Abstract
A 55-year-old male presented with abdominal pain that had begun about 5 days ago. Physical examination revealed oral aphtha, genital aphthosis, and pseudofolliculitis, and the patient was diagnosed with incomplete Behçet's disease (BD). Contrast-enhanced computed tomography (CECT) showed dilation of the superior mesenteric artery and mesenteric infiltration of inflammation, indicating vasculo-BD. The symptoms were improved by 3-day of intravenous methylprednisolone pulse therapy followed by oral prednisolone. A literature review suggested that vasculo-BD should be included as a differential diagnosis in cases with unexplained abdominal pain, arterial dilation, and mesenteric invasion, and CECT examination and steroid therapy should be considered.
Keywords: Behçet's disease, vasculo-Behçet's disease, vascular involvement, superior mesenteric artery
Introduction
Behçet's disease (BD) is a systemic, chronic, inflammatory disease that is characterized by oral/genital aphthous ulcers and vasculitis (1). Although BD exists worldwide, its incidence is highest in the Mediterranean, Middle East, and the Far East (2). The incidence of BD in Japan is 22 per 100,000 people (3). Diagnostic criteria for BD have no specific examination findings, and complete BD only comprises about 30% of BD cases, which makes diagnosis difficult (3).
The underlying cause of BD is unknown; however, infectious, autoimmune, and genetic etiologies have been considered (4). BD can affect the vascular system by causing inflammatory destruction of vessels, which can be fatal (5). The frequency of vascular involvement in BD is 7-38% (6), while arterial complications such as thrombosis, stenosis, occlusion, and aneurysm occur in only 1-7% of patients with BD (7). The arterial complications are commonly isolated, but can be multiple (8), with the abdominal aorta as the most common site (9). BD lesions of superior mesenteric artery (SMA) are very rare, but can result in arterial rupture and death (5,10). Although the general epidemiological features of vascular manifestations in patients with BD have been reported (11), the features of SMA lesions have not fully been summarized. We herein described a case of vasculo-BD with a SMA lesion, and review the relevant literature.
Case Report
A 55-year-old Japanese male was admitted to our emergency outpatient room due to fever, headache, back pain, crotch and knee joint pain, and abdominal pain that had been present for about 5 days. Although headache was recognized, meningeal irritation sign or neurological symptoms were not recognized. The abdominal pain was in the upper abdomen, and there was no nausea, vomiting, diarrhea, and/or tarry stools. The patient had previously had hyperuricemia, but had otherwise been basically healthy, expect for a vague feeling of sickness a few months ago. The patient had multiple, painful, oral aphthous ulcers (Fig. 1a). A painful ulcer was found in the right scrotum, and pseudofolliculitis was observed in the lower limbs and trunk (Fig. 1b and c).
Figure 1.
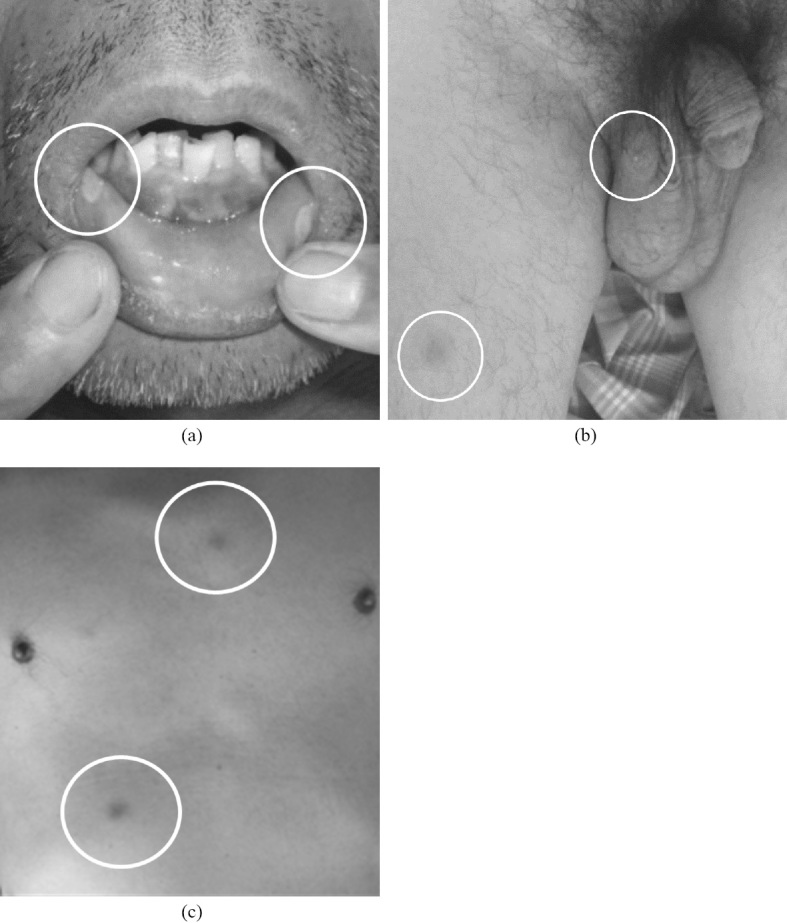
Images of a 55-year-old male with vasculo-Behçet’s disease. Multiple painful oral aphthous ulcers were observed (a). A painful ulcer was found in the right scrotum (b), and pseudofolliculitis was observed in the lower limbs and trunk (b, c).
Blood and cerebrospinal fluid test results are shown in Table 1. Blood tests on admission showed elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels, while the WBC count was within normal range. A biochemical examination showed elevation of hepatobiliary enzymes. Blood culture resulted in no bacterial growth. Viral hepatitis was not detected. Further investigations showed normal immunoglobulin levels, normal C3, C4, and CH50 levels, and negative results for antinuclear antibodies. The patient tested positive for human leukocyte antigen B51, and negative for A26. Cerebrospinal pressure was slightly elevated. No increase in cell count was observed. Cerebrospinal fluid culture was negative. No increase in the concentration of IL-6 in the spinal fluid was observed. No abnormalities were recognized in a chest radiography, electrocardiography, and/or echocardiography. No abnormalities were recognized in head computed tomography (CT) and magnetic resonance imaging. Contrasted-enhancement CT (CECT) showed dilation of the SMA and its jejunal branch, and mesenteric infiltration of inflammation (Fig. 2a and b). Deep venous thrombosis was not observed in lower limb venous echo examination. A clinical diagnosis of BD was made in accordance with the International Criteria for BD, due to the history of genital aphthosis, oral aphthosis, and skin lesions (pseudofolliculitis) (1). A skin biopsy also revealed histological findings of necrotizing vasculitis. Dilatation of the SMA and its jejunal branch led to a diagnosis of vasculo-BD (10).
Table 1.
Laboratory Findings in a 55-year-old Male with Vasculo- Behçet’s Disease.
| Complete blood count | Immunology | ||||||||||||
| WBC | 8,700 | /μL | ALT | 60 | IU/L | IgG | 776 | mg/dL | |||||
| Neu | 7,400 | /μL | LDH | 209 | IU/L | IgA | 130 | mg/dL | |||||
| Lym | 570 | /μL | ALP | 601 | IU/L | IgM | 46 | mg/dL | |||||
| Mono | 680 | /μL | γGT | 421 | IU/L | C3 | 209 | mg/dL | |||||
| Eo | 10 | /μL | CRP | 23.24 | mg/dL | C4 | 44 | mg/dL | |||||
| Ba | 10 | /μL | PCT | 0.33 | ng/mL | CH50 | 84 | U/mL | |||||
| RBC | 403 | ×104/μL | ESR | 106 | mm/h | ANA | <40 | ||||||
| Hb | 13.8 | g/dL | M2 Ab | 1.7 | U/mL | ||||||||
| MCV | 97 | fL | Coagulation | ||||||||||
| Hct | 39.2 | % | PT | 12.0 | % | Human leukocyte antigen | |||||||
| Plt | 16.9 | ×104/μL | PT-INR | 1.05 | B51 | + | |||||||
| APTT | 32.9 | s | A26 | - | |||||||||
| Biochemical markers | D-dimer | 1.2 | μg/mL | ||||||||||
| TP | 6.8 | g/dL | Cerebrospinal fluid | ||||||||||
| ALB | 3.8 | g/dL | Infection | Liquor pressure | 26 | cmH2O | |||||||
| UA | 3.6 | mg/dL | HBs Ag | - | Appearance | watery fluid | |||||||
| BUN | 13.1 | mg/dL | HBs Ab | - | Cl | 120 | meq/L | ||||||
| Cr | 0.86 | mg/dL | HBc Ab | - | Protein | 67 | mg/dL | ||||||
| T-Bil | 2.5 | mg/dL | HCV Ab | - | Glucose | 59 | mg/dL | ||||||
| D-Bil | 1.5 | mg/dL | RPR | - | Cell count | 26 | /μL | ||||||
| Na | 135 | meq/L | TPHA | - | Neu : Lym | 3 : 7 | |||||||
| K | 4.2 | meq/L | IFN-γ | - | RBC | 0 | ×104/μL | ||||||
| Cl | 99 | meq/L | Blood culture | - | IL-6 | 3.8 | pg/mL | ||||||
| PG | 81 | mg/dL | Fluid culture | - | |||||||||
| AMY | 54 | mg/dL | Tumor markers | ||||||||||
| CPK | 79 | IU/L | Ferritin | 526.9 | ng/mL | ||||||||
| AST | 60 | IU/L | sIL-2R | 681 | U/mL | ||||||||
Figure 2.
Contrast-enhanced computed tomography images showing dilation of the superior mesenteric artery and its jejunal branch (↓), and mesenteric infiltration of inflammation (○) (a, b). After treatment, these lesions were improved (c).
After confirmation of negative results of an interferon-ganmma release assay and hepatitis B antigen and antibodies, the patient was initially started on 1 g per day of intravenous methylprednisolone for 3 days, followed by 60 mg per day of oral prednisone. After starting treatment, the symptoms improved. The prednisone dose was able to be gradually decreased to less than 20 mg per day. There was remission of the oral aphthosis, painful ulcer in the right scrotum, and pseudofolliculitis. CRP and ESR levels were within normal ranges. The dilation of the SMA and its jejunal branch was improved (Fig. 2c). The patient was discharged in good condition after approximately 1 month.
Literature review
A search of the PubMed database was performed using the terms “Behçet's disease” AND “superior mesenteric artery” for all articles published up to January 2018; this search retrieved 15 articles. Table 2 summarized the characteristics of previous patients diagnosed with vasculo-BD involving the SMA; the average age was 35 years (range, 23-50 years), and there were 14 males (12-14,16-26) and one female (15). The complete type of BD was seen in one case (13). Three patients had ocular lesions (12,13,26), five had genital aphthosis (13-17), 11 had oral ulcers (12-18,21,24-26), and five had skin lesions (13,15,16,24,26). There were no articles reporting neurological findings in a patient with BD. A positive pathergy test was reported in three cases (16,17,25). Abdominal pain was initial chief complaint for most patients with BD (approximately 80%) (13,15-17,19,21-25).
Table 2.
Clinical Diagnosis and Treatment of Behçet’s Disease with Superior Mesenteric Artery Vasculitis.
| No | References | Age | Sex | Previous diagnosis of BD | Time to diagnosis of vascular BD | Diagnostic method | Vascular manifestations in SMA | Other affected vascular areas |
|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 43 | M | + | 9 | Angiography | Thrombosis | Aneurysms of the left anterior communicating artery and celiac trunk Thrombosis of subclavian arteries and inferior mesenteric arteries |
| 2 | 13 | 37 | M | - | 0 | Angiography | Aneurysm | Aneurysm of right renal artery |
| 3 | 14 | 23 | M | - | 0 | US, angiography | Aneurysms | Aneurysm of inferior mesenteric artery |
| 4 | 15 | 26 | F | - | 0 | Angiography | Thrombosis | NR |
| 5 | 16 | 41 | M | + | 15 | Surgical exploration | Thrombosis | NR |
| 6 | 17 | 32 | M | - | 0 | US, CECT | Aneurysm | NR |
| 7 | 18 | 24 | M | + | 4 | Angiography | Aneurysm | Aneurysms of superior branch of right renal and celiac artery Thrombosis of light anterior tibial and right popliteal artery |
| 8 | 19 | 39 | M | + | 5 | US, CECT | Aneurysm | Aneurysms of left renal, left brachial, and anterior left anterior descending coronary arteries DVT of lower extremities |
| 9 | 20 | 26 | M | + | 2 | ND | Aneurysm | Aneurysms of left radial and left intracranial internal carotid arteries |
| 10 | 21 | 50 | M | - | -4 | CT | Aneurysm | Aneurysms of thoracic, abdominal, and right common iliac arteries |
| 11 | 22 | 40 | M | + | 9 | CECT | Dissection | NR |
| 12 | 23 | 44 | M | + | 6 | US, CECT | Aneurysm | NR |
| 13 | 24 | 35 | M | - | 0 | CECT | Aneurysm and Thrombosis | Aneurysms of jejunum, splenic and renal arteries |
| 14 | 25 | 25 | M | - | 0 | US, CECT | Aneurysm | Aneurysm of iliac artery |
| 15 | 26 | 30 | M | + | ND | CT | Aneurysm | Aneurysms of internal carotid, coronary, celiac trunk, renal, splenic, and iliac arteries |
BD: Behçet's disease, SMA: superior mesenteric artery, NR: not reported, US: ultrasonography, CT: computed tomography, CECT: contrast-enhanced CT, DVT: deep vein thrombosis, TCAE: transcatheter arterial embolization
The treatment and clinical outcome data from patients diagnosed with vasuculo-BD are summarizes in Table 3. Six patients were first diagnosed with BD at the time of the onset of vasculo-BD (13-15,17,24,25). The duration of the period from the diagnosis of BD to the onset of vasculo-BD in the SMA was 2-15 years (12,16,18-20,22,23). One case had an aneurysm in the SMA 4 years before the diagnosis of BD (21). Diagnostic methods were CT or CECT in eight patients (17,19,21-26), and angiography in five (12-15,18). Ultrasonography was used for the initial evaluation in five patients (14,17,19,23,25). Vascular pathologies included aneurysmal changes in 11 cases (13,14,17-21,23-26), thrombosis in four (12,13,16,24), and dissection in one (22). Five patients solely had a lesion in the SMA (15-17,22,23), while 10 had lesions in multiple arteries (12-14,18-21,24-26). Treatment was steroid therapy in seven cases (12,13,15,17,18,23,24), laparotomy in six (13,16,17,23-25), bypass surgery in two (20,21), and endovascular treatment in one (19). Two cases were improved with immunosuppressant therapy alone (15,18). In one case, steroid treatment alone resulted in worsening of symptoms, and so surgery was performed (13). Immunosuppressive drugs were used after laparotomy in two cases (17,24).
Table 3.
Treatment and Clinical Outcome of Data from Patients with Behçet’s Disease with Superior Mesenteric Artery Vasculitis.
| No | References | Treatment | Outcome |
|---|---|---|---|
| 1 | 12 | Prednisolone | NR |
| 2 | 13 | Prednisolone Laparotomy |
Symptoms worsened with prednisolone monotherapy, and continued laparotomy was successfully performed. |
| 3 | 14 | NR | NR |
| 4 | 15 | Prednisolone and antiplatelet agents | Corticosteroid and antiplatelet treatment was successful. |
| 5 | 16 | Laparotomy | Laparotomy was successfully performed. 3 year after surgical intervention, the preserved parts of the SMA were radiologically normal. |
| 6 | 17 | Laparotomy Prednisolone and antiplatelet agents |
Laparotomy was successfully performed, and corticosteroid and antiplatelet were prescribed. The patient was in good health at 1 year follow up. |
| 7 | 18 | Prednisolone | Prednisolone treatment was successfully performed. |
| 8 | 19 | TCAE | Endovascular treatment was successfully performed. |
| 9 | 20 | Bypass surgery | The bypass graft between the SMA and right iliac artery was successfully performed. After 2 years, the aneurysm of intracranial internal carotid artery was diagnosed and endovascular embolization was successfully performed. |
| 10 | 21 | Bypass surgery | The aneurysm of SMA was resection and bypass surgery through left radial artery was successful. After 4 years, the size of aneurysm of thoracic and abdominal increased, and stent graft treatment was successfully performed. |
| 11 | 22 | NR | NR |
| 12 | 23 | Laparotomy Prednisolone |
Laparotomy was performed successfully, and corticosteroid was prescribed. The patient was in good health at six months follow up. |
| 13 | 24 | Laparotomy and anti-inflammatory medications (Prednisolone, Tripterygium wilfordii and thalidomide) | The patient was in good health at eight months follow up. |
| 14 | 25 | Laparotomy | Laparotomy was successfully performed. |
| 15 | 26 | NR | NR |
NR: not reported, TCAE: transcatheter arterial embolization, SMA: superior mesenteric artery
The literature review revealed that vasuculo-BD in the SMA can occur in both the chronic phase and the acute phase of BD. Multiple vascular lesions were more common than single lesions. CECT seems to be useful for whole-body blood vessel evaluation. Early initiation of steroid therapy is indicated if the situation does not require surgical treatment.
Discussion
We reported a case of a patient with vasculo-BD who experienced abnormal pain due to a lesion in the SMA (jejunal branch), and mesenteric infiltration of inflammation. The present patient experienced unexplained pain without a previous history of BD. When there is unexplained abdominal pain in patients with BD, vasculo-BD should be considered as a differential diagnosis. Taken together with the previous literature, we consider that CECT is useful for the diagnosis of vasculo-BD, and rapid initiation of treatment with methylprednisolone pulse therapy is useful.
Our literature review revealed that vasculo-BD in the SMA was more common in males than females, which is consistent with a prior report (11). Vascular involvements are thought to develop in the chronic phase after the diagnosis of BD (11). However, our literature review revealed that there were six cases with an acute onset of vasculo-BD in the SMA. The present case showed such an acute onset. This may be related to the fact that many patients with BD show an incomplete type of BD (3), and vasculo-BD can be the first manifestation of BD.
In the present case, CECT revealed the SMA lesion. Diagnostic modalities used to investigate vascular involvement in patients with BD include ultrasonography, CT, CECT, and angiography. Although abdominal ultrasonography combining color and pulse Doppler can be performed initially, this method is unsuitable for evaluation of the intraperitoneal cavity, and so evaluation of the SMA is difficult. Angiography seems to be the best method for the diagnosis of aneurysms and pseudoaneurysms; however, the risk of new aneurysm formation is high in BD (27). CECT is suitable, as it can easily detect vascular abnormality and search for multiple lesions. CECT is used in many medical facilities, as it has the advantages of cost effectiveness and safety (5). Therefore, even though extensive methods like digital subtraction angiography and magnetic resonance angiography are usable, CECT is useful for the diagnosis of vasculo-BD in the SMA.
The main therapeutic aim in vasculo-BD is to avoid arterial rupture (28). In the acute phase of aneurysm, high-dose prednisolone (1 mg per kg per day) or intravenous methylprednisolone pulse (1 g per day for 3 days) are recommended, and furthermore, the combined use of cyclophosphamide, methotrexate, azathioprine, cyclosporine A is positively considered (10). In addition, effective cases of infliximab, a biological agent, have also been reported (29), so considering combination therapy. Although early prednisone therapy was successful in the present case, prednisone therapy alone is more likely to result in recurrence of vasculo-BD than combined treatment with methotrexate or other immunosuppressive agents. Although HLA-B51 was positive in this case, the patient did not meet the diagnosis criteria of acute type neuro-BD (without neurological symptoms) (30). As cyclosporine A induces neuro-BD, it is not used when neuro-BD is suspected (31). The traditional treatment in vasculo-BD is also surgical treatment, but complications include occlusion of transplantation and formation of anastomotic pseudoaneurysm (32).
As in the present case, early-stage steroid therapy without other immunosuppressants or biologic agents can be successful when vasculo-BD is diagnosed early. A careful follow-up is necessary to diagnosis the recurrence of vasculo-BD or onset of neuro-BD. If possible, steroid therapy should be considered for vasculo-BD in the SMA.
Conclusion
We described a patient with abdominal pain who was diagnosed with BD and vasculo-BD at the same time. Vasculo-BD should be considered in patients with BD who experience unexplained severe abdominal pain, and CECT examination and steroid therapy should be considered.
The authors state that they have no Conflict of Interest (COI).
References
- 1. International Team for the Revision of the International Criteria for Behçet's Disease (ITR-ICBD). The International Criteria for Behçet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 28: 338-347, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Davatchi F, Chams-Davatchi C, Shams H, et al. Behcet's disease: epidemiology, clinical manifestations, and diagnosis. Expert Rev Clin Immunol 13: 57-65, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Suzuki Kurokawa M, Suzuki N. Behcet's disease. Clin Exp Med 4: 10-20, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Mendoza-Pinto C, García-Carrasco M, Jiménez-Hernández M, et al. Etiopathogenesis of Behcet's disease. Autoimmun Rev 9: 241-245, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Owlia MB, Mehrpoor G. Behcet's disease: new concepts in cardiovascular involvements and future direction for treatment. ISRN Pharmacol 760484, 2012(Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakane T, Takeno M, Suzuki N, Inaba G. Behçet's disease. N Engl J Med 341: 1284-1291, 1999. [DOI] [PubMed] [Google Scholar]
- 7. Jayachandran NV, Rajasekhar L, Chandrasekhara PK, Kanchinadham S, Narsimulu G. Multiple peripheral arterial and aortic aneurysms in Behcet's syndrome: a case report. Clin Rheumatol 27: 265-267, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Calamia KT, Schirmer M, Melikoglu M. Major vessel involvement in Behçet disease. Curr Opin Rheumatol 17: 1-8, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Kojima N, Sakano Y, Ohki S, Misawa Y. Rapidly growing aortic arch aneurysm in Behcet's disease. Interact Cardiovasc Thorac Surg 12: 502-504, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Merashli M, Eid RE, Uthman I. A review of current management of vasculo-Behcet's. Curr Opin Rheumatol 30: 50-56, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Ideguchi H, Suda A, Takeno M, Ueda A, Ohno S, Ishigatsubo Y. Behçet disease: evolution of clinical manifestations. Medicine (Baltimore) 90: 125-132, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Hassen Khodja R, Declemy S, Batt M, Daune B, Avril G, Le Bas P. Behçet's disease with multiple arterial lesions and voluminous hemangioma of the brain. J Mal Vasc 16: 383-386, 1991. [PubMed] [Google Scholar]
- 13. Chubachi A, Saitoh K, Imai H, et al. Case report: intestinal infarction after an aneurysmal occlusion of superior mesenteric artery in a patient with Behçet's disease. Am J Med Sci 306: 376-378, 1993. [DOI] [PubMed] [Google Scholar]
- 14. Men S, Ozmen MN, Balkanci F, Boyacigil S, Akbari H. Superior mesenteric artery aneurysm in Behçet's disease. Abdom Imaging 19: 333-334, 1994. [DOI] [PubMed] [Google Scholar]
- 15. Mercié P, Constans J, Tissot B, et al. Thrombosis of the superior mesenteric artery and Behçet's syndrome. Rev Med Interne 17: 470-473, 1996. [DOI] [PubMed] [Google Scholar]
- 16. Bayraktar Y, Soylu AR, Balkanci F, Gedikoğlu G, Cakmakçi M, Sayek I. Arterial thrombosis leading to intestinal infarction in a patient with Behçet's disease associated with protein C deficiency. Am J Gastroenterol 93: 2556-2558, 1998. [DOI] [PubMed] [Google Scholar]
- 17. Hafsa C, Kriaa S, Zbidi M, et al. Superior mesenteric artery aneurysm revealing a Behçet disease: a case report. Ann Cardiol Angeiol (Paris) 55: 291-293, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Yokota K, Akiyama Y, Sato K, et al. Vasculo-Behçet's disease with non-traumatic subcapsular hematoma of the kidney and aneurysmal dilatations of the celiac and superior mesenteric arteries. Mod Rheumatol 18: 615-618, 2008. [DOI] [PubMed] [Google Scholar]
- 19. Güven K, Rozanes I, Kayabali M, Minareci O. Endovascular treatment of a superior mesenteric artery aneurysm secondary to Behcet's disease with Onyx (ethylene vinyl alcohol copolymer). Cardiovasc Intervent Radiol 32: 159-162, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Ozveren MF, Matsumoto Y, Kondo R, Takahashi A. Coil embolization of an unruptured intracranial aneurysm associated with Behcet's disease: case report. Neurol Med Chir (Tokyo) 49: 471-473, 2009. [DOI] [PubMed] [Google Scholar]
- 21. Saiki M, Nakamura Y, Fujiwara Y, et al. Single-stage endovascular treatment performed on multiple aortic aneurysms in a patient with Behçet's disease-report of a case. Ann Vasc Dis 6: 734-737, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ogul H, Pirimoglu B, Colak A, Kantarci M. Dissection of superior mesenteric artery associated with Behcet's disease. Joint Bone Spine 81: 450, 2014. [DOI] [PubMed] [Google Scholar]
- 23. Tijani Y, Chtata H, Elkaoui H, et al. The aneurysms of digestive system arteries: three cases. Ann Cardiol Angeiol (Paris) 64: 109-112, 2015. [DOI] [PubMed] [Google Scholar]
- 24. Wu XY, Wei JP, Zhao XY, et al. Spontaneous intra-abdominal hemorrhage due to rupture of jejunal artery aneurysm in Behcet disease: case report and literature review. Medicine (Baltimore) 94: e1979, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bibiche Y, Kanjaa N. Aneurysm of the superior mesenteric artery revealing Behçet's disease: report of a case. Pan Afr Med J 20: 312, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen JY, Tsai YS, Li YH. Multiple arterial aneurysms in a patient with Behçet's disease. Eur Heart J Cardiovasc Imaging 17: 587, 2016. [DOI] [PubMed] [Google Scholar]
- 27. Ozeren M, Mavioglu I, Dogan OV, Yucel E. Reoperation results of arterial involvement in Behçet's disease. Eur J Vasc Endovasc Surg 20: 512-519, 2000. [DOI] [PubMed] [Google Scholar]
- 28. Hatemi G, Silman A, Bang D, et al. Management of Behçet disease: a systematic literature review for the European League Against Rheumatism evidence-based recommendations for the management of Behçet disease. Ann Rheum Dis 68: 1528-1534, 2009. [DOI] [PubMed] [Google Scholar]
- 29. Hibi T, Hirohata S, Kikuchi H, et al. Infliximab therapy for intestinal, neurological, and vascular involvement in Behcet disease: efficacy, safety, and pharmacokinetics in a multicenter, prospective, open-label, single-arm phase 3 study. Medicine (Baltimore) 95: e3863, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hirohata S, Kikuchi H, Sawada T, et al. Clinical characteristics of neuro-Behcet's disease in Japan: a multicenter retrospective analysis. Mod Rheumatol 22: 405-413, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hirohata S, Kikuchi H, Sawada T, et al. Analysis of various factors on the relapse of acute neurological attacks in Behçet's disease. Mod Rheumatol 24: 961-965, 2014. [DOI] [PubMed] [Google Scholar]
- 32. Li S, Chen AJ, Huang K, Li H. Successful treatment of vasculo-Behcet's disease presenting as recurrent pseudoaneurysms: the importance of medical treatment. Dermatol Ther (Heidelb) 3: 107-112, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]



