Abstract
It is known that plant arboviruses infect insect vector cells by endocytosis; however, the cellular receptors that mediate endocytosis have not been well defined. In our recently published work and this study, by clarifying the vertical transmission mechanism of Rice stripe virus (RSV) in Laodelphax striatellus, we provide a novel paradigm for how arboviruses enter insect germ-line cells. Instead of direct interaction with a viral receptor, the virus binds to a secreted ligand protein, hitchhiking the ligand-receptor pathway to achieve cell entry. Vitellogenin (Vg) is an indispensable protein for embryo development that is synthesized extra-ovarially and taken up by germ-line cells through Vg receptor (VgR)-mediated endocytosis. After revealing that RSV invades L. striatellus ovary by a specific molecular interaction with the insect Vg in haemolymph, this study addressed VgR's function in mediating the RSV invasion of the germarium nurse cells, further confirming the ligand's receptor-mediated viral cell-invasion mechanism. Understanding the viral ovary-entry pathways in vectors will help to find suitable measures to block the trans-generation transmission of the viruses.
This article is part of the theme issue ‘Biotic signalling sheds light on smart pest management’.
Keywords: plant arbovirus, insect vector, cellular receptor, ligand-receptor pathway, anti-viral strategies
1. Introduction
Viral entry into specific host or tissue cells is regarded as the early stage of a viral infection, and viral attachment is the first step for cell entry. Attachment factors serve to bind the viral particles and thus help to concentrate virus onto the surface of susceptible cells. On the cell surface, the virus specifically binds to the viral receptor and initiates cell entry by activating signalling pathways and promoting endocytic internalization. The interactions between a virus and its cellular receptors are usually highly specific, thus the types of receptors present determine, to a large degree, which cell types and species can be infected [1–6]. Unravelling the cellular receptors that specifically mediate viral cell entry will provide the basis for the development of strong anti-viral strategies [2,7,8].
For the viruses that infect mammals, hundreds of attachment factors and receptors have been identified [1,9]. For several important human viruses, the detailed molecular mechanisms of viral cell entry have been elucidated and have led to efficient therapeutic methods [10–12]. For example, maraviroc, an FDA-approved HIV cell-entry inhibitor, binds to the CCR5 receptor, an essential co-receptor for most HIV strains, blocks binding of the viral envelope glycoprotein, gp120, to CCR5 to prevent the membrane fusion events necessary for viral entry. HIV is then unable to enter human macrophages or T cells [2,7].
For viruses that infect plant cells, the rigid and fairly thick cell wall presents a unique barrier for entry. Initial entry into plant cells is believed to take advantage of breaks in the integrity of the cell wall. Most plant viruses are carried by insect vectors and are inoculated into the plant tissues or cells through the breaks made by the insect's stylet [13–15]. Since the virus can enter the plant tissues or cells through such a break, interactions with specific plant cellular receptors may not be necessary.
Some human viruses of medical importance are also transmitted by arthropod vectors [16,17]. The viral transmission routes within the arthropod vectors, independent of animal or plant host, are highly conserved [18]. A complete circulation route for viruses that are transmitted by arthropod vector in a persistent-propagative manner starts from the viral entry into midgut epithelia. Viruses then replicate inside the infected cells and spread to adjacent gut cells, passing through the gut barrier to disseminate into the haemolymph. From the haemolymph, viruses can invade various insect tissues, including salivary glands, which lead to the delivery of viruses into healthy hosts through the saliva. Some arboviruses can be vertically transmitted to offspring and the transmission initiates from the viral entry of germ-line cells in female ovaries [14,18]. Microscope-based techniques have clarified the infection routes of viruses within the vector body; however, for each transmission barrier, especially midgut, salivary glands and ovaries, the cellular receptors that mediate the initial viral entry are not well defined or characterized [16,19].
Arthropods have cellular membrane structures that are comparable with those of mammals. Viral entry into arthropod cells by receptor-mediated endocytosis has been detected using electron microscopy [20,21]. For example, Rice dwarf virus (RDV), which is transmitted by Nephotettix cincticeps, enters N. cincticeps cells by endocytosis through coated pits [21]. The treatment of N. cincticeps cells with drugs that block either receptor- or clathrin-mediated endocytosis confirmed the clathrin-dependent receptor-mediated endocytosis [21]. Electron tomography clearly showed that the RDV minor outer-capsid protein P2 connects the viral particle to the host's cellular membrane during cell entry [22]. However, the cellular receptor for RDV's cell entry remained unidentified [19]. At present, no insect cellular receptor for viral entry has been well defined [16,19]. By contrast, considerable numbers of attachment factors and receptor candidates have been identified [16,19,23–26]. For example, Aedes aegypti transmits West Nile virus (WNV). A secreted mosquito C-type lectin captures WNV in the haemolymph by binding to the WNV envelope glycoprotein. A CD45-like protein tyrosine phosphatase, mosPTP-1, located at the cell surface recruits the C-type lectin through a molecular interaction, which enables WNV to attach onto the cell surface and facilitates viral cell entry [24]. However, whether mosPTP-1 participates in viral endocytosis was not studied, and it is not clear if it acts as an attachment factor or as an entry receptor [27]. Identifying the cellular receptors involved in the initial recognition of arboviruses by their insect vectors at each critical transmission barrier is still a major challenge.
Building on our previous studies [28,29], we reveal that a plant arbovirus hitchhikes a well-defined insect ligand-receptor interaction pathway to achieve its cell entry. This represents a previously undescribed mechanism for the arboviral invasion of vector cells. Rice stripe virus (RSV) is the causative agent of rice stripe disease and is completely dependent on Laodelphax striatellus (small brown planthopper, SBPH) for transmission from RSV-infected rice to healthy plants. RSV is able to infect L. striatellus ovaries and is vertically transmitted to offspring. We have demonstrated that the insect vitellogenin (Vg) protein plays a key role in helping RSV to invade L. striatellus ovaries. Following the Vg synthesis and transport route, we have found that RSV binds to Vg in haemocytes and then travels in the haemolymph to the nurse cells in the germarium zone. Taking into consideration the Vg-uptake mechanism that has been clarified in detail, we hypothesized that RSV may break the L. striatellus ovarian barrier by entering nurse cells through Vg receptor (VgR)-mediated endocytosis. In this study, we provide new evidence of VgR's function in mediating the RSV invasion of nurse cells, further confirming this novel viral cell-entry mechanism.
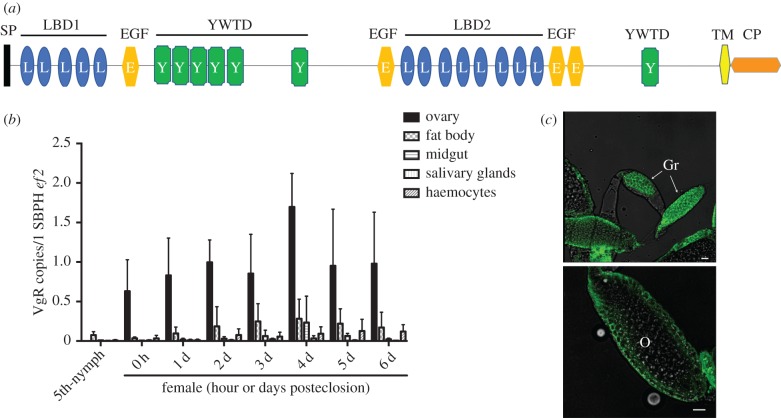
By searching the L. striatellus genome ([30] and personal communications) with our previously obtained VgR sequence (GenBank number KJ452775), a single-copy VgR gene was identified (figure 1a, sequence and annotation). The VgR open reading frame is 5784 bp in length, encoding 1928 amino acids. A BLAST algorithm-based analysis [31] against sequence databases revealed that the full-length amino acid sequence of L. striatellus VgR shared a 76% identity with VgR from Nilaparvata lugens. A SignalP 4.1 analysis [32] revealed a signal peptide of 20 amino acids at the N-terminus (figure 1a). The transmembrane (TM) prediction using Hidden Markov Models (http://www.cbs.dtu.dk/services/TMHMM/) identified a TM helix from amino acids 1774–1796. Thus, VgR appears to be a TM protein with an N-terminal extracellular region and a C-terminal cytoplasmic tail. An analysis of the deduced protein sequence with the SMART algorithm revealed conserved domains similar to those of the low-density lipoprotein receptor family [33,34], including two ligand-binding domains with insect VgR characteristics [35], epidermal growth factor-like domains and YWXD amino acid repeats (figure 1a).
Figure 1.
The Laodelphax striatellus VgR gene and its expression profile. (a) Schematic composition of L. striatellus VgR, as illustrated by the SMART algorithm. SP, signal peptide sequence; LBD, ligand-binding domain; EGF, epidermal growth factor-like domain; YWTD, YWTD amino acid repeats; TM, transmembrane domain; CP, cytoplasmic region. (b) RT-qPCR to quantify VgR mRNA levels in L. striatellus tissues at different developmental stages. Ef2, L. striatellus elongation factor 2 gene. Means and s.d. were calculated from three independent experiments, with five mRNA samples per experiment. (c) Immunofluorescence assay to localize VgR protein in L. striatellus ovaries. VgR was probed with a mouse anti-VgR polyclonal antibody and stained with Alexa Fluor 488 (shown in green). Samples were examined using a Leica TCS SP8 confocal microscope. Images are representatives of three independent experiments each with five insects analysed. Gr, germarium; O, oocyte. Scale bar, 20 µm. (Online version in colour.)
VgR expression was measured by quantitative reverse transcription PCR (RT-qPCR) with the VgR-specific primers VgR-QF/VgR-QR (primer sequences listed in electronic supplementary material, table S1 and protocols described in electronic supplementary material). Temporal expression measurements indicated that the VgR transcript was produced in females at all stages of ovarian development (figure 1b). The VgR transcript level rapidly increased in immature female ovaries of final-instar nymphs and in the early previtellogenic period (before 12 h posteclosion), and continued its dramatic rise during the vitellogenic periods, reaching its peak at 96-h posteclosion (figure 1b). In other tested tissues of female adults, including fat body, midgut, salivary glands and haemocytes, VgR was at very low or undetectable levels (figure 1b). We next determined the cellular distribution of VgR. Immunofluorescence staining with a VgR-specific antibody indicated that the protein was distributed in both the germarium and developing oocytes (figure 1c).
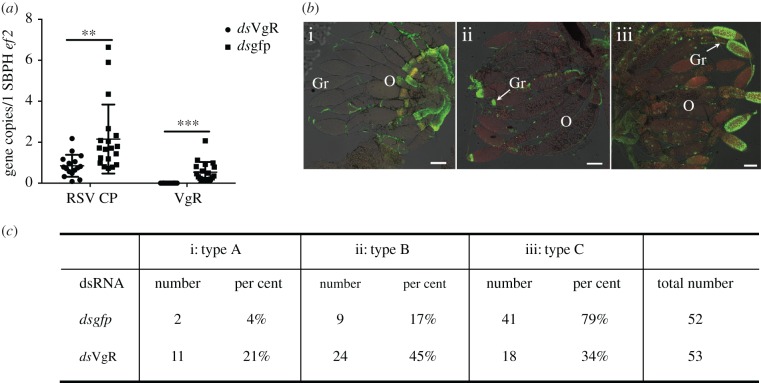
To determine whether the RSV–Vg interaction mediates RSV cell entry through VgR-mediated endocytosis or the interaction just enriched the virus amount reaching the germarium assuming RSV enters the cells through an interaction with another receptor, we investigated VgR's function during the RSV infection of insect ovaries. We knocked down VgR transcripts using RNA interference (RNAi) through the microinjection of dsRNA of VgR (dsVgR). Dsgfp was microinjected as a control. The fifth-instar nymphs, at 24 h before eclosion, were injected with dsRNA. Both the VgR-knockdown efficiency and the RSV titres were measured 48-h posteclosion. RT-qPCR indicated that treatment with dsVgR resulted in a 75% lower VgR transcript abundance in ovaries compared with the dsgfp treatment. We then assessed whether the RSV titre in the ovaries was affected by the RNAi-mediated VgR deficiency and found that, compared with the control group injected with dsgfp, dsVgR-treated L. striatellus showed a 73% lower RSV titre in the ovaries (figure 2a). Immunofluorescence staining was performed to observe the RSV infection route in ovaries. At 48-h posteclosion in the dsgfp-treated group, 79% of the insects showed RSV infecting most parts of the germarium (type C infection level), 17% showed RSV RNPs (filamentous ribonucleoprotein particles) accumulating in the anterior part of the germarium (type B), while only 4% showed no RSV RNPs in the germarium (type A). By contrast, in the dsVgR-treated group, a significantly lower proportion of the insect population exhibited a heavier RSV infection. In total, 21% of the insects had a type A ovarian infection level, 45% had type B and 34% had type C (figure 2b,c). Thus, VgR appears to be required for the RSV invasion of L. striatellus ovaries. When the VgR-deficient RSV-infected L. striatellus females were mated with RSV-infected males, no offspring were produced, indicating that the VgR-deficiency also impeded Vg uptake and oocyte development. We then compared the influence of dsVg [28] and dsVgR on RSV ovarian infection levels and found that the RNAi-mediated silencing of either associated gene resulted in similar decreases in the viral infection level in the ovaries, suggesting that VgR is the dominant downstream receptor for the Vg–RSV complex. Cellular receptors usually determine the cell types that viruses can invade, while the lack of a viral receptor leads to a complete block of viral cell entry [1]. dsRNA-mediated gene silencing does not eliminate all gene transcripts; therefore, it remains uncertain whether the Vg–VgR endocytic machine is the only receptor pathway required for RSV to enter L. striatellus nurse cells. A CRISPR/Cas9-mediated gene knockout or cultivable L. striatellus cell line will be used in the future to clarify the function of the Vg–VgR endocytic machine in RSV cell entry.
Figure 2.
Influence of a VgR deficiency on RSV infection of the L. striatellus ovary. RSV-infected fifth-instar nymphs at 24 h before eclosion were injected with dsRNA. Both VgR knockdown efficiency and the RSV titre were measured 48-h posteclosion. (a) Quantitative assessment of RSV in dsRNA-injected ovaries. Each dot represents one RSV-infected L. striatellus ovary. Both means and s.d. were calculated from three independent experiments, with 5–7 mRNA samples per experiment. Ef2, L. striatellus elongation factor 2 gene. **p < 0.01; ***p < 0.001. (b) Immunofluorescence assay to define different RSV infection levels in L. striatellus ovarioles. (i) Type A, RSV did not invade the ovariole; (ii) type B, RSV began to invade the tip of the germaria of only a few ovarioles and (iii) type C, RSV present in most of the germaria of nearly all ovarioles. Arrows indicate the RSV-infected area. The RSV antibody was conjugated to Alexa Fluor 488. Gr, germarium; O, oocyte; scale bar, 100 µm. (c) Statistical summary of L. striatellus individuals with different RSV infection levels. The dsVgR-treatment of L. striatellus impeded the ability of RSV to infect L. striatellus ovarioles when compared with the dsgfp-treatment. Each number represents one ovary dissected from one insect. The total 52 or 53 analysed ovaries were collected from three independent dsRNA injection experiments; in each experiment, 15–20 insects per group were analysed. (Online version in colour.)
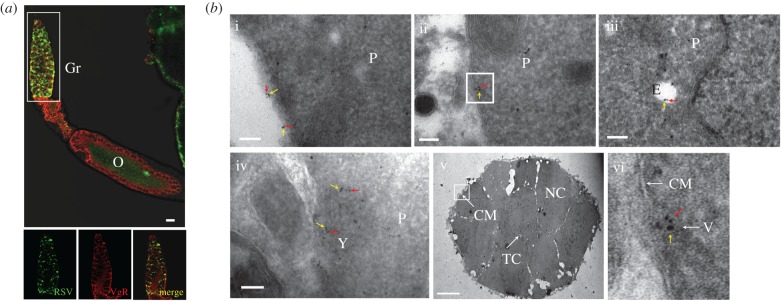
To further confirm that RSV enters the germarium nurse cells using the VgR-mediated endocytic machinery, we investigated the co-localization patterns of RSV and VgR in nurse cells. Insect VgR localizes in clathrin-coated pits on the surface of germarium nurse cells or growth-competent oocytes. Vg binding to the membrane-bound VgR results in the accumulation of Vg in clathrin-coated pits, which subsequently invaginate and pinch off to form intracellular coated vesicles. These vesicles then carry the Vg–VgR to an endosome. Vg is then accumulated in early endosomes, which are fused into late endosomes, forming the yolk granules [36]. Using an immunofluorescence assay, we found that RSV and VgR co-localize in the germinative zone (figure 3a) where RSV–Vg co-localization occurred. By immunoelectron microscopy, the RSV RNPs and VgR were found to co-localize on the surfaces of the nurse cells, inside the intracellular vesicle-like structures and in the yolk granules at high abundances (figure 3b). Thus, VgR is required for RSV to enter the nurse cells. When Vg is deficient and VgR remains normally expressed, RSV cannot efficiently invade the germarium [28,29], indicating that the influence of VgR on the ability of RSV to invade an ovary is dependent on Vg.
Figure 3.
Immunofluorescence and immunoelectron micrographs showing the distributions of RSV and VgR in the L. striatellus germaria. (a) Immunofluorescence assay to show the co-localization of RSV and VgR in the germarium. RSV was probed with a rabbit anti-RSV polyclonal antibody and stained with Alexa 488 (shown in green), and VgR was probed with a mouse anti-VgR polyclonal antibody and stained with Alexa Fluor 594 (shown in red). Co-localization of RSV and VgR is shown in yellow. Images were examined using a Leica TCS SP8 confocal microscope. Images are representative of three independent experiments, with a total of 15 SBPHs analysed. Gr, germarium; O, oocyte. Scale bar, 20 µm. (b) Immunoelectron micrographs showing the distribution of RSV and VgR in the germarium. Both RSV (red arrow) and VgR (yellow arrow) antigens were found on the surfaces of nurse cells (i); inside the nurse cells within a vesicle-like structure (ii); associated with an endosome (iii) and within the yolk granule (iv). (v) The cross-sectional view of the germarium zone with a white rectangle indicating the region for detection of RSV-VgR co-localization close to the cytomembrane, scale bar is 10 µm. (vi) The enlarged vesicle-like structure detected in (ii). Red arrow, 6-nm gold-conjugated goat-anti-rabbit IgG against RSV used to detect the virus; yellow arrow, 10-nm gold-conjugated goat-anti-mouse IgG against VgR. P, cytoplasm; E, endosome; Y, yolk granule; CM, cytomembrane; TC, trophic core; NC, nurse cell; V, vesicle-like structure. Scale bar in all figures except for (v) represents 100 nm. (Online version in colour.)
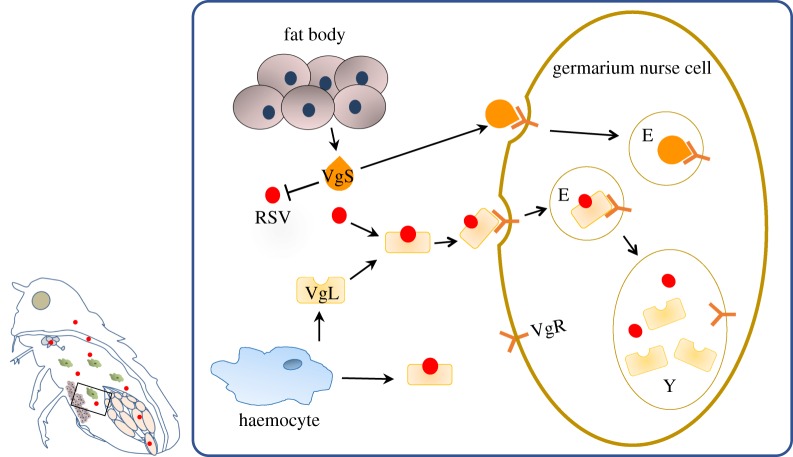
Based on our obtained data regarding Vg transport, the VgR-mediated endocytic machine and RSV invasion of the insect ovarian germarium, we proposed a model in which RSV crosses the ovarian barrier to enter the nurse cells of the germarium, resulting in successful vertical transmission (figure 4). Laodelphax striatellus fat body and haemocytes produce Vg in different molecular forms, but only the haemocyte-produced Vg is able to interact with RSV capsid protein [29]. When Vg is produced in haemocytes, RSV binds to Vg. RSV is then secreted into the haemolymph through the Vg secretion pathway and is transported to the germarium. It is enriched onto the surface of the nurse cells by Vg binding to the membrane-bound VgR to induce endocytosis. The endocytic vesicles then carry RSV to an endosome and then to the yolk granules, and finally RSV spreads into the developing oocytes through the nutritive cords.
Figure 4.
Working model in which RSV breaches the L. striatellus ovarian barrier. L. striatellus fat body and haemocytes produce vitellogenin (Vg) in different molecular forms. When L. striatellus Vg is produced in haemocytes (named VgL), RSV binds to VgL. The RSV–VgL complex is then secreted into the haemolymph, transported to the germarium and concentrated onto the surfaces of nurse cells by VgL binding to VgR. RSV enters the nurse cell through VgR-mediated endocytosis. The endocytic vesicles carry RSV to an endosome and then the yolk granule. VgS, fat body-produced Vg molecular form; VgL, haemocyte-produced Vg molecular form; Y, yolk granule. (Online version in colour.)
This is the first work to describe an insect ligand's receptor-mediated endocytosis which carries a virus into insect cells. Unlike the typical virus–receptor interaction, in this case, the virus hitchhikes an existing ligand-receptor pathway, and the ligand's receptor mediates the endocytic process to uptake the bound virus. In insects, other atypical pathways have also been reported to facilitate viral cell entry [37,38]. For example, the green rice leafhopper N. cincticeps vertically transmits both RDV and the symbiotic bacterium Sulcia. RDV achieves its own transmission into insect offspring by binding to the Sulcia outer membrane protein [37]. In the RSV–L. striatellus interaction, the L. striatellus sugar transporter 6 interacts with RSV CP, and this interaction mediates RSV's entry into L. striatellus midgut epithelial cells [38].
Because of the indispensability of insect Vg for embryo development, the Vg–VgR-mediated viral attachment and cell entry might be an evolutionarily conserved mechanism for a virus to overcome the ovarian barrier to achieve vertical transmission. Insect Vg aids in ovarian infection by other viruses or endosymbionts [39,40]. For example, the Spiroplasma endosymbiont can be vertically transmitted by Drosophila. The yolk-uptake machinery, mainly containing yolk protein and yolk receptor, helps the bacteria to colonize the insect germ line [40]. The whitefly Bemisia tabaci transmits Tomato yellow leaf curl virus (TYLCV). The TYLCV coat protein can interact with whitefly Vg, and these interacting parties play vital roles in TYLCV's entry into whitefly ovaries. Whether TYLCV invades the whitefly germ-line cells through VgR-mediated endocytic machinery remains to be investigated. Moreover, both L. striatellus and B. tabaci transmit arboviruses that do not invade ovaries, including Rice black-streaked dwarf virus by L. striatellus and Papaya leaf curl China virus by B. tabaci. In each case, the viral coat protein does not bind to the insect Vg, and the virus is unable to invade the insect ovaries [28,39].
Overall, our studies described a novel molecular mechanism for how an arbovirus hitchhikes a required insect ligand-receptor pathway to achieve cell entry and vertical transmission. This mechanism may serve as a paradigm applying to other arboviruses of medical and agricultural importance.
Supplementary Material
Acknowledgements
We sincerely thank Prof. Feng Cui (State Key Laboratory of Integrated Management of Pest Insects and Rodents, Institute of Zoology, Chinese Academy of Sciences, Beijing, China) for kindly providing the Laodelphax striatellus genomic DNA sequence for alignment with the sequence of the vitellogenin receptor gene. We sincerely thank Prof. Xueping Zhou (Institute of Biotechnology, Zhejiang University, China) for kindly providing RSV-specific monoclonal antibodies.
Data accessibility
All primers and methods supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
Conception and design: Y.H., X.C., R.F. and L.Z. Data acquisition: Y.H., Y.Y., Q.L., M.Z. and D.L. Data analysis and interpretation: Y.H., Y.Y. and L.Z. Article writing: L.Z. and R.F. Final approval: Y.H., Y.Y., Q.L., M.Z., D.L., X.C., L.Z. and R.F.
Competing interests
We have no competing interests.
Funding
This work was supported by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDB11040100) to R.F., from the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDB11040800) to L.Z., and from the National Natural Science Foundation of China (no. 31601605) to Y.H.
References
- 1.Marsh M, Helenius A. 2006. Virus entry: open sesame. Cell 124, 729–740. ( 10.1016/j.cell.2006.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilen CB, Tilton JC, Doms RW. 2012. HIV: cell binding and entry. Cold Spring Harb. Perspect. Med. 2, a006866 ( 10.1101/cshperspect.a006866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenouillet E, Barbouche R, Jones IM. 2007. Cell entry by enveloped viruses: redox considerations for HIV and SARS-coronavirus. Antioxid. Redox Signal. 9, 1009–1034. ( 10.1089/ars.2007.1639) [DOI] [PubMed] [Google Scholar]
- 4.Johnson JE, Vogt PK. 2010. Cell entry by non-enveloped viruses. Curr. Top. Microbiol. Immunol. 343, v–vii. [PubMed] [Google Scholar]
- 5.Cosset FL, Lavillette D. 2011. Cell entry of enveloped viruses. Adv. Genet. 73, 121–183. ( 10.1016/B978-0-12-380860-8.00004-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plemper RK. 2011. Cell entry of enveloped viruses. Curr. Opin. Virol. 1, 92–100. ( 10.1016/j.coviro.2011.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorr P, et al. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 49, 4721–4732. ( 10.1128/AAC.49.11.4721-4732.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teissier E, Penin F, Pecheur EI. 2010. Targeting cell entry of enveloped viruses as an antiviral strategy. Molecules 16, 221–250. ( 10.3390/molecules16010221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young JTY. 2001. Virus entry and uncoating. In Fields virology (eds Knipe DM, Howley PM), pp. 87–103, 4th edn Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- 10.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393, 648–659. ( 10.1038/31405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skehel JJ, Wiley DC. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69, 531–569. ( 10.1146/annurev.biochem.69.1.531) [DOI] [PubMed] [Google Scholar]
- 12.Rossmann MG, He Y, Kuhn RJ. 2002. Picornavirus-receptor interactions. Trends Microbiol. 10, 324–331. [DOI] [PubMed] [Google Scholar]
- 13.Ng JC, Falk BW. 2006. Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 44, 183–212. ( 10.1146/annurev.phyto.44.070505.143325) [DOI] [PubMed] [Google Scholar]
- 14.Hogenhout SA, Ammar el D, Whitfield AE, Redinbaugh MG. 2008. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 46, 327–359. ( 10.1146/annurev.phyto.022508.092135) [DOI] [PubMed] [Google Scholar]
- 15.Jia D, Chen Q, Mao Q, Zhang X, Wu W, Chen H, Yu X, Wang Z, Wei T. 2018. Vector mediated transmission of persistently transmitted plant viruses. Curr. Opin. Virol. 28, 127–132. ( 10.1016/j.coviro.2017.12.004) [DOI] [PubMed] [Google Scholar]
- 16.Neelakanta G, Sultana H. 2016. Viral receptors of the gut: vector-borne viruses of medical importance. Curr. Opin. Insect Sci. 16, 44–50. ( 10.1016/j.cois.2016.04.015) [DOI] [PubMed] [Google Scholar]
- 17.Gubler DJ. 2001. Human arbovirus infections worldwide. Ann. N Y Acad. Sci. 951, 13–24. [DOI] [PubMed] [Google Scholar]
- 18.Gray SM, Banerjee N. 1999. Mechanisms of arthropod transmission of plant and animal viruses. Microbiol. Mol. Biol. R 63, 128–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q, Wei T. 2016. Viral receptors of the gut: insect-borne propagative plant viruses of agricultural importance. Curr. Opin. Insect Sci. 16, 9–13. ( 10.1016/j.cois.2016.04.014) [DOI] [PubMed] [Google Scholar]
- 20.Gray S, Gildow FE. 2003. Luteovirus-aphid interactions. Annu. Rev. Phytopathol. 41, 539–566. ( 10.1146/annurev.phyto.41.012203.105815) [DOI] [PubMed] [Google Scholar]
- 21.Wei TY, Chen HY, Ichiki-Uehara T, Hibino H, Omura T. 2007. Entry of Rice dwarf virus into cultured cells of its insect vector involves clathrin-mediated endocytosis. J. Virol. 81, 7811–7815. ( 10.1128/Jvi.00050-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazaki N, Higashiura A, Higashiura T, Akita F, Hibino H, Omura T, Nakagawa A, Iwasaki K. 2016. Electron microscopic imaging revealed the flexible filamentous structure of the cell attachment protein P2 of Rice dwarf virus located around the icosahedral 5-fold axes. J. Biochem. 159, 181–190. ( 10.1093/jb/mvv092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JL, de Wet BJ, Martinez-Pomares L, Radcliffe CM, Dwek RA, Rudd PM, Gordon S. 2008. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 4, e17 ( 10.1371/journal.ppat.0040017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng G, Cox J, Wang PH, Krishnan MN, Dai JF, Qian F, Anderson JF, Fikrig E. 2010. A C-type lectin collaborates with a CD45 phosphatase homolog to facilitate West Nile virus infection of mosquitoes. Cell 142, 714–725. ( 10.1016/j.cell.2010.07.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroschewski H, Allison SL, Heinz FX, Mandl CW. 2003. Role of heparan sulfate for attachment and entry of tick-borne encephalitis virus. Virology 308, 92–100. [DOI] [PubMed] [Google Scholar]
- 26.Kuadkitkan A, Wikan N, Fongsaran C, Smith DR. 2010. Identification and characterization of prohibitin as a receptor protein mediating DENV-2 entry into insect cells. Virology 406, 149–161. ( 10.1016/j.virol.2010.07.015) [DOI] [PubMed] [Google Scholar]
- 27.Perera-Lecoin M, Meertens L, Carnec X, Amara A. 2013. Flavivirus entry receptors: an update. Viruses 6, 69–88. ( 10.3390/v6010069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huo Y, et al. 2014. Transovarial transmission of a plant virus is mediated by vitellogenin of its insect vector. PLoS Pathog. 10, e1003949 ( 10.1371/journal.ppat.1003949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huo Y, Yu Y, Chen L, Li Q, Zhang M, Song Z, Chen X, Fang R, Zhang L. 2018. Insect tissue-specific vitellogenin facilitates transmission of plant virus. PLoS Pathog. 14, e1006909 ( 10.1371/journal.ppat.1006909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, et al. 2017. Genome sequence of the small brown planthopper, Laodelphax striatellus. Gigascience 6, 1–12. ( 10.1093/gigascience/gix109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786. ( 10.1038/nmeth.1701) [DOI] [PubMed] [Google Scholar]
- 33.Cong L, Yang WJ, Jiang XZ, Niu JZ, Shen GM, Ran C, Wang JJ. 2015. The essential role of vitellogenin receptor in ovary development and vitellogenin uptake in Bactrocera dorsalis (Hendel). Int. J. Mol. Sci. 16, 18 368–18 383. ( 10.3390/ijms160818368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tufail M, Takeda M. 2005. Molecular cloning, characterization and regulation of the cockroach vitellogenin receptor during oogenesis. Insect. Mol. Biol. 14, 389–401. ( 10.1111/j.1365-2583.2005.00570.x) [DOI] [PubMed] [Google Scholar]
- 35.Tufail M, Takeda M. 2009. Insect vitellogenin/lipophorin receptors: molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect. Physiol. 55, 87–103. ( 10.1016/j.jinsphys.2008.11.007) [DOI] [PubMed] [Google Scholar]
- 36.Sappington TW, Raikhel AS. 1998. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect. Biochem. Mol. Biol. 28, 277–300. [DOI] [PubMed] [Google Scholar]
- 37.Jia D, et al. 2017. Insect symbiotic bacteria harbour viral pathogens for transovarial transmission. Nat. Microbiol. 2, 17025 ( 10.1038/nmicrobiol.2017.25) [DOI] [PubMed] [Google Scholar]
- 38.Qin F, Liu W, Wu N, Zhang L, Zhang Z, Zhou X, Wang X. 2018. Invasion of midgut epithelial cells by a persistently transmitted virus is mediated by sugar transporter 6 in its insect vector. PLoS Pathog. 14, e1007201 ( 10.1371/journal.ppat.1007201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei J, He YZ, Guo Q, Guo T, Liu YQ, Zhou XP, Liu SS, Wang XW. 2017. Vector development and vitellogenin determine the transovarial transmission of begomoviruses. Proc. Natl Acad. Sci. USA 114, 6746–6751. ( 10.1073/pnas.1701720114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herren JK, Paredes JC, Schupfer F, Lemaitre B. 2013. Vertical transmission of a Drosophila endosymbiont via cooption of the yolk transport and internalization machinery. MBio 4, e00532–12 ( 10.1128/mBio.00532-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All primers and methods supporting this article have been uploaded as part of the electronic supplementary material.