Abstract
It has long been known that phytopathogenic bacteria react to plant-specific stimuli or environmental factors. However, how bacterial cells sense these environmental cues remains incompletely studied. Recently, three kinds of histidine kinases (HKs) were identified as receptors to perceive plant-associated or quorum-sensing signals. Among these kinases, HK VgrS detects iron depletion by binding to ferric iron via an ExxE motif, RpfC binds diffusible signal factor (DSF) by its N-terminal peptide and activates its autokinase activity through relaxation of autoinhibition, and PcrK specifically senses plant hormone–cytokinin and elicits bacterial responses to oxidative stress. These HKs are critical sensors that regulate the virulence of a Gram-negative bacterium, Xanthomonas campestris pv. campestris. Research progress on the signal perception of phytopathogenic bacterial HKs suggests that inter-kingdom signalling between host plants and pathogens controls pathogenesis and can be used as a potential molecular target to protect plants from bacterial diseases.
This article is part of the theme issue ‘Biotic signalling sheds light on smart pest management’.
Keywords: bacteria, histidine kinase, plant pathogen, receptor, phosphorylation, two-component signal transduction
1. Introduction
Discriminating ‘self’ and ‘non-self’ is the primary step during host–microbe interaction [1,2]. Eukaryotic cells employ the so-called pattern recognition receptors (PRRs) to detect the pathogen-associated molecular pattern to elicit immune responses, whereas pathogenic prokaryotes must monitor and respond to hostile environments or various stresses in the host tissues [3,4]. Studies in this area became more popular in the areas of both animal and plant diseases after 1990. Rapid progress was made and in 2011, when Bruce A. Beutler and Jules A. Hoffmann won the Nobel Prize in Physiology and Medicine for their discoveries concerning the activation of innate immunity in animals via TLR-like receptors sensing bacterial lipopolysaccharides [5,6], which emphasizes the importance of these investigations.
In the past two decades, scientists studying plant–microbe interactions have identified a number of plant PRRs that function in the recognition of microbial pathogens and modulate innate immune responses [7], including FLS2-perceiving flg22, a short peptide of bacterial flagella [8]; EFR-sensing bacterial elongation factor Tu [9]; CERK1-detecting chitin of fungal cells [10]; LYM-sensing peptidoglycan [11] and LORE-sensing lipopolysaccharides [12]. Although it is well known that plant pathogenic bacteria react to various stimulations of host plants [13,14], the biochemical mechanism by which bacterial cells sense plant chemicals or plant-derived cues remains largely unknown. Especially, the specific receptor–ligand interaction between host and pathogen has not been well established through studies that combine multi-discipline approaches. In fact, knowledge about bacterial sensing will provide insights into the molecular mechanism of disease pathogenesis, which facilitates the rational design of novel approaches to fight these important pathogens.
2. Histidine kinases of Xanthomonas campestris pv. campestris
With the exception of species belonging to the genus Mycoplasma, most bacteria use several to hundreds of two-component signal transduction systems (TCS) as the predominant mechanism to detect and respond to environmental stimuli [15,16]. TCS typically contains a membrane-bound histidine kinase (HK) and a cytosolic response regulator (RR). A typical HK has a variable N-terminal sensor domain that detects a specific signal and a C-terminal that contains a conserved transmitter domain to hydrolyze ATP and that can be autophosphorylated. Upon detection of a stimulus, HK is phosphorylated, and then the phosphoryl group is transferred to the receiver (REC) domain of its cognate RR. The RR performs downstream regulation via its C-terminal output domain, mainly acting as transcription factors [17]. Additionally, a hybrid type of HK (HyHK) that contains the REC domain is widely distributed in various bacteria [18]. HyHK is involved in a multiple-step phosphorelay with other HKs and RRs, adding more regulatory checkpoints to the signal transduction [19–21]. The first TCS was identified in 1986 [22]. After 30 years of research, there is now a full understanding on the biochemistry of the phosphoryltransfer process and the regulatory function of RRs. However, how HK senses environmental stimuli is incompletely understood. Only a few studies have reported the biological functions of HK–signal or HK–ligand interactions [23,24]. For example, in enterohaemorrhagic Escherichia coli, HKs QseE and QseC perceive animal hormone epinephrine [25], and PhoQ detects the concentrations of Mg2+ and Ca2+, antimicrobial peptides and pH [26,27]. These HKs modulate bacterial virulence. In phytopathogenic bacteria, although a number of HKs are involved in regulating virulence, with the exception of the study cases reviewed here, the signals detected by these HKs remain unclear.
Various scenarios can explain this finding: (i) in contrast to the receptor serine/tyrosine/threonine kinases of eukaryotes, phosphorylated HK is typically unstable and has a very short half-life [15,28], which makes it technically difficult to test the phosphorylated states of HKs in vivo by means of Western blot using specific antibodies or Phos-tag gels [29]. Generally, the phosphorylation of HKs or RRs is studied in vitro using radio-autography. (ii) The majority of HKs have hydrophobic transmembrane regions that function in signal perception. The standard study strategy that removes transmembrane regions to obtain recombinant, soluble proteins precludes the possibility of investigating HK–signal interactions [23]. (iii) The nature of transmembrane proteins makes it difficult to express and purify recombinant HKs unless full-length HKs are embedded into lipid bilayer [30].
Xanthomonas campestris pv. campestris is one of the model organisms used in studies of plant–pathogen interactions given that it is the causative agent of black rot disease in cruciferous vegetables, it is genetically amendable and a Gram-negative, aerobic, rod-shaped bacterium. This bacterium causes serious yield loss worldwide and is listed as one of the disastrous pathogens in agricultural production. The complete genomes of several strains of X. campestris pv. campestris were reported in the early years of the new millennium [31–33], thus providing genetic information to conduct in-depth research.
The genome of X. campestris pv. campestris encodes 53 HKs and 54 RRs [34,35], including a HWE-family (histidine–tryptophan–glutamate) HK that was recently identified [36]. These HKs are mainly classified into three major structural groups based on their membrane topologies [34]: Group I, membrane-bound HK with a well-defined periplasmic sensor domain to perceive extracytoplasmic stimuli; Group II, membrane-bound HK without an apparent periplasmic sensory domain, which is believed to employ an unidentified sensory domain and transmembrane regions to perceive extracytoplasmic stimuli or detect membrane-associated stimuli derived from membrane integrity factors, such as ion gradients and electric potential; Group III, soluble, cytoplasmic HK without recognizable transmembrane helices. These HKs of X. campestris pv. campestris encode a limited number of sensor domains, including Per–Arnt–Sim domain (PAS)/PAC, KdpD, AAA, CHASE, CHASE3, GAF and HAMP domains, prompting an interesting question on how these HKs detect numerous stimuli when the bacterium is free-living or survives in the host environment. Among them, RpfC (Group II), VgrS (or named ColS), PhoQ, PcrK and HpaS (Group I) are critical to regulate virulence of X. campestris pv. campestris [35,37–40]. Thus, exploration of the stimuli sensed by these virulence-associated HKs is important for the understanding of the molecular pathogenesis of this bacterial pathogen.
3. Techniques for the study of biochemistry of full-length histidine kinases
Most of the HKs of X. campestris pv. campestris contain 1–12 transmembrane helices and are located in the inner membrane of bacterial cells [34]. As previously mentioned, to investigate the enzymatic activities of HKs, including autokinase, phosphatase and phosphotransferase, full-length HKs must be expressed and embedded into lipid bilayers to reconstruct correct conformations. Currently, three approaches can be used, including inverted membrane vesicle (IMV), liposome and nanodisc. Among them, the IMV is a vesicle formed by fractioned plasma membrane with an inverted direction [41]. The vesicle is extracted from the membrane fraction of E. coli cells expressing HKs by ultracentrifugation. Although IMV is the simplest method among these approaches, it has a limitation in the purity of the HK. In fact, IMV is typically a mixture of various proteins embedded in the membrane. The purity of HK ranges from 5% to 30% in total protein, and affinity chromatograph can occasionally be used to increase the purity of HK embedded in IMV [42].
The level of purity obtained with liposomes and nanodiscs is improved compared with IMV, but these systems are more experimentally complicated. A liposome is an artificial spherical vesicle with at least one lipid bilayer that could be inserted by transmembrane HKs to construct a membrane system [43,44]. Full-length HKs are purified, dissolved in appropriate detergents and then integrated into the phospholipid bilayer after self-assembly and dialysis. To study HK biochemistry, one of the considerations for the use of liposomes is the direction of HKs in the lipid bilayer. Some of the HKs are incorrectly embedded in the liposome such that their sensor regions are located in the ‘ball’ of phospholipid, which blocks the interaction between sensor regions and chemicals. However, this problem is solved by nanodiscs. Nanodisc is also a synthetic membrane system with a phospholipid bilayer with a planar structure [45]. Two membrane-scaffolding proteins are used to assist the assembly of membrane proteins into the phospholipid bilayer. In addition, selection of appropriate scaffolding proteins can control the number of protein molecules that are integrated into the membrane, thus producing various oligomers, such as dimers, trimers and tetramers [46]. The application of these membrane protein techniques is a prerequisite to study the perception function of bacterial HKs.
4. Bacterium employs the VgrSR system to sense iron depletion and iron repletion
Iron is an essential metal for cellular organisms that participate in crucial biological processes, including photosynthesis, respiration, enzymatic activity and redox reactions [47]. In addition, excess iron is toxic to cells given that it catalyses the Fenton reaction in the presence of oxygen to deliver reactive oxygen species that are harmful to multiple biomacromolecules [48]. Although iron is the fourth most abundant element in the Earth's crust, upon infection, host plants and animals tend to govern iron by chelating the metal or retaining it in macromolecules. Therefore, pathogenic bacteria must survive in an iron-depletion niche after infection. To maintain iron homeostasis, these pathogens must sense extracellular iron depletion to absorb iron into the cells and sense an intracellular iron-replete environment to avoid the destructive role of iron excess.
VgrS–VgrR of X. campestris pv. campestris is a regulatory TCS of virulence [35,49]. VgrS is a HK that has a transmembrane region and a putative periplasmic sensor, whereas VgrR is an OmpR-family RR that contains a C-terminal helix-turn-helix output region. VgrR exhibits transcription factor activity to bind an AT-rich, palindromic motif in the promoter regions of approximately 400 genes as revealed by chromatin immunoprecipitation (ChIP-seq) analysis [50]. VgrS–VgrR constitutes a typical TCS given that the full-length VgrS can be autophosphorylated and then phosphorylates VgrR. Given that the half-life of the phosphorylated VgrR is as short as approximately 10 s, whether VgrS exhibits phosphatase activity is currently unknown for technical reasons. Inactivation of vgrR in various Xanthomonas species resulted in significant attenuation of virulence, and vgrS mutations caused a slight reduction in virulence. In addition, vgrS–vgrR mutations caused deficiencies in bacterial stress responses, metal tolerance, bacterial growth and the ability to elicit hypersensitivity on non-host plants [51,52]. VgrS–VgrR is an important, evolutionarily conserved virulence regulator in pathogenic bacteria belonging to Xanthomonas.
A recent study revealed that VgrS–VgrR is involved in the critical regulatory system in sensing and responding to iron repletion and depletion [50]. VgrS harbours a typical ExxE (EPQE) motif within its periplasmic sensor region to specifically bind ferric iron rather than ferrous iron. The Fe3+–VgrS interaction remarkably inhibits VgrS autophosphorylation. In iron-depleted environments, dissociation of Fe3+ from the sensor domain of VgrS thus activates VgrS autokinase activity and promotes phosphotransfer from VgrS to VgrR (figure 1). Consequently, the phosphorylated VgrR regulates the transcription of hundreds of genes, especially those involved in iron uptake from the host plant. Interestingly, phosphorylated VgrR behaves as a transcriptional repressor to inhibit the transcription of tdvA, a TonB-dependent receptor gene that is detrimental to iron uptake and virulence. Genetic inactivation of tdvA significantly increased bacterial virulence and iron uptake, suggesting that TdvA must exhibit a special function that differs from most of the TonB-dependent receptors [50].
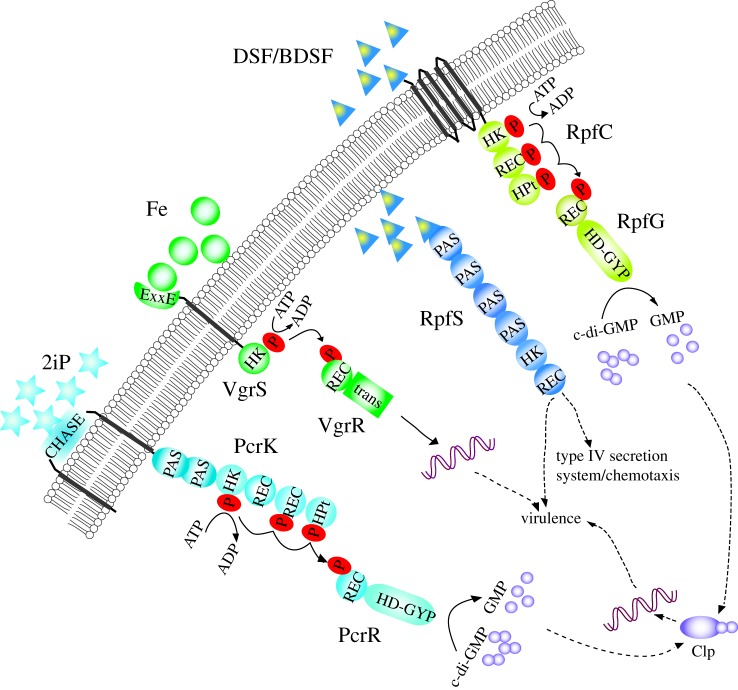
Figure 1.
Biochemical mechanisms underlying histidine kinase-mediated perception of host plant and environmental signals of Xanthomonas campestris pv. campestris. VgrSR, RpfCG, RpfS and PcrKR systems are depicted. P in red circles indicate phosphoryl group. Domains are reported based on the Pfam and SMART databases. HD-GYP, histidine–aspartic acid–glycine–tyrosine–proline (HD-GYP) domain; HK: HisKA domain; pfam acc. no. PF07730; PAS, Per–Arnt–Sim domain; REC: response reg domain, PF00072; HPt: HPT domain, PF01627; CHASE: CHASE domain, PF03924; PAS: PAS/PAS4/PAS8 domain, PF00989/08448/13188; HD-GYP: HD-GYP domain, SM000471; Trans: trans_reg_C domain, SM000862. The known signals and regulation pathways were designated.
Otherwise, when the intracellular iron is in excess, Fe2+ binds to the RR VgrR to dissociate it from the promoters and VgrS. This process inactivates the transcription factor activity of VgrR and leads to the transcription initiation of tdvA. Expression of tdvA exhibits a negative effect on iron uptake of X. campestris pv. campestris. In contrast to VgrS, VgrR could bind ferrous iron (Fe3+). However, the physiological significance of VgrR–Fe3+ binding is unknown given that Fe3+ is generally reduced to Fe2+ within cells (figure 1) [50].
To our knowledge, VgrS is the first biochemically identified iron-sensing HK in phytopathogenic bacteria. Its function is more like the ExxE motif-carrying iron receptor of Pseudomonas aeruginosa BqsS [53], given that Fe3+ binding by BqsS and VgrS decreased their autokinase activities. This performance is quite different from the PmrB of Salmonella enterica. PmrB autophosphorylation levels are increased in the presence of high iron concentrations, which might be beneficial to bacteria surviving in the intestine of animals [54]. VgrS and PmrB contain iron-binding ExxE motifs. PmrB can bind both Fe3+ and Fe2+, but VgrS specifically interacts with Fe3+. The molecular mechanism determining the specificity remains unknown.
5. Bacterium employs the RpfCG system to sense the quorum-sensing signal
Quorum sensing is an ecological process by which microbes measure their population size and elicit crucial physiological responses that are different from the planktonic lifestyle. A number of chemical signals used in bacterial cell–cell communication were identified, including homoserine lactones, quinolones and peptides [55]. In Xanthomonas spp. and their close relatives, a group of moderate chain, unsaturated fatty acids act as chemical signals to regulate quorum-sensing [56]. These fatty acids were named diffusible signal factors (DSFs), including DSF (cis-11-methyl-2-dodecenoic acid), BDSF (cis-2-dodecenoic acid), CDSF (cis,cis-11 methyldodeca-2,5-dienoic acid), IDSF (cis-10-methyl-2-dodecenoic acid) and SDSF (trans-2-decenoic acid). DSF family signals not only regulate the quorum sensing of Xanthomonas spp. but also are produced or detected by other organisms, such as Xylella fastidosa, Stenotrophomonas maltophilia, Burkholderia cenocepacia, P. aeruginosa and even the fungus Candida albicans, suggesting a role in inter-kingdom signalling. The biological roles and turnover of DSF family chemicals have been extensively reviewed elsewhere and will not be discussed here [57–60].
In X. campestris pv. campestris, it has long been predicted that the HK RpfC is the receptor of DSF. RpfC is a HyHK with additional REC and histidine-containing phosphotransfer (HPt) domains [61,62]. RpfC has five transmembrane helices, so it belongs to the Group II HKs. The cognate RR of RpfC is RpfG, a histidine–aspartic acid–glycine–tyrosine–proline (HD-GYP) domain-containing protein that exhibits phosphodiesterase activity to degrade the second messenger cyclic di-GMP into GMP. RpfC–RpfG is also a central system to modulate bacterial virulence. Inactivation of rpfC or rpfG severely decreases bacterial virulence, production of extracellular polysaccharides and various extracellular enzymes, but increases the production of DSF [58,61]. In addition to the aforementioned problems in studying DSF–RpfC interactions, the chemical nature of DSF, i.e. a fatty acid, made it more difficult to determine the HK–fatty acid interaction. For example, two proteins RpfS and RpfR were identified as DSF receptors [63,64]. However, these two proteins lack transmembrane regions and might act as intracellular receptors of DSF.
Recently, Cai et al. [65] successfully constructed an IMV and liposome that embedded the full-length RpfC. DSF stimulation remarkably doubled the autophosphorylation level of RpfC liposomes compared with the negative control. A series of deletion analyses revealed that both the transmembrane region and an N-terminal, short sensor of RpfC that is 22 amino acids in length, are important in DSF perception. The study then combined several biophysics methods, including microscale thermophoresis (MST), nanodifferential scanning fluorimetry (NanoDSF) and circular dichroism (CD) spectra, to demonstrate that DSF binds to the 22-amino acid peptide of RpfC (figure 1). The binding affinity measured by MST analysis is at the nanomole level, suggesting a relatively strong interaction between DSF and RpfC. In addition, critical amino acid residues impacting DSF binding were identified, revealing five residues near the first transmembrane helix. In addition, the study used alanine-scanning mutagenesis to identify the conserved juxtamembrane region of RpfC, which links the transmembrane region and the transmitter region of RpfC and autoinhibits its autokinase activity in the absence of DSF. DSF stimulation releases this autoinhibition in an allosteric manner and activates the RpfC. In the juxtamembrane region, substitutions of two residues, Leu172 and Ala178, could constitutively activate RpfC autophosphorylation regardless of the absence or presence of DSF stimulation [65]. Therefore, these results demonstrate that RpfC is the bona fide receptor of DSF and that the juxtamembrane region of HK exhibits an autoinhibition function, which is similar to that noted in the eukaryotic tyrosine kinases (figure 1). The establishment of the DSF–RpfC relationship and related research approaches will promote in-depth investigations on the specificity of other DSF family signals in various bacteria.
6. Bacterium employs the PcrKR system to sense plant hormones
In pathogenic bacteria infecting animals, perception of host signalling chemicals by HKs was discovered, revealing that inter-kingdom signalling is critical in the regulation of bacterial virulence. For example, E. coli senses animal adrenaline or epinephrine via the HK QseC and senses enteric fucose via the HK FusK [25,66]. Will plant pathogenic bacteria in parallel sense host-derived signalling chemicals, such as plant hormones?
In plants, two types of hormone receptors are HKs [67]: cytokinin and ethylene receptors (such as AHK2/AHK3/AHK4 and ETR1/ETR2/EIN4 of Arabidopsis thaliana). This finding prompts an interesting question given that plant pathogenic bacteria have the potential ability to sense these two hormones, especially considering that there are a large number of HKs in the bacterial cell. To challenge this hypothesis, Wang et al. identified the HK PcrK from a protein expression library containing all of the 52 HKs of Xanthomonas campestris pv. campestris [39]. PcrK is a HyHK with an experimentally confirmed periplasmic CHASE domain, two additional REC domains and a HPt domain. PcrK and its cognate RR PcrR control bacterial virulence (figure 1). In vitro phosphorylation assays revealed that plant cytokinin 2-isopentenyladenine (2iP) remarkably inhibited PcrK autokinase activity. This inhibition is specific given that other plant cytokinins, such as trans-zeatin (tZT) and cis-zeatin (cZT) and kintin, did not exhibit a similar effect. In addition, 2iP binds firmly to the periplasmic CHASE domain of PcrK with a dissociation constant at the nanomole level [39]. The 2iP–PcrK interaction promoted bacterial resistance to oxidative stress via the 3′,5′-cyclic diguanylic acid (c-di-GMP) regulatory pathway. Briefly, 2iP stimulation decreased PcrK–PcrR phosphorylation levels, whereas dephosphorylated PcrR exhibits increased phosphoesterase activity to degrade c-di-GMP, which elicits the expression of more than 50 genes (figure 1). Among them, a TonB-dependent receptor CtrA is critical in the oxidative stress response [58].
PcrK is the first experimentally identified prokaryotic receptor of plant hormones [68,69]. This result thus prompts two questions. Do other microbial HKs sense plant cytokinin? PcrK contains a CHASE domain as the plant cytokinin receptor. CHASE-like domains are widely distributed protein motifs in prokaryotes, including a number of plant-associated bacteria. The CHASE domain is differentiated into CHASE, CHASE2, CHASE3 and CHASE4 domains [70]. Therefore, it is possible that other CHASE domain-containing HKs also sense cytokinins. For example, although the receptor remains unidentified, cytokinin is important for the virulence of the animal pathogen Mycobacterium tuberculosis [71]. The second question is associated with evolution. How did pathogenic bacteria evolve receptors to detect plant cytokinin? Cytokinins are adenine derivatives that have an ancient evolutionary history, whereas the genus of Xanthomonas originated before 1600 million years ago, which is much earlier than the appearance of the original plants in the Earth. We tend to believe that PcrK-like receptors originated early to detect cytokinins, and these receptors then coevolved and hijacked cytokinin signalling after the bacteria–host plant relationship was established in evolutionary history. Future studies are needed to clarify these problems.
7. Concluding remarks and future perspectives
One of the fundamental scientific questions that puzzles plant pathologists is how phytopathogenic bacteria recognize host plants or plant-associated environmental factors. Recent biochemical studies on the relationships between VgrS-iron depletion, RpfC-DSF and PcrK-cytokinin then revealed that bacterial HKs not only monitor and detect environmental stimuli but also sense important plant chemicals, suggesting that inter-kingdom communication between host plant and bacteria is mutual. Although most of the studies were performed in X. campestris pv. campestris, it is anticipated that other cases will soon be reported given the diversity of plant–bacteria interactions. The results of such studies not only decipher the molecular pathogenesis of pathogens but also can identify lead compounds and ideal molecular targets to develop creative approaches to combat bacterial pathogens.
Acknowledgements
We thank members of our laboratory for their helpful comments on this manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
F.-F.W. and W.Q. conceived, designed and drafted the original manuscript and approved the final version to be published.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB11040700 to W.Q.), the National Science Foundation of China (grant nos 31870119 and 31400071 to F.-F.W. and 31671989 and 31671989 to W.Q.), the Ministry of Science and Technology of China (grant no. 2016YFD0100602) and the State Key Laboratory of Plant Genomics.
References
- 1.Ronald PC, Beutler B. 2010. Plant and animal sensors of conserved microbial signatures. Science 330, 1061–1064. ( 10.1126/science.1189468) [DOI] [PubMed] [Google Scholar]
- 2.Jones JD, Dangl JL. 2006. The plant immune system. Nature 444, 323–329. ( 10.1038/nature05286) [DOI] [PubMed] [Google Scholar]
- 3.Zipfel C. 2014. Plant pattern-recognition receptors. Trends Immunol. 35, 345–351. ( 10.1016/j.it.2014.05.004) [DOI] [PubMed] [Google Scholar]
- 4.Smale ST. 2012. Transcriptional regulation in the innate immune system. Curr. Opin. Immunol. 24, 51–57. ( 10.1016/j.coi.2011.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Neill LA, Golenbock D, Bowie AG. 2013. The history of Toll-like receptors — redefining innate immunity. Nat. Rev. Immunol. 13, 453–460. ( 10.1038/nri3446) [DOI] [PubMed] [Google Scholar]
- 6.Volchenkov R, Sprater F, Vogelsang P, Appel S. 2012. The 2011 Nobel Prize in physiology or medicine. Scand. J. Immunol. 75, 1–4. ( 10.1111/j.1365-3083.2011.02663.x) [DOI] [PubMed] [Google Scholar]
- 7.Couto D, Zipfel C. 2016. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552. ( 10.1038/nri.2016.77) [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Gomez L, Boller T. 2000. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 5, 1003–1011. ( 10.1016/S1097-2765(00)80265-8) [DOI] [PubMed] [Google Scholar]
- 9.Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. 2006. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125, 749–760. ( 10.1016/j.cell.2006.03.037) [DOI] [PubMed] [Google Scholar]
- 10.Miya A, et al. 2007. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl Acad. Sci. USA 104, 19 613–19 618. ( 10.1073/pnas.0705147104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willmann R, et al. 2011. Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc. Natl Acad. Sci. USA 108, 19 824–19 829. ( 10.1073/pnas.1112862108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranf S, et al. 2015. A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat. Immunol. 16, 426–433. ( 10.1038/ni.3124) [DOI] [PubMed] [Google Scholar]
- 13.Venturi V, Fuqua C. 2013. Chemical signaling between plants and plant-pathogenic bacteria. Annu. Rev. Phytopathol. 51, 17–37. ( 10.1146/annurev-phyto-082712-102239) [DOI] [PubMed] [Google Scholar]
- 14.Glick BR. 2012. Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012, 963401 ( 10.6064/2012/963401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stock AM, Robinson VL, Goudreau PN. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69, 183–215. ( 10.1146/annurev.biochem.69.1.183) [DOI] [PubMed] [Google Scholar]
- 16.Hoch JA. 2000. Two-component and phosphorelay signal transduction. Curr. Opin Microbiol. 3, 165–170. ( 10.1016/S1369-5274(00)00070-9) [DOI] [PubMed] [Google Scholar]
- 17.Bourret RB, Silversmith RE. 2010. Two-component signal transduction. Curr. Opin Microbiol. 13, 113–115. ( 10.1016/j.mib.2010.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khorchid A, Ikura M. 2006. Bacterial histidine kinase as signal sensor and transducer. Int. J. Biochem. Cell Biol. 38, 307–312. ( 10.1016/j.biocel.2005.08.018) [DOI] [PubMed] [Google Scholar]
- 19.Thomason P, Kay R. 2000. Eukaryotic signal transduction via histidine-aspartate phosphorelay. J. Cell Sci. 113, 3141–3150. [DOI] [PubMed] [Google Scholar]
- 20.Herivaux A, So YS, Gastebois A, Latge JP, Bouchara JP, Bahn YS, Papon N. 2016. Major sensing proteins in pathogenic fungi: the hybrid histidine kinase family. PLoS Pathog. 12, e1005683 ( 10.1371/journal.ppat.1005683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Defosse TA, et al. 2015. Hybrid histidine kinases in pathogenic fungi. Mol. Microbiol. 95, 914–924. ( 10.1111/mmi.12911) [DOI] [PubMed] [Google Scholar]
- 22.Ninfa AJ, Magasanik B. 1986. Covalent modification of the GlnG product, Nri, by the Glnl product, NRii, regulates the transcription of the GlnAlg operon in Escherichia coli. Proc. Natl Acad. Sci. USA 83, 5909–5913. ( 10.1073/pnas.83.16.5909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascher T. 2014. Bacterial (intramembrane-sensing) histidine kinases: signal transfer rather than stimulus perception. Trends Microbiol. 22, 559–565. ( 10.1016/j.tim.2014.05.006) [DOI] [PubMed] [Google Scholar]
- 24.Jung K, Fried L, Behr S, Heermann R. 2012. Histidine kinases and response regulators in networks. Curr. Opin Microbiol. 15, 118–124. ( 10.1016/j.mib.2011.11.009) [DOI] [PubMed] [Google Scholar]
- 25.Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. 2006. The QseC sensor kinase: a bacterial adrenergic receptor. Proc. Natl Acad. Sci. USA 103, 10 420–10 425. ( 10.1073/pnas.0604343103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groisman EA. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183, 1835–1842. ( 10.1128/Jb.183.6.1835-1842.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SY, Groisman EA. 2014. Signal-specific temporal response by the Salmonella PhoP/PhoQ regulatory system. Mol. Microbiol. 91, 135–144. ( 10.1111/mmi.12449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trajtenberg F, et al. 2014. Allosteric activation of bacterial response regulators: the role of the cognate histidine kinase beyond phosphorylation. mBio 5, e02105 ( 10.1128/mBio.02105-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scharf BE. 2010. Summary of useful methods for two-component system research. Curr. Opin Microbiol. 13, 246–252. ( 10.1016/j.mib.2010.01.006) [DOI] [PubMed] [Google Scholar]
- 30.Pfluger T, et al. 2018. Signaling ammonium across membranes through an ammonium sensor histidine kinase. Nat. Commun. 9, 164 ( 10.1038/s41467-017-02637-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Silva ACR, et al. 2002. Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417, 459–463. ( 10.1038/417459a) [DOI] [PubMed] [Google Scholar]
- 32.Qian W, et al. 2005. Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris. Genome Res. 15, 757–767. ( 10.1101/gr.3378705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vorholter FJ, et al. 2008. The genome of Xanthomonas campestris pv. campestris B100 and its use for the reconstruction of metabolic pathways involved in xanthan biosynthesis. J. Biotechnol. 134, 33–45. ( 10.1016/j.jbiotec.2007.12.013) [DOI] [PubMed] [Google Scholar]
- 34.Qian W, Han ZJ, He C. 2008. Two-component signal transduction systems of Xanthomonas spp.: a lesson from genomics. Mol. Plant Microbe Interact. 21, 151–161. ( 10.1094/MPMI-21-2-0151) [DOI] [PubMed] [Google Scholar]
- 35.Qian W, Han ZJ, Tao J, He C. 2008. Genome-scale mutagenesis and phenotypic characterization of two-component signal transduction systems in Xanthomonas campestris pv. campestris ATCC 33913. MPMI 21, 1128–1138. ( 10.1094/MPMI-21-8-1128) [DOI] [PubMed] [Google Scholar]
- 36.Karniol B, Vierstra RD. 2004. The HWE histidine kinases, a new family of bacterial two-component sensor kinases with potentially diverse roles in environmental signaling. J. Bacteriol. 186, 445–453. ( 10.1128/JB.186.2.445-453.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang JL, Liu YN, Barber CE, Dow JM, Wootton JC, Daniels MJ. 1991. Genetic and molecular analysis of a cluster of Rpf genes involved in positive regulation of synthesis of extracellular enzymes and polysaccharide in Xanthomonas campestris pathovar campestris. Mol. Gen. Genet. 226, 409–417. [DOI] [PubMed] [Google Scholar]
- 38.Peng BY, Pan Y, Li RJ, Wei JW, Liang F, Wang L, Wang FF, Qian W. 2017. An essential regulatory system originating from polygenic transcriptional rewiring of PhoP-PhoQ of Xanthomonas campestris. Genetics 206, 2207–2223. ( 10.1534/genetics.117.200204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang FF, Cheng ST, Wu Y, Ren BZ, Qian W. 2017. A bacterial receptor PcrK senses the plant hormone cytokinin to promote adaptation to oxidative stress. Cell Rep. 21, 2940–2951. ( 10.1016/j.celrep.2017.11.017) [DOI] [PubMed] [Google Scholar]
- 40.Li RF, et al. 2014. Identification of a putative cognate sensor kinase for the two-component response regulator HrpG, a key regulator controlling the expression of the hrp genes in Xanthomonas campestris pv. campestris. Environ. Microbiol. 16, 2053–2071. ( 10.1111/1462-2920.12207) [DOI] [PubMed] [Google Scholar]
- 41.Rosen BP, Mcclees JS. 1974. Active-transport of calcium in inverted membrane-vesicles of Escherichia coli. Proc. Natl Acad. Sci. USA 71, 5042–5046. ( 10.1073/pnas.71.12.5042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung K, Tjaden B, Altendorf K. 1997. Purification, reconstitution, and characterization of KdpD, the turgor sensor of Escherichia coli. J. Biol. Chem. 272, 10 847–10 852. ( 10.1074/jbc.272.16.10847) [DOI] [PubMed] [Google Scholar]
- 43.Reading NC, Rasko D, Torres AG, Sperandio V. 2010. A transcriptome study of the QseEF two-component system and the QseG membrane protein in enterohaemorrhagic Escherichia coli O157:H7. Microbiology-SGM 156, 1167–1175. ( 10.1099/mic.0.033027-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reading NC, Rasko DA, Torres AG, Sperandio V. 2009. The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis. Proc. Natl Acad. Sci. USA 106, 5889–5894. ( 10.1073/pnas.0811409106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bayburt TH, Sligar SG. 2010. Membrane protein assembly into Nanodiscs. FEBS Lett. 584, 1721–1727. ( 10.1016/j.febslet.2009.10.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritchie TK, Grinkova YV, Bayburt TH, Denisov IG, Zolnerciks JK, Atkins WM, Sligar SG. 2009. Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Method Enzymol. 464, 211–231. ( 10.1016/S0076-6879(09)64011-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cassat JE, Skaar EP. 2013. Iron in infection and immunity. Cell Host Microbe 13, 510–520. ( 10.1016/j.chom.2013.04.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nairz M, Haschka D, Demetz E, Weiss G. 2014. Iron at the interface of immunity and infection. Front. Pharmacol. 5, 152 ( 10.3389/Fphar.2014.00152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang SS, et al. 2008. A putative colR(XC1049)-col(XC1050) two-component signal transduction system in Xanthomonas campestris positively regulates hrpC and hrpE operons and is involved in virulence, the hypersensitive response and tolerance to various stresses. Res. Microbiol. 159, 569–578. ( 10.1016/j.resmic.2008.06.010) [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Pan Y, Yuan ZH, Zhang H, Peng BY, Wang FF, Qian W. 2016. Two-component signaling system VgrRS directly senses extracytoplasmic and intracellular iron to control bacterial adaptation under iron depleted stress. PLoS Pathog. 12, e1006133 ( 10.1371/journal.ppat.1006133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu RQ, et al. 2008. AvrAC(Xcc8004), a type III effector with a leucine-rich repeat domain from Xanthomonas campestris pathovar campestris confers avirulence in vascular tissues of Arabidopsis thaliana ecotype Col-0. J. Bacteriol. 190, 343–355. ( 10.1128/Jb.00978-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan Q, Wang NA. 2011. The ColR/ColS two-component system plays multiple roles in the pathogenicity of the citrus canker pathogen Xanthomonas citri subsp citri. J. Bacteriol. 193, 1590–1599. ( 10.1128/Jb.01415-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kreamer NN, Wilks JC, Marlow JJ, Coleman ML, Newman DK. 2012. BqsR/BqsS constitute a two-component system that senses extracellular Fe(II) in Pseudomonas aeruginosa. J. Bacteriol. 194, 1195–1204. ( 10.1128/JB.05634-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen HD, Groisman EA. 2013. The biology of the PmrA/PmrB two-component system: the major regulator of lipopolysaccharide modifications. Annu. Rev. Microbiol. 67, 83–112. ( 10.1146/annurev-micro-092412-155751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rutherford ST, Bassler BL. 2012. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harbor Perspect. Med. 2, a012427 ( 10.1101/cshperspect.a012427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang LH, et al. 2004. A bacterial cell–cell communication signal with cross-kingdom structural analogues. Mol. Microbiol. 51, 903–912. ( 10.1046/j.1365-2958.2003.03883.x) [DOI] [PubMed] [Google Scholar]
- 57.He YW, Zhang LH. 2008. Quorum sensing and virulence regulation in Xanthomonas campestris. FEMS Microbiol. Rev. 32, 842–857. ( 10.1111/j.1574-6976.2008.00120.x) [DOI] [PubMed] [Google Scholar]
- 58.Deng YY, Wu JE, Tao F, Zhang LH. 2011. Listening to a new language: DSF-based quorum sensing in Gram-negative bacteria. Chem. Rev. 111, 160–173. ( 10.1021/cr100354f) [DOI] [PubMed] [Google Scholar]
- 59.Zhou L, Zhang LH, Camara M, He YW. 2017. The DSF family of quorum sensing signals: diversity, biosynthesis, and turnover. Trends Microbiol. 25, 293–303. ( 10.1016/j.tim.2016.11.013) [DOI] [PubMed] [Google Scholar]
- 60.Ryan RP, An SQ, Allan JH, McCarthy Y, Dow JM. 2015. The DSF family of cell–cell signals: an expanding class of bacterial virulence regulators. PLoS Pathog. 11, e1004986 ( 10.1371/journal.ppat.1004986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He YW, Wang C, Zhou L, Song HW, Dow JM, Zhang LH. 2006. Dual signaling functions of the hybrid sensor kinase RpfC of Xanthomonas campestris involve either phosphorelay or receiver domain-protein interaction. J. Biol. Chem. 281, 33 414–33 421. ( 10.1074/jbc.M606571200) [DOI] [PubMed] [Google Scholar]
- 62.Slater H, Alvarez-Morales A, Barber CE, Daniels MJ, Dow JM. 2000. A two-component system involving an HD-GYP domain protein links cell–cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol. Microbiol. 38, 986–1003. ( 10.1046/j.1365-2958.2000.02196.x) [DOI] [PubMed] [Google Scholar]
- 63.Deng YY, et al. 2012. Cis-2-dodecenoic acid receptor RpfR links quorum-sensing signal perception with regulation of virulence through cyclic dimeric guanosine monophosphate turnover. Proc. Natl Acad. Sci. USA 109, 15 479–15 484. ( 10.1073/pnas.1205037109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.An SQ, Allan JH, McCarthy Y, Febrer M, Dow JM, Ryan RP. 2014. The PAS domain-containing histidine kinase RpfS is a second sensor for the diffusible signal factor of Xanthomonas campestris. Mol. Microbiol. 92, 586–597. ( 10.1111/mmi.12577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cai Z, Yuan ZH, Zhang H, Pan Y, Wu Y, Tian XQ, Wang FF, Wang L, Qian W. 2017. Fatty acid DSF binds and allosterically activates histidine kinase RpfC of phytopathogenic bacterium Xanthomonas campestris pv. campestris to regulate quorum-sensing and virulence. PLoS Pathog. 13, e1006304 ( 10.1371/journal.ppat.1006304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. 2012. Fucose sensing regulates bacterial intestinal colonization. Nature 492, 113–117. ( 10.1038/nature11623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanstraelen M, Benkova E. 2012. Hormonal interactions in the regulation of plant development. Annu. Rev. Cell Dev. Biol. 28, 463–487. ( 10.1146/annurev-cellbio-101011-155741) [DOI] [PubMed] [Google Scholar]
- 68.Kabbara S, Schmulling T, Papon N. 2018. CHASEing cytokinin receptors in plants, bacteria, fungi, and beyond. Trends Plant Sci. 23, 179–181. ( 10.1016/j.tplants.2018.01.001) [DOI] [PubMed] [Google Scholar]
- 69.Naseem M, Bencurova E, Dandekar T. 2018. The cytokinin-activating LOG-family proteins are not lysine decarboxylases. Trends Biochem. Sci. 43, 232–236. ( 10.1016/j.tibs.2018.01.002) [DOI] [PubMed] [Google Scholar]
- 70.Anantharaman V, Aravind L. 2001. The CHASE domain: a predicted ligand-binding module in plant cytokinin receptors and other eukaryotic and bacterial receptors. Trends Biochem. Sci. 26, 579–582. ( 10.1016/S0968-0004(01)01968-5) [DOI] [PubMed] [Google Scholar]
- 71.Samanovic MI, et al. 2015. Proteasomal control of cytokinin synthesis protects Mycobacterium tuberculosis against nitric oxide. Mol. Cell 57, 984–994. ( 10.1016/j.molcel.2015.01.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.