Abstract
Many hemipteran insects that can transmit plant viruses in a persistent and transovarial manner are generally associated with a common obligate bacterial symbiont Sulcia and its β-proteobacterial partner. Rice dwarf virus (RDV), a plant reovirus, can bind to the envelope of Sulcia through direct interaction of the viral minor outer capsid protein P2 with the bacterial outer membrane protein, allowing the virus to exploit the ancient oocyte entry path of Sulcia in rice leafhopper vectors. Here, we show that RDV can hitchhike with both Sulcia and its β-proteobacterial partner Nasuia to ensure their simultaneous transovarial transmission. Interestingly, RDV can move through the outer envelope of Nasuia and reside in the periplasmic space, which is mediated by the specific interaction of the viral major outer capsid protein P8 and the porin channel on the bacterial outer envelope. Nasuia porin-specific antibody efficiently interferes with the binding between RDV and the Nasuia envelope, thus strongly preventing viral transmission to insect offspring. Thus, RDV has evolved different strategies to exploit the ancient oocyte entry paths used by two obligate bacterial symbionts in rice leafhoppers. Our results thus reveal that RDV has formed complex, cooperative interactions with both Sulcia and Nasuia during their joint transovarial transmission.
This article is part of the theme issue ‘Biotic signalling sheds light on smart pest management’.
Keywords: rice dwarf virus, rice leafhopper, transovarial transmission, obligate bacterial symbionts, Nasuia and Sulcia, porin channel
1. Introduction
At least 10% of all insect species harbour obligate symbionts that supply essential nutrients and other benefits to their hosts [1,2]. Obligate bacterial symbionts generally reside intracellularly within specialized organs and cells (bacteriomes and bacteriocytes, respectively) that regulate symbiont functions and ensure vertical transmission to subsequent generations [3]. Sap-sucking insects in the hemipteran suborder Auchenorrhyncha, including treehoppers, leafhoppers, and planthoppers, are global pests and generally associated with the obligate bacterium ‘Candidatus Sulcia muelleri’ (hereafter Sulcia) and one β-proteobacterial partner, which is usually replaced by other bacterial symbionts or yeast-like microorganisms [4–6]. The gram-negative bacteria Sulcia and β-proteobacteria each comprise two envelopes with an outer and an inner membrane and the periplasmic space [7]. Various protein channels on the bacterial outer membrane (OM) are involved in the transport, uptake or efflux of diverse compounds including nutrients and toxins [8]. Porins, a class of outer membrane proteins (OMPs) of gram-negative bacteria, are essentially trimeric β-barrels that form channels, possibly facilitating metabolic exchanges between the symbiont and the host cell [9]. Although the structural features and functions of porins in bacteria–host interactions have been well studied [10,11], their roles in the interactions of bacterial symbionts with other microorganisms are still poorly understood. Previous investigations showed that some bacterial symbionts can pass through the outer envelopes of other bacterial symbionts in insects. For example, in leafhopper Cicadella viridis, the secondary γ-proteobacterium symbiont Sodalis, as well as Arsenophonus in the leafhopper Macrosteles laevis, can pass through the envelope of the obligate symbiont Sulcia [12,13]. In consequence, Sodalis and Arsenophonus can directly enter the cytoplasm of the primary bacteria to ensure their simultaneous transmission through insect generations [12,13]. These two bacterial symbionts presumably must rely on the porin channels on the outer envelopes for transport into Sulcia. Thus, understanding how the outer envelopes of insect bacterial symbionts respond to other microorganisms would offer important evolutionary insights into numerous interactions among microorganisms in nature.
Most obligate bacterial symbionts are transovarially transmitted by female insects through eggs [14]. Similarly, many plant viruses can be transovarially transmitted by female hemipteran insects to their offspring via infection to ovaries for sustaining virus populations in nature [15]. We hypothesized that the long-term coexistence of bacterial symbionts and plant viruses on their pathway into the oocyte may lead to specific evolutionary outcomes for cross-kingdom interaction between a virus and a bacterium in nature. In general, bacterial symbionts can indirectly affect viral transmission by enhancing immunity and resistance to viruses in insects [16]. In the haemolymph, the GroEL homologue proteins encoded by bacterial symbionts of vector aphids or whiteflies have been reported to interact with the capsid proteins of luteoviruses or begomoviruses and thus ensure their entry into the salivary glands [17,18]; however, the evidence is contradictory. Rice leafhoppers, the hosts of obligate bacteria Sulcia and a β-proteobacterium ‘Ca. Nasuia deltocephalinicola’ (hereafter Nasuia), can vertically transmit rice dwarf virus (RDV, plant reovirus) to offspring [19]. RDV causes disease outbreaks and extensive rice yield losses in Asian rice-growing countries and is the first recorded plant virus to be transovarially transmitted by an insect vector [19,20]. RDV, belonging to the genus Phytoreovirus in the family Reoviridae [21], is an icosahedral, double-layered particle 70 nm in diameter with one minor outer capsid protein P2 and one major outer capsid protein P8 [22,23]. We previously reported that RDV virions can attach to the outer envelope of Sulcia and induce the formation of virus-containing invaginations or vesicles, allowing RDV to hitchhike on the transovarial transmission route used by obligate symbionts in the rice green leafhopper Nephotettix cincticeps [24]. The specific interaction of the viral minor outer capsid protein P2 and the conserved bacterial surface antigen (BSA) domain of Sulcia OMP mediates this virus–bacterium attachment [24]. However, RDV cannot pass through the Sulcia envelope and enter the cytoplasm [24]. Alternatively, RDV exploits only the Sulcia envelope as a transfer vehicle into insect offspring [24]. Here, we report evidence for a new model for the direct interaction of a plant virus with its vector's bacterial symbionts, wherein RDV particles directly pass through the porin channels on the outer envelopes of Nasuia and accumulate in the periplasmic space, enabling their joint vertical transmission to insect generations. The specific interaction of the viral major outer capsid protein P8 and Nasuia porin mediates this novel virus–bacterium association. Our results suggest that RDV, Sulcia and Nasuia form complex tripartite interactions during their joint transovarial transmission to the next insect generation.
2. Material and methods
(a). Insects, virus and antibodies
The rice green leafhopper N. cincticeps was reared on rice plants in insect-proof cages. The RDV isolate was collected from infected field rice in Sanming, Fujian, China, and its identity was confirmed by reverse-transcription polymerase chain reaction (RT-PCR). The virus was transmitted to TN-1 rice plants using N. cincticeps and methods described previously [25]. IgGs isolated from polyclonal antibodies against major outer capsid P8 of RDV were conjugated to fluorescein isothiocyanate (FITC) according to the manufacturer's instructions, as described previously [24].
(b). Electron microscopy
For observing the subcellular localization of RDV, Sulcia and Nasuia within insect ovaries, 50 ovaries from leafhoppers inoculated with RDV at different days (6 and 8 d) post-emergence were excised, fixed, dehydrated and embedded as described previously [24]. Thin sections were examined with an H-7650 Hitachi transmission electron microscope.
(c). Immunofluorescence microscopy and fluorescence in situ hybridization
The distributions of RDV, Sulcia or Nasuia in excised ovaries of N. cincticeps were observed by using short oligonucleotide DNA probes Sulcia-cy5 (5′-CTGAATTACAACGTACAAAACCC-3′) and Nasuia-cy3 (5′-GTACTAATTCTTTTACAAGCACTT-3′) (Sangon Biotech) and RDV P8-specific antibody directly conjugated to fluorescein isothiocyanate (P8-FITC). Two hundred second-instar nymphs of N. cincticeps were allowed a 2-day acquisition access period on rice plants infected with RDV, then allowed to feed on rice seedlings. At different days post-emergence, 30 insect ovaries were excised and fixed in 4% v/v paraformaldehyde and immunolabelled with P8-FITC. The ovaries were then fixed again for 20 min, pretreated in hybridization buffer (20 mM Tris–HCl, 180 mM NaCl, 10% v/v SDS, 30% v/v formamide) for 15 min, and incubated in hybridization buffer containing 10 nM oligonucleotide DNA probes targeting the 16S rRNA sequence of Sulcia and Nasuia, as described previously with slight modifications [24]. After 4 h incubation at 50°C, the samples were thoroughly washed in washing buffer (0.15 M NaCl, 0.015 M sodium citrate) and then observed with a Leica TCS SP5 confocal microscope. As controls, excised ovaries or salivary glands from rice leafhoppers that fed on healthy plants were prepared in the same way.
(d). Yeast two-hybrid assay
A yeast two-hybrid assay was performed to test the interaction between Nasuia porin and outer capsid proteins P2 or P8 of RDV, using the Matchmaker Gal4 Two-Hybrid System 3 (Clontech). Based on the sequence of the Nasuia porin gene from treehopper Entylia carinata (GenBank accession no. CP021173.1), the fragment of Nasuia porin gene from leafhopper N. cincticeps had been obtained by RT-PCR process. Nasuia porin and Sulcia OMP were constructed in the prey plasmid pGADT7 (electronic supplementary material, table S1). The full segment of RDV P8 gene and N-terminal segment of RDV P2 gene were constructed in the bait plasmid pGBKT7. The bait and prey plasmids were used to co-transform yeast strain AH109, and transformants were plated on DDO mediums to test protein interactions. Transformants were also streaked on QDO medium supplemented with 40 µg ml−1 5-bromo-4-chloro-3-indoxyl-α-d-galactopyranoside (X-α-Gal) to further confirm interaction of different co-transformants. The positive control pGBKT7-53/pGADT7-T and negative control pGBKT7-Lam/pGADT7-T were transformed in the same way.
(e). Glutathione S-transferase pull-down assay
Nasuia porin cDNA fragment was cloned into pGEX-3X for fusion with glutathione S-transferase (GST) (electronic supplementary material, table S1). RDV P8 cDNA fragment was cloned into pDEST17 for fusion with His-tag. All recombinant proteins were expressed in Escherichia coli strain Rosetta and purified. The GST-tag fused protein, GST-Nasuia porin, was first bound to GST-Sepharose 4B beads (GE) for 3 h at 4°C, then the mixture was centrifuged for 5 min at 500g, and the supernatant was discarded. His-RDV P8 was added to the beads and incubated for 2 h at 4°C. After being centrifuged and washed five times with washing buffer (300 mM NaCl, 10 mM Na2HPO3, 2.7 mM KCl and 1.7 M KH2PO4), the bead-bound proteins were separated by SDS-PAGE and detected by western blot with His-tag antibody and GST-tag antibody (Sigma).
(f). Nasuia porin-specific antibody preparation and interference with Rice dwarf virus–Nasuia binding
Mouse polyclonal antibody against Nasuia porin was prepared as described previously [26]. Briefly, the Nasuia porin gene was amplified and inserted into plasmid pH4 (electronic supplementary material, table S1). The recombinant plasmid was used to transform E. coli strain Rosetta to express the targeted protein, which was then injected into mice to produce antibodies. To test the specificity of antibody against Nasuia porin, we extracted total proteins separately from female adult bacteriomes, abdomen I (segments 1 and 2), abdomen II (segments 3–5), and thorax and head, separated them by SDS-PAGE, and probed a western blot assay with porin-specific antibody.
To determine whether the antibody against Nasuia porin neutralized the direct interaction between RDV P8 and Nasuia porin, the abdomens of 30 adult female leafhoppers at 1 day after emergence were microinjected with 64 nl of a mixture of purified viruses (0.01 µg µl−1) and Nasuia porin-specific antibody (0.5 µg µl−1), and the insects were then allowed to feed on healthy rice seedlings. At 5 and 7 days post microinjection, the ovaries were immunolabelled with P8-FITC and hybridized with the probes Sulcia-cy5 and Nasuia-cy3, and then processed for immunofluorescence microscopy as described earlier [24]. As controls, adult female leafhoppers were microinjected with purified virions (0.01 µg µl−1) and pre-immune serum (0.5 µg µl−1) and treated in the same way. To further detect the effects of porin-specific antibody on transovarial transmission, we microinjected female leafhoppers with a mixture of RDV virions and porin-specific antibody and maintained them on rice seedlings. At 15 days post-emergence, 100 eggs were collected from rice seedlings and tested for RDV P8 transcript level by RT-qPCR assay. As controls, the female leafhoppers were microinjected with RDV virions and pre-immune serum and treated in the same way. In addition, the levels of RDV, Nasuia and Sulcia were detected by RT-PCR and western blot assays, respectively, after being treated with porin-specific antibody or pre-immune serum.
3. Results
(a). Rice dwarf virus virions move with Sulcia and Nasuia from the haemolymph into the oocytes of female N. cincticeps
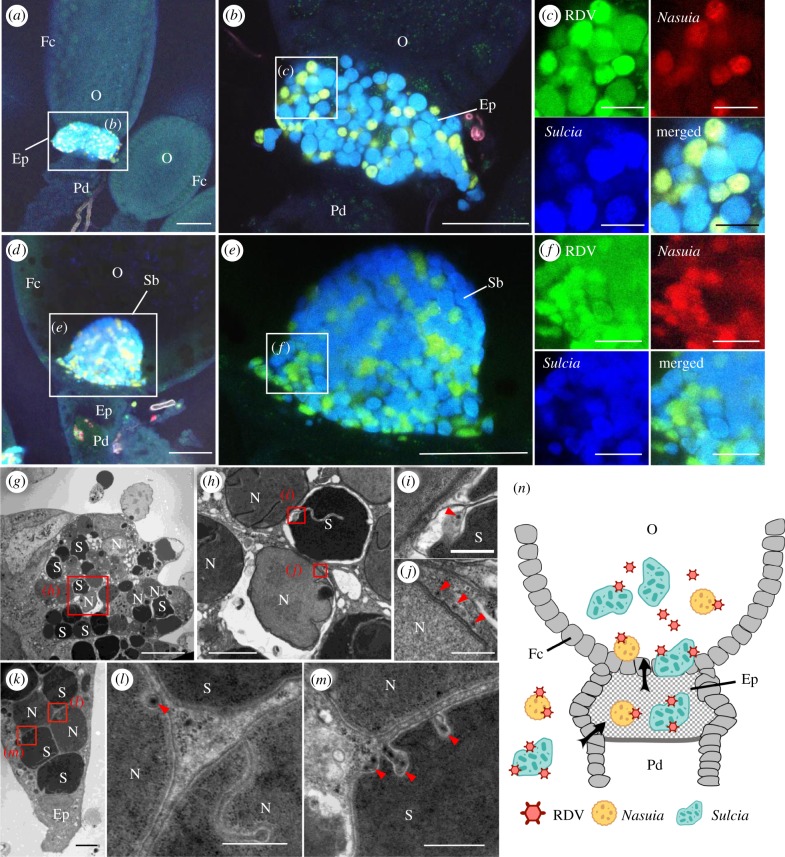
As the main vector of RDV, the green rice leafhopper N. cincticeps is associated with two types of obligate bacterial symbionts, the electron-dense Sulcia and electron-translucent Nasuia (electronic supplementary material, figure S1), which follow a common path to enter the ovaries of female leafhoppers [24]. In female insects, the ovary consists of several ovarioles, each of which contains a germarium, vitellarium and pedicel, from apex to base [27]. Oocytes produced by the germarium are linearly arranged within the vitellarium, and surrounded by a layer of follicular cells [27]. Electron microscopy showed that the two obligate bacterial symbionts simultaneously entered the same follicular epithelial cells surrounding the posterior pole of the oocyte, i.e. the epithelial plug, and then accumulated in the oocytes to form a characteristic ‘symbiont ball’ in the female adults of N. cincticeps (electronic supplementary material, figure S1) [28]. RT-qPCR assay revealed that the two obligate bacterial symbionts started to multiply at the vitellogenesis stage in the female adults of N. cincticeps (electronic supplementary material, figure S2). Their abundance in male insects was extremely low, and the copy number of Sulcia in the female adults was almost twice that of Nasuia (electronic supplementary material, figure S2a,b), suggesting that these two symbionts are essential for female reproduction. To observe the distribution of RDV virions and the two types of symbionts during oocyte invasion, the ovaries excised from viruliferous female adults of N. cincticeps were labelled with P8-FITC using immunofluorescence and specific oligonucleotide probes targeting the 16S rRNA of Sulcia or Nasuia (Sulcia-cy5 and Nasuia-cy3) using fluorescence in situ hybridization (FISH). At 6 days after emergence of adult females of N. cincticeps, RDV virions accompanied Sulcia and Nasuia into the epithelial plug (figure 1a–c). At 8 days after emergence of N. cincticeps, RDV accompanied Sulcia and Nasuia into the oocyte (figure 1d–f). We examined at least 50 virus-infected ovaries and found that RDV always colocalized with both symbionts. Electron microscopy confirmed that viral particles were distributed along the envelopes of Sulcia or Nasuia in the same follicular epithelial cells at the epithelial plug (figure 1g–m). Thus, RDV virions accompany both Sulcia and Nasuia to be transported from the haemolymph into the epithelial plug, and finally into the oocytes of N. cincticeps (figure 1n).
Figure 1.
RDV virions moved with the obligate bacterial symbionts Sulcia and Nasuia into the oocyte of female N. cincticeps. (a–c) Confocal micrographs show the colocalization of RDV with Sulcia or Nasuia in the epithelial plug of female insects at 6 days post-emergence. Panel b is an enlargement of boxed area in a. Panels in c are enlargements of boxed area in b. (d–f) Colocalization of RDV and Sulcia or Nasuia in the symbiont ball in the oocyte of female insects at 8 days post-emergence. Panel e is an enlargement of boxed area in d. Panels in f are enlargements of boxed area in e. Scale bars in a, b, d and e: 100 µm; c and f: 25 µm. (g–m) Transmission electron micrographs showing RDV particles distributed along the envelopes of Nasuia or Sulcia at the same follicular epithelial cells in the epithelial plug. Panel h is an enlargement of boxed area in g. Panels i and j are enlargements of boxed areas in h. Panels l and m are enlargements of boxed areas in k. Scale bars in g: 10 µm; h and k: 2 µm; i, j, l and m: 500 nm. (n) Model for the exploitation of bacterial symbionts Sulcia and Nasuia by RDV to enter the oocyte. RDV virions directly attach to the envelopes of Nasuia or Sulcia and then enter the oocyte from the epithelial plug along with Nasuia or Sulcia. Ep, epithelial plug; Fc, follicular cell; N, Nasuia, O, oocyte; Pd, pedicel; S, Sulcia; Sb, symbiont ball. Black arrows mark the entry route of virus-associated bacterial symbionts. Red arrows indicate RDV virions. All electron micrographs and immunofluorescence figures are representative of at least three replications.
(b). Distinct binding strategies for RDV virions with the envelopes of Sulcia and Nasuia
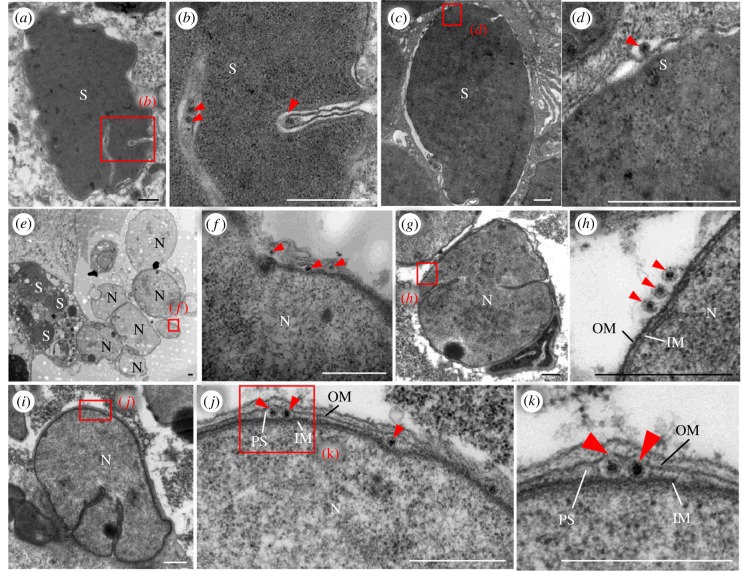
We then used electron microscopy to further observe how RDV virions were associated with Sulcia and Nasuia during their entry into the oocyte. As described previously [24], RDV virions attached to the outer envelopes of Sulcia, which finally formed virus-containing invaginations or vesicles (figure 2a–d). The envelope of Nasuia is surrounded by two layers of membrane structures with a few big invaginations (figure 2). Frequently, RDV particles can be seen on the outer envelopes of Nasuia, in the invagination or between the neighbouring symbionts (figure 2e–h). Interestingly, RDV virions were also found within the periplasmic space between the outer and inner membranes (IMs) of Nasuia (figure 2i–k). Among at least 40 virus-associated Nasuia samples, we calculated that about 20% had viruses in the periplasmic space (electronic supplementary material, figure S3). It appears that RDV virions may directly pass through the protein channels on the outer envelope of Nasuia and accumulate in the periplasmic space, which enables their joint vertical transmission to the next insect generation.
Figure 2.
Electron micrographs showing distinct interaction strategies for RDV virions with the envelopes of Sulcia or Nasuia. (a–d) RDV virions in the invaginations formed by Sulcia envelopes. Panels b and d are enlargements of boxed areas in panels a and c, respectively. Scale bars in a and c: 2 µm; b and d: 500 nm. (e–h) RDV virions attached to the envelope of Nasuia close to the epithelial plug. Panels f and h are enlargements of boxed areas in panels e and g, respectively. Scale bar: 500 nm. (i–k) RDV virions embedded in the periplasmic space between outer and IMs of Nasuia in the epithelial plug. Panels j and k are enlargements of boxed areas of panels i and j, respectively. Scale bars: 500 nm. IM, inner membranes; N, Nasuia; OM, outer membranes; PS, periplasmic space; S, Sulcia. Red arrowheads indicate RDV virions. All micrographs are representative of at least three replications.
(c). Distinct interaction strategies for Rice dwarf virus virions with the envelopes of Sulcia and Nasuia
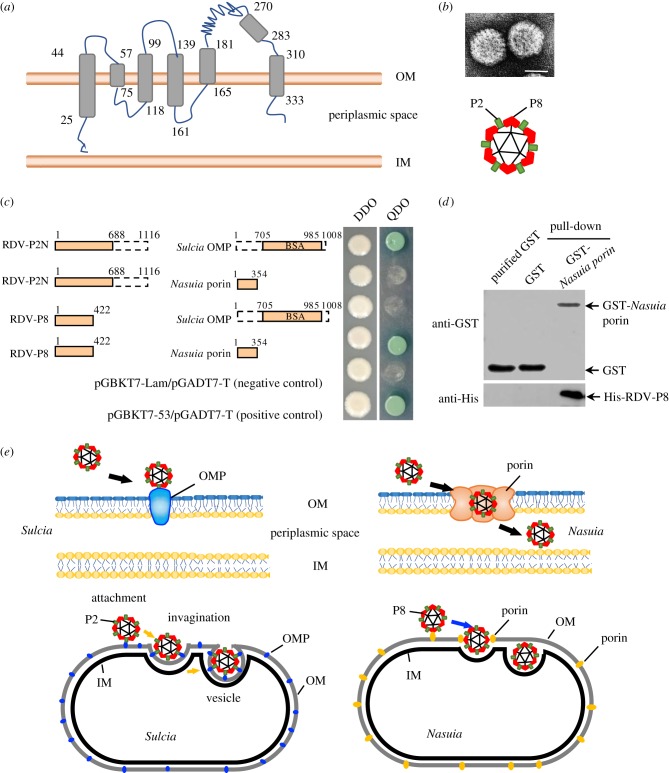
The above observations indicate that RDV can pass through the protein channels in the OM of Nasuia into the periplasmic space. Because porins are abundant OMPs in bacterial OM and form channels [9], we assume that porins are involved in the entry of RDV into the periplasmic space of Nasuia. Based on the sequence of the Nasuia porin gene from the treehopper E. carinata (GenBank accession no. CP021173.1), the fragment of Nasuia porin gene from the leafhopper N. cincticeps had been obtained by RT-PCR process. The complete porin gene of Nasuia from N. cincticeps contained an open reading frame of 1065 bp (GenBank accession no. MH724815) encoding 354 amino acids (aa) with 93% or 86% similarity with that of Nasuia from the treehopper E. carinata or Burkholderia ambifaria, but only 30% or 28% similarity with that of E. coli or Salmonella enterica (electronic supplementary material, figure S4). Secondary structure prediction showed that Nasuia porin consists of 18 β-strands, six transmembrane regions and a 332-aa OM channel domain (figure 3a). The intact RDV virion consists of one minor outer capsid protein P2 and one major outer protein P8 (figure 3b). The minor outer capsid protein P2, which protrudes from the surface of the outer shell of virions, is responsible for initial contact with vector receptors [23]. The yeast two-hybrid assay confirmed that RDV P8 but not P2 specifically interacted with the MS-channel domain of the Nasuia porin (figure 3c). A GST pull-down assay also confirmed that the GST-fused MS-channel domain of Nasuia porin specifically bound to the His-fused RDV P8 (figure 3d). Moreover, we generated a Nasuia porin-specific antibody to localize porin (electronic supplementary material, figure S5a, b), and we found that RDV P8 and Nasuia porin colocalized with each other in ovary (electronic supplementary material, figure S5a–c). Thus, the association of RDV virions with the Nasuia envelope was mediated by the specific interaction between RDV P8 and Nasuia porin. Previously, we have shown that the 15-nm domain of RDV P2 (P2 N) rather than P8 directly interacts with the BSA domain of Sulcia OMP, inducing the formation of virus-containing invaginations or vesicles of the outer envelope of Sulcia [24]. Therefore, it is clear that RDV has evolved to form different interaction strategies with the envelopes of Sulcia and Nasuia (figure 3e).
Figure 3.
RDV virion entry into Nasuia periplasmic space was mediated by specific interaction between RDV P8 and Nasuia porin. (a) Secondary structure prediction showed that Nasuia porin consisted of 18 β-strands and six transmembrane regions. (b) RDV virion consists of one minor outer capsid protein P2 and one major outer protein P8. Scale bar; 50 nm. (c) Yeast two-hybrid assay to detect interactions between P2N (15 nm domain, 1–688 aa) or RDV P8 and Sulcia OMP (BSA domain, 706–985 aa) or Nasuia porin. (d) GST pull-down assay to detect interactions between RDV P8 and Nasuia porin. Nasuia porin-GST acted as a bait protein with GST as a control. Pull-down samples were probed with GST antibody in a western blot. (e) Proposed model for the different binding strategies for RDV virions with the envelopes of Sulcia and Nasuia. IM, inner membranes; OM, outer membranes; OMP, outer membrane protein; PS, periplasmic space.
(d). Inhibition of interaction between Rice dwarf virus P8 and Nasuia porin reduces viral vertical transmission to next insect generation
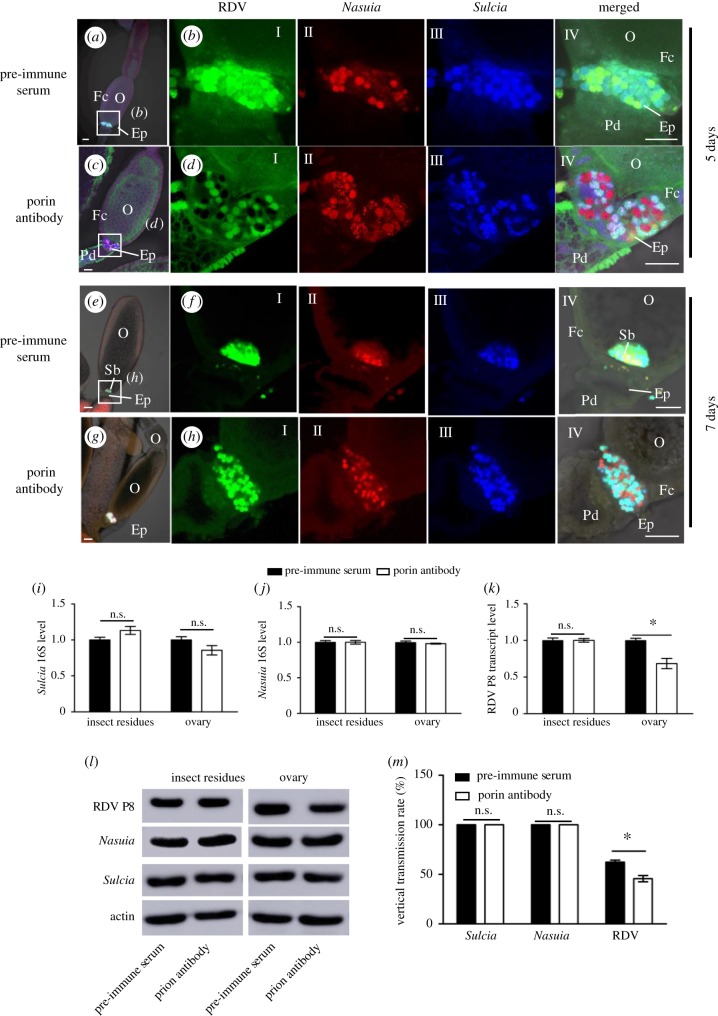
Our results implied that both Sucia and Nasuia can mediate vertical virus transmission to the next insect generation. To further determine the role of Nasuia porin in the transovarial transmission of RDV, we microinjected Nasuia porin antibody into abdomens of leafhoppers to block the adsorption of RDV virions with the envelopes of Nasuia. At 1 day after insect emergence, adult N. cincticeps females were microinjected with the purified RDV virions and either the porin-specific antibody or pre-immune serum to trace the distributions of RDV, Sulcia and Nasuia in the ovaries using immunofluorescence and FISH. At 5 and 7 days after microinjection, confocal microscopy showed that the treatment with the Nasuia porin-specific antibody strongly inhibited the association of RDV with Nasuia, but not with Sucia in the ovaries (figure 4a–h). Moreover, we found that Nasuia porin-specific antibody did not affect the invasion efficiency of Sulcia and Nasuia into the epithelial plug or oocytes, but caused an approximately 30–40% reduction of viruses into the epithelial plug or oocytes among 50 ovaries tested (table 1). RT-qPCR assay indicated that the transcript levels of 16S rRNA from either Sulcia or Nasuia in the ovaries and other parts of the insect bodies were not significantly affected after treatment with the porin-specific antibody (figure 4i–j). However, the porin-specific antibody caused a significant reduction of the transcript level of RDV P8 in the ovaries, but not in other parts of the insect bodies (figure 4k). Subsequently, western blot assay confirmed that the microinjection of porin-specific antibody significantly reduced the accumulation of RDV in the ovaries but not in the other parts of insect bodies (figure 4l). Nor was the vertical transmission rate of Sulcia and Nasuia altered by porin-specific antibody treatment; 100% of the tested eggs laid by females treated with the porin-specific antibody contained Sulcia and Nasuia. However, the porin-specific antibody treatment significantly decreased the rates of RDV transmission to eggs (figure 4m). Our results clearly indicated that Nasuia porin-specific antibody efficiently neutralized the ability of RDV to bind to Nausia envelope, thereby partially inhibiting the efficiency of RDV entry into the oocytes and its transovarial transmission to the next generation. Thus, the direct interaction of RDV with the envelope of the two obligate bacterial symbionts mediated viral transmission from females to their offspring.
Figure 4.
Microinjection of Nasuia porin-specific antibody reduced RDV vertical transmission to the next insect generation. Newly emerged adult insects were injected with the purified RDV virions together with porin-specific antibody or pre-immune serum, and ovaries were dissected to detect RDV, Nasuia and Sulcia in 5 days. (a–d) At 5 days after microinjection, confocal micrographs showed the porin-specific antibody treatment inhibited the association of RDV with Nasuia, but not with Sulcia, in the epithelial plug. Scale bars: 100 µm. (e–h) At 7 days after microinjection, confocal microscopy showed that the Nasuia porin-specific antibody treatment strongly inhibited the association of RDV with Nasuia, but not with Sucia, in the ovaries. (i–k) Transcript levels of Nasuia 16S rRNA, Sulcia 16S rRNA and RDV P8 in ovaries or other parts of insects treated with porin-specific antibody or pre-immune serum, as determined by RT-qPCR assay in three independent experiments. (l) Accumulation levels of Nasuia, Sulcia and RDV P8 in ovaries or other parts of insects treated with porin-specific antibody or pre-immune serum, as determined by western blot using Nasuia porin-, Sulcia OMP- or RDV P8-specific IgGs, respectively. Insect actin was detected with actin-specific IgG as a control. (m) Vertical transmission rates of Nasuia, Sulcia and RDV after treatment with porin-specific antibody or pre-immune serum. Ep, epithelial plug; Fc, follicular cell; O, oocyte; Pd, pedicel. All images are representative of at least three replications. (i, j, k and m) Data are means ± s.e. from three independent experiments (independent-sample t-test at 0.05 level).
Table 1.
Distribution of RDV, Sulcia and Nasuia in epithelial plug, symbiont ball and oocyte of viruliferous leafhoppers, as revealed by confocal microscopy.
| days post microinjection | microbe | percentage of tissues with RDV, Nasuia and Sulcia signals (mean ± s.e.)a |
|||||
|---|---|---|---|---|---|---|---|
| epithelial plug |
symbiont ball |
oocyte |
|||||
| pre-immune serum | porin antibody | pre-immune serum | porin antibody | pre-immune serum | porin antibody | ||
| 5 days | RDV | 0.38 ± 0.01b | 0.20 ± 0.01c | 0.13 ± 0.01b | 0.07 ± 0.01b | 0.47 ± 0.01b | 0.26 ± 0.01c |
| Sulcia and Nasuia | 0.53 ± 0.01b | 0.49 ± 0.01b | 0.21 ± 0.01b | 0.19 ± 0.01b | 0.69 ± 0.02b | 0.63 ± 0.01b | |
| 7 days | RDV | 0.52 ± 0.02b | 0.42 ± 0.02c | 0.33 ± 0.02b | 0.23 ± 0.01c | 0.53 ± 0.01b | 0.42 ± 0.01c |
| Sulcia and Nasuia | 0.97 ± 0.02b | 0.91 ± 0.02b | 0.50 ± 0.02b | 0.47 ± 0.02b | 0.98 ± 0.02b | 0.93 ± 0.02b | |
aFor each treatment, 50 samples were collected, and three replicates were performed.
b,cData for the same tissue in the same row that are followed by different letters are significantly different (P < 0.05, one-way ANOVA, Tukey's HSD test).
4. Discussion
Viruses have apparently developed diverse strategies to enter oocytes for transovarial transmission during long-term associations with insect vectors. For example, viruses can exploit the existing pathways to enter oocytes, as used by a major yolk protein precursor, vitellogenin (Vg) and obligate bacterial symbionts [24,29–32]. Here, we show that RDV virions migrate to the ovaries and enter the eggs by hitchhiking on the envelopes of Sulcia and Nasuia, and they simply benefit from the vertical transmission routes used by the two obligate bacterial symbiont partners in female N. cincticeps (figures 1 and 2). RDV exploits its minor outer capsid protein P2 to interact with the BSA domain of the OMP in the Sulcia envelope, inducing the formation of virus-containing invaginations or membrane-enclosed vesicles [24]. In the case of Nasuia, RDV exploits its major outer capsid protein P8 to interact with the porin in the bacterial envelope (figure 3), possibly inducing the opening of porin channels for virions to pass through the outer membrane into the periplasmic space. Additionally, inhibition of the interaction between Sulcia and RDV by using Sulcia OMP antibody reduced RDV accumulation in the ovary to 50% [24]; in our study, our findings indicated that inhibition of the interaction between Nasuia and RDV by using Nasuia porin antibody reduced the RDV accumulation in the ovary to 70% (figure 4k). Therefore, these findings suggested that, compared with Nasuia, Sulcia may play the main role in RDV transovarial transmission. These two strategies for the acquisition of viruses by the outer envelopes of Sulcia and Nasuia represent different cross-kingdom interactions between viruses and bacteria, which enable their joint transovarial transmission. Our results suggest that the RDV, Sulcia and Nasuia have developed complex tripartite interactions and provide novel insights into the development of efficient approaches to attenuate viral epidemics by targeting such symbiont-mediated maternal transmission.
In general, the porin channels on the OMs of gram-negative bacteria are well known as receptors for bacteriocins and phages [33,34]. For example, two transducing bacteriophages require an outer membrane porin of the symbiotic nitrogen-fixing bacterium Sinorhizobium meliloti as a receptor for infection [35]. Some bacteriophages also exploit both porins and lipopolysaccharides in the outer leaflet of the outer membrane of the bacterial cell for their adsorption [36–38]. Herein, we reveal for the first time that a viral pathogen has evolved to pass through the porin channels on the OMs of a symbiotic bacterium into the periplasmic space for viral vertical transmission. However, it remains unknown whether this phenomenon also occurs in other insect symbiont–virus systems. Further investigations are warranted to study diverse systems to understand the differences and similarities between the roles and mechanisms of symbionts in viral transmission.
Mutualistic intracellular symbiosis between obligate bacterial symbionts and insects is a widespread phenomenon that has contributed to the global success of insects [39]. The obligate bacterial symbionts, by provisioning nutrients lacking in plant sap, allow the hemipteran species to occupy or dominate new ecological niches [40]. Sulcia and its partner Nasuia in the female adults of N. cincticeps can also be expected to interact cooperatively during their cotransmission to the subsequent insect generations. As revealed by its genomic sequence, Sulcia synthesizes homoserine, which is then used by Nasuia to synthesize the essential amino acid methionine for the host [41]. The two symbionts appear to show functional complementarity and form a symbiotic relationship, which can also be seen from their simultaneous entry into the same follicular epithelial cells at the epithelial plug and transport into the oocytes to form the characteristic symbiont ball (electronic supplementary material, figure S1). RDV has evolved a unique strategy for transovarial transmission by hitchhiking on Sulcia and its partner Nasuia, but the evolution of such multiple interactions among microorganisms in nature is poorly understood. Obligate endosymbionts are generally maternally inherited by their insect hosts and require their host to survive and have a high reproductive output to ensure the endosymbiont persists within the host species over generations [3]. By contrast, vector-borne viral pathogens require immediate access to host resources to complete their life cycle, which often reduces insect fecundity [42–44]. Coexistence of a maternally inherited symbiont and a virus within the same host may result in a conflict in survival requirements. It appears that maternally inherited symbionts may protect their hosts from viruses to maintain the symbiont within host populations [3]. Several reports have shown that flies are protected from viral infection by the endosymbiont Wolbachia by interfering with viral replication, and in turn, decreasing virus-induced mortality [45–47]. Why the obligate bacterial symbionts cooperate with RDV, rather than competing or conflicting with the virus during their vertical transmission is puzzling, but not surprising. Clearly, there is still much to understand about the molecular basis of symbiont–virus interactions in nature.
5. Conclusion
Our results reveal the diverse strategies exploited by a plant reovirus for vertical transmission by interacting with proteins on the OMs of its vector's bacterial symbiont. These results substantially extend our understanding of the vertical transmission mechanisms of plant reoviruses by leafhoppers and provide invaluable clues to the interactions between insect symbionts and plant viruses.
Supplementary Material
Data accessibility
Data inquiries can be directed to the corresponding author.
Authors' contributions
All authors read and approved the manuscript. W.W., J.W. and T.W. conceived and designed the study, and wrote the paper. W.W. performed most experiments and helped with data analysis. L.H. performed yeast two-hybrid assay and immunofluorescence microscopy. Q.M. performed the transmission electron microscopy. J.L. and Y.Z. performed gene cloning and GST pull-down. Q.Z. and D.J. performed RT-qPCR experiments. T.W. discussed the data and revised the manuscript and also organized and directed the project.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (grant nos 31730071, 31571979, 31770166 and 31501602).
References
- 1.Ferrari J, Vavre F. 2011. Bacterial symbionts in insects or the story of communities affecting communities. Phil. Trans. R. Soc. B 366, 1389–1400. ( 10.1098/rstb.2010.0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas AE. 2011. Lessons from studying insect symbioses. Cell Host Microbe 10, 359–367. ( 10.1016/j.chom.2011.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlie JC, Johnson KN. 2009. Symbiont-mediated protection in insect hosts. Trends Microbiol. 17, 348–354. ( 10.1016/j.tim.2009.05.005) [DOI] [PubMed] [Google Scholar]
- 4.Moran NA, Tran P, Gerardo NM. 2005. Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Appl. Environ. Microbiol. 71, 8802–8810. ( 10.1128/AEM.71.12.8802-8810.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koga R, Bennett GM, Cryan JR, Moran NA. 2013. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ. Microbiol. 15, 2073–2081. ( 10.1111/1462-2920.12121) [DOI] [PubMed] [Google Scholar]
- 6.Sacchi L, et al. 2008. Multiple symbiosis in the leafhopper Scaphoideus titanus (Hemiptera: Cicadellidae): details of transovarial transmission of Cardinium sp. and yeast-like endosymbionts. Tissue Cell 40, 231–242. ( 10.1016/j.tice.2007.12.005) [DOI] [PubMed] [Google Scholar]
- 7.Costerton J, Ingram J, Cheng K. 1974. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol. Rev. 38, 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bos MP, Robert V, Tommassen J. 2007. Biogenesis of the gram-negative bacterial outer membrane. Annu. Rev. Microbiol. 61, 191–214. ( 10.1146/annurev.micro.61.080706.093245) [DOI] [PubMed] [Google Scholar]
- 9.Achouak W, Heulin T, Pagès J-M. 2001. Multiple facets of bacterial porins. FEMS Microbiol. Lett. 199, 1–7. ( 10.1016/S0378-1097(01)00127-6) [DOI] [PubMed] [Google Scholar]
- 10.Osborn M, Wu H. 1980. Proteins of the outer membrane of gram-negative bacteria. Annu. Rev. Microbiol. 34, 369–422. ( 10.1146/annurev.mi.34.100180.002101) [DOI] [PubMed] [Google Scholar]
- 11.Koebnik R, Locher KP, Van Gelder P.. 2000. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37, 239–253. ( 10.1046/j.1365-2958.2000.01983.x) [DOI] [PubMed] [Google Scholar]
- 12.Kobiałka M, Michalik A, Walczak M, Junkiert Ł, Szklarzewicz T. 2016. Sulcia symbiont of the leafhopper Macrosteles laevis (Ribaut, 1927)(Insecta, Hemiptera, Cicadellidae: Deltocephalinae) harbors Arsenophonus bacteria. Protoplasma 253, 903–912. ( 10.1007/s00709-015-0854-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michalik A, Jankowska W, Kot M, Gołas A, Szklarzewicz T. 2014. Symbiosis in the green leafhopper, Cicadella viridis (Hemiptera, Cicadellidae). Association in statu nascendi? Arthropod. Struct. Dev. 43, 579–587. ( 10.1016/j.asd.2014.07.005) [DOI] [PubMed] [Google Scholar]
- 14.Bright M, Bulgheresi S. 2010. A complex journey: transmission of microbial symbionts. Nat. Rev. Microbiol. 8, 218–230. ( 10.1038/nrmicro2262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia D, Chen Q, Mao Q, Zhang X, Wu W, Chen H, Yu X, Wang Z, Wei T. 2018. Vector mediated transmission of persistently transmitted plant viruses. Curr. Opin. Virol. 28, 127–132. ( 10.1016/j.coviro.2017.12.004) [DOI] [PubMed] [Google Scholar]
- 16.Pinheiro PV, Kliot A, Ghanim M, Cilia M. 2015. Is there a role for symbiotic bacteria in plant virus transmission by insects? Curr. Opin. Insect Sci. 8, 69–78. ( 10.1016/j.cois.2015.01.010) [DOI] [PubMed] [Google Scholar]
- 17.Morin S, Ghanim M, Zeidan M, Czosnek H, Verbeek M, van den Heuvel JF. 1999. A GroEL homologue from endosymbiotic bacteria of the whitefly Bemisia tabaciis implicated in the circulative transmission of tomato yellow leaf curl virus. Virology 256, 75–84. ( 10.1006/viro.1999.9631) [DOI] [PubMed] [Google Scholar]
- 18.Bouvaine S, Boonham N, Douglas AE. 2011. Interactions between a luteovirus and the GroEL chaperonin protein of the symbiotic bacterium Buchnera aphidicola of aphids. J. Gen. Virol. 92, 1467–1474. ( 10.1099/vir.0.029355-0) [DOI] [PubMed] [Google Scholar]
- 19.Fukushi T. 1933. Transmission of the virus through the eggs of an insect vector. Proc. Imperial Acad. 9, 457–460. ( 10.2183/pjab1912.9.457) [DOI] [Google Scholar]
- 20.Hibino H. 1996. Biology and epidemiology of rice viruses. Annu. Rev. Phytopathol. 34, 249–274. ( 10.1146/annurev.phyto.34.1.249) [DOI] [PubMed] [Google Scholar]
- 21.Lu G, Zhou ZH, Baker ML, Jakana J, Cai D, Wei X, Chen S, Gu X, Chiu W. 1998. Structure of double-shelled rice dwarf virus. J. Virol. 72, 8541–8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omura T, Ishikawa K, Hirano H, Ugaki M, Minobe Y, Tsuchizaki T, Kato H. 1989. The outer capsid protein of rice dwarf virus is encoded by genome segment S8. J. Gen. Virol. 70, 2759–2764. ( 10.1099/0022-1317-70-10-2759) [DOI] [PubMed] [Google Scholar]
- 23.Omura T, et al. 1998. The P2 protein of rice dwarf phytoreovirus is required for adsorption of the virus to cells of the insect vector. J. Virol. 72, 9370–9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia D, et al. 2017. Insect symbiotic bacteria harbour viral pathogens for transovarial transmission. Nat. Microbiol. 2, 17025 ( 10.1038/nmicrobiol.2017.25) [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Xu D, Pu L, Zhou G. 2014. Southern rice black-streaked dwarf virus alters insect vectors' host orientation preferences to enhance spread and increase rice ragged stunt virus co-infection. Phytopathology 104, 196–201. ( 10.1094/PHYTO-08-13-0227-R) [DOI] [PubMed] [Google Scholar]
- 26.Jia D, Chen H, Zheng A, Chen Q, Liu Q, Xie L, Wu Z, Wei T. 2012. Development of an insect vector cell culture and RNA interference system to investigate the functional role of fijivirus replication protein. J. Virol. 86, 5800–5807. ( 10.1128/JVI.07121-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonhag PF. 1958. Ovarian structure and vitellogenesis in insects. Annu. Rev. Entomol. 3, 137–160. ( 10.1146/annurev.en.03.010158.001033) [DOI] [Google Scholar]
- 28.Hummel NA, Zalom FG, Peng CY. 2006. Anatomy and histology of reproductive organs of female Homalodisca coagulata (Hemiptera: Cicadellidae: Proconiini), with special emphasis on categorization of vitellogenic oocytes. Ann. Entomol. Soc. Amer. 99, 920–932. ( 10.1603/0013-8746(2006)99[920:AAHORO]2.0.CO;2) [DOI] [Google Scholar]
- 29.Whitfield AE, Falk BW, Rotenberg D. 2015. Insect vector-mediated transmission of plant viruses. Virology 479, 278–289. ( 10.1016/j.virol.2015.03.026) [DOI] [PubMed] [Google Scholar]
- 30.Gubler DJ. 2001. Human arbovirus infections worldwide. Ann. N Y Acad. Sci. 951, 13–24. ( 10.1111/j.1749-6632.2001.tb02681.x) [DOI] [PubMed] [Google Scholar]
- 31.Adams B, Boots M. 2010. How important is vertical transmission in mosquitoes for the persistence of dengue? Insights from a mathematical model. Epidemics 2, 1–10. ( 10.1016/j.epidem.2010.01.001) [DOI] [PubMed] [Google Scholar]
- 32.Huo Y, et al. 2014. Transovarial transmission of a plant virus is mediated by vitellogenin of its insect vector. PLoS Pathog. 10, e1003949 ( 10.1371/journal.ppat.1003949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeth K, Thein M. 2010. Porins in prokaryotes and eukaryotes: common themes and variations. Biochem. J. 431, 13–22. ( 10.1042/BJ20100371) [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee S, Rothenberg E. 2012. Interaction of bacteriophage l with its E. coli receptor, LamB. Viruses 4, 3162–3178. ( 10.3390/v4113162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crook MB, Draper AL, Guillory RJ, Griffitts JS. 2013. The Sinorhizobium meliloti essential porin RopA1 is a target for numerous bacteriophages. J. Bacteriol. 195, 3663–3671. ( 10.1128/JB.00480-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silverman J, Benson S. 1987. Bacteriophage K20 requires both the OmpF porin and lipopolysaccharide for receptor function. J. Bacteriol. 169, 4830–4833. ( 10.1128/jb.169.10.4830-4833.1987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sukupolvi S. 1984. Role of lipopolysaccharide in the receptor function for bacteriophage Ox2. FEMS Microbiol. Lett. 21, 83–87. ( 10.1111/j.1574-6968.1984.tb00190.x) [DOI] [Google Scholar]
- 38.Yu F, Mizushima S. 1982. Roles of lipopolysaccharide and outer membrane protein OmpC of Escherichia coli K-12 in the receptor function for bacteriophage T4. J. Bacteriol. 151, 718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldhaar H. 2011. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 36, 533–543. ( 10.1111/j.1365-2311.2011.01318.x) [DOI] [Google Scholar]
- 40.Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T. 2007. Obligate symbiont involved in pest status of host insect. Proc. R. Soc. B 274, 1979–1984. ( 10.1098/rspb.2007.0620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Douglas AE. 2016. How multi-partner endosymbioses function. Nat. Rev. Microbiol. 14, 731 ( 10.1038/nrmicro.2016.151) [DOI] [PubMed] [Google Scholar]
- 42.Styer LM, Meola MA, Kramer LD. 2007. West Nile virus infection decreases fecundity of Culex tarsalis females. J. Med. Entomol. 44, 1074–1085. ( 10.1093/jmedent/44.6.1074) [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Lu C, Li M, Wu W, Zhou G, Wei T. 2016. Adverse effects of rice gall dwarf virus upon its insect vector Recilia dorsalis (Hemiptera: Cicadellidae). Plant Dis. 100, 784–790. ( 10.1094/PDIS-06-15-0713-RE) [DOI] [PubMed] [Google Scholar]
- 44.Nakasuji F, Kiritani K. 1970. Effects of rice dwarf virus upon its vector, Nephotettix cincticeps Uhler (Hemiptera: Deltocephalidae), and its significance for changes in relative abundance of infected individuals among vector populations. Appl. Entomol. Zool. 5, 1–12. ( 10.1303/aez.5.1) [DOI] [Google Scholar]
- 45.Hedges LM, Brownlie JC, O'Neill SL, Johnson KN. 2008. Wolbachia and virus protection in insects. Science 322, 702 ( 10.1126/science.1162418) [DOI] [PubMed] [Google Scholar]
- 46.Moreira LA, et al. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278. ( 10.1016/j.cell.2009.11.042) [DOI] [PubMed] [Google Scholar]
- 47.Teixeira L, Ferreira Á, Ashburner M. 2008. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6, e1000002 ( 10.1371/journal.pbio.1000002) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data inquiries can be directed to the corresponding author.