Abstract
Mice and rats are animals commonly used in research and laboratory testing. Compared with other animal species, they harbor many more zoonotic agents. Hymenolepis nana (H. nana) is a common tapeworm that parasitizes both humans and rodents. Although this tapeworm is of socio-economic importance worldwide, information related to its mitochondrial genome is limited. The present study examined the sequence diversity of two mitochondrial (mt) genes, subunit I of cytochrome oxidase (cox1) and NADH dehydrogenase subunit 5 (pnad5), of H. nana in mice and rats from two geographical regions of Saudi Arabia (Makkah and Riyadh). Partial sequences of cox1 and pnad 5 from individual H. nana isolates were separately amplified using polymerase chain reaction (PCR) and sequenced. The GC contents of the sequences ranged between 31.6–33.5% and 27.2–28.6% for cox1 and pnad5, respectively. The genomic similarity among specimens determined via cox1 primer and pnad5 primer was 97.1% and 99.7%, respectively. Based on these primers, our data did not indicate any differences between H. nana from rat and mice isolates. Results demonstrated that the present species are deeply embedded in the genus Hymenolepis with close relationship to other Hymenolepis species, including H. nana as a putative sister taxon, and that the isolates cannot be categorized as belonging to two different groups with origins in Makkah and Riyadh.
Keywords: Hymenolepis nana, Mitochondrial genome, Phylogenetic analysis
Introduction
Hymenolepis nana, a common tapeworm that is distributed worldwide, is found mostly in young children in developing countries [1]. Human hymenolepiasis, caused by H. nana and H. diminuta, is a globally prevalent zoonosis. It is endemic in Asia, Southern and Eastern Europe, Central and South America, and Africa, and produces many health problems such as headaches, weakness, anorexia, abdominal pain, and diarrhea [2]. The mature worm lives in the small intestine of humans, mice, and rats [3]. It is mostly transmitted by contamination with fecal matter containing eggs or via insect vectors acting as intermediate hosts. Hymenolepis nana, is also able to complete its entire life cycle in a single host, and is therefore capable of auto-infection [4]. Hymenolepis nana, in different rodents, such as rats and mice, is morphologically similar to human H. nana. Thus, establishing the identity of these two species is epidemiologically important [5]. Despite revised nomenclature, speciation and host specificity of H. nana continues to be problematic [6]. Hence, a biological, taxonomic and epidemiological investigation of H. nana in various hosts may be useful in order to better understand endemic strains [6].
Hymenolepidids have been categorized into several genera based on morphological characteristics [7, 8]. Mitochondrial (mt) genomes are small (usually less than 20,000 bp), circular and maternally inherited [9]. The property of having a high copy number per cell makes them attractive and more amenable targets for studies related to characterization, population genetics, and phylogenetics [10]. Mitochondrial DNA (mtDNA) sequences are reliable genetic markers that have been useful in studies on population genetics and systematics [11]. Genetic diversity of H. nana has been studied using genetic makers, such as the mt cytochrome oxidase subunit 1(cox1) and the entire first and second internal transcribed spacer (ITS-1 and ITS-2) regions of nuclear ribosomal DNA (rDNA) [6, 12, 13]. These studies indicated the presence of genetic variation in H. nana from different domestic and wildlife host species, as well as from different areas, suggesting that H. nana comprises ‘cryptic’ species, which are morphologically identical but genetically distinct. Although mitochondrial (mt) genes, such as NADH dehydrogenase subunit 5 (pnad5), small subunit ribosomal RNA (rrnS) and ATPase subunit6 (atp6), of H. nana in mice from different geographical regions of China have been studied, information on the sequence variability in other mt genes of H. nana isolates, is rare [14].
The objective of the present study was to analyze cox1 and pnad5 in H. nana isolated from naturally infected mice and rats in Makkah and Riyadh, Saudi Arabia.
This work was based on my previous study, “Gene-based molecular analysis of cox1 in Echinococcus granulosus cysts isolated from naturally infected livestock in Riyadh, Saudi Arabia,” which was a part of a major research project. This project is conducted by the Zoology Department, Faculty of Science, King Saud University. The project aims to analyze genetic sequences of different parasites that are found spread out over Saudi Arabia, in order to help differentiate between the genetic sequences of local parasites and parasites of other regions, both inside and outside Saudi Arabia. Such information is expected to facilitate the development of methods for the prevention and control of these parasites.
Materials and methods
Sample collection
During the period between March and April of 2017, a total of 100 BALB/c mice (50 from Makkah and 50 from Riyadh) and 120 Rattusu norvegis rats (70 from Makkah and 50 from Riyadh) were obtained from the Female Center for Scientific and Medical Colleges, Riyadh, Saudi Arabia. The animals were kept in wire-bottomed cages in a room under conditions of standard illumination with a 12-h light–dark cycle, at a temperature of 25 ± 1°C for 1 week, until the commencement of treatment. Animals were provided with tap water and a balanced diet ad libitum. Mice were killed via decapitation. Worms were collected and extracted from all mice and rats, washed with normal saline and examined under a microscope to determine the type of worm. Worms were stored at −20°C until molecular analysis. All experiments were conducted according to specifications of the animal ethics committee outlined by the University of Sattam Bin Abdulaziz University (IRB number: SAU-2017-LAB-523/PI), which also included the joint efforts of Parasitology Department, Sattam Bin Abdulaziz University, and the College of Science, King Saud University.
DNA extraction
Worms obtained from mice and rats were washed with distilled water and ethanol before they were centrifuged. Genomic DNA (gDNA) was then extracted using a High Pure PCR Template Preparation Kit (Qiagen GmbH, Hilden, Germany Cat. No.51304). Amplification of cox1 and pnad5 was performed using specific primers (cox1: F:5′ AGAGTGATCCGGTGATATGGTGA 3′ R:5′ ACCATTCACCCTTGGTATAAGCAGA 3′, pnad5: F:5′ GAAGCGTTAATTATGGGTT 3′ R:5′ GATTACAAGTTGATAGAGCCC 3′) [14] in a 40 μl reaction mixture containing 8 μl of master mix, 25.6 μl of deoxynucleotides (dNTPs), 2.4 μl of primers, and 4 μl of DNA template. The PCR program consisted of an initial denaturation step at 94°C for 5 min followed by 40 cycles of denaturation at 94°C for 45 s, annealing at 50°C for 45 s, extension at 72°C for 10 min, and a final extension step at 72°C for 10 min. PCR products were analyzed via 1% agarose gel electrophoresis.
DNA sequencing and phylogenetic analysis
PCR products of cox1 and pnad5 were purified and sequenced using both forward and reverse complements by Genetic Analyzer at the Central Lab of King Saud University. A multiple sequence alignment was generated for the samples using the ClustalW [15] algorithm with a gap opening penalty of 10 and a gap extension penalty of 1. All sequences were truncated slightly using an error probability method with a limit of 0.05 at both ends. A BLAST search was performed for each sequence to locate related sequences. A phylogenetic tree was generated using MrBayes 3.2.6 [16], a Bayesian inference algorithm. Bootstrap method was used for resampling with the number of replicates set to 1000.
Results
Amplification of cox1 and pnad5
Partial PCR amplification of cox1 and pnad5 yielded the expected 800 bp fragments for all DNA samples from both mice and rats.
Analysis of Cox1
The sequences of 25 samples, including those of 10 Makkah mice, 13 Riyadh mice and 2 Makkah rats, were analyzed. The final sequences were 776–793 nucleotides in length (Table 1). A BLAST search was performed for each sequence to locate related sequences. All samples except one showed a pairwise identity of 99–99.60% and a 62–100% coverage relative to the genome of H. nana, Japan, with the accession number LM402005. In addition, all samples showed a pairwise identity of 99.00–99.60% and a 62–100% coverage relative to H. nana mt genome with accession numbers LM403673 and AP017666. All samples showed a pairwise identity of 97.90–98.60% and a 62–100% coverage to H. nana mt genome with the accession number KT951722.
Table 1. Genetic sequences from the isolated H. nana with variable lengths and GC contents (cox1).
| Name | Host location | Host species | %GC | Post-Trim | Length |
|---|---|---|---|---|---|
| 1MR | Riyadh, Saudi Arabia | Mus musculus | 31.80% | 756 | 790 |
| 2MR | Riyadh, Saudi Arabia | Mus musculus | 31.80% | 757 | 786 |
| 3MM | Makkah, Saudi Arabia | Mus musculus | 32.00% | 754 | 785 |
| 8MM | Makkah, Saudi Arabia | Mus musculus | 31.80% | 773 | 789 |
| 11MR | Riyadh, Saudi Arabia | Mus musculus | 31.70% | 765 | 793 |
| 12MR | Riyadh, Saudi Arabia | Mus musculus | 32.20% | 760 | 786 |
| 13MM | Makkah, Saudi Arabia | Mus musculus | 31.60% | 754 | 787 |
| 14MM | Makkah, Saudi Arabia | Mus musculus | 32.00% | 762 | 791 |
| 15MM | Makkah, Saudi Arabia | Mus musculus | 31.90% | 767 | 789 |
| 15MR | Riyadh, Saudi Arabia | Mus musculus | 31.90% | 755 | 786 |
| 16MM | Makkah, Saudi Arabia | Mus musculus | 32.30% | 748 | 787 |
| 16MR | Riyadh, Saudi Arabia | Mus musculus | 31.80% | 756 | 786 |
| 18MR | Riyadh, Saudi Arabia | Mus musculus | 32.00% | 758 | 776 |
| 19MR | Riyadh, Saudi Arabia | Mus musculus | 32.20% | 757 | 777 |
| 20MM | Makkah, Saudi Arabia | Mus musculus | 31.90% | 761 | 791 |
| 20MR | Riyadh, Saudi Arabia | Mus musculus | 32.00% | 760 | 788 |
| 24MR | Riyadh, Saudi Arabia | Mus musculus | 32.00% | 759 | 785 |
| 26MR | Riyadh, Saudi Arabia | Mus musculus | 31.90% | 759 | 788 |
| 27MM | Makkah, Saudi Arabia | Mus musculus | 31.60% | 779 | 787 |
| 32MR | Riyadh, Saudi Arabia | Mus musculus | 32.10% | 757 | 787 |
| 36MR | Riyadh, Saudi Arabia | Mus musculus | 32.20% | 758 | 786 |
| 37MM | Makkah, Saudi Arabia | Mus musculus | 31.90% | 763 | 789 |
| 42MM | Makkah, Saudi Arabia | Mus musculus | 31.80% | 759 | 787 |
| 23RM | Makkah, Saudi Arabia | Rattus norvegius | 33.50% | 759 | 786 |
| 40RM | Makkah, Saudi Arabia | Rattus norvegius | 33.40% | 203 | 293 |
All samples showed a pairwise identity of 98.80–99.60% and a 62.60–86.48% coverage with H. nana mt cox1 gene, encoding cytochrome c oxidase subunit 1, partial cds, isolate: H. nana with accession number LC063187.
All samples showed a pairwise identity of more than 97.60% and 54% coverage with H. nana cox1 partial cds, mitochondrial with accession numbers GU433102, GU433103, and GU433104. All of the samples showed a pairwise identity of 98.80–99.60% and 62.60–74% coverage to H. nana cox1 mitochondrial gene, partial cds with accession number AB033412.
The multiple sequence alignment of the 25 samples and related sequences retrieved from Genbank was generated. The sequence LM402005 was set as the reference sequence. We found the following one-nucleotide substitutions (SNP) transitions: T to C at position 9998 of the reference sequence in 25% of the samples; C to T at position 10264 of the reference sequence in 92% of the samples; G to A at position 10495 of the reference sequence in all samples; and A to C at position 10591 of the reference sequence in all samples. In addition, one insertion of T was found at position 10766 of the reference sequence in 80% of the samples. Finally, two deletions of A were observed at positions 10760 and 10004, in 31% and 55% of the samples respectively (note: the alignment is provided in FASTA and Nexus formats).
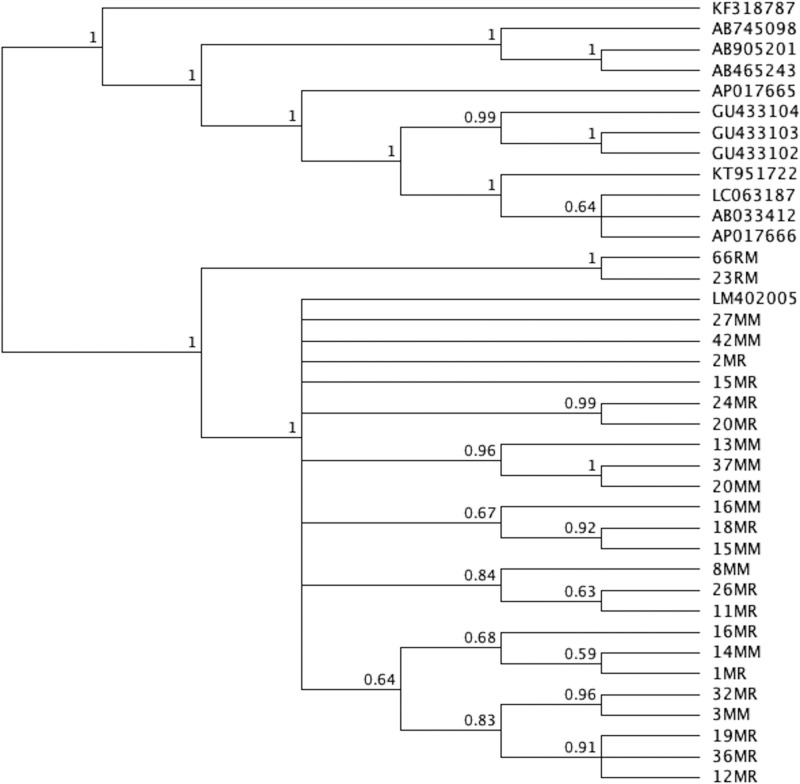
The Phylogenetic tree was generated using MrBayes. Dicrocoelium dendriticum (Accession number KF318787) was used as the outgroup. The Phylogenetic tree with posterior probability values is shown (Figure 1). All the samples in the present study were grouped in a clade with H. nana genome assembly H. nana_Japan with accession number LM402005 (note: the tree is provided in Nexus format).
Figure 1. Phylogenetic tree of the 25 mice and rats samples in the present study along with similar sequences published in Genbank.
Analysis of pnad5
Sequences of 31 samples, including, 10 Makkah mice, 17 Riyadh mice and 4 Makkah rats, were analyzed. The final sequences were 816–846 nucleotides in length (Table 2). A BLAST search was performed for each sequence in order to locate related sequences. All samples showed a pairwise identity of 98.70–99.50% and coverage 82.40–100% with H. nana sequences with accession numbers: LM403673, LM402005, KT951722, and AP017666.
Table 2. Genetic sequences from the isolated H. nana with variable lengths and GC contents (pnad5).
| Name | Host location | Host species | %GC | Post-Trim | Length |
|---|---|---|---|---|---|
| 1MM | Makkah, Saudi Arabia | Mus musculus | 27.80% | 814 | 834 |
| 1MR | Riyadh, Saudi Arabia | Mus musculus | 28.20% | 801 | 838 |
| 2MR | Riyadh, Saudi Arabia | Mus musculus | 27.60% | 800 | 837 |
| 3MM | Makkah, Saudi Arabia | Mus musculus | 27.70% | 798 | 837 |
| 8MM | Makkah, Saudi Arabia | Mus musculus | 28.20% | 803 | 837 |
| 10MR | Riyadh, Saudi Arabia | Mus musculus | 27.90% | 802 | 834 |
| 11MR | Riyadh, Saudi Arabia | Mus musculus | 27.90% | 799 | 838 |
| 12MR | Riyadh, Saudi Arabia | Mus musculus | 27.80% | 802 | 837 |
| 13MM | Makkah, Saudi Arabia | Mus musculus | 27.60% | 799 | 836 |
| 15MR | Riyadh, Saudi Arabia | Mus musculus | 27.70% | 803 | 838 |
| 16MM | Makkah, Saudi Arabia | Mus musculus | 27.50% | 798 | 816 |
| 16MR | Riyadh, Saudi Arabia | Mus musculus | 27.20% | 807 | 843 |
| 19MR | Riyadh, Saudi Arabia | Mus musculus | 27.80% | 802 | 834 |
| 20MR | Riyadh, Saudi Arabia | Mus musculus | 27.60% | 801 | 837 |
| 21MM | Makkah, Saudi Arabia | Mus musculus | 27.70% | 802 | 839 |
| 23RM | Riyadh, Saudi Arabia | Mus musculus | 28.20% | 799 | 837 |
| 25MR | Riyadh, Saudi Arabia | Mus musculus | 27.50% | 803 | 834 |
| 26MR | Riyadh, Saudi Arabia | Mus musculus | 27.40% | 803 | 819 |
| 27MM | Makkah, Saudi Arabia | Mus musculus | 27.60% | 801 | 836 |
| 27MR | Riyadh, Saudi Arabia | Mus musculus | 27.40% | 797 | 820 |
| 32MR | Riyadh, Saudi Arabia | Mus musculus | 28.10% | 802 | 839 |
| 35MR | Riyadh, Saudi Arabia | Mus musculus | 28.40% | 754 | 846 |
| 36MR | Riyadh, Saudi Arabia | Mus musculus | 28.10% | 805 | 837 |
| 37MM | Makkah, Saudi Arabia | Mus musculus | 28.60% | 746 | 840 |
| 40MM | Makkah, Saudi Arabia | Mus musculus | 27.70% | 818 | 840 |
| 40MR | Riyadh, Saudi Arabia | Mus musculus | 27.50% | 806 | 835 |
| 41MR | Riyadh, Saudi Arabia | Mus musculus | 28.60% | 811 | 844 |
| 42MM | Makkah, Saudi Arabia | Mus musculus | 27.80% | 800 | 837 |
| 66RM | Makkah, Saudi Arabia | Rattus norvegius | 28.00% | 801 | 837 |
| 40RM | Makkah, Saudi Arabia | Rattus norvegius | 27.40% | 801 | 819 |
| 22RM | Makkah, Saudi Arabia | Rattus norvegius | 27.90% | 800 | 838 |
| 23RM | Makkah, Saudi Arabia | Rattus norvegius | 28.2% | 799 | 837 |
These samples were also similar to the sequence of H. nana isolate y1 pnad5, partial cds, mitochondrial (accession number KT589891), H. nana isolate shz1 pnad5, partial cds, mitochondrial (accession number KT589901), and H. nana isolate s2 gene, partial cds, mitochondrial (accession number KT589905) with identity of 98.70–99.40% and coverage of 81–89%.
A multiple sequence alignment was generated for the 31 samples and related sequences using ClustalW algorithm. The sequence LM402005 was set as the reference sequence. The SNPs are shown (Table 3).
Table 3. Variations found in the sequences in the present study relative to sequence LM402005.
| Name | Minimum | Maximum | Length | Change | Coverage | Polymorphism type | Variant frequency |
|---|---|---|---|---|---|---|---|
| C | 5616 | 5616 | 1 | A -> C | 4 | SNP (transversion) | 25.00% |
| CA | 6442 | 6443 | 2 | TC->CA | 3 -> 4 | Substitution | 33.3% -> 50.0% |
| 5634 | 5634 | 1 | (A)3 -> (A)2 | 29 | Deletion (tandem repeat) | 37.90% | |
| C | 6439 | 6439 | 1 | A -> C | 5 | SNP (transversion) | 40.00% |
| T | 6440 | 6440 | 1 | A -> T | 5 | SNP (transversion) | 40.00% |
| 5621 | 5621 | 1 | #NAME? | 20 | Deletion | 45.00% | |
| T | 6426 | 6426 | 1 | A -> T | 5 | SNP (transversion) | 60.00% |
| 6439 | 6439 | 1 | #NAME? | 5 | Deletion | 60.00% | |
| 6440 | 6440 | 1 | #NAME? | 5 | Deletion | 60.00% | |
| 5617 | 5618 | 2 | (AA)3 -> (AA)2 | 10 | Deletion (tandem repeat) | 80.00% | |
| A | 5885 | 5885 | 1 | G -> A | 31 | SNP (transition) | 100.00% |
| A | 5956 | 5956 | 1 | G -> A | 31 | SNP (transition) | 100.00% |
| G | 6273 | 6273 | 1 | A -> G | 31 | SNP (transition) | 100.00% |
| T | 6295 | 6295 | 1 | C -> T | 31 | SNP (transition) | 100.00% |
| 6427 | 6432 | 6 | #NAME? | 5 | Deletion | 100.00% | |
| C | 6435 | 6435 | 1 | A -> C | 5 | SNP (transversion) | 100.00% |
| 6438 | 6438 | 1 | #NAME? | 5 | Deletion | 100.00% | |
| T | 6441 | 6441 | 1 | C -> T | 5 | SNP (transition) | 100.00% |
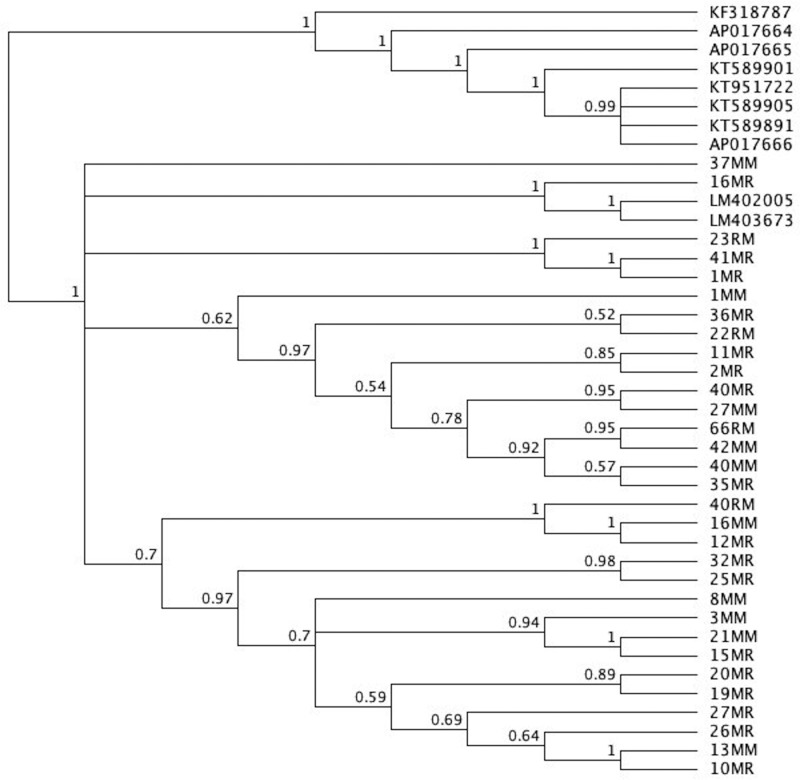
MrBayes was used in order to generate the Phylogenetic tree. Dicrocoelium dendriticum (accession number KF318787) was used as the outgroup. The Phylogenetic tree with posterior probability values is shown (Figure 2). The sequences placed in a clade of H. nana sequences LM402005 and LM403673 (note: the tree is provided Nexus format).
Figure 2. Phylogenetic tree of 31 mice and rats samples in the current study along with similar sequences published in Genbank.
Discussion
Laboratory animal models, especially rodents of the family, Muridae, share important links in food chains in their ecosystems, due to their life style and great biotic potential [17–19]. Compared with most animal species, rodents have a greater ability to harbor many zoonotic agents [20–23]. Due to their broad distribution and close contact with different animals as well as with humans, rodents may act as reservoir hosts for vector-borne disease agents [24]. In conventional animal facilities, rodent colonies either are frequently infected with helminth parasites or become infected in places where they are maintained while waiting to be experimented on [2,25,26]. Based on morphological characters, the Hymenolepidid species that were analyzed had all the characteristic features of genus Hymenolepis and were identified as H. nana.
Molecular phylogenetic approaches in association with traditional morphological techniques are used extensively for identification, phylogenetic analysis, and differentiation of highly similar Hymenolepidid species infecting laboratory rodents [27–33]. Mitochondria play an essential role in metabolism, apoptosis, illness, and aging [34]. They facilitate oxidative phosphorylation, ATP production and other biochemical functions. Mitochondria contain their own genome, consisting of mitochondrial DNA (mtDNA), often used as a part of molecular phylogenetics studies [35]. Mitochondrial genomes of helminths display unique characteristics such as all genes being coded on the same strand [36].
In the present study, mt genes of H. nana were amplified using species-specific primers. A descriptive analysis of H. nana mt genes may enable the use of genetic markers in the diagnosis of hymenolepiasis and facilitate epidemiological studies of H. nana at a molecular level. Furthermore, the use of mtDNA markers to examine genetic variability in cryptic/sibling species and larval stages of H. nana may be vital as morphological descriptions of H. nana are still rare [6, 37, 38]. For purposes of the present study, genomic DNA was extracted from 31 specimens of H. nana, from two different geographical locations in Saudi Arabia. The lengths of cox1 and pnad5 sequences, obtained separately from the specimens, were 850 bp, and the GC contents of the sequences were 31.6–33.5%, for cox1, and 27.2%-28.6% for pnad5. The range of genomic similarity determined among specimens by cox1 primer was 97.1% and by pnad5 primer was 99.7%.
The inter-specific sequence differences between cox1 and pnad5 were found to be low and recorded between H. nana (present isolates) and H. nana (accession number LM402005, LM403673, KT951722, AP017666, LC063187, GU433102, GU433103, GU433104, AB033412, KT589891, KT589901, and KT589905). These results agreed with those of a previous study that reported lower divergence values between the Hymenolepis species that are most related to each other [39].
In the phylogenetic tree, H. nana isolates did not exhibit an obvious geographical distinction based on the sequences of the two mtDNA regions. All H. nana isolates from Makkah and Riyadh grouped together, indicating that all H. nana samples from Makkah and Riyadh were strongly related. Furthermore, isolates from both mice and rats displayed genomic similarity. These results were similar to those of a previous study [14]. Subsequent analyses of genetic sequences of Hymenolepid species have strongly supported monophyly with strong bootstrap values within the cestoda clade. These results substantiated those obtained in previous studies indicating that Hymenolepididae species of the genus Hymenolepis may be monophyletic in origin [40–43].
Supported by existing data, the present study, investigated the placement of Hymenolepid species within Hymenolepididae. Results indicated that the present species were deeply embedded in the genus Hymenolepis with close relationships to other Hymenolepis species, including previously described H. nana, as a putative sister taxon. Our results indicate that more indepth phylogenetic studies, which include more taxa and different molecular markers of Hymenolepid species, may be needed in the future. A recent field study provided useful tools for the rapid identification and phylogenetic analysis of Hymenolepidids infecting laboratory rodents. In addition, cox1 and pnad5 of H. nana that were analyzed by the present study yielded a unique sequence that confirmed their taxonomic position within the family of Hymenolepid species. Also, laboratory rodents should be considered potential natural reservoirs of different parasite species, which require further monitoring in order to improve the awareness of researchers, in order to prevent possible transmission of parasitic zoonosis from laboratory animals.
Abbreviation
- cox1
cytochrome oxidase
Funding
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research through the Research Group project no. RG-1439-034.
Author Contribution
Conceptualization: D.M.M. Data curation: H.M.Y. Formal analysis: H.A.A. Funding acquisition: M.F.E. Investigation: D.M.M. Methodology: H.A.A. and T.T.A. Project administration: D.M.M. Resources: H.A.A. and T.T.A. Software: I.M.T. Supervision: D.M.M. Validation: D.M.M. Visualization: H.M.Y. Writing: original draft: D.M.M. Writing, review and editing: MFE, DMM, and IMT.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Willcocks B., McAuliffe G.N. and Baird R.W. (2015) Dwarf tapeworm (Hymenolepis nana): characteristics in the Northern Territory 2002-2013. Aust. Paediatr. J. 51, 982–987 [DOI] [PubMed] [Google Scholar]
- 2.Thompson R.A. (2015) Neglected zoonotic helminths: Hymenolepis nana, Echinococcus canadensis and Ancylostoma ceylanicum. Clin. Microbiol. Infect. 21, 426–432 10.1016/j.cmi.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 3.Marquardt W.C., Demaree R.S. and Grieve R.B. (2000) Parasitology and Vector Biology, Harcoart Academic Press, London [Google Scholar]
- 4.Sirivichayakul C., Radomyos P., Praevanit R., Jojjaroen-Anant C. and Wisetsing P. (2000) Hymenolepis nana infection in Thai children. J. Med. Assoc. Thailand 83, 1035–1038 [PubMed] [Google Scholar]
- 5.Ferretti G., Gabriele F. and Palmas C. (1981) Development of human and mouse strain of Hymenolepis nana in mice. Int. J. Parasitol. 11, 425–430 10.1016/0020-7519(81)90060-6 [DOI] [PubMed] [Google Scholar]
- 6.Macnish M.G., Morgan-Ryan U.M., Monis P.T., Behnke J.M. and Thompson R.C.A. (2002) A molecular phylogeny of nuclear and mitochondrial sequences in Hymenolepis nana (Cestoda) supports the existence of a cryptic species. Parasitology 125, 567–575 10.1017/S0031182002002366 [DOI] [PubMed] [Google Scholar]
- 7.Widmer V., Boyko G. and Mariaux J. (2013) A new genus of the family Hymenolepididae (Cestoda) from Sephanoides sephaniodes (Apodiformes, Trochilidae) in Northern Patagonia (Chile). Acta Parasitol. 58, 105–111 10.2478/s11686-013-0117-y [DOI] [PubMed] [Google Scholar]
- 8.Makarikov A.A., Mel’nikova Y.A. and Tkach V.V. (2015) Description and phylogenetic affinities of two new species of Nomadolepis (Eucestoda, Hymenolepididae) from Eastern Palearctic. Parasitol. Int. 64, 453–463 10.1016/j.parint.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 9.Boore J. L. (1999) Animal mitochondrial genomes. Nucleic Acids Res. 27, 1767–1780 10.1093/nar/27.8.1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McManus D.P., Le T.H. and Blair D. (2004) Genomics of parasitic flatworms. Int. J. Parasitol. 34, 153–158 10.1016/j.ijpara.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 11.Li J.Y., Liu G.H., Wang Y., Song H.Q., Lin R.Q., Zou F.C.. et al. (2013) Sequence variation in three mitochondrial DNA genes among isolates of Ascaridia galli originating from Guangdong, Hunan and Yunnan provinces, China. J. Helminthol. 87, 371–375 10.1017/S0022149X12000326 [DOI] [PubMed] [Google Scholar]
- 12.Okamoto M., Agatsuma T., Kurosawa T. and Ito A. (1997) Phylogenetic relationships of three hymenolepidid species inferred from nuclear ribosomal and mitochondrial DNA sequences. Parasitol 115, 661–666 10.1017/S0031182097001741 [DOI] [PubMed] [Google Scholar]
- 13.Mirjalali H., Kia E.B., Kamranrashani B., Hajjaran H. and Sharifdini M. (2016) Molecular analysis of isolates of the cestode Rodentolepis nana from the great gerbil, Rhombomysopimus. J. Helminthol. 90, 252–255 10.1017/S0022149X15000115 [DOI] [PubMed] [Google Scholar]
- 14.Cheng T., Gao D. Z., Zhu W. N., Fang S. F., Chen N., Zhu X.. et al. (2016) Genetic variability among Hymenolepis nana isolates from different geographical regions in China revealed by sequence analysis of three mitochondrial genes. Mitochondrial DNA Part A 27, 4646–4650 10.3109/19401736.2015.1101595 [DOI] [PubMed] [Google Scholar]
- 15.Thompson J.D., Higgins D.G. and Gibson T.J. (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huelsenbeck J.P. and Ronquist F. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- 17.Rosas G.A. (1997) Diagnóstico: parasitosis intestinal por Aspiculuris tetraptera. Anim. Experiment. 2, 9–11 [Google Scholar]
- 18.Gonçalves L., Pinto R.M., Vicente J.J., Noronha D. and Gomes D.C. (1998) Helminth parasites of conventionally maintained laboratory mice - II. Inbred strains with an adaptation of the anal swab technique. Memorias Do Instituto Oswaldo Cruz. 93, 121–126 10.1590/S0074-02761998000100023 [DOI] [PubMed] [Google Scholar]
- 19.Perec-Matysiak A., Okulewicz A., Hildebrand J. and Zaleśny G. (2006) Helminth parasites of laboratory mice and rats. Wiadomosci parazytologiczne 52, 99–102 [PubMed] [Google Scholar]
- 20.Neifer S., Kremsner P.G., Weinig M., Harms G., Sahlmüller G., Bienzle U.. et al. (1991) Interferon-gamma treatment in mice experimentally infected with Trichinella spiralis. Parasitol. Res. 77, 437–442 10.1007/BF00931641 [DOI] [PubMed] [Google Scholar]
- 21.Durden L.A., Hu R., Oliver J.H. Jr and Cilek J.E. (2000) Rodent ectoparasites from two locations in northwestern Florida. J. Vector Ecol. 25, 222–228 [PubMed] [Google Scholar]
- 22.Mehlhorn H., Schmahl G. and Mevissen I. (2005) Efficacy of a combination of imidacloprid and moxidectin against parasites of reptiles and rodents: case reports. Parasitol. Res. 97, S97–S101 10.1007/s00436-005-1451-2 [DOI] [PubMed] [Google Scholar]
- 23.Pakdel N., Naem S., Rezaei F. and Chalehchaleh A.A. (2013) A survey on helminthic infection in mice (Mus musculus) and rats (Rattus norvegicus and Rattus rattus) in Kermanshah, Iran. Vet. Res. Forum. 4, 105–109 [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmoud A.E., Attia R. A.H., Eldeek H.E.M., Abdel B.L. and Oshaish H.A. (2009) Oxyurid nematodes detected by colonoscopy in patients with unexplained abdominal pain. Parasitol. Unit J. 2, 93–102 [Google Scholar]
- 25.Sato Y., Ooi H.K., Nonaka N., Oku Y. and Kamiya M. (1995) Antibody production in Syphacia obvelata infected mice. J. Parasitol. 8, 559–562 10.2307/3283853 [DOI] [PubMed] [Google Scholar]
- 26.Rehbinder C., Baneux P., Forbes D., van Herck H., Nicklas W., Rugaya Z.. et al. (1996) FELASA recommendations for the health monitoring of mouse, rat, hamster, gerbil, guinea pig and rabbit experimental units. Lab. Animal. 30, 193–208 10.1258/002367796780684881 [DOI] [PubMed] [Google Scholar]
- 27.Jacobs K.A., Collins-Racie L.A., Colbert M., Duckett M., Golden-Fleet M., Kelleher K.. et al. (1997) A genetic selection for isolating cDNAs encoding secreted proteins. Gene 198, 289–296 10.1016/S0378-1119(97)00330-2 [DOI] [PubMed] [Google Scholar]
- 28.Zhu X.Q., Jacobs D.E., Chilton N.B., Sani R.A., Cheng N. and Gasser R.B. (1998) Molecular characterization of Toxocara variant from cats in Kuala Lumpur, Malaysia. Parasitol 117, 155–164 10.1017/S0031182098002856 [DOI] [PubMed] [Google Scholar]
- 29.Vermund S.H. and Wilson C.M. (2000) Pinworm (Enterobius vermicularis). Semin. Pediatr. Infect. Dis. WB Saunders 11, 252–256 10.1053/spid.2000.9639 [DOI] [Google Scholar]
- 30.Morales-Hojas R., Post R.J., Shelley A.J., Maia-Herzog M., Coscarón S. and Cheke R.A. (2001) Characterization of nuclear ribosomal DNA sequences from Onchocerca volvulus and Mansonella ozzardi (Nematoda: Filarioidea) and development of a PCR-based method for their detection in skin biopsies. Int. J. Parasitol. 31, 169–177 10.1016/S0020-7519(00)00156-9 [DOI] [PubMed] [Google Scholar]
- 31.Nakano T., Okamoto M., Ikeda Y. and Hasegawa H. (2006) Mitochondrial cytochrome c oxidase subunit 1 gene and nuclear rDNA regions of Enterobius vermicularis parasitic in captive chimpanzees with special reference to its relationship with pinworms in humans. Parasitol. Res. 100, 51–57 10.1007/s00436-006-0238-4 [DOI] [PubMed] [Google Scholar]
- 32.Zhu X.Q., Amelio S.D., Gasser R.B., Yang T.B., Paggi L., He F.. et al. (2007) Practical PCR tools for the delineation of Contracaecum rudolphii A and Contracaecum rudolphii B (Ascaridoidea: Anisakidae) using genetic markers in nuclear ribosomal DNA. Mol. Cell. Probes 21, 97–102 10.1016/j.mcp.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 33.Chang T.K., Liao C.W., Haung Y.C., Chang C.C., Chou C.M., Tsay H.C.. et al. (2009) Prevalence of Enterobius vermicularis infection among preschool children in kindergartens of Taipei City, Taiwan in 2008. Korean J. Parasitol. 47, 185–187 10.3347/kjp.2009.47.2.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin M.T. and Beal M.F. (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- 35.Avise J.C. (2000) Phylogeography: The History and Formation of Species 56, p. 7, Harvard University Press [Google Scholar]
- 36.von Nickisch-Rosenegk M., Brown W.M. and Boore J.L. (2001) Complete sequence of the mitochondrial genome of the tapeworm Hymenolepis diminuta: gene arrangements indicate that Platyhelminths are Eutrochozoans. Mol. Biol. Evol. 18, 721–730 10.1093/oxfordjournals.molbev.a003854 [DOI] [PubMed] [Google Scholar]
- 37.Morgan J.A.T. and Blair D. (1995) Nuclear rDNA ITS sequence variation in the trematode genus Echinostoma: an aid to establishing relationships within the 37-collar-spine group. Parasitology 111, 609–615 10.1017/S003118200007709X [DOI] [PubMed] [Google Scholar]
- 38.Sorensen R.E., Curtis J. and Mindhela D.J. (1998) Intraspecific variation in the rDNA ITS loci of 37-collar-spined Echinostomes from North America: Implications for sequence-based diagnoses and phylogenetics. J. Parasitol. 84, 992–997 10.2307/3284633 [DOI] [PubMed] [Google Scholar]
- 39.Nkouawa A., Haukisalmi V., Li T., Nakao M., Lavikainen A., Chen X.. et al. (2016) Cryptic diversity in hymenolepidid tapeworms infecting humans. Parasitol. Intl. 65, 83–86 10.1016/j.parint.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 40.De Ley P. and Blaxter M.L. (2002) Systematic position and phylogeny. The Biol. Nematodes 1, 1–30 [Google Scholar]
- 41.Bert W., Messiaen M., Manhout J., Houthoofd W. and Borgonie G. (2006) Evolutionary loss of parasitism by nematodes? Discovery of a free-living fiG. (20 nematode) J. Parasitol. 92, 645–647 10.1645/GE-672R.1 [DOI] [PubMed] [Google Scholar]
- 42.Holterman M., van der Wurff A., van den Elsen S., van Megen H., Bongers T., Holovachov O.. et al. (2006) Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 23, 1792–1800 10.1093/molbev/msl044 [DOI] [PubMed] [Google Scholar]
- 43.Wijová M., Moravec F., Horák A. and Lukeš J. (2006) Evolutionary relationships of Spirurina (Nematoda: Chromadorea: Rhabditida) with special emphasis on dracunculoid nematodes inferred from SSU rRNA gene sequences. Int. J. Parasitol. 36, 1067–1075 10.1016/j.ijpara.2006.04.005 [DOI] [PubMed] [Google Scholar]