Abstract
Background
Predicting treatment response to chemo-radiotherapy (CRT) in esophageal cancer remains an unrealized goal despite studies linking constellations of genes to prognosis. We aimed to determine if specific expression profiles are associated with pathologic complete response (pCR) after neoadjuvant CRT.
Methods
Eleven genes previously associated with esopprognosishageal cancer prognosis were identified. Esophageal adenocarcinoma (EAC) patients treated with neoadjuvant CRT and esophagectomy were included. Patients were classified into two groups: pCR and no-or-incomplete response (NR). Polymerase chain reaction was used to evaluate gene expression. Omnibus testing was applied to overall gene expression differences between groups, and log-rank tests compared individual genes.
Results
Eleven pCR and eighteen NR patients were analyzed. Combined expression profiles were significantly different between pCR and NR groups (p<0.01). The gene CCL28 was over-expressed in pCR patients (Log-HR: 1.53, 95%CI: 0.46–2.59, p=0.005), and DKK3 was under-expressed in pCR (Log-HR: −1.03 95%CI: −1.97, −0.10, p=0.031).
Conclusion
EAC tumors that demonstrated a pCR have genetic profiles that are significantly different from typical NR profiles. The genes CCL28 and DKK3 are potential predictors of treatment response.
Keywords: Esophageal cancer, biomarker, pathologic complete response, neoadjuvant therapy, gene expression
Introduction
Even with optimal treatment, the median 5-year survival for patients diagnosed with esophageal cancer remains less than 50%.(1) Current multimodality regimens often carry significant risk despite advancements in therapy. It is estimated that 3% of patients treated with neoadjuvant chemoradiotherapy (CRT) will die before surgery, and 37% will suffer grade 3 or worse toxicity when undergoing treatment.(2) Subsequent esophagectomy has an operative mortality rate ranging from 3 to 12% and up to 50% morbidity.(3–6) Given these data, it is clear that any improvements in current treatment regimens would be welcomed in the face of an alarming increase in the incidence of esophageal adenocarcinoma (EAC) in the United States (7, 8) .
Clinical trials have demonstrated that CRT followed by esophagectomy has a survival benefit compared to surgery alone. This has led to the adoption of trimodality therapy as standard treatment for loco-regional disease.(9) Unfortunately, individual treatment response to neoadjuvant therapy is highly variable. Between 25–30% of esophageal cancer patients will have no residual malignant cells present on final pathologic exam, termed a pathologic complete response (pCR).(9, 10) More importantly, it has been demonstrated that achieving a pCR to neoadjuvant therapy portends significantly improved survival.(11) Thus, novel treatments targeted at improving pCR rate have the potential to significantly improve outcomes.
Many studies have attempted to identify histopathologic markers associated with pCR, but the data regarding such markers has been inconclusive.(12–14) A possible explanation for the heterogeneity in treatment response may be explained by differing gene expression profiles of the individual tumors. Evaluating tumor genetics has proven useful in other cancers to assess likelihood of treatment response.(15) These technologies are now being used clinically to individualize treatment plans, and provide patients and clinicians with prognostic information. Similar approaches to esophageal cancer have not yet been proven to provide consistent and definite predictive power in the clinical setting. Prognosis prediction in EAC remains an unrealized goal despite rapid growth in the fields of genomics and proteomics. Our hope in identifying clear prognostic indicators is that clinicians can reliably predict treatment response, then individualized treatment plans can be developed to minimize adverse effects while providing the best possible chance at a cure. The aim of this study was to evaluate existing candidate genes using our EAC tissue repository for association with a favorable treatment response in the context of current standard of care neoadjuvant CRT.
Methods
Patient Selection
This study was approved by the Oregon Health and Science University (OHSU) IRB (#1759). The OHSU Esophageal Cancer and Related Diseases database (ECRD) is a prospectively maintained registry of clinical and pathologic data on all patients treated with esophageal cancer at our institution. We identified patients from the ECRD with the diagnosis of EAC treated with neoadjuvant CRT and esophagectomy between January 2011 and July 2015. Given our focus on EAC, patients with squamous cell carcinoma (SCC) histology were excluded. EAC patients with pretreatment formalin-fixed, paraffin-embedded (FFPE) esophageal biopsies in our tissue repository were included. Medical records were reviewed for pathologic classification based on final surgical pathology as evaluated an expert pathologist within the OHSU department of surgical pathology. A pCR was defined as no evidence of tumor on final pathologic exam in the primary specimen or associated lymph nodes. Any evidence of residual tumor cells was classified as non-or-incomplete responder (NR), regardless of down staging or degree of primary tumor reduction.
Gene Selection, RNA Isolation, and qPCR
Candidate genes for investigation were identified based on previous publications and our own preliminary studies. Eleven genes were selected for investigation in our cohort due to their association with esophageal cancer prognosis.(16–21) The target genes investigated were CCL28(20), SPARC(18), S100A2(19), SPRR3(19), SIRT2(21), NOV(20), PERP(19), PAPSS2(21), DCK(21), DKK3(22), ALDH1(16). The gene ACTB was selected as the endogenous control based on published work.(17)
Two ten-micron sections were taken from each specimen block and RNA was isolated from FFPE tissue using the RNeasy Mini Kit FFPE (Qiagen). Following RNA isolation, RNA quality assessment was performed using the Agilent 2100 Bioanalyzer with a Eukaryote total RNA Nano chip. Reverse transcription was performed using the SuperScript VILO cDNA synthesis kit (Life Technologies) with 100ng of input RNA per 20μl reaction. Following reverse transcription, 200ng of cDNA was used in a pre-amplification reaction using the TaqMan pre-amplification master mix (Life Technologies) which included a pool of all 12 primer/probe sets at a 0.2X concentration. The qPCR assays were performed on the QuantStudio RealTime PCR System (Life Technologies) using TaqMan probes for the eleven target genes and ACTB. Data was collected using Applied Biosystems QuantStudio™ 12K Flex Software v1.0.
Statistical Analysis of Gene Expression
Cycle threshold (Ct) values were used as a measure of gene expression level, with lower values representing higher levels of gene expression. Individual sample variation in Ct values for the control, ACTB, was normalized using regression models to adjust for mean expression across all genes. The normalized Ct values of the target genes were interpreted as expression relative to ACTB. The gene expression profiles of pCR versus NR groups were then compared in order to identify differential expression patterns between groups. An omnibus test using Mahalanobis distance from the multigene centroid of the NR group was applied to evaluate overall gene expression differences between pCR and NR. Log-rank tests were applied to compare the differential expression of individual genes between groups, and comparisons made based on hazard ratios. Statistical significance was set at a p-value of 0.05. Statistical analysis was carried out using STATA statistical software (Version 14, College Station, TX, USA).
Results
From January 2011 to July 2015, 29 patients had pre-treatment biopsy specimens available within our FFPE tissue bank for inclusion in the study. The average age of the patient population was 67.7 years and ranged from 56 to 79 years. There was only one female (3%) in the cohort, and the entire sample population was Caucasian. Clinical stage of disease ranged from IB to IIIB. Of the 29 patients, eleven (38%) were identified as having a pCR to neoadjuvant therapy, and eighteen (62%) were classified as NR with residual tumor on final pathology. There were no statistically significant differences between groups with respect to age at diagnosis, race, gender, clinical stage, or chemotherapy regimen. Baseline characteristics are shown in Table 1. All pCR specimens had significantly different combined genetic profiles versus the prototypical NR, comparing the combined genetic profiles between groups (Figure 1). This is demonstrated by the fact that all specimens from patients with a pCR have a Mahalanobis distance greater than the 99th percentile of the estimated distance distribution of the NR samples. Expression levels between groups for each individual gene is shown in Figure 2. CCL28 was over-expressed in pCR (Log-HR: 1.53, 95%CI: 0.46–2.59, p=0.005). Expression of CCL28 was increased by a factor of 2.28 in pCR specimens. Conversely, DKK3 was under-expressed in pCR tumors (Log-HR: −1.03 95%CI: −1.97, −0.10, p=0.031). Expression of DKK3 in pCR specimens was 0.85 times that of the NR group. None of the other nine target genes demonstrated statistically significant differences between groups (Table 2).
Table 1.
Baseline Patient Demographics for all patients in our study.
| No or Incomplete Response (NR) n=18 |
Pathologic Complete Response (pCR) n=11 |
Combined n=29 |
P-value | |
|---|---|---|---|---|
|
| ||||
| Age, years (std dev) | 67.1(±7.7) | 68.1 (±4.8) | 67.7 (±5.9) | 0.67a |
|
| ||||
| Gender | ||||
| M | 18 (100) | 10 (91) | 28 (97) | 0.38b |
| F | 0 (0) | 1 (9) | 1 (3) | |
|
| ||||
| Clinical Stage, N(%) | ||||
| IB | 0 (0) | 1 (9) | 1 (3) | 0.74b |
| IIA | 2 (11) | 0 (0) | 2 (7) | |
| IIB | 7 (39) | 5 (45) | 12 (41) | |
| IIIA | 5 (28) | 3 (27) | 8 (28) | |
| IIIB | 4 (22) | 2 (18) | 6 (21) | |
|
| ||||
| Chemotherapy Regimen | ||||
| Carboplatin/Paclitaxel | 17 (94) | 10 (91) | 27 (93) | 0.62b |
| Carboplatin/Paclitaxel/5-FU | 0 (0) | 1 (9) | 1 (3) | |
| Cisplatinum/5-FU | 1 (6) | 0 (0) | 1 (3) | |
Students T-test,
Fisher’s Exact Test
Figure 1. Overall genetic variation of pCR samples compared to NR samples.
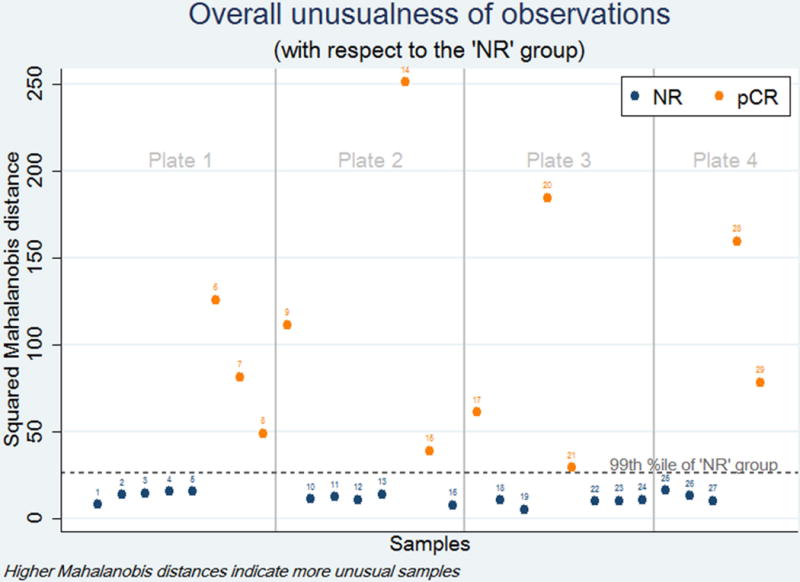
Omnibus testing using Mahalanobis distance demonstrates that the overall genetic variation between all pCR patients is significantly different than NR patients (p<0.01).
Figure 2. Comparison of mean expression level of each individual gene between pCR and NR groups.
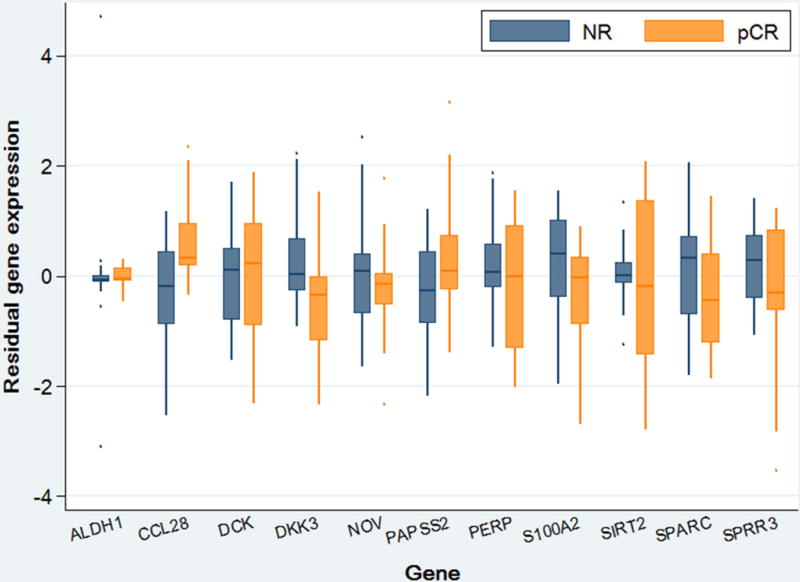
Box plot depicts mean of gene expression levels and interquartile range of each gene-responder group combination relative to the endogenous control (ACTB).
Table 2.
Log-hazard ratio and fold change of individual gene expression levels of pCR versus NR.
| Gene | Log hazard ratio (95% CI) | p-valuea | Fold Change (95% CI) pCR:NR |
|---|---|---|---|
| ACTB | 0 (reference gene) | 1.000 | 1.00 (reference gene) |
| ALDH1 | −0.35 (−2.63, 1.94) | 0.766 | 2.33 (0.77, 7.07) |
| CCL28 | 1.53 (0.46, 2.59) | 0.005 | 2.28 (1.12, 4.66) |
| DCK | 0.06 (−0.85, 0.97) | 0.895 | 1.75 (0.92, 3.31) |
| DKK3 | −1.03 (−1.97, −0.10) | 0.031 | 0.85 (0.50, 1.45) |
| NOV | −0.27 (−1.34, 0.79) | 0.615 | 1.43 (0.50, 4.12) |
| PAPSS2 | 0.55 (−0.42, 1.53) | 0.263 | 1.37 (0.83, 2.28) |
| PERP | −0.80 (−1.66, 0.06) | 0.067 | 0.52 (0.29, 0.94) |
| S100A2 | −0.66 (−1.67, 0.35) | 0.203 | 0.36 (0.10, 1.28) |
| SIRT2 | −0.63 (−1.63, 0.36) | 0.211 | 1.23 (0.79, 1.94) |
| SPARC | −0.54 (−1.45, 0.36) | 0.241 | 0.80 (0.44, 1.45) |
| SPRR3 | −0.00 (−0.91, 0.90) | 0.995 | 0.44 (0.08, 2.45) |
Log-rank test
Discussion
Outcomes in patients treated with multimodality treatment for EAC are highly variable. Even when matched for stage and demographics, patients receiving the same treatment can have vastly different outcomes. Early identification of treatment response group could aid in determining the best treatment plans in order to avoid over- or undertreating. Unfortunately, there is currently no method to reliably predict which patients will manifest a pCR prior to undergoing an esophagectomy. Because of the difficulty in identifying pCR prior to surgery, esophagectomy is recommended for all patients. In certain high risk surgical patients who demonstrate a clinical complete response, predictive biomarkers could aid in the decision to pursue watchful waiting. Conversely, patients with a low likelihood of pCR could be started on alternative neoadjuvant regimens or proceed directly to surgery.
Predictive gene assays are currently used in other solid tumors to determine prognosis and predict treatment response.(15, 23) Most notably, the field of breast oncology has adopted these techniques leading to a number of commercially available assays that are now widely used in clinical practice for designing personalized treatment plans for breast cancers.18 These concepts are now being applied to many other solid tumors like colon and prostate.(24, 25) Since no similar gene assays exist for esophageal cancers, we wanted to investigate whether gene expression could aid in identifying EAC patients who will respond favorably to current neoadjuvant therapy. From our analysis of eleven candidate genes, we found that two genes demonstrated differential expression between pCR patients and incomplete or non-responders.
CCL28 encodes for a mucosae associated epithelial chemokine that is involved in regulating inflammation and immune response and was over expressed in pCR patients.(26) It is recognized that tumor microenvironment and systemic inflammation play an important role in tumor behavior. Accordingly, CCL28 seems like a rational candidate gene for our investigations.(26, 27) An association between CCL28 and pCR in esophageal cancer was first shown in a 2009 study, that carried out microarray analyses of over 27,000 individual human genes on 27 patients with both EAC and SCC of the esophagus. (20) From the microarray analysis the authors able to create a panel of 5 genes, including CCL28, which could predict response to therapy with 95% accuracy in 74% of their patients.(20) Interestingly, they demonstrated that CCL28 was downregulated in their responder group, and this is opposite to our findings in this study. One possible explanation for these differences is the heterogeneity in the pathology found in the 2009 study which included both SCC and EAC tumors. It is well known that EAC and SCC respond differently to neoadjuvant therapy. For example the pCR rate for SCC tumors has been as high as 49% compared to 23% for EAC.(9) Overexpression of CCL28 has been shown to be associated with tumor progression in other studies of SCC, (26) but to our knowledge, this gene has not been studied exclusively in EAC tumors. In addition, all patients in the 2009 study received the older chemotherapy regimen of Cisplatinum and 5-FU, (20) while the vast majority of patients in our study received Carboplatin and Paclitaxel based on the results of the 2012 CROSS trial.(9) The different chemotherapy regimens could be a potential source for the differences in the CCL28 gene expression profiles between the two studies. Our cohort consisted of only EAC patients who were treated with contemporary CRT regimens, which may account for these observed differences, but further study of CCL28 in EAC patients should be undertaken to understand the specific role this gene plays in treatment response.
The gene DKK3 was under-expressed in our pCR tumor cohort. DKK3 is thought to act as a tumor suppressor in many cancer cell lines. Interestingly overexpression of DKK3 is seen in some other malignancies such as hepatocellular carcinoma.(22) Previous studies involving DKK3 and esophageal cancer have demonstrated similar overexpression in EAC cell lines.(22) DKK3 transfected cell lines demonstrated increased angiogenesis, invasion, and proliferation.(22) Most importantly, DKK3 transfected cell lines were significantly more chemo-resistant.(22) Moreover, the authors demonstrated DKK3 transfected cell lines over-expressed the Transforming Growth Factor-β (TGFβ) pathway, which has been associated with metastasis and invasion in many advanced cancers.(28, 29) The previous studies of DKK3 support the view that overexpression is associated with worse prognosis in EAC, which is consistent with our data. In our study, an unfavorable treatment response to neoadjuvant CRT was associated with higher levels of DKK3. Taken together these data suggest that DKK3 the TGFβ pathway may offer potential future targets to decrease chemo-resistance.
The relatively few genes studied and small sample size are limitations in this study. Large-scale studies of esophageal cancers are complicated by a number of issues. First, in order to evaluate tumor response to neoadjuvant therapy, researchers must obtain pre-treatment biopsy tissue. The small volume of pre-treatment tumor tissue relative to post treatment tissue can be limiting. Since a small volume of tissue taken at initial diagnostic biopsy, this tissue may be completely consumed for clinical use with little or none available for research. Secondly, esophageal cancer is a complex malignancy that often requires referral to a specialized treatment center. Initial diagnosis and subsequent treatment are often carried out at different institutions, and this further complicates attaining and storing treatment naïve tissue. Despite the sample size limitation, our data demonstrate that genetic profiling of esophageal tumors can be carried out with relative ease and our findings do point to differences between treatment response groups. We were not able to develop predictive models based on our findings since only two genes demonstrated significant differences. Validation in larger patient samples and whole-genome studies are needed to develop models that can accurately predict treatment response.
Conclusions
This study demonstrated that EAC patients with a pCR after neoadjuvant therapy have genetic profiles that are significantly different from typical NR profiles. In our population, the genes CCL28 and DKK3 are potential predictors of treatment response. In addition to predicting treatment response, these two genes offer potential targets for alternate systemic therapies that could potentially improve treatment of EAC. Large-scale whole-genome studies with rigorous validation are needed in order to create ideal predictive genetic assays for clinical use to offer an improved standard of care in EAC.
Acknowledgments
This research was made possible by funding provided by the Michael J. Newton Esophageal Cancer Foundation (McLaren), the Oregon Clinical and Translational Research Institute (OCTRI), Knight Cancer Institute Clinical Scholar Award (Vaccaro) and a grant (no. UL1TR000128) from the National Center for Advancing Translational Sciences (NACATS) of the National Institutes of Health (Dolan). The authors would like to acknowledge the Oregon Health and Sciences Histopathology Shared Resource and Gene Profiling Shared Resource for their contributions to this study.
Grant Support: The funding for this research was provided by the Michael J. Newton Esophageal Cancer Foundation, Knight Cancer Institute Clinical Scholar Award, and the National Center for Advancing Translational Sciences (NACATS) of the National Institutes of Health (grant no. UL1TR000128).
Footnotes
No author has any financial conflict of interest to disclose.
The first author, Dr. McLaren, and corresponding author, Dr. Dolan, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Previous Presentation: Presented at the North Pacific Surgical Association Annual Meeting November, 12, 2016.
References
- 1.Holscher AH, Drebber U, Schmidt H, Bollschweiler E. Prognostic classification of histopathologic response to neoadjuvant therapy in esophageal adenocarcinoma. Ann Surg. 2014;260(5):779–84. doi: 10.1097/SLA.0000000000000964. discussion 84–5. [DOI] [PubMed] [Google Scholar]
- 2.Honing J, Smit JK, Muijs CT, Burgerhof JG, de Groot JW, Paardekooper G, et al. A comparison of carboplatin and paclitaxel with cisplatinum and 5-fluorouracil in definitive chemoradiation in esophageal cancer patients. Ann Oncol. 2014;25(3):638–43. doi: 10.1093/annonc/mdt589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rentz J, Bull D, Harpole D, Bailey S, Neumayer L, Pappas T, et al. Transthoracic versus transhiatal esophagectomy: a prospective study of 945 patients. J Thorac Cardiovasc Surg. 2003;125(5):1114–20. doi: 10.1067/mtc.2003.315. [DOI] [PubMed] [Google Scholar]
- 4.Bailey SH, Bull DA, Harpole DH, Rentz JJ, Neumayer LA, Pappas TN, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75(1):217–22. doi: 10.1016/s0003-4975(02)04368-0. discussion 22. [DOI] [PubMed] [Google Scholar]
- 5.McCulloch P, Ward J, Tekkis PP, surgeons Ago, British Oesophago-Gastric Cancer G Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ. 2003;327(7425):1192–7. doi: 10.1136/bmj.327.7425.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhungel B, Diggs BS, Hunter JG, Sheppard BC, Vetto JT, Dolan JP. Patient and peri-operative predictors of morbidity and mortality after esophagectomy: American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), 2005–2008. J Gastrointest Surg. 2010;14(10):1492–501. doi: 10.1007/s11605-010-1328-2. [DOI] [PubMed] [Google Scholar]
- 7.Abrams JA, Sharaiha RZ, Gonsalves L, Lightdale CJ, Neugut AI. Dating the rise of esophageal adenocarcinoma: analysis of Connecticut Tumor Registry data, 1940–2007. Cancer Epidemiol Biomarkers Prev. 2011;20(1):183–6. doi: 10.1158/1055-9965.EPI-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Pardo BJ, Bronson NW, Diggs BS, Thomas CR, Jr, Hunter JG, Dolan JP. The Global Burden of Esophageal Cancer: A Disability-Adjusted Life-Year Approach. World J Surg. 2016;40(2):395–401. doi: 10.1007/s00268-015-3356-2. [DOI] [PubMed] [Google Scholar]
- 9.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 10.Nabavizadeh N, Shukla R, Elliott DA, Mitin T, Vaccaro GM, Dolan JP, et al. Preoperative carboplatin and paclitaxel-based chemoradiotherapy for esophageal carcinoma: results of a modified CROSS regimen utilizing radiation doses greater than 41.4 Gy. Dis Esophagus. 2016;29(6):614–20. doi: 10.1111/dote.12377. [DOI] [PubMed] [Google Scholar]
- 11.Berger AC, Farma J, Scott WJ, Freedman G, Weiner L, Cheng JD, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23(19):4330–7. doi: 10.1200/JCO.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Bronson NW, Diggs BS, Bakis G, Gatter KM, Sheppard BC, Hunter JG, et al. Molecular Marker Expression Is Highly Heterogeneous in Esophageal Adenocarcinoma and Does Not Predict a Response to Neoadjuvant Therapy. J Gastrointest Surg. 2015;19(12):2105–10. doi: 10.1007/s11605-015-2944-7. [DOI] [PubMed] [Google Scholar]
- 13.Slotta-Huspenina J, Wolff C, Drecoll E, Feith M, Bettstetter M, Malinowsky K, et al. A specific expression profile of heat-shock proteins and glucose-regulated proteins is associated with response to neoadjuvant chemotherapy in oesophageal adenocarcinomas. Br J Cancer. 2013;109(2):370–8. doi: 10.1038/bjc.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang SS, Huang QY, Yang H, Xie X, Luo KJ, Wen J, et al. Correlation of p53 status with the response to chemotherapy-based treatment in esophageal cancer: a meta-analysis. Ann Surg Oncol. 2013;20(7):2419–27. doi: 10.1245/s10434-012-2859-4. [DOI] [PubMed] [Google Scholar]
- 15.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–34. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 16.Ajani JA, Wang X, Song S, Suzuki A, Taketa T, Sudo K, et al. ALDH-1 expression levels predict response or resistance to preoperative chemoradiation in resectable esophageal cancer patients. Mol Oncol. 2014;8(1):142–9. doi: 10.1016/j.molonc.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bronkhorst AJ, Aucamp J, Wentzel JF, Pretorius PJ. Reference gene selection for in vitro cell-free DNA analysis and gene expression profiling. Clin Biochem. 2016;49(7–8):606–8. doi: 10.1016/j.clinbiochem.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Kim SM, Park YY, Park ES, Cho JY, Izzo JG, Zhang D, et al. Prognostic biomarkers for esophageal adenocarcinoma identified by analysis of tumor transcriptome. PLoS One. 2010;5(11):e15074. doi: 10.1371/journal.pone.0015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luthra R, Wu TT, Luthra MG, Izzo J, Lopez-Alvarez E, Zhang L, et al. Gene expression profiling of localized esophageal carcinomas: association with pathologic response to preoperative chemoradiation. J Clin Oncol. 2006;24(2):259–67. doi: 10.1200/JCO.2005.03.3688. [DOI] [PubMed] [Google Scholar]
- 20.Maher SG, Gillham CM, Duggan SP, Smyth PC, Miller N, Muldoon C, et al. Gene expression analysis of diagnostic biopsies predicts pathological response to neoadjuvant chemoradiotherapy of esophageal cancer. Ann Surg. 2009;250(5):729–37. doi: 10.1097/SLA.0b013e3181bce7e1. [DOI] [PubMed] [Google Scholar]
- 21.Peters CJ, Rees JR, Hardwick RH, Hardwick JS, Vowler SL, Ong CA, et al. A 4-gene signature predicts survival of patients with resected adenocarcinoma of the esophagus, junction, and gastric cardia. Gastroenterology. 2010;139(6):1995–2004 e15. doi: 10.1053/j.gastro.2010.05.080. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Lin L, Thomas DG, Nadal E, Chang AC, Beer DG, et al. The role of Dickkopf-3 overexpression in esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2015;150(2):377–85 e2. doi: 10.1016/j.jtcvs.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markopoulos C, van de Velde C, Zarca D, Ozmen V, Masetti R. Clinical evidence supporting genomic tests in early breast cancer: Do all genomic tests provide the same information? Eur J Surg Oncol. 2016 doi: 10.1016/j.ejso.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Klein EA, Cooperberg MR, Magi-Galluzzi C, Simko JP, Falzarano SM, Maddala T, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol. 2014;66(3):550–60. doi: 10.1016/j.eururo.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 25.You YN, Rustin RB, Sullivan JD. Oncotype DX((R)) colon cancer assay for prediction of recurrence risk in patients with stage II and III colon cancer: A review of the evidence. Surg Oncol. 2015;24(2):61–6. doi: 10.1016/j.suronc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Zou HY, Lv GQ, Dai LH, Zhan XH, Jiao JW, Liao LD, et al. A truncated splice variant of human lysyl oxidase-like 2 promotes migration and invasion in esophageal squamous cell carcinoma. Int J Biochem Cell Biol. 2016;75:85–98. doi: 10.1016/j.biocel.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Kang Y. Pro-metastasis function of TGFbeta mediated by the Smad pathway. J Cell Biochem. 2006;98(6):1380–90. doi: 10.1002/jcb.20928. [DOI] [PubMed] [Google Scholar]
- 29.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]


