Abstract
Insecticide resistance has been reported to impact the interactions between mosquitoes and the pathogens they transmit. However, the effect on vector competence for arboviruses still remained to be investigated. We examined the influence of two insecticide resistance mechanisms on vector competence of the mosquito Culex quinquefasciatus for two arboviruses, Rift Valley Fever virus (RVFV) and West Nile virus (WNV). Three Cx. quinquefasciatus lines sharing a common genetic background were used: two insecticide-resistant lines, one homozygous for amplification of the Ester2 locus (SA2), the other homozygous for the acetylcholinesterase ace-1 G119S mutation (SR) and the insecticide-susceptible reference line Slab. Statistical analyses revealed no significant effect of insecticide-resistant mechanisms on vector competence for RVFV. However, both insecticide resistance mechanisms significantly influenced the outcome of WNV infections by increasing the dissemination of WNV in the mosquito body, therefore leading to an increase in transmission efficiency by resistant mosquitoes. These results showed that insecticide resistance mechanisms enhanced vector competence for WNV and may have a significant impact on transmission dynamics of arboviruses. Our findings highlight the importance of understanding the impacts of insecticide resistance on the vectorial capacity parameters to assess the overall consequence on transmission.
Keywords: insecticide resistance, vector competence, arboviruses, Culex quinquefasciatus
1. Introduction
Over the last decades, arthropod-borne viruses (arboviruses) have taken the centre stage owing to re-emergence in endemic regions and new epidemic outbreaks in naive countries. There are numerous arboviruses spanning different viral families and genera such as Dengue, West Nile and Zika viruses (family Flaviviridae, genus Flavivirus), Chikungunya virus (family Togaviridae, genus Alphavirus) and Rift Valley Fever virus (RVFV) (family Phenuiviridae; genus Phlebovirus) that affect human health worldwide [1]. In the absence of vaccines and specific treatments, the control of mosquito populations is the only affordable measure to disrupt the transmission of arboviruses. For this concern, insecticide treatments have been and are still highly used to control mosquito populations. However, the overuse of these insecticides for public health and agricultural concerns increases selective pressures, leading to the selection and spread of resistance genes in mosquito populations [2–4].
Two main mechanisms are responsible for high levels of resistance to insecticides in mosquitoes: overproduction of metabolic enzymes (i.e. metabolic resistance) and the modification of the insecticide target (i.e. target-site resistance) [5]. Metabolic resistance regroups the various defence mechanisms against xenobiotics that sequestrate and degrade the insecticide in less or non-toxic products, thus decreasing the quantity of toxic molecules likely to reach the target. Three major families of enzymes are involved in this type of resistance: glutathione S-transferases, cytochrome P450 monooxygenases and carboxylesterases [5]. Resistance by target-site modification is owing to point mutations in the gene coding of the insecticide target that limits the insecticide binding. Three essential target proteins, all of them being expressed in the nervous system, are the target of insecticides of distinct families: the acetylcholinesterase (target of carbamates and organophosphates), the γ-aminobutyric acid receptor (organochlorine) and the voltage-gated sodium channels (pyrethroids and dichlorodiphenyltrichloroethane (DDT) [6–8]). The selection of one of these mechanisms leads to increased vector survival in treated environments and to a greater population size, which could increase vectorial capacity.
Insecticide resistance genes are often associated with negative pleiotropic effects that lead to fitness disadvantage or cost. In insecticide-resistant Culex quinquefasciatus mosquitoes, numerous life-history traits can be modified including increased larval development time, reduced predation avoidance and reduced male reproductive success [9–14]. Such negative impacts lead to the reduction of resistant allele frequency in the mosquito population when the insecticide selective pressure is absent or very low [15,16]. Insecticide resistance and their associated costs may interfere with the development and the diversity of symbionts hosted by mosquito vectors. In Cx. quinquefasciatus, the density of the endosymbiotic bacteria Wolbachia was found to be significantly higher in resistant mosquitoes compared to susceptible ones [13,17], although this interaction is very dynamic [18]. Pyrethroid-resistant Anopheles gambiae carrying the kdr mutation were shown to be more susceptible to infection by the fungi Metharhizium anisopliae and Beauveria bassiana [19]. Lastly, a recent study on Anopheles albimanus showed a higher bacterial diversity in resistant compared with susceptible specimens [20]. Insecticide resistance may also affect interactions between mosquito vectors and pathogens they transmit, which may have an impact on vectorial capacity. In Cx. quinquefasciatus, insecticide-resistant mosquitoes with higher carboxylesterase activity were less parasitized by the filaria parasite Wuchereria bancrofti than their insecticide-susceptible counterparts [21]. In An. gambiae, the main malaria vector, target-site mutations responsible for insecticide resistance (ace-1 G119S and kdr L1014F) increased the prevalence of Plasmodium falciparum infections in the mosquito salivary glands, which could lead to increased parasite transmission [22,23]. Consistently, pyrethroid-resistant An. gambiae from Tanzania (kdr-east, L1014S) was found to be more competent for malaria than susceptible vectors [24]. However, to our knowledge, there is no study describing the potential effects of insecticide resistance on arbovirus transmission.
Here, we aimed at characterizing the impact of the two main organophosphate (OP) insecticide resistance mechanisms (carboxylesterase overproduction and insensitive acetylcholinesterase) on the vector competence of Cx. quinquefasciatus mosquitoes for two arboviruses RVFV and West Nile virus (WNV). For this purpose, four parameters (infection rate (IR), dissemination rate (DR), transmission rate (TR) and transmission efficiency (TE)) were compared between resistant and susceptible mosquito lines sharing a common genetic background to determine the influence of insecticide resistance alleles. We determined the respective contributions of insecticide resistance mechanisms, the arbovirus, the time from blood feeding and the interactions between these variables in the dissemination and transmission of RVFV and WNV.
2. Material and methods
(a). Mosquito lines
We used three isogenic lines of Cx. quinquefasciatus; one susceptible (named Slab) and two lines resistant to OP insecticides. The OP resistant lines were: SA2 homozygous for the amplification of the Ester2 locus (leading to overproduction of carboxylesterase) [25] and SR homozygous for the ace-1 G119S mutation [26]. The two resistant lines share a common genetic background with Slab. Each line was backcrossed for at least 14 generations with Slab with the recurrent selection with OP insecticides [9]. Eggs of the three mosquito lines were obtained from the Institut des Sciences de l'Evolution de Montpellier (ISEM) and set up to hatch under standard insectary conditions (27 ± 1°C, 70 ± 8% relative humidity and 12 L : 12 D photoperiod). Just after hatching, larvae were randomly seeded into plastic trays containing 1 l of tap water at a constant density of about 500 individuals per tray. Larvae were fed ad libitum with a mixture of rabbit and fish food while adults were fed with 10% sucrose solution (w/v).
(b). Viral strains
We used the RVFV SH172805 strain from the lineage East/Central Africa isolated from a human case in Mauritania in 2003 [27] and a WNV strain belonging to the lineage 1a and isolated from a horse in France (Camargue) in 2000 [28]. All virus stocks were produced on Aedes albopictus C6/36 cells, after four passages for RVFV and after three passages for WNV. For all virus stocks, supernatants were harvested and stored at −80°C until experimental infections.
(c). Oral infections of mosquitoes
Seven to 10-day-old females were fed on an infectious blood meal containing 1.4 ml of washed rabbit erythrocytes and 700 µl of viral suspension supplemented with a phagostimulant (ATP) at a final concentration of 5 mM. The titres of infectious blood meals were 107 plaque-forming units (PFU) ml−1 for both RVFV and WNV. Mosquitoes were allowed to feed for 1 h. Afterwards, fully engorged females were transferred in cardboard containers and maintained with 10% sucrose at 27 ± 1°C for 21 days. The three mosquito lines were infected once with the RVFV, while three experimental infections were performed with the WNV (three with the Slab and SR lines and two with the SA2 line).
(d). Vector competence analysis
At 3, 7, 14 and 21 days post-infection (dpi), saliva was collected from individual mosquitoes (15–51 per mosquito line and per experimental infection) by forced salivation as previously described [29]. Briefly, legs and wings of each mosquito were removed and the mosquito's proboscis was inserted into a micropipette tip containing 5 µl of foetal bovine serum (FBS). After 45 min, the saliva-containing FBS was expelled into 45 µl of Dulbecco's modified Eagle medium (DMEM). Following salivation, the head and the body of each mosquito were separated and individually homogenized in 300 µl of DMEM that was supplemented with 2% FBS.
Vector competence was assessed based on four parameters: IR, DR, TR and TE. The IR corresponds to the proportion of mosquitoes with a body (abdomen and thorax) containing infectious viral particles among fully engorged mosquitoes; the DR was calculated as the proportion of females with infected head tissues (i.e. in which the virus successfully disseminated from the midgut) among mosquitoes presenting infection in their bodies; the TR represents the proportion of mosquitoes with infectious saliva among mosquitoes able to disseminate the virus; and the TE corresponds to the proportion of mosquitoes whose saliva contains infectious viral particles among all blood-fed mosquitoes.
(e). Virus titration
The detection of infectious viral particles in bodies, heads and saliva extracts was performed by titration on Vero cells. For this, six-well plates containing confluent monolayers of Vero cells were infected with serial 10-fold dilutions of body, head homogenates or saliva and incubated for 1 h at 37°C. Thereafter, cells were covered with an overlay consisting of DMEM, 2% FBS, 1% antibiotic-antimycotic mix (Invitrogen, Gibco) and 1% agarose and incubated at 37°C. Cells were incubated 4 days for samples infected with WNV or 5 days for those infected with RVFV. Lytic plaques were then counted after staining with a solution of crystal violet (0.2% in 10% formaldehyde and 20% ethanol).
(f). Statistical analyses
We analysed the RVFV and WNV infection outcome on Cx. quinquefasciatus using four parameters as response variables: the IR, the DR, the TR and the TE. To this aim, we examined the effects of three explanatory variables: ‘mosquito line’ (a three-level categorical variable: Slab, SA2 and SR), ‘arbovirus’ (a two-level categorical variable: RVFV and WNV) and ‘dpi’ the day post-infection (a numerical variable). All statistical analyses were performed with R software 3.4.0 [30] using a generalized linear model with a binomial error structure. Maximal models included the variables ‘mosquito line’, ‘arbovirus’ and ‘dpi’ and all their interactions. Significance of variables and selection of the minimal model has been assessed using the ‘ANOVA’ procedure within the package ‘car’ [31], which performs a type III hypothesis. Estimates of each three parameters were computed and post hoc tests (package ‘lsmeans’, [32]) were carried out to assess the differences between estimates, and Bonferroni corrections were applied for multiple comparisons. For each mosquito tissue (body, head and saliva), the viral loads were compared between mosquito lines using a Kruskal–Wallis test.
3. Results
(a). Comparing vector competence for Rift Valley Fever virus and West Nile virus
We examined the effects of two insecticide resistance mechanisms on the transmission of two arboviruses, RVFV and WNV, by comparing vector competence of three Cx. quinquefasciatus lines sharing a similar genetic background: two insecticide-resistant lines (SA2 and SR) and the insecticide-susceptible reference line Slab. Overall, 801 blood-fed females (383 with RVFV-infected blood and 418 with WNV-infected blood) were analysed to compare RVFV and WNV infection dynamics in mosquitoes over time. This analysis includes mosquitoes infected only once with one of the two viruses.
(i). Infection rate
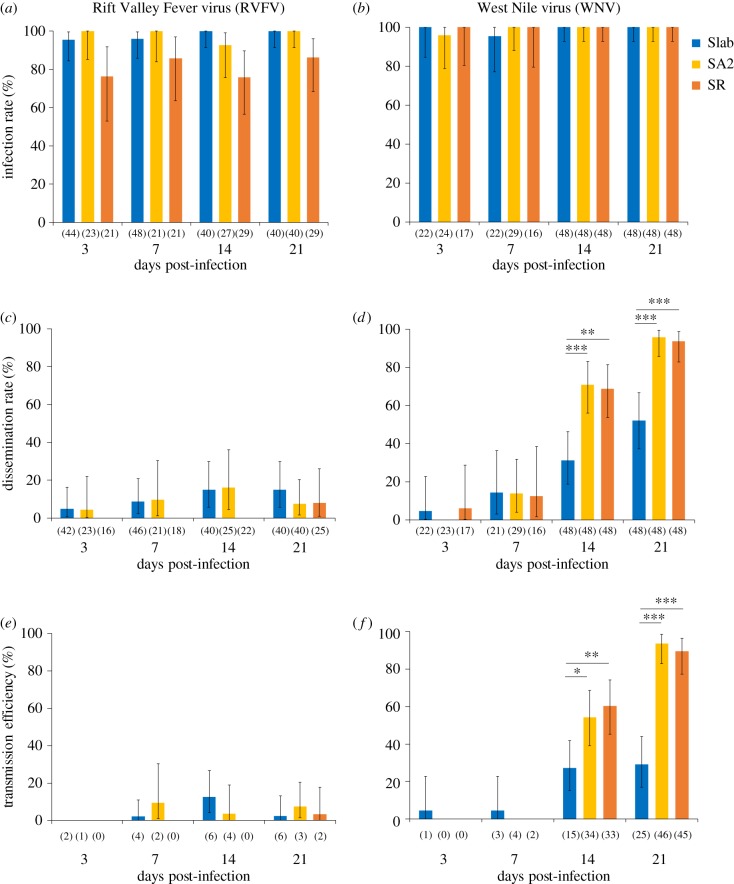
The IR was significantly influenced by the mosquito line (χ2 = 59.80, p < 0.0001; table 1), by the mosquito line × arbovirus interaction (χ2 = 11.47, p = 0.003; table 1) and by the arbovirus × dpi interaction (χ2 = 5.45, p = 0.019; table 1). These significant two-way interactions revealed that the effect of insecticide resistance was different according to the tested arbovirus and that the kinetic of midgut infection was distinct for RVFV and WNV. When mosquitoes were infected with RVFV, IRs ranged from 76% to 100% (figure 1a). Regardless of dpi, a significant decrease in IR was observed in SR compared to Slab and SA2 (p < 0.0001 and p < 0.0001 for pairwise comparisons between SR/Slab and SR/SA2, respectively) but no significant difference was observed between Slab and SA2 (p = 0.96). By contrast, the three mosquito lines showed similar IRs when challenged with the WNV (IRs > 95%, all p > 0.99; figure 1b).
Table 1.
Statistical analyses of vector competence parameters after infections with Rift Valley Fever virus (RVFV) and West Nile virus lineage 1a (WNV). (In these analyses, the influence of mosquito lines (Slab, SA2 and SR), arboviruses (RVFV and WNV) and day post-infections (3, 7, 14 and 21 dpi) were tested. d.f. is the degree of freedom and χ2 is the Chi-square value. Significance of variables was obtained after downward selection based on Akaike information criterion (AIC). Variables with significant impact in the minimal model are shown in bold.)
| infection rate |
dissemination rate |
transmission rate |
transmission efficiency |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| source | χ2 | d.f. | p-value | χ2 | d.f. | p-value | χ2 | d.f. | p-value | χ2 | d.f. | p-value |
| mosquito line (ML) | 59.8 | 2 | <0.0001 | 6.32 | 2 | 0.042 | 0.04 | 1 | 0.843 | 3.04 | 2 | 0.219 |
| arbovirus | 0.53 | 1 | 0.467 | 4.13 | 1 | 0.042 | 2.37 | 1 | 0.124 | 2.87 | 1 | 0.09 |
| days post-infection (dpi) | 3.07 | 1 | 0.061 | 0.11 | 1 | 0.746 | 1.04 | 1 | 0.309 | 0.65 | 1 | 0.421 |
| ML × arbovirus | 11.47 | 1 | 0.003 | 8.54 | 2 | 0.014 | 4.93 | 1 | 0.026 | 6.96 | 2 | 0.031 |
| ML × dpi | — | — | — | 3.91 | 2 | 0.142 | 0.49 | 1 | 0.486 | 1.6 | 2 | 0.448 |
| arbovirus × dpi | 5.45 | 1 | 0.019 | 27.83 | 1 | <0.0001 | 3.55 | 1 | 0.059 | 15.92 | 1 | <0.0001 |
| ML × arbovirus × dpi | — | — | — | 15.25 | 2 | 0.0005 | 4.20 | 1 | 0.040 | 11.37 | 2 | 0.003 |
Figure 1.
Vector competence parameters of the mosquito lines Slab, SA2 and SR infected with RVFV (a,c,e) and WNV (b,d,f). At 3, 7, 14 and 21 days after a blood meal containing RVFV or WNV (titre of 107 PFU ml−1 for both RVFV and WNV), mosquitoes were examined for the presence of infectious viral particles detected by titration on Vero cells. The infection rate (a,b) corresponds to the proportion of mosquitoes whose bodies (thorax and abdomen) contain infectious viral particles among infected mosquitoes; the dissemination rate (c,d) is the proportion of females with infected head tissues among mosquitoes presenting infection in their bodies and the transmission efficiency (e,f) corresponds to the proportion of mosquitoes whose saliva contains infectious viral particles among all infected mosquitoes. The number of mosquitoes analysed is indicated in brackets. Error bars represent the 95% confidence interval. Tests of significance were corrected for multiple testing using the Bonferroni procedure. Only tests with significant difference are represented. Asterisks indicate the significance level: *p < 0.05; **p < 0.01; ***p < 0.001.
(ii). Dissemination rate
We then investigated whether insecticide resistance mechanisms affect the viral dissemination beyond the midgut barrier after infectious blood meals through the estimation of the DR. The effects of mosquito line and arbovirus were significant (χ2 = 6.32, p = 0.042 and χ2 = 4.13, p = 0.042, respectively; table 1). The mosquito line by arbovirus interaction (χ2 = 8.54, p = 0.014; table 1), the arbovirus by dpi interaction (χ2 = 27.83, p < 0.0001; table 1) and the three-way interaction mosquito line × arbovirus × dpi (χ2 = 15.25, p = 0.0005; table 1) significantly influenced the DR. This shows that insecticide resistance affected the level and the kinetic of dissemination of WNV compared to susceptible mosquitoes but no difference was observed between the resistant lines SR and SA2 (p = 0.0001, p = 0.0004 and p = 0.86 for pairwise comparisons between Slab/SA2, Slab/SR and SA2/SR, respectively; figure 1d). By contrast, there was no significant difference in the DR of RVFV between the three lines (pairwise comparisons, all p > 0.84; figure 1c).
(iii). Transmission rate and transmission efficiency
We then evaluated the TR and the TE. The statistical analysis showed that both the TR and the TE were dependent on the three-way interaction mosquito line × arbovirus × dpi (χ2 = 4.2, p = 0.04 and χ2 = 11.37, p = 0.003; respectively, for TR and TE; table 1). For TR, the mosquito line by arbovirus interaction was also significant (χ2 = 4.93, p = 0.026; table 1). Concerning TE, the mosquito line × arbovirus (χ2 = 6.96, p = 0.031; table 1) and arbovirus × dpi (χ2 = 15.9, p < 0.0001; table 1) interactions were significant but not the mosquito line × dpi (χ2 = 1.6, p = 0.448; table 1). The significant mosquito line × arbovirus × dpi interaction suggests that the insecticide-resistant lines influenced viral transmission. The significant interaction mosquito line × arbovirus showed that insecticide resistance impacted the transmission of both arboviruses differently as observed in figure 1e,f. The significant arbovirus × dpi interaction indicated that the kinetic of viral propagation was arbovirus-specific.
Very low TEs were observed with the RVFV regardless of the dpi (all TEs less than 14%; figure 1e). Moreover, no significant difference was found between the three mosquito lines (pairwise comparisons, all p > 0.94). By contrast, TEs of WNV were very low at 3 and 7 dpi for the three mosquito lines; and, a significant increase was observed from 14 to 21 dpi for both resistant lines: from 54% (±0.07) to 94% (±0.04) in SA2 and from 60% (±0.07) to 90% (±0.04) in SR. For the Slab line, TE increased at day 14 and then was found steady between 14 and 21 dpi with 27% (±0.06) at 14 dpi and 29% (±0.07) at 21 dpi (figure 1f). Overall, the insecticide-resistant lines SA2 and SR were significantly more competent to transmit the WNV, but not RVFV, than their susceptible counterpart (p = 0.01, p = 0.038 and p = 0.9 for pairwise comparisons between Slab/SA2, Slab/SR and SA2/SR respectively).
(b). Influence of insecticide resistance on West Nile virus vector competence
To confirm the higher capacity of insecticide-resistant mosquitoes to transmit WNV compared to their susceptible counterparts, we analysed data of three independent experimental assays where Slab, SA2 and SR were infected with WNV and the mosquitoes examined only at 14 dpi. A total of 324 mosquitoes (124, 86 and 114 from the Slab, SA2 and SR lines, respectively) were examined and we determined the effects of mosquito line, experimental assay and the interactions between the two variables on IR, DR, TR and TE. On the four vector competence parameters examined, the experimental assay had a significant effect on three parameters (χ2 = 91.85, p < 0.0001; χ2 = 17.47, p = 0.00016 and χ2 = 12.31, p = 0.002 for IR, DR and TE, respectively; table 2).
Table 2.
Statistical analyses of vector competence parameters after infections with WNV obtained from different experimental replications. (To this end, the effects of mosquito line (Slab, SA2 and SR) and experimental assay (three sets of the experiment) were tested. d.f. is the degree of freedom and χ2 is the Chi-square value. Significance of variables was obtained after downward selection based on AIC. Variables with significant impact in the minimal model are shown in bold.)
| infection rate |
dissemination rate |
transmission rate |
transmission efficiency |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| source | χ2 | d.f. | p-value | χ2 | d.f. | p-value | χ2 | d.f. | p-value | χ2 | d.f. | p-value |
| mosquito line (ML) | — | — | — | 18.38 | 2 | 0.0001 | 1.70 | 2 | 0.428 | 12.15 | 2 | 0.0023 |
| experimental assay (Exp.) | 91.85 | 2 | p < 0.0001 | 17.47 | 2 | 0.00016 | 0.04 | 2 | 0.981 | 12.31 | 2 | 0.002 |
| ML × Exp. | — | — | — | — | — | — | 7.81 | 3 | 0.05 | 12.89 | 3 | 0.005 |
(i). Infection and dissemination rates
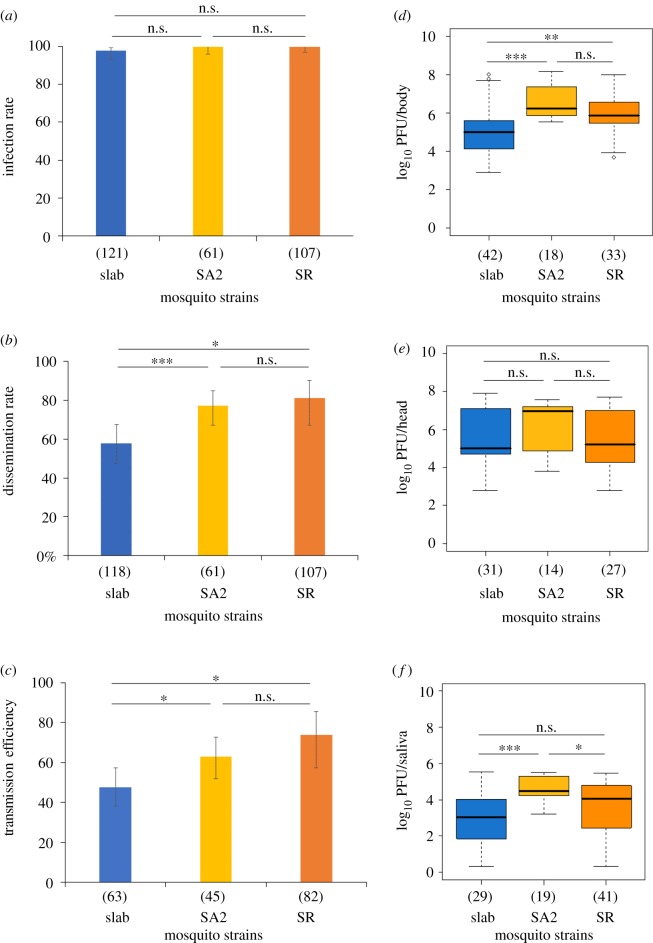
When analysing the IR, no significant difference was observed between the three mosquito lines (all p > 0.99; figure 2a) while for the DR, the main variable mosquito line had a significant influence (χ2 = 18.38, p = 0.0001; table 2). The two insecticide-resistant lines were more permissive for WNV dissemination than susceptible mosquitoes (p = 0.0008, p = 0.022 and p = 0.63 for pairwise comparisons between Slab/SA2, Slab/SR and SA2/SR, respectively; figure 2b).
Figure 2.
Vector competence parameters and viral loads in bodies (thorax and abdomen), heads and saliva of mosquitoes from the Slab, SA2 and SR lines infected with WNV (titre of 107 PFU ml−1). Three different experiments were performed independently and infected mosquitoes were analysed at 14 days post-infection. The presence of infectious viral particles was detected by titration on Vero cells. (a) corresponds to the infection rate, (b) to the dissemination rate (c) to the transmission efficiency, (d) to viral load in the bodies, (e) to viral load in the heads, and (f) to viral loads in the saliva. The number of mosquitoes analysed is indicated in brackets. Error bars represent the 95% confidence interval. Tests of significance were corrected for multiple testing using the Bonferroni procedure. Asterisks indicate the significance level: *p < 0.05; **p < 0.01; ***p < 0.001. n.s.: no significant difference.
(ii). Transmission rate and transmission efficiency
Both the TR and the TE were dependent on the mosquito line by experimental assay interaction (χ2 = 7.81, p = 0.05 and χ2 = 12.89, p = 0.005 for TR and TE, respectively; table 2) indicating that the difference between mosquito lines varied according to the experimental assay. In addition, a significant effect of mosquito line on TE was observed (χ2 = 12.15, p = 0.0023; table 2). Overall, TEs of SA2 and SR lines were significantly higher than that of Slab (p = 0.03, p = 0.02 and p = 0.6 for pairwise comparisons between Slab/SA2, Slab/SR and SA2/SR, respectively; figure 2c).
(iii). West Nile virus load
Finally, we compared the viral loads measured in bodies, heads and saliva of mosquitoes from Slab, SA2 and SR infected with WNV and examined at 14 dpi. Among the mosquito lines, Slab individuals had the lowest viral loads in their bodies (mean viral load of 5.09 ± 1.32, 6.63 ± 0.97 and 6.02 ± 1.10 log10PFU for Slab, SA2 and SR, respectively; Kruskal–Wallis rank sum test = 23.91, p < 0.0001, figure 2d) and saliva (mean viral load of 2.90 ± 1.50, 4.48 ± 0.72 and 3.64 ± 1.36 log10PFU for Slab, SA2 and SR, respectively; Kruskal–Wallis rank sum test = 16.28, p = 0.0003; figure 2f) compared to SA2 and SR. However, no significant difference of viral loads in heads was noted between the three mosquito lines (mean viral load of 5.57 ± 1.4, 6.29 ± 1.23 and 5.52 ± 1.46 log10PFU for Slab, SA2 and SR, respectively; Kruskal–Wallis rank sum test = 2.27, p = 0.32; figure 2e).
4. Discussion
Insecticide resistance has been shown to affect vector competence for pathogens such as the filarial parasite W. bancrofti [21] and the malaria parasite P. falciparum [22–24]. Here, we provide, to our knowledge, the first evidence of the impact of insecticide resistance mechanisms on the transmission of arboviruses. Using experimental infections, we compared four vector competence parameters (IR, DR, TR and TE) of insecticide-resistant (SA2 and SR) and -susceptible (Slab) Cx. quinquefasciatus lines for RVFV and WNV. These mosquito lines shared a common genetic background through introgression of the Slab genome and differed only by the insecticide selected loci which include the insecticide resistance alleles [9]. Therefore, any phenotypic changes between the insecticide-resistant and the susceptible specimens could be associated with the presence of insecticide resistance loci or with any linked loci hitchhiked during introgression. Moreover, Cx. quinquefasciatus is among the main vectors of WNV (reviewed in [33]) and can also transmit RVFV [34], both arboviruses with an increasing risk of emergence and extending geographical range [35]. The findings presented here show that insecticide resistance mechanisms did not affect vector competence for RVFV probably because the Cx. quinquefasciatus lines tested were poorly competent for this arbovirus [36]. However, both insecticide resistance mechanisms significantly impacted vector competence for WNV by increasing the DR, the TE and the viral loads in bodies and saliva of insecticide-resistant mosquitoes compared to their susceptible counterparts.
When infected with RVFV, very low transmission efficiencies were observed for the three mosquito lines (all values less than 14%) and no significant difference was found between the insecticide-resistant and -susceptible lines. To be transmitted by mosquitoes, arboviruses must overcome several tissue barriers associated with the midgut and the salivary glands [37]. So, we asked whether the observed low transmission of RVFV was the result of low IR and/or DR. Infection rates were high and quite similar between the three mosquito lines (all values greater than 76%). However, dissemination rates were very low (all values less than 17%) even for longer incubation periods (i.e. at 14 and 21 dpi). Thus, RVFV was able to infect and replicate in the midgut epithelial cells but showed low ability to disseminate in the mosquito general cavity and then, to infect salivary glands for subsequent transmission. The observed low dissemination and transmission of RVFV are consistent with previous investigations showing that the mosquito Cx. quinquefasciatus was less able to disseminate and to transmit RVFV compared to other mosquito species such as Aedes vexans [27,38,39]. Therefore, the presence of insecticide resistance mechanisms did not appear to change the interactions between the RVFV and Cx. quinquefasciatus mosquitoes in our conditions.
Unlike RVFV, WNV dissemination was significantly affected by insecticide resistance mechanisms. At 14 and 21 dpi, higher dissemination rates and transmission efficiencies were noted for SA2 and SR compared to Slab. Variations among the experimental replicates were observed, highlighting the importance of performing several experimental replicates to better estimate the factors influencing arbovirus transmission. Collectively, insecticide-resistant mosquitoes showed higher transmission potentials owing to a higher DR compared with their susceptible counterparts. In addition, viral loads in saliva and bodies of resistant individuals were also higher than in Slab individuals. Interestingly, both insecticide-resistant mechanisms (i.e. the esterase overproduction in SA2 and a modified acetylcholinesterase in SR) showed similar effects on WNV vector competence. Different insecticide resistance mechanisms were also found to increase the vector competence of mosquitoes for human and rodents malaria parasites [22,24,40] but not for avian malaria parasites vectored by Cx. quinquefasciatus and Culex pipiens [41,42].
Several non-exclusive mechanisms that could explain the observed impact of the carboxylesterases overproduction and the insensitive acetylcholinesterase on vector competence for WNV were thus explored. There are no data indicating that these overproduced/mutated proteins could directly affect vector competence but it is likely that other loci in linkage disequilibrium could directly modulate vector competence, as demonstrated in pyrethroid-resistant An. gambiae [43]. Further work is ongoing to identify the resistance-linked loci and to characterize them functionally. Among these resistant mechanisms, indirect effects of insecticide resistance loci (and/or linked loci) on (i) energetic resources, (ii) immune genes and (iii) microbiota may modulate the infection and dissemination of WNV. Concerning energetic resources, the overproduction of carboxylesterase enzymes in the SA2 line may deplete the energy reserves, thus reducing the resources available to cover other biological functions. The energetic resources hypothesis is consistent with a previous study in Cx. quinquefasciatus where insecticide-resistant mosquitoes carrying carboxylesterase overproduction alleles have been found to contain less energetic reserves (lipids, glycogen and glucose) than their susceptible counterparts [44]. This carboxylesterases overproduction may also lead to unbalanced redox equilibrium and to oxidative stress, which could affect immunity [45,46]. Concerning immunity, gene expression analysis in insecticide-resistant and -susceptible An. gambiae revealed upregulation of Defensin and Cecropin genes [47,48], two anti-microbial peptides involved in the anti-Plasmodium [49] and antiviral [50] immune responses. Ultimately, the higher competence of SA2 and SR to transmit the WNV compared with Slab could be the difference in the composition of their microbiota. Indeed, there is an important bacterial diversity in mosquito midgut that can modulate vector competence (reviewed in [51]). Moreover, in the mosquito An. albimanus, insecticide-resistant specimens were found to harbour lower bacterial diversity compared with susceptible mosquitoes [20].
In conclusion, we showed that the two main insecticide resistance mechanisms affect the vector competence of Cx. quinquefasciatus for WNV. The selection of resistance mechanisms resulting from the widespread use of insecticides (in vector and pest control) may thus influence the epidemiology of arboviruses. Such information is crucial because it can help evaluate the impact of insecticide resistance and vector control on the risk of emergence and on the spread of arboviruses. Further studies using diverse mosquito field populations should help understand the effects of insecticide resistance on vector competence under different environmental contexts.
Acknowledgements
We are very grateful to Sandra Unal, Patrick Makoundou and Jocelyne Alexandre for technical assistance.
Ethics
Animals were housed in the Institut Pasteur animal facilities accredited by the French Ministry of Agriculture for performing experiments on live rodents. Work on animals was performed in compliance with French and European regulations on care and protection of laboratory animals (EC Directive 2010/63, French Law 2013–118, 6 February 2013). All experiments were approved by the Ethics Committee no. 89 and registered under the reference APAFIS no. 6427-2016061411435359 vI.
Data accessibility
This article has no additional data.
Authors' contributions
C.M.A., M.W. and A.-B.F. conceived and designed the experiments; C.M.A., L.M. and M.V. performed the experiments; H.A. carried out the statistical analyses; C.M.A., H.A., M.W., M.D. and A.-B.F. wrote the paper. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This study was supported by the AXA research fund and the Pasteur-Cantarini postdoctoral fellowships. H.A. is supported by Marie Skołodowska-Curie under the grant agreement number 749897.
References
- 1.Gould E, Pettersson J, Higgs S, Charrel R, de Lamballerie X. 2017. Emerging arboviruses: why today? One Health 4, 1–13. ( 10.1016/j.onehlt.2017.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lines JD. 1988. Do agricultural insecticides select for insecticide resistance in mosquitoes? A look at the evidence. Parasitol. Today 4, S17–S20. ( 10.1016/0169-4758(88)90083-X) [DOI] [PubMed] [Google Scholar]
- 3.Raymond M, Berticat C, Weill M, Pasteur N, Chevillon C. 2001. Insecticide resistance in the mosquito Culex pipiens: what have we learned about adaptation? Genetica 112–113, 287–296. ( 10.1023/A:1013300108134) [DOI] [PubMed] [Google Scholar]
- 4.Labbe P, Lenormand T, Raymond M. 2005. On the worldwide spread of an insecticide resistance gene: a role for local selection. J. Evol. Biol. 18, 1471–1484. ( 10.1111/j.1420-9101.2005.00938.x) [DOI] [PubMed] [Google Scholar]
- 5.Labbe P, Alout H, Djogbénou L, Weill M, Pasteur N.. 2017. Evolution of resistance to insecticide in disease vectors. In Genetics and evolution of infectious diseases (ed. Tibayrenc M.), p. 686 Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 6.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Bergé JB, Devonshire AL, Guillet P, Pasteur N, Pauron D. 1998. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect. Mol. Biol. 7, 179–184. ( 10.1046/j.1365-2583.1998.72062.x) [DOI] [PubMed] [Google Scholar]
- 7.ffrench-Constant RH, Anthony N, Aronstein K, Rocheleau T, Stilwell G. 2000. Cyclodiene insecticide resistance: from molecular to population genetics. Annu. Rev. Entomol. 45, 449–466. ( 10.1146/annurev.ento.45.1.449) [DOI] [PubMed] [Google Scholar]
- 8.Weill M, et al. 2003. Comparative genomics: insecticide resistance in mosquito vectors. Nature 423, 136–137. ( 10.1038/423136b) [DOI] [PubMed] [Google Scholar]
- 9.Berticat C, Boquien G, Raymond M, Chevillon C. 2002. Insecticide resistance genes induce a mating competition cost in Culex pipiens mosquitoes. Genet. Res. 79, 41–47. ( 10.1017/S001667230100547X) [DOI] [PubMed] [Google Scholar]
- 10.Berticat C, Duron O, Heyse D, Raymond M. 2004. Insecticide resistance genes confer a predation cost on mosquitoes Culex pipiens. Genet. Res. 83, 189–196. ( 10.1017/S0016672304006792) [DOI] [PubMed] [Google Scholar]
- 11.Bourguet D, Guillemaud T, Chevillon C, Raymond M. 2004. Fitness costs of insecticide resistance in natural breeding sites of the mosquito Culex pipiens. Evolution 58, 128–135. ( 10.1111/j.0014-3820.2004.tb01579.x) [DOI] [PubMed] [Google Scholar]
- 12.Agnew P, Berticat C, Bedhomme S, Sidobre C, Michalakis Y. 2004. Parasitism increases and decreases the costs of insecticide resistance in mosquitoes. Evolution 58, 579–586. ( 10.1111/j.0014-3820.2004.tb01680.x) [DOI] [PubMed] [Google Scholar]
- 13.Duron O, Labbe P, Berticat C, Rousset F, Guillot S, Raymond M, Weill M. 2006. High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution 60, 303–314. ( 10.1111/j.0014-3820.2006.tb01108.x) [DOI] [PubMed] [Google Scholar]
- 14.Milesi P, Assogba BS, Atyame CM, Pocquet N, Berthomieu A, Unal S, Makoundou P, Weill M, Labbé P. 2017. The evolutionary fate of heterogeneous gene duplications: a precarious overdominant equilibrium between environment, sublethality and complementation. Mol. Ecol. 27, 493–507. ( 10.1111/mec.14463) [DOI] [PubMed] [Google Scholar]
- 15.Lenormand T, Bourguet D, Guillemaud T, Raymond M. 1999. Tracking the evolution of insecticide resistance in the mosquito Culex pipiens. Nature 400, 861–864. ( 10.1038/23685) [DOI] [PubMed] [Google Scholar]
- 16.Milesi P, Lenormand T, Lagneau C, Weill M, Labbe P. 2016. Relating fitness to long-term environmental variations in Natura. Mol. Ecol. 25, 5483–5499. ( 10.1111/mec.13855) [DOI] [PubMed] [Google Scholar]
- 17.Berticat C, Rousset F, Raymond M, Berthomieu A, Weill M. 2002. High Wolbachia density in insecticide-resistant mosquitoes. Proc. R. Soc. Lond. B 269, 1413–1416. ( 10.1098/rspb.2002.2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Echaubard P, Duron O, Agnew P, Sidobre C, Noel V, Weill M, Michalakis Y. 2010. Rapid evolution of Wolbachia density in insecticide resistant Culex pipiens. Heredity (Edinb.) 104, 15–19. ( 10.1038/hdy.2009.100) [DOI] [PubMed] [Google Scholar]
- 19.Howard AF, Koenraadt CJ, Farenhorst M, Knols BG, Takken W. 2010. Pyrethroid resistance in Anopheles gambiae leads to increased susceptibility to the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Malar. J. 9, 168 ( 10.1186/1475-2875-9-168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dada N, Sheth M, Liebman K, Pinto J, Lenhart A. 2018. Whole metagenome sequencing reveals links between mosquito microbiota and insecticide resistance in malaria vectors. Sci. Rep. 8, 2084 ( 10.1038/s41598-018-20367-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarroll L, Hemingway J. 2002. Can insecticide resistance status affect parasite transmission in mosquitoes? Insect. Biochem. Mol. Biol. 32, 1345–1351. ( 10.1016/S0965-1748(02)00097-8) [DOI] [PubMed] [Google Scholar]
- 22.Alout H, Ndam NT, Sandeu MM, Djegbe I, Chandre F, Dabiré RK, Djogbenou LS, Corbel V, Cohuet A. 2013. Insecticide resistance alleles affect vector competence of Anopheles gambiae s.s. for Plasmodium falciparum field isolates. PLoS One 8, e63849 ( 10.1371/journal.pone.0063849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alout H, Yameogo B, Djogbenou LS, Chandre F, Dabire RK, Corbel V, Cohuet A. 2014. Interplay between Plasmodium infection and resistance to insecticides in vector mosquitoes. J. Infect. Dis. 210, 1464–1470. ( 10.1093/infdis/jiu276) [DOI] [PubMed] [Google Scholar]
- 24.Kabula B, Tungu P, Rippon EJ, Steen K, Kisinza W, Magesa S, Mosha F, Donnelly MJ. 2016. A significant association between deltamethrin resistance, Plasmodium falciparum infection and the Vgsc-1014S resistance mutation in Anopheles gambiae highlights the epidemiological importance of resistance markers. Malar. J. 15, 289 ( 10.1186/s12936-016-1331-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berticat C, Dubois MP, Marquine M, Chevillon C, Raymond M. 2000. A molecular test to identify resistance alleles at the amplified esterase locus in the mosquito Culex pipiens. Pest Manag. Sci. 56, 727–731. () [DOI] [Google Scholar]
- 26.Weill M, et al. 2004. Insecticide resistance: a silent base prediction. Curr. Biol. 14, R552–R553. ( 10.1016/j.cub.2004.07.008) [DOI] [PubMed] [Google Scholar]
- 27.Ndiaye EH, et al. 2016. Vector competence of Aedes vexans (Meigen), Culex poicilipes (Theobald) and Cx. quinquefasciatus Say from Senegal for West and East African lineages of Rift Valley Fever virus. Parasit. Vectors 9, 94 ( 10.1186/s13071-016-1383-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murgue B, Murri S, Zientara S, Durand B, Durand JP, Zeller H. 2001. West Nile outbreak in horses in southern France, 2000: the return after 35 years. Emerg. Infect. Dis. 7, 692–696. ( 10.3201/eid0704.017417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux AB. 2009. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One 4, e5895 ( 10.1371/journal.pone.0005895) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 31.Fox J, Weisberg S.. 2011. An R companion to applied regression (ed. London S.), p. 449 Thousand Oaks, CA: SAGE Publications. [Google Scholar]
- 32.Lenth RV. 2016. Least-squares means: the R Package lsmeans. J. Stat. Softw. 69, 1–33. ( 10.18637/jss.v069.i01) [DOI] [Google Scholar]
- 33.Ciota AT. 2017. West Nile virus and its vectors. Curr. Opin. Insect Sci. 22, 28–36. ( 10.1016/j.cois.2017.05.002) [DOI] [PubMed] [Google Scholar]
- 34.Turell MJ, Wilson WC, Bennett KE. 2010. Potential for North American mosquitoes (Diptera: Culicidae) to transmit Rift Valley Fever virus. J. Med. Entomol. 47, 884–889. ( 10.1093/jmedent/47.5.884) [DOI] [PubMed] [Google Scholar]
- 35.Balenghien T, et al. 2013. Towards a better understanding of Rift Valley Fever epidemiology in the south-west of the Indian Ocean. Vet. Res. 44, 78 ( 10.1186/1297-9716-44-78) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moutailler S, Krida G, Schaffner F, Vazeille M, Failloux AB. 2008. Potential vectors of Rift Valley Fever virus in the Mediterranean region. Vector Borne Zoonotic Dis. 8, 749–753. ( 10.1089/vbz.2008.0009) [DOI] [PubMed] [Google Scholar]
- 37.Franz AW, Kantor AM, Passarelli AL, Clem RJ. 2015. Tissue barriers to arbovirus infection in mosquitoes. Viruses 7, 3741–3767. ( 10.3390/v7072795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turell MJ, Dohm DJ, Mores CN, Terracina L, Wallette DL, Hribar LJ, Pecor JE, Blow JA. 2008. Potential for North American mosquitoes to transmit Rift Valley Fever virus. J. Am. Mosq. Control Assoc. 24, 502–507. ( 10.2987/08-5791.1) [DOI] [PubMed] [Google Scholar]
- 39.Moutailler S, Krida G, Madec Y, Bouloy M, Failloux AB. 2010. Replication of Clone 13, a naturally attenuated avirulent isolate of Rift Valley Fever virus, in Aedes and Culex mosquitoes. Vector Borne Zoonotic Dis. 10, 681–688. ( 10.1089/vbz.2009.0246) [DOI] [PubMed] [Google Scholar]
- 40.Lo TM, Coetzee M. 2013. Marked biological differences between insecticide resistant and susceptible strains of Anopheles funestus infected with the murine parasite Plasmodium berghei. Parasit. Vectors 6, 184 ( 10.1186/1756-3305-6-184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vezilier J, Nicot A, Gandon S, Rivero A. 2010. Insecticide resistance and malaria transmission: infection rate and oocyst burden in Culex pipiens mosquitoes infected with Plasmodium relictum. Malar. J. 9, 379 ( 10.1186/1475-2875-9-379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zele F, Vezilier J, L'Ambert G, Nicot A, Gandon S, Rivero A, Duron O. 2014. Dynamics of prevalence and diversity of avian malaria infections in wild Culex pipiens mosquitoes: the effects of Wolbachia, filarial nematodes and insecticide resistance. Parasit. Vectors 7, 437 ( 10.1186/1756-3305-7-437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitri C, et al. 2015. The kdr-bearing haplotype and susceptibility to Plasmodium falciparum in Anopheles gambiae: genetic correlation and functional testing. Malar. J. 14, 391 ( 10.1186/s12936-015-0924-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivero A, Magaud A, Nicot A, Vezilier J. 2011. Energetic cost of insecticide resistance in Culex pipiens mosquitoes. J. Med. Entomol. 48, 694–700. ( 10.1603/ME10121) [DOI] [PubMed] [Google Scholar]
- 45.Molina-Cruz A, DeJong RJ, Charles B, Gupta L, Kumar S, Jaramillo-Gutierrez G, Barillas-Mury C. 2008. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J. Biol. Chem. 283, 3217–3223. ( 10.1074/jbc.M705873200) [DOI] [PubMed] [Google Scholar]
- 46.Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z. 2012. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl Acad. Sci. USA 109, E23–E31. ( 10.1073/pnas.1116932108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vontas J, Blass C, Koutsos AC, David JP, Kafatos FC, Louis C, Hemingway J, Christophides GK, Ranson H, 2005. Gene expression in insecticide resistant and susceptible Anopheles gambiae strains constitutively or after insecticide exposure. Insect. Mol. Biol. 14, 509–521. ( 10.1111/j.1365-2583.2005.00582.x) [DOI] [PubMed] [Google Scholar]
- 48.Vontas J, David JP, Nikou D, Hemingway J, Christophides GK, Louis C, Ranson H. 2007. Transcriptional analysis of insecticide resistance in Anopheles stephensi using cross-species microarray hybridization. Insect. Mol. Biol. 16, 315–324. ( 10.1111/j.1365-2583.2007.00728.x) [DOI] [PubMed] [Google Scholar]
- 49.Vizioli J, et al. 2000. Cloning and analysis of a cecropin gene from the malaria vector mosquito, Anopheles gambiae. Insect. Mol. Biol. 9, 75–84. ( 10.1046/j.1365-2583.2000.00164.x) [DOI] [PubMed] [Google Scholar]
- 50.Xi Z, Ramirez JL, Dimopoulos G. 2008. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 4, e1000098 ( 10.1371/journal.ppat.1000098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hegde S, Rasgon JL, Hughes GL. 2015. The microbiome modulates arbovirus transmission in mosquitoes. Curr. Opin. Virol. 15, 97–102. ( 10.1016/j.coviro.2015.08.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.