 8.0 with 7.5 % (v/v) DMSO, resulting in concentrations of 5 mM DEHP, MEHP and MEOHP as well as 2.4 mM 15Δ-PGJ2. For each experiment 0.5 μg (1.6 μl) PPARγ was used. As reference PPARγ was mixed with water and subsequently subjected to the automated Acquity UPLC M-Class System with HDX Technology (Waters Corporation), where the sample was passed through an immobilized pepsin column (ENZYMATE BEH Pepsin Column 2.1×30 mm, Waters Corporation) at 70 μl/min and 20 °C. The resulting peptides were trapped on a C18 column (Acquity UPLC BEH C18 1.7 μm VanGuard Pre-Column 2.1×30 mm, Waters Corporation) and then gradient-eluted (8–35 % (v/v) acetonitrile and 0.1 % (v/v) formic acid) across another C18 column (Acquity UPLC BEH C18 1.7 μm 1×100 mm Column, Waters Corporation) for 7 min at 40 μl/min and 0 °C. The eluted peptides were subsequently subjected to electrospray ionization (Z-Spray Source, Waters Corporation) directly coupled to the Xevo G2-S Tof (Waters Corporation). Each experiment was carried out in triplicates. The peptide identification was performed using Protein Lynx Global Server 3.0.2 (Waters Corporation).22
To obtain deuteration data for the free PPARγ, the protein was incubated in 50 μl D2O (resulting in >95 % (v/v) D2O) for 60 s. For investigation of ligand binding, the protein solution was preincubated with 1 μl ligand solution for at least one hour, followed by deuteration with 50 μl D2O for 60 s. Deuteration was stopped by addition of 16.5 μl quenching solution (1 M TCEP, 3 M urea, 182 mM Tris-HCl), resulting in pH 2.5. Samples were transferred to the already described automated system and the deuteration level of each identified peptide was determined by DynamX 3.0 (Waters Corporation).
2.2. Docking
To evaluate the binding sites of the ligands, a docking study using Rosetta24, 25 was conducted. Structures of ligands for docking (15Δ-PGJ2, DEHP, MEHP, and MEOHP) were obtained in SMILES format and converted to SDF format with 3D coordinates and hydrogens appropriate for pH 7.4 using OpenBabel.26 A conformer library for each ligand was generated with BCL27, resulting in 1000, 370, 175, and 351 conformers for 15Δ-PGJ2, DEHP, MEHP, and MEOHP, respectively. Rosetta ligand parameter files were generated for each conformer library, with setting to keep the neighbor atom (the “center” of the molecule) attached to the ring. Structures of PPARγ were downloaded from the RCSB protein database (PDB: 1PRG, 2ZK1 and 4EMA), and trimmed to be a single chain containing the ligand binding site. The apo structures were then subjected separately to both fixed-backbone repacking with the Rosetta fixbb application and an all-heavy atom constrained relax.28
Due to the large size of the binding site, multiple starting points were applied. Each ligand heavy atom position in the 2ZK1 and 4EMA structure was employed as a separate starting point for the ligand center. The RosettaLigand Transform protocol29 was used to dock the ligands into each of the four prepared apo structures. Due to the size of the ligand, an expanded ligand grid size (30 Å) was employed.
2.3. Surface plasmon resonance spectroscopy
A Biacore T100 (GE Healthcare) was used for interaction analyses of 15Δ-PGJ2, DEHP, MEHP and MEOHP (molecular weights are listed in Table S12) with PPARγ. Series S Sensor Chip CM5, amine coupling kit and HBS-EP (10x) were obtained from GE Healthcare Europe GmbH (Freiburg, Germany). PPARγ was immobilized on Series S Sensor Chip CM5 surfaces at 25 °C via amine coupling according to the manufacturer´s protocol (GE Healthcare), leading to an average immobilization level of 5284 RU (Table S13) when using a concentration of 50 μg/ml PPARγ in 10 mM sodium acetate buffer (pH 4.5) at a flow rate of 5 μl/min. A surface, which was directly deactivated with 1 M ethanolamine-HCl (pH 8.5) for 420 s (10 μl/min) after activation with a mixture containing 50 mM N-hydroxysuccinimide (NHS) and 195.6 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) for 420 s (10 μl/min), served as reference without immobilized receptor.
HBS-EP (0.01 M HEPES (pH 7.4), 3 mM EDTA, 0.15 M sodium chloride, 0.05 % surfactant P20) with 3 % DMSO served as running buffer for all interaction analyses performed at 25 °C. The samples were diluted with the same buffer. Binding studies were performed by injecting the analytes (15Δ-PGJ2, MEHP, MEOHP, DEHP) for 120 s at 30 μl/min. 50 μM and 100 μM were used as analyte concentrations to maximize the binding response since they were expected to be low for small analytes. After a dissociation phase of 600 s, 5 M sodium chloride with 5 mM sodium hydroxide was used for regeneration. Binding levels were recorded 10 s before injection stop. Prior to further injection, the chip surfaces were allowed to stabilize for 1000 s with running buffer. Specific Biacore sensorgrams were obtained by reference and blank (running buffer) subtraction according to Myszka.30 A range of DMSO concentrations (2.5 – 3.8 % DMSO in HBS-EP) was injected every 20 cycles to apply a solvent correction via the Biacore T100 evaluation software 2.03 if necessary. This allows the correction of potential differences in bulk response between samples that can occur due to small variations of the DMSO concentration. The same software was applied to evaluate the binding parameters. The obtained values (Table S14) were afterwards normalized to the binding response of 100 μM 15Δ-PGJ2 (Table S15) and corrected for the respective molecular weight of the analyte (Table S16) to rank the binding strength.
Single cycle kinetics were performed to characterize the 15Δ-PGJ2, MEHP and MEOHP interactions with PPARγ. Therefore, five increasing concentrations of 15Δ-PGJ2 (6.25 – 100 μM), MEHP (6.25 – 100 μM) or MEOHP (25 – 200 μM) were injected for 240 s per injection over a PPARγ surface without regeneration between the injections prior to a 1000 s dissociation phase. Kinetic parameters were calculated via the Biacore T100 evaluation software applying the heterogeneous ligand model. To assess the kinetic fit, Χ2 as statistical parameter was used. Steady state affinity analyses were used to estimate Kd value ranges for MEHP and MEOHP binding to PPARγ.
2.4. Reporter gene assay
To verify the biological activity of PPARγ due to ligand binding, the GeneBLAzer® PPAR gamma-UAS-bla 293H Cell-based Assay (Invitrogen) was used. The assay was conducted in agonistic mode and cytotoxicity was quantified in parallel. Each experiment was carried out in duplicate on three different days. The method has been previously described in detail.31
DEHP and MEOHP were dissolved in DMSO resulting in 14 mM and 33 mM stock solutions, respectively. MEHP was dissolved in methanol and deprotonated with a 0.1 M NaOH solution to produce a pH 7 stock of 86 mM. Rosiglitazone was used as reference compound (stock solution 4 μM) and a DMSO control was run that showed that the IC10 for cytotoxicity was 1.5 % DMSO and that DMSO did not activate PPARγ at or below cytotoxic concentrations. Therefore, not more than 0.15 % DMSO was used for the experiments. The highest methanol content was 1.4 %, which is permissible. The obtained dose response curves of the DMSO control and the reference compound rosiglitazone are presented in Figure S5 of the Supplement.
The cells were seeded in 384-well plates (4000 cells per well in 30 μl medium) and incubated for 24 h at 37 °C and 5 % CO2. 10 μl of dosing medium containing the dilution series of the test compound were added per well and incubated for 22 h at 37 °C and 5 % CO2. 8 μl of FRET detection including ToxBlazer mixture were added per well followed by 2 h incubation at room temperature. Blue (activated) and green signals (inactive) were detected using an excitation filter of 409 nm and emission filters of 460 nm and 530 nm, respectively. Induction of the transcription factor or, more precisely, production of β-lactamase was measured by the ratio of blue to green (B/G) FRET fluorescence signal. For the cytotoxicity, an excitation filter of 600 nm and an emission filter of 665 nm were used.
The evaluation of dose response curves for activation and cytotoxicity has been previously described.32 The concentration-response model was a log-logistic model of the B/G ratios which were normalized to the maximum effect induced by the reference compound and the minimum effect of the controls. Only the effect data at concentrations that did not cause more than 10 % cytotoxicity (IC10) were included in the data evaluation of the activation of PPARγ, which was derived as EC10 (the effect concentration for 10 % of activation).
3. Results and Discussion
3.1. Hydrogen/deuterium exchange mass spectrometry
To investigate the binding of ligands (structures are presented in Figure 1) to PPARγ and to verify the binding sites, HDX-MS was applied. This method can provide information on dynamic effects caused by ligand binding and thus allows the determination of ligand binding sites.
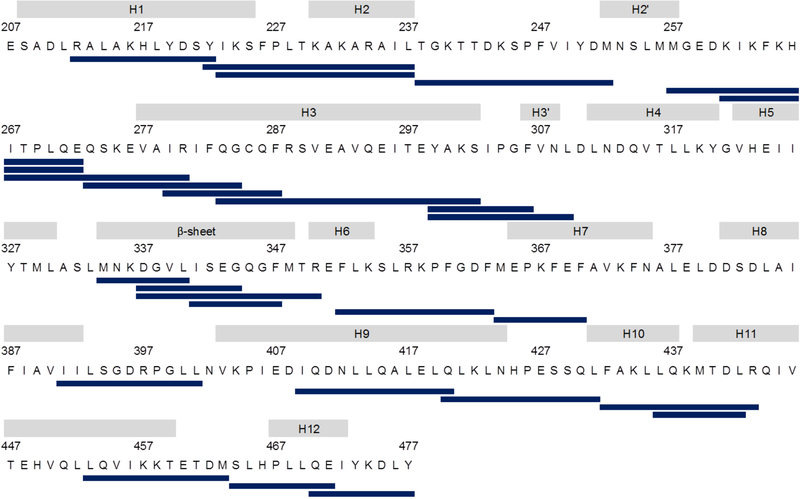
The coverage map presented in Figure 2 shows that for each experiment 26 peptides could be detected that covered 72 % of the PPARγ LBD (raw data are summarized in Table S1 - S4). Deuteration data for the free PPARγ are visualized in a heatmap (Figure S1). The relative H/D exchange kinetics of PPARγ in complex with different ligands were compared to the data of the free PPARγ and summarized in a heat map as well (Figure S2). A t-test was executed to ascertain the peptides with the most significant differences with respect to the peptides of the free PPARγ (p-values are summarized in Table S5). Peptides with the most significant differences are listed in Table 1 and show that the endogenous ligand 15Δ-PGJ2 as well as the DEHP metabolites MEHP and MEOHP resulted in protection of specific regions within the LBD of PPARγ. In contrast, DEHP caused no protection. Figure 3C shows these most significantly protected peptides in the PPARγ structure using the example of 15Δ-PGJ2.
Figure 2: Sequence coverage map of the PPARγ LBD.
Presented are the 26 identified peptides that cover 72 % of the sequence of the PPARγ LBD (PDB: 1PRG).
Table 1: Percentage deuteration of significantly protected peptides after 1 min deuteration.
The percentage deuteration values of peptides that were significantly protected (p-value < 0.001) for at least one of the compounds are shown as well as the corresponding standard deviations and p-values obtained by performing a t-test against the free PPARγ. The cells are colored based on the percentage deuteration values. The peptides in this table are visualized in Figure 3C using the example of 15Δ-PGJ2 and were used for the cluster analysis presented in Figure 5.
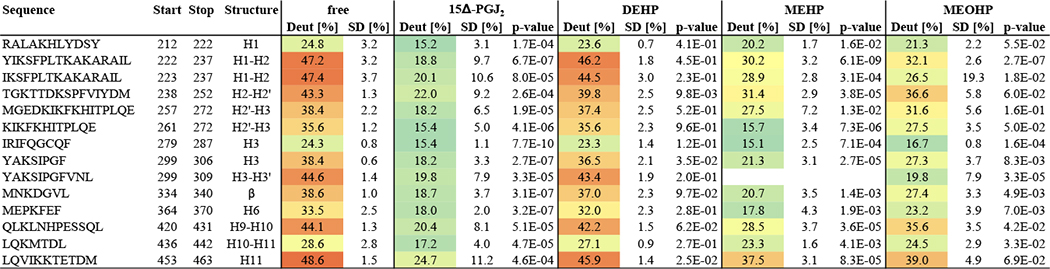 |
Figure 3: LBD of PPARγ in complex with 15Δ-PGJ2 colored by the relative deuteration levels after 1 min of deuteration.
A shows the PPARγ LBD (PDB: 2ZK1) colored with respect to the obtained relative deuteration levels after 1 min of deuteration. 15Δ-PGJ2 is colored orange and the amino acids that are supposed to be involved in ligand binding33 are labeled in B. C presents the peptides that showed significant protection (p-value < 0.001) for at least one of the tested compounds. These peptides are furthermore listed in Table 1.
In accordance with previously described data for binding of 15Δ-PGJ2 to the LBD of PPARγ33 it could be revealed that especially for peptides being located in the ligand binding pocket, lower H/D exchange values were determined (Figure 3A), e.g. both Val-339 and Lys-367 are reported to be directly involved in hydrophobic interactions with 15Δ-PGJ2 and peptides containing these amino acids showed significant protection. Furthermore, the peptide containing Tyr-473, which is supposed to build a hydrogen bond with the 15Δ-PGJ2, was protected due to the binding of 15Δ-PGJ2. Based on these observations and the fact that the obtained deuteration patterns were similar to previously reported ones34, we concluded that the observed binding was specific.
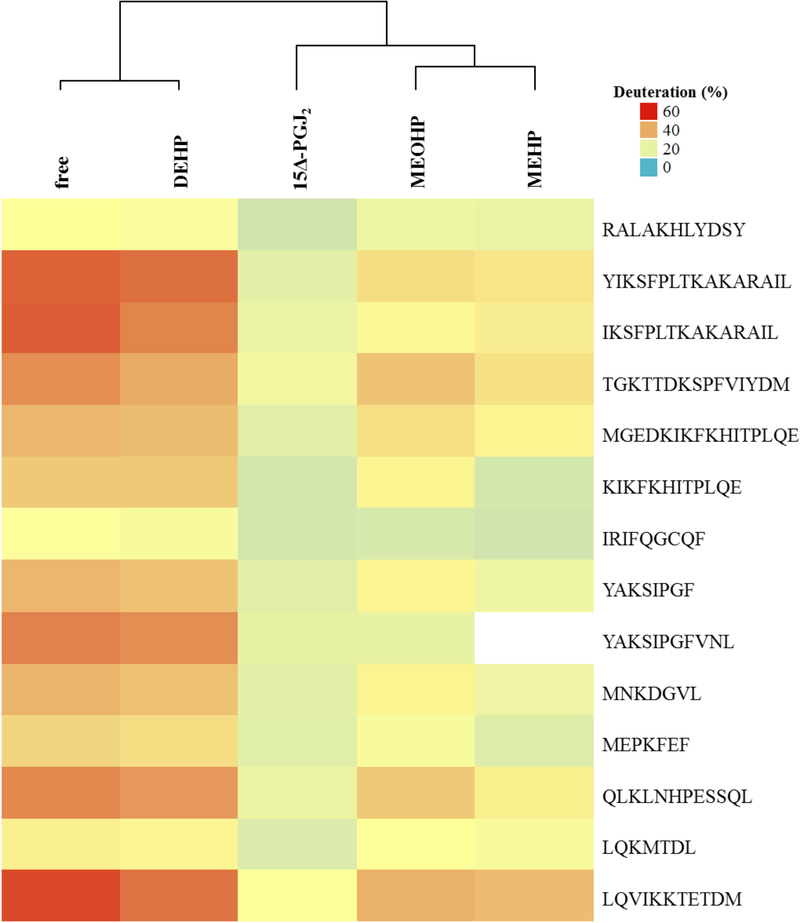
Furthermore, comparing HDX data obtained with the endogenous ligand 15Δ-PGJ2 with those from DEHP, MEHP and MEOHP treatment (Figure 4), suggests that binding of MEHP and MEOHP was specific as well. However, no binding effects were observed with the phthalate DEHP itself.
Figure 4: Structural overview of the obtained relative deuteration levels after 1 min of deuteration and corresponding docking results.
Structures of the PPARγ LBD (PDB: 1PRG (free), 2ZK1), colored with respect to the relative deuteration levels after 1 min of deuteration and showing the docking results for the respective top scored structures of docking DEHP, MEHP and MEOHP are presented. Ligands are shown in orange.
For all three binding ligands, the regions with the most significant binding effects (Table 1) were within H1-H2, H3-H4 and H6-H7, which are known to be also involved in the formation of the binding pocket within the LBD of PPARγ.35
The peptide containing Cys-285, which is especially relevant for the activation of PPARγ33, displayed protection not only with 15Δ-PGJ2, but also with MEHP and MEOHP as ligands. Importantly, these effects were not significant. Peptides comprising Ser-245 and Ser-342, which can be stabilized by ligand binding36, showed protection as well with significant effects for the Ser-245 including peptide under treatment with 15Δ-PGJ2 and MEHP, respectively. To confirm that DEHP does not bind to the PPARγ LBD, while MEHP and MEOHP do bind, an Euclidean cluster analysis with the percentage deuteration data after 1 min of deuteration for the peptides summarized in Table 1 was performed. This analysis showed that DEHP clusters with the free PPARγ, while both MEHP and MEOHP cluster with the positive control 15Δ-PGJ2 (Figure 5), thus confirming the observation that DEHP does not bind while its metabolites do bind. Furthermore, this leads to the assumption that the obtained deuteration data are valuable to distinguish between binding and not binding compounds.
Figure 5: Cluster analysis of percentage deuteration data after one minute of deuteration.
The cluster analysis was performed with R-3.3.140, 41 under use of the euclidean algorithm. The obtained percentage deuteration levels of those peptides that showed significant (p-value < 0.001) differences for at least one ligand with respect to the obtained values for the free PPARγ were used for the analysis. This cluster analysis confirms the assumption that DEHP does not bind to the PPARγ LBD while 15Δ-PGJ2, MEHP and MEOHP do bind because DEHP clustered with the free PPARγ and MEHP and MEOHP clustered with the positive control 15Δ-PGJ2.
3.2. Docking
To structurally explain that MEHP and MEOHP specifically bind to PPARγ and DEHP does not, a docking study was conducted. The three top scored structures obtained from docking DEHP, MEHP and MEOHP into the PPARγ LBD (PDB: 2ZK1) are included in Figure 4. A sense of the binding mode can be obtained by examining typical protein-ligand contacts. Residue contacts common to all compounds (Table S9) cluster on the H11 side of the pocket, whereas the DEHP-specific residues (Table S10) are found at the β-sheet, indicating that MEHP and MEOHP prefer binding at the H11 side of the pocket. The location of polar heavy atom contacts with side chains (Table S11) indicates that the greater hydrogen bonding opportunities on MEHP and MEOHP compared to DEHP contribute to the differential localization. Particularly, residues His-323, Tyr-327, His-449 and Tyr-473 are predicted to be more involved in polar heavy atom contacts for MEHP and MEOHP, which is consistent with placing the free carboxylate in a binding pocket formed by those residues. Furthermore, the contacts that have been predicted (e.g. around H3 and H7) by docking are consistent with the observed protection in the regions of H3, H6-H7 and H11. Protected regions that could not be explained by atom contacts, such as the effects in the H8-H9 region or the H9-H10 region, might arise from conformational changes or even a global rigidification induced by ligand binding. Taking into account B-factors (Figure S2), it is observable that these factors indicate high flexibility of some regions as the H2’-H3 region, so that even minor binding effects might result in significant protection.
While HDX and docking experiments give an indication of the binding mode of MEHP and MEOHP, the current results still leave uncertainties as to the atomistic details of the interactions. Further mutational studies could give insights into the role of individual residues in MEHP and MEOHP binding. Methyl esters of MEHP and MEOHP could further elulcidate the critical features of MEHP and MEOHP which lead to differential binding and activation of PPARγ, including the role of polar interactions to the free carboxylate of MEHP and MEOHP.
3.3. Surface plasmon resonance spectroscopy
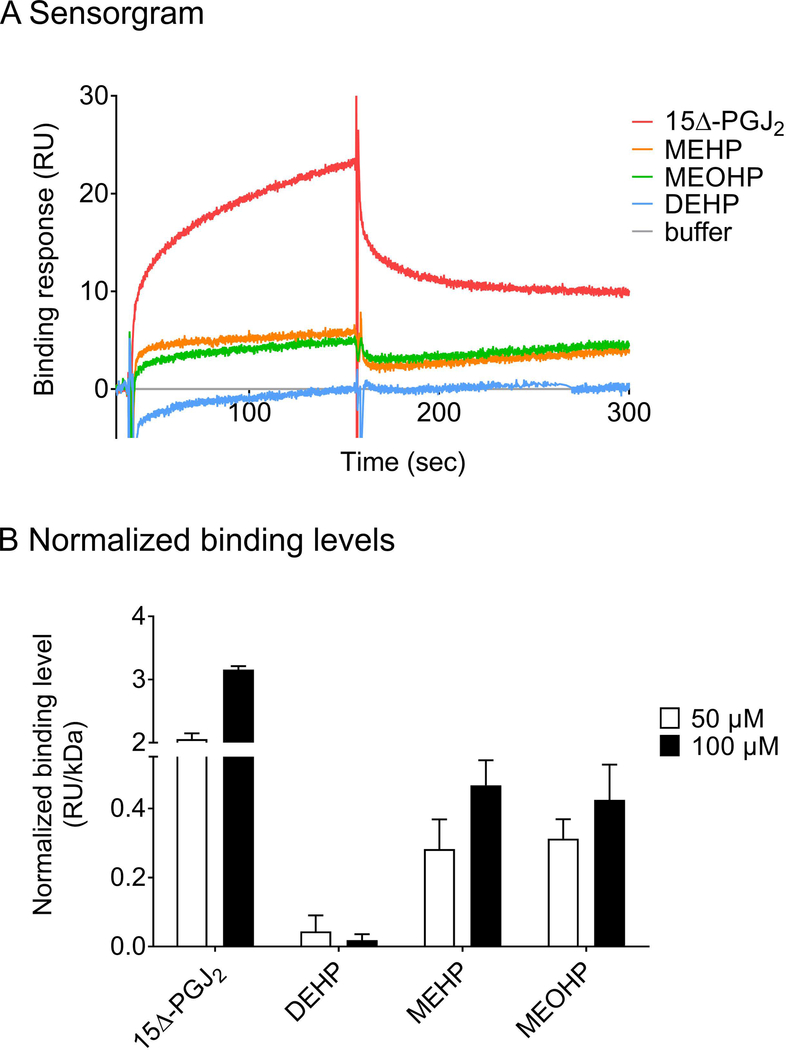
To compare the binding strength of the different ligands to PPARγ in vitro, the direct interaction of 15Δ-PGJ2, DEHP, MEHP and MEOHP to immobilized PPARγ was analyzed via SPR (Figure 6). Their respective binding levels were ranked in comparison to the natural PPARγ ligand 15Δ-PGJ2. Binding analyses revealed direct binding of 15Δ-PGJ2, MEHP and MEOHP to PPARγ (Figure 6A). DEHP showed almost no detectable binding, while the binding strength of MEHP, MEOHP as well as 15Δ-PGJ2 increased in a concentration-dependent manner (Figure 6B). In summary, the binding strength increased in the following order: DEHP < MEHP ≈ MEOHP << 15Δ-PGJ2.
Figure 6: Interaction of 15Δ-PGJ2, DEHP, MEHP and MEOHP with PPARγ.
A representative sensorgram showing the binding response of 100 μM analyte to immobilized PPARγ (5255 RU) is displayed in A. Binding levels of 15Δ-PGJ2, DEHP, MEHP and MEOHP (50 μM and 100 μM) to PPARγ receptor surfaces (2876 RU; 5255 RU; 7721 RU) normalized to the response of 100 μM 15Δ-PGJ2 as determined via SPR binding analysis at 25°C are shown in B.
Single cycle kinetics showed Kd values for 15Δ-PGJ2 binding (Kd1 = 10 μM, Kd2 = 311 μM, Χ2 = 3.97 RU2) (Figure S4A) which are in a comparable μM range as reported previously.37 Kd values for the interactions of phthalates and PPARγ could not be determined even with the highest phthalate concentrations experimentally accessible due to the limited solubility of the phthalates in the running buffer. However, steady state affinity analyses allow for estimating them to be in the higher micromolar range (Figure S4B, C).
3.4. Reporter gene assay
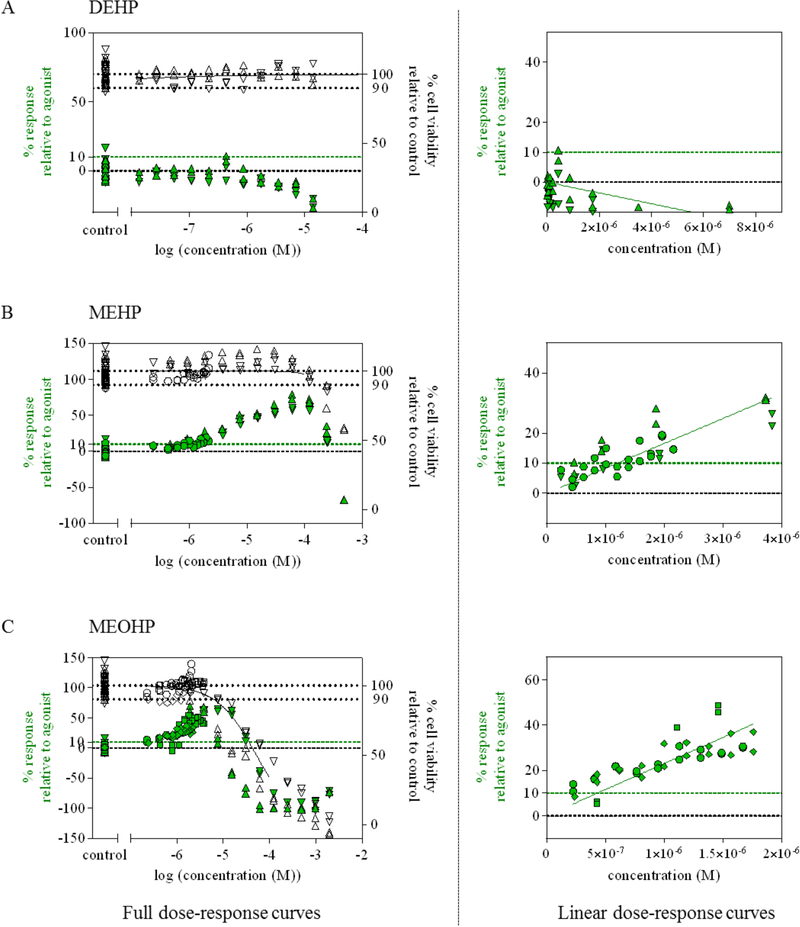
To confirm that the observed binding of MEHP and MEOHP also leads to the activation of PPARγ on the cellular level, the GeneBLAzer® bioassay was performed. The dose response curves for cytotoxicity and activation are plotted in Figure 7. Up to the highest tested concentration of 14 μM, DEHP did not show any cytotoxicity, or activation of PPARγ. The IC10 value for cytotoxicity of MEHP was 203 μM and the EC10 value for activation of PPARγ was 1.2 μM. MEOHP was cytotoxic at 100 times lower concentration than MEHP with an IC10 of 2.9 μM. Its EC10 value was 0.45 μM. Thus, the results of the GeneBLAzer® bioassay confirmed the binding order that was observed by HDX and SPR and additionally verified that the binding data that were obtained by HDX and SPR actually lead to PPARγ activation.
Figure 7: Dose response curves for DEHP and its metabolites MEHP and MEOHP.
Presented are the observed cytotoxicity (white symbols, right y-axis) and activation of PPARγ (green symbols, left y-axis) relative to the agonist rosiglitazone for DEHP (A), MEHP (B) and MEOHP (C). The activation of PPARγ is shown as full dose-response curve (left) and as linear dose-response curve (right). The EC10 of each compound was derived from the linear dose response curve. Different symbols represent three independent experiments.
Due to the fact that the GeneBLAzer® bioassay gave EC10 values in the lower micromolar range for both ligands and neither the HDX data, nor the SPR data showed significant differences between MEHP and MEOHP binding, it can be assumed, that the differences in MEHP and MEOHP binding are negligible. In contrast, the differences between binding of the endogenous ligand 15Δ-PGJ2 and MEHP or MEOHP are significant for HDX (Table S6 - S8, Figure S3) and SPR (Table S17), confirming that HDX data can be used as indicators in matters of binding affinities.38, 39
4. Conclusions
This study shows that the DEHP metabolites MEHP and MEOHP bind to and activate PPARγ. HDX data indicate binding of MEHP and MEOHP to specific regions within the LBD that match the binding site of the endogenous ligand 15Δ-PGJ2. Using the GeneBLAzer® bioassay, it was additionally shown that the binding of MEHP and MEOHP resulted in biological activity of PPARγ.
In summary, this study gives structural insights into the direct interaction of PPARγ with MEHP and MEOHP and shows that the DEHP metabolites may modulate the lipid metabolism through the PPARγ pathway.
Supplementary Material
6 Acknowledgments
This work was supported by the BMBF (03XP0008B), the Max Kade Foundation, the German Research Council CRC TRR67 (subprojects A3, Z4), the subproject Z3 of the CRC 1052, Mechanisms of Adiposity, as well as the ESF Investigator group GPCR 2. Work in the Meiler laboratory is supported through NIH (R01 GM080403, R01 GM073151) and NSF (CHE 1305874). Furthermore, we thank Christin Kühnert and Maria König for laboratory assistance.
Abbreviations:
- PPARγ
peroxisome proliferator-activated receptor gamma
- 15Δ-PGJ2
15-deoxy-delta-12,14-prostaglandin J2
- DEHP
di-(2-ethylhexyl)phthalate
- MEHP
mono(2-ethylhexyl)phthalate
- MEOHP
mono(2-ethyl-5-oxohexyl)phthalate
- HDX-MS
Hydrogen/deuterium exchange mass spectrometry
Footnotes
Supplementary Material
Raw data from HDX experiments and SPR are listed in the Supplementary Material together with results from significance tests and docking. Furthermore, the Supplementary Material contains additional visualizations of data from the reporter gene assay, namely of DMSO (control) and rosiglitazone (positive control).
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
7 References
- 1.Chinetti G, Fruchart JC, Staels B Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflammation research : official journal of the European Histamine Research Society 2000;49(10):497–505. [DOI] [PubMed] [Google Scholar]
- 2.Ahrends R, Ota A, Kovary KM, Kudo T, Park BO, Teruel MN Controlling low rates of cell differentiation through noise and ultrahigh feedback. Science 2014;344(6190):1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willson TM, Lambert MH, Kliewer SA Peroxisome proliferator-activated receptor gamma and metabolic disease. Annual review of biochemistry 2001;70:341–367. [DOI] [PubMed] [Google Scholar]
- 4.Bishop-Bailey D, Hla T Endothelial Cell Apoptosis Induced by the Peroxisome Proliferator-activated Receptor (PPAR) Ligand 15-Deoxy-Δ12,14-prostaglandin J2. Journal of Biological Chemistry 1999;274(24):17042–17048. [DOI] [PubMed] [Google Scholar]
- 5.Nolte RT, Wisely GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature 1998;395(6698):137–143. [DOI] [PubMed] [Google Scholar]
- 6.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell 1995;83(5):813–819. [DOI] [PubMed] [Google Scholar]
- 7.Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR). Toxicological sciences 2006;90(2):269–295. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Waltenberger B, Pferschy-Wenzig E-M, Blunder M, Liu X, Malainer C, et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochemical pharmacology 2014;92(1):73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, et al. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environmental health perspectives 2003;111(9):1148–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbin A, Östelius J Determination by electron-capture gas chromatography of mono- and di(2-ethylhexyl) phthalate in intravenous solutions stored in poly(vinyl chloride) bags. Journal of Chromatography A 1980;193(3):405–412. [Google Scholar]
- 11.Kato K, Silva MJ, Reidy JA, Hurtz D, Malek NA, Needham LL, et al. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environmental health perspectives 2004;112(3):327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klöting N, Hesselbarth N, Gericke M, Kunath A, Biemann R, Chakaroun R, et al. Di-(2-Ethylhexyl)-Phthalate (DEHP) Causes Impaired Adipocyte Function and Alters Serum Metabolites. PloS one 2015;10(12):e0143190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt J-S, Schaedlich K, Fiandanese N, Pocar P, Fischer B Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environmental health perspectives 2012;120(8):1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engel A, Buhrke T, Imber F, Jessel S, Seidel A, Volkel W, et al. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors ERalpha, ERbeta, and AR. Toxicol Lett 2017;277:54–63. [DOI] [PubMed] [Google Scholar]
- 15.Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH Concentrations of Urinary Phthalate Metabolites Are Associated with Increased Waist Circumference and Insulin Resistance in Adult U.S. Males. Environmental health perspectives 2007;115(6):876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engen JR Analysis of Protein Conformation and Dynamics by Hydrogen/Deuterium Exchange MS. Analytical Chemistry 2009;81(19):7870–7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katta V, Chait BT, Carr S Conformational changes in proteins probed by hydrogen-exchange electrospray-ionization mass spectrometry. Rapid Communications in Mass Spectrometry 1991;5(4):214–217. [DOI] [PubMed] [Google Scholar]
- 18.Wales TE, Engen JR Hydrogen exchange mass spectrometry for the analysis of protein dynamics. Mass Spectrometry Reviews 2006;25(1):158–170. [DOI] [PubMed] [Google Scholar]
- 19.Burns KM, Rey M, Baker CA, Schriemer DC Platform dependencies in bottom-up hydrogen/deuterium exchange mass spectrometry. Mol Cell Proteomics 2013;12(2):539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rand KD, Zehl M, Jorgensen TJ Measuring the hydrogen/deuterium exchange of proteins at high spatial resolution by mass spectrometry: overcoming gas-phase hydrogen/deuterium scrambling. Acc Chem Res 2014;47(10):3018–3027. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann T, Samsonov SA, Pichert A, Lemmnitzer K, Schiller J, Huster D, et al. Structural analysis of the interleukin-8/glycosaminoglycan interactions by amide hydrogen/deuterium exchange mass spectrometry. Methods 2015;89:45–53. [DOI] [PubMed] [Google Scholar]
- 22.Rother S, Samsonov SA, Hofmann T, Blaszkiewicz J, Kohling S, Moeller S, et al. Structural and functional insights into the interaction of sulfated glycosaminoglycans with tissue inhibitor of metalloproteinase-3 - A possible regulatory role on extracellular matrix homeostasis. Acta Biomater 2016;45:143–154. [DOI] [PubMed] [Google Scholar]
- 23.König M, Escher BI, Neale PA, Krauss M, Hilscherová K, Novák J, et al. Impact of untreated wastewater on a major European river evaluated with a combination of in vitro bioassays and chemical analysis. Environmental Pollution 2017;220:1220–1230. [DOI] [PubMed] [Google Scholar]
- 24.Leaver-Fay A, Tyka M, Lewis SM, Lange OF, Thompson J, Jacak R, et al. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods in enzymology 2011;487:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meiler J, Baker D ROSETTALIGAND: Protein–small molecule docking with full side‐chain flexibility. Proteins: Structure, Function, and Bioinformatics 2006;65(3):538–548. [DOI] [PubMed] [Google Scholar]
- 26.O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR Open Babel: An open chemical toolbox. Journal of Cheminformatics 2011;3(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kothiwale S, Mendenhall JL, Meiler J BCL::Conf: small molecule conformational sampling using a knowledge based rotamer library. Journal of Cheminformatics 2015;7(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nivón LG, Moretti R, Baker D A Pareto-Optimal Refinement Method for Protein Design Scaffolds. PloS one 2013;8(4):e59004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeLuca S, Khar K, Meiler J Fully Flexible Docking of Medium Sized Ligand Libraries with RosettaLigand. PloS one 2015;10(7):e0132508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myszka DG Improving biosensor analysis. J Mol Recognit 1999;12(5):279–284. [DOI] [PubMed] [Google Scholar]
- 31.Neale PA, Altenburger R, Aït-Aïssa S, Brion F, Busch W, de Aragão Umbuzeiro G, et al. Development of a bioanalytical test battery for water quality monitoring: Fingerprinting identified micropollutants and their contribution to effects in surface water. Water Research 2017;123:734–750. [DOI] [PubMed] [Google Scholar]
- 32.Escher BI, Allinson M, Altenburger R, Bain PA, Balaguer P, Busch W, et al. Benchmarking Organic Micropollutants in Wastewater, Recycled Water and Drinking Water with In Vitro Bioassays. Environmental Science & Technology 2014;48(3):1940–1956. [DOI] [PubMed] [Google Scholar]
- 33.Waku T, Shiraki T, Oyama T, Fujimoto Y, Maebara K, Kamiya N, et al. Structural insight into PPARgamma activation through covalent modification with endogenous fatty acids. Journal of molecular biology 2009;385(1):188–199. [DOI] [PubMed] [Google Scholar]
- 34.Chalmers MJ, Busby SA, Pascal BD, Southern MR, Griffin PR A Two-Stage Differential Hydrogen Deuterium Exchange Method for the Rapid Characterization of Protein/Ligand Interactions. Journal of Biomolecular Techniques 2007;18(4):194–204. [PMC free article] [PubMed] [Google Scholar]
- 35.Kroker AJ, Bruning JB Review of the Structural and Dynamic Mechanisms of PPAR γ Partial Agonism. PPAR research 2015;2015(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes TS, Chalmers MJ, Novick S, Kuruvilla DS, Chang MR, Kamenecka TM, et al. Ligand and Receptor Dynamics Contribute to the Mechanism of Graded PPARγ Agonism. Structure 2012;20(1):139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler R, Mitchell SH, Tindall DJ, Young C Nonapoptotic cell death associated with S-phase arrest of prostate cancer cells via the peroxisome proliferator-activated receptor g ligand, 15-deoxy-D12, 14-prostaglandin J2. Cell Growth and Differentiation-Publication American Association for Cancer Research 2000;11(1):49–62. [PubMed] [Google Scholar]
- 38.Zhu MM, Rempel DL, Du Z, Gross ML Quantification of protein− ligand interactions by mass spectrometry, titration, and H/D exchange: PLIMSTEX. Journal of the American Chemical Society 2003;125(18):5252–5253. [DOI] [PubMed] [Google Scholar]
- 39.Zhu MM, Rempel DL, Gross ML Modeling data from titration, amide H/D exchange, and mass spectrometry to obtain protein-ligand binding constants. Journal of the American Society for Mass Spectrometry 2004;15(3):388–397. [DOI] [PubMed] [Google Scholar]
- 40.R. C. Team. R: A Language and Environment for Statistical Computing. 2016.
- 41.Gu Z, Eils R, Schlesner M Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.