Abstract
Thousands of species of ambrosia beetles excavate tunnels in wood to farm fungi. They maintain associations with particular lineages of fungi, but the phylogenetic extent and mechanisms of fidelity are unknown. We test the hypothesis that selectivity of their mycangium enforces fidelity at coarse phylogenetic scales, while permitting promiscuity among closely related fungal mutualists. We confirm a single evolutionary origin of the Xylosandrus complex—a group of several xyleborine genera that farm fungi in the genus Ambrosiella. Multi-level co-phylogenetic analysis revealed frequent symbiont switching within major Ambrosiella clades, but not between clades. The loss of the mycangium in Diuncus, a genus of evolutionary cheaters, was commensurate with the loss of fidelity to fungal clades, supporting the hypothesis that the mycangium reinforces fidelity. Finally, in vivo experiments tracked symbiotic compatibility throughout the symbiotic life cycle of Xylosandrus compactus and demonstrated that closely related Ambrosiella symbionts are interchangeable, but the probability of fungal uptake in the mycangium was significantly lower in more phylogenetically distant species of symbionts. Symbiont loads in experimental subjects were similar to wild-caught beetles. We conclude that partner choice in ambrosia beetles is achieved in the mycangium, and co-phylogenetic inferences can be used to predict the likelihood of specific symbiont switches.
Keywords: Ascomycota, cooperation, forest health, partner choice, Xyleborini, symbiosis
1. Introduction
Partner fidelity and partner choice are prevailing models for explaining the evolutionary stability of mutualisms. Partner fidelity describes associations where individuals or genetic lines of mutualists repeatedly interact and enforce mutually positive outcomes through punishment, reciprocation and positive fitness feedbacks. Partner choice applies to associations where mutualists might not interact repeatedly, but positive outcomes are favoured when one partner can select good cooperators from multiple potential partners [1,2]. Although typically presented as alternative scenarios, the two models are not necessarily mutually exclusive. Partner choice as a means of partner specificity may actually drive fidelity when transmission is often, but not always vertical [3]. Specificity of partner choice is rarely perfect and the implications of variable specificity for long-term fidelity and coevolutionary processes are largely unknown.
Specificity in mutualisms is a continuum, and many systems are not as specific as they may seem at first look [4]. Previous inferences of strict specificity have been overturned in some of the best studied mutualistic systems as a result of improved molecular-based taxonomy and co-phylogenetic studies that reveal host and symbiont switches over evolutionary time [5–9]. Similarly, strict specificity has also been refuted as a result of species invasion and subsequent new encounters between host and symbiont species [10–12]. These departures from strict specificity may be common because they are advantageous. While a degree of specificity may stabilize mutualisms by enforcing partner fidelity [1–3], strict specificity can be an evolutionary dead end. When species are absolutely dependent on other species, the extinction of one is inevitably followed by the extinction of the other [13]. Strict specificity also diminishes species' abilities to adapt to changing conditions, or oppositely, non-specificity allows populations and individuals to form associations that are best suited for current local conditions and maintain a broad niche breadth [14–17]. The degree of specificity in symbioses need to be considered in a phylogenetic framework because symbionts are more likely to switch among closely related hosts than distantly related hosts, and vice versa [18,19]. We hypothesize that this phylogenetic bias in specificity maintains coarse-scale phylogenetic congruence between hosts and symbionts in systems where long-term fidelity is enforced by the phylogenetic specificity of partner choice. Testing this prediction requires combined experimental and phylogenetic studies to characterize the degree of fidelity at ecological and evolutionary time scales, and identify their underlying mechanisms.
Few symbiotic systems are as amenable to comparative evolutionary studies as the ambrosia beetle–fungus system. Ambrosia beetles engage in the most diverse and widespread fungus-farming mutualism known. They comprise approximately 3400 species within two subfamilies of weevils (Curculionidae; Scolytinae and Platypodinae) [20]. Several clades of ambrosia beetles have independently evolved specialized pouch- or pit-like organs termed mycangia (singular: mycangium) which are used to transport actively growing and reproducing fungal propagules to newly established galleries [21–23]. In return for dispersal, the fungi produce specialized enlarged nutritious spores that comprise the majority or entirety of the beetle diet [21–23]. While some ambrosia beetles carry multiple related species within their mycangium [24,25], more commonly ambrosia beetles carry a single primary symbiont in their mycangium [26–28]. The phylogenetic extent of fidelity in ambrosia beetles and fungi and the underlying mechanism(s) are unknown. Likewise, the mechanisms that control fidelity are also poorly understood in other insect–fungus symbioses such as bark beetles [29], ants [5,30] and wood wasps [11,31].
In this study, we address multiple mechanisms that have been hypothesized to support fidelity in ambrosia symbioses using a newly developed experimental ambrosial study system, the Xylosandrus complex (Coleoptera, Curculionidae, Scolytinae) and Ambrosiella fungi (Fungi, Microascales, Ceratocystidaceae). The Xylosandrus complex is one of the most successful, widespread, well-studied and economically relevant lineages of ambrosia beetles, consisting of 111 described species in six genera in the tribe Xyleborini (Anisandrus, Cnestus, Diuncus, Eccoptopterus, Hadrodemius and Xylosandrus) that live in tropical to temperate forests around the world. While some studies have suggested that the Xylosandrus complex is polyphyletic [32–34], others have found monophyly albeit with limited taxon sampling [35,36]. The Xylosandrus complex also includes several species of widely introduced forest health and agricultural pests [37]. Females in this group possess large mesothoracic glandular mycangia just beneath the scutellum [21]; (figure 1). Xylosandrus complex species have each been found consistently associated with a single fungus species in the genus Ambrosiella (Microascales: Ceratocystidaceae) across multiple continents, even in areas where multiple Xylosandrus complex species co-infest the same trees [23,26,39–42]. It has been hypothesized that the mycangium is selective in the sense that it is an environment that is only habitable or accessible to particular lineages of symbiotic fungi [26], though there have been no previous experimental tests of the selectivity of the mycangium.
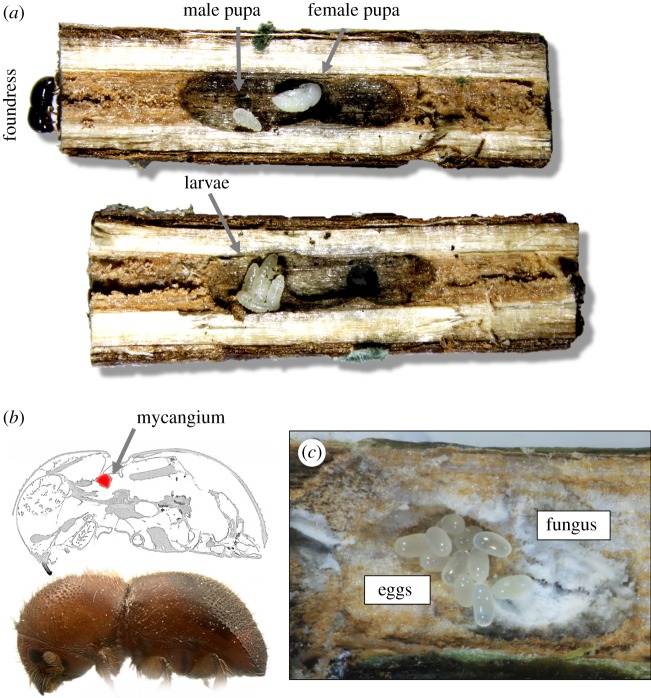
Figure 1.
Typical Xylosandrus ambrosia beetle life history. (a) A foundress excavates a gallery in the pith or xylem of a twig or branch. She produces daughters and one to several haploid sons. Males are small and flightless, and mate with their sisters and mothers [38]. Mated diploid females disperse and establish new galleries. Just prior to dispersal the females' mycangia (b) rapidly fills with budding fungal spores, which are used to propagate a fungal garden in newly established galleries. Shown is a micro CT scan cross section of Xylosandrus crassiusculus with the mycangium highlighted in red. (c) Dispersed females initiate new galleries and oviposit after the establishment of ambrosia fungi, visible as a dense mat of enlarged nutritious spores surrounding a clutch of Xylosandrus compactus eggs in a natural gallery in red bay Persea borbonia. (Online version in colour.)
One genus in the Xylosandrus complex, Diuncus, lacks a mycangium [35]. Diuncus exemplify an evolutionary cheater strategy because they exploit the nutritious spores produced by ambrosia fungi, but do not reciprocate as fungal vectors. Instead, they bore their gallery near the gallery of other ambrosia beetles, and feed on the other beetle's fungus as the hyphae extend into their gallery [35]. The existence of fungal cheaters, fungi that infiltrate the mycangium of ambrosia beetles but do not produce nutritious spores in the gallery, has been suggested but not yet demonstrated [28].
We constructed the most extensive to date phylogenies of the Xylosandrus complex and Ambrosiella fungi, and statistically tested for the co-phylogenetic structure at multiple phylogenetic scales. Informed by observed co-phylogenetic patterns, we conducted the first symbiont switching experiments using laboratory raised aposymbiotic Xylosandrus ambrosia beetles to test the hypothesis that the mycangium provides a mechanism for partner choice and an intermediate degree of fidelity that is contingent on phylogenetic distances among potential symbionts. Our results reveal a driving mechanism behind the co-phylogenetic structure and patterns in ecological associations in the world's widest ranging and diverse fungus-farming mutualism and illustrate the utility of co-phylogenetic analysis for predicting lateral transmission of fungal symbionts among vector species.
2. Material and methods
(a). Cophylogenetic analysis
Sequences for five marker genes for beetles were obtained from specimens and databases: mitochondrial gene cytochrome oxidase I (COI), nuclear large subunit ribosomal gene (28S), elongation factor 1-alpha (EF1α), a gene which encodes carbamoylphosphate synthetase, aspartate transcarbamylase and dihydroorotase (CAD) and arginine kinase (ArgK). For Ambrosiella fungi, we targeted three marker genes: the nuclear large subunit ribosomal gene (28S/LSU), trans-elongation factor 1-alpha (TEF1α) and RNA polymerase II subunit 1 (RPB1). Because the monophyly of Xylosandrus and similar genera has not been consistently found in previous taxon-rich phylogenetic studies of Xyleborini [32,33] we included 17 other Xyleborini genera to test for monophyly of the Xylosandrus complex. We also included Coccotrypes (Dryocoetini), which is probably the ancestral group of all Xyleborini, and Dryocoetiops (Dryocoetini) to root our beetle phylogeny. Monophyly of Ambrosiella associated with Xylosandrus has been previously demonstrated [26].
In addition to all available sequences on GenBank, we obtained 19 beetle sequences and 12 fungus sequences from specimens of seven beetle species stored in the University of Florida Forest Entomology cryo-preserved collection (see electronic supplementary material, tables S1 and S2). See electronic supplementary material for DNA extraction methods. GenBank accession numbers are listed in the electronic supplementary material, tables S1 and S2.
Phylogenies for ambrosia beetles and ambrosia fungi were inferred with the same methods (see electronic supplementary material). The beetle phylogeny was pruned to only include beetles for which observations of fungal associations were available, and reciprocally the fungus tree was pruned to only fungi associated with represented beetles. Associations for beetles in the genus Diuncus were inferred from published and unpublished observations of gallery parasitism on other species in the Xylosandrus complex with known fungal associates [35]. Even though they lack mycangia, Diuncus were included in the graphical representation of cophylogenetic relationships to see if the species that they parasitized were restricted to those that farm a particular lineage of fungi, or conversely if fidelity to fungal lineages was lost with the mycangium.
Phylogenetic congruence between the Xylosandrus complex and Ambrosiella fungi was visually assessed by constructing a co-phylogenetic tanglegram using the cophylo() function of the ape package [43] for R [44], and rotating nodes to find the paired topologies with fewest intersecting ties. Pruned trees were converted to pairwise patristic branch length distance matrices for co-phylogenetic analysis using the cophenetic() function in ape [43]. We tested for significant congruence using a null model test of cophylogenetic congruence based on Procrustean analysis (paco [45]), implemented using the PACo() function, of the paco package [46] for R. After preliminary analysis indicated that all or most of the phylogenetic congruence between beetles and fungi corresponded to the deepest division in the fungal tree, we conducted subsequent paco analyses for all links within each of two major fungal clades (hereafter the beaveri clade and the xylebori clade) separately to determine if there was congruence at finer phylogenetic scales within the major clades of Ambrosiella. For all paco analyses, we applied a symmetrical Procrustean analysis and used the quasi-swap matrix permutation algorithm [47], which maintains row and column sums, to create 1000 permutations of the interaction matrix, and provides a conservative test of the null hypothesis of no congruence, and does not require prior assumptions about which lineage has tracked the other over their coevolutionary history [46,48].
(b). Symbiont switching experiments
(i). Beetle collection and fungal isolation
During the spring of 2016, we obtained isolates of Ambrosiella fungi from the mycangia of X. compactus, X. amputatus and X. crassiusculus in Gainesville, Florida, USA. We also obtained isolates from the mycangia of X. discolor in Tam Dao National Park, Vinh Phuc Province, Vietnam and from X. germanus collected in Madison, Wisconsin, USA. Dispersing Xylosandrus beetles were collected alive using bottle traps [49] and Lindgren funnel traps baited with 95% ethanol. Fungal isolation, estimation of spore abundance, purification, DNA extraction, PCR and DNA barcode identification followed the methods of Bateman et al. [50]. To extract fungi from the mycangium, beetles were impaled through the head and pronotum with insect mounting pins. Gentle pressure was applied to the scutellum to evert the prothoracic mycangium, expelling the paste-like fungal spore mass when present. Mycangia were confirmed to be empty when fully everted without expelling a visible spore mass (see electronic supplementary material, video supplement V2). We considered a mycangium with any visible spore mass to be ‘full’, recognizing that full mycangia will vary in the number of propagules they contain. Spore masses were aseptically transferred to a 1.5 ml tube with 500 µl sterile phosphate buffered saline, vortexed for 30 s, and plated on standard potato dextrose agar at 0.1, 0.01 and 0.001 spore dilutions. Plates were incubated for one week at 25°C prior to colony morphotype assignment, estimation of colony forming units (CFU) and subculture. Subcultures were incubated one week further, inspected for purity and used for extraction of genomic DNA. From genomic DNA, we amplified and sequenced translation elongation factor one alpha (TEF1α) using the primers EFCF1.5 and EFCF6 [51] for identification by comparison to sequences of type materials available on GenBank (electronic supplementary material, table S1). We recovered isolates of Ambrosiella xylebori, A. nakashimae and A. roeperi from Xylosandrus compactus, X. amputatus and X. crassiusculus, respectively. We isolated A. beaveri from X. discolor and A. grosmanniae from X. germanus. All of these associations match previous accounts [26,39,40,42,50].
(ii). Beetle development and mycangium assays
Beetles were reared from eggs and first instar larvae in artificial galleries experimentally inoculated with one of three Ambrosiella species. See electronic supplementary material for artificial gallery construction and inoculation methods. We tested the typical symbiont A. xylebori, the Vietnamese isolate from X. discolor (A. beaveri) and A. grossmaniae from X. germanus collected in Wisconsin, USA. Eggs and larvae were harvested from wild active galleries collected in Gainesville Florida, USA (n = 74). Eggs and first instar larvae were placed on sterile moistened filter paper, washed by dripping sterile PBS via micropipette and incubated for 24 h before being washed again. We placed three to five randomly selected eggs and first instar larvae (in combination) in each colonized experimental gallery, then incubated at 25–30°C and 90–100% RH, with a 12 h d−1 indirect full spectrum LED light and monitored daily for emergent adults.
In the mycangium assay, we reared adult beetles from pupae in experimental galleries to determine if the mycangium of X. compactus could support the growth and transportation of alternative ambrosial species. Beetles were reared on the typical symbiont (A. xylebori; n = 14), one of four alternative symbionts A. roeperi (n = 10), A. grosmanniae (n = 12), A. beaveri (n = 7), A. nakashimae (n = 11), or in negative control galleries without any inoculated fungi (n = 5). Pupae were harvested from laboratory reared broods (see electronic supplementary material). We placed two to three female pupae in each experimental gallery and monitored daily for emergent adults. The mycangia of emergent adults were immediately examined for a fungal spore mass as described above. When a spore mass was present, spore abundance and identity were determined by dilution plating and DNA barcode identification as described above. Between 4 and 12 isolates were sequenced from every emergent adult beetle with a full mycangium to determine if mycangial contents were uncontaminated monocultures. After eight weeks, galleries were opened to confirm the mortality of remaining beetles. To determine if spore loads in our experiment were similar to naturally reared beetles, we used the same methods to identify and quantify spore loads in six wild-collected dispersing black twig borers.
For the beetle development assay, we tested for an effect of fungus species on the probability of survival and development to adult using a binomial generalized linear model (GLM). We used the typical symbiont (A. xylebori) as the reference group and the number of adults recovered versus the number of mortalities per gallery as our response variable. We also used binomial GLM to test for differences in the proportions of emerging adults that had full mycangia among fungal species treatments in the development and mycangium assays. For these analyses, our response variable was the number of emergent adults with full versus empty mycangia, per gallery. We conducted binomial GLM at the fungus species level to test for individual differences between the typical symbiont, A. xylebori (reference group), and each other Ambrosiella species. We also conducted a clade-level generalized linear mixed model (GLMM), in which all alternative symbionts from the same clade of Ambrosiella as the typical symbiont were compared to Ambrosiella from the alternative clade, with a random intercept for fungal species. We also tested for differences in symbiont survival and abundance by comparing colony forming unit (CFU) counts estimated from full mycangia between all experimental treatments, and beetles captured wild in flight. For this, we used a negative binomial GLM implemented by the glm.nb() function of the MASS package [52] with field collected beetles as the reference group. A negative binomial model was chosen because of high overdispersion in the CFU data. GLM analyses were implemented using the glm() function in the R stats package [44] and GLMM using the glmer() function of the lme4 package [53].
3. Results
(a). Phylogenetic analysis
Phylogenetic analysis found monophyly of the Xylosandrus complex containing Anisandrus, Cnestus, Diuncus, Eccoptopterus, Hadrodemius and Xylosandrus (electronic supplementary material, figure S1). All genera were found to be monophyletic except Anisandrus. The mycocleptic genus Diuncus was nested within mycangia-possessing species, indicating that the mycangium and associated tuft of setae on the pronotum were secondarily lost in this lineage of evolutionary cheaters. The phylogeny of Ambrosiella revealed two major clades, the xylebori clade and the beaveri clade. The xylebori clade contained A. catenulata, A. cleistominuta, A. batrae, A. grossmaniae, A. hartigii, A. roeperi and A. xylebori, as well as two undescribed Ambrosiella species sequenced directly from the mycangia of X. morigerus and E. gracilipes. The beaveri clade contained several genetically similar fungi including A. nakashimae and multiple genotypes identified as A. beaveri in previous work [26,39], which may represent multiple closely related species.
(b). Co-phylogenetic analysis
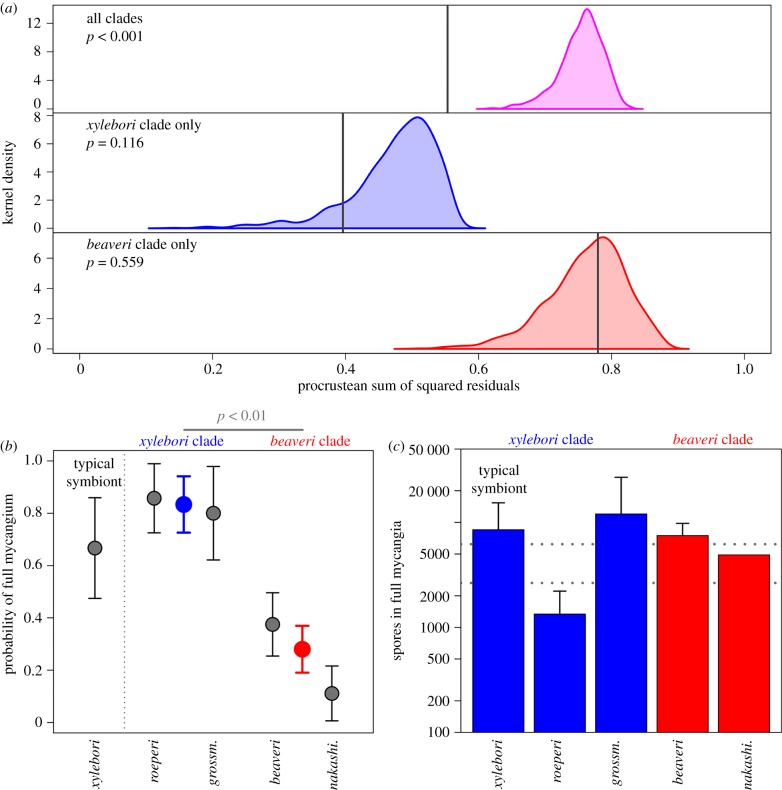
Co-phylogenetic congruence between the ambrosia beetle and ambrosia fungus phylogenies was highly significant when analysed across all clades (procrustean test of phylogenetic congruence; p < 0.001; figures 2 and 3a). Most of the congruence was derived from beetle genera that had exclusive associations to members of either of the two major fungal clades; all members of two clades of beetles were associated with fungi in the beaveri clade, one comprises Hadrodemius and Cnestus, the other comprises four species of Xylosandrus: X. amputatus, X. brevis, X. discolor, and X. mancus. All other beetles, excluding the mycocleptic parasites in Diuncus, were exclusively associated with xylebori clade fungi. In contrast to the strong coarse-scale co-phylogenetic pattern, we found no significant congruence at finer phylogenetic scales within xylebori clade associations (p = 0.116), nor within beaveri clade associations (p = 0.559).
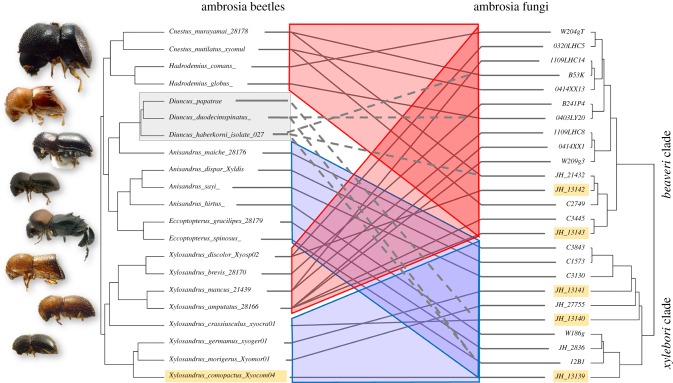
Figure 2.
Coarse phylogenetic congruence in beetles of the Xylosandrus complex and Ambrosiella fungi. Tanglegram shows phylogenetic congruence among ambrosia beetles and ambrosia fungi; two clades of beetles are exclusively associated with the beaveri clade of Ambrosiella fungi (highlighted in red). The remainder is associated with the xylebori clade (blue), except for the fungus-stealing cheaters in the genus Diuncus. Dotted lines indicate inferred relationships with Ambrosiella farmed by parasitized beetle species. Unlike all other Xylosandrus complex beetles with mycangia, Diuncus was associated with both major Ambrosiella clades. Names of fungal isolates and beetle used in symbiont switching experiments are highlighted; JH_13142 = A. beaveri, JH_13143 = A. nakashimae, JH_13141 = A. grosmanniae, JH_13140 = A. roeperi, JH_13139 = A. xylebori. (Online version in colour.)
Figure 3.
Mycangium selectivity explains coarse phylogenetic congruence in Xylosandrus and Ambrosiella. (a) Ambrosia beetle lineages have maintained fidelity to either fungal clade but have been promiscuous within those clades. Permutations-based null model hypothesis test of phylogenetic congruence based on pairwise distances: coloured areas show null distributions of Procrustean sums of squares (higher values indicate less congruence) for 1000 permutations of the association matrix. Grey vertical lines show observed sums of squares. The observed sums of squares for all associations were lower than all permutations of the association matrix, indicating highly significant congruence. There was no significant congruence when the analysis was restricted to only association involving either the xylebori clade fungi or the beaveri clade. (b) In symbiont switching experiments, ambrosia fungi from the alternative beaveri clade were significantly less likely to be taken up by the mycangium of X. compactus than the typical symbiont (A. xylebori) and others in the xylebori clade. Model estimates for each clade (excluding typical symbiont) are shown as coloured symbols. (c) Experimental subjects had fungal spore loads similar to wild collected beetles for all Ambrosiella species except A. roeperi. Error bars represent 95% CI. Horizontal dotted lines show 95% CI for estimates from wild X. compactus caught while flying. (Online version in colour.)
(c). Development assay
Adult X. compactus beetles were successfully reared from eggs and first instar larvae to normal adults on Ambrosiella species from both major clades. An average of 40.5% of beetles from each gallery survived to the adult stage. There was no significant difference in beetle survival between the typical symbiont, A. xylebori, and either alternative symbionts; A. grossmanniae (binomial GLM; z = 1.539, p = 0.12) and A. beaveri (z = 1.291, p = 0.20).
(d). Mycangium assay
The mycangium of emerging X. compactus adults was significantly more likely to be full when the beetle was incubated with an Ambrosiella fungus from the xylebori clade (the same clade as their typical symbiont, A. xylebori) than when reared on Ambrosiella from beaveri clade fungi (binomial GLMM, z = −2.825, p = 0.004; figure 3b). At the species level, emergent females reared with the beaveri clade fungus A. nakashimae were significantly less likely to have a full mycangium (z = −2.025, p = 0.043), while all other species were not different from the reference group. Once a fungus was established in the mycangium of X. compactus, there were no significant differences among Ambrosiella species in the number of propagules produced, except for A. roeperi which was significantly lower than all other Ambrosiella species tested (p < 0.05 for all pairwise comparisons that included A. roeperi; Tukey's HSD post hoc test; figure 3b). The significantly lower propagule production of A. roeperi in vitro is consistent with previous experiments that showed variable and often low propagule production of this species within the mycangium of its typical vector X. crassiusculus [54]. Excluding A. roeperi, mycangial growth under our experimental conditions was comparable to natural conditions (i.e. the CFU estimates for all other fungal treatments were not significantly different from wild-caught dispersing X. compactus females and were similar to previous surveys of X. compactus caught in flight [50]).
4. Discussion
The diverse and globally distributed mutualism between beetles in the Xylosandrus complex and Ambrosiella fungi stemmed from a single evolutionary origin. Throughout the co-diversification of the Xylosandrus complex and Ambrosiella, this mutualism has not been species-specific, nor has it been entirely promiscuous. Instead, this association displays coarse-scale phylogenetic fidelity, such that each genus of beetle (except Diuncus) has remained exclusively associated with only one of the major clades of Ambrosiella fungi. However, the loss of co-phylogenetic signal at finer phylogenetic scales indicates that Xylosandrus beetles have frequently switched fungi within the xylebori and beaveri fungal clades, but switches between these clades have occurred much less frequently, perhaps only before the two Ambrosiella lineages had diverged. We can infer only one such switch, in the ancestor of X. brevis and X. amputatus after the divergence of X. crassiusculus. Phylogenetic analysis placed Diuncus within the Xylosandrus complex, confirming the hypothesis that the mycangium was secondarily lost when this lineage began stealing the fungal cultivars of other ambrosia beetles [35].
We provide the first experimental demonstration that the ambrosia beetle mycangium is selective among congeneric lineages of ambrosia fungi and that selectivity explains coevolutionary patterns between ambrosia beetles and ambrosia fungi. Beetles incubated with their typical symbiont or alternative symbionts from the same clade (i.e. the xylebori clade) were approximately three times more likely to emerge from their galleries with mycangia bearing fungal propagules than beetles incubated with fungi from an alternative clade. Therefore, the mycangium in this system is not strictly species-specific in selecting fungal symbionts, nor does it discriminate completely at any level within Ambrosiella, but instead it makes acquisition of novel fungal partners increasingly unlikely with increasing phylogenetic distance from the typical symbiont. In doing so, the mycangium reinforces fidelity at coarse co-phylogenetic scales, while allowing promiscuity among closely related fungal symbionts. The specific mechanisms that drive selectivity in the mycangium are still unknown but could include negative feedbacks such as chemical or immunological exclusion of non-target fungi and positive feedbacks such as nutritional sources that can only be used by target fungi, and may involve host behaviours that make the mycangium more or less accessible to various fungi. Future theoretical and empirical research is needed to determine if the maintenance of this intermediate degree of fidelity that is based on phylogenetic relatedness is an adaptive trait and a stabilizing mechanism in the evolution of mutualisms.
Other proposed mechanisms for fidelity between ambrosia beetles and their fungi were not supported. Though transmission of ambrosia fungi appears vertical in effect [55], it is actually transmitted indirectly from parent to offspring. Ambrosia beetles do not obtain their symbionts until they have completed development to the adult stage when the mycangium develops, and the fungal symbiont is acquired from the gallery, not through direct contact with the mother [54]. This transmission mode provides ample opportunity for horizontal transmission because fungal symbionts extend throughout the wood and invade neighbouring galleries [12,28,56]. We found evidence of frequent horizontal symbiont switching at fine phylogenetic scales as loss of phylogenetic congruence within major clades of symbionts. Our result is congruent with a recent co-phylogenetic study of Euwallacea ambrosia beetles and ambrosial Fusarium [6]. Therefore, strict vertical transmission cannot explain co-phylogenetic congruence in ambrosia beetles. Geographic isolation also cannot explain co-phylogenetic congruence. Many beetles in the Xylosandrus complex have extensive geographic ranges, and the co-occurrence of several to many species in dead trees of tropical and sub-tropical forests is typical [57]. It has been recently hypothesized that Xylosandrus beetles maintain fidelity to their fungi by seeking wood substrates that are rich in ethanol which provides a selectively harsh environment that screens out antagonistic non-ambrosial fungi such as Penicillium and Aspergillus [58]. However, all species in the Xylosandrus complex, as well as many other ambrosia beetle groups (e.g. Ambrosiodmus, Corthylus, Euwallacea, Xyleborus and Xyleborinus), are attracted to the ethanol released by stressed or dying trees. Owing to this shared attraction, dying trees in the tropics and subtropics are typically perforated by intermingled galleries, each inoculated with various species of ambrosia fungi. Consequently, ethanol screening cannot explain the co-phylogenetic congruence between Xylosandrus and Ambrosiella clades over evolutionary time shown here, or broader clade-level fidelity among independently derived ambrosia systems [59].
Nutritional specificity also does not explain fidelity in ambrosia symbioses. Larval X. compactus placed in experimental galleries with alternative fungal symbionts from either Ambrosiella clade began grazing on fungal spores within minutes (electronic supplementary material, video supplement V1). Xylosandrus compactus were successfully raised from eggs to adults on fungi from both major clades of Ambrosiella, and there were no significant differences among the tested Ambrosiella species. Xylosandrus compactus and its mycangial symbiont have been the focus of extensive study [23,50,60,61], and in every case the symbionts isolated from the mycangium were identified as A. xylebori. Thus, our experimental results demonstrate that even a beetle which displays very high fidelity for its fungal symbionts in nature, can consume and develop normally on alternative fungi, and thus symbiont specificity cannot be reliably inferred from even extensive field observations of host/symbiont associations alone.
Observations from Diuncus confirmed several important points. First, the parasitic lifestyle of Diuncus demonstrates that the transmission of ambrosia fungi is not necessarily vertical because every generation of Diuncus larvae is fed by horizontally transmitted ambrosia fungi that infiltrate their galleries through the xylem from neighbouring galleries [35]. Second, Diuncus was observed parasitizing a diversity of beetles in the Xylosandrus complex, including farmers of either major Ambrosiella clade, confirming that beetles in the Xylosandrus complex are not nutritionally limited to specific species or broader clades of Ambrosiella. Finally, the loss of mycangia in Diuncus was commensurate with a unique lack of fidelity to either Ambrosiella clade, further implicating the mycangium as a mechanism for coarse-scale fidelity among species within the Xylosandrus complex and Ambrosiella fungi.
While selectivity of the ambrosia beetle mycangium maintains fidelity at coarse phylogenetic scales, geography, ecology and climatic differences have stronger influences on symbiotic associations at finer scales. Historical shifts in distribution may have allowed co-speciation, which may reflect finer patterns within clades. Within the Ambrosiella xylebori clade, a monophyletic fungal clade is associated with a paraphyletic group of beetles that share biogeographic histories. The beetles Anisandrus dispar, A. sayi, A. maiche and X. germanus represent different genera, but are unusual among the Xylosandrus complex because they are all found in temperate areas as opposed to the tropics. Interestingly, these beetles are together associated with one monophyletic clade of Ambrosiella. We speculate that this clade of fungi may be particularly cold hardy and acquired separately by each temperate beetle lineage as they progressed northward from the tropics. There is also a clade of Australasian Xylosandrus (X. russulus, X. monteithi and X. rotundicollis) in which the symbionts are entirely unknown and may have evolved independently with their own Ambrosiella lineage.
The results reported here have important practical implications for species introductions, global silviculture and forest health. Anthropogenic introductions are increasingly bringing together novel combinations of insects and fungal plant pathogens, with potentially massive negative impacts. While most introduced ambrosia beetles have had little to no negative impact, a few have been devastating [37,62]. For example, a single introduction of the red bay ambrosia beetle (Xyleborus glabratus) and its symbiont Raffaelea lauricola, the causal agent of laurel wilt disease, has caused the death of more than 300 million lauraceous trees and altered forest community structure throughout the southeastern United States [63,64]. While attacks from the red bay ambrosia beetle are mostly limited to the red bay (Persea borbonia), horizontal transmission of the pathogen to alternative ambrosia beetle species with broader host tree preferences is negatively impacting avocado (Persea americana) industries [62]. Our results demonstrate that knowledge of the co-phylogenetic relationships of ambrosia beetles and their symbionts determines their risk level and can inform future invasive species management decisions. Our capacity to identify which invading beetles are likely to serve as alternative vectors for introduced pathogenic fungi, and which ones are likely to remain harmless, allows agencies to focus their limited resources on monitoring and controlling the most relevant species.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Carolina Hernandez for valuable assistance in the field and laboratory and Robert P. Creed for valuable feedback on previous versions of this manuscript.
Data accessibility
The data supporting this article are available in the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.4bm536k [65]. All sequence data are available on GenBank (see electronic supplementary material, tables S1 and S2 for accession numbers).
Authors' contributions
J.S., A.J.J., M.A.J., C.C.B., Y.L. and J.H. collected specimens and data, assisted in the intellectual development of the project and contributed to final manuscript preparation. J.S. and A.J.J. analyzed data, produced figures and prepared initial manuscript draft.
Competing interests
We declare we have no competing interests.
Funding
This work was financially supported by USDA Forest Service cooperative agreements, the USDA APHIS Farm Bill §10007, NSF DEB, the Florida Department of Agriculture—Division of Plant Industry and the Florida Forest Service.
References
- 1.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. 2004. The evolution of cooperation. Q. Rev. Biol. 79, 135–160. ( 10.1086/383541) [DOI] [PubMed] [Google Scholar]
- 2.Bull J, Rice W. 1991. Distinguishing mechanisms for the evolution of co-operation. J. Theor. Biol. 149, 63–74. ( 10.1016/S0022-5193(05)80072-4) [DOI] [PubMed] [Google Scholar]
- 3.Kaltenpoth M, Roeser-Mueller K, Koehler S, Peterson A, Nechitaylo TY, Stubblefield JW, Herzner G, Seger J, Strohm E. 2014. Partner choice and fidelity stabilize coevolution in a Cretaceous-age defensive symbiosis. Proc. Natl Acad. Sci. USA 111, 6359–6364. ( 10.1073/pnas.1400457111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronstein JL. 2015. Mutualism. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Mueller UG, Rehner SA, Schultz TR. 1998. The evolution of agriculture in ants. Science 281, 2034–2038. ( 10.1126/science.281.5385.2034) [DOI] [PubMed] [Google Scholar]
- 6.O'Donnell K, et al. 2015. Discordant phylogenies suggest repeated host shifts in the Fusarium–Euwallacea ambrosia beetle mutualism. Fung. Genet. Biol. 82, 277–290. ( 10.1016/j.fgb.2014.10.014) [DOI] [PubMed] [Google Scholar]
- 7.Machado CA, Robbins N, Gilbert MTP, Herre EA. 2005. Critical review of host specificity and its coevolutionary implications in the fig/fig-wasp mutualism. Proc. Natl Acad. Sci. USA 102(Suppl. 1), 6558–6565. ( 10.1073/pnas.0501840102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakita A, Kato M. 2006. Assessment of the diversity and species specificity of the mutualistic association between Epicephala moths and Glochidion trees. Mol. Ecol. 15, 3567–3581. ( 10.1111/j.1365-294X.2006.03037.x) [DOI] [PubMed] [Google Scholar]
- 9.Darwell CT, al-Beidh S, Cook JM. 2014. Molecular species delimitation of a symbiotic fig-pollinating wasp species complex reveals extreme deviation from reciprocal partner specificity. BMC Evol. Biol. 14, 189 ( 10.1186/s12862-014-0189-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wooding AL, Wingfield MJ, Hurley BP, Garnas JR, De Groot P, Slippers B.. 2013. Lack of fidelity revealed in an insect–fungal mutualism after invasion. Biol. Lett. 9, 20130342 ( 10.1098/rsbl.2013.0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajek AE, Nielsen C, Kepler RM, Long SJ, Castrillo L. 2013. Fidelity among Sirex woodwasps and their fungal symbionts. Microb. Ecol. 65, 753–762. ( 10.1007/s00248-013-0218-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrillo D, Duncan R, Ploetz J, Campbell A, Ploetz R, Peña J. 2014. Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathol. 63, 54–62. ( 10.1111/ppa.12073) [DOI] [Google Scholar]
- 13.Dunn RR, Harris NC, Colwell RK, Koh LP, Sodhi NS. 2009. The sixth mass coextinction: are most endangered species parasites and mutualists? Proc. R. Soc. B 276, 3037–3045. ( 10.1098/rspb.2009.0413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker DM, Andras JP, Jordán-Garza AG, Fogel ML. 2013. Nitrate competition in a coral symbiosis varies with temperature among Symbiodinium clades. ISME J. 7, 1248–1251. ( 10.1038/ismej.2013.12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer TM, Doak DF, Stanton ML, Bronstein JL, Kiers ET, Young TP, Goheen JR, Pringle RM. 2010. Synergy of multiple partners, including freeloaders, increases host fitness in a multispecies mutualism. Proc. Natl Acad. Sci. USA 107, 17 234–17 239. ( 10.1073/pnas.1006872107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojeda ADI, et al. 2017. Genetic and genomic evidence of niche partitioning and adaptive radiation in mountain pine beetle fungal symbionts. Mol. Ecol. 26, 2077–2091. ( 10.1111/mec.14074) [DOI] [PubMed] [Google Scholar]
- 17.Batstone RT, Carscadden KA, Afkhami ME, Frederickson ME. 2018. Using niche breadth theory to explain generalization in mutualisms. Ecology 99, 1039–1050. ( 10.1002/ecy.2188) [DOI] [PubMed] [Google Scholar]
- 18.Poulin R, Krasnov BR, Mouillot D. 2011. Host specificity in phylogenetic and geographic space. Trends Parasitol. 27, 355–361. ( 10.1016/j.pt.2011.05.003) [DOI] [PubMed] [Google Scholar]
- 19.Poulin R, Mouillot D. 2003. Parasite specialization from a phylogenetic perspective: a new index of host specificity. Parasitology 126, 473–480. ( 10.1017/S0031182003002993) [DOI] [PubMed] [Google Scholar]
- 20.Kirkendall LR, Biedermann PH, Jordal BH. 2015. Evolution and diversity of bark and ambrosia beetles. In Bark beetles: biology and ecology of native and invasive species (eds Vega FE, Hofstetter RW), pp. 85–156. London, UK: Academic Press. [Google Scholar]
- 21.Francke-Grosmann H. 1956. Hautdrüsen als träger der pilzsymbiose bei ambrosiakäfern. Zoomorphology 45, 275–308. [Google Scholar]
- 22.Six DL. 2003. Bark beetle-fungus symbioses. Insect Symbiosis 1, 97–114. ( 10.1201/9780203009918.ch7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batra LR. 1963. Ecology of ambrosia fungi and their dissemination by beetles. Trans. Kansas Acad. Sci. 66, 213–236. ( 10.2307/3626562) [DOI] [Google Scholar]
- 24.Saucedo-Carabez JR, Ploetz RC, Konkol JL, Carrillo D, Gazis R. 2018. Partnerships between ambrosia beetles and fungi: lineage-specific promiscuity among vectors of the laurel wilt pathogen, Raffaelea lauricola. Microb. Ecol. 20, 1–6. ( 10.1007/s00248-018-1188-y) [DOI] [PubMed] [Google Scholar]
- 25.Campbell AS, Ploetz RC, Dreaden TJ, Kendra PE, Montgomery WS. 2016. Geographic variation in mycangial communities of Xyleborus glabratus. Mycologia 108, 657–667. ( 10.3852/15-133) [DOI] [PubMed] [Google Scholar]
- 26.Mayers CG, McNew DL, Harrington TC, Roeper RA, Fraedrich SW, Biedermann PH, Castrillo LA, Reed SE. 2015. Three genera in the Ceratocystidaceae are the respective symbionts of three independent lineages of ambrosia beetles with large, complex mycangia. Fung. Biol. 119, 1075–1092. ( 10.1016/j.funbio.2015.08.002) [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Bateman CC, Skelton J, Jusino MA, Nolen ZJ, Simmons DR, Hulcr J. 2017. Wood decay fungus Flavodon ambrosius (Basidiomycota: Polyporales) is widely farmed by two genera of ambrosia beetles. Fung. Biol. 121, 984–989. ( 10.1016/j.funbio.2017.08.004) [DOI] [PubMed] [Google Scholar]
- 28.Skelton J, Jusino MA, Li Y, Bateman C, Thai PH, Wu C, Lindner DL, Hulcr J. 2018. Detecting symbioses in complex communities: the fungal symbionts of bark and ambrosia beetles within Asian pines. Microb. Ecol. 76, 839–850. ( 10.1007/s00248-018-1154-8) [DOI] [PubMed] [Google Scholar]
- 29.Harrington TC. 2005. Ecology and evolution of mycophagous bark beetles and their fungal partners. In Ecological and evolutionary advances in insect-fungal associations (eds Vega FE, Blackwell M), pp. 257–291. Oxford, UK: Oxford University Press. [Google Scholar]
- 30.Mehdiabadi NJ, Mueller UG, Brady SG, Himler AG, Schultz TR. 2012. Symbiont fidelity and the origin of species in fungus-growing ants. Nat. Commun. 3, 840 ( 10.1038/ncomms1844) [DOI] [PubMed] [Google Scholar]
- 31.Talbot P. 1977. The Sirex-Amylostereum-Pinus association. Annu. Rev. Phytopathol. 15, 41–54. ( 10.1146/annurev.py.15.090177.000353) [DOI] [Google Scholar]
- 32.Cognato AI, Hulcr J, Dole SA, Jordal BH. 2011. Phylogeny of haplo–diploid, fungus-growing ambrosia beetles (Curculionidae: Scolytinae: Xyleborini) inferred from molecular and morphological data. Zool. Script. 40, 174–186. ( 10.1111/j.1463-6409.2010.00466.x) [DOI] [Google Scholar]
- 33.Dole SA, Jordal BH, Cognato AI. 2010. Polyphyly of Xylosandrus Reitter inferred from nuclear and mitochondrial genes (Coleoptera: Curculionidae: Scolytinae). Mol. Phylogenet. Evol. 54, 773–782. ( 10.1016/j.ympev.2009.11.011) [DOI] [PubMed] [Google Scholar]
- 34.Jordal B. 2002. Elongation Factor 1 α resolves the monophyly of the haplodiploid ambrosia beetles Xyleborini (Coleoptera: Curculionidae). Insect. Mol. Biol. 11, 453–465. ( 10.1046/j.1365-2583.2002.00354.x) [DOI] [PubMed] [Google Scholar]
- 35.Hulcr J, Cognato AI. 2010. Repeated evolution of crop theft in fungus-farming ambrosia beetles. Evolution 64, 3205–3212. ( 10.1111/j.1558-5646.2010.01055.x) [DOI] [PubMed] [Google Scholar]
- 36.Gohli J, Kirkendall LR, Smith SM, Cognato AI, Hulcr J, Jordal BH. 2017. Biological factors contributing to bark and ambrosia beetle species diversification. Evolution 71, 1258–1272. ( 10.1111/evo.13219) [DOI] [PubMed] [Google Scholar]
- 37.Hulcr J, Dunn RR. 2011. The sudden emergence of pathogenicity in insect–fungus symbioses threatens naive forest ecosystems. Proc. R. Soc. B 278, 2866–2873. ( 10.1098/rspb.2011.1130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkendall L. 1983. The evolution of mating systems in bark and ambrosia beetles (Coleoptera: Scolytidae and Platypodidae). Zool. J. Linn. Soc. 77, 293–352. ( 10.1111/j.1096-3642.1983.tb00858.x) [DOI] [Google Scholar]
- 39.Lin Y-T, Shih H-H, Hulcr J, Lin C-S, Lu S-S, Chen C-Y. 2017. Ambrosiella in Taiwan including one new species. Mycoscience 58, 242–252. ( 10.1016/j.myc.2017.02.004) [DOI] [Google Scholar]
- 40.Bateman C, Kendra PE, Rabaglia R, Hulcr J. 2015. Fungal symbionts in three exotic ambrosia beetles, Xylosandrus amputatus, Xyleborinus andrewesi, and Dryoxylon onoharaense (Coleoptera: Curculionidae: Scolytinae: Xyleborini) in Florida. Symbiosis 66, 141–148. ( 10.1007/s13199-015-0353-z) [DOI] [Google Scholar]
- 41.Kostovcik M, Bateman CC, Kolarik M, Stelinski LL, Jordal BH, Hulcr J. 2015. The ambrosia symbiosis is specific in some species and promiscuous in others: evidence from community pyrosequencing. ISME J. 9, 126–138. ( 10.1038/ismej.2014.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrington TC, McNew D, Mayers C, Fraedrich SW, Reed SE. 2014. Ambrosiella roeperi sp. nov. is the mycangial symbiont of the granulate ambrosia beetle, Xylosandrus crassiusculus. Mycologia 106, 835–845. ( 10.3852/13-354) [DOI] [PubMed] [Google Scholar]
- 43.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 44.R Developement Core Team. 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 45.Balbuena JA, Míguez-Lozano R, Blasco-Costa I. 2013. PACo: a novel procrustes application to cophylogenetic analysis. PLoS ONE 8, e61048 ( 10.1371/journal.pone.0061048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hutchinson MC, Cagua EF, Balbuena JA, Stouffer DB, Poisot T. 2017. paco: implementing procrustean approach to cophylogeny in R. Methods Ecol. Evol. 8, 932–940. ( 10.1111/2041-210X.12736) [DOI] [Google Scholar]
- 47.Oksanen J, et al. 2013. vegan: Community Ecology Package. R package version 2.0-10.
- 48.Gotelli NJ. 2000. Null model analysis of species co-occurrence patterns. Ecology 81, 2606–2621. ( 10.1890/0012-9658(2000)081[2606:NMAOSC]2.0.CO;2) [DOI] [Google Scholar]
- 49.Steininger M, Hulcr J, Šigut M, Lucky A. 2015. Simple and efficient trap for bark and ambrosia beetles (Coleoptera: Curculionidae) to facilitate invasive species monitoring and citizen involvement. J. Econ. Entomol. 108, 1115–1123. ( 10.1093/jee/tov014) [DOI] [PubMed] [Google Scholar]
- 50.Bateman C, Šigut M, Skelton J, Smith KE, Hulcr J. 2016. Fungal associates of the Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) are spatially segregated on the insect body. Environ. Entomol. 45, 883–890. ( 10.1093/ee/nvw070) [DOI] [PubMed] [Google Scholar]
- 51.Oliveira LS, Harrington TC, Ferreira MA, Damacena MB, Al-Sadi AM, Al-Mahmooli IH, Alfenas AC. 2015. Species or genotypes? Reassessment of four recently described species of the Ceratocystis wilt pathogen, Ceratocystis fimbriata, on Mangifera indica. Phytopathology 105, 1229–1244. ( 10.1094/PHYTO-03-15-0065-R) [DOI] [PubMed] [Google Scholar]
- 52.Venables WN, Ripley BD. 2002. Modern applied statistics with S. New York, NY: Springer. [Google Scholar]
- 53.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7 ed. See http://CRAN.R-project.org/package=lme4. [Google Scholar]
- 54.Li Y, Ruan Y, Stanely E, Skelton J, Hulcr J. 2018. Plasticity of mycangia in Xylosandrus ambrosia beetles. Insect Sci. ( 10.1111/1744-7917.12590) [DOI] [PubMed] [Google Scholar]
- 55.van de Peppel L, Aanen D, Biedermann P.. 2018. Low intraspecific genetic diversity indicates asexuality and vertical transmission in the fungal cultivars of ambrosia beetles. Fungal Ecol. 32, 57–64. ( 10.1016/j.funeco.2017.11.010) [DOI] [Google Scholar]
- 56.Six DL. 2012. Ecological and evolutionary determinants of bark beetle–fungus symbioses. Insects 3, 339–366. ( 10.3390/insects3010339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hulcr J, Mogia M, Isua B, Novotny V. 2007. Host specificity of ambrosia and bark beetles (Col., Curculionidae: Scolytinae and Platypodinae) in a New Guinea rainforest. Ecol. Entomol. 32, 762–772. ( 10.1111/j.1365-2311.2007.00939.x) [DOI] [Google Scholar]
- 58.Ranger CM, et al. 2018. Symbiont selection via alcohol benefits fungus farming by ambrosia beetles. Proc. Natl Acad. Sci. USA 115, 4447–4452. ( 10.1073/pnas.1716852115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hulcr J, Stelinski L. 2017. The ambrosia symbiosis: from evolutionary ecology to practical management. Annu. Rev. Entomol. 62, 285–303. ( 10.1146/annurev-ento-031616-035105) [DOI] [PubMed] [Google Scholar]
- 60.Daehler CC, Dudley N. 2002. Impact of the black twig borer, an introduced insect pest, on Acacia koa in the Hawaiian Islands. Micronesica-Agana 35, 35–53. [Google Scholar]
- 61.Hara AH, Beardsley JW. 1979. The biology of the black twig borer, Xylosandrus compactus (Eichhoff), in Hawaii. Proc. Hawaiian Entomol. Soc. 8, 57–70. [Google Scholar]
- 62.Ploetz RC, Hulcr J, Wingfield MJ, de Beer ZW.. 2013. Destructive tree diseases associated with ambrosia and bark beetles: black swan events in tree pathology? Plant Dis. 97, 856–872. ( 10.1094/PDIS-01-13-0056-FE) [DOI] [PubMed] [Google Scholar]
- 63.Hughes M, Riggins J, Koch F, Cognato A, Anderson C, Formby J, Dreaden T, Ploetz R, Smith J. 2017. No rest for the laurels: symbiotic invaders cause unprecedented damage to southern USA forests. Biol. Invasions 19, 2143–2157. ( 10.1007/s10530-017-1427-z) [DOI] [Google Scholar]
- 64.Harrington TC, Fraedrich S, Aghayeva D. 2008. Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon 104, 399–404. [Google Scholar]
- 65.Skelton J, Johnson A, Jusino M, Bateman C, Li Y, Hulcr J. 2018. Data from: A selective fungal transport organ (mycangium) maintains coarse phylogenetic congruence between fungus-farming ambrosia beetles and their symbionts Dryad Digital Repository. ( 10.5061/dryad.4bm536k) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Skelton J, Johnson A, Jusino M, Bateman C, Li Y, Hulcr J. 2018. Data from: A selective fungal transport organ (mycangium) maintains coarse phylogenetic congruence between fungus-farming ambrosia beetles and their symbionts Dryad Digital Repository. ( 10.5061/dryad.4bm536k) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting this article are available in the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.4bm536k [65]. All sequence data are available on GenBank (see electronic supplementary material, tables S1 and S2 for accession numbers).